Abstract
Objective
Arterial stiffness may be an intermediary biological pathway involved in the association between cardiovascular health (CVH) and cardiovascular disease. We aimed to evaluate the effect of CVH on progression of brachial–ankle pulse wave velocity (baPWV) over approximately 4 years.
Methods
We included 1315 cardiovascular disease-free adults (49±12 years) who had two checkups from 2010 to 2019. CVH metrics (current smoking, body mass index, total cholesterol, blood pressure, and fasting plasma glucose) were assessed at baseline, and the number of ideal CVH metrics and CVH score were calculated. Additionally, baPWV was examined at baseline and follow-up.
Results
Median baPWV increased from 1340 cm/s to 1400 cm/s, with an average annual change in baPWV of 15 cm/s. More ideal CVH metrics and a higher CVH score were associated with lower baseline and follow-up baPWV, and the annual change in baPWV, even after adjustment for confounding variables. Associations between CVH parameters and baseline and follow-up baPWV remained robust in different sex and age subgroups, but they were only able to predict the annual change in baPWV in men and individuals older than 50 years.
Conclusions
Our findings highlight the benefit of a better baseline CVH profile for progression of arterial stiffness.
Keywords: Cardiovascular health, pulse wave velocity, arterial stiffness, risk factor, smoking, body mass index, blood pressure, cholesterol, fasting plasma glucose
Introduction
Cardiovascular disease (CVD), including coronary heart disease and cerebrovascular disease, is the leading cause of death in China. Additionally, China is one of the countries that have the highest burden of CVD internationally.1,2 The CVD epidemic can be curtailed by identification and management of risk factors for CVD. Communicating this risk in a more understandable and easier manner will have a greater emotional impact and be able to motivate a population to make lifestyle changes.3 To reduce CVD mortality and the prevalence of risk factors, the American Heart Association proposed a simple and useful concept called cardiovascular health (CVH).4 This new concept developed definitions of “ideal”, “intermediate”, and “poor” CVH on the basis of seven health behaviors (current smoking, body mass index [BMI], physical activity, and healthy diet score) and health factors (total cholesterol, blood pressure, and fasting plasma glucose[FPG]).4 Previous evidence has suggested that the presence of more ideal CVH metrics is associated with a lower incidence and mortality of CVD,5–10 but the mechanisms underlying this association warrant investigation.
Vascular structure and function become damaged over time. Vascular aging provides a more comprehensive explanation for development of CVD events, especially for those with little atherosclerosis.11 Arterial stiffness is a major age-related, arterial, degenerative phenotype and is considered a physiological method for quantifying vascular aging.12–15 Accumulating studies have shown that arterial stiffness is an independent predictor for CVD events and deaths.16,17 Therefore, arterial stiffness has been proposed as a surrogate endpoint for CVD. We hypothesized that the protective effects of CVH on the incidence and mortality of CVD are mediated by the favorable effects of CVH on arterial stiffness.
Many studies have reported the association between CVD risk factors and arterial stiffness, but evidence of their joint effect on arterial stiffness is lacking, particularly in longitudinal studies. Limited prospective studies have investigated the association between CVH and arterial stiffness.18–23 However, these studies were based on measurement of arterial stiffness at a single time point at follow-up and ignored the change in arterial stiffness during the study period. Therefore, this study aimed to examine the predictive value of the baseline CVH profile in relation to the progression rate of arterial stiffness over 4.2 years of follow-up.
Patients and methods
Subjects
This retrospective, longitudinal study was conducted at the physical examination center of the Geriatric Department of Tongji Hospital and was approved by the medical ethics committee of Tongji Hospital (TJ-IRB20190410). We screened 21,627 brachial–ankle pulse wave velocity (baPWV) database records from November 2010 to October 2019. A total of 1564 individuals had a second baPWV measurement after a delay of more than 3 years, and their second baPWV measurement depended on their own personal arrangements. The exclusion criteria were as follows: age younger than 18 years, coronary disease (n = 60), stroke (n = 4), obvious arrhythmia (persistent atrial fibrillation, frequent premature beats, or wearing a pacemaker) (n = 33), cardiomyopathy (n = 2), valvular heart disease, chronic liver or kidney disease (n = 8), cancer (n = 36), ankle–brachial index < 0.9 (n = 3), and missing data (n = 103). Verbal informed consent was obtained from all participants.
