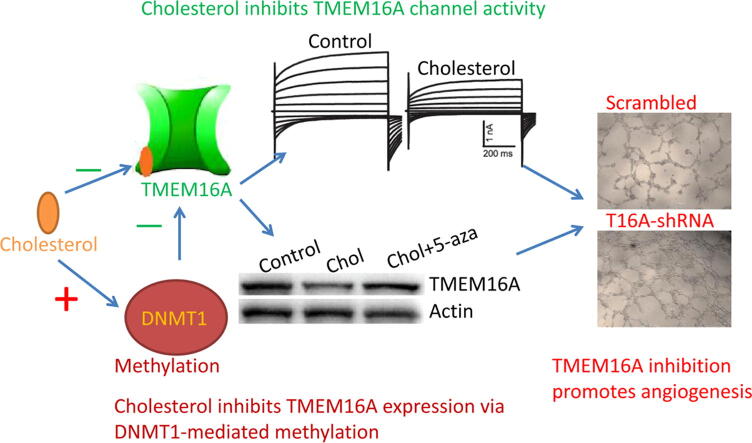
Graphical abstract
Cholesterol reduces TMEM16A expression via DNMT1-mediated promoter methylation and directly inhibits channel activities. TMEM16A inhibition promotes endothelial angiogenesis.
Keywords: TMEM16A, Cholesterol, Endothelial cells, Angiogenesis
Abbreviations: HAECs, human aortic endothelial cells; DNMT1, DNA methyltransferase 1; MβCD, methyl-β cyclodextrin; DMEM, Dulbecco’s Modified Eagle Medium; FBS, fetal bovine serum; shRNAs, short hairpin RNAs; HEPES, N-2-hydroxyethil-piperazine-N'-2-ethanesulfonic acid; EGTA, ethylene glycol-bis(2-aminoethyl ether)-N,N,N',N'-tetraacetic acid; NMDG, N-methyl-D-glucamine; RIPA, radio immunoprecipitation assay; PVDF, polyvinylidene fluoride; CCK-8, Cell Counting Kit-8; SE, standard error; ANOVA, analysis of variance; CaCCs, Ca2+-activated Cl− currents; 5-aza, 5-Aza-2′-deoxycytidine; ROS, reactive oxygen species
Abstract
Introduction
Ca2+-activated Cl− channel TMEM16A is expressed in endothelial cells, and contributes to many diseases such as hypertension, blood-brain barrier dysfunction, and pulmonary hypertension. It remains unclear whether TMEM16A regulates endothelial angiogenesis, which participates in many physiological and pathological processes. Cholesterol regulates many ion channels including TMEM16A, and high cholesterol levels contribute to endothelial dysfunction. It remains to be determined whether cholesterol regulates TMEM16A expression and function in endothelial cells.
Objective
This study aimed to investigate whether cholesterol regulated TMEM16A expression and function in endothelial angiogenesis.
Methods
Whole-cell patch clamp techniques were used to record Ca2+-activated Cl− currents in human aortic endothelial cells (HAECs) and HEK293 cells transfected with TMEM16A-overexpressing plasmids. Western blot was used to examine the expression of TMEM16A and DNA methyltransferase 1 (DNMT1) in HAECs. CCK-8 assay, would healing assay, and tube formation assay were used to test endothelial cell proliferation, migration and angiogenesis, respectively.
Results
TMEM16A mediates the Ca2+-activated Cl− channel in HAECs. Cholesterol treatment inhibited TMEM16A expression via upregulation of DNMT1 in HAECs, and the inhibitory effect of cholesterol on TMEM16A expression was blocked by 5-aza, the DNMT1 inhibitor. In addition, direct application of cholesterol inhibited TMEM16A currents in heterologous HEK293 cells with an IC50 of 0.1209 μM. Similarly, cholesterol directly inhibited TMEM16A currents in HAECs. Furthermore, TMEM16A knockdown increased in vitro tube formation, cell migration and proliferation of HAECs, and TMEM16A overexpression produced the opposite effect.
Conclusion
This study reveals a novel mechanism of cholesterol-mediated TMEM16A inhibition, by which cholesterol reduces TMEM16A expression via DNMT1-mediated methylation and directly inhibits channel activities. TMEM16A channel inhibition promotes endothelial cell angiogenesis.
Introduction
Vascular endothelial cells play a wide range of physiological functions including maintenance of vascular tone, involvement in blood coagulation, participation in inflammatory process, regulation of vascular permeability, and angiogenesis [1]. Endothelial dysfunction is often the initial event to trigger the development of atherosclerosis, which is a known risk factor for cardiovascular diseases [2]. In addition, endothelial dysfunction also contributes to the development of many cardiovascular diseases such as hypertension [3]. Therefore, maintenance of the normal endothelial cell function may exert protective effects against cardiovascular diseases.
Ion channels participate in many endothelial cell functions such as release of vasoactive substances, endocytosis and exocytosis of macromolecules, and proliferation and angiogenesis of endothelial cells [4]. Among various ion channels in endothelial cells, Ca2+-activated Cl− channel is expressed in endothelial cells [4], [5], and is implicated in regulating endothelial functions, including membrane potential control, Ca2+ signaling regulation, and cell proliferation [4], [6]. Since TMEM16A (anoctamin 1) was discovered as a Ca2+-activated Cl− channel in 2008 [7], [8], [9], it has been found in many endothelial cells [10], [11], [12], [13]. TMEM16A contributes to endothelial dysfunction in hypertension by promoting the generation of reactive oxygen species [10]. In addition, hypoxia induces TMEM16A expression in cardiac vascular endothelial cells [13], and TMEM16A overexpression contributes to increased blood–brain barrier permeability in ischemic stroke [12]. Furthermore, TMEM16A activation inhibits pulmonary endothelial cell proliferation by promoting P38-dependent apoptosis [11].
As a major lipid component in the plasma membrane, cholesterol is important for maintenance of membrane fluidity and compartment of lipid domains for signaling transduction at physiological levels, and contributes to normal endothelial cell function [14], [15]. It is well known that hypercholesterolemia is a major risk factor associated with endothelial dysfunction [16]. Cholesterol regulates a variety of ion channels via direct interaction with cholesterol binding motif in many ion channels [15]. Sones et al. reported that cholesterol inhibited TMEM16A Ca2+-activated Cl− currents in vascular myocytes [17]. Recently, De Jesus-Perez et al. found that removal of cholesterol with methyl-β cyclodextrin (MβCD) increased TMEM16A channel activities with decreased channel rundown [18]. However, it remains unclear whether cholesterol regulates TMEM16A expression and function in endothelial cells.
