Graphical abstract
Endogenous sulfur dioxide is a novel inhibitor of hypoxia-induced mast cell degranulation. Endogenous SO2/AAT pathway exists in MCs. Mast cell-derived SO2 upregulates cAMP level through activation of AC and inhibition of PDE, thereby suppressing degranulation of MCs.
Keywords: Endogenous sulfur dioxide, Mast cells, Stabilization, Degranulation, cAMP
Highlights
-
•
Endogenous SO2/AAT pathway exists in mast cells (MCs).
-
•
Endogenous SO2 is a novel MC membrane stabilizer under hypoxic circumstance.
-
•
MC-derived SO2 upregulates cAMP level, thereby suppressing MC degranulation.
Abstract
Introduction
Mast cell (MC) degranulation is an important step in the pathogenesis of inflammatory reactions and allergies; however, the mechanism of stabilizing MC membranes to reduce their degranulation is unclear.
Methods
SO2 content in MC culture supernatant was measured by HPLC-FD. The protein and mRNA expressions of the key enzymes aspartate aminotransferase 1 (AAT1) and AAT2 and intracellular AAT activity were detected. The cAMP level in MCs was detected by immunofluorescence and ELISA. The release rate of MC degranulation marker β-hexosaminidase was measured. The expression of AAT1 and cAMP, the MC accumulation and degranulation in lung tissues were detected.
Objectives
To exam whether an endogenous sulfur dioxide (SO2) pathway exists in MCs and if it serves as a novel endogenous MC stabilizer.
Results
We firstly show the existence of the endogenous SO2/AAT pathway in MCs. Moreover, when AAT1 was knocked down in MCs, MC degranulation was significantly increased, and could be rescued by a SO2 donor. Mechanistically, AAT1 knockdown decreased the cyclic adenosine monophosphate (cAMP) content in MCs, while SO2 prevented this reduction in a dose-independent manner. Pretreatment with the cAMP-synthesizing agonist forskolin or the cAMP degradation inhibitor IBMX significantly blocked the increase in AAT1 knockdown-induced MC degranulation. Furthermore, in hypoxia-stimulated MCs, AAT1 protein expression and SO2 production were markedly down regulated, and MC degranulation was activated, which were blunted by AAT1 overexpression. The cAMP synthesis inhibitor SQ22536 disrupted the suppressive effect of AAT1 overexpression on hypoxia-induced MC degranulation. In a hypoxic environment, mRNA and protein expression of AAT1 was significantly reduced in lung tissues of rats. Supplementation of SO2 elevated the cAMP level and reduced perivascular MC accumulation and degranulation in lung tissues of rats exposed to a hypoxic environment in vivo.
Conclusion
SO2 serves as an endogenous MC stabilizer via upregulating the cAMP pathway under hypoxic circumstance.
Introduction
Mast cells (MCs) play important physiological and pathological roles in host defense, immune regulation, allergic reactions and chronic inflammation [1]. During MC degranulation, activated mast cells rapidly release particles stored in the cytoplasm into the extracellular environment in response to external stimuli [2]. The release of IgE-dependent degranulation and the release of proinflammatory mediators during an allergic reaction play important roles in host defense and nonallergic inflammatory diseases [3]. Studies have shown that the activation of MC degranulation is also an important step in the pathogenesis of nonallergic chronic diseases such as myocardial infarction [4], cardiomyopathy [5], cirrhosis [6], [7] and other chronic diseases. Clarifying the mechanisms for MC degranulation has important implications in the life science and medical science fields.
The activation of MC degranulation is mainly induced by immune stimulation caused by antigens and nonimmune stimulation by drugs, which in turn causes the activation of downstream signaling pathways. This activation ultimately leads to the release of inflammatory mediators such as histamine, heparin and various cytokines stored in MCs. During allergic reactions, IgE binds to the IgE receptor and induces MC activation [8], [9]. MC degranulation depends on the action of calcium ions. An increase in the cytosolic calcium ion concentration leads to changes in calmodulin (CaM) configuration, which in turn activates CaM protein kinase, and activated tubulin and myosin promote significant fusion and efflux of vesicle particles and cell membranes in MCs [10], [11]. After MC activation, protein kinase C (PKC) is significantly activated and phosphorylates cell membrane proteins and cytoskeletal proteins, thereby triggering MC degranulation [12]; however, the mechanisms underlying MC degranulation and the responsible signaling pathways are unclear. Therefore, exploring endogenous factors that steadily regulate MC activation and stabilization, as well as the regulatory mechanisms, would be of great significance for the control of allergic diseases and inflammation.