Baseline clinical characteristics
When the subjects visited the physical examination center, trained personnel conducted standardized in-person interviews with the subjects to collect information regarding age, sex, current smoking and alcohol drinking status, medical history, and medication use. Anthropometric indices, including height and weight, were measured. BMI was computed as the weight in kilograms divided by the square of the height in meters. Blood pressure and heart rate were measured using an Omron sphygmomanometer (Omron Corporation, Kyoto, Japan). Systolic blood pressure, diastolic blood pressure, and heart rate used in the analysis were calculated as the average of three measured values. Fasting venous blood samples were collected and sent to the hospital’s clinical chemistry laboratory. Total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), FPG, glycated hemoglobin (HbA1c), creatinine, and uric acid levels were measured using standard certified assays.
Number of ideal CVH metrics and CVH score at baseline
In the present study, we included five CVH metrics (current smoking, BMI, total cholesterol, blood pressure, and FPG). Each metric was categorized as ideal, intermediate, or poor according to American Heart Association criteria (Table 1). For each participant, the number of ideal CVH metrics was calculated (total scale: 0–5 points). We also constructed a CVH score on the basis of the five CVH metrics (poor = 0 points; intermediate = 1 point; ideal = 2 points; total scale: 0–10 points).5,24
Table 1.
Distribution of individual baseline CVH metrics.
| Metrics | Definition* | Total sample (n = 1315) |
|---|---|---|
| Current smoking | ||
| Ideal | Never or quit for > 12 months | 943 (71.7) |
| Intermediate | Former for ≤ 12 months | 48 (3.7) |
| Poor | Yes | 324 (24.6) |
| BMI (kg/m2) | ||
| Ideal | < 25 | 716 (54.4) |
| Intermediate | 25–29.9 | 551 (41.9) |
| Poor | ≥ 30 | 48 (3.7) |
| Total cholesterol (mmol/L) | ||
| Ideal | < 5.18 | 893 (67.9) |
| Intermediate | 5.18–6.20 or treated to goal | 345 (26.2) |
| Poor | ≥ 6.21 | 77 (5.9) |
| Blood pressure | ||
| Ideal | < 120/< 80 mmHg | 511 (38.9) |
| Intermediate | SBP of 120–139, DBP of 80–89 mmHg, or treated to goal | 447 (34.0) |
| Poor | SBP ≥ 140 or DBP ≥ 90 mmHg | 357 (27.1) |
| FPG (mmol/L) | ||
| Ideal | < 5.56 | 1032 (78.5) |
| Intermediate | 5.56–6.99 or treated to goal | 215 (16.3) |
| Poor | ≥ 7.00 | 68 (5.2) |
Data are shown as number (%).
CVH, cardiovascular health; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting plasma glucose.
*According to reference 4.
Arterial stiffness at baseline and follow-up
The ankle–brachial index and baPWV were measured using the Vascular Profiler BP-203RPEIII system (Omron Corporation). Trained technicians placed the pressure cuffs on the subjects (i.e., one on the upper part of each arm and one on each ankle). The subjects were then examined after 10 minutes of rest in the supine position. The device simultaneously recorded the bilateral pulse waves of the brachial and posterior tibial arteries using an oscillometric method. baPWV was calculated as the ratio of the traveled distance, which was automatically estimated from the body height divided by the transit time of the pulse wave between the brachial and posterior tibial arteries. The annual change in baPWV was calculated by dividing the difference between baseline and follow-up baPWV in cm/s by the interval time in years.
