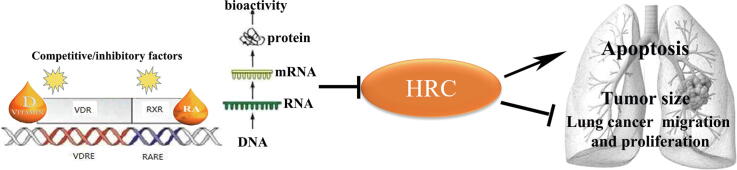
Graphical abstract
Keywords: Vitamin D, Lung cancer, Histidine-rich calcium-binding protein, Vitamin D receptor
Abstract
Introduction
Intrinsic vitamin D affects the proliferation, apoptosis, invasion, metastasis, and tumorigenesis of lung cancer by regulating tumor signaling pathways. Histidine-rich calcium-binding protein (HRC) maintains Ca2+ homeostasis, which plays crucial roles in the occurrence and development of cancer.
Objectives
Our study aims to investigate the ability of vitamin D in the regulation of HRC and the role of HRC playing in lung cancer.
Methods
We investigated the effects of vitamin D on lung cancer and the underlying mechanisms, by measuring HRC and vitamin D receptor (VDR) expression in lung cancer, paracancer, and normal tissues from patients using immunohistochemistry, western blotting, and real time RT-PCR. We transfected H460 lung cancer cells (supplemented or not with vitamin D) with PX458-HRC and pcDNA3.1-HRC plasmids and injected mice with lung cancer cells harboring pcDNA3.1-vector or pcDNA3.1-HRC plasmids.
Results
Vitamin D inhibited HRC expression and H460 cell migration and proliferation, and promoted apoptosis compared with controls. The expression of HRC and VDR was significantly upregulated and downregulated, respectively, in lung cancer versus paracancer or normal tissues. Cell proliferation and migration were reduced, apoptotic cells were more and tumors were smaller in mice treated with vitamin D/cholecalciferol cholesterol emulsion (CCE) than in vitamin D/CCE+HRC+/+ mice.
Conclusion
Vitamin D inhibited lung cancer tumor growth, migration, and proliferation by downregulating HRC.
Introduction
The global incidence and mortality of lung cancer are higher than those of other tumors. About 85% of lung cancers are non-small cell lung cancer, large cell cancer, adenocarcinoma, and squamous cell carcinoma, all of which are prone to local lymph node metastasis and blood transmission. Epidemiological studies in 2018 identified 2.09 million new cases and 1.76 million deaths due to lung cancer [1]. Although the treatment of lung cancer has improved over the past decade, the 5-year survival rate for patients remains <17% [2]. Therefore, basic research is required to understand the molecular events that occur during lung cancer and determine more effective treatments.
Histidine-rich calcium-binding protein (HRC) maintains Ca2+ homeostasis [3], which regulates the uptake, storage, and release of Ca2+ from the sarcoplasmic reticulum [4]. HRC overexpression decreases the rate of Ca2+ uptake in the sarcoplasmic reticulum, but it also increases Ca2+ extrusion by increasing the levels of Na+/Ca2+ exchange protein (NCX) [5], [6], [7]. Ca2+ plays a key role in tumor invasion and metastasis [8], [9], [10]. Many calcium binding proteins such as S100A4 are involved in the emergence and development of cancer [11], [12]. HRC increases the expression of cyclin D1 and cyclin-dependent kinase 2 (CDK2), promotes the G1/S cell cycle progression, and accelerates the invasion, migration, and metastasis of hepatocellular carcinoma (HCC) cells [13]. A deficiency of HRC inhibits the invasion and metastasis of HCC cells in vitro [14]. The anti-apoptotic effect of HRC is associated with endoplasmic reticulum stress, which can help cells to restore homeostasis to some extent and induce persistent apoptosis [15]. The pro-invasion and pro-migration effects of HRC are closely associated with focal adhesion turnover, which is the result of FAK phosphorylation [14]. Investigations into the role of HRC in promoting the invasion, migration, and metastasis of hepatocellular carcinoma indicate that HRC might serve as an anti-tumor target. However, the status of HRC expression and the mechanism of HRC action in lung cancer remain unclear.
Vitamin D is a steroid hormone that maintains calcium and phosphorus homeostasis. Calcitriol, the most important active vitamin D analog, is mainly used to treat hypoparathyroidism and osteoporosis [16]. Cholecalciferol cholesterol emulsion (CCE) becomes active vitamin D through secondary hydroxylation in the liver and kidneys in vivo, and it is clinically used to treat vitamin D deficiency [17]. After binding to the vitamin D receptor (VDR), vitamin D forms a dimer with the retinol X receptor (RXR), and combines with vitamin D reaction elements (VDREs) in the promoter of a target gene to form complexes and recruit co-modulators to further regulate the transcription of the downstream genes [18], [19], [20], [21], [22]. Vitamin D status also negatively correlates with morbidity and mortality from various cancers [23], [24]. Intrinsic vitamin D inhibits the growth of lung cancer cells [25], [26], and active vitamin D3, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3; calcitriol) reduces their proliferation, apoptosis, invasion, metastasis, and tumorigenesis by regulating various tumor signaling pathways [27], [28], [29], [30]. Srinivasan et al. suggested that levels of VDR negatively correlated with survival rates in a study of 73 patients with lung cancer [31]. Vitamin D has broad-spectrum inhibitory effects; they inhibit the growth of colon cancer and many other tumor cells by inhibiting the Wnt/β-catenin signaling pathway [32]. However, the ability of vitamin D to regulate HRC remains obscure.
We hypothesized that HRC downregulation and VDR activation might be novel treatment approaches for lung cancer. Therefore, we determined whether HRC overexpression attenuates the inhibitory effect of calcitriol on the metastasis and proliferation of lung cancer cells.
Materials and methods
Tissue specimens and cell culture
All patients provided written informed consent to the use of their specimens for research purposes, and the Clinical Research Ethics Committee of Shengjing Hospital of China Medical University approved the study (Approval No: 2019PS533K). Specimens of lung cancer, paracancer and normal tissues were obtained from the biological specimen banks of Shengjing Hospital of China Medical University. We also cultured the non-small cell lung cancer H460 cells in RPMI1640 medium containing 10% fetal bovine serum (FBS), and 1% penicillin-streptomycin under a 5% CO2 atmosphere at 37 °C. We applied calcitriol at a concentration of 2 × 10−8 M, which we previously determined to be effective.
Chemicals and reagents
Calcitriol and CCE were supplied by Shengjing Hospital of China Medical University. The plasmid pcDNA3.1 was a kind gift from Dr. Yuan Zhengwei at the Key Laboratory of Congenital Malformation, Ministry of Health of Shengjing Hospital. Anti-HRC and anti-VDR antibodies were respectively purchased from Proteintech (Chicago, IL, USA) and Santa Cruz Biotechnology Inc. (Dallas, TX, USA).
