Abstract
Background
Although many cardiovascular risk factors for stroke have been reported before, it has not been investigated comprehensively for all major stroke types in large datasets. This study aimed to evaluate the associations of healthy behaviors, biological phenotypes and cardiovascular health (CVH) with long-term risks of strokes events, overall and stroke subtypes (ischemic stroke [IS], intracerebral hemorrhage [ICH], subarachnoid hemorrhage [SAH], and unspecified stroke).
Methods
Between 2006 and 2010, a total of 354,976 participants (age 40–70 years) in the UK Biobank free of stroke and coronary heart disease were examined and thereafter followed up to 2020. According to American Heart Association guideline, the global CVH included four behavioral (smoking, diet, physical activity, body mass index) and three biological (blood glucose, blood cholesterol, blood pressure) metrics. The behavioral, biological and global CVH score was the sum of four, three, and seven metrics, respectively, and then was categorized into poor, intermediate and ideal group. Cox proportional hazard models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of stroke events.
Findings
A total of 5804 incident stroke cases, including 3664 IS, 714 ICH and 453 SAH, were documented over a median follow-up of 11 years. The risk of stroke decreased significantly and linearly with both increasing behavioral CVH score and biological CVH score. Ideal behavioral CVH group was significantly associated with lower risks of all stroke subtypes, biological CVH was related to stroke events except for SAH. Additionally, the 1-point increment in global CVH score was associated with 11%,13%, 8% and 13% lower risks of stroke, IS, ICH and unspecified stroke, however, there was no significant dose-dependent association between global CVH and SAH.
Interpretation
Our findings suggest inverse linear associations of behavioral, biological and global CVH with long-term risks of stroke and stroke subtypes, except for SAH, highlighting the benefits of maintaining better CVH status as a primordial prevention strategy of stroke.
Funding
The National Natural Science Foundation of China (71,910,107,004, 91,746,205).
Keywords: Cardiovascular health, Stroke, Cohort study, Primordial prevention
Research in context.
Evidence before this study
Previous studies searching for PubMed showed that better CVH was associated with lower risks of stroke, however, there were also some studies found no significant association between global CVH score and stroke. Moreover, data are sparse regarding the influence of behavioral and biological CVH status on the risks of stroke subtypes, including ischemic stroke, intracerebral hemorrhage and subarachnoid hemorrhage.
Added value of this study
To the best of our knowledge, this is one of the largest single cohort studies of its kind to date that quantifies the comprehensive associations of healthy behaviors, biological phenotypes and CVH with long-term risks of strokes events. We provide evidence that adherence to AHA 7-item CVH recommendations, including healthy behaviors and biological phenotypes might be associated with substantially lower risks of stroke events in general population.
Implications of all the available evidence
Prevention is an important element in tackling the challenge posed by a growing number of stroke cases. Our study highlights the benefits of maintaining better CVH across the life course.
Alt-text: Unlabelled box
Introduction
Stroke presents major global public health problems accounting for the leading causes of death, disability, and disability-adjusted life years [1,2]. Due to its high mortality and recurrence rate, the early detection of risk factors and prevention of recurrent stroke become an urgent issue. Primordial prevention that reemphasized by the American Heart Association (AHA) is considered the most effective strategy to minimize the cardiovascular diseases-related health burden among the general population [3]. To this end, the AHA had developed a 7-item cardiovascular health (CVH) tool including 4 health behaviors (physical activity, smoking status, healthy weight and diet pattern at optimal levels) and 3 biological measures (blood pressure, blood glucose, and blood cholesterol at optimal levels) to promote optimal CVH. Recognition of CVH risk factors associated with stroke is essential for decreasing incidence of stroke events [4], [5], [6].
Much of the current evidence are on individual CVH risk factors considered one at a time, although the importance of their clustering is increasingly recognized. There is cumulative evidence suggesting that better CVH was associated with lower risks of diabetes, cardiovascular disease and mortality [7], [8], [9], [10]. However, results of the association between global CVH score and stroke were inconsistent in several previous studies [7,11,12]. For example, a meta-analysis including 193,126 individuals indicated that ideal CVH status can result in substantial reduction in the risk of stroke [11], whereas a recent study found no significant association between global CVH score and stroke [7]. Moreover, data are sparse regarding the influence of behavioral and biological CVH status on the risks of stroke subtypes, such as ischemic stroke (IS), intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH), which have different etiologies and risk factors.
Therefore, using data from large-scale UK Biobank cohort over a more than 10 years follow-up, our objective was mainly to prospectively examine comprehensive associations of behavioral, biological and global CVH with risks of stroke and stroke subtypes in UK general population.
Methods
Study design and population
This was a prospective, population-based cohort study of participants enrolled in the UK Biobank. Between April 2006 and December 2010, the UK Biobank recruited 502,528 adults (40–70 years old) from the general population. Participants attended one of 22 assessment centers across England, Scotland, and Wales, where they completed touchscreen and nurse-led questionnaires, had physical measurements taken, and provided biological samples [13]. In the current study, behavioral and biological CVH was used as exposures and stroke events as outcomes. The present study sample was restricted to the 403,243 participants who had complete data on either biological samples or self-reported physical activity. Participants were excluded if they had history of cardiovascular events (stroke and coronary heart disease) before recruitment, leaving 354,976 participants included in final study (Supplemental Fig. S1).
Behavioral, biological and global CVH
The global CVH score included four behavioral (smoking, diet, physical activity, body mass index) and three biological (blood glucose, blood cholesterol, blood pressure) metrics. Each level of these 7 metrics was categorized as poor (scored as 0), intermediate (scored as 1), and ideal (scored as 2) according to the AHA criteria (Supplemental Table S1). The CVH metrics adopted in this study conformed with the CVH metrics developed by the AHA guideline and consisted of seven health behavioral and biological factors, including smoking status, physical activity, diet pattern, BMI, serum cholesterol, blood pressure and blood glucose. Smoking status (current, past, never), physical activity (hours of moderate and vigorous physical activity per week), and diet (the amount of fish, fruit and vegetable consumption) were assessed using questionnaires. A trained nurse used standard protocols to collect data on BMI (weight/height2 in kg/m2), glucose concentration, blood cholesterol concentration, and blood pressure. Systolic and diastolic blood pressure was taken as the mean of two measurements. Blood samples in storage at the UK Biobank (both −80 °C and LN2) are grouped on storage racks by sample type and by participant in line with the sample processing and storage protocols in place at the time of sample collection. Specially, given that previous studies and data available in current study, we tiny adjusted corresponding components, for example, fiber-rich whole grains, sodium and sugar-sweetened beverages could not be evaluated accurately across the whole population at recruitment in the UK Biobank, so the diet metric was defined on intakes of fruit and vegetables, and fish [14], which were available in the whole cohort and which have shown robust associations with stroke in previous studies [15,16]. We combined fasting plasma glucose with HbA1c to determine the level of blood glucose.