Statistical analysis
Data were analyzed using R version 3.6.2 and R Studio version 1.2.5033 (https://www.R-project.org/). Continuous variables are presented as the mean ± standard deviation or median (interquartile range), as appropriate for the distribution. Categorical variables are shown as counts (%). Log-transformation was conducted to achieve normality. We compared the baseline variables between groups using analysis of variance or the chi-square test accordingly. We used univariate linear regression or the Mantel–Haenszel test to calculate the p value for trend. Paired t-tests were used to determine if baPWV changed over the follow-up period. Crude and multivariable linear regression models were developed to estimate βs and 95% confidence intervals (CIs) for baseline baPWV, follow-up baPWV, and the annual change in baPWV associated with CVH. Age and sex were controlled for in model 1. Further adjustments were made for heart rate, LDL-C, HDL-C, triglycerides, HbA1c, current alcohol drinking status, creatinine, and uric acid in model 2. Baseline baPWV was adjusted in all models using the annual change in baPWV as the dependent variable. We conducted subgroup analyses to examine whether the effects of CVH differed with sex (male vs female) or age (< 50 years vs > 50 years). Two-tailed p values < 0.05 were considered significant.
Results
Baseline characteristics and the CVH profile
A total of 1315 subjects were included in this study. The proportions of participants in the total sample who had ideal levels of individual CVH metrics at baseline were as follows: current smoking, 71.7%; BMI, 54.4%; total cholesterol, 67.9%; blood pressure, 38.9%; and FPG, 78.5% (Table 1). Among the total sample, 194 (14.8%) subjects had five ideal CVH metrics (Figure 1). The median number of ideal CVH metrics was 3 points and the median CVH score was 8 points.
Figure 1.
Histograms of the number of ideal CVH metrics and the CVH score at baseline.
CVH, cardiovascular health.
Table 2 shows the baseline demographic and metabolic characteristics of the 1315 participants free of CVD, stratified by the number of ideal CVH metrics. The subjects’ mean age was 49 ± 12 years, and 1005 (76.4%) were men and 310 (23.6%) were women. The presence of more ideal CVH metrics was significantly associated with a younger age, female sex, non-smokers, non-alcohol drinkers, lower BMI, blood pressure, heart rate, total cholesterol levels, LDL-C levels, triglyceride levels, FBG levels, HbA1c levels, creatinine levels, and uric acid levels, and higher HDL-C levels (all p < 0.001). Therefore, CVD risk factors (older age, obesity, smoking, high blood pressure, dyslipidemia, and elevated glucose) were clustered together in subjects with less ideal CVH metrics.
Table 2.
Baseline characteristics and progression of arterial stiffness of the study sample in relation to CVH categories.
| Variables | All participants (n = 1315) |
Number of ideal CVH metrics |
p | p for trend | ||
|---|---|---|---|---|---|---|