Angiogenesis is a process in which endothelial cells coordinate proliferation and migration to form new blood vessel. Although the role of TMEM16A on endothelial angiogenesis has not been studied in the literature, TMEM16A have been found to regulate proliferation and migration of cancer cells in a cell type-dependent manner [19], [20]. For example, TMEM16A overexpression promotes cell proliferation and migration in some cancers such as breast cancer [21], lung cancer [22] and hepatocellular carcinoma [23], whereas TMEM16A overexpression inhibits cell proliferation or migration in other cancer cells such as MDA-MB-435S cells [24] and UM-SCC1 cells [25]. In non-tumor cells, TMEM16A appears to inhibit cell proliferation. For example, TMEM16A activation inhibits proliferation in vascular smooth muscle cells following angiotensin II treatment [26], [27], and inhibits pulmonary endothelial cell proliferation [11]. It appears that TMEM16A exerts a cell-specific effect on cell proliferation and migration, depending on diverse cellular environments or different TMEM16A interactomes among different cells [19], [20]. Whether TMEM16A regulates endothelial cell proliferation, migration, and angiogenesis remains to be determined.
This study investigated the effect of cholesterol on TMEM16A in human aortic endothelial cells (HAECs). Cholesterol treatment downregulated TMEM16A expression by promoting DNA methyltransferase 1 (DNMT1)-mediated methylation of the TMEM16A gene. In addition, cholesterol also directly inhibited TMEM16A currents both in heterologous HEK293 cells overexpressing TMEM16A and in native endothelial cells. Furthermore, TMEM16A knockdown promoted proliferation, migration and tube formation of HAECs. Therefore, TMEM16A inhibition by cholesterol may be important for endothelial angiogenesis.
Materials and methods
Cell culture and drug treatments
HAECs were obtained from ScienCell, USA. HAECs were cultured in DMEM (Dulbecco’s Modified Eagle Medium, HyClone) supplemented with 10% FBS (fetal bovine serum, Biological Industries, Israel) and 1% penicillin–streptomycin in a humid incubator (37℃, 5% CO2). To examine the effect of cholesterol or MβCD on TMEM16A expression, HAECs were treated with water-soluble cholesterol (0–25 μM; Sigma-Aldrich) or MβCD (10 mM; Sigma-Aldrich) for 0–48 h.
Cell transfection
To identify whether TMEM16A mediated Ca2+-activated Cl− currents in HAECs, HAECs were transfected with scrambled shRNAs (short hairpin RNAs) and TMEM16A-shRNAs in the pGPU6-EGFP (enhanced green fluorescent protein) vector (Shanghai GenePharma, Shanghai, China). To investigate the effect of DNTM1 on TMEM16A expression and currents in HAECs, HAECs were transfected with empty vector or DNMT1-overexpressing plasmids (Biomedical Technology Laboratory, China). To demonstrate that cholesterol directly inhibited TMEM16A channels, HEK293 cells were transfected with control pEGFP-N1 vectors or the TMEM16A-containing plasmid in pEGFP-N1 vectors. Lipofectamine 2000 (Invitrogen, USA) was used for transfection. For patch clamp experiments, transfected cells were identified by green fluorescence under a fluorescence microscope.
Adenoviral infection
To test the effect of TMEM16A inhibition on angiogenic activities, HAECs were infected using adenovirus containing TMEM16A-shRNAs (Ad-TMEM16A shRNAs). Ad-TMEM16A shRNAs were constructed by Shanghai GenePharma (Shanghai, China) with the following sequence: 5′-TCACTAACTTGGTCTCCAT-3′. Cells were infected with Ad-TMEM16A shRNAs for 48 h, and cultured in the DMEM with 1 μg/ml puromycin before experimentation.
Patch clamp recordings
Whole-cell patch clamp technique was performed to record Ca2+-activated Cl− currents in HAECs. Recording pipettes with resistances of 2–4 MΩ were made using a Sutter P97 puller (Sutter Instruments, USA). Recordings were performed with an Axopatch 200B amplifier via a Digidata 1322A (Molecular Device, USA). Clampex 10 software was used for data acquisition. Cells were clamped at a holding potential of 0 mV, and currents were elicited by voltage steps (20 mV increment, 750-ms duration) from −100 to +100 mV. To record the effect of acute application of the TMEM16A inhibitors T16Ainh-A01 and CaCCinhh-A01 or cholesterol on Ca2+-activated Cl− currents, HAECs were clamped from ramps (750-ms duration, 10 s interval) from −100 to +100 mV. The external solution contained (in mM): 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES (N-2-hydroxyethil-piperazine-N'-2-ethanesulfonic acid) (pH 7.3). The “high” Ca2+ pipette solution contained (in mM): 146 CsCl, 2 MgCl2, 5 Ca2+-EGTA (ethylene glycol-bis(2-aminoethyl ether)-N,N,N',N'-tetraacetic acid) and 8 HEPES, pH 7.3, with a free Ca2+ concentration of 25 µM. The “0” Ca2+ pipette solution contained 5 mM EGTA instead of Ca2+-EGTA. Pipette solutions with Ca2+ concentrations between 0 and 25 μM were made by mixing the “0” Ca2+ and “high” Ca2+ solutions as we previously reported [21], [28].
Western blot
HAECs were lysed in radio immunoprecipitation assay (RIPA) buffer (Beyotime Biotechnology, China). After proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto polyvinylidene fluoride (PVDF) membranes, the membranes were incubated with TMEM16A antibodies (1:5000) or DNMT1 (1:1000) overnight at 4℃. Then, membranes were incubated with secondary antibodies (1:10,000) at room temperature for 1 h. All antibodies were obtained from Abcam Biotechnology, UK. Bands were visualized using enhanced chemiluminescence detection agents.
Cell Counting Kit-8 (CCK-8) assay
CCK-8 assay (Biosharp, China) was used to measure cell proliferation. After the indicated treatments, HAECs were cultured on 96-well plates for 48 h. After CCK-8 solution was added and incubated for 2 h, the optical density was measured at 450 nm wavelength by a microplate reader (Molecular Devices, USA).
Wound healing assay
Wound healing assay was used to measure cell migration. Briefly, after HAECs were seeded onto a 6-well plate and grew to reach confluency, cells were gently scratched with a 200 μl pipette tip, followed by gently washing with culture medium. After different drug treatments, images were obtained at 48 h under a light microscope. The scratch width was recorded for comparison.
