Abstract
Background
Low sodium intake stimulates the production and activity of renin. The aim is to analyse the association between a large range of sodium intake and the plasma renin activity (PRA).
Methods
We performed electronic searches for articles published between January 1st 1946 and March 18th 2020 and updated on January 21st 2021. Randomized controlled trials (RCTs) allocating participants to different sodium diets were included. Data were extracted from published reports. Meta-regression analyses of mean PRA versus mean sodium intake estimated by 24-hour urinary sodium excretion were performed. PROSPERO Registration number is CRD42020150355.
Findings
93 RCTs (102 interventions) were identified. In populations with usual/high sodium intake PRA was not associated with sodium intake. In populations with low sodium intake this association was mean -2·91 ng/ml/h per 100 mmol sodium (95% CI: -5·41– -0·42) in 60 studies of normotensive populations (n = 1769) and -1·91 ng/ml/h per 100 mmol sodium (-3·24 – -0·58) in 42 studies of hypertensive populations (n = 1267). The association of the change in PRA with the change in sodium intake was 1·32 ng/ml/h per 100 mmol sodium (0·47–2·18) in normotensive populations and 0·82 ng/ml/h per 100 mmol sodium (0·39–1·24) in hypertensive populations. Contrasting over-all bias assessments and potential effect modifiers had no independent impact on the sodium-PRA relationship. The variability between studies was considerable (I2 > 90%).
Interpretation
The accelerating effect of sodium reduction on PRA towards a sodium intake of zero mmol/24 h probably explains the interstudy variability. Further studies are needed to test whether this stimulating effect on PRA reflects a physiological disadvantage potentially associated with increased mortality
Funding
None
Research in context.
Evidence before this study
In 1963 Brown et al. in 6 participants showed that low sodium intake stimulated renin secretion, and in 1972 a cross-sectional study of 219 participants showed an inverse relationship between sodium intake and pl-renin. Amongst 4994 references not limited to English language searched in electronic databases and reference lists from January 1st 1946 to January 21st 2021 we identified RCTs allocating participants to different sodium diets, which measured pl-renin as outcome.
Study populations were included irrespective of sex, age and blood pressure. Studies including participants with treated hypertension or concomitant diseases, or which reported renin in unusable units were excluded.
We used the search terms: 1) salt or sodium, 2) restriction or dietary, 3) blood pressure or hypertension, 4) randomized or random. For hormones and lipids, the third search term was changed with the hormone or lipid term.
We identified 231 studies and for the present analysis we selected 93, which measured pl-renin as outcome. Risk of bias was estimated by the Cochrane risk of bias assessment tool.
Added value of this study
The present study shows that a stable sodium intake during several days causes a stable renin response, which, as shown in a previous cross-sectional study, accelerates at sodium intake below 100 mmol, especially when sodium intake approaches zero mmol.
Implications of all the available evidence
The renin-angiotensin-aldosterone system is involved in the regulation of inflammatory and thrombogenic mechanisms, which may explain why high plasma renin levels have been associated with increased mortality.
Therefore, the cut-off point of sodium intake for an increase in renin, which is about 100 mmol, might explain why sodium intake below 100 mmol in some studies has been associated with increased mortality
Alt-text: Unlabelled box
1. Introduction
In 1898 Tigerstedt and Bergman discovered renin [1,2]. The experiments of Goldblatt [3] accelerated this field of research in the subsequent decades [2] leading to the discovery of angiotensin [4,5] and characterization of the renin-angiotensin-aldosterone system (RAAS) [6]. In 1963 Brown et al. showed that low sodium intake stimulated the renin secretion [7], and in 1965 Thurau and Schnermann demonstrated that this stimulation took place in the macula densa of the juxtaglomerular apparatus [8]. In 1972 a cross-sectional study of 219 participants showed that plasma renin activity (PRA) increased with decreasing 24-h urine sodium excretion (a proxy for sodium intake) [9]. In 1975 Oliver et al. showed that PRA was persistently increased in a population with a lifelong low sodium intake [10]. Since then many randomized controlled trials (RCTs) have confirmed that a reduction in sodium intake leads to an increase in PRA [11]. As PRA is associated with mortality [12], this effect may be more important than assessed in recent sodium intake guidelines [13]. Consequently, the aim of the present study is to analyse the association between sodium intake and level of PRA and to associate the change in sodium intake with the change in PRA through a large range of sodium intake on the population level by means of meta-regression analyses of RCT data.
2. Methods
A protocol is registered in PROSPERO (Registration number: CRD42020150355,
https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=150355). The review is conducted in accordance with the PRISMA guidelines [14] and is based on an updated (March 18th 2020) identification of studies included in a previous meta-analysis (updated March 10th 2016) [15].
2.1. Search strategy and selection criteria
Types of studies: RCTs, double-blind or open, allocating participants to diets with different sodium contents, and in which the sodium intake was estimated by the 24-hour urinary sodium excretion.
Types of participants: Study populations were included irrespective of sex and age and stratified by blood pressure (BP) (normotension/hypertension). Hypertension was defined as systolic BP (SBP) ≥ 140 mmHg and/or diastolic (DBP) ≥ 90 mmHg. Study populations, in which participants were treated with antihypertensive treatments, were excluded, as such treatments have an influence on PRA. Studies systematically investigating participants with comorbidities, for instance diabetes or heart failure, were excluded. Studies, which tested for sodium sensitivity and excluded participants, who were either sodium sensitive or sodium resistant, were excluded.