Previous studies reported that endogenously generated gaseous signal molecules are widely present in mammals and involved in the fine and continuous regulation of disease development [13], [14]. Sulfur dioxide (SO2), a novel endogenous sulfur-containing gaseous signaling molecule catalyzed by aspartate aminotransferase (AAT) with the substrate of cysteine [15], significantly reduced the levels of inflammatory factors in endothelial cells [16]. Also, it was reported that SO2 pretreatment reduced serum IL-1 and IL-6 levels in rats with acute lung injury induced by lipopolysaccharide [17]. The abovementioned reports suggest that endogenous SO2 has the ability to control the inflammatory response. Previous findings demonstrated that the endogenous sulfur-containing gaseous signaling molecule hydrogen sulfide (H2S) is produced in MCs [18] and that both H2S and SO2 are products of the same sulfur-containing amino acid metabolic family [19], [20]. Therefore, we hypothesized that a potential endogenous SO2 production pathway exists in MCs. In the case of such a pathway, whether endogenous SO2 regulates allergic or inflammation-induced degranulation of MCs is an important mechanism to address.
After the activation of human MCs, active substances such as β-hexosaminidase and histamine, release and participate in the inflammatory reaction, and the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA)/phosphodiesterases (PDEs) pathway impacts the activation of human MCs [21]. The intracellular cAMP was found to inhibit excessive inflammatory or allergic reactions. cAMP activates cAMP-dependent PKA to inhibit IgE/antigen-dependent signaling activation and calcium influx, thereby inhibiting MC degranulation [21], [22]. Increased intracellular cAMP levels caused by stimuli, such as adenosine and beta-adrenergic receptors, were shown to inhibit MC degranulation [23]. Previous studies on smooth muscle cell proliferation showed that endogenous SO2 activates cAMP/PKA and thereby inhibits the activation of Raf/MEK/ERK and cell proliferation [24]. Whether endogenous SO2 controls the degranulation of MCs and the underlying mechanism remains unclear. Therefore, we investigated the existence of an endogenous SO2/AAT pathway in MCs, its possible role in MC degranulation and its potential mechanisms.
Materials and methods
Reagents
MCs, vascular smooth muscle cells (VSMCs) and vascular endothelial cells (VECs) were acquired from the China Infrastructure of Cell Line Resources Center. Sodium bisulfite and sodium sulfite (NaHSO3/Na2SO3, freshly mixed at a mole ratio 1:3, pH 7.4) was used as the SO2 donor. Lentivirus carrying AAT1 shRNA, AAT2 shRNA, scramble shRNA and AAT1 cDNA, and vehicle lentivirus were constructed by Cyagen (Guangzhou, China). The AAT activity detection kit was purchased from Jiancheng (Nanjing, China). The cAMP ELISA kit was from Neweast(PA, USA). The L-glutamine, streptomycin penicillin and fetal bovine serum were purchased from Gibco (USA). SYBR Green was purchased from Thermofisher (USA). The Oligo(dT)15 primer and reverse transcription kit were purchased from Promega (USA). Toluidine blue dye was purchased from Solarbio (Beijing, China).
Cell culture and treatment
The MCs and VECs used in the experiments were human-derived, and the VSMCs were rat-derived. The MCs were cultured in Iscove's Modified Dulbecco's Medium (IMDM), the VECs were cultured in F12/Dulbecco’s modified Eagle’s medium (F12/DMEM) and the VSMCs were cultured in DMEM. To prepare complete culture medium for MCs, IMDM was supplemented with the fetal bovine serum (10%), streptomycin penicillin (1%) and L-glutamine (2 mM). The cell synchronization culture medium IMDM was the same as the complete culture medium except for the addition of fetal bovine serum. The MCs were cultured in a moist environment at 37 °C and 5% CO2.