| Low, 0–1 (n = 138) | Medium, 2–3 (n = 655) | High, 4–5 (n = 522) | ||||
| Age, years | 49 ± 12 | 52 ± 9 | 51 ± 11 | 46 ± 12 | <0.001 | <0.001 |
| Male sex | 1005 (76.4%) | 134 (97.1%) | 572 (87.3%) | 299 (57.3%) | <0.001 | <0.001 |
| BMI, kg/m2 | 24.6 ± 3.0 | 27.4 ± 2.1 | 25.6 ± 2.6 | 22.5 ± 2.4 | <0.001 | <0.001 |
| SBP, mmHg | 123 ± 15 | 131 ± 12 | 128 ± 14 | 114 ± 13 | <0.001 | <0.001 |
| DBP, mmHg | 75± 11 | 82 ± 9 | 79 ± 9 | 69 ± 10 | <0.001 | <0.001 |
| Heart rate, beats/minute | 67 ± 10 | 69 ± 10 | 67 ± 10 | 66 ± 10 | 0.03 | 0.02 |
| Hypertension | 438 (33.3%) | 80 (58.0%) | 296 (45.2%) | 62 (11.9%) | <0.001 | <0.001 |
| Antihypertensive medication | 303 (23.0%) | 58 (42.0%) | 206 (31.5%) | 39 (7.5%) | <0.001 | <0.001 |
| Total cholesterol, mmol/L | 4.71 ± 0.86 | 5.18 ± 0.97 | 4.82 ± 0.87 | 4.45 ± 0.71 | <0.001 | <0.001 |
| LDL-C, mmol/L | 2.86 ± 0.73 | 3.08 ± 0.85 | 2.95 ± 0.75 | 2.69 ± 0.63 | <0.001 | <0.001 |
| HDL-C, mmol/L | 1.23 ± 0.30 | 1.12 ± 0.23 | 1.17 ± 0.28 | 1.32 ± 0.31 | <0.001 | <0.001 |
| Triglycerides, mmol/L | 1.30 (1.01) | 2.06 (1.47) | 1.49 (1.08) | 0.98 (0.65) | <0.001 | <0.001 |
| Lipid-lowering medication | 66 (5.0%) | 19 (13.8%) | 45 (6.9%) | 2 (0.4%) | <0.001 | <0.001 |
| FPG, mmol/L | 5.18 ± 0.91 | 6.26 ± 1.45 | 5.19 ± 0.75 | 4.89 ± 0.66 | <0.001 | <0.001 |
| HbA1c, % | 5.7 ± 0.5 | 6.2 ± 0.8 | 5.7 ± 0.4 | 5.5 ± 0.4 | <0.001 | <0.001 |
| Diabetes mellitus | 108 (8.2%) | 50 (36.2%) | 53 (8.1%) | 5 (1.0%) | <0.001 | <0.001 |
| Diabetes medication | 47 (3.6%) | 27 (19.6%) | 19 (2.9%) | 1 (0.2%) | <0.001 | <0.001 |
| Current smoking | <0.001 | <0.001 | ||||
| Yes | 324 (24.6%) | 84 (60.9%) | 197 (30.1%) | 43 (8.2%) | ||
| Former for ≤ 12 months | 48 (3.7%) | 13 (9.4%) | 24 (3.7%) | 11 (2.1%) | ||
| Never or quit for >12 months | 943 (71.7%) | 41 (29.7%) | 434 (66.3%) | 468 (89.7%) | ||
| Current alcohol drinking | <0.001 | <0.001 | ||||
| Yes | 247 (18.8%) | 57 (41.3%) | 158 (24.1%) | 32 (6.1%) | ||
| Former for ≤ 12 months | 400 (30.4%) | 41 (29.7%) | 210 (32.1%) | 149 (28.5%) | ||
| Never or quit for >12 months | 668 (50.8%) | 40 (29.0%) | 287 (43.8%) | 341 (65.3%) | ||
| Creatinine, µmol/L | 75.6 ± 15.2 | 77.2 ± 11.7 | 79.0 ± 14.8 | 70.9 ± 15.4 | <0.001 | <0.001 |
| Uric acid, µmol/L | 357 ± 90 | 398 ± 86 | 380 ± 85 | 317 ± 81 | <0.001 | <0.001 |
| Number of ideal CVH metrics | 3 (2) | 1 (0) | 3 (1) | 4 (1) | <0.001 | <0.001 |
| CVH score | 8 (3) | 4 (2) | 7 (2) | 9 (1) | <0.001 | <0.001 |
| ABI | 1.11 ± 0.08 | 1.12 ± 0.08 | 1.12 ± 0.08 | 1.09 ± 0.08 | <0.001 | <0.001 |
| Baseline baPWV, cm/s | 1340 (321) | 1430 (214) | 1390 (322) | 1220 (242) | <0.001 | <0.001 |
| Follow-up baPWV, cm/s | 1400 (336) | 1500 (319) | 1470 (352) | 1290 (274) | <0.001 | <0.001 |
| Annual change in baPWV, cm/s per year | 15.0 (43.3) | 20.4 (50.5) | 15.0 (45.9) | 14.5 (39.5) | 0.17 | 0.93 |
Data are mean ± standard deviation, median (interquartile range), or number (%).
CVH, cardiovascular health; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; ABI, ankle–brachial index; baPWV, brachial–ankle pulse wave velocity.