Types of interventions: The intervention was a change in sodium intake, the study populations randomly being divided into a group eating a “low” sodium diet or a "usual/high" sodium diet. Confounding was not allowed, i.e. studies treating persons with a concomitant intervention were only included if the concomitant intervention was identical during the low and the high-sodium diet and the concomitant intervention did not have an impact on PRA. Studies, which reported renin in units, which cannot be directly translated to the most frequent unit (ng/ml/h) were also excluded.
Types of outcome measures: The outcome was the effect on PRA.
Search methods for identification of studies: The Cochrane Hypertension Information Specialist conducted systematic searches in MEDLINE, MEDLINE In-Process, EMBASE, CENTRAL, the Hypertension Group Specialised Register, and ClinicalTrials.gov using the following combinations of search terms: 1) salt or sodium, 2) restriction or dietary, 3) blood pressure or hypertension, 4) randomized or random. Similar searches were made for hormones and lipids changing the third search term (blood pressure or hypertension) with the hormone or lipid term. The search string is shown in the appendix.
Selection of studies: Two review authors (NG and GJ or NG and THG) independently performed study selection. Discrepancies were resolved by agreement.
2.2. Data analysis
Data extraction and management: Two authors (NG and GJ or NG and THG) independently recorded data from each trial. Discrepancies were resolved by agreement. The following data were recorded: The sample size (N); the mean age of participants; the mean weight of participants; the duration of the intervention; level of renin in plasma and standard deviations during low-sodium and usual/high-sodium diets. Other renin units than ng/mL/hour were when possible transformed to ng/mL/hour; the sodium reduction measured as the difference between 24-hour urinary sodium excretion during low-sodium and usual/high-sodium diets and standard deviation (SD); SBP (SD) and DBP (SD) before and after intervention; Assessment of risk of bias in included studies: This was performed using the Cochrane Risk of bias tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions [16] including selection bias, performance bias, detection bias and attrition bias. Measures of treatment effect: PRA measurements are continuous. Consequently, the outcome measure was defined as the mean difference (MD) with 95% confidence intervals (CI) between the changes from baseline to end of treatment during low- and high-sodium diets.
Dealing with missing data: If the SD was not reported, it was calculated from a given SE, 95% confidence interval (CI), P value or t value, estimated from a figure or imputed from the formula SD (change) = square root (SD1sq + SD2sq), SD1 is SD on blood pressure before intervention and SD2 is SD on blood pressure after intervention.
Assessment of heterogeneity: A Chi2 test was used to assess whether observed differences in results are compatible with chance alone. A low P value (or a large Chi2 statistic relative to its degree of freedom) provides evidence of heterogeneity of intervention effects (variation in effect estimates beyond chance). The Chi2 statistic can be transformed to a statistic (I2), which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error [16]. The I2 value can be interpreted as less important (0–40%), moderate 30–60%, substantial 50–90% and considerable (75–100%) [16]. The I2 value was used to describe the percentage of heterogeneity in the individual analyses. Data synthesis: The mean difference (MD) was calculated for PRA with identical units in the included studies. As we accumulated data from a series of studies that had been performed by researchers operating independently, and as the goal of the analysis was to extrapolate to other populations, we used a random-effects model to estimate the summary measure as the mean of a distribution of effects. The following associations were analysed with PRA as the dependant variable: 1) Association between 24-h urinary sodium excretion and PRA in study populations randomized to normal/high sodium intake; 2) Association between 24-h urinary sodium excretion and PRA in study populations randomized to low sodium intake; 3) Association between sodium reduction (difference in sodium intake between low and high sodium intake dietary groups) and effects on PRA calculated as the difference between the change during intervention in the low and high sodium intake dietary groups. For each of these 3 datasets, we quantified the association between sodium intake and PRA using univariable meta-regression analyses weighted by the inverse variance of the effect on PRA. Furthermore, we quantified the association between potential effect modifying covariates (study duration, age, SBP, DBP and weight) and PRA. Finally, we quantified the association between PRA and sodium intake and selected effect modifying covariates in multi variable regression analysis weighted by the inverse variance of the effect on PRA. We used “R” [17] version 3.6.3.
Risk of bias across studies: To investigate possible heterogeneity due to methodological diversity, subgroup analyses was performed for groups with contrasting assessments of overall-bias appearing from the risk of bias analysis. The stratification by BP may prevent some heterogeneity and meta-regression analyses of potential effect modifiers may reveal other sources of heterogeneity.
Additional analyses: To investigate the potential effect of the method of sodium administration we stratified the studies into a group, which ingested sodium by diet alone and into a group, which was treated with salt tablets or placebo tablets.
2.3. Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
The search date for identification of studies was March 18, 2020. A post-hoc search to identify recent studies not included in the analysis was performed January 21st, 2021, but this search did not reveal further eligible studies.