RNA extraction and real time RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). First-strand cDNA templates were synthesized using the Prime Script RT reagent kit (Takara Bio Inc., Kusatsu, Japan). Real time RT-PCR was conducted using a 7500 Fast sequence detector to quantify mRNA expression levels. Differences between samples were determined using the 2−△△Ct method. Primer pairs designed for real time RT-PCR were as follows:
HRC: forward, 5′-CGCTTCACCATCATCCCCAAC-3′ and reverse, 5′-CTGGCTGGTAGTTCCCATACT-3′;
GAPDH: forward, 5′-AAATCAAGTGGGGCGATGCT-3′ and reverse, 5′-TGGTTCACACCCATGACGAA-3′.
Plasmids and transfection
We amplified the CDS sequence of the HRC gene and inserted it into the pcDNA3.1 vector to construct plasmids overexpressing HRC. The forward and reverse primers used for amplification were respectively: 5′-ATGGGCCACCATAGGCCA-3′ and 5′-TCAGGGTTCCGGCGTTTC-3′. We confirmed that the plasmids overexpressed HRC by sequencing (Invitrogen). The pcDNA3.1-HRC plasmid and pcDNA3.1 vector were transfected into H460 cells using Lipofectamine 3000 (Invitrogen) as described by the manufacturer. This experiment included vitamin D (VD), HRC+/+, VD+HRC+/+, and control (Con) groups.
CRISPR/Cas9 gene knockout in cells
We used CRISPR designer (http://crispr.mit.edu/) to design the following single guide (sg) RNA primers to knock out HRC genes in H460 cells: forward: 5′-CACCGACAACAGCACTGGAGTCGCC-3′, and reverse: 5′-AAACGGCGACTCCAGTGCTGTTGTC-3′. The sgRNA sequences were annealed and inserted into the PX458-vector after digestion with the restriction enzyme BpiI (Thermo Fisher Scientific Inc., Waltham, MA, USA). The construct was checked by sequencing (Invitrogen). This experiment included VD, HRC−/−, VD+HRC−/−, and Con groups.
Western blotting
Cells were collected at 24 h after transfection, lysed with RIPA buffer, then lysates were resolved by electrophoresis on 10% agar gels. Separated proteins were transferred to PVDF membranes (Millipore Sigma Co., Ltd., Burlington, MA, USA), then non-specific binding was blocked in 5% skimmed milk for 1 h. Thereafter, the blots were incubated with primary antibody overnight followed by the secondary antibody for 1 h. Bound proteins were detected using Amersham ECL reagents (Cytiva, Little Chalfont, UK).
Wound healing assays
Monolayers of H460 cells transfected or not with plasmids in 6-well dishes were wounded by scratching the surface with a plastic 200 μL pipet tip. The cells were cultured under experimental condition medium mentioned above with or without calcitriol at 37 °C for 24 h. The area of the remaining wound was assessed using ImageJ software, and the distance that the cells migrated was estimated by the calculation.
Cell migration
Cell migration was assessed using Transwell chambers with 8 μm pores (Corning Inc., Corning, NY, USA). Four hours after transfection, H460 cells were digested with trypsin and suspended in serum-free 1640 medium. Cells (1 × 105) in 100 μL of medium were seeded into the upper chamber and 1640 medium containing 10% FBS was used as the chemotactic agent in the lower chamber. After 24 h, non-migrated cells were removed from the upper surface of the Transwell using a cotton swab. The Transwell membranes were fixed in methanol for 20 min, dried, then stained with crystal violet for 30 min. Cells that had migrated through the cell membrane to the lower surface were examined by optical microscopy and counted using Image Pro software.
Cell proliferation assays
Transfected H460 cells were digested with trypsin, suspended in medium, and seeded in 96-well plates at a density of 1 × 103/100 μL. Cell viability was detected using the Cell Titer 96® AQueous One Solution Reagent (Promega Corp., Madison, WI, USA). After incubating the cells for 3 h with 20 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTS) per well, the degree of cell proliferation represented by absorbance at 490 nm was measured with a microplate reader.
Xenograft tumor models
All animal studies were conducted in accordance with the guidelines of the Ethics Committee of Shengjing Hospital and the local animal care and use committee (Approval No: 2017PS311K). Male, 3–4-week-old Balb/c nude mice were randomly assigned to groups that were injected with 5 × 106 cells harboring pcDNA3.1 (group V), or pcDNA3.1-HRC+CCE (group C), HRC (H), or CCE+HRC (C+H). The CCE+vector and the HRC+CCE groups received CCE in drinking water (1 μL/mL) for 28 days.
Immunohistochemistry (IHC)
Tumor tissue sections were routinely dewaxed, boiled in 10 mM Na citrate solution for 10 min, then incubated with HRC or VDR antibody and stained with 3,30-diaminobenzidine (DAB) as described [33]. Images were acquired by optical microscopy.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assays
Apoptotic cells were detected using TUNEL assays as described by the manufacturer (WLA029a; Wanleibio Co., Ltd., Shenyang, China) [34], [35]. Apoptotic nuclei in tumor cells were detected by staining cells with DAB.
Apoptosis assays
The induction of apoptosis was determined using Annexin V- PE Apoptosis Detection Kit I (BD Pharmingen, San Diego, CA, USA). Cells were washed twice with cold PBS, then resuspended in 1× binding buffer at a density of 1 × 106 cells/mL. Suspensions (100 μL containing 1 × 105 cells) were transferred to 5 mL tubes and incubated with annexin V-phycoerythrin (PE) and 7-amino-actinomycin (7-AAD) for 15 min at 25 °C in darkness. Thereafter, 400 μL of 1× binding buffer was added to the tubes and apoptotic cells were assessed by flow cytometry.
Chromatin immunoprecipitation assays (ChIP)
Protein-bound DNA was assayed using Simple ChIP Plus Sonication Chromatin IP Kits (Cell Signaling Technology, Danvers, MA, USA) and the forward and reverse PCR primer sequences: 5′-GCACGTATCATTCCAGC-3′ and 5′- ACCAGTGAGGGGTCTCTCC -3′, respectively.
Luciferase assays
The transcriptional sites of VDREs on the HRC promoter were predicted using the University of California, Santa Cruz genome browser, and primers containing these transcriptional sites were designed. The HRC-PGL3 recombinant plasmid was constructed by inserting PCR products into the pGL3 basic vector and confirmed by sequencing (Invitrogen). Recombinant plasmids containing the HRC promoter were transfected into Cos-7 cells with Lipofectamine™ 3000 (Invitrogen). Renilla and firefly luciferase reporter activities were quantified using Dual-Glo™ Luciferase Assay Systems (Promega).