The concentration of forskolin [25] and SQ22536 used in the experiment was 30 μmol/L. The concentration of IBMX was 500 μmol/L [26]. The oxygen concentration in the hypoxic treatment group was set at 1%.
The plasma SO2 concentration in rats was approximately 0–30 μmol/L [27]. A previous study showed that the SO2 concentration in rat vascular tissues was approximately 127 μM [28]. Therefore, the SO2 donor in the present study was used at concentrations of 50, 100 and 200 μmol/L for dose-dependent experiments and at 100 μmol/L for the remaining cellular experiments.
Lentivirus transfection
The lentivirus carrying AAT1 or AAT2 shRNA (Cyagen, China) was transfected into the MCs to construct AAT1 or AAT2 knockdown MCs according to manufacturer's instructions. Briefly, the MCs were seeded in 25 cm2 culture flasks and infected with lentiviral AAT1 or AAT2 shRNA at a multiplicity of infection of 10 when the cell density was about 60%-70%. After a 24-hour transfection, the medium was replaced with fresh complete culture medium and incubated at 37 °C overnight. Then, the infected cells were screened with puromycin (4 μg/mL) for 1 week to acquire the stably transfected AAT1 or AAT2 shRNA MCs. At the same time, the lentivirus carrying scramble shRNA was used to infect the MCs as the control according to the same protocol. Similarly, overexpression of AAT1 in MCs was achieved by transfecting with lentivirus containing AAT1 cDNA and vehicle lentivirus was used as the control.
Animal model
Twenty-four Wistar male rats (150–160 g) were randomly divided into normoxic, hypoxic and hypoxic + SO2 groups (n = 6 each group). The rats in hypoxic group and hypoxic + SO2 group breathed in the normobaric hypoxic air (10% O2) in a hypoxia chamber (Biospherix, USA) for 3 weeks and 6 h every day, while the rats in the normoxic group breathed in the room air (21% O2) for the same period. Moreover, rats in the hypoxic + SO2 group were injected with NaHSO3/Na2SO3 mixture (0.18 mmol and 0.54 mmol per kilogram body weight) before hypoxia exposure each day. The rats of the normoxic and hypoxic groups were injected with the same volume of physiological saline. The dose of SO2 donor was chosen based on the previous finding that the administration of this dosage of NaHSO3/Na2SO3 mixture could significantly restore the hypoxia-inhibited pulmonary SO2 content and alleviate pulmonary hypertension and pulmonary vascular remodeling in hypoxic rats [29]. The experimental rat hypoxic model was established according to the previous literatures [29], [30], [31], [32]. In the abovementioned studies [29], [30], [31], [32], the rats exposed to hypoxia for 21 days showed a significant pulmonary hypertension and pulmonary vascular inflammation.
All rats were euthanized after 21 days of hypoxic exposure and the lung tissues were collected. One side of the lung lobe was removed and fixed with 10% formalin. Then, paraffin-embedded lung tissues were sectioned at a thickness of 5 μm for toluidine blue staining and immunohistochemistry. The remaining lung tissue was rapidly frozen in liquid nitrogen and stored at − 80 °C for subsequent examinations.
Determination of aspartate aminotransferase (AAT) 1 and AAT2 mRNA by real-time RT-PCR
The AAT1 and AAT2 mRNA expression in MCs were measured by RT-PCR. The following primers were used:
AAT1-human, 5′-GTCAGAATCCGGTCAGCCATT-3′ (R),
5′-GTGGCCAGCACCCTCTCTAA-3′ (F);
AAT2-human, 5′-CGCAGGCATGCAGAAGAA-3′ (R),
5′-GTGGCCAGCACCCTCTCTAA-3′ (F);
GAPDH-human, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ (R),
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ (F).
AAT1-rat, 5′-GCCATTGTCTTCACGTTTCCTT-3′ (R),
5′-CCAGGGAGCTCGGATCGT-3′ (F);
AAT2-rat, 5′-GTTTCCCCAGGATGGTTTGG-3′ (R),
5′-GAGGGTCGGAGCCAGCTT-3′ (F);
Actin-rat, 5′-TATCGTCATCCATGGCGAACT-3′ (R),
5′-ACCCGCGAGTACAACCTTCTT-3′ (F).