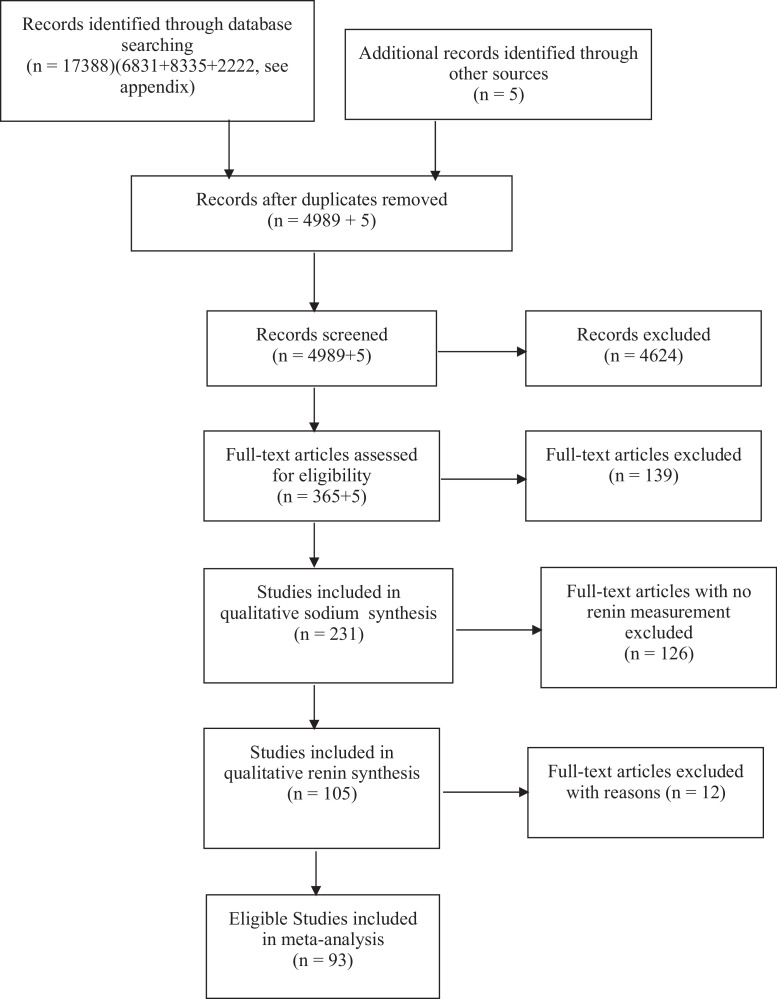
A flow chart of 93 included studies (appendix reference list 17-109) is shown in Fig. 1. Reasons for exclusion of studies are explained in the appendix. The trend of the PRA response to sodium intake in the excluded studies did not differ from the trend in the included studies. Nine studies supplied data on both hypertensive and normotensive individuals resulting in 102 study interventions. Characteristics of included studies are shown in appendix table 1. The median 24-h sodium excretion decreased from 213 mmol/24 h (range:110–386) in the usual/high sodium group to 36 mmol/24 h (range: 6–113) in the low sodium group in 60 studies of normotensive populations (n = 1769) and from 199 mmol/24 h (range 94–343) to 43 mmol/24 h (range 5–110) in 42 studies of hypertensive populations (n = 1267). The individual study outcomes, the overall effects and the heterogeneity analyses are shown in appendix Fig. 1, revealing considerable heterogeneity across studies (94% and 97%). The bias assessments of the individual studies are shown in appendix table 2.
Fig. 1.
Study selection.
Table 1.
Univariate weighted meta-regression analysis of plasma renin activity versus 24-h urinary sodium excretion and potential effect modifiers (duration, age, SBP, DBP and weight).
| A: Low sodium intake, Normotension, association with pl-renin activity (weighted) | ||||||||||
| Coefficient | SE | t | p | 2.5% | 97.5% | Intercept | SE | t | p | |
| Sodium intake (low) | −0,0291 | 0,0125 | −2,3354 | 0,0230 | −0,0541 | −0,0042 | 4,1870 | 0,5895 | 7,1022 | 0,0000 |
| Duration | −0,0929 | 0,0419 | −2,2173 | 0,0305 | −0,1768 | −0,0090 | 3,8742 | 0,4963 | 7,8061 | 0,0000 |
| Age | 0,0152 | 0,0309 | 0,4924 | 0,6243 | −0,0467 | 0,0772 | 2,4154 | 1,0373 | 2,3285 | 0,0235 |
| SBP (low sodium) | −0,0106 | 0,0498 | −0,2123 | 0,8326 | −0,1104 | 0,0892 | 4,1910 | 5,8160 | 0,7206 | 0,4742 |
| DBP (low sodium) | −0,0109 | 0,0533 | −0,2054 | 0,8380 | −0,1177 | 0,0958 | 3,7516 | 3,7529 | 0,9996 | 0,3219 |
| Weight (kg) | −0,0056 | 0,0422 | −0,1317 | 0,8958 | −0,0906 | 0,0795 | 3,1420 | 3,0882 | 1,0174 | 0,3144 |
| B: Low sodium intake, Hypertension, association with pl-renin activity (weighted) | ||||||||||
| Coefficient | SE | t | p | 2.5% | 97.5% | Intercept | SE | t | p | |
| Sodium intake (low) | −0,0191 | 0,0066 | −2,9013 | 0,0060 | −0,0324 | −0,0058 | 3,3476 | 0,4138 | 8,0905 | 0,0000 |
| Duration | −0,0569 | 0,0177 | −3,2113 | 0,0026 | −0,0927 | −0,0211 | 3,2910 | 0,3675 | 8,9561 | 0,0000 |
| Age | −0,0306 | 0,0207 | −1,4786 | 0,1471 | −0,0725 | 0,0112 | 3,7748 | 1,0150 | 3,7188 | 0,0006 |
| SBP (end) | −0,0149 | 0,0195 | −0,7635 | 0,4496 | −0,0543 | 0,0245 | 4,4364 | 2,7933 | 1,5882 | 0,1201 |
| DBP (end) | −0,0088 | 0,0291 | −0,3042 | 0,7626 | −0,0676 | 0,0499 | 3,0986 | 2,6009 | 1,1914 | 0,2407 |
| Weight (kg) | 0,0056 | 0,0322 | 0,1732 | 0,8635 | −0,0598 | 0,0710 | 1,7460 | 2,4556 | 0,7111 | 0,4818 |
| C: Usual/high sodium intake, Normotension, association with pl-renin activity (weighted) | ||||||||||
| Coefficient | SE | t | p | 2.5% | 97.5% | Intercept | SE | t | p | |
