Graphical abstract
Keywords: Luffa aegyptiaca, GC/MS, UHPLC/MS, Metabolomics, SPME
Abstract
Introduction
Luffa aegyptiaca Mill, sponge gourd or Egyptian cucumber, is grown worldwide for its edible fruit consumed as a vegetable like cucumber. Unlike young fruit (YF), the fully mature ripened fruit (MF) is strongly fibrous and is used as a cleanser to make scrubbing bath sponges. YF undergoes a complex series of physiological and biochemical changes during fruit ripening. However, the chemical compositional differences between YF and MF in Luffa aegyptiaca have not been distinguished to date.
Objectives
Comprehensively compare the metabolites profile of YF and MF to give insight on how maturation stage affects chemical composition.
Methods
Mass-based metabolomics comprising GC/MS and UHPLC/MS were adopted in this study targeting its volatile and non-volatile metabolites coupled with chemometrics to rationalize for the differences.
Results
A total of 53 volatile metabolites were identified via headspace solid phase microextraction (SPME) comprising 66.2% aldehydes/furans, 51.6% alcohols, 38.2% ketones, 15.1% acids and 10.1% aromatics of which aldehydes/ furans were dominant at both fruit stages. Young fruit was in general more erniched in metabolites as revealed from UHPLC/MS and GC/MS analyses. The YF group encompassed higher levels of short chain alcohols (1-octen-3-ol) and aldehydes ((E)-2-hexenal and cucumber aldehyde) in addition to terpenoids (linalool). In contrast, fatty acids (octanoic acid) predominated MF specimens. UHPLC/MS analysis revealed for several oleanene triterpene glycosides as major secondary bioactive compounds, dihydroxy-oxo-oleanenoic acid glycoside found more abundant in YF versus MF as revealed from multivariate data analyses.
Conclusions
Our results reveal for the distinct metabolite changes in L. aegyptiaca fruit in its different stages and to rationalize for its different usage.
Introduction
Luffa aegyptiaca (Mill), a synonym of Luffa cylindrica (Linn) M.Roem, is a unique vegetable member of the Cucurbitaceae family, native to Asia and widely cultivated in several tropical and subtropical regions worldwide for its economical, medicinal and nutritional uses [1]. It iscommonly named sponge gourd, vegetable sponge, bath sponge, dish cloth gourd and loofa [2], [3].
The green young fruit (YF) is edible either as raw like cucumber or after being cooked as in squash. A myriad of metabolites are reported in its YF. i.e., phenolic acids, flavonoids, vitamins, carotenoids, saponins and triterpenes which add to its health effects [4]. In contrast, the abrasive nature of the fibrous sponge gourd mature fruit (MF) allows for its use in skin exfoliation, body cleansing and or stimulation of blood circulation [5]. Traditionally, in Japan, the “Hechimasui” or the water extract of the plant’s vascular bundle, is used as skin lotion owing to its saponins content aside from amino acids and minerals which help maintain the skin healthy [6]. Industrially, L. aegyptiaca is used for water purification acting as adsorbent for heavy metals from waste water [3]. With regards to its health benefit effects, L. aegyptiaca fruits exhibit diverse traditional biological activities such as anthelmentic, stomachic, purgative, emollient, tonic, galactagogue and antipyretic, also useful intreatment of syphilis, tumours, lung complaints, splenopathy and leprosy along with nephritis and jaundice [5], [7]. While externally it is used for rheumatism, backache, as well as in hemorrhoids [5]. Chemical content mediating for luffa fruit uses include flavonoids, saponins and steroidal compounds [8]. Many phenolic and flavonoid glycosides were separated using antioxidant-guided assay suggesting that consumption of sponge gourds can mitigate against oxidative stress in human body [9]. Polyphenolic metabolites in luffa may also contribute to its anti-inflammatory action by inhibiting LPS-induced NO generation [9], [10]. Another two compounds named, 3-hydroxy-1-methylene-tetrahydroxy-napthalene-2-carbaldehyde and dihydroxy spinasterol, were isolated from L. aegyptiaca fruit petroleum ether extract and found to exhibit moderate antimicrobial action [11].
Seeds encompassed within L. Aegyptiaca fruit are also recognized with several effects. For example, luffin-a and luffin-b are two proteins isolated from L. aegyptiaca seeds to exhibit ribosome-inactivating cytotoxic action along with an abortifacient effect [12]. Luffacylin is another peptide isolated from sponge gourd seeds with anti-fungal activity against Mycosphaerella arachidicola and Fusarium oxysporum [13]. Two triterpenoids isolated from L. aegyptiaca seeds i.e., oleanolic acid and echinocystic acid exhibited immune-stimulatory effect [14]. An investigation by Muthumani, Meera [15] assessed L. aegyptiaca seeds anti- inflammatory, bronchodilator and antimicrobial activities against S. aureus and Candida albicans. In addition, the polypeptide’’luffin P1′’isolated from seeds of L. aegyptiaca exhibited anti-HIV-1 activity [16].
Luffa peel was also found to be enriched in phytonutrients at higher levels relative to its pulp with more potent anti-inflammatory effect similar to citrus peel [17]. Considering that fruit undergoes major changes in its composition upon ripening to affect its effects and usage warrants for monitoring such changes using analytical tools. Indeed, no detailed study has uncovered compositional difference in metabolites of L. aegyptiaca fruits represented by young and mature stages.
Analytical techniques that aid in profiling a complex mixture of metabolites in biological systems are increasingly applied in food research and to employ chromatographic separation using either gas chromatography (GC) or liquid chromatography (LC) coupled to mass spectrometry (MS) targeting its volatile and non volatile metabolites, respectively.
In this study, headspace solid phase micro-extraction (SPME) was used to collect volatile constituents of Luffa aegyptiaca prior to its analysis using GC–MS. SPME is a fast, non-solvent technique that enables volatiles sampling emitted from the plants at low levels and is superior to hydro-distillation in being sensitive due to thermal heating without the generation of artifacts [18], [19], [20].
For analysis of non-volatile high molecular weight secondary metabolites, ultra high performance liquid chromatography (UHPLC) coupled to ESI-mass detector is a well developed technology that provides monitoring of a large number of metabolite peaks at high sensitivity [21], [22]. Considering the complexity of metabolites dataset and to assess for differences in L. aegyptiaca fruits at two maturation stages in an untargeted manner, multivariate data analyses were employed. Principal component analysis (PCA) as an unsupervised clustering way without dataset knowledge alongside supervised OPLS were employed for analysing GC–MS and UHPLC-MS datasets [23], [24].
To the best of our knowledge, detailed metabolites profile of L. aegyptiaca and impact of maturity stage upon its chemical composition have never been addressed before, issues of value to rationalize for its different uses and or biological effects.
Materials & methods
Plant material
Luffa aegyptiaca fruits were collected fresh from the field at Behira Governerate, Egypt at the two different ripening stages, young (YF) and old mature (MF) during the month of May 2016. The description regarding old versus mature is based on the fruit pulp (interior part) in case of young is a soft vegetable part whereas in case of old mature the pulp is almost completely fibrous. Voucher specimens are deposited at the Department of Pharmacognosy, College of Pharmacy, Cairo University.
Chemicals and fibers
SPME fiber of stableflex coated with divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS, 50/30 µm) was purchased by Supelco (Oakville, ON, Canada). All chemicals and standards were purchased from Sigma Aldrich (St. Louis, MO, USA). Acetonitrile and formic acid (LC–MS grade) were obtained from J. T. Baker (Netherlands), milliQ water was used for UHPLC/PDA/ESI–Orbitrap HRMS analysis.