As described previously [14,[17], [18], [19]], we used the sum of each metric to calculate the composite CVH score, ranging from 0 to 8 for behavioral CVH, 0 to 6 for biological CVH, and 0 to 14 for global CVH, with higher scores corresponding to better CVH. We categorized global CVH score as poor for scores ranging from 0 to 7 (corresponding to less than one standard deviation (SD) from the mean), intermediate for scores ranging from 8 to 11 (+/−1 SD from the mean), and ideal for scores between 12 and 14 (>1 SD from the mean). Accordingly, the behavioral CVH score was categorized as poor (0 to 3), intermediate (4 to 6) and ideal (7 to 8) group, biological CVH score as 0 to 2, 3 to 4, and 5 to 6.
Outcome ascertainment
The primary outcomes for this study were the incidence of fatal and nonfatal stroke as well as stroke subtypes, including IS, ICH, SAH or unspecified stroke. All residents in England, Scotland, and Wales have a unique National Health Service (NHS) identification number, which we used to link all participants to electronic health records. Stroke events were ascertained using ICD-10 (international classification of disease, 10th revision) codes I60-I64 from hospital inpatient records containing data on admissions and diagnoses obtained from the Hospital Episode Statistics for England, Scottish Morbidity Record data for Scotland, and the Patient Episode Database for Wales. The cause of death of the UK Biobank participants was obtained by linkage to the national death registers. The latest record linkage was available until 31 March 2020.
Covariates
Covariates of our analysis included exact age, sex, ethnicity (White, Black, South Asian, Mixed background), socioeconomic status (Townsend Deprivation Index, quintiles), employment status (worked, unemployed, retired, others), education attainment (college or university degree, professional qualifications, others), family history of stroke (yes, no), consumption of alcohol intaking (continuous, g), and C-reactive protein (continuous, mg/dL). Further details for each variable are available on the UK Biobank Website (https://www.ukbiobank.ac.uk/).
Statistical analysis
We summarized baseline characteristics by global CVH category using descriptive statistics, reporting the mean and standard deviation (SD) of normal distribution or median and interquartile ranges of non-normal distribution for continuous variables, and proportions for categorical variables. We compared the baseline characteristics by global CVH category using Chi-square test for categorical or One-Way ANOVA or Mann-Whitney U for continuous variables.
Person-year was calculated from the date of recruitment to date of stroke diagnosis, death or the end of follow-up on March 31, 2020, whichever event occurred first. Incidence rate and absolute rate difference per 1000 person-year of CVH category were calculated. Cox proportional hazards models with age as timescale were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) of stroke and stroke subtypes for behavioral, biological and global CVH. We grouped study participants into 3 categories of global CVH score and compared the HRs with the lowest category as the referent. Behavioral, biological and global CVH score as a linear variable were used to examine the risk reduction associated with a 1-point increment. Additionally, we created a variable with 9 categories, which combines behavioral CVH (poor, intermediate, ideal) with biological CVH (poor, intermediate, ideal) to investigate their joint effect on risk of stroke, and interaction effect between behavioral and biological CVH was tested by included an interaction terms in Cox model. We then examined the shape of the associations of the continuous global CVH score with risk of stroke by using restricted cubic spline regressions with score 0 as the reference (HR = 1). The proportional hazard assumption was checked by tests based on Schoenfeld residuals, and the results indicated that the assumptions had not been violated (Supplemental Fig. S2).
In the sensitivity analysis, we applied a series of analyses to test the robustness of our findings. First, the global CVH level was also ascertained through the number of CVH metrics at recommended optimal level, ranging from 0 to 7. Second, stratified analyses were further conducted to examine possible effect modification of sex and age on the associations of behavioral, biological and global CVH score with stroke and stroke subtypes. Third, the possible influence of worse health condition on CVH-stroke associations was evaluated by excluding the participants with cancer or long-illness. Fourth, to minimize the potential contribution of reverse causality to these findings, we did a landmark analysis excluding stroke events occurring within the two years after recruitment. Fifth, the competing risk of non-stroke death on the association between behavioral, biological and global CVH and stroke events was investigated using the subdistribution method proposed by Fine and grey [20]. Finally, missing covariates were imputed with multiple imputation procedure using the chained equations method, five imputed datasets were generated and results were combined using Rubin's rules.
All analyses were performed using STATA 15 statistical software (StataCorp) and R i386 3.4.3 (R Foundation for Statistical Computing). To maximize the likelihood of reporting true findings, we set the α at 0·05 and used Bonferroni correction to adjust for multiple testing. We considered two sided P values less than 0.05 (P value of less than 0.05 divided by the number of tests, i.e. 0.05/5) statistically significant.
Ethics
All participants provided written informed consent to investigators of UK Biobank team and the study was approved by the NHS National Research Ethics Service (Ref: 11/NW/0382). This research has been conducted using the UK Biobank Resource under the project number of 45,676.
Role of the funding source
The sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We included 354,976 participants (54.7% female) who took part in the 2006–2010 examination, during a median follow-up of 11 years, a total of 5804 (1.64%) participants had incident stroke, of which 3664 (1.03%) IS, 714 (0.20%) ICH, 453 (0.13%) SAH, 426 (0.12%) unspecified stroke. Table 1 shows the baseline characteristics of the study population by global CVH score. The characteristics of excluded participants were generally similar to those included (Supplemental Table S2).
Table 1.