| Sodium intake (high) | 0,0000 | 0,0018 | −0,0073 | 0,9942 | −0,0036 | 0,0036 | 0,9309 | 0,3942 | 2,3617 | 0,0216 |
| Duration | −0,0048 | 0,0121 | −0,3954 | 0,6940 | −0,0290 | 0,0194 | 0,9726 | 0,1434 | 6,7842 | 0,0000 |
| Age | 0,0010 | 0,0093 | 0,1060 | 0,9160 | −0,0176 | 0,0195 | 0,8911 | 0,3107 | 2,8679 | 0,0058 |
| SBP (high sodium) | 0,0148 | 0,0134 | 1,1046 | 0,2741 | −0,0120 | 0,0415 | −0,8078 | 1,5842 | −0,5099 | 0,6121 |
| DBP (high sodium) | −0,0131 | 0,0160 | −0,8189 | 0,4164 | −0,0451 | 0,0189 | 1,8653 | 1,1307 | 1,6496 | 0,1047 |
| Weight (kg) | 0,0095 | 0,0145 | 0,6543 | 0,5163 | −0,0197 | 0,0386 | 0,2182 | 1,0586 | 0,2061 | 0,8376 |
| D: Usual/high sodium intake, Hypertension, association with pl-renin activity (weighted) | ||||||||||
| Coefficient | SE | t | p | 2.5% | 97.5% | Intercept | SE | t | p | |
| Sodium intake (high) | −0,0006 | 0,0017 | −0,3855 | 0,7019 | −0,0041 | 0,0028 | 1,1019 | 0,3608 | 3,0537 | 0,0040 |
| Duration | 0,0038 | 0,0087 | 0,4318 | 0,6682 | −0,0139 | 0,0214 | 0,9033 | 0,1812 | 4,9844 | 0,0000 |
| Age | −0,0116 | 0,0092 | −1,2629 | 0,2140 | −0,0302 | 0,0070 | 1,5234 | 0,4506 | 3,3809 | 0,0016 |
| SBP (high sodium) | 0,0037 | 0,0086 | 0,4355 | 0,6656 | −0,0136 | 0,0210 | 0,4101 | 1,2859 | 0,3189 | 0,7514 |
| DBP (high sodium) | 0,0081 | 0,0126 | 0,6400 | 0,5259 | −0,0175 | 0,0337 | 0,2316 | 1,1720 | 0,1976 | 0,8444 |
| Weight (kg) | 0,0027 | 0,0167 | 0,1639 | 0,8707 | −0,0312 | 0,0367 | 0,7512 | 1,2739 | 0,5897 | 0,5592 |
| E: Change in sodium intake, Normotension, association with pl-renin activity difference (weighted) | ||||||||||
| Coefficient | SE | t | p | 2.5% | 97.5% | Intercept | SE | t | p | |
| Sodium reduction | 0,0132 | 0,0043 | 3,0957 | 0,0030 | 0,0047 | 0,0218 | −0,2171 | 0,7836 | −0,2771 | 0,7827 |
| Duration | −0,0881 | 0,0347 | −2,5378 | 0,0139 | −0,1576 | −0,0186 | 2,9016 | 0,4113 | 7,0554 | 0,0000 |
| Age | 0,0142 | 0,0253 | 0,5625 | 0,5760 | −0,0365 | 0,0650 | 1,5246 | 0,8490 | 1,7957 | 0,0778 |
| SBP (high sodium) | −0,0349 | 0,0365 | −0,9576 | 0,3424 | −0,1080 | 0,0382 | 6,1419 | 4,3275 | 1,4193 | 0,1614 |
| DBP (high sodium) | −0,0303 | 0,0434 | −0,6985 | 0,4878 | −0,1174 | 0,0567 | 4,1682 | 3,0768 | 1,3547 | 0,1810 |
| Weight (kg) | −0,0150 | 0,0326 | −0,4604 | 0,6475 | −0,0808 | 0,0507 | 2,9233 | 2,3866 | 1,2248 | 0,2270 |
| F: Change in sodium intake, Hypertension, association with pl-renin activity difference (weighted) | ||||||||||
| Coefficient | SE | t | p | 2.5% | 97.5% | Intercept | SE | t | p | |
| Sodium reduction | 0,0082 | 0,0021 | 3,8625 | 0,0004 | 0,0039 | 0,0124 | 0,1081 | 0,3583 | 0,3018 | 0,7644 |
| Duration | −0,0607 | 0,0134 | −4,5410 | 0,0001 | −0,0877 | −0,0337 | 2,3877 | 0,2771 | 8,6169 | 0,0000 |
| Age | −0,0190 | 0,0174 | −1,0961 | 0,2796 | −0,0541 | 0,0161 | 2,2513 | 0,8502 | 2,6481 | 0,0115 |
| SBP (high sodium) | −0,0177 | 0,0159 | −1,1152 | 0,2714 | −0,0498 | 0,0144 | 3,9924 | 2,3836 | 1,6749 | 0,1017 |
| DBP (high sodium) | −0,0197 | 0,0238 | −0,8281 | 0,4127 | −0,0679 | 0,0284 | 3,1520 | 2,2065 | 1,4285 | 0,1611 |
| Weight (kg) | 0,0028 | 0,0260 | 0,1094 | 0,9135 | −0,0499 | 0,0556 | 0,9949 | 1,9801 | 0,5024 | 0,6185 |
Table 2.