Statistical analysis
Data are presented as means ± SD. Data were statistically analyzed using unpaired two-tailed Student t-tests or one-way analysis of variance followed by Bonferroni post hoc tests, as appropriate. Values with P < 0.05 were considered statistically significant.
Results
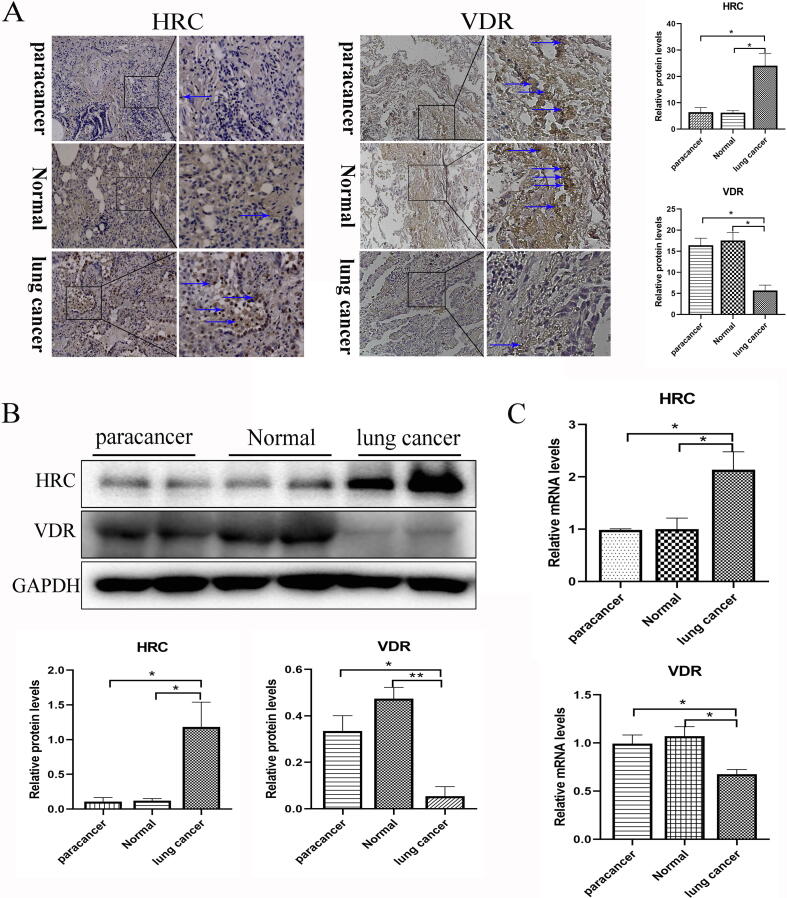
HRC was upregulated and VDR was downregulated in lung cancer tissues from patients
Compared with corresponding normal and paracancer tissues, HRC protein and mRNA expression was significantly upregulated in the lung cancer tissues, whereas that of VDR was remarkedly downregulated (Fig. 1A–C), suggesting that the expression of VDR was inhibited, whereas that HRC was increased in lung cancer specimens.
Fig. 1.
Expression of VDR and HRC respectively decreased and increased in lung cancer. (A) Immunohistochemical staining and (B) western blot of HRC and VDR protein expression in lung cancer, paracancer and normal tissues. The expression is shown as relative to GAPDH protein levels determined using Prism 5. Blue arrow, positive area (original magnification, ×200; partial magnification of ×400). (C) Levels of HRC and VDR mRNA expression in lung cancer, paracancer and normal tissues measured using real time TR-PCR. Values are shown as means ± SD. Statistical significance is shown as *P < 0.05, **P < 0.01, ***P < 0.001. HRC, histidine-rich calcium binding protein; VDR, vitamin D receptor. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
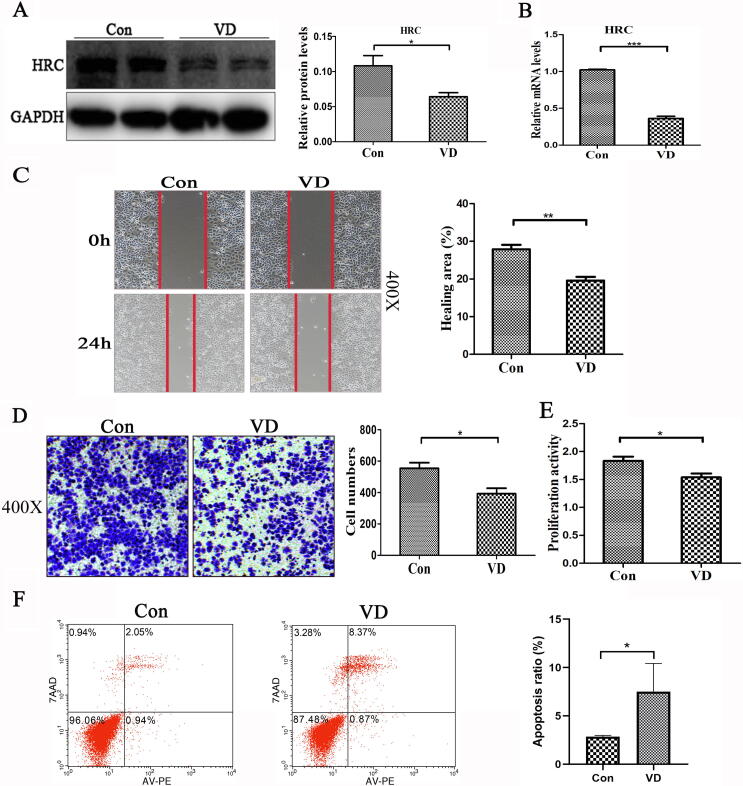
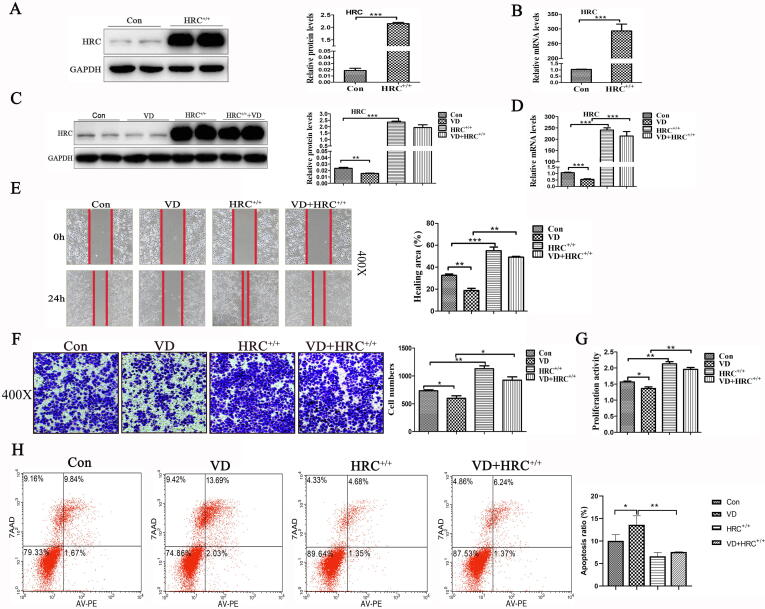
Vitamin D inhibited HRC expression and H460 cell migration and proliferation and promoted H460 cell apoptosis
We explored the effects of 2 × 10−8 M vitamin D on HRC in H460 cells. Western blot and real time RT -PCR findings showed that vitamin D downregulated the expression of HRC compared with the control group (Fig. 2A–B). Wound healing, Transwell, and MTS assays showed that vitamin D inhibited the migration (Fig. 2C–D) and proliferation (Fig. 2E) of H460 cells. The ratio (%) of apoptotic cells was increased by vitamin D in comparison with Control group (Fig. 2F). These results might probably indicate that vitamin D could inhibit the migration and proliferation of H460 cells, and promote apoptosis.