Progression of arterial stiffness over the follow-up period
The median duration of follow-up was 4.2 years (interquartile range: 2.8 years). There was a significant increase in arterial stiffness, as evaluated by baPWV from baseline to follow-up (p < 0.001), with an annual change in baPWV of 15 cm/s (Table 2). Participants with four to five ideal CVH metrics had significantly lower baseline and follow-up baPWV compared with those with a low or medium amount of ideal CVH metrics (both p < 0.001, Figure 2). The annual change in baPWV also appeared to be lower in individuals with a high number of ideal CVH metrics, but this was not significant. Although grouping may help data presentation in tables, categorization leads to loss of information and the statistical power to detect a relation between variables is reduced.25
Figure 2.
Boxplots of baseline baPWV, follow-up baPWV, and the annual change in baPWV in relation to baseline CVH categories. Horizontal lines indicate the median value and boxes contain 50% of the data.
CVH, cardiovascular health; baPWV, brachial–ankle pulse wave velocity.
Association between CVH and progression of arterial stiffness
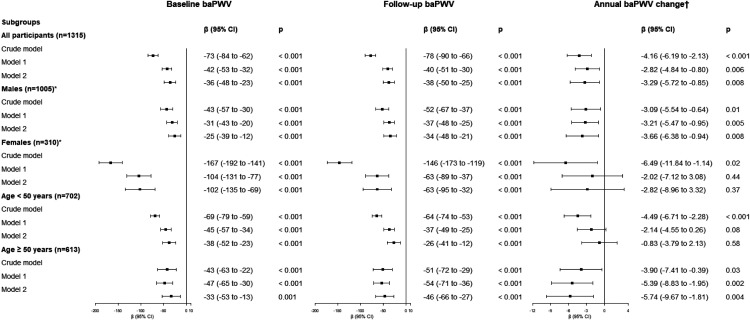
Figures 3 and 4 present the results of crude and multiple linear regression analyses using the number of ideal CVH metrics or the CVH score as the independent variable, respectively. The presence of more ideal CVH metrics and a higher CVH score were independently associated with lower baseline baPWV, follow-up baPWV, and the annual change in baPWV. Every additional ideal CVH metric corresponded to a 36 cm/s (95% CI: 23–48; p < 0.001) decrease in baseline baPWV, a 38 cm/s (95% CI: 25–50; p < 0.001) decrease in follow-up baPWV, and a 3.29 cm/s (95% CI: 0.85–5.72; p = 0.008) decrease in the annual change in baPWV, after adjustment for all confounding variables. Every increase in the CVH score by 1 point corresponded to a 35 cm/s (95% CI: 26–43; p < 0.001) decrease in baseline baPWV, a 31 cm/s (95% CI: 23–39; p < 0.001) decrease in follow-up baPWV, and a 2.13 cm/s (95% CI: 0.46–3.80; p = 0.01) decrease in the annual change in baPWV, after adjustment for all confounding variables.
Figure 3.
Associations between the baseline number of ideal cardiovascular health metrics and progression of arterial stiffness in different study population subgroups. β represents the change in the dependent variable (cm/s for baseline baPWV and follow-up baPWV, or cm/s/year for the annual change in baPWV) for a 1 point increase in the baseline number of ideal cardiovascular health metrics. Model 1: adjusted for baseline age and sex; model 2: adjusted for baseline age, sex, heart rate, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, glycated hemoglobin, current alcohol drinking status, creatinine, and uric acid. *Adjusted for the same independent parameters as those in models 1 and 2, except for sex. †Adjusted for baseline baPWV plus the same independent parameters as those in models 1 and 2
baPWV, brachial–ankle pulse wave velocity; CI, confidence interval.
Figure 4.
Associations between the baseline CVH score and progression of arterial stiffness in different study population subgroups. β represents the change in the dependent variable (cm/s for baseline baPWV and follow-up baPWV, or cm/s/year for the annual change in baPWV) for a 1 point increase in the CVH score. Model 1: adjusted for baseline age and sex; model 2: adjusted for baseline age, sex, heart rate, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, glycated hemoglobin, current alcohol drinking status, creatinine, and uric acid. *Adjusted for the same independent parameters as those in models 1 and 2, except for sex. †Adjusted for baseline baPWV plus the same independent parameters as those in models 1 and 2 baPWV, brachial–ankle pulse wave velocity; CI, confidence interval.