Headspace volatiles analysis of L. aegyptiaca
The HS-SPME volatile analysis was carried as stated in [20] with slight modifications. Dried finely ground fruits (100 mg) were placed in SPME screw cap vials (1.5 ml) and spiked with (Z)-3-hexneyl acetate dissolved in water at a final concentration of 2 µg per vial. The SPME fiber was inserted manually into vial containing samples placed in an oven kept at 50 °C for 30 min. The fiber was subsequently withdrawn into the needle and then injected into the injection port of the gas chromatography-mass spectrometer (GC–MS). GC–Ms analysis was performed on a Schimadzu GC-17A gas chromatogram equipped with DB-5 column (30 m × 0.25 mm i.d. ×0.25 µm film thickness; Supelco) and coupled to Schimadzu QP5050A mass spectrometer. The interface and the injector tempreatures were both set at 220 °C. The following gradient temperature program was used for volatiles analysis. The oven temperature was kept first at 40 °C for 3 min, then increased to 180 °C at a rate of 12 °C min−1, kept at 180 °C for 5 min, and finally ramped at a rate of 40 °C min−1 to 240 °C and kept at this temperature for 5 min. The carrier gas Helium was used at a total flow rate of 0.9 ml/min. Splitless injection mode was used for analysis considering the lower levels of volatiles in samples. SPME fiber was prepared to the next analysis by placing it in the injection port for 2 min at 220 °C to ensure complete elution of volatiles. Blank runs were made during samples analyses. The HP quadruple mass spectrometer was operated in EI mode at 70 eV. A scan range was set at m/z 40–500. Volatile components were identified by comparing their retention indices (RI) relative to n-alkanes (C6-C20), mass matching to NIST, WILEY library database and with standards whenever available. Peaks were first deconvoluted using AMDIS software (www.amdis.net) prior to mass spectral matching. GCMS files shall be made available upon readers request.
GC–MS data processing for multivariate analysis
Volatile metabolites abundance data were prepared for multivariate data analysis by extraction using MET-IDEA software [19] for data extraction. Peaks mass abundance were first normalized to the amount of spiked (Z)-3-hexneyl acetate then subjected to principal component analysis (PCA) and partial least squares-discriminant analysis (OPLS-DA) using SIMCA-P version 13.0 software package (Umetrics, Umeå, Sweden). All variables were mean centered and scaled to Pareto variance.
Metabolites extraction for UHPLC- Orbitrap HRMS analysis
Extraction of luffa fruits for UHPLC/MS was made by homogenizing freeze dried powder (120 mg) with 5 ml 100% MeOH containing umbelliferone (an internal standard for relative quantification using UPLC-MS) present at a concentration of 10 µg/mL using a Turrax mixer (11,000 RPM). Extracts were then vortexed vigorously and centrifuged at 3000g for 30 min to remove plant debris. For UHPLC-MS analyses, 500 μL was aliquot and filtered through 20 µm size filter. Three microliters were used for UPLC-MS analysis. For each specimen, three biological replicates from each fruit stage were provided and extracted in parallel under identical conditions.
UHPLC-Orbitrap HRMS analysis
The negative ion high-resolution ESI and collision-induced dissociation (CID) MSn spectra were obtained from an Orbitrap Elite mass spectrometer (Thermo Fisher Scientific, Germany) equipped with a heated electrospray ion source (negative spray voltage of 3 kV, capillary temperature of 300 °C, source heater temperature of 250 °C, FTMS resolution of 30.000). Nitrogen was used as sheath and auxiliary gas. The MS system was coupled to an UHPLC system (Dionex UltiMate 3000, Thermo Fisher Scientific), equipped with a RP-18 column (particle size 1.8 µm, pore size 100 Å, 150 × 1 mm ID,Acquity HSS T3, Waters; column temperature of 40 °C) and a photodiode array detector (220–600 nm, Thermo Fisher Scientific). The mobile phases were H2O and CH3CN with 0.1% formic acid by using the following binary gradient at a flow rate of 150 μL/min: 0–1 min, isocratic 95% A, 5% B; 1–11 min, linear from 5 to 100% B; 11–19 min, isocratic 100% B; and 19–30 min, isocratic 5% B. The injection volume was 2 μL. The CID mass spectra (buffer gas: helium) were recorded using normalized collision energy (NCE) of 35%. Metabolites were characterized by their UV–VIS spectra (220–600 nm), retention times relative to external standards, mass spectra and comparison to phytochemical dictionary of natural products database (CRC) and reference literature. UHPLC-MS files are uploaded in study number MTBLS1305 in Metabolights https://www.ebi.ac.uk/metabolights/database.
UHPLC/MS data processing for multivariate data analysis
Relative Luffa metabolites analyzed after UHPLC/MS was performed using XCMS data analysis software, which can be downloaded freely from https://bioconductor.org/packages/release/bioc/html/xcms.html [22], [25]. Data were subjected to PCA and OPLS-DA, using the SIMCA-P 13.0 software package (Umetrics, Umea, Sweden). Markers were subsequently identified by analyzing the S-plot, which was declared with covariance (p) and correlation (pcor). All variables were mean centered and scaled to Pareto variance.
Results & discussion
L. aegyptiaca volatiles profiling using SPME coupled to GC–MS.
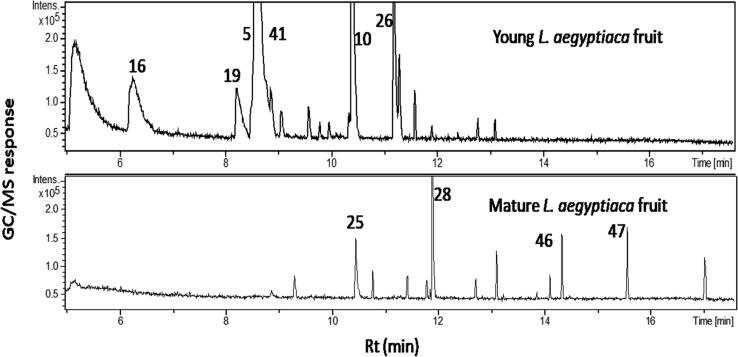
In this study, the metabolic profiles of L. aegyptiaca fruits were characterized at two different maturity stages represented by young and old mature fruits. Three independent biological replicates from different fruits representing each stage were extracted and analyzed under identical conditions. The volatile analysis of L. aegyptiaca (Fig. 1) revealed for the presence of 53 volatiles listed in Table 1, categorized into acids, alcohols, aldehydes/furan, aromatics, esters, ketones, aliphatic hydrocarbons and mono& sesqui-terpene hydrocarbons. Both fruit ripening stages showed relatively a comparable aroma composition as shown in their gas chromatograms (Fig. 2). This is the first report on aroma compositional differences of L. aegyptiaca fruits at two different stages of growth world wide.
Fig. 1.
A photo of L. aegyptiaca fruit collected at the 2 different ripening stages (YF) and (MF).
Table 1.
Relative percentile levels of volatile components detected in young and old mature L. aegyptiaca fruit using SPME-GC–MS measurements (n = 3).