Participants’ characteristics according to global cardiovascular health (CVH) Score. Values are numbers (percentages) unless stated otherwise.
| Characteristic | Total | Global CVH score |
P value | ||
|---|---|---|---|---|---|
| Poor (0–7) | Intermediate (8–11) | Optimal (12–14) | |||
| Total | 354,976 | 75,172 (21·2) | 228,453 (64·3) | 51,351 (14·5) | |
| Sex | <0·001 | ||||
| Male | 160,938 (45·3) | 45,535 (60·4) | 103,971 (45·5) | 11,532 (22·5) | |
| Female | 194,038 (54·7) | 29,737 (39·6) | 124,482 (54·5) | 39,819 (77·5) | |
| Age (years), mean (SD) | 56·2 (8·1) | 56·9 (7·91) | 56·4 (8·10) | 54·7 (8·08) | <0·001 |
| Ethnicity | <0·001 | ||||
| White | 337,447 (95·1) | 70,765 (94·1) | 217,222 (95·1) | 49,460 (9·3) | |
| Black | 5376 (1·5) | 1423 (1·9) | 3502 (1·5) | 451 (0·9) | |
| South Asian | 7041 (2·0) | 1798 (2·4) | 4475 (2·0) | 768 (1·5) | |
| Mixed background | 5112 (1·4) | 1186 (1·6) | 3254 (1·4) | 672 (1·3) | |
| Townsend deprivation index | <0·001 | ||||
| 1 (Least deprived) | 73,609 (20·7) | 12,471 (16·6) | 48,979 (21·4) | 12,159 (23·7) | |
| 2 | 73,193 (20·6) | 13,487 (17·9) | 48,318 (21·2) | 11,388 (22·2) | |
| 3 | 71,663 (20·2) | 14,400 (19·2) | 46,717 (20·5) | 10,546 (20·5) | |
| 4 | 71,184 (20·0) | 16,017 (21·3) | 45,193 (19·8) | 9974 (19·4) | |
| 5 (Most deprived) | 65,327 (18·4) | 18,797 (25·0) | 39,246 (17·2) | 7284 (14·2) | |
| Education attainment | <0·001 | ||||
| College or university degree | 121,319 (34·2) | 17,177 (24·2) | 79,709 (34·9) | 23,433 (45·6) | |
| Professional qualifications | 180,314 (50·8) | 40,030 (53·3) | 116,318 (50·9) | 23,966 (46·7) | |
| Others | 53,343 (15·0) | 16,965 (22·6) | 32,426 (14·2) | 3952 (7·7) | |
| Employment | <0·001 | ||||
| Worked | 210,953 (59·4) | 42,096 (56·0) | 136,207 (59·6) | 32,650 (63·6) | |
| Retired | 114,028 (32·1) | 24,340 (32·4) | 75,244 (32·9) | 14,444 (28·1) | |
| Unemployed | 24,779 (7·0) | 7724 (10·3) | 13,749 (6·0) | 3306 (6·4) | |
| Others | 5216 (1·5) | 1012 (1·4) | 3253 (1·4) | 951 (1·8) | |
| Alcohol consumption (g/day), mean (SD) | 14·86 (18·24) | 16·41 (22·13) | 14·95 (17·77) | 12·22 (12·90) | <0·001 |
| C-response protein (mg/dL), mean (SD) | 2·52 (4·22) | 3·86 (5·20) | 2·35 (4·00) | 1·36 (2·94) | <0·001 |
| Family history of stroke | 89,460 (25·2) | 19,103 (25·4) | 58,081 (25·4) | 12,276 (23·9) | <0·001 |
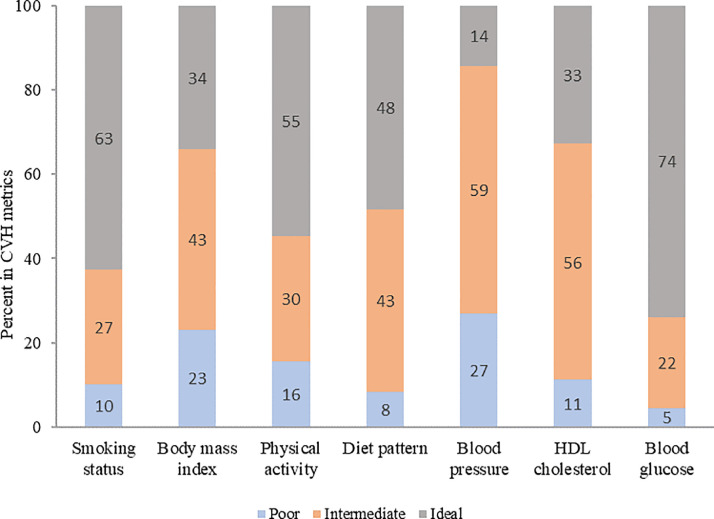
The distribution of each behavioral and biological CVH metric is shown in Fig. 1. For each CVH metric, we found that the risks of stroke and IS were significantly decreased for all ideal CVH groups including smoking status, physical activity, diet pattern, BMI, HDL-cholesterol, blood glucose and blood pressure (Table 2).
Fig. 1.
Distribution of AHA 7-item among participants in poor, intermediate and ideal CVH categories. Poor CVH metric status included current smokers, no moderate or vigorous physical activity, <1 portion per day of fresh fruit, raw vegetables, cooked fruit/vegetables and <2 portions per week of fish, BMI ≥ 30 kg/m2, HDL-C < 40 mg/dL, SBP ≥140 mm Hg or DBP ≥90 mm Hg, FPG ≥126 mg/dL or HbA1c ≥6.5. Intermediate CVH metric status included quit smoking < 5 months, 1–149 min/week of moderate activity or 1–74 min/week of vigorous activity or 1–149 min/ week of moderate and vigorous activity, ≥1 portion per day of fresh fruit, raw vegetables, cooked fruit/vegetables or ≥2 portions per week of fish, BMI 25–29.9 kg/m2, HDL-C ≥ 60 treated or 40–60 mg/dL, SBP <120 mm Hg and DBP <80 mm Hg treated or SBP 120–139 or DBP 80–89 mm Hg, FPG <100 mg/dL treated or 100–125 mg/dL or HbA1c <5.7 treated or 5.7–6.5. Ideal CVH metric status included never or quit smoking ≥ 5 months, ≥150 min/week of moderate activity or ≥75 min/week of vigorous activity or ≥150 min/week of moderate and vigorous activity, ≥1 portion per day of each of fresh fruit, raw vegetables, cooked fruit/vegetables and ≥ 2 portions per week of fish, BMI < 25 kg/m2, HDL-C ≥ 60 mg/dL untreated, SBP <120 mm Hg and DBP <80 mm Hg untreated, FPG <100 mg/dL untreated or HbA1c <5.7 untreated.
Table 2.