Tube formation assay
In vitro angiogenesis was evaluated using tube formation assay. Briefly, HAECs were placed on the 96-well plate pre-coated with Matrigel (50 μl/well; Corning), and cultured in DMEM with 10% FBS for 24 h. Capillary-like tube formation was photographed under an inverted microscope. Tube length and branching points were calculated using the NIH ImageJ software.
Statistical analysis
SPSS 13.0 software was used for statistical analysis. Data are represented as mean ± standard error (SE). One-way analysis of variance (ANOVA) was used to compare the difference among groups. P value < 0.05 was considered to be statistically significant.
Results
TMEM16A Ca2+-activated Cl− channel is expressed in endothelial cells
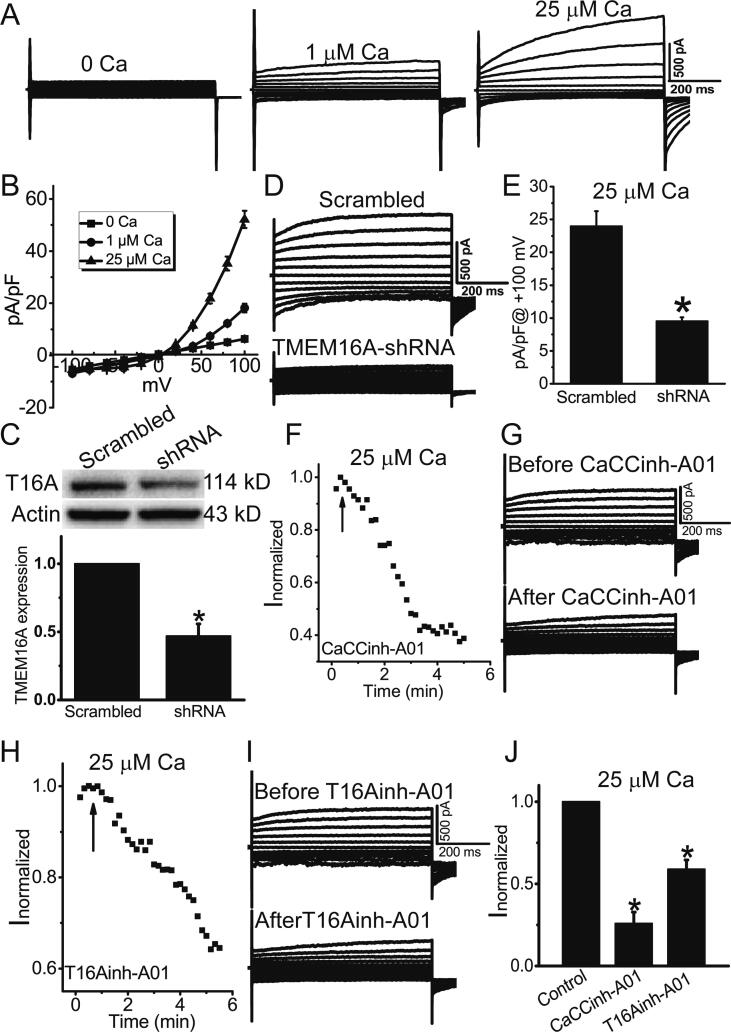
Whole-cell patch clamp recordings were performed to record Ca2+-activated Cl− currents in HAECs. Whole-cell Cl− currents in HAECs were increased with increasing Ca2+ concentrations in the pipette solution (Fig. 1A), suggesting that HAECs expressed Ca2+-activated Cl− channels. The Ca2+-activated Cl− currents (CaCCs) in HAECs exhibited the characteristic feature of TMEM16A currents, including outward rectification, slow activation with time on depolarization, and deactivating tail currents on repolarization (Fig. 1A, B) [28], suggesting that TMEM16A may mediate the CaCCs in HAECs. Furthermore, Western blot analysis showed that TMEM16A expression was reduced by knockdown of TMEM16A by shRNAs (Fig. 1C), and the CaCCs activated by 25 μM Ca2+ were significantly reduced by TMEM16A-shRNA treatment (Fig. 1D, E). The TMEM16A inhibitors T16Ainh-A01 and CaCCinhh-A01 significantly reduced CaCCs in HAECs (Fig. 1F-J), further confirming that the CaCCs recorded in HAECs were mediated by TMEM16A channels.
Fig. 1.
TMEM16A mediated Ca2+-activated Cl− currents in HAECs. A. Representative whole-cell Cl− currents activated by different Ca2+ concentrations (0, 1, and 25 μM). The currents were elicited with 750-ms voltage steps from –100 mV to + 100 mV in 20 mV increments. B. The current–voltage relationship of Cl− currents activated by 0, 1, and 25 μM Ca2+. n = 4–5 cells. C. Western blot results of TMEM16A expression in HAECs treated with scrambled shRNAs and TMEM16A-shRNAs. n = 3. *p < 0.05 vs scrambled shRNA. D. Representative whole-cell Cl− currents activated by 25 μM Ca2+ in HAECs treated with scrambled shRNAs and TMEM16A-shRNAs. E. Mean current densities at + 100 mV in D. n = 5–6 cells. *p < 0.05 vs scrambled shRNA. F. H. The time course of Cl− currents activated by 25 μM Ca2+ in cells treated with the TMEM16A inhibitors CaCCinh-A01 (20 μM) (F) or T16Ainh-A01 (20 μM) (H). Cells were clamped from ramps from − 100 to + 100 mV with a 750-ms duration at 10 s intervals. The current was normalized to the peak current before CaCCinh-A01 or T16Ainh-A01 application. The application of CaCCinh-A01 or T16Ainh-A01 is indicated by the arrow. G. I. Representative current traces before (top) and after (bottom) CaCCinh-A01 (G) or T16Ainh-A01 (I) treatment. J. The normalized currents (Inormalized) before and after CaCCinh-A01 or T16Ainh-A01. The currents were normalized to those before application of these inhibitors. n = 4–6 cells. *p < 0.05 vs control (before treatment).