| Peak | Rt (min) | KI | Identification | Class | Mature average ± Std. Dev. | Young average ± Std. Dev. |
|---|---|---|---|---|---|---|
| Fatty acid | ||||||
| 1 | 8.51 | 951 | Pentanoic acid | 2.67 ± 2.01 | 0.46 ± 0.37 | |
| 2 | 10.01 | 1071 | Heptanoic acid | 0.85 ± 0.55 | 0.24 ± 0.20 | |
| 3 | 11.42 | 1180 | Octanoic acid | 3.93 ± 2.30 | 1.46 ± 0.77 | |
| 4 | 12.71 | 1270 | Nonanoic acid | 3.86 ± 2.02 | 1.60 ± 0.84 | |
| Total Fatty Acids | 11.30 | 3.76 | ||||
| 5 | 8.49 | 949 | 1-Octen-3-ol | Alcohol | 6.85 ± 9.55 | 15.94 ± 1.01 |
| 6 | 8.78 | 971 | 3-Octanol | 0.74 ± 0.73 | 2.15 ± 0.16 | |
| 7 | 9.29 | 1010 | 6,6-Dimethyl-1,3-heptadien-5-ol | 3.45 ± 2.77 | 0.91 ± 0.52 | |
| 8 | 9.43 | 1045 | Benzyl alcohol | 0.49 ± 0.19 | 0.27 ± 0.09 | |
| 9 | 9.98 | 1080 | Dihydromyrcenol | 2.23 ± 1.30 | 0.10 ± 0.05 | |
| 10 | 10.39 | 1107 | β-Linalool | 1.62 ± 0.09 | 9.86 ± 3.38 | |
| 11 | 10.67 | 1127 | Phenylethyl Alcohol | 0.34 ± 0.12 | 0.29 ± 0.21 | |
| 12 | 11.19 | 1164 | β-Terpineol | 0.54 ± 0.30 | 0.40 ± 0.12 | |
| 13 | 11.57 | 1191 | Camphol | 0.52 ± 0.25 | 1.88 ± 0.61 | |
| 14 | 11.85 | 1211 | α-Terpineol | 1.99 ± 1.45 | 0.16 ± 0.08 | |
| 15 | 13.23 | 1317 | Z-2-Dodecenol | 0.81 ± 0.41 | 0.13 ± 0.06 | |
| Total Alcohols | 19.59 | 32.08 | ||||
| 16 | 6.19 | 773 | 2-Hexenal | Aldehyde/furan | 0.17 ± 0.50 | 10.58 ± 1.26 |
| 17 | 6.56 | 879 | 3-Furanmethanol | 0.19 ± 0.32 | 1.69 ± 0.19 | |
| 18 | 7.08 | 906 | Heptanal | 0.68 ± 0.41 | 0.29 ± 0.07 | |
| 19 | 8.22 | 929 | Benzaldehyde | 0.06 ± 0.40 | 4.09 ± 1.38 | |
| 20 | 8.63 | 960 | 2-n-Pentylfuran | 0.02 ± 0.01 | 0.01 ± 0.01 | |
| 21 | 8.86 | 1009 | Octanal | 0.50 ± 0.21 | 0.36 ± 0.08 | |
| 22 | 9.57 | 1054 | Benzenacetaldehyde | 0.83 ± 0.67 | 2.57 ± 0.59 | |
| 23 | 9.65 | 1038 | Cumene aldehyde | 0.60 ± 0.34 | 0.17 ± 0.18 | |
| 24 | 9.78 | 1047 | 2-Octenal, (E)- | 0.85 ± 0.21 | 1.06 ± 0.44 | |
| 25 | 10.45 | 1111 | Nonanal | 8.76 ± 1.84 | 1.67 ± 0.38 | |
| 26 | 11.18 | 1163 | Nonadienal(cucumberaldehyde) | 1.03 ± 0.99 | 5.39 ± 2.83 | |
| 27 | 11.28 | 1170 | 2-Nonenal, (E)- | 1.97 ± 0.30 | 2.37 ± 0.66 | |
| 28 | 11.90 | 1214 | Decanal | 8.98 ± 3.08 | 0.77 ± 0.05 | |
| 29 | 12.16 | 1234 | p-Phthalaldehyde | 0.27 ± 0.24 | 0.03 ± 0.01 | |
| Total Aldehyde/Furans | 35.14 | 31.06 | ||||
| 30 | 6.49 | 878 | p-Xylene | Aromatic | 1.93 ± 0.88 | 0.76 ± 0.54 |
| 31 | 8.73 | 967 | Mesitylene (Benzene, 1,3,5-trimethyl-) | 2.22 ± 1.07 | 0.70 ± 0.25 | |
| 32 | 9.25 | 1033 | m-Cymene | 0.26 ± 0.07 | 0.38 ± 0.06 | |
| 33 | 10.09 | 1087 | Dimethylethylbenzene | 0.61 ± 0.30 | 0.13 ± 0.03 | |
| 34 | 10.50 | 1115 | p cymene | 0.14 ± 0.09 | 0 ± 0 | |
| 35 | 11.21 | 1165 | Unknown aromatic | 0.47 ± 0.24 | 0.07 ± 0.04 | |
| 36 | 13.27 | 1320 | β-Methylnaphthalene | 1.66 ± 0.52 | 0.18 ± 0.25 | |
| 37 | 15.71 | 1511 | β-Vinylnaphthalene | 0.54 ± 0.27 | 0.06 ± 0.10 | |
| Total Aromatics | 7.82 | 2.28 | ||||
| 38 | 13.08 | 1304 | Bornyl acetate | Ester | 0.16 ± 0.04 | 0.59 ± 0.22 |
| 39 | 14.09 | 1376 | 3-Hydroxy-2,4,4-trimethylpentyl 2-methylpropanoate | 1.90 ± 1.54 | 0.22 ± 0.05 | |
| 40 | 14.50 | 1420 | Cyclopentanecarboxylic acid, isopropyl ester | 0.09 ± 0.04 | 0.01 ± 0.02 | |
| Total Esters | 2.16 | 0.83 | ||||
| 41 | 8.57 | 955 | 3-Octanone | Ketone | 4.74 ± 3.73 | 26.95 ± 2.02 |
| 42 | 9.20 | 1003 | Acetophenone | 0.74 ± 0.32 | 0.21 ± 0.06 | |
| 43 | 11.78 | 1205 | 2,4-Dimethyl-3-hexanone | 5.02 ± 0.66 | 0.50 ± 0.56 | |
| Total ketones | 10.50 | 27.66 | ||||
| 44 | 10.36 | 1104 | n-Undecane | Aliphatic hydrocarbon | 0.33 ± 0.19 | 0.18 ± 0.07 |
| 45 | 13.09 | 1305 | Tridecane | 1.65 ± 1.11 | 0.22 ± 0.06 | |
| 46 | 14.32 | 1393 | Tetradecane | 4.49 ± 2.72 | 0.60 ± 0.27 | |
| 47 | 15.54 | 1505 | Pentadecane | 3.17 ± 2.10 | 0.40 ± 0.25 | |
| 48 | 17.01 | 1601 | Hexadecane | 2.96 ± 2.09 | 0.42 ± 0.41 | |
| Total Aliphatic hydrocarbons | 12.60 | 1.83 | ||||
| 49 | 9.33 | 1014 | Limonene | Monoterpene hydrocarbon | 0.40 ± 0.15 | 0.28 ± 0.07 |
| 50 | 10.77 | 1134 | Isomyocorene | 0.19 ± 0.15 | 0.04 ± 0.03 | |
| 51 | 14.74 | 1440 | Unknown | Sesquiterpene hydrocarbon | 0.06 ± 0.04 | 0 ± 0 |
| Total Terpene hydrocarbons | 0.66 | 0.32 | ||||
| 52 | 11.07 | 1155 | Allyl dithioacetate | sulphur compound | 0.20 ± 0.08 | 0.06 ± 0.05 |
| 53 | 13.87 | 1369 | Eugenol | phenolic ether | 0.03 ± 0.04 | 0.13 ± 0.04 |
| Total Miscellaneous | 0.23 | 0.18 | ||||
Fig. 2.
SPME-GC–MS chromatograms of headspace volatiles collected from young (YF) and old mature (MF) L. aegyptiaca fruit.
Aldehydes
Aldehydes/furans amounted for the major class present in mature fruit at ca 32–35%. Decanal (28) & nonanal (25) were the higher volatiles signal intensity percentile detected in MF (8.9% & 8.7%), respectively, and to reach (0.7 & 1.6%) in YF, respectively. Both aldehydes were reported to exhibit antimicrobial/antifungl activity and to possess a citrus but waxy/fatty odor [26], [27]. In contrast, hexenal (16), with an odor of freshly cut grass [28] was the major aldehyde component in YF represents 10.6%. Other aldehydes included nonadienal (cucumberaldehyde) that predominated in young luffa aroma at 5.4% versus 1% in mature fruit [29]. Cucumberaldehyde was reported for it potential antimicrobial activities [30] and is used as commercial food flavoring agent, as well as in cosmetics and perfumes fragrance [31]. Benzaldehyde (19) with its characteristic almond like odour [32], represents 4.1% in YF rather than MF.
Previous volatile analysis in the closely related vegetable ‘’cucumber’’ suggested for alihpatic aldehydes i.e., hexenal, nonenal and nonadienal (cucumber aldehyde), to account for its characteristic pleasant aroma and to change upon ripening as observed in luffa [33]. Generation of these compounds is believed to be via enzymatic activity after mechanical rupture or cutting of the fruit “as in chewing proccess” to yield the characteristic flavour of fresh young cucumber fruit i.e., nonadienal [29]. Extending such aroma profile to also encompass L. aegyptiaca fruit being detected a higher levels at the edible stage with notable decrease upon ripening and to contribute majorly for young fruits aroma.
Alcohols
Next to aldehydes/furans class, alcohols presented the second major volatile class and also showing same pattern being higher in young fruits at 32.1% versus 19.6% at the mature stage. Octenol (5), β-linalool (10) and camphol (13) were the major components in YF present at (15.9, 9.9 & 1.9%) versus (6.8, 1.6 & 0.5%) in MF, respectively.