Multivariate weighted meta-regression analysis of plasma renin activity versus 24-h urinary sodium excretion, age and SBP.
| A: Low sodium intake, Normotension, association with pl-renin activity (weighted) | ||||||
| Estimate | SE | t | p | 2.5% | 97.5% | |
| Sodium intake (low) | −0,0314 | 0,0130 | −2,4197 | 0,0191 | −0,0575 | −0,0054 |
| Age | 0,0250 | 0,0301 | 0,8299 | 0,4104 | −0,0355 | 0,0855 |
| SBP (low sodium) | 0,0291 | 0,0488 | 0,5970 | 0,5531 | −0,0688 | 0,1270 |
| (Intercept) | −0,1057 | 5,4402 | −0,0194 | 0,9846 | −11,0223 | 10,8108 |
| B: Low sodium intake, Hypertension, association with pl-renin activity (weighted) | ||||||
| Estimate | SE | t | p | 2.5% | 97.5% | |
| Sodium intake (low) | −0,0193 | 0,0075 | −2,5875 | 0,0136 | −0,0344 | −0,0042 |
| Age | −0,0201 | 0,0230 | −0,8744 | 0,3874 | −0,0666 | 0,0264 |
| SBP (low sodium) | 0,0165 | 0,0220 | 0,7513 | 0,4571 | −0,0279 | 0,0609 |
| (Intercept) | 1,9637 | 2,7276 | 0,7199 | 0,4760 | −3,5581 | 7,4855 |
| C: Usual/high sodium intake, Normotension, association with pl-renin activity (weighted) | ||||||
| Estimate | SE | t | p | 2.5% | 97.5% | |
| Sodium intake (high) | 0,0010 | 0,0019 | 0,5123 | 0,6106 | −0,0029 | 0,0049 |
| Age | −0,0049 | 0,0102 | −0,4848 | 0,6298 | −0,0254 | 0,0155 |
| SBP (high sodium) | 0,0199 | 0,0155 | 1,2818 | 0,2055 | −0,0112 | 0,0509 |
| (Intercept) | −1,4705 | 1,8988 | −0,7744 | 0,4421 | −5,2790 | 2,3380 |
| D: Usual/high sodium intake, Hypertension, association with pl-renin activity (weighted) | ||||||
| Estimate | SE | t | p | 2.5% | 97.5% | |
| Sodium intake (high) | −0,0017 | 0,0017 | −0,9829 | 0,3319 | −0,0052 | 0,0018 |
| Age | −0,0247 | 0,0117 | −2,1053 | 0,0419 | −0,0484 | −0,0009 |
| SBP (high sodium) | 0,0159 | 0,0103 | 1,5495 | 0,1296 | −0,0049 | 0,0366 |
| (Intercept) | 0,1207 | 1,3536 | 0,0892 | 0,9294 | −2,6194 | 2,8608 |
| E: Change in sodium intake, Normotension, association with pl-renin activity (weighted) | ||||||
| Estimate | SE | t | p | 2.5% | 97.5% | |
| Sodium reduction | 0,0137 | 0,0037 | 3,6817 | 0,0005 | 0,0062 | 0,0211 |
| Age | 0,0134 | 0,0217 | 0,6176 | 0,5395 | −0,0301 | 0,0569 |
| SBP (high sodium) | 0,0142 | 0,0347 | 0,4100 | 0,6835 | −0,0553 | 0,0837 |
| (Intercept) | −2,5696 | 4,2267 | −0,6079 | 0,5458 | −11,0472 | 5,9081 |
| F: Change in sodium intake, Hypertension, association with pl-renin activity (weighted) | ||||||