Fig. 2.
Vitamin D downregulated HRC expression and inhibited H460 cell growth. (A) Levels of HRC protein expression in H460 cells incubated with or without vitamin D. The histogram shows that vitamin D significantly reduced HRC expression. (B) Real time RT-PCR results show that vitamin D significantly reduced HRC mRNA levels. (C) Wound healing assay. Vitamin D significantly reduced cell mobility, expressed as the ratio (%) of healed areas after 24 h. Red line, edge of cell migration. (D) Transwell migration assays. The histogram shows vitamin D-inhibited cell migration and numbers of migrated cells. (E) Cell proliferation assays. Vitamin D inhibited H460 cell proliferation. (F) Apoptosis induction assessed by flow cytometry after Annexin V-PE/7-AAD staining. Vitamin D promoted apoptosis. Values are expressed as means ± SD. Statistical significance is shown as *P < 0.05, **P < 0.01, and ***P < 0.001. HRC, Histidine-rich calcium binding protein. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
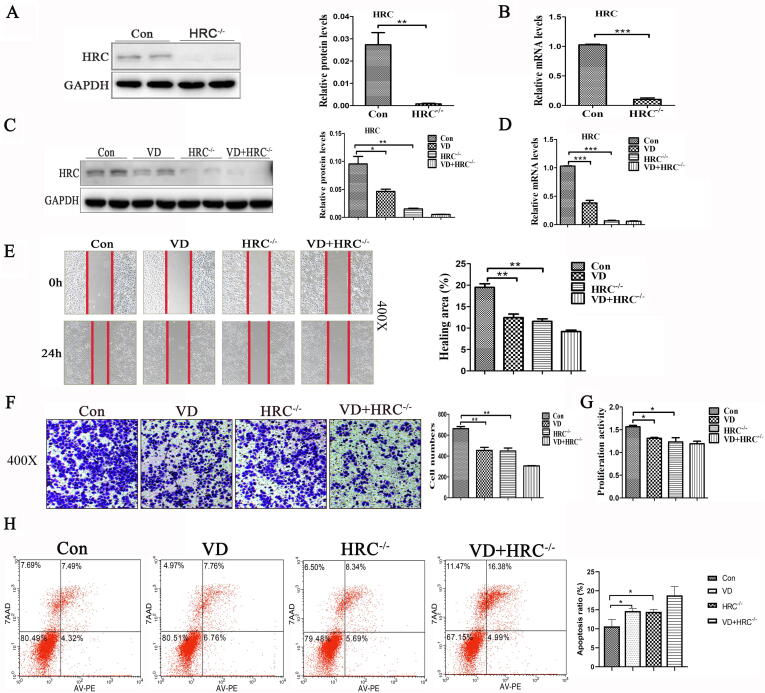
Synergistic effects of vitamin D and HRC−/− on cancer cell migration, proliferation, and apoptosis
We investigated the effects of HRC on the migration and proliferation of H460 cells. We knocked down the HRC gene in H460 cells using CRISPR-Cas9 technology. Fig. 3A–D shows the knockdown efficiency of HRC gene at the protein and mRNA levels. The results of migration and proliferation experiments showed that both HRC−/− and vitamin D inhibited cell migration and proliferation and that the effect was synergistic (Fig. 3E–G). Furthermore, HRC−/− and vitamin D significantly and synergistically increased the ratio of apoptotic cells (Fig. 3H). These results suggested that VD and HRC−/− have synergistic effects on lung cancer cell metastasis, replication, and apoptosis.
Fig. 3.
Synergistic effect of vitamin D and HRC−/− on cell migration, proliferation and apoptosis. (A) Protein expression of HRC in H460 cell lines with HRC knockdown. Western blots show that HRC has been knocked out. (B) HRC was detected at the level of mRNA by real time RT-PCR. (C) Western blots of HRC expression show that vitamin D and HRC−/− further reduced HRC expression. (D) Levels of HRC mRNA detected by real time RT-PCR. (E) Wound healing assay. Vitamin D and HRC knockdown both reduced cell mobility, but together, the reduction in cell migration was further reduced. (F) Transwell migration assays revealed that vitamin D and HRC−/− synergistically inhibited H460 cell migration. (G) Proliferation of H460 cells. Results of MTS assays showed good synergistic effects. (H) Apoptosis induction assessed by flow cytometry after annexin V-PE/7-AAD staining. Vitamin D and HRC−/− synergistically promoted apoptosis. Values are expressed as means ± SD. Statistical significance is shown as *P < 0.05, **P < 0.01, and ***P < 0.001. HRC, Histidine-rich calcium binding protein.
Vitamin D-induced inhibition of lung cancer was attenuated by HRC+/+
We constructed full-length plasmids (pcDNA3.1-HRC) to stabilize HRC gene expression and investigate how vitamin D inhibits H460 cell migration and proliferation. Fig. 4A–D shows that the HRC gene was overexpressed at the protein and mRNA levels. The mobility and proliferation of H460 cells transfected with plasmids overexpressing HRC and incubated with vitamin D were partially reduced in the VD+HRC+/+ group compared with those in the HRC+/+ group, whereas the difference did not reach statistical significance. However, mobility and proliferation capacity of cells were higher in the VD+HRC+/+ group than in the VD group (Fig. 4E–G). The ratio (%) of apoptotic cells was lower in the VD+HRC+/+ group than in the VD group, whereas that in the HRC+/+ and VD+HRC+/+ groups did not significantly differ (Fig. 4H). The above results indicated that HRC+/+ partially abolished the regulating effects of vitamin D on migration, proliferation and apoptosis of lung cancer cells.