Octenol is one of the major volatiles detected in YF volatiles (15.9%) characterized by mushroom like yet sweet aroma and flavor [34]. Whereas, linalool abundance in young fruit (10%) might contribute for the fruit antimicrobial effect [35], [36], in addition to its potent fungitoxic action against Fusicladium effusum [37]. Other volatiles identified in L. aegyptiaca volatile blend of potential antimicrobial activity included cymene, naphthalene, benzyl alcohol, phenylethyl alcohol and eugenol [38], [39] and likely to contribute for its MF use as bath cleanser. In terms of aroma perception, β-linalool contributes strongly to orange oil aroma [40] and might also contribute to luffa overall aroma.
Ketones
The highest % ketone peak intensity in YF was for 3-octanone (41), detected at 27% and dropping to 5% upon fruit maturity. 3-octanone alongside octenol are involved in attracting insects for pollintation due to its fruity sweet aroma [34]. In contrast, 2,4-dimethyl-3-hexanone (43) was detected in MF at 5% while almost diminished in the YF to 0.5%.
Aromatics
Aromatics were present at 7.8 and 2.3% in MF vs. YF, respectively with xylene (30), mesitylene (31) and methylnaphthalene (36) as major components. Naphthalene exhibits a strong unpleasant odour and insecticidal action [41]. Interestingly, naphthalene showed higher % peak intensity in mature fibrous inedible stage of fruit rather than its edible fruit.
Terpenoids
Terpenoids accounted for minor levels as volatile class as both stages with limonene as the predominant monoterpene present in both fruit stages ca 0.35%.
Fatty acids/hydrocarbons
Two major classes found extensively in MF (11.3 & 12.6%) and to decline in YF (3.7 & 1.8%) were short fatty acids and hydrocarbons, respectively and contrary to most other volatile classes showed higher peak intensity level in YF and suggesting that lypophilic VOCs i.e. fatty acids/hydrocarbons contribute more to MF aroma.
Multivariate data analysis of volatiles dataset in the different fruit ripening stages
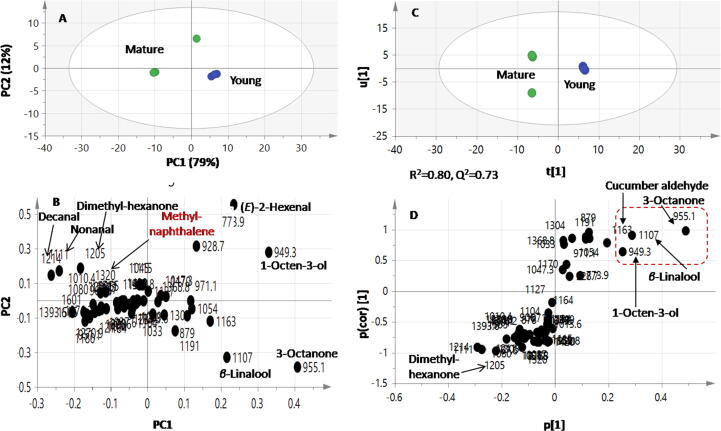
Although difference in volatile patterns could be observed, we attempted to employ mutivariate data analysies to help identify markers for each fruit ripening stage via principle component analysis (PCA) and supervised orthogonal partial least squares (OPLS). PCA score plot was prescribed by two orthogonal PCs with PC1 accounting for 79% of the total variance versus PC2 to account for 12%, (Fig. 3A). Most notably, replicates representing volatiles analysis of each of the two ripening stages showed segreggation of specimens to some extent based on ripening stage with YF exhibting negative score values along PC1 versus positioning of mature fruits on the other side.
Fig. 3.
Principal component analysis (PCA) and orthogonal projection to latent structures-discriminant analysis (OPLS) supervised data analysis of modelling youngversus old mature fruit specimens analysed via SPME GC–MS for their volatile metabolites. PCA score (A) and loading plot (B) (n = 3); OPLS-DA score plot (C) and loading S-plot (D). Segregation in both score plots showed enrichment of alcohols, aldehyde and ketone compounds in young fruit.
PCA loading plot examination (Fig. 3B) revealed that oxygenated volatiles i.e., aldehydes/ketones were the most variant volatiles and contribtuing for specimens segregation. 2-Hexenal (16), cucumber aldehyde (26), octen-3-ol (5), 3-octanone (41) and the terpenoid alcohol β-linalool (10) in YF and to contribute for its characteristic aroma compared to the abundance of nonanal (25), decanal (28), dimethylhexanone (43) and β-methylnaphthalene (36) in mature fruits (Fig. 3B). Such segregation pattern suggest that qualitative difference in aldehydes and ketones that accounted for the different fruits stages aroma composition, structures of the major key volatiles are illustrated in Suppl. Fig. S1.
Further, supervised multivariate data analysis, i.e., OPLS, was employed for samples classification. OPLS-DA outscores PCA in the separation of the predictive variation from orthogonal variation and enhances data interpretation [42]. The OPLS-DA score plot (Fig. 3C) showed better discrimination between the sample groups with the young group clustering separately from the mature one with variance coverage (R2 = 0.80) and prediction power of (Q2 = 0.73).
The S-loading plot derived from the OPLS-DA model (Fig. 3D) revealed that linalool and cucumber aldehyde could be identified as the most discriminatory aroma compounds for YF. To assess for validity of the GC–MS based OPLS models, Q2, R2X and R2Y values were close to. Permutation diagnostic analysis of 20 iterations provided reference distribution of R2/Q2 values and hence indicated statistical significance of these parameters, with most models showing a regression line crossing zero, which signifies the models validation. Also, the p-value for OPLS-DA model was calculated using CV-ANOVA was estimated at 0.03 below p-value of 0.05 (Suppl. Fig. S2).
Secondary metabolites profiling of L. aegyptiaca via UHPLC/PDA/Orbitrap HRMS
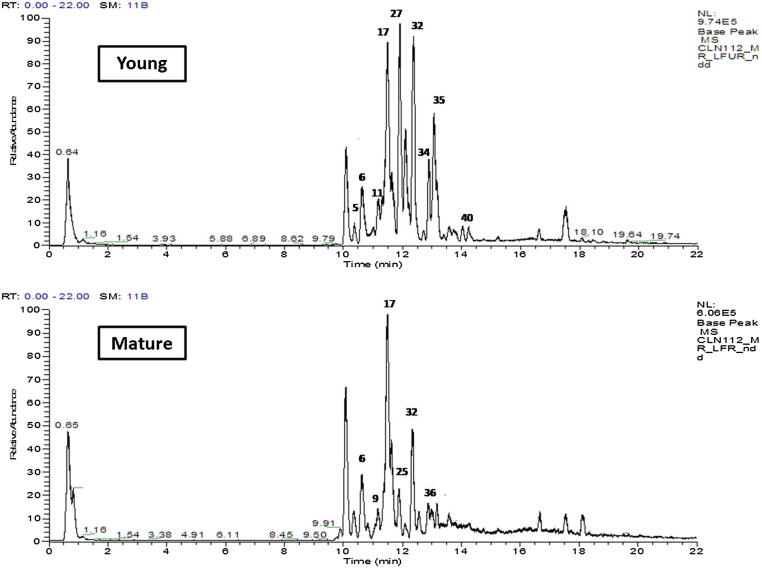
Different studies investigated the chemical composition of L. aegyptiaca organs [5], [43], [44], [45], [46]. However, this study provides the first comprehensive secondary metabolites profile in luffa fruits at the different ripening stages via high resolution UHPLC/PDA/Orbitrap HRMS (Fig. 4) and coupled to multivariate data analyses (Fig. 5).
Fig. 4.
UHPLC-MS base peak chromatograms of secondary metabolites analysed from young (YF) and old mature (MF) L. aegyptiaca fruit.
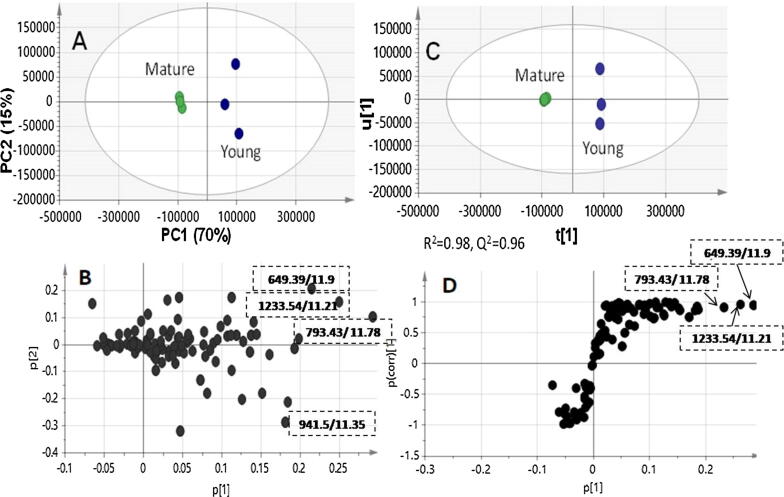
Fig. 5.