Hazard ratios (HRs) of individual behavioral and biological cardiovascular health (CVH) metrics for stroke and stroke subtypes.
| Individual cardiovascular health metric | Stroke |
Ischemic stroke |
Intracerebral hemorrhage |
Subarachnoid hemorrhage |
Unspecified stroke |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI)* | HR (95% CI) † | HR (95% CI)* | HR (95% CI) † | HR (95% CI)* | HR (95% CI) † | HR (95% CI)* | HR (95% CI) † | HR (95% CI)* | HR (95% CI) † | |
| Behavioral CVH subscales | ||||||||||
| Smoking status | ||||||||||
| Poor | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Intermediate | 0·68 (0·63–0·75) | 0·69 (0·64–0·75) | 0·63 (0·56–0·69) | 0·63 (0·56–0·69) | 1·07 (0·82–1·39) | 1·10 (0·84–1·44) | 0·35 (0·27–0·47) | 0·38 (0·29–0·50) | 0·74 (0·55–1·00) | 0·71 (0·53–0·97) |
| Ideal | 0·57 (0·53–0·62) | 0·59 (0·54–0·64) | 0·56 (0·50–0·61) | 0·57 (0·52–0·63) | 0·84 (0·64–1·09) | 0·86 (0·66–1·13) | 0·34 (0·27–0·44) | 0·36 (0·28–0·46) | 0·58 (0·44–0·78) | 0·58 (0·43–0·78) |
| P for trend | <0·001 | <0·001 | <0·001 | <0·001 | 0·015 | 0·022 | <0·001 | <0·001 | <0·001 | <0·001 |
| Body mass index | ||||||||||
| Poor | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Intermediate | 0·85 (0·80–0·91) | 0·93 (0·87–0·99) | 0·81 (0·75–0·87) | 0·89 (0·82–0·96) | 0·98 (0·81–1·19) | 1·08 (0·89–1·31) | 1·26 (0·97–1·64) | 1·19 (0·92–1·55) | 0·77 (0·61–0·96) | 0·87 (0·69–1·10) |
| Ideal | 0·82 (0·77–0·88) | 0·97 (0·90–1·05) | 0·72 (0·66–0·79) | 0·86 (0·78–0·94) | 1·00 (0·82–1·24) | 1·21 (0·97–1·51) | 1·58 (1·21–2·06) | 1·45 (1·10–1·92) | 0·69 (0·53–0·89) | 0·84 (0·63–1·11) |
| P for trend | <0·001 | 0·652 | <0·001 | 0·007 | 0·939 | 0·147 | 0·001 | 0·002 | 0·005 | 0·277 |
| Physical activity | ||||||||||
| Poor | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Intermediate | 0·83 (0·77–0·90) | 0·87 (0·81–0·94) | 0·83 (0·75–0·91) | 0·88 (0·79–0·97) | 0·75 (0·60–0·95) | 0·78 (0·62–0·97) | 1·06 (0·79–1·42) | 1·12 (0·83–1·50) | 0·85 (0·64–1·13) | 0·89 (0·67–1·18) |
| Ideal | 0·85 (0·79–0·91) | 0·92 (0·86–0·99) | 0·85 (0·77–0·92) | 0·94 (0·86–1·02) | 0·84 (0·69–1·02) | 0·87 (0·71–1·07) | 1·10 (0·84–1·43) | 1·15 (0·88–1·51) | 0·80 (0·62–1·04) | 0·87 (0·67–1·13) |
| P for trend | <0·001 | 0·124 | 0·002 | 0·349 | 0·287 | 0·51 | 0·506 | 0·365 | 0·111 | 0·328 |
| Diet pattern | ||||||||||
| Poor | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Intermediate | 0·82 (0·75–0·90) | 0·86 (0·79–0·95) | 0·81 (0·72–0·91) | 0·86 (0·76–0·96) | 0·88 (0·67–1·17) | 0·91 (0·68–1·20) | 0·61 (0·45–0·84) | 0·67 (0·49–0·92) | 1·15 (0·79–1·67) | 1·22 (0·84–1·78) |
| Ideal | 0·76 (0·69–0·84) | 0·81 (0·74–0·90) | 0·76 (0·68–0·86) | 0·82 (0·73–0·92) | 0·78 (0·59–1·03) | 0·80 (0·60–1·06) | 0·50 (0·36–0·68) | 0·55 (0·40–0·76) | 1·07 (0·74–1·67) | 1·16 (0·79–1·69) |
| P for trend | <0·001 | <0·001 | <0·001 | 0·001 | 0·036 | 0·059 | <0·001 | <0·001 | 0·88 | 0·824 |
| Biological CVH subscales | ||||||||||
| HDL cholesterol | ||||||||||
| Poor | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Intermediate | 0·78 (0·73–0·84) | 0·75 (0·78–0·91) | 0·77 (0·71–0·85) | 0·86 (0·78–0·94) | 0·86 (0·68–1·08) | 0·87 (0·69–1·10) | 0·85 (0·61–1·18) | 0·83 (0·59–1·15) | 0·65 (0·50–0·85) | 0·74 (0·56–0·96) |
| Ideal | 0·64 (0·58–0·70) | 0·73 (0·66–0·81) | 0·58 (0·52–0·65) | 0·71 (0·63–0·80) | 0·80 (0·61–1·04) | 0·81 (0·60–1·10) | 0·95 (0·66–1·37) | 0·87 (0·60–1·27) | 0·57 (0·41–0·79) | 0·71 (0·50–1·00) |
| P for trend | <0·001 | <0·001 | <0·001 | <0·001 | 0·117 | 0·199 | 0·722 | 0·706 | 0·002 | 0·061 |
| Blood pressure | ||||||||||
| Poor | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Intermediate | 0·79 (0·75–0·83) | 0·79 (0·74–0·83) | 0·78 (0·73–0·83) | 0·78 (0·73–0·83) | 0·78 (0·67–0·91) | 0·78 (0·67–0·91) | 1·02 (0·83–1·26) | 0·98 (0·80–1·20) | 0·69 (0·56–0·84) | 0·69 (0·56–0·84) |
| Ideal | 0·57 (0·51–0·65) | 0·58 (0·52–0·66) | 0·60 (0·52–0·70) | 0·64 (0·55–0·74) | 0·36 (0·24–0·54) | 0·35 (0·24–0·52) | 0·81 (0·56–1·17) | 0·68 (0·47–0·99) | 0·56 (0·37–0·85) | 0·60 (0·40–0·91) |
| P for trend | <0·001 | <0·001 | <0·001 | <0·001 | <0·001 | <0·001 | 0·492 | 0·121 | <0·001 | <0·001 |
| Blood glucose | ||||||||||
| Poor | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Intermediate | 0·68 (0·61–0·75) | 0·71 (0·64–0·78) | 0·63 (0·56–0·70) | 0·66 (0·59–0·75) | 0·91 (0·67–1·24) | 0·94 (0·69–1·28) | 2·41 (1·26–4·60) | 2·25 (1·18–4·32) | 0·51 (0·36–0·71) | 0·55 (0·39–0·78) |
| Ideal | 0·58 (0·53–0·64) | 0·65 (0·59–0·71) | 0·52 (0·47–0·58) | 0·59 (0·53–0·66) | 0·77 (0·57–1·04) | 0·82 (0·60–1·10) | 2·56 (1·36–4·82) | 2·46 (1·30–4·65) | 0·46 (0·34–0·62) | 0·53 (0·39–0·73) |
| P for trend | <0·001 | <0·001 | <0·001 | <0·001 | 0·017 | 0·051 | 0·016 | 0·024 | <0·001 | 0·003 |
HRs were adjusted for age (timescale), sex, ethnicity, education attainment, employment status, Townsend deprivation index, consumption of alcohol intaking, C-response protein, family history of stroke at baseline, using the poor level as the reference exposure category.