Fig. 4.
HRC+/+ attenuated the effects of vitamin D on cell migration, proliferation and apoptosis. (A) Western blots show HRC overexpression in H460 cells; a 200-fold increase in HRC expression was noted. (B) Overexpressed HRC detected by real time RT-PCR. (C) The histogram shows HRC protein expression after 24 h incubation with vitamin D. (D) Effects of vitamin D on HRC mRNA expression determined by real time RT-PCR. (E) Wound healing assay. Vitamin D with HRC+/+ partially reduced cell mobility compared with that with HRC+/+, but the effect was weaker than that in the VD group. (F) Transwell migration assays. The histogram shows that vitamin D with HRC+/+ partially inhibited H460 cell migration. (G) Assays of H460 cell proliferation showed that HRC+/+ attenuates the effects of vitamin D on cell migration and proliferation. (H) Apoptosis induction assessed by flow cytometry after staining with annexin V-PE/7-AAD. Vitamin D-induced H460 cell apoptosis was significantly reduced by HRC+/+. Values are expressed as means ± SD. Statistical significance is shown as *P < 0.05, **P < 0.01, and ***P < 0.001. HRC, Histidine-rich calcium binding protein.
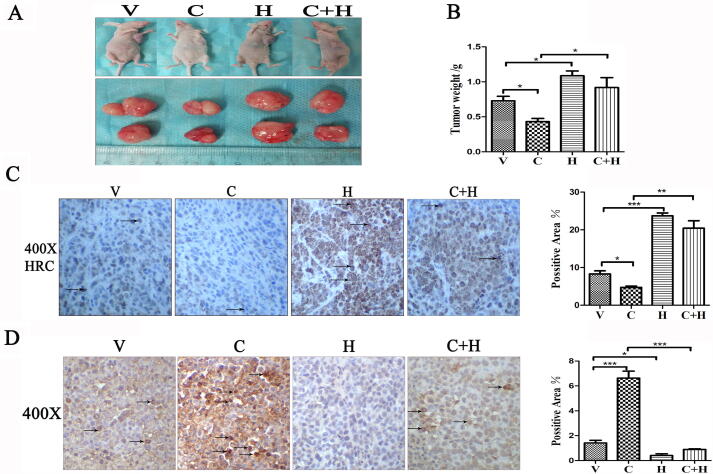
Tumor growth was partially inhibited in the CCE+HRC group compared with that in the HRC group
Inhibition of CCE to tumor growth was further confirmed by subcutaneous xenotransplantation model. H460 cells transfected with an empty vector and pcDNA3.1-HRC plasmids were subcutaneously injected under axillae of left front paws into nude mice. The tumor volume (Fig. 5A) and weight (Fig. 5B) were lower in the CCE than in the Con group, whereas the inhibitory effects on tumors were lower in the CCE+HRC group than in the CCE group. Immunohistochemical staining revealed more HRC expression in the HRC and CCE+HRC groups than in the Con and CCE groups (Fig. 5C). TUNEL staining results similarly indicated that the apoptosis of tumor cells promoted by CCE was significantly reduced by HRC overexpression (Fig. 5D). These results suggested that CCE could inhibit tumor growth in vivo by affecting the expression of HRC.
Fig. 5.
Tumor growth was partially inhibited in the CCE+HRC group compared with the HRC group in vivo. (A) Images of tumors in the xenograft nude mice. Tumors were the smallest in the CCE group, and tumor reduction by CCE was weakened by HRC. (B) Mean tumor weight of nude mice. Tumor growth was partially inhibited by CCE under HRC overexpression, but the effect was weaker than that under CCE treatment alone. (C) Immunohistochemical staining shows HRC expression among groups. (D) TUNEL staining was more intense in the CCE group than in the CCE+HRC group. Arrow, site of apoptosis. Values are expressed as means ± SD. Statistical significance is shown as *P < 0.05, **P < 0.01, and ***P < 0.001. CCE, cholecalciferol cholesterol emulsion; HRC, histidine-rich calcium binding protein; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
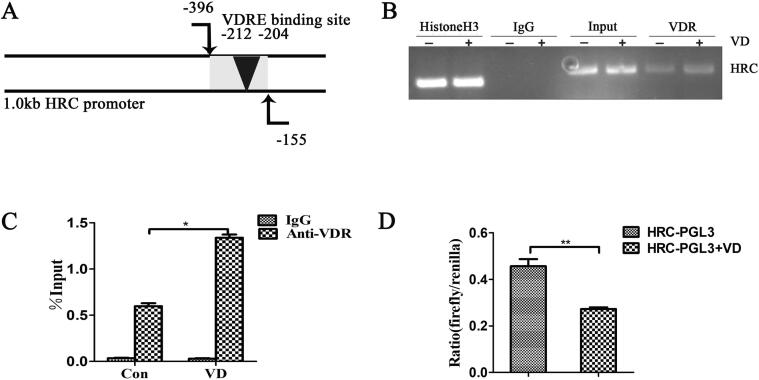
HRC is a direct target gene of vitamin D
We predicted a VDRE site (HRC-VDRE) in 2000 bp upstream of the HRC gene promoter using the PROMO database (Fig. 6A). The ChIP assays showed that VDR bound to the VDRE on the HRC promoter, indicating that vitamin D could transcriptionally regulate HRC expression (Fig. 6B). Real time RT-PCR findings showed that vitamin D promoted the binding of VDR to HRC-VDRE (Fig. 6C). Vitamin D reduced HRC transcriptional activity in Cos-7 cells transfected with HRC-PGL3 plasmids (Fig. 6D). Based on these findings, we concluded that the regulatory effect of vitamin D on HRC may directly target VDRE on HRC promoter.
Fig. 6.
HRC is the direct target of vitamin D. (A) Schema of the HRC promoter region. (B) ChIP assays of H460 cells using anti-VDR antibody. Vitamin D response elements in the HRC promoter amplified by PCR. (C) Immunoprecipitated DNA with protein detected by ChIP-qPCR. The bar graph shows the ratio (%) of relative fold enrichment of VDR across the HRC promoter region. (D) Cos-7 cells transfected with HRC-PGL3 reporter plasmid. Vitamin D reduced the transcriptional activity of HRC. Values are expressed as means ± SD. Statistical significance is shown as *P < 0.05 and **P < 0.01. HRC, histidine-rich calcium binding protein; VDR, vitamin D receptor.