Principal component analysis (PCA) and orthogonal projection to latent structures-discriminant analysis (OPLS) supervised data analysis of modelling young versus mature fruit specimens analysed viaUHPLC-MS for their secondary metabolites. PCA score (A) and loading plot (B) (n = 3); OPLS-DA score plot (C) and loading S-plot (D). Segregation in both score plots showed marker metabolites for YF as Mol.ion/Rt, namely: Lucyoside I (649.39/11.9), Dihydroxy-23-oxo-12-oleanen-28-oic acid-O-dipentosyl deoxyhexosyl-O- hexosyl glucuronoside (1233.54/11.21); Dihydroxy-23-oxo-12-oleanen-28-oic acid-O-deoxyhexosyl-hexoside (793.43/11.78) and Dihydroxy-23-oxo-12-oleanen-28-oic acid-O-pentosyl dihexoside (941.5/11.35).
Chemical constituents of young and mature fruits were analysed using gradient elution mobile phase (H2O: ACN) buffered with formic acid allowing for peaks elution. 40 Metabolites were detected belonging to 17 saponins, 9 fatty acids, 6 flavonoids and other miscellaneous compounds (Table 2, Fig. 6). Identification was based on molecular formulae, fragmentation patterns, UV absorbance, and comparison with compounds reported in the literature, mass bank and phytochemical dictionary of natural product database. The nature of the attached sugars in glycosides i.e. saponins and flavonoids was revealed from the lost fragment moieties that is −162 amu; C6H10O5− for hexose (galactose or glucose), −146 amu; C6H10O4− for deoxyhexose (rhamnose), −132 amu; C5H8O4− for pentose and −176 amu; C6H8O6− for hexauronic acid [47], [48].
Table 2.
Secondary metabolites detected in young YF and old mature MF L. aegyptiaca fruit using UHPLC–PDA-MS.
| No. | Rt (min.) | Identification | (M-H)− | Element composition | Error (ppm) | MSn ions m/z (-) ppm | Fruit |
|
|---|---|---|---|---|---|---|---|---|
| Young | Mature | |||||||
| 1 | 0.66 | Unknown | 215.0324 | C12H7O4− | 6.8 | 179, 161, 89 | (+) | (+) |
| 2 | 0.87 | (Iso)citric acid | 191.0193 | C6H7O7− | 3.7 | 173, 111 | (+) | (+) |
| 3 | 9.9 | Gibberellin A8 | 363.1437 | C19H23O7− | 0.3 | 345, 319, 301, 275, 257, 239, 119 | (+) | |
| 4 | 10.08 | Umbelliferone (internal standard) | 161.0241 | C9H5O3− | 4.8 | 133, 117 | (+) | (+) |
| 5 | 10.32 | Luteolin-O-glucuronide | 461.0709 | C21H17O12− | 1.1 | 285, 153 | (+) | (+) |
| 6 | 10.55 | Apigenin-O-glucuronide | 445.0757 | C21H17O11− | 1.7 | 269 | (+) | (+) |
| 7 | 10.7 | Diosmetin-O-glucuronide | 475.0867 | C22H19O12− | 0.78 | 299, 285, 270 | (+) | (+) |
| 8 | 10.8 | Trihydroxy-oxo-octadecenoic acid | 343.2116 | C18H31O6− | 0.2 | 325, 307, 289, 209, 201, 171, 153, 135 | (+) | |
| 9 | 11.04 | Lucyoside Nϯ | 1395.6016 | C64H99O33− | 2.8 | 1377 , 1233, 1101, 969, 823, 643, 485 | (+) | |
| 10 | 11.12 | Dihydroxy-23-oxo-12-oleanen-28-oic acid-O-tripentosyl-deoxyhexosyl -O-hexosyl glucuronide | 1365.5899 | C63H97O32− | 3.3 | 1347, 1233, 1101, 969, 823, 661, 643, 485 | (+) | |
| 11 | 11.14 | Lucyoside A | 811.4470 | C42H67O15− | 0 | 691, 649, 487 | (+) | (+) |
| 12 | 11.21 | Dihydroxy-23-oxo-12-oleanen-28-oic acid-O-dipentosyl deoxyhexosyl-O- hexosyl glucuronoside | 1233.5491 | C58H89O28− | 2.7 | 1101, 955, 823, 661, 643, 485 | (+) | |
| 13 | 11.28 | Lucyoside J. | 809.4301 | C42H65O15− | 2 | 647, 485 | (+) | (+) |
| 14 | 11.29 | Luteolin | 285.03998 | C15H9O6− | 1.5 | (+) | (+) | |
| 15 | 11.35 | Hydroxy-23-oxo-12-oleanen-28-oic acid-O-tripentosyl-deoxyhexosyl-O-hexosyl glucuronide | 1349.5969 | C63H97O31− | 2 | 1331, 1217, 807, 645, 627, 469 | (+) | |
| 16 | 11.35 | Dihydroxy-23-oxo-12-oleanen-28-oic acid-O-pentosyl dihexoside | 941.5097 | C48H77O18− | 0.7 | 779, 485 | (+) | |
| 17 | 11.43 | Lucyoside C | 795.4522 | C42H67O14− | 0.3 | 633, 471, 385 | (+) | (+) |
| 18 | 11.48 | Apigenin | 269.04477 | C15H9O5− | 1.1 | (+) | (+) | |
| 19 | 11.53 | Diosmetin | 299.05548 | C16H11O6− | 1.1 | (+) | (+) | |
| 20 | 11.55 | Lucyoside G | 795.4516 | C42 H67 O14− | 1.09 | 633, 471, 405 | (+) | (+) |
| 21 | 11.6 | Trihydroxy-octadecadienoic acid | 327.2166 | C18H31O5− | 3.3 | 299 | (+) | |
| 22 | 11.66 | Trihydroxy-octadecenoic acid | 329.2323 | C18H33O5− | 3 | 311, 293, 229, 211, 171 | (+) | (+) |
| 23 | 11.7 | Dihydroxy-12-oleanen-28-oic acid-O-deoxyhexoside-O-di-hexoside | 925.5141 | C48H77O17− | 1.4 | 779, 763, 617, 471 | (+) | (+) |
| 24 | 11.78 | Dihydroxy-23-oxo-12-oleanen-28-oic acid-O-deoxyhexosyl-hexoside | 793.4367 | C42H65O14− | 0.1 | 631, 485 | (+) | |
| 25 | 11.8 | Lucyoside H | 779.4566 | C42H67O13− | 1.3 | 617, 455 | (+) | (+) |
| 26 | 11.81 | Hydroxy-23-oxo-12-oleanen-28-oic acid pentoside | 601.3734 | C35H53O8− | 0.1 | 469 | (+) | |
| 27 | 11.9 | Lucyoside I | 649.3947 | C36H57O10− | 0.22 | 487 | (+) | |
| 28 | 11.98 | Dihydroxyoctadecatrienoic acid | 309.2063 | C18H29O4− | 1.8 | 291, 269, 249, 175 | (+) | |
| 29 | 12.01 | Dihydroxyoctadecadienoic acid | 307.191 | C18H27O4− | 1.5 | 289 | (+) | |
| 30 | 12.1 | Dihydroxy-23-oxo-12-oleanen-28-oic acid-O-hexoside | 647.3797 | C36H55O10− | 1 | 485 | (+) | (+) |
| 31 | 12.32 | Lucyoside O/ Q | 633.4000 | C36H57O9− | 0 | 471 | (+) | (+) |
| 32 | 12.48 | Acetylated lucyoside O/Q | 675.4088 | C38H59O10− | 2.2 | 633, 615, 513, 471, 467 | (+) | (+) |
| 33 | 12.78 | Lucyin A | 485.3252 | C30H45O5− | 0 | 474, 463, 439, 423, 405, 393 | (+) | |
| 34 | 12.86 | Oxo-octadecadienoic acid | 293.2113 | C18H29O3− | 0.8 | 275, 265, 249, 235, 211, 183 | (+) | |
| 35 | 13.02 | Phosphatidyl ethanolamine hexanoic acid derivative | 452.2769 | C21H43NO7P− | 0.4 | 391, 255, 214, 196 | (+) | |
| 36 | 13.18 | Oxo-octadecenoic acid | 295.2271 | C18H31O3− | 1.1 | 277, 233, 195, 171 | (+) | (+) |
| 37 | 13.2 | Octadecadienoic acid | 279.2325 | C18H32O2− | 1.2 | (+) | ||
| 38 | 13.37 | Phosphatidyl inositol hexanoic acid derivative | 571.2869 | C25H48O12P − | 1.4 | 391, 315, 255, 241 | (+) | |
| 39 | 13.5 | Hexanoic acid | 255.232 | C16H32O2− | 3 | (+) | ||
| 40 | 13.99 | Phosphatidyl inositol hexanoic acid derivative | 483.2714 | C22H44O9P− | 0.6 | 391, 255, 227 | (+) | |
(+) denotes presence of metabolite at certain fruit stage.