| Estimate | SE | t | p | 2.5% | 97.5% | |
| Sodium reduction | 0,0084 | 0,0023 | 3,5853 | 0,0009 | 0,0036 | 0,0131 |
| Age | 0,0113 | 0,0199 | 0,5663 | 0,5745 | −0,0290 | 0,0516 |
| SBP (high sodium) | −0,0112 | 0,0172 | −0,6483 | 0,5207 | −0,0461 | 0,0237 |
| (Intercept) | 1,2086 | 2,2905 | 0,5277 | 0,6008 | −3,4282 | 5,8455 |
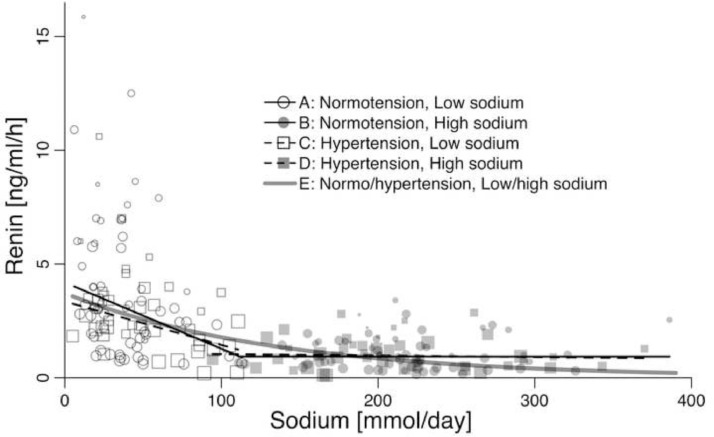
In populations with low sodium intake the association of PRA with sodium intake was −2·91 ng/ml/h per 100 mmol sodium (95% CI: −5·41– −0·42) in normotensive populations (Fig. 2A, table 1A) and −1·91 ng/ml/h per 100 mmol sodium (−3·24 – −0·58) in hypertensive populations (Fig. 2C, table 1B). PRA was also associated with duration of sodium intake (table 1A and 1B), but not with age, SBP, DBP, or weight (table 1A and 1B). In populations with a usual/high sodium intake PRA was not associated with sodium intake (Fig. 2B and 2D), the duration of sodium intake, age, SBP, DBP, or weight (table 1C and 1D). A non-linear least squares analysis of all data indicates that the increase in PRA with decreasing sodium intake is of exponential nature (Fig. 2E).
Fig. 2.
The relationship between the mean PRA and mean sodium intake/day assessed as 24-h urinary sodium excretion in 60 studies of normotensive individuals and 42 studies of untreated hypertensive individuals.
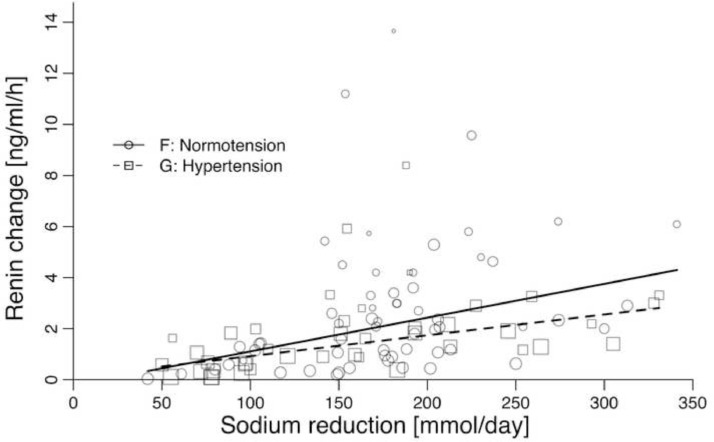
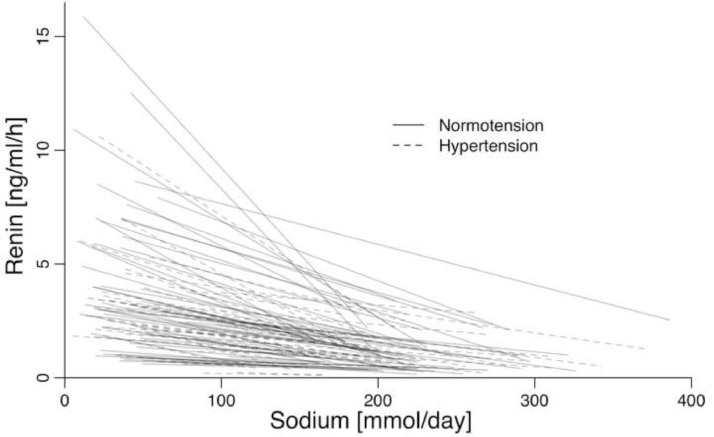
The association of the difference in PRA between usual/high sodium intake and low sodium intake with the difference in sodium intake was 1·32 ng/ml/h per 100 mmol sodium (0·47–2·18) in normotensive populations (table 1E, Fig. 3F) and 0·82 ng/ml/h per 100 mmol sodium (0·39–1·24) in hypertensive populations (table 1F, Fig. 3G). PRA was also associated with duration of sodium intake (table 1E and 1F), but not with age, SBP, DBP, or weight (table 1E and 1F). Fig. 4 shows the relationship between the change in PRA and the change in sodium intake in each of the 102 studies.
Fig. 3.
The relationship between the change in mean PRA and the change in mean sodium intake/day assessed as 24-h urinary sodium excretion in 60 studies of normotensive individuals and 42 studies of untreated hypertensive individuals.
Fig. 4.
The within-study relationship between the change in renin and the change in sodium intake in 60 studies of normotensive individuals and 42 studies of untreated hypertensive individuals.