Cholesterol inhibits TMEM16A expression in HAECs via DNMT1-mediated methylation
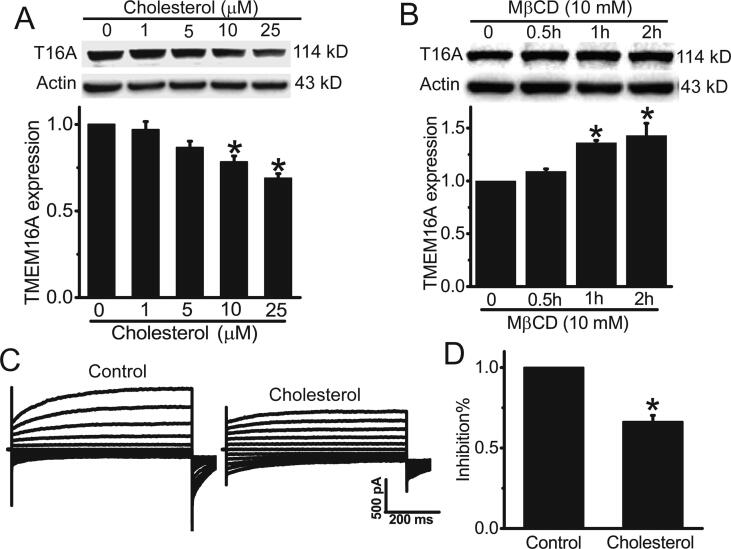
We then examined whether cholesterol regulated TMEM16A expression in HAECs. Western blot results showed that cholesterol treatment dose-dependently inhibited TMEM16A expression (Fig. 2A), whereas the cholesterol chelator MβCD increased TMEM16A expression in HAECs (Fig. 2B). Furthermore, cholesterol treatment for 48 h significantly inhibited TMEM16A Cl− currents (Fig. 2C, D). These results suggested that cholesterol treatment inhibited TMEM16A expression in HAECs.
Fig. 2.
Cholesterol treatment inhibited TMEM16A expression in HAECs. A. B. Western blot results of TMEM16A expression in HAECs treated with different concentrations (0–25 μM) of cholesterol for 48 h (A) or MβCD (10 mM) for 0–2 h (B). n = 3. *p < 0.05 vs control. C. Representative TMEM16A Cl− currents activated by 25 μM Ca2+ in HAECs treated with or without 25 μM cholesterol for 48 h. D. The inhibitory percentage (inhibition%) of cholesterol in TMEM16A currents in HAECs in C. n = 4–5. *p < 0.05 vs control.
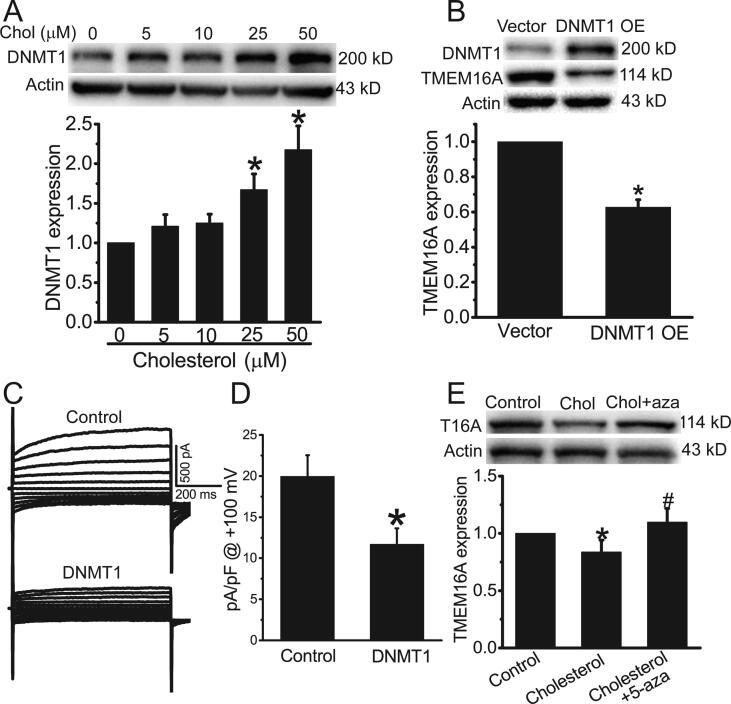
Since cholesterol increases DNMT1 expression in endothelial cells [29], and promotes DNA methylation of many genes [30], we further investigated whether cholesterol induced TMEM16A downregulation in HAECs via DNMT1-mediated methylation. Western blot results showed that at concentrations > 25 μM, cholesterol treatment significantly increased DNMT1 expression in HAECs (Fig. 3A). DNMT1 overexpression inhibited TMEM16A protein expression and currents in HAECs (Fig. 3B–D). In addition, DNMT1 inhibition by 10 μM 5-Aza-2′-deoxycytidine (5-aza) blocked cholesterol-induced decrease in TMEM16A expression (Fig. 3E). These results suggested that cholesterol inhibited TMEM16A expression via DNMT1-mediated methylation in HAECs.
Fig. 3.
Cholesterol treatment inhibited TMEM16A expression in HAECs by upregulating DNMT1 expression. A. Western blot results of DNMT1 expression in HAECs treated with different concentrations of cholesterol. n = 3. *p < 0.05 vs control. B. Western blot results showed TMEM16A expression in HAECs treated with empty vector or DNMT1-overexpressing plasmids (DNMT1 OE). n = 3. *p < 0.05 vs vector. C. Representative whole-cell Cl− currents activated by 25 μM Ca2+ in HAECs treated with empty vector or DNMT1 OE plasmids. D. Mean current densities at + 100 mV in C. n = 5 cells. *p < 0.05 vs vector. E. Western blot results of TMEM16A expression in control HAECs or HAECs treated with 25 μM cholesterol in the presence or absence of 5-aza (10 μM). n = 3. *p < 0.05 vs control; #p < 0.05 vs cholesterol.