Fig. 6.
Structures of major secondary metabolites detected by UHPLC/MS and discussed throughout the manuscript.
Saponins
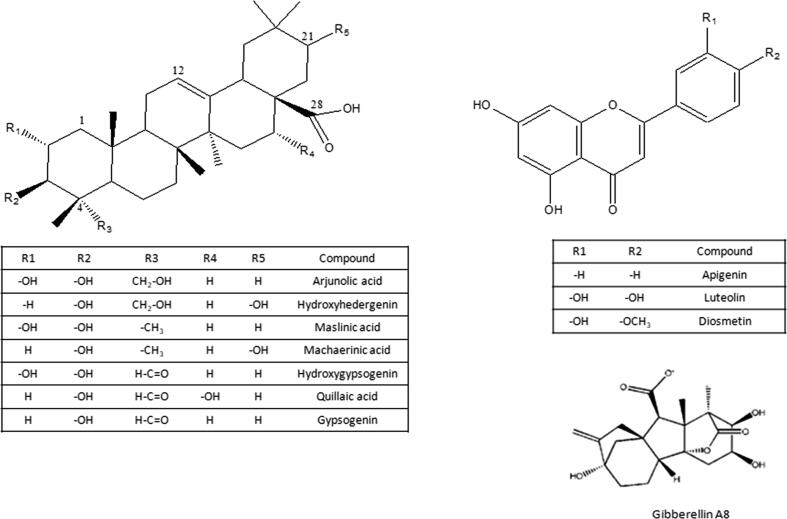
As a rich source of saponins, 17 compounds originating from 5 triterpene sapogenins were identified (Fig. 6). Saponins were previously reported in luffa [43]and revealed to exhibit biological actions as immunostimulatory, anti-inflammatory and fibrinolysis [5], [7], [14]. Saponins of the triterpene oleanene type identified in the current study included trihydroxy-12-oleanen-28-oic acid (hydroxyhederagenin/arjunolic acid) at m/z 487; C30H47O5− in peaks (11 and 27), dihydroxy-12-oleanen-28-oic acid (maslinic/machaerinic acid) at m/z 471; C30H48O4−in peaks (17, 20, 23, 31 and 32), dihydroxy-23-oxo-12-oleanen-28-oic acid (hydroxygypsogenin/quillaic acid) at m/z 485; C30H45O5− in peaks (9, 10, 12, 13, 16, 24 and 30), hydroxy-23-oxo-12-oleanen-28-oic acid (gypsogenin) at m/z 469; C30H46O4− in peaks (15 and 26) [5], [7], [43], [49], [50] and hydroxy-8-oleanen-29-oic acid (bryonolic acid) at m/z 455; C30H48O3− as in peak (25) that was reported before fom L. cylindrica [51].
A fibrinolytic saponin lucyoside Nϯ (peak 9) was detected in MF [5], with a molecular ion (M−H)− at m/z 1395.6017, C64H99O33−. Tandem MS/MS revealed sequential loss of its attached sugars from fragments appearing at m/z 1233 (−162 amu, hexose); m/z 1101 (−132 amu, pentose); m/z 969 (−132 amu, pentose); m/z 823; C42H63O16− (−146 amu, rhamnose); m/z 643; C36H51O10− (−162 amu, hexose and 18 amu, H2O) and quillaic acid at m/z 485; C30H45O5− (−176 amu, glucuronic acid) as showed in (Suppl. Fig. S3).
Flavonoids
6 Flavones were detected herein as confirmed from its UV absorbance spectra in both fruit stages as glucuronoide conjugates at m/z 461.0710 (λmax268, 346 nm), 445.0758 (λmax 269, 338 nm) and 475.0867 (λmax 270, 344 nm) in peaks (5, 6 and 7) along with their corresponding aglycones at m/z 285.0399, 269.0447 and 299.0554 for luteolin, apigenin and diosmetin, respectively in peaks (14, 18 and 19), illustrated in Suppl. Fig. S (4, 5 & 6). L. aegyptiaca is reported previously for the presence of flavone glycosides either as glucuronides or as methyl ester of glucuronic acid conjugates and to possess antioxidant, anti-emetic and anti-inflammatory activities [9], [45].
Fatty acids
Several fatty acids (FA) were detected and found most abundant in YF (peaks 8, 21, 22, 28, 29, 34, 36, 37 and 39). Their order of elution was consistent with their degree of oxygenation starting with trihydroxylated and dihydroxylated unsaturated FA then finally saturated FA. Fatty acids have been reported as bioactive metabolites to lower diabetes incidence [52]. In contrast, biological actions reported for hydroxylated fatty acids (oxylipids) included cytotoxic, anti-inflammatory and antimicrobial effects [53], previously reported in luffa fruits and seeds [17], [54].
Miscellaneous
Other detected compounds included citric acid (peak 2) at m/z 191.0193; C6H7O7−found at both fruit stages and a lactone diterpenoid plant growth hormone; Gibberellin A8 (peak 3) (Suppl. Fig. S7) at m/z 363.1437; C19H23O7− with fragment ions at m/z 319 (−44 amu, M-H-COO−), m/z 275 (−88 amu, M-H-COO-COO) and m/z 257 (−106 amu, M-H-C2O4-H2O) [55] confirming its structure detected exclusively in MF. In contrast, 3 phosphatidyl- hexanoic acid derivatives were detected only in YF samples (peaks 35, 38 and 40) that are involved in plant cell membrane regulation [56], [57]. In addition, phosphatidyl-ethanolamine derivatives are incorporated with leukemic antiproliferative agent (methotrexate) to provide effective transport system of cytoxoic drug [58].
Multivariate PCA and OPLS data analyses of the UPLC/MS dataset:
In an attempt to distinguish between young and mature L. aegyptiaca fruits, PCA and OPLS were employed as in GC/MS dataset as analysed via UHPLC/MS (Fig. 5). PCA score plot showed samples segregation along PC1 and PC2 both accounting for more than 85% of the variance, whereas OPLS model exhibited good prediction power of (Q2 = 0.96) and (R2 = 0.98). Both PCA loading plot and OPLS S-plot revealed for secondary metabolites markers for YF as dihydroxy-23-oxo-12-oleanen-28-oic acid-O-dipentosyl deoxyhexosyl-O- hexosyl glucuronide, dihydroxy-23-oxo-12-oleanen-28-oic acid-O-pentosyl dihexoside, dihydroxy-23-oxo-12-oleanen-28-oic acid-O-deoxyhexosyl-hexoside and trihydroxy-12-oleanen-28-oic acid hexoside (lucyoside I) (peaks 12, 16, 24 and 27). It is obvious that all YF markers contribute to polyoxygenated sapogenins i.e., (dihydroxylated-oxo-sapogenins along with trihydroxylated sapogenin for lucyoside I), while no markers were detected for MF. Such metabolites abundance is likely attributed to metabolic changes that occur upon fruit lignification for fibrous vascular bundles hardening and channeling metabolic pathways towards production of higher molecular weight metabolites not detected by UHPLC/MS. Cross validation of UHPLC/MS derived OPLS-DA is presented in Suppl. Fig. S8.
Conclusion
In this study, detailed compositional variation in sensory and secondary metabolitesis presented herein for the first time in L. aegyptiaca fruits at different developmental stages via an untargeted metabolomics approach based on GC/MS and UPLC–PDA/ESI–Orbitrap HRMS MS Datasets were further analyzed using chemometric tools to determine metabolites heterogeneity for both fruit stages and to account for its different morphology or biological effects.