HRs were further adjusted for the other CVH metrics.
The distributions of behavioral and biological CVH score are presented in Supplemental Figure S3. Analysis by global CVH subscales showed that the risk of stroke decreased significantly and linearly with both increasing behavioral CVH score (HR = 0·90, 95% CI: 0.88–0.91 per 1-point increment) and biological CVH score (HR = 0·82, 95% CI: 0.80–0.84 per 1-point increment) (Table 3). Ideal behavioral CVH was significantly associated with lower risks of all stroke subtypes. Biological CVH was related to stroke events except for SAH (HR = 1·24, 95% CI: 0.88–1.76). Joint effect of behavioral and biological CVH on the risk of stroke was also examined, participants with poor biological and behavioral CVH had 2.78-fold higher risk of stroke compared with those with ideal biological and behavioral CVH (HR = 2.78, 95% CI: 2.36–3.28) (Fig. 2), and there was no interaction between biological and behavioral CVH on risk of stroke (P for interaction = 0.95).
Table 3.
Behavioral and biological cardiovascular health (CVH) and risks of stroke and stroke subtypes.
| Cardiovascular health subscales | Stroke |
Ischemic stroke |
Intracerebral hemorrhage |
Subarachnoid hemorrhage |
Unspecified stroke |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events/total | HR (95% CI) | Events/total | HR (95% CI) | Events/total | HR (95% CI) | Events/total | HR (95% CI) | Events/total | HR (95% CI) | |
| Behavioral CVH | ||||||||||
| Poor (0–3) | 959/40,659 | 1 (Reference) | 624/40,659 | 1 (Reference) | 94/40,659 | 1 (Reference) | 76/40,659 | 1 (Reference) | 79/40,659 | 1 (Reference) |
| Intermediate (4–6) | 3658/221,201 | 0·72 (0·67–0·78) | 2332/221,201 | 0·71 (0·65–0·78) | 454/221,201 | 0·85 (0·68–1·07) | 264/221,201 | 0·59 (0·45–0·76) | 263/221,201 | 0·68 (0·53–0·89) |
| Ideal (7–8) | 1187/93,116 | 0·59 (0·54–0·65) | 708/93,116 | 0·56 (0·50–0·62) | 166/93,116 | 0·75 (0·57–0·97) | 113/93,116 | 0·57 (0·42–0·77) | 84/93,116 | 0·57 (0·41–0·78) |
| P for trend | <0·001 | <0·001 | <0·001 | 0·003 | <0·001 | |||||
| 1-point increment in behavioral scale | 5804/354,976 | 0·90 (0·88–0·91) | 3664/354,976 | 0·88 (0·86–0·90) | 714/354,976 | 0·94 (0·89–0·99) | 453/354,976 | 0·92 (0·87–0·98) | 426/354,976 | 0·88 (0·83–0·94) |
| Biological CVH | ||||||||||
| Poor (0–2) | 1341/46,069 | 1 (Reference) | 913/46,069 | 1 (Reference) | 142/46,069 | 1 (Reference) | 52/46,069 | 1 (Reference) | 115/46,069 | 1 (Reference) |
| Intermediate (3–4) | 3660/212,275 | 0·72 (0·68–0·77) | 2313/212,275 | 0·60 (0·64–0·74) | 463/212,275 | 0·83 (0·68–1·01) | 292/212,275 | 1·31 (0·97–1·77) | 251/212,275 | 0·58 (0·46–0·73) |
| Ideal (5–6) | 803/96,632 | 0·49 (0·45–0·54) | 438/96,632 | 0·43 (0·38–0·48) | 109/96,632 | 0·58 (0·45–0·76) | 109/96,632 | 1·24 (0·88–1·76) | 60/96,632 | 0·43 (0·31–0·60) |
| P for trend | <0·001 | <0·001 | <0·001 | 0·365 | <0·001 | |||||
| 1-point increment in biological scale | 5804/354,976 | 0·82 (0·80–0·84) | 3664/354,976 | 0·79 (0·77–0·82) | 714/354,976 | 0·84 (0·79–0·90) | 453/354,976 | 1·05 (0·96–1·15) | 426/354,976 | 0·77 (0·71–0·84) |
Hazard ratio estimated using Cox regression models with age as timescale and adjusted for sex, ethnicity, education attainment, employment status, Townsend deprivation index, consumption of alcohol intaking, C-response protein, family history of stroke.
Fig. 2.
Joint effect of behavioral and biological cardiovascular health on the risk of stroke. Behavioral CVH included smoking, diet, physical activity, body mass index. Biological CVH metrics included blood glucose, blood cholesterol, blood pressure.