Cholesterol directly inhibits TMEM16A currents
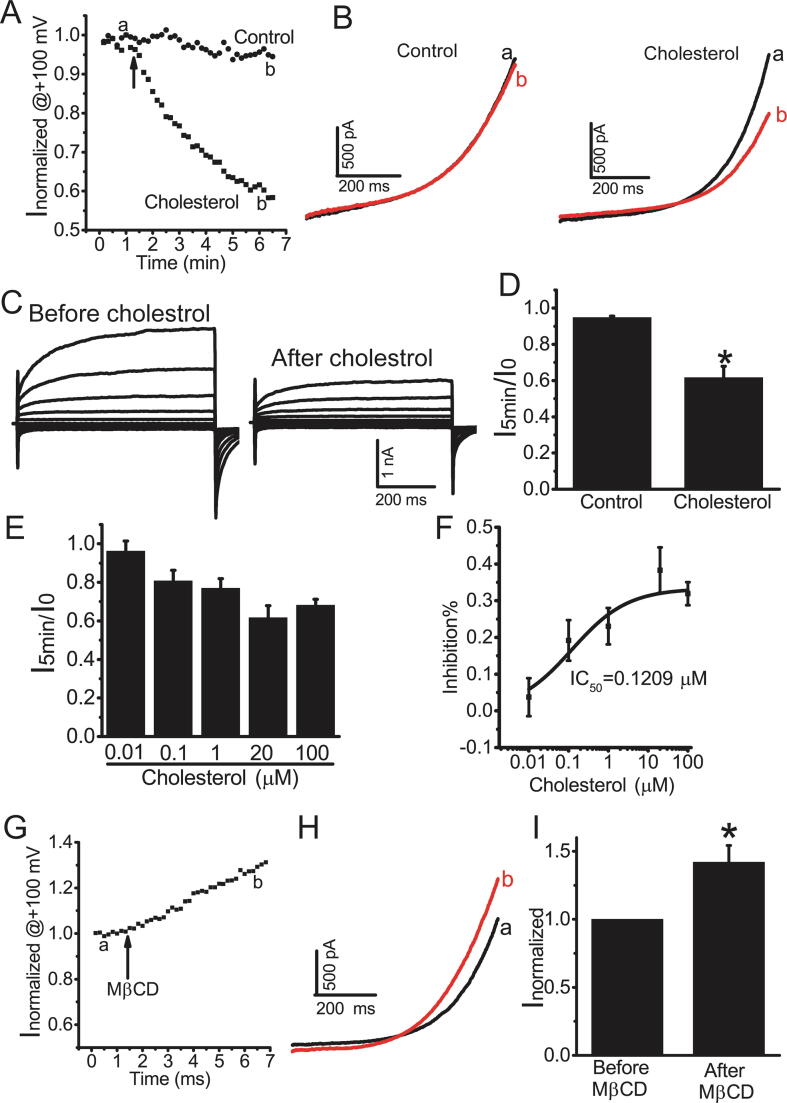
We then investigated whether cholesterol directly inhibited TMEM16A currents. In HEK293 cells transfected with TMEM16A-expressing plasmids, TMEM16A currents activated by 200 nM Ca2+ remained stable without obvious rundown after application of vehicle control (Fig. 3A, B). Acute application of cholesterol (20 μM) resulted in a decrease in TMEM16A currents by approximately 40% at 5 min after cholesterol application (Fig. 4A–D). Cholesterol dose-dependently inhibited TMEM16A currents, with an IC50 of 0.1209 μM (Fig. 4E, F). In addition, acute application of MβCD (2 mM) significantly increased TMEM16A currents (Fig. 4G–I). These results suggested that cholesterol directly inhibited TMEM16A currents in heterologous system.
Fig. 4.
Cholesterol inhibited TMEM16A currents in HEK293 cells transfected with TMEM16A-overexpressing plasmids. A. The time course of TMEM16A currents at + 100 mV activated by 200 nM Ca2+ in HEK293 cells before (a) and after (b) cholesterol (20 μM) or vehicle control. Cells were clamped from ramps from − 100 to + 100 mV with a 750-ms duration at 10 s intervals. The currents were normalized to peak current before application of cholesterol or vehicle. B. Representative currents recorded before (a) and after (b) application of cholesterol or vehicle control in A. C. The representative currents before (a) and after (b) cholesterol treatment (20 μM). Currents were elicited with 750-ms voltage step from –100 mV to + 100 mV in 20 mV increments. D. Current ratio (I5min/I0) at 5 min after cholesterol application. I5min indicates the current at 5 min after the application of cholesterol. I0 indicates the current immediately before cholesterol application. n = 4 cells. *p < 0.05 vs control. E. I5min/I0 for different concentrations of cholesterol (0.01–100 μM). n = 4–6 cells. F. The inhibitory ratio (inhibition%) is plotted vs the cholesterol concentrations. The inhibition% was calculated as follows: inhibition%=(I0-I5min)/I0. The plot was fitted to the Hill equation with an IC50 = 0.1209 μM. G. The time course of TMEM16A currents activated by 200 nM Ca2+ in HEK293 cells before (a) and after (b) MβCD (2 mM). The voltage protocol is the same in A. The currents were normalized to the current before MβCD application. H. Representative currents recorded before (a) and after (b) MβCD application in G. I. The normalized currents (Inormalized) before and after MβCD application in G. n = 4. *p < 0.05 vs before MβCD application.
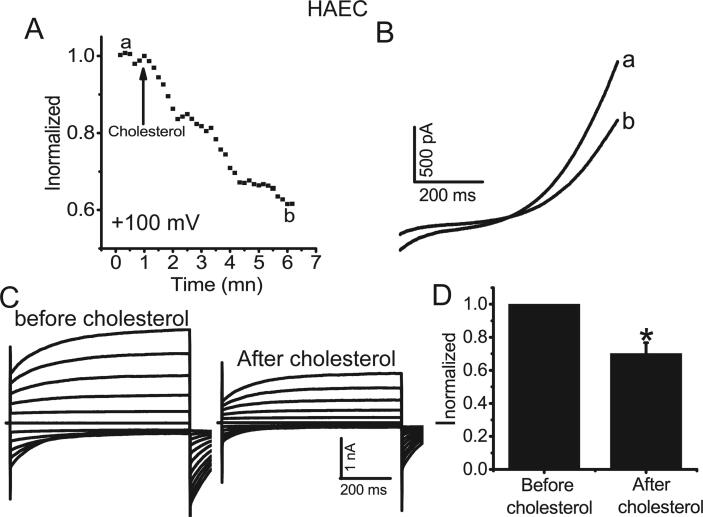
We then investigated the acute effect of cholesterol on TMEM16A Ca2+-activated Cl− currents in native HAECs. Acute application of cholesterol (20 μM) inhibited Ca2+-activated Cl− currents in HAECs by approximately 40% (Fig. 5), similar to the effect of cholesterol on TMEM16A currents in HEK293 cells overexpressing TMEM16A-containing plasmids. These results suggested that cholesterol directly inhibited TMEM16A currents in both HAECs and in HEK293 cells heterologously expressing TMEM16A.
Fig. 5.