A total of 53 volatiles were detected and to account for this fruit aroma categorized into diverse chemical classes, edible YF showed richness in sensory metabolites to mediate for its aroma viz., hexenal, nonadienal (cucumber aldehyde) along with terpenoidal alcohol β-linalool compared to the inedible mature fruit. It could be speculated that enzymes catalyzing these metabolites biosynthetic pathways are down-regulating in luffa fruit upon ripening.
UHPLC/MS revealed 40 metabolites with saponins of oleanene triterpene glycosides as major bioactive secondary metabolites in both fruit stages, and with polyoxygenated sapogenins glycosides; dihydroxy-23-oxo-12-oleanen-28-oic acid derivaties and lucyoside I as metabolic markers in YF versus no marker to contribute for unedible lignified MF. Such chemical differences between the two Luffa aegyptiaca stages could lead to variation in biological effects, yet though to be determined in future work to exploit uses specific for each fruit maturity stage.
Compliance with Ethics requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Dr. Mohamed Farag would like to acknowledge the funding received from Cairo university and Jesour program grant number 30 from ASRT, Egypt.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2019.10.009.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Lawal I., Uzokwe N., Igboanugo A., Adio A., Awosan E., Nwogwugwu J. Ethno medicinal information on collation and identification of some medicinal plants in Research Institutes of South-west Nigeria. Afr J Pharm Pharmacol. 2010;4(1):001–7. [Google Scholar]
- 2.Oboh I., Aluyor E. Luffa cylindrica-an emerging cash crop. Afr J Agric Res. 2009;4(8):684–688. [Google Scholar]
- 3.Demir H., Top A., Balköse D., Ülkü S. Dye adsorption behavior of Luffa cylindrica fibers. J Hazard Mater. 2008;153(1–2):389–394. doi: 10.1016/j.jhazmat.2007.08.070. [DOI] [PubMed] [Google Scholar]
- 4.Azeez M.A., Bello O.S., Adedeji A.O. Traditional and medicinal uses of Luffa cylindrica: a review. J Med Plants. 2013;1(5):102–111. [Google Scholar]
- 5.Partap S., Kumar A., Sharma N.K., Jha K.K. Luffa Cylindrica : An important medicinal plant. J Nat Prod Plant Resour. 2012;2(1):127–134. [Google Scholar]
- 6.Lee S, Yoo J. Method for preparing transformed Luffa cylindrica Roem (World Intellectual property organization); 2006.
- 7.Du Q., Gao S. Preparative separation of Saponins from the Luffa Cylindeica (L.) Roem. by slow rotary countercurrent chromatography. J Liq Chromatogr Relat Technol. 2006;29:2451–2456. [Google Scholar]
- 8.Irshad IA M., Goel H.C., Moshahid M., Rizvi A. Phytochemical screening and high performance TLC analysis of some cucurbits. Res J Phytochem. 2010;4(4):242–247. [Google Scholar]
- 9.Du Q., Xu Y., Li L., Zhao Y., Jerz G., Winterhalter P. Antioxidant constituents in the fruits of Luffa cylindrica (L.) Roem. J Agric Food Chem. 2006;54(12):4186–4190. doi: 10.1021/jf0604790. [DOI] [PubMed] [Google Scholar]
- 10.Bor J.-Y., Chen H.-Y., Yen G.-C. Evaluation of antioxidant activity and inhibitory effect on nitric oxide production of some common vegetables. J Agric Food Chem. 2006;54(5):1680–1686. doi: 10.1021/jf0527448. [DOI] [PubMed] [Google Scholar]
- 11.Ismail M., Hussain M.M., Dastagir M.G., Billah M., Quader A. Phytochemical and antimicrobial investigation of Luffa cylindrica. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 2010;9(5) [Google Scholar]
- 12.Ng T., Wong R., Yeung H. Two proteins with ribosome-inactivating, cytotoxic and abortifacient activities from seeds of Luffa cylindrica roem (Cucurbitaceae) Biochem Int. 1992;27(2):197–207. [PubMed] [Google Scholar]
- 13.Parkash A., Ng T., Tso W. Isolation and characterization of luffacylin, a ribosome inactivating peptide with anti-fungal activity from sponge gourd (Luffa cylindrica) seeds. Peptides. 2002;23(6):1019–1024. doi: 10.1016/s0196-9781(02)00045-1. [DOI] [PubMed] [Google Scholar]
- 14.Khajuria A., Gupta A., Garai S., Wakhloo B.P. Immunomodulatory effects of two sapogenins 1 and 2 isolated from Luffa cylindrica in Balb/C mice. Bioorg Med Chem Lett. 2007;17(6):1608–1612. doi: 10.1016/j.bmcl.2006.12.091. [DOI] [PubMed] [Google Scholar]
- 15.Muthumani P., Meera R., Subin M., Devi P., Kameswari B., Priya B. Phytochemical screening and anti inflammatory, bronchodilator and antimicrobial activities of the seeds of Luffa cylindrica. Res J Pharmaceut, Biol Chem Sci. 2010;1(4):11–22. [Google Scholar]
- 16.Ng Y.-M., Yang Y., Sze K.-H., Zhang X., Zheng Y.-T., Shaw P.-C. Structural characterization and anti-HIV-1 activities of arginine/glutamate-rich polypeptide Luffin P1 from the seeds of sponge gourd (Luffa cylindrica) J Struct Biol. 2011;174(1):164–172. doi: 10.1016/j.jsb.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Kao T., Huang C., Chen B. Functional components in Luffa cylindrica and their effects on anti-inflammation of macrophage cells. Food Chem. 2012;135(2):386–395. doi: 10.1016/j.foodchem.2012.04.128. [DOI] [PubMed] [Google Scholar]
- 18.Farag M.A., Maamoun A.A., Ehrlich A., Fahmy S., Wesjohann L.A. Assessment of sensory metabolites distribution in 3 cactus Opuntia ficus-indica fruit cultivars using UV fingerprinting and GC/MS profiling techniques. LWT. 2017;80:145–154. [Google Scholar]
- 19.Farag M.A., Rasheed D.M., Kamal I.M. Volatiles and primary metabolites profiling in two Hibiscus sabdariffa (roselle) cultivars via headspace SPME-GC-MS and chemometrics. Food Res Int. 2015;78:327–335. doi: 10.1016/j.foodres.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Khalil M.N., Fekry M.I., Farag M.A. Metabolome based volatiles profiling in 13 date palm fruit varieties from Egypt via SPME GC–MS and chemometrics. Food Chem. 2017;217:171–181. doi: 10.1016/j.foodchem.2016.08.089. [DOI] [PubMed] [Google Scholar]
- 21.Gumustas M., Kurbanoglu S., Uslu B., Ozkan S.A. UPLC versus HPLC on drug analysis: advantageous, applications and their validation parameters. Chromatographia. 2013;76(21–22):1365–1427. [Google Scholar]
- 22.Farag M.A., Wessjohann L.A. Volatiles profiling in medicinal licorice roots using steam distillation and solid-phase microextraction (SPME) coupled to chemometrics. J Food Sci. 2012;77(11):C1179–C1184. doi: 10.1111/j.1750-3841.2012.02927.x. [DOI] [PubMed] [Google Scholar]
- 23.Goodacre R., Shann B., Gilbert R.J., Timmins E.M., McGovern A.C., Alsberg B.K. Detection of the dipicolinic acid biomarker in Bacillus spores using Curie-point pyrolysis mass spectrometry and Fourier transform infrared spectroscopy. Anal Chem. 2000;72(1):119–127. doi: 10.1021/ac990661i. [DOI] [PubMed] [Google Scholar]
- 24.Farag M.A., Maamoun A.A., Meyer A., Wessjohann L.A. Salicylic acid and its derivatives elicit the production of diterpenes and sterols in corals and their algal symbionts: a metabolomics approach to elicitor SAR. Metabolomics. 2018;14(10):127. doi: 10.1007/s11306-018-1416-y. [DOI] [PubMed] [Google Scholar]
- 25.Smith C.A., Want E.J., O'Maille G., Abagyan R., Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78(3):779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 26.Fernando W.D., Ramarathnam R., Krishnamoorthy A.S., Savchuk S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem. 2005;37(5):955–964. [Google Scholar]
- 27.Burdock G.A. 6th ed. CRC Press; Boca Raton: 2010. Fenaroli's Handbook of Flavor Ingredients; p. 2159. [Google Scholar]
- 28.Simon C. Hexenal - Molecule of the Month March 2005 [Archived version]; 2017.