Restricted cubic splines showed the association between global CVH score and stroke to be linear (P for linearity < 0.001) (Supplemental Figure S4). The Kaplan-Meier curves indicated a graded decreased risk of stroke associated with higher global CVH score. The incidence rates of stroke and stroke subtypes decreased with increasing global CVH score category (Table 4). Compared with the incident rate of stroke of 2.37 (95% CI 2.27–2.48) per 100 person-years among those with poor global CVH, the absolute rate differences per 100 person-years were −0.97 (−0.85 to −1.09) for intermediate global CVH and −1.64 (−1.51 to −1.77) for ideal global CVH. Similar patterns were also observed in stroke subtypes. In multivariable analysis, the risks of stroke and stroke subtypes decreased significantly across CVH category (all P for trend < 0.001) (Table 4). The HRs of stroke, IS, ICH and unspecified stroke for ideal CVH were 0.48 (95% CI 0.43–0.53), 0·42 (95% CI 0.36–0.48), 0.50 (95% CI 0.37–0.69) and 0·48 (95% CI 0.32–0.72) compared with poor CVH, respectively. Whereas, ideal CVH was not significantly associated with the risk of SAH (HR = 0.73, 95% CI: 0.52–1.02). The 1-point CVH score increment was associated with 11%, 13%, 8% and 13% lower risks of stroke, IS, ICH and unspecified stroke.
Table 4.
Global cardiovascular health (CVH) and risks of stroke and stroke subtypes.
| Outcomes | Events/total | Incidence rate per 1000 person-year (95% CI) | Absolute rate difference per 1000 person-year (95% CI) | HR (95% CI) |
|---|---|---|---|---|
| Stroke | ||||
| Poor CVH score (0–7) | 1908/75,172 | 2·37 (2·27–2·48) | 1 (Reference) | 1 (Reference) |
| Intermediate CVH score (8–11) | 3483/228,453 | 1·40 (1·36–1·45) | −0·97 (−1·09 to-0·85) | 0·69 (0·65–0·73) |
| Ideal CVH score (12–14) | 413/51,351 | 0·73 (0·67–0·81) | −1·64 (−1·77 to-1·51) | 0·48 (0·43–0·53) |
| P for trend | <0·001 | |||
| 1-point increment in CVH score (range 0–14) | 5804/354,976 | 1·51 (1·47–1·55) | 0·89 (0·88–0·90) | |
| Ischemic stroke | ||||
| Poor CVH score (0–7) | 1259/75,172 | 1·56 (1·48–1·65) | 1 (Reference) | 1 (Reference) |
| Intermediate CVH score (8–11) | 2182/228,453 | 0·88 (0·84–0·91) | −0·68 (−0·78 to-0·59) | 0·67 (0·63–0·72) |
| Ideal CVH score (12–14) | 223/51,351 | 0·40 (0·35–0·45) | −1·16 (−1·27 to-1·07) | 0·42 (0·36–0·48) |
| P for trend | <0·001 | |||
| 1-point increment in CVH score (range 0–14) | 3664/354,976 | 0·95 (0·92–0·98) | 0·87 (0·86–0·89) | |
| Intracerebral hemorrhage | ||||
| Poor CVH score (0–7) | 205/75,172 | 0·25 (0·22–0·29) | 1 (Reference) | 1 (Reference) |
| Intermediate CVH score (8–11) | 455/228,453 | 0·18 (0·17–0·20) | −0·07 (−0·10 to-0·03) | 0·78 (0·66–0·92) |
| Ideal CVH score (12–14) | 54/51,351 | 0·10 (0·07–0·12) | −0·15 (−0·20 to-0·11) | 0·50 (0·37–0·69) |
| P for trend | <0·001 | |||
| 1-point increment in CVH score (range 0–14) | 714/354,976 | 0·18 (0·17–0·20) | 0·92 (0·89–0·95) | |
| Subarachnoid hemorrhage | ||||
| Poor CVH score (0–7) | 117/75,172 | 0·14 (0·12–0·17) | 1 (Reference) | 1 (Reference) |
| Intermediate CVH score (8–11) | 281/228,453 | 0·11 (0·10–0·13) | −0·03 (−0·06 to-0·02) | 0·77 (0·62–0·96) |
| Ideal CVH score (12–14) | 55/51,351 | 0·10 (0·07–0·13) | −0·04 (−0·08 to-0·01) | 0·73 (0·52–1·02) |
| P for trend | 0·029 | |||
| 1-point increment in CVH score (range 0–14) | 453/354,976 | 0·12 (0·11–0·13) | 0·97 (0·93–1·02) | |
| Unspecified stroke | ||||
| Poor CVH score (0–7) | 147/75,172 | 0·18 (0·15–0·21) | 1 (Reference) | 1 (Reference) |
| Intermediate CVH score (8–11) | 249/228,453 | 0·10 (0·09–0·11) | −0·08 (−0·11 to-0·05) | 0·68 (0·55–0·84) |
| Ideal CVH score (12–14) | 30/51,351 | 0·05 (0·04–0·08) | −0·13 (−0·16 to-0·09) | 0·48 (0·32–0·72) |
| P for trend | <0·001 | |||
| 1-point increment in CVH score (range 0–14) | 426/354,976 | 0·11 (0·10–0·12) | 0·87 (0·83–0·91) |
Hazard ratios were estimated using Cox regression models with age as timescale and adjusted for sex, ethnicity, education attainment, employment status, Townsend deprivation index, consumption of alcohol intaking, C-response protein, family history of stroke.
The robustness of the associations between CVH and stroke events was examined by several sensitivity analyses. Ideal global CVH metric at optimal level (5 to 7 ideal metrics) were significantly associated with lower risks of stroke, IS, ICH and unspecified stroke compared with poor (0 to 2 ideal metrics) CVH (P for trend <0.0001). In line with the main analysis, this association was not found for SAH outcome (Supplemental Table S3). Further, we explored the associations of CVH score with stroke events by sex and age subgroups, and the results were similar (Supplemental Table S4 and Table S5). No significant interactions between CVH score and either sex or age on stroke risk were found. We repeated the main analysis among participants with at least 2 years of follow-up, and the HRs of stroke and stroke subtypes for were similar (Supplemental Table S6). Simultaneously, the results were generally consistent in the sensitivity analyses that excluded individuals who had a history of cancer or long-illness (Supplemental Table S7). The associations of CVH score, behavioral CVH and biological CVH with stroke and stroke subtypes with multiple imputed data showed that the HRs remained essentially unchanged (Supplemental Table S8). Finally, the results of competing risk analysis were also consistent with the main analyses that CVH was associated with stroke ant its subtypes, except for SAH (Supplemental Table S9).