Total RNA was extracted from the MCs and lung tissues using Invitrogen TRIzol reagent. A total of 2 μg of RNA was used for complementary deoxyribonucleic acid (cDNA) synthesis by reverse transcription with an oligo(dT)15 primer. cDNA synthesis conditions included a 25 μl of reaction system for denaturation at 70 °C for 5 min followed by 42 °C for 60 min. The real-time PCR reaction conditions were set at 95 °C pre-denaturation for 10 min, 95 °C denaturation for 15 s, 60 °C annealing for 1 min, and 72 °C polymerization extension for 30 s, with a total of 40 cycles. The relative mRNA expression of AAT1 or AAT2 was analyzed using the formula 2−ΔΔCT method. GAPDH or β-actin mRNA was used as internal reference, respectively.
Determination of SO2 concentration by HPLC-FD
The SO2 content in the cell supernatant and rat lung tissues was detected by high performance liquid chromatography with fluorescence detection (HPLC-FD) [22]. This experiment was quantified by the standard sodium sulfite. Briefly, sodium borohydride (70 μl, 0.212 mol/L) was added to the 100 μl of sample or standard. The mixtures were then incubated at 37 °C for 30 min. Next, mBrB (5 μl, 70 mmol/L) was added to the above mixtures, which were then incubated at 42 °C for 10 min. Perchloric acid (40 μl, 1.5 mol/L) was added to the mixture. The samples were centrifuged (12,400 × g) at 23 °C for 10 min to remove the protein precipitates. Tris-HCl (10 μl, 2 mol/L, pH 3.0) was used to neutralize the mixture. Finally, the mixture was collected and then analyzed by HPLC.
Determination of AAT1 and AAT2 protein expression by Western blot analysis
The MCs were collected by centrifuging the cell culture medium, and washed twice with PBS. The cells were lysed in RIPA lysis buffer (50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% NP40, 0.25% sodium deoxycholate and protease inhibitor cocktail) for 20 min in an ice bath, vortexed, ultrasonicated and centrifuged. The supernatant was collected as the protein lysate. The lung tissues were ground into a homogenate according to the mass volume ratio 1:10 (mg/μL) with RIPA lysis buffer, and then the homogenate was centrifuged for 10 min at 4 °C to separate the supernatant. The protein lysate was separated on a 10% SDS polyacrylamide gel, and then transferred to a membrane. Following ponceau staining and using the reference protein marker, the target protein band was labeled. The membrane was blocked and then incubated with primary antibody. The primary antibodies used for the experiment were as follows: anti-AAT1 from Sigma, 1:1000; anti-AAT2 from Sigma, 1:1000; and anti-GAPDH from KangCheng, 1:4000. The following day, the membrane was removed from 4 °C and incubated with the following secondary antibodies: goat anti-mouse IgG, 1:5000; rabbit anti-goat IgG, 1:2000; and goat anti-rabbit IgG, 1:2000. Exposure was performed using the Alpha Imager 3400 gel image acquisition system. The strip gray value was analyzed by the Alpha Ease FS software and corrected by the gray value of the internal reference GAPDH strip.
AAT activity detected by colorimetric assay
The colorimetric method was used to test AAT protein activity in MCs [27]. The MCs were collected and lysed in 80 μl of 0.01 M PBS by ultrasound under ice water bath conditions. AAT activity was detected following the manufacturer instructions. The substrate solution was added into the 96-well plate and briefly placed at 37 °C. A total of 20 μl of each sample was added to the corresponding well, and the plate was placed at 37 °C for 30 min. The sodium hydroxide solution (200 μl, 0.4 mol/L) was added and gently mixed into each well. The plate was incubated for 15 min at room temperature. The optical density (OD) value was detected at the absorption wavelength of 510 nm.