Cholesterol inhibited TMEM16A currents in HAECs. A. The time course of TMEM16A currents at +100 mV activated by 25 μM Ca2+ in HAECs before (a) and after (b) cholesterol (20 μM) application. Cells were clamped from ramps from −100 to +100 mV with a 750-ms duration at 10 s intervals. The currents were normalized to peak current before cholesterol application. B. Representative currents recorded before (a) and after (b) cholesterol application in A. C. Representative currents before (a) and after (b) cholesterol treatment (20 μM). Currents were elicited with 750-ms voltage step from –100 mV to +100 mV in 20 mV increments. D. The normalized currents (Inormalized) before and after cholesterol application. n = 4 cells.*p < 0.05 vs before cholesterol application.
TMEM16A channels inhibit angiogenic activities of HAECs
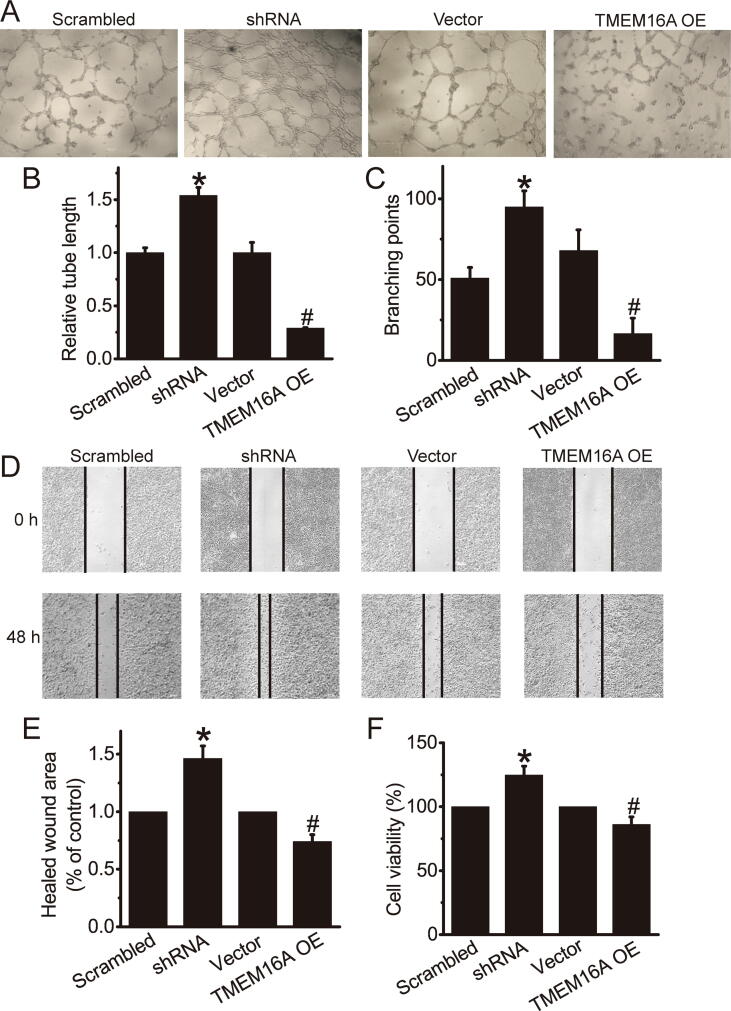
We examined the effect of TMEM16A channels on the angiogenic activities of HAECs. Knockdown of TMEM16A by shRNAs significantly increased tube formation, whereas TMEM16A overexpression significantly inhibited tube formation in HAECs (Fig. 6A–C). TMEM16A-shRNA treatment significantly increased cell migration, whereas TMEM16A overexpression significantly inhibited migration of HAECs (Fig. 6D, E). In addition, CCK-8 assays showed that endothelial cell proliferation was also increased by TMEM16A-shRNA treatment, and inhibited by TMEM16A oeverexpression (Fig. 6F, G). Thus, TMEM16A channels inhibited angiogenic activities of HAECs.
Fig. 6.
TMEM16A channels inhibited angiogenic activities of HAECs. A. Representative images of tubular networks in Matrigel in HAECs transfected with Ad-TMEM16A-shRNAs (shRNA) and its scrambled control (Scrambled) or TMEM16A-overexpressing plasmids (TMEM16A OE) or empty vector (vector) control. B. C. Relative tube length (B) and the number of branching points (C) in A. n = 3. *p < 0.05 vs control. D. Representative images in wound healing assay showing the migration of HAECs transfected with Ad-TMEM16A-shRNAs (shRNA) and its scrambled control (Scrambled) or TMEM16A-overexpressing plasmids (TMEM16A OE) or empty vector (vector). E. Quantification results of wound healing assay in D. n = 4. *p < 0.05 vs control. F. CCK-8 assay showing the cell viability of HAECs transfected with Ad-TMEM16A-shRNAs (shRNA) and its scrambled control (Scrambled) or TMEM16A-overexpressing plasmids (TMEM16A OE) or empty vector (vector). n = 4. *p < 0.05 vs control.
Discussion
This study found that cholesterol inhibited TMEM16A expression in HAECs via DNMT1-mediated methylation of the TMEM16A promoter. Cholesterol also directly inhibited TMEM16A Ca2+-activated Cl− currents in HAECs. These results suggest that cholesterol reduced TMEM16A function by downregulating protein expression and inhibiting channel activities. In addition, TMEM16A knockdown increased cell proliferation, migration and tube formation in HAECs, suggesting that TMEM16A inhibition may promote angiogenic activities of HAECs. Taken together, this study suggests that TMEM16A channel inhibition by cholesterol may contribute to increased angiogenic activities of endothelial cells.
Changes in TMEM16A expression have been reported in many pathological conditions, such as cancer, hypertension, and inflammation [10], [20], [31], [32]. The mechanisms underlying TMEM16A overexpression in cancers include gene amplification, transcriptional regulation, and microRNAs [20], [21], [33], [34]. Since many CpG islands are identified in the TMEM16A promoter [35], DNA methylation could be an important mechanism for regulation of TMEM16A expression. Shiwarski et al. reported that TMEM16A downregulation via increased promoter methylation promoted HNSCC cells in a metastatic state [25]. High cholesterol has been found to promote DNA methylation of a diverse genes involved in atherosclerosis such as Kruppel-like factor 2, and lectin-like oxLDL receptor [30]. In this study, we found that cholesterol-induced TMEM16A downregulation was mediated by DNMT1-mediated methylation, since DNMT1 inhibition by 5-aza reduced TMEM16A protein expression induced by cholesterol. High LDL-cholesterol treatment increases intracellular cholesterol levels and increases DNMT1 expression in endothelial cells [29]. Similarly, we found that cholesterol treatment increased DNMT1 expression and DNMT1 overexpression inhibited TMEM16A expression in HAECs, further confirming that cholesterol downregulated TMEM16A expression via DNMT1-mediated methylation of the TMEM16A promoter.