In subgroup analysis, the effects of the number of ideal CVH metrics and the CVH score on arterial stiffness were similar (Figures 3 and 4). The associations between CVH parameters and baseline and follow-up baPWV remained robust in different sex and age subgroups. However, in subgroup analysis, CVH parameters were only able to predict the annual change in baPWV change in men and individuals older than 50 years, after adjustment for all confounding variables (all p < 0.05).
Discussion
Arterial aging-associated structural and functional changes are accelerated by cardiovascular risk factors, such as adiposity, hypertension, hyperlipidemia, and diabetes.26 Arterial stiffness is considered a cumulative measure of the damaging effects of these risk factors on the arterial wall with aging.27 We confirmed the joint effect of cardiovascular risk factors on arterial stiffness in cross-sectional and longitudinal analysis. The presence of more ideal CVH metrics and a higher CVH score at baseline were not only associated with baseline baPWV in cross-sectional analysis, but also independently predicted follow-up baPWV and the annual change in baPWV. The significant and inverse relationship between CVH status and arterial stiffness remained robust in different subgroups, especially in men and individuals older than 50 years. These findings may provide some evidence of the intermediary biological pathways through which ideal CVH results in a lower incidence and mortality of CVD.
In line with our study, three other cross-sectional studies also showed that ideal CVH had an independent favorable effect on vascular elasticity, as measured by baPWV or carotid–femoral pulse wave velocity (cfPWV) in Chinese, Spanish, and Australian adults.28–30 Furthermore, each additional point in the ideal CVH score was also associated with slower cfPWV in a population of Australian children aged 11 to 12 years.30 This previous finding suggested the importance of interventions to improve CVH behavior and factors at a young age.
Although previous longitudinal studies examined the relationship between CVH and arterial stiffness, they only had one measurement of arterial stiffness at follow-up and lacked baseline baPWV values.18–23 Therefore, these studies may have been insufficient to accurately assess the change in arterial stiffness. An increasing number of ideal CVH metrics at baseline were significantly associated with decreased follow-up cfPWV in American adolescents with type 1 diabetes and American adults in the SEARCH CVD study19 and the Maine–Syracuse Longitudinal Study.18 This finding is in concordance with our study, which showed that a higher baseline number of ideal CVH metrics and CVH score independently predicted a lower baPWV after 4.2 years. Additionally, this relationship remained robust across all different sex and age subgroups. Previous studies aimed to examine the association between long-term patterns in the CVH trajectory and follow-up arterial stiffness.20–23 These studies showed that attainment of ideal CVH typically declined with age, and higher long-term attainment of ideal CVH and improvement in the CVH profile throughout life were associated with low baPWV or cfPWV at follow-up.
None of the above-mentioned studies took into account the long-term change in arterial stiffness. In the current study, we calculated the annual change in baPWV to overcome the limitations of previous studies. We found that the presence of more ideal CVH metrics predicted a lower progression rate of baPWV in men and individuals older than 50 years, even after adjustment for all confounding factors. This conclusion is consistent with two other Chinese studies, and both of them only included middle-aged and elderly adults.31,32 In contrast to our findings, Wang et al.32 showed that the dose–response effect of ideal CVH on elevated baPWV was attenuated in men and elderly individuals in subgroup analysis. Therefore, because of this discrepancy between studies, further studies are required.
Some limitations of our study warrant consideration. First, the proposed CVH metrics include seven components. However, data on physical activity and diet were not available in the present study, and this might have decreased the external validity of the study. A similar limitation can be found in other studies.33,34 However, notably, physical activity and diet pattern might also have had effects on progression of arterial stiffness.35–38 This might lead to underestimation of the effect of CVH on arterial stiffness. Second, we only included baseline CVH status. Therefore, we were not able to quantify the effects of changes in the CVH profile on arterial stiffness. Additionally, selection bias might have occurred because whether individuals came for a follow-up visit and had a second baPWV measurement depended on their own personal arrangements. Finally, this study was conducted at a single center, resulting in a sample that comprised more men than women. Further large, community-based research is still required.