Discussion
We found that HRC and VDR were significantly upregulated and downregulated, respectively, in lung cancer tissues from patients. Others have similarly shown that vitamin D/VDR levels are inversely related to lung cancer progression [36], [37]. Furthermore, another study indicated that the expression of VDR is significantly lower in hepatocellular carcinoma compared with non-tumorous liver [38]. Moreover, HRC expression positively correlates with the migration and proliferation of HCC cells [14]. Therefore, we investigated the roles of VDR and HRC in the occurrence and development of lung cancer. We used H460 cells in subsequent studies to further validate the underlying mechanism described above.
Vitamin D levels in the blood negatively correlate with the incidence of breast, colorectal, prostate, and lung cancers [39], [40]. Studies suggested that vitamin D could reduce carcinogenesis by inducing apoptosis [41], [42], [43]. We found that vitamin D along with decreased HRC expression in vitro inhibited the migration and proliferation of H460 cells, which has not previously been reported. The expression of HRC was significantly decreased in the groups treated with vitamin D. Therefore, we predicted that the invasion, tumorigenesis, and metastasis of HCC cell lines would be inhibited by vitamin D, playing an antagonistic role by regulating the HRC pathway [13].
We knocked down HRC and used pcDNA3.1 to overexpress HRC in H460 cells to determine whether vitamin D affects tumor cell metastasis, proliferation and apoptosis through the HRC pathway. Vitamin D was involved in regulating the migration, proliferation and apoptosis of cancer cells and was synergistic with HRC knockdown and antagonistic toward HRC expression. These findings indicated that vitamin D is relevant to not only limiting tumor invasion, metastasis and proliferation but also promoting tumor apoptosis, possibly by regulating HRC. Vitamin D alters the expression of many cancer-related genes, including HDAC2 and VEGF-A, which are involved in cancer cell migration and proliferation [14]. Here, we identified the impact of vitamin D on HRC gene regulation. High levels of HRC expression significantly weakened the ability of vitamin D to inhibit the migration and proliferation of H460 lung cancer cells and promote their apoptosis. Therefore, inhibition of lung cancer by vitamin D depends on decreased HRC expression. Consistent with in vitro findings, CCE inhibited tumorigenesis in nude mice. However, the effect was partially reduced by HRC +/+, which further supports the notion that HRC could serve as a new target of vitamin D for tumor cell inhibition. The effects of HRC on tumor growth in nude mice may be due to its ability to upregulate the expression of cyclin D1 and CDK2 and promote the G1/S cell cycle progression [13]. In contrast, vitamin D transcriptionally downregulates the cyclin D1 site, which not only attenuates the expression of this gene during mucosal cell maturation and tumorigenesis but also abrogates the conserved sequence in cyclin D1 intron 3 [44]. Vitamin D might inhibit the harmful signal transduction of cancer pathways such as cyclin D1 and many others, by inhibiting the calcium binding role of HRC protein. Notably, HRC downregulation significantly reduces the invasion, metastasis, and proliferation of cancer cells [45], [46], [47].
We predicted VDRE sites using a PROMO database, and ChIP analysis showed that vitamin D bound to VDRE sites in the HRC promoter region. Luciferase assay showed that vitamin D reduces the transcriptional activity of HRC, indicating that a VDR-binding site in the HRC gene promoter transcriptionally regulates the expression of HRC. Since the discovery of VDR as a transcription factor in steroid nuclei, the regulatory effect of vitamin D on genes has become more apparent. Vitamin D regulates the transcriptional activity of target genes, such as SLC1A5, by binding to the VDRE, which is the locus where the vitamin D complex binds to specific DNA on the target gene promoter [48]. Therefore, we believe that VDRE is vital to the regulation of HRC gene expression by vitamin D and the subsequent inhibition of cancer.
Conclusion
Vitamin D inhibits tumor growth, migration and proliferation, and promotes apoptosis by downregulating HRC. Therefore, the HRC gene could serve as a novel potential therapeutic target of vitamin D to inhibit tumor growth.
Compliance with Ethics requirements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by Clinical projects in ShengJing Hospital in 2019 - Clinical study on homocysteine and vitamin/trace elements in blood; the Newly Established Nationwide Medical Experts Committee Funded Program in 2018; ShengJing Hospital 345 Talent Project and the National Natural Science Foundation of China (protocol no. 81570811, 81170065).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2020.08.013.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
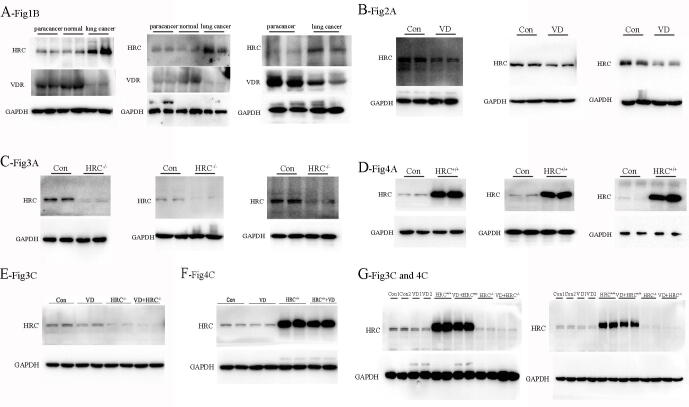
Supplementary fig. 1.
Replicates of all western blots have been shown in this figure. Replicates of (A) fig.1B, (B) fig.2A, (C) fig.3A, (D) fig.4A, (E) fig.3C, (F) fig.4C, and (G) fig.3C and 4C.
Supplementary fig. 2.
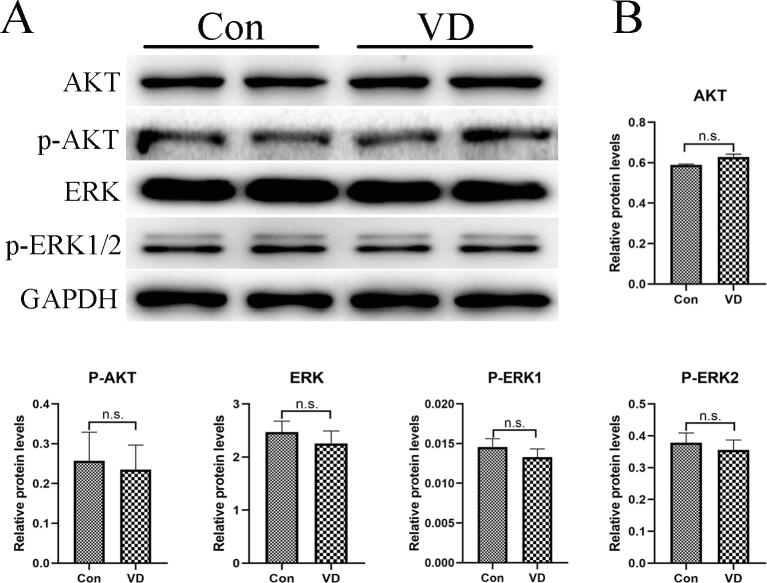
Vitamin D does not regulate the protein expression of AKT, p-AKT, ERK and p-ERK1/2 in H460 cells. (A) Western blots show that levels of AKT, P-AKT, ERK and P-ERK protein expression in H460 cells incubated with or without vitamin D. (B) The histograms show that the differences between the Con and VD groups are not statistically significant. Values are expressed as means ± SD. Statistical significance is shown as n.s. (P >0.05, not significant). AKT, protein kinase B; p-AKT, phospho-protein kinase B; ERK; extracellular regulated protein kinases; p-ERK, phospho-extracellular regulated protein kinases.