- 29.Buescher R., Buescher R. Production and stability of (E, Z)-2, 6-Nonadienal, the major flavor volatile of cucumbers. J Food Sci. 2001;66(2):357–361. [Google Scholar]
- 30.Scriven R, Meloan CE. (E, Z)-2, 6-nonadien-1-al and (E)-2-nonen-1-al Present in Crushed Cucumbers are Natural Repellents for the American Cockroach (Periplaneta Americana); 1984.
- 31.Kula J., Sadowska H. Unsaturated aliphatic C9-aldehydes as natural flavorants:(E, Z)-2, 6-nonadienal. Perfumer Flavorist. 1993;18(5):23–25. [Google Scholar]
- 32.Fan W., Qian M.C. Identification of aroma compounds in Chinese ‘Yanghe Daqu’liquor by normal phase chromatography fractionation followed by gas chromatography [sol] olfactometry. Flavour Fragrance J. 2006;21(2):333–342. [Google Scholar]
- 33.Schieberle P., Ofner S., Grosch W. Evaluation of potent odorants in cucumbers (Cucumis sativus) and muskmelons (Cucumis melo) by aroma extract dilution analysis. J Food Sci. 1990;55(1):193–195. [Google Scholar]
- 34.Combet E., Henderson J., Eastwood D.C., Burton K.S. Eight-carbon volatiles in mushrooms and fungi: properties, analysis, and biosynthesis. Mycoscience. 2006;47(6):317–326. [Google Scholar]
- 35.Park S.-N., Lim Y.K., Freire M.O., Cho E., Jin D., Kook J.-K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe. 2012;18(3):369–372. doi: 10.1016/j.anaerobe.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Herman A., Tambor K., Herman A. Linalool affects the antimicrobial efficacy of essential oils. Curr Microbiol. 2016;72(2):165–172. doi: 10.1007/s00284-015-0933-4. [DOI] [PubMed] [Google Scholar]
- 37.Langhans V, Hedin P, Graves Jr C. Fungitoxic chemicals in pecan tissue [Fusicladium effusum, Carya illinoensis]. Plant Disease Reporter; 1978.
- 38.Zhang Z.-Z., Li Y.-B., Qi L., Wan X.-C. Antifungal activities of major tea leaf volatile constituents toward Colletorichum camelliae Massea. J Agric Food Chem. 2006;54(11):3936–3940. doi: 10.1021/jf060017m. [DOI] [PubMed] [Google Scholar]
- 39.Krist S., Halwachs L., Sallaberger G., Buchbauer G. Effects of scents on airborne microbes, part I: thymol, eugenol, trans-cinnamaldehyde and linalool. Flavour Fragrance J. 2007;22(1):44–48. [Google Scholar]
- 40.Högnadóttir Á., Rouseff R.L. Identification of aroma active compounds in orange essence oil using gas chromatography–olfactometry and gas chromatography–mass spectrometry. J Chromatogr A. 2003;998(1–2):201–211. doi: 10.1016/s0021-9673(03)00524-7. [DOI] [PubMed] [Google Scholar]
- 41.Griego F.Y., Bogen K.T., Price P.S., Weed D.L. Exposure, epidemiology and human cancer incidence of naphthalene. Regul Toxicol Pharm. 2008;51(2):22–26. doi: 10.1016/j.yrtph.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Rasheed D.M., Porzel A., Frolov A., El Seedi H.R., Wessjohann L.A., Farag M.A. Comparative analysis of Hibiscus sabdariffa (roselle) hot and cold extracts in respect to their potential for α-glucosidase inhibition. Food Chem. 2018;250:236–244. doi: 10.1016/j.foodchem.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Shmuel Y. Dictionary of food compounds with CD-ROM, 2nd ed: CRC Press, Taylor and Francis Group; 2012. 2346 p.
- 44.Zhou J, Xie G, Yan X. Encyclopedia of Traditional Chinese Medicines - Molecular Structures, Pharmacological Activities, Natural Sources and Applications: Springer-Verlag Berlin Heidelberg; 2011. 730 p.
- 45.Kanwal W., Syed A.W., Salman A., Mohtasheem H.M. Antiemetic and anti-inflammaotry activity of fruit peel of Luffa cylindrica (L.) Roem. J Ethno Trad Med Photon. 2013;118:258–263. [Google Scholar]
- 46.Liang L, E Lu L, C Cai Y. [Studies on the chemical components from leaves of Luffa cylinderica Roem]; 1993. 836–9 p. [PubMed]
- 47.Farag M.A., Abou Zeid A.H., Hamed M.A., Kandeel Z., El-Rafie H.M., El-Akad R.H. Metabolomic fingerprint classification of Brachychiton acerifolius organs via UPLC-qTOF-PDA-MS analysis and chemometrics. Nat Prod Res. 2015;29(2):116–124. doi: 10.1080/14786419.2014.964710. [DOI] [PubMed] [Google Scholar]
- 48.Farag M.A., Khattab A.R., Maamoun A.A., Kropf M., Heiss A.G. UPLC-MS metabolome based classification of Lupinus and Lens seeds: A prospect for phyto-equivalency of its different accessions. Food Res Int. 2019;115:379–392. doi: 10.1016/j.foodres.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Tang W, GE. Luffa cylindrica (L.) Roem. In: Chinese Drugs of Plant Origin: Springer, Berlin, Heidelberg; 1992.
- 50.Azimova S. Physicochemical and Pharmacological Properties of Triterpene Glycosides.
- 51.Tanaka S., Uno C., Akimoto M., Tabata M., Honda C., Kamisako W. Anti-allergic effect of bryonolic acid from Luffa cylindrica cell suspension cultures. Planta Med. 1991;57(6):527–530. doi: 10.1055/s-2006-960199. [DOI] [PubMed] [Google Scholar]
- 52.Salmerón J., Hu F.B., Manson J.E., Stampfer M.J., Colditz G.A., Rimm E.B. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutrit. 2001;73(6):1019–1026. doi: 10.1093/ajcn/73.6.1019. [DOI] [PubMed] [Google Scholar]
- 53.Abou-ElWafa G.S.E., Shaaban M., Shaaban K.A., El-Naggar M.E.E., Laatsch H. Three new unsaturated fatty acids from the marine green alga ulva fasciata delile. Zeitschrift Fur Naturforschung Section B J Chem Sci. 2009;64(10):1199–1207. [Google Scholar]
- 54.Muthumani P., Meera R., Subin Mary, Jeenamathew, Devi P., Kameswari B., Priya B.E. Phytochemical screening and anti inflammatory, bronchodilator and antimicrobial activities of the seeds of Luffa cylindrica. Res J Pharmaceut, Biol Chem Sci. 2010;1(4):11–22. [Google Scholar]
- 55.Urbanová T., Tarkowská D., Novák O., Hedden P., Strnad M. Analysis of gibberellins as free acids by ultra performance liquid chromatography–tandem mass spectrometry. Talanta. 2013;112:85–94. doi: 10.1016/j.talanta.2013.03.068. [DOI] [PubMed] [Google Scholar]
- 56.Hsu F.-F., Kuhlmann F.M., Turk J., Beverley S.M. Multiple-stage linear ion-trap with high resolution mass spectrometry towards complete structural characterization of phosphatidylethanolamines containing cyclopropane fatty acyl chain in Leishmania infantum. J Mass Spectrometry: JMS. 2014;49(3):201–209. doi: 10.1002/jms.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsu F.-F., Turk J. Characterization of phosphatidylinositol, phosphatidylinositol-4-phosphate, and phosphatidylinositol-4,5-bisphosphate by electrospray ionization tandem mass spectrometry: a mechanistic study. J Am Soc Mass Spectrom. 2000;11(11):986–999. doi: 10.1016/S1044-0305(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 58.Kinsky S.C., Loader J.E. Circumvention of the methotrexate transport system by methotrexate-phosphatidylethanolamine derivatives: effect of fatty acid chain length. Biochim Biophys Acta (BBA)-Lipids Lipid Metabolism. 1987;921(1):96–103. doi: 10.1016/0005-2760(87)90175-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.