In conclusion, our study shows that there is a significant and inverse association between baseline CVH status and progression of arterial stiffness in 4.2 years, especially in men and individuals older than 50 years. This finding indicates that optimizing CVH metrics could delay the onset and progression of arterial stiffness and it might be a potential intervention to reduce the burden of CVD. Assessment of the CVH profile at the population level should be advocated in China.
Acknowledgements
We greatly acknowledge the assistance of physicians and nurses at our physical examination center.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Major Technology Innovation of Hubei Province (Program No. 2019ACA141).
ORCID iD: Yu Sang https://orcid.org/0000-0001-5895-4585
References
- 1.Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol 2019; 16: 203–212. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Nguyen G, Cercy K, et al. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N Engl J Med 2018; 379: 2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Gonzalez AA, Aguilo A, Frontera M, et al. Effectiveness of the Heart Age tool for improving modifiable cardiovascular risk factors in a Southern European population: a randomized trial. Eur J Prev Cardiol 2015; 22: 389–396. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 2010; 121: 586–613. [DOI] [PubMed] [Google Scholar]
- 5.Kulshreshtha A, Vaccarino V, Judd SE, et al. Life's Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke 2013; 44: 1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA 2012; 307: 1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong C, Rundek T, Wright CB, et al. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation 2012; 125: 2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xanthakis V, Enserro DM, Murabito JM, et al. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation 2014; 130: 1676–1683. [DOI] [PubMed] [Google Scholar]
- 9.Ying Y, Lin S, Kong F, et al. Ideal Cardiovascular Health Metrics and Incidence of Ischemic Stroke Among Hypertensive Patients: A Prospective Cohort Study. Front Cardiovasc Med 2020; 7: 590809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isiozor NM, Kunutsor SK, Voutilainen A, et al. American heart association’s cardiovascular health metrics and risk of cardiovascular disease mortality among a middle-aged male Scandinavian population. Ann Med 2019; 51: 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Rourke MF, Safar ME, Dzau V. The Cardiovascular Continuum extended: aging effects on the aorta and microvasculature. Vasc Med 2010; 15: 461–468. [DOI] [PubMed] [Google Scholar]
- 12.Bhuva AN, D'Silva A, Torlasco C, et al. Training for a First-Time Marathon Reverses Age-Related Aortic Stiffening. J Am Coll Cardiol 2020; 75: 60–71. [DOI] [PubMed] [Google Scholar]
- 13.Niiranen TJ, Lyass A, Larson MG, et al. Prevalence, Correlates, and Prognosis of Healthy Vascular Aging in a Western Community-Dwelling Cohort: The Framingham Heart Study. Hypertension 2017; 70: 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sang Y, Wu X, Miao J, et al. Determinants of Brachial-Ankle Pulse Wave Velocity and Vascular Aging in Healthy Older Subjects. Med Sci Monit 2020; 26: e923112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Sanchez M, Gomez-Sanchez L, Patino-Alonso MC, et al. Vascular aging and its relationship with lifestyles and other risk factors in the general Spanish population: Early Vascular Ageing Study. J Hypertens 2020; 38: 1110–1122. [DOI] [PubMed] [Google Scholar]