TMEM16A is localized in cholesterol-rich lipid rafts [17]. Depletion of cholesterol by MβCD increases TMEM16A currents in vascular myocytes and in HEK293 cells transfected with TMEM16A plasmids [17], [18]. Here, we found that in TMEM16A-overexpressing HEK293 cells, direct application of cholesterol inhibited TMEM16A currents with an IC50 of 0.1209 μM. Similarly, TMEM16A Cl− currents in endothelial cells were inhibited by directly application of cholesterol. These results suggest that TMEM16A channel activities are sensitive to changes in cholesterol concentrations in endothelial cells. Since hypercholesterolemia is associated with endothelial dysfunction, TMEM16A inhibition by cholesterol may contribute to endothelial dysfunction induced by hypercholesterolemia, which is an important event to induce atherosclerosis [2].
Angiogenesis is the process to form new blood vessel involving endothelial cell proliferation, migration and tube formation, and contributes to many pathological processes such as cardiovascular diseases, atherosclerosis and tumor. TMEM16A has been identified to be the Ca2+-activated Cl− channel in various endothelial cells including human umbilical vein endothelial cells [10], pulmonary endothelial cells [11], brain endothelial cells [12], and cardiac vascular endothelial cells [13]. TMEM16A is expressed in the plasma membrane as well as in the mitochondria [11], [36], and TMEM16A overexpression inhibits cell proliferation by promoting mitochondria-dependent apoptosis via increased reactive oxygen species (ROS) in pulmonary endothelial cells [11] and in vascular smooth muscle cells [36]. In addition, TMEM16A overexpression promotes ROS generation via Nox2-containing NADPH oxidase in vascular endothelial cells [10]. We found that TMEM16A channel inhibition by shRNAs increased cell proliferation in HAECs. However, to date, the role of TMEM16A channels in endothelial angiogenesis has not been investigated yet. Here, we showed that TMEM16A knockdown increased proliferation, migration and tube formation of HAECs, and TMEM16A overexpression produced the opposite effects. These findings suggest that TMEM16A channels are important for inhibiting angiogenesis in endothelial cells, and TMEM16A channel inhibition (e.g. by cholesterol) may contribute to pathological angiogenesis.
Conclusions
In summary, this study revealed novel mechanisms underlying the regulation of TMEM16A by cholesterol, by which cholesterol reduced TMEM16A expression via DNMT1-mediated promoter methylation and directly inhibited channel activities. In addition, TMEM16A inhibition by shRNAs increased endothelial cell proliferation, migration and tube formation, suggesting TMEM16A channels are important for endothelial cell angiogenesis. Since it is known that high cholesterol levels in endothelial cells promote angiogenesis [37], TMEM16A channel activators may be used to treat angiogenesis under the pathological condition of high cholesterol such as atherosclerosis.
Declaration of Competing Interest
All authors declare no conflicts of interest.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81572613 and No. 31371145 to Qinghuan Xiao; No. 81900390 to Ke Ma), and the Liaoning Pandeng Scholar (to Qinghuan Xiao).
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Marchio P., Guerra-Ojeda S., Vila J.M., Aldasoro M., Victor V.M., Mauricio M.D. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev. 2019;2019:8563845. doi: 10.1155/2019/8563845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P., Buring J.E., Badimon L., Hansson G.K., Deanfield J., Bittencourt M.S. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 3.Konukoglu D., Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511–540. doi: 10.1007/5584_2016_90. [DOI] [PubMed] [Google Scholar]
- 4.Nilius B., Prenen J., Voets T., Van den Bremt K., Eggermont J., Droogmans G. Kinetic and pharmacological properties of the calcium-activated chloride-current in macrovascular endothelial cells. Cell Calcium. 1997;22:53–63. doi: 10.1016/s0143-4160(97)90089-0. [DOI] [PubMed] [Google Scholar]
- 5.Nilius B., Prenen J., Szucs G., Wei L., Tanzi F., Voets T. Calcium-activated chloride channels in bovine pulmonary artery endothelial cells. J Physiol. 1997;498(Pt 2):381–396. doi: 10.1113/jphysiol.1997.sp021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartzell C., Putzier I., Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y.D., Cho H., Koo J.Y., Tak M.H., Cho Y., Shim W.S. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 8.Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder B.C., Cheng T., Jan Y.N., Jan L.Y. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma M.M., Gao M., Guo K.M., Wang M., Li X.Y., Zeng X.L. TMEM16A contributes to endothelial dysfunction by facilitating Nox2 NADPH oxidase-derived reactive oxygen species generation in hypertension. Hypertension. 2017;69:892–901. doi: 10.1161/HYPERTENSIONAHA.116.08874. [DOI] [PubMed] [Google Scholar]
- 11.Allawzi A.M., Vang A., Clements R.T., Jhun B.S., Kue N.R., Mancini T.J. Activation of anoctamin-1 limits pulmonary endothelial cell proliferation via p38-mitogen-activated protein kinase-dependent apoptosis. Am J Respir Cell Mol Biol. 2018;58:658–667. doi: 10.1165/rcmb.2016-0344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu P.Y., Zhang Z., Liu Y., Tang X.L., Shu S., Bao X.Y. TMEM16A inhibition preserves blood-brain barrier integrity after ischemic stroke. Front Cell Neurosci. 2019;13:360. doi: 10.3389/fncel.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu M.M., Lou J., Song B.L., Gong Y.F., Li Y.C., Yu C.J. Hypoxia augments the calcium-activated chloride current carried by anoctamin-1 in cardiac vascular endothelial cells of neonatal mice. Br J Pharmacol. 2014;171:3680–3692. doi: 10.1111/bph.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broadley C., Dawidowicz E., Chong P.L., Hoover R. Modulation of membrane cholesterol levels: effects on endothelial cell function. Exp Cell Res. 1991;193:144–150. doi: 10.1016/0014-4827(91)90548-9. [DOI] [PubMed] [Google Scholar]