Discussion
In this prospective cohort study of 354,976 individuals with a 11-year median follow-up period, those with ideal global CVH status had markedly lower incidence rate for stroke and stroke subtypes compared with individuals with intermediate and poor CVH status. Our findings suggested that ideal behavioral, biological and global CVH score were significantly associated with lower risks of stroke, IS, ICH and unspecified stroke, but not SAH.
CVH is a construct developed by the AHA to define the ideal state of the cardiovascular system and includes 7 of the most important modifiable risk factors and health behaviors for the prevention of cardiovascular diseases. Several previous studies had examined the association of global CVH with stroke, as parts of analyses on total cardiovascular diseases, but these results were inconsistent. A cohort study of 2981 participants from the Northern Manhattan Study identified an inverse association between the number of CVH metric and the risk of total stroke over a 11-year median follow-up [12]. A prospective study including 30,239 participants who were followed for 4·9 years suggested that greater global CVH has been associated with lower risk of total stroke whether in black or white Americans [6]. Moreover, a meta-analysis combining results from 6 prospective studies with follow-up duration ranging from 4·0 to 11 years, identified a linear association between global CVH score and risk of total stroke [11]. The association between trajectories of global CVH score over time and the risk of stroke was also revealed in the Framingham Offspring Study [21] and The Kailuan Study [9]. However, no significant association between global CVH score and total stroke was found among 9294 participants within the Three-City Study, reporting the HR for ideal CVH compared with poor CVH was 0.45 (95% CI: 0.20–1.03) [7]. In the EPIC—Norfolk Study, similarly, the highest CVH category (score 12–14) was not statistically associated with the risk of total stroke compared with the lowest CVH category (score 0–2) among 10,043 participants [22]. Our study from a contemporary cohort clearly observed that ideal global CVH score as well as behavioral and biological CVH were significantly associated with a lower risk of total stroke. A linear gradient in the adjusted HRs across behavioral, biological and global CVH categories for total stroke were also presented.
In addition, our results complement several previous analyses of stroke demonstrating the association between behavioral, biological and global CVH and stroke subtypes, suggesting that CVH status was statistically associated with stroke subtypes including IS and ICH, except for SAH. To the best of our knowledge, only one study reported the relationship between CVH and stroke subtypes. In this study on 91,698 participants with a 4-year follow-up from the Kailuan Study [23], the results showed that greater number of ideal global CVH metric was only associated with a lower risk of IS but not ICH. However, the study was of modest incident stroke cases (<1500), and was limited by its shorter follow-up period and failed to differentiate ICH and SAH. It is of worth noting that the associations between CVH and stroke subtypes were corroborated by our findings over a longer follow-up duration and among a larger sample. One explanation for the inconsistency of ICH and SAH is that they have different pathogenesis and risk factors [24,25]. IS occurs owing to the occlusion of a blood vessel, whereas the hemorrhagic stroke is inclined to a rupture of a blood vessel. Hemorrhagic strokes include ICH and SAH. Different from the ICH, non-traumatic parenchymal hemorrhage in the brain, SAH results from the rupture of a blood vessel connecting the arachnoid membrane to the surface of the brain an performs more severe clinical symptoms including headache, nausea and vomiting [26]. ICH is related to hypertension, diabetes, and other atherosclerosis risk factors, SAH prefer to link with intracranial aneurysms or arteriovenous malformations and high blood pressure promotes the vessels burst [24,27]. As described previously, the 7-item CVH metrics tend to increase the oxidative stress, vascular inflammation and microcirculation disorder in the blood vessels [28]. For example, hypertension contributes to the arteriole spasms, proliferation of smooth muscle cells, vascular wall remodeling and fibrosis, thus leading to arterial sclerosis and stenosis [29]. Meanwhile, increased serum cholesterol level is directly combined with the atheromatous plaque formation, developing into the stenosis or occlusion of the lumen and increasing the incidence of cardiovascular and cerebrovascular events [30]. In current study, we found that the difference of CVH metric for ICH and SAH lie in smoking status, BMI, diet pattern and blood glucose.
By combining behavioral and biological cardiovascular risk factors using the AHA 7-item tool, we investigated the relationship between an extended CVH profile and stroke events. Our results showed a graded and substantial reductions in the risks of stroke events across the continuum of behavioral, biological and global CVH score, highlighting the importance of clustering of CVH risk factors for risk of incident stroke. It should be emphasized that the association between CVH and stroke was linear, so that even intermediate CVH or the gain of 1 additional ideal metric was systematically associated with favorable outcomes. Moreover, each 1-point increment in the CVH score was associated with a similar reduction in the risk of stroke events to each additional CVH metric at optimal level. Use of a finely graded 14-point scale, which also considers each metric at an intermediate level, suggests that even small improvements in CVH metrics, without necessarily reaching the optimal level for each metric, are likely to be beneficial in reducing the risk of stroke events. From a pragmatic and public health perspective, promoting change in CVH metrics from poor to intermediate levels with increasing CVH scores may be more achievable and is likely to have a greater population-level effect for preventing risk factors associated with stroke events than the much more challenging change from poor to optimal level. These results contribute to quantify the potential long-term implications of meeting high CVH recommendations.
This study has several limitations. First, CVH metrics were obtained only at baseline, and changes over time were not accounted for in this study. Second, although analyses were adjusted for known potential sources of bias and participants were followed up for a median of 11 years, the possibility of unmeasured confounding and reverse causation remains. Third, as in many large prospective studies, healthy lifestyle information (e.g. physical activity, diet metrics) was subjectively measured by self-reported, which are known to cause possible recall bias. However, recall-based assessment methods remain reasonable representations for health behaviors with alternative biases and problems inherent in observed assessment methods. Besides, the definition of our diet metrics was not complete, since information on fiber-rich whole grains, sodium and sugar-sweetened beverages was lack, future study should include all metrics using another large-scale cohort according to AHA criteria. Final, the study sample was recruited from a community set ting but there is evidence of participation bias with study participants being more affluent and healthier than the average UK population [31]. Therefore, UK Biobank is not a representative sample of the UK population, we should be cautious in generalizing summary statistics to the general population. However, it can be used to provide valid estimates of exposure–disease relationships due to its large sample size and multitude of exposures [31,32]. Estimated relative risks derived from UK Biobank are consistent with more representative population cohorts [32].