- 16.Ato D. Brachial-ankle pulse wave velocity, cardio-ankle vascular index, and prognosis. Vasc Health Risk Manag 2018; 14: 321–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohkuma T, Ninomiya T, Tomiyama H, et al. Brachial-Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular Disease: An Individual Participant Data Meta-Analysis. Hypertension 2017; 69: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 18.Crichton GE, Elias MF, Robbins MA. Cardiovascular health and arterial stiffness: the Maine-Syracuse Longitudinal Study. J Hum Hypertens 2014; 28: 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alman AC, Talton JW, Wadwa RP, et al. Cardiovascular health in adolescents with type 1 diabetes: the SEARCH CVD study. Pediatr Diabetes 2014; 15: 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aatola H, Hutri-Kähönen N, Juonala M, et al. Prospective relationship of change in ideal cardiovascular health status and arterial stiffness: the Cardiovascular Risk in Young Finns Study. J Am Heart Assoc 2014; 3: e000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah AM, Claggett B, Folsom AR, et al. Ideal Cardiovascular Health During Adult Life and Cardiovascular Structure and Function Among the Elderly. Circulation 2015; 132: 1979–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng X, Zhang R, Liu X, et al. Association between cumulative exposure to ideal cardiovascular health and arterial stiffness. Atherosclerosis 2017; 260: 56–62. [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, Xie J, Yang R, et al. Association between ideal cardiovascular health score trajectories and arterial stiffness: the Kailuan Study. Hypertens Res 2020; 43: 140–147. [DOI] [PubMed] [Google Scholar]
- 24.Huffman MD, Capewell S, Ning H, et al. Cardiovascular health behavior and health factor changes (1988-2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation 2012; 125: 2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006; 332: 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kucharska-Newton AM, Stoner L, Meyer ML. Determinants of Vascular Age: An Epidemiological Perspective. Clin Chem 2019; 65: 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: A tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension 2009; 54: 3–10. [DOI] [PubMed] [Google Scholar]
- 28.Yan N, Zhou Y, Wang Y, et al. Association of Ideal Cardiovascular Health and Brachial-Ankle Pulse Wave Velocity: A Cross-Sectional Study in Northern China. J Stroke Cerebrovasc Dis 2016; 25: 41–48. [DOI] [PubMed] [Google Scholar]
- 29.García-Hermoso A, Martínez-Vizcaíno V, Gomez-Marcos M, et al. Ideal Cardiovascular Health and Arterial Stiffness in Spanish Adults-The EVIDENT Study. J Stroke Cerebrovasc Dis 2018; 27: 1386–1394. [DOI] [PubMed] [Google Scholar]
- 30.Liu RS, Wake M, Grobler A, et al. Cross-sectional associations between Ideal Cardiovascular Health scores and vascular phenotypes in 11- to 12-year-olds and their parents: The Longitudinal Study of Australian Children. Int J Cardiol 2019; 277: 258–265. [DOI] [PubMed] [Google Scholar]
- 31.Gao J, Bao M, Liu Y, et al. Changes in cardiovascular health score and atherosclerosis progression in middle-aged and older persons in China: a cohort study. BMJ Open 2015; 5: e007547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Niu JY, Zhao ZY, et al. Ideal Cardiovascular Health is Inversely Associated with Subclinical Atherosclerosis: A Prospective Analysis. Biomed Environ Sci 2019; 32: 260–271. [DOI] [PubMed] [Google Scholar]
- 33.Wilsgaard T, Loehr LR, Mathiesen EB, et al. Cardiovascular health and the modifiable burden of incident myocardial infarction: the Tromsø Study. BMC Public Health 2015; 15: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao B, Wang F, Zhu M, et al. Cardiovascular health metrics and all-cause mortality and mortality from major non-communicable chronic diseases among Chinese adult population. Int J Cardiol 2020; 313: 123–128. [DOI] [PubMed] [Google Scholar]
- 35.Madden KM, Lockhart C, Cuff D, et al. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care 2009; 32: 1531–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aatola H, Koivistoinen T, Hutri-Kähönen N, et al. Lifetime fruit and vegetable consumption and arterial pulse wave velocity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation 2010; 122: 2521–2528. [DOI] [PubMed] [Google Scholar]
- 37.Cavero-Redondo I, Tudor-Locke C, Álvarez-Bueno C, et al. Steps per Day and Arterial Stiffness. Hypertension 2019; 73: 350–363. [DOI] [PubMed] [Google Scholar]
- 38.Jennings A, Berendsen AM, De Groot L, et al. Mediterranean-Style Diet Improves Systolic Blood Pressure and Arterial Stiffness in Older Adults. Hypertension 2019; 73: 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]