References
- 1.Nasim F., Sabath B.F., Eapen G.A. Lung cancer. Med Clin North Am. 2019;103:463–473. doi: 10.1016/j.mcna.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Maj E., Filip-Psurska B., Milczarek M., Psurski M., Kutner A., Wietrzyk J. Vitamin D derivatives potentiate the anticancer and anti-angiogenic activity of tyrosine kinase inhibitors in combination with cytostatic drugs in an A549 non-small cell lung cancer model. Int J Oncol. 2018;52:337–366. doi: 10.3892/ijo.2017.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picello E., Damiani E., Margreth A. Low-affinity Ca(2+)-binding sites versus Zn(2+)-binding sites in histidine-rich Ca(2+)-binding protein of skeletal muscle sarcoplasmic reticulum. Biochem Biophys Res Commun. 1992;186:659–667. doi: 10.1016/0006-291x(92)90797-o. [DOI] [PubMed] [Google Scholar]
- 4.Arvanitis D.A., Vafiadaki E., Johnson D.M., Kranias E.G., Sanoudou D. The histidine-rich calcium binding protein in regulation of cardiac rhythmicity. Front Physiol. 2018;9:1379. doi: 10.3389/fphys.2018.01379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvanitis D.A., Vafiadaki E., Fan G.C., Mitton B.A., Gregory K.N., Del Monte F. Histidine-rich Ca-binding protein interacts with sarcoplasmic reticulum Ca-ATPase. Am J Physiol Heart Circulatory Physiol. 2007;293:H1581–H1589. doi: 10.1152/ajpheart.00278.2007. [DOI] [PubMed] [Google Scholar]
- 6.Arvanitis D.A., Vafiadaki E., Sanoudou D., Kranias E.G. Histidine-rich calcium binding protein: the new regulator of sarcoplasmic reticulum calcium cycling. J Mol Cell Cardiol. 2011;50:43–49. doi: 10.1016/j.yjmcc.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory K.N., Ginsburg K.S., Bodi I., Hahn H., Marreez Y.M., Song Q. Histidine-rich Ca binding protein: a regulator of sarcoplasmic reticulum calcium sequestration and cardiac function. J Mol Cell Cardiol. 2006;40:653–665. doi: 10.1016/j.yjmcc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y.F., Hsu K.F., Shen M.R. The store-operated Ca(2+) entry-mediated signaling is important for cancer spread. BBA. 1863;2016:1427–1435. doi: 10.1016/j.bbamcr.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Jardin I., Rosado J.A. STIM and calcium channel complexes in cancer. BBA. 1863;2016:1418–1426. doi: 10.1016/j.bbamcr.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Mo P., Yang S. The store-operated calcium channels in cancer metastasis: from cell migration, invasion to metastatic colonization. Front Biosci. 2018;23:1241–1256. doi: 10.2741/4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Link T., Kuhlmann J.D., Kobelt D., Herrmann P., Vassileva Y.D., Kramer M. Clinical relevance of circulating MACC1 and S100A4 transcripts for ovarian cancer. Mol Oncol. 2019;13:1268–1279. doi: 10.1002/1878-0261.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L., Qi L., Knifley T., Piecoro D.W., Rychahou P., Liu J. S100A4 alters metabolism and promotes invasion of lung cancer cells by up-regulating mitochondrial complex I protein NDUFS2. J Biol Chem. 2019;294:7516–7527. doi: 10.1074/jbc.RA118.004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J., Han P., Li M., Yan W., Liu J., He J. Histidine-rich calcium binding protein promotes growth of hepatocellular carcinoma in vitro and in vivo. Cancer Sci. 2015;106:1288–1295. doi: 10.1111/cas.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J., Han P., Li M., Yan W., Liu J., Liu J. The histidine-rich calcium binding protein (HRC) promotes tumor metastasis in hepatocellular carcinoma and is upregulated by SATB1. Oncotarget. 2015;6:6811–6824. doi: 10.18632/oncotarget.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav R.K., Chae S.W., Kim H.R., Chae H.J. Endoplasmic reticulum stress and cancer. J Cancer Prevent. 2014;19:75–88. doi: 10.15430/jcp.2014.19.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leere J.S., Vestergaard P. Calcium metabolic disorders in pregnancy: primary hyperparathyroidism, pregnancy-induced osteoporosis, and vitamin D deficiency in pregnancy. Endocrinol Metab Clin North Am. 2019;48:643–655. doi: 10.1016/j.ecl.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Liu N., Zhang Y., Su H., Wang J., Liu Z., Kong J. Effects of cholecalciferol cholesterol emulsion on renal fibrosis and aquaporin 2 and 4 in mice with unilateral ureteral obstruction. Biomed Pharmacother. 2018;102:633–638. doi: 10.1016/j.biopha.2018.03.093. [DOI] [PubMed] [Google Scholar]
- 18.Bouillon R., Carmeliet G., Verlinden L., van Etten E., Verstuyf A., Luderer H.F. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleet J.C., DeSmet M., Johnson R., Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441:61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haussler M.R., Whitfield G.K., Kaneko I., Haussler C.A., Hsieh D., Hsieh J.C. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen J., Katz L.H., Munoz N.M., Gu S., Shin J.H., Jogunoori W.S. Vitamin D deficiency promotes liver tumor growth in transforming growth factor-beta/Smad3-deficient mice through Wnt and toll-like receptor 7 pathway modulation. Sci Rep. 2016;6:30217. doi: 10.1038/srep30217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hossain S., Beydoun M.A., Beydoun H.A., Chen X., Zonderman A.B., Wood R.J. Vitamin D and breast cancer: A systematic review and meta-analysis of observational studies. Clin Nutr ESPEN. 2019;30:170–184. doi: 10.1016/j.clnesp.2018.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keum N., Lee D.H., Greenwood D.C., Manson J.E., Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol Off J Eur Soc Med Oncol. 2019;30:733–743. doi: 10.1093/annonc/mdz059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawa K., Kawaura A., Kato S., Takeda E., Okano T. Metastatic growth of lung cancer cells is extremely reduced in Vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2004;89–90:545–547. doi: 10.1016/j.jsbmb.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 26.Norton R., O'Connell M.A. Vitamin D: potential in the prevention and treatment of lung cancer. Anticancer Res. 2012;32:211–221. [PubMed] [Google Scholar]