Table 2 shows the outcomes of the multivariable analyses. We included SBP and excluded DBP because the association for SBP with PRA generally was less weak than the association for DBP with PRA (Table 1). Weight was excluded because of the very weak associations in the univariable analyses (Table 1), and because weight was not recorded in all studies. Duration was not included in the model because of strong collinearity with sodium intake (Appendix Fig. 2, Fig. 3, Fig. 4). The coefficients in the multivariable analyses (table 2) are very similar to the univariable coefficients (table 1), indicating that the interaction between the effect modifiers is negligible.
4. Discussion
The present analysis of RCTs with a duration of 4–42 days shows that at sodium-intake higher than 100 mmol PRA was not associated with decreasing sodium intake (Fig. 2), but at sodium-intake lower than 100 mmol an inverse relationship of exponential nature appeared (Fig. 2). Accordingly, sodium reduction leads to an increase in PRA (Fig. 3), which is higher, when the target of sodium intake is low compared with a moderate target (Fig. 4). PRA was inversely associated with study duration (table 1, A-B), probably because sodium intake generally did not reach low levels in studies with longer duration (appendix Fig. 2, Fig. 3). Similarly, the association of the change in PRA with duration (table 1, E-F) probably reflects the inverse association between sodium reduction and duration (appendix Fig. 4). These findings may reflect that researchers generally avoided to expose participants to extreme sodium intakes for a longer period. An observational study of a low sodium culture has verified that the increase in PRA is persistent, if the sodium intake is continuously low [10]. Whether the long-term increase in PRA is attenuated compared with the short-term increase in PRA needs to be clarified in longer-term RCTs.
Fig. 3 shows the association between a change in sodium intake and the corresponding change in PRA. As this relationship probably depends more on the target of low sodium intake, than on a biological relationship with the amount of sodium reduction, we did not find it meaningful to explore non-linear functions. Fig. 3 indicates that PRA responses seem stronger in normotensive than in hypertensive populations. The normotensive studies reduce sodium intake to a median of 36 mmol, whereas the hypertensive studies reduce to a median of 43 mmol. This could in part explain the difference between the two regression lines. An additional explanation could be that hypertensive individuals may have a weaker PRA response to sodium depletion.
The level of sodium intake at which a marked increase in PRA is initiated seems to be about 100 mmol, which may reflect a physiologically important limit of sodium intake and may in part explain why 95% of the World's populations have a sodium intake above 100 mmol [18]. As a marker of RAAS activity this PRA increase may explain why the effect of sodium reduction on BP is small, especially in healthy individuals with normal BP [11,15] and may contribute to an increased risk of mortality [12], although an increase in PRA is not always a predictor of increased mortality. For instance, some antihypertensive treatments reduce mortality despite an increase in PRA. However, in hypertensive patients it is likely that the BP lowering advantage of an antihypertensive treatment is bigger than the disadvantage of an increase in PRA. In contrast, sodium reduction may create an inappropriate balance between a relatively weak BP lowering effect and a stronger increase in PRA, which may explain the association of low sodium intake with increased mortality [19,20].
It is a strength that only RCTs were included and both the intervention (24-h urinary sodium excretion) and the outcome (PRA) were measured by laboratories supposed to be ignorant of the randomization status of the participants, i.e. the risk of detection bias is assessed to be low. Potential effect modifiers could not explain the considerable heterogeneity (>90%), because either they were not independently associated with PRA (age, SBP, DBP, weight), or they were concurrently associated with sodium intake (duration, bias assessment, method of sodium reduction). PRA may be influenced by acute water loading [21] or furosemide stimulation [22] but none of the included PRA values were obtained after water loading or furosemide stimulation. Thus, the main explanation for the high degree of heterogeneity was probably that the association of sodium reduction with PRA was not linear through the whole range of sodium intake, but accelerated when the target of sodium intake was very low compared with a moderate sodium intake target. A contributing factor to the observed heterogeneity could be that most of the studies included in this meta-regression analysis used the PRA method (PRA: measurement of renin by its enzymatic, angiotensin I-generating activity on its endogenous substrate), which generally have poor intra- and interlaboratory reproducibility compared with the direct measurement of renin by immunoradiometric assay [23]. There was a high degree of consistency, as all studies showed the same trend towards an increase in PRA, when sodium intake was reduced (Fig. 4, appendix Fig. 1). The grade of evidence is therefore considered to be high. The number of studies included in the PRA analyses (n = 102) is substantial as is the number of participants (1769 normotensive and 1267 hypertensive individuals) further emphasizing the robustness of the analyses. Although it is a limitation that individual study data were not available, it is worth noticing that the only study, which have presented individual PRA values as a function of a large range of sodium intake, found an association similar to the one presented here [9]. In conclusion the present results confirm the original findings [7], [8], [9], [10] that sodium intake is the essential independent factor, which is associated with PRA in study populations of untreated healthy and hypertensive individuals. Sodium reduction leads to proportional increase in PRA, which accelerates at sodium intakes below 100 mmol. Future guidelines should take such associations into consideration, when assessing dietary reference intakes for sodium.
Data sharing statement
Study protocol with statistical analysis plan is available in PROSPERO. All data used in the present analyses are available in appendix Fig. 1 and appendix tables 1 and 2. Requests to use the data for other purposes than control of the present publication should be directed to graudal@dadlnet.dk.