- 15.Levitan I., Singh D.K., Rosenhouse-Dantsker A. Cholesterol binding to ion channels. Front Physiol. 2014;5:65. doi: 10.3389/fphys.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su J.B. Vascular endothelial dysfunction and pharmacological treatment. World J Cardiol. 2015;7:719–741. doi: 10.4330/wjc.v7.i11.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sones W.R., Davis A.J., Leblanc N., Greenwood I.A. Cholesterol depletion alters amplitude and pharmacology of vascular calcium-activated chloride channels. Cardiovasc Res. 2010;87:476–484. doi: 10.1093/cvr/cvq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Jesus-Perez J.J., Cruz-Rangel S., Espino-Saldana A.E., Martinez-Torres A., Qu Z., Hartzell H.C. Phosphatidylinositol 4,5-bisphosphate, cholesterol, and fatty acids modulate the calcium-activated chloride channel TMEM16A (ANO1) Biochim Biophys Acta Mol Cell Biol Lipids. 1863;2018:299–312. doi: 10.1016/j.bbalip.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crottes D., Jan L.Y. The multifaceted role of TMEM16A in cancer. Cell Calcium. 2019;82 doi: 10.1016/j.ceca.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H., Zou L., Ma K., Yu J., Wu H., Wei M. Cell-specific mechanisms of TMEM16A Ca(2+)-activated chloride channel in cancer. Mol Can. 2017;16:152. doi: 10.1186/s12943-017-0720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H., Yao F., Luo S., Ma K., Liu M., Bai L. A mutual activation loop between the Ca(2+)-activated chloride channel TMEM16A and EGFR/STAT3 signaling promotes breast cancer tumorigenesis. Cancer Lett. 2019;455:48–59. doi: 10.1016/j.canlet.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Jia L., Liu W., Guan L., Lu M., Wang K. Inhibition of calcium-activated chloride channel ANO1/TMEM16A suppresses tumor growth and invasion in human lung cancer. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0136584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng L., Yang J., Chen H., Ma B., Pan K., Su C. Knockdown of TMEM16A suppressed MAPK and inhibited cell proliferation and migration in hepatocellular carcinoma. Onco Targets Ther. 2016;9:325–333. doi: 10.2147/OTT.S95985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H., Wang H., Guan S., Zhang J., Chen Q., Wang X. Cell-specific regulation of proliferation by Ano1/TMEM16A in breast cancer with different ER, PR, and HER2 status. Oncotarget. 2017;8:84996–85013. doi: 10.18632/oncotarget.18662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiwarski D.J., Shao C., Bill A., Kim J., Xiao D., Bertrand C.A. To “grow” or “go”: TMEM16A expression as a switch between tumor growth and metastasis in SCCHN. Clin Cancer Res. 2014;20:4673–4688. doi: 10.1158/1078-0432.CCR-14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X.H., Zheng B., Yang Z., He M., Yue L.Y., Zhang R.N. TMEM16A and myocardin form a positive feedback loop that is disrupted by KLF5 during Ang II-induced vascular remodeling. Hypertension. 2015;66:412–421. doi: 10.1161/HYPERTENSIONAHA.115.05280. [DOI] [PubMed] [Google Scholar]
- 27.Wang M., Yang H., Zheng L.Y., Zhang Z., Tang Y.B., Wang G.L. Downregulation of TMEM16A calcium-activated chloride channel contributes to cerebrovascular remodeling during hypertension by promoting basilar smooth muscle cell proliferation. Circulation. 2012;125:697–707. doi: 10.1161/CIRCULATIONAHA.111.041806. [DOI] [PubMed] [Google Scholar]
- 28.Xiao Q., Yu K., Perez-Cornejo P., Cui Y., Arreola J., Hartzell H.C. Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop. Proc Natl Acad Sci USA. 2011;108:8891–8896. doi: 10.1073/pnas.1102147108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A., Kumar S., Vikram A., Hoffman T.A., Naqvi A., Lewarchik C.M. Histone and DNA methylation-mediated epigenetic downregulation of endothelial Kruppel-like factor 2 by low-density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol. 2013;33:1936–1942. doi: 10.1161/ATVBAHA.113.301765. [DOI] [PubMed] [Google Scholar]
- 30.Grimaldi V., Vietri M.T., Schiano C., Picascia A., De Pascale M.R., Fiorito C. Epigenetic reprogramming in atherosclerosis. Curr Atheroscler Rep. 2015;17:476. doi: 10.1007/s11883-014-0476-3. [DOI] [PubMed] [Google Scholar]
- 31.Forrest A.S., Joyce T.C., Huebner M.L., Ayon R.J., Wiwchar M., Joyce J. Increased TMEM16A-encoded calcium-activated chloride channel activity is associated with pulmonary hypertension. Am J Physiol Cell Physiol. 2012;303:C1229–1243. doi: 10.1152/ajpcell.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunzelmann K., Ousingsawat J., Cabrita I., Dousova T., Bahr A., Janda M. TMEM16A in cystic fibrosis: activating or inhibiting? Front Pharmacol. 2019;10:3. doi: 10.3389/fphar.2019.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Britschgi A., Bill A., Brinkhaus H., Rothwell C., Clay I., Duss S. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci USA. 2013;110:E1026–1034. doi: 10.1073/pnas.1217072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cha J.Y., Wee J., Jung J., Jang Y., Lee B., Hong G.S. Anoctamin 1 (TMEM16A) is essential for testosterone-induced prostate hyperplasia. Proc Natl Acad Sci USA. 2015;112:9722–9727. doi: 10.1073/pnas.1423827112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzone A., Gibbons S.J., Bernard C.E., Nowsheen S., Middha S., Almada L.L. Identification and characterization of a novel promoter for the human ANO1 gene regulated by the transcription factor signal transducer and activator of transcription 6 (STAT6) FASEB J. 2015;29:152–163. doi: 10.1096/fj.14-258541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng J.W., Chen B.Y., Lv X.F., Sun L., Zeng X.L., Zheng H.Q. Transmembrane member 16A participates in hydrogen peroxide-induced apoptosis by facilitating mitochondria-dependent pathway in vascular smooth muscle cells. Br J Pharmacol. 2018;175:3669–3684. doi: 10.1111/bph.14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyu J., Yang E.J., Shim J.S. Cholesterol trafficking: an emerging therapeutic target for angiogenesis and cancer. Cells. 2019;8 doi: 10.3390/cells8050389. [DOI] [PMC free article] [PubMed] [Google Scholar]