- 27.Thyer L., Ward E., Smith R., Fiore M.G., Magherini S., Branca J.J. A novel role for a major component of the vitamin D axis: vitamin D binding protein-derived macrophage activating factor induces human breast cancer cell apoptosis through stimulation of macrophages. Nutrients. 2013;5:2577–2589. doi: 10.3390/nu5072577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanoirbeek E., Eelen G., Verlinden L., Carmeliet G., Mathieu C., Bouillon R. PDLIM2 expression is driven by vitamin D and is involved in the pro-adhesion, and anti-migration and -invasion activity of vitamin D. Oncogene. 2014;33:1904–1911. doi: 10.1038/onc.2013.123. [DOI] [PubMed] [Google Scholar]
- 29.Jeon S.M., Shin E.A. Exploring vitamin D metabolism and function in cancer. Exp Mol Med. 2018;50:20. doi: 10.1038/s12276-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rai V., Abdo J., Agrawal S., Agrawal D.K. Vitamin D receptor polymorphism and cancer: an update. Anticancer Res. 2017;37:3991–4003. doi: 10.21873/anticanres.11784. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan M., Parwani A.V., Hershberger P.A., Lenzner D.E., Weissfeld J.L. Nuclear vitamin D receptor expression is associated with improved survival in non-small cell lung cancer. J Steroid Biochem Mol Biol. 2011;123:30–36. doi: 10.1016/j.jsbmb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira F., Larriba M.J., Munoz A. Vitamin D and colon cancer. Endocr Relat Cancer. 2012;19:R51–R71. doi: 10.1530/erc-11-0388. [DOI] [PubMed] [Google Scholar]
- 33.Fu Y., Zhu J., Zhang Y., Liu Z., Su H., Kong J. Vitamin D regulates the expressions of AQP-1 and AQP-4 in mice kidneys. Biomed Res Int. 2019;2019:3027036. doi: 10.1155/2019/3027036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christina M., Angelika H.L., Bernd P., Martina P. Simultaneous detection of a cell surface antigen and apoptosis by microwave-sensitized TUNEL assay on paraffin sections. J Immunol Methods. 2006;316:163–166. doi: 10.1016/j.jim.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Liu N., Su H., Zhang Y., Liu Z., Kong J. Cholecalciterol cholesterol emulsion attenuates experimental autoimmune myocarditis in mice via inhibition of the pyroptosis signaling pathway. Biochem Biophys Res Commun. 2017;493:422–428. doi: 10.1016/j.bbrc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Haznadar M., Krausz K.W., Margono E., Diehl C.M., Bowman E.D., Manna S.K. Inverse association of vitamin D3 levels with lung cancer mediated by genetic variation. Cancer Med. 2018;7:2764–2775. doi: 10.1002/cam4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X., Cheng J., Yang K. Vitamin D-related gene polymorphisms, plasma 25-hydroxy-vitamin D, cigarette smoke and non-small cell lung cancer (NSCLC) risk. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horvath E., Balla B., Kosa J., Lakatos P.A., Lazary A., Nemeth D. Vitamin D metabolism and signaling in human hepatocellular carcinoma and surrounding non-tumorous liver. Orv Hetil. 2016;157:1910–1918. doi: 10.1556/650.2016.30592. [DOI] [PubMed] [Google Scholar]
- 39.Cai L., Luo L., Tang Z., Meng X. Combined antitumor effects of 1,25dihydroxy vitamin D3 and Notch inhibitor in liver cancer. Oncol Rep. 2018;40:1515–1524. doi: 10.3892/or.2018.6549. [DOI] [PubMed] [Google Scholar]
- 40.Huang J., Yang G., Huang Y., Zhang S. 1,25(OH)2D3 induced apoptosis of human hepatocellular carcinoma cells in vitro and inhibited their growth in a nude mouse xenograft model by regulating histone deacetylase 2. Biochimie. 2018;146:28–34. doi: 10.1016/j.biochi.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Mondul A.M., Weinstein S.J., Moy K.A., Mannisto S., Albanes D. Circulating 25-hydroxyvitamin D and prostate cancer survival. Cancer Epidemiol Biomarkers Prev. 2016;25:665–669. doi: 10.1158/1055-9965.EPI-15-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song Z.Y., Yao Q., Zhuo Z., Ma Z., Chen G. Circulating vitamin D level and mortality in prostate cancer patients: a dose-response meta-analysis. Endocr Connect. 2018;7:R294–R303. doi: 10.1530/EC-18-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaughan-Shaw P.G., O'Sullivan F., Farrington S.M., Theodoratou E., Campbell H., Dunlop M.G. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: systematic review and meta-analysis. Br J Cancer. 2017;116:1092–1110. doi: 10.1038/bjc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maier S., Daroqui M.C., Scherer S., Roepcke S., Velcich A., Shenoy S.M. Butyrate and vitamin D3 induce transcriptional attenuation at the cyclin D1 locus in colonic carcinoma cells. J Cell Physiol. 2009;218:638–642. doi: 10.1002/jcp.21642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiang J.M., Tan R., Wang J.Y., Chen J.S., Lee Y.S., Hsieh P.S. S100P, a calcium-binding protein, is preferentially associated with the growth of polypoid tumors in colorectal cancer. Int J Mol Med. 2015;35:675–683. doi: 10.3892/ijmm.2015.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koh S.A., Lee K.H. HGF-mediated S100A11 overexpression enhances proliferation and invasion of gastric cancer. Am J Transl Res. 2018;10:3385–3394. [PMC free article] [PubMed] [Google Scholar]
- 47.Meng M., Sang L., Wang X. S100 calcium binding protein A11 (S100A11) promotes the proliferation, migration and invasion of cervical cancer cells, and activates Wnt/beta-catenin signaling. Onco Targets Ther. 2019;12:8675–8685. doi: 10.2147/OTT.S225248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou X., Zheng W., Nagana Gowda G.A., Raftery D., Donkin S.S., Bequette B. 1,25-dihydroxyvitamin D inhibits glutamine metabolism in Harvey-ras transformed MCF10A human breast epithelial cell. J Steroid Biochem Mol Biol 163. 2016:147–156. doi: 10.1016/j.jsbmb.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]