Authors’ contributions
NG created the original search strategy, which was elaborated by the Cochrane Hypertension Group. NG, THG and GJ screened articles and collected data. THG and NG did the statistical analyses. NG wrote the first draft with input from THG and GJ. All authors verified the data and had responsibility for the decision to submit the manuscript.
Funding
There was no funding source for this study.
Declaration of Competing Interest
We declare no competing interests
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100750.
Appendix. Supplementary materials
References
- 1.Tigerstedt R., Bergman P.G. Niere und Kreislauf. Skand Arch Physiol. 1898;8:223–271. [Google Scholar]
- 2.Basso N., Terragno N.A. History about the discovery of the renin-angiotensin system. Hypertension. 2001;38:1246–1249. doi: 10.1161/hy1201.101214. [DOI] [PubMed] [Google Scholar]
- 3.Goldblatt H., Lynch J., Hanzal R.F., Summerville W.W. Studies on experimental hypertension, I: the production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun-Menéndez E., Fasciolo J.C., Leloir L.F., Muñoz J.M. La sustancia hipertensora de la sangre del riñón isquemiado. Rev Soc Arg Biol. 1939;15:420–425. [Google Scholar]
- 5.Page I.H., Helmer O.M. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. J Exp Med. 1940;71:29–42. doi: 10.1084/jem.71.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun-Menéndez E., Page I.H. Suggested revision of nomenclature: angiotensin. Science. 1958;127:242. doi: 10.1126/science.127.3292.242-a. [DOI] [PubMed] [Google Scholar]
- 7.Brown J.J., Davies D.L., Lever A.F., Robertson J.I. Influence of sodium loading and sodium depletion on plasma-renin in man. Lancet. 1963;2(7302):278–279. doi: 10.1016/s0140-6736(63)90176-4. [DOI] [PubMed] [Google Scholar]
- 8.Thurau K., Schnermann J. Die Natriumkonzentration an den Macula Densa-Zellen als regulierender Faktor für das Glomerulumfiltrat (Mikropunktionsversuche) Klin Wochenschr. 1965;43:410–413. doi: 10.1007/BF01483845. [DOI] [PubMed] [Google Scholar]
- 9.Brunner H.R., Laragh J.H., Baer L. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med. 1972;286:441–449. doi: 10.1056/NEJM197203022860901. [DOI] [PubMed] [Google Scholar]
- 10.Oliver W.J., Cohen E.L., Neel J.V. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a "no-sodium" culture. Circulation. 1975;52:146–151. doi: 10.1161/01.cir.52.1.146. [DOI] [PubMed] [Google Scholar]
- 11.Graudal N.A., Galløe A.M., Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta-analysis. JAMA. 1998;279:1383–1391. doi: 10.1001/jama.279.17.1383. [DOI] [PubMed] [Google Scholar]
- 12.De Boer R.A., Schroten N.F., Bakker S.J. Plasma renin and outcome in the community: data from PREVEND. Eur Heart J. 2012;33:2351–2359. doi: 10.1093/eurheartj/ehs198. [DOI] [PubMed] [Google Scholar]
- 13.National Academies of Sciences, Engineering, and Medicine . The National Academies Press; Washington, DC: 2019. Dietary reference intakes for sodium and potassium. [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group PRISMA. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 15.Graudal N., Hubeck-Graudal T., Jürgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Datab Syst Rev. 2017;4 doi: 10.1002/14651858.CD004022.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011] Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]Cochrane Collab. 2011 www.cochrane-handbook.org Available from. [Google Scholar]
- 17.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: a language and environment for statistical computing. [Google Scholar]
- 18.McCarron D.A., Kazaks A.G., Geerling J.C., Stern J.S., Graudal N.A. Normal range of human dietary sodium intake: a perspective based on 24-hour urinary sodium excretion worldwide. Am J Hypertens. 2013;26:1218–1223. doi: 10.1093/ajh/hpt139. [DOI] [PubMed] [Google Scholar]
- 19.Mente A., O'Donnell M., Rangarajan S. PURE, EPIDREAM and ONTARGET/TRANSCEND Investigators. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388(10043):465–475. doi: 10.1016/S0140-6736(16)30467-6. [DOI] [PubMed] [Google Scholar]
- 20.Graudal N., Jürgens G., Baslund B., Alderman M.H. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens. 2014;27:1129–1137. doi: 10.1093/ajh/hpu028. [DOI] [PubMed] [Google Scholar]
- 21.Burnier M., Pechère-Bertschi A., Nussberger J., Waeber B., Brunner H.R. Studies of the renal effects of angiotensin II receptor blockade: the confounding factor of acute water loading on the action of vasoactive systems. Am J Kidney Dis. 1995;26:108–115. doi: 10.1016/0272-6386(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 22.Koolen M.I., Brummelen P. Sodium sensitivity in essential hypertension: role of the renin-angiotensin-aldosterone system and the predictive value of an intravenous frusemide test. J Hypertens. 1984;2:55–59. doi: 10.1097/00004872-198402000-00010. [DOI] [PubMed] [Google Scholar]
- 23.De Bruin R.A., Bouhuizen A., Diederich S., Perschel F.H., Boomsma F., Deinum J. Validation of a new automated renin assay. Clin Chem. 2004;50:2111–2116. doi: 10.1373/clinchem.2004.032052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.