In conclusion, in this large population-based sample, ideal behavioral, biological and global CVH score, as defined by the AHA 7-item tool, were associated with substantially lower risks of stroke, IS, ICH and unspecified stroke, but not SAH. Our study highlights the benefits of maintaining better CVH across the life course and call attention to the need for comprehensive strategies to preserve and restore high CVH score to prevent stroke events.
Declaration of Competing Interest
The authors declare that they have no conflicts of interests.
Acknowledgments
Funding
This study was supported by the National Natural Science Foundation of China (71910107004, 91746205).
Author's Contributions
YGW and ZC conceived the study. ZC and SL, HXY wrote the first and successive drafts of the manuscript. ZC, HXY, CJX and SL analyzed the data. ZC and SL, YZ had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. XLY, TY, TL and YGW reviewed the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Acknowledgements
We thank the participants of the UK Biobank. This research has been conducted using the UK Biobank Resource under the project number of 45676.
Data sharing statement
The data that support the findings of the study are available from the UK Biobank (https://www.ukbiobank.ac.uk/). Data will be available from the corresponding author upon reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100791.
Appendix. Supplementary materials
References
- 1.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray C.J., Vos T., Lozano R. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D.M., Hong Y., Labarthe D. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American heart association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 4.Carnethon Mr Fau - Pu J., Pu J Fau - Howard G., Howard G Fau - Albert M.A. Cardiovascular health in African Americans: a scientific statement from the American heart association. Circulation. 2018 doi: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 5.Bundy J.D., Ning H., Zhong V.W. Cardiovascular health score and lifetime risk of cardiovascular disease: the cardiovascular lifetime risk pooling project. Circ Cardiovasc Qual Outcomes. 2020 doi: 10.1161/CIRCOUTCOMES.119.006450. Circoutcomes119006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulshreshtha A., Vaccarino V., Judd S.E. Life's Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44:1909–1914. doi: 10.1161/STROKEAHA.111.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaye B., Canonico M., Perier M.C. Ideal cardiovascular health, mortality, and vascular events in elderly subjects: the three-city study. J Am Coll Cardiol. 2017;69:3015–3026. doi: 10.1016/j.jacc.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Joseph J.J., Bennett A., Echouffo Tcheugui J.B. Ideal cardiovascular health, glycaemic status and incident type 2 diabetes mellitus: the reasons for geographic and racial differences in stroke (REGARDS) study. Diabetologia. 2019;62:426–437. doi: 10.1007/s00125-018-4792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu S., An S., Li W. Association of trajectory of cardiovascular health score and incident cardiovascular disease. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MI N., Kunutsor S.K., Voutilainen A. Association between ideal cardiovascular health and risk of sudden cardiac death and all-cause mortality among middle-aged men in Finland. Eur J Prev Cardiol. 2021 doi: 10.1177/2047487320915338. 20202047487320915338. [DOI] [PubMed] [Google Scholar]
- 11.Guo L., Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: a meta-analysis of prospective studies. Clin Cardiol. 2017;40:1339–1346. doi: 10.1002/clc.22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong C., Rundek T., Wright C.B. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125:2975–2984. doi: 10.1161/CIRCULATIONAHA.111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudlow C., Gallacher J., Allen N. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samieri C., Perier M.C., Gaye B. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA. 2018;320:657–664. doi: 10.1001/jama.2018.11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao W., Tang H., Yang X. Fish consumption and stroke risk: a meta-analysis of prospective cohort studies. J Stroke Cerebrovasc Dis. 2019;28:604–611. doi: 10.1016/j.jstrokecerebrovasdis.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 16.He K., Song Y., Daviglus M.L. Fish consumption and incidence of stroke: a meta-analysis of cohort studies. Stroke. 2004;35:1538–1542. doi: 10.1161/01.STR.0000130856.31468.47. [DOI] [PubMed] [Google Scholar]
- 17.Wang T., Lu J., Su Q. Ideal cardiovascular health metrics and major cardiovascular events in patients with prediabetes and diabetes. JAMA Cardiol. 2019;4:874–883. doi: 10.1001/jamacardio.2019.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Y., Hao G., Wang Z. Ideal cardiovascular health status and risk of cardiovascular disease or all-cause mortality in chinese middle-aged population. Angiology. 2019;70:523–529. doi: 10.1177/0003319718813448. [DOI] [PubMed] [Google Scholar]
- 19.Climie R.E., van Sloten T.T., Périer M.C. Change in cardiovascular health and incident type 2 diabetes and impaired fasting glucose: the Whitehall II study. Diabetes Care. 2019;42:1981–1987. doi: 10.2337/dc19-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 21.Corlin L., Short M.I., Vasan R.S. Association of the duration of ideal cardiovascular health through adulthood with cardiometabolic outcomes and mortality in the framingham offspring study. JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lachman S., Peters R.J., Lentjes M.A. Ideal cardiovascular health and risk of cardiovascular events in the EPIC-Norfolk prospective population study. Eur J Prev Cardiol. 2016;23:986–994. doi: 10.1177/2047487315602015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q., Zhou Y., Gao X. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013;44:2451–2456. doi: 10.1161/STROKEAHA.113.678839. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S., Zhang W., Zhou G. Extended risk factors for stroke prevention. J Natl Med Assoc. 2019;111:447–456. doi: 10.1016/j.jnma.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Campbell B.C.V., Khatri P. Stroke. Lancet. 2020;396:129–142. doi: 10.1016/S0140-6736(20)31179-X. [DOI] [PubMed] [Google Scholar]
- 26.Dorsch N.W. Haemorrhagic stroke. Intracerebral and subarachnoid haemorrhage. Aust Fam Physician. 1997;26:1145–1150. [PubMed] [Google Scholar]
- 27.O'Donnell M.J., Chin S.L., Rangarajan S. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 28.Boehme A.K., Esenwa C., Elkind M.S. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120:472–495. doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buonacera A., Stancanelli B., Malatino L. Stroke and hypertension: an appraisal from pathophysiology to clinical practice. Curr Vasc Pharmacol. 2019;17:72–84. doi: 10.2174/1570161115666171116151051. [DOI] [PubMed] [Google Scholar]
- 30.Sandesara P.B., Virani S.S., Fazio S. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. 2019;40:537–557. doi: 10.1210/er.2018-00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fry A., Littlejohns T.J., Sudlow C. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batty G.D., Gale C.R., Kivimaki M. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ-Br Med J. 2020:368. doi: 10.1136/bmj.m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.