The β-hexosaminidase release rate detected by colorimetric assay
The release of β-hexosaminidase was used as a marker for MC degranulation. β-acetylglucosaminidase is an acid hydrolase in the cell lysosome. The substrate is hydrolyzed by β-acetylglucosaminidase to release free p-nitrophenol. The reaction was terminated by adding an alkaline solution, in which the p-nitrophenol was stained. The MCs were separated from the cell culture medium and resuspended in 4 °C PBS. The cells were sonicated and centrifuged, and the supernatant was collected for analysis. A total of 100 μl of the sample (cell culture supernatant or cell solution) was mixed with 500 μl of substrate buffer. After thoroughly mixing, the samples were incubated for 15 min at 37 °C. After stopping the reaction, the absorbance was detected at 400 nm to calculate the β-acetylglucosaminidase activity. Each liter of the sample was incubated with the substrate for 1 min at 37 °C. The enzyme activity unit was 1 μmol p-nitrophenol formed by hydrolysis. In this experiment, 0.6 mmol/L p-nitrophenol was used as the standard.
Immunofluorescence detection of cAMP expression in mast cells
A confocal laser scanning microscope was used to acquire the immunofluorescence images. The MCs were gently rinsed and fixed. The anti-cAMP antibody (1:50, CST, USA) was added to the cells then the secondary antibody (1:200, Thermo, USA) was added to conjugate to the primary antibody. Finally, the cells were observed under a confocal microscope [33].
The level of cAMP detected by enzyme-linked immunosorbent assay (ELISA)
The level of cAMP in the MCs was measured using an ELISA kit. The number of MCs was counted using a cytometer. The MCs were then centrifuged to remove the cell culture medium, and lysed by the addition of HCl (100 μl, 0.1 M). The standard sample had a known concentration of cAMP. A total of 100 μl of the standard and samples were added to a microplate that was coated with polyclonal goat anti-mouse antibody. A total of 50 μl of cAMP-conjugated horseradish peroxidase and 50 μl of anti-cAMP antibody were then added to the microplate, which was then shaken for 2 h. After washing, the substrate was added to develop color for 30 min. Immediately after the reaction was stopped, an OD value at the absorption wavelength of 450 nm was measured with a microplate reader, and the cAMP concentration of each sample was calculated.
Toluidine blue staining
Toluidine blue O is a commonly used synthetic basic dye. The cations in toluidine blue have a dyeing effect. The acidic substances of tissue cells are combined with the cations to be dyed, and the nucleus can be stained blue. Heparin and histamine in the MC cytoplasm can be stained purplish red when encountered with toluidine blue, and as such was used to detect MCs. The rat lung tissue sections were dewaxed and incubated with toluidine blue dye solution for 10 min. The excess staining solution was washed away and the color was separated with 95% alcohol for 5 min. Then, images were captured with a Leica inverted microscope. The number of toluidine blue-positive cells in the rat lung tissue sections was counted at 400× high power field (HFP).
Detection of cAMP and AAT1 expression by immunohistochemistry
The lung tissue slides were dewaxed by dimethylbenzene and then rehydrated by graded ethanol. After rinsing with PBS, the slides were incubated with 3% H2O2 for 10 min at room temperature in a humidified chamber. The slides were washed with PBS and incubated with goat serum for 30 min at 37 °C. The slide was incubated with the primary antibody (anti-cAMP, 1:100; anti-AAT1, 1:100) overnight at 4 °C. The slide was then rinsed with PBS and incubated with the secondary antibody for 1 h at 37 °C. 3,3-diaminobenzidine (DAB) was added to develop color, and the sections were stained with hematoxylin for 5 s. The slides were dehydrated by graded ethanol and made transparent in dimethylbenzene. Images were captured with a Leica inverted microscope.
Statistical analysis
The SPSS (20.0) software was used for statistical analysis. The data were presented as the mean ± standard deviation. The difference between 2 groups was analyzed by an independent Student’s t-test. A one-way analysis of variance (ANOVA) was used for the comparison of the differences among multiple groups following the Bonferroni post hoc test if the data were normally distributed, while the Dunnett T3 post hoc test followed ANOVA if the data were not normally distributed. Pearson’s correlation analysis was used to identify the dose-dependence effect. P-values were set to 0.05, which was considered statistically significant.
Results
An endogenous SO2/AAT pathway is present in mast cells
In this study, VECs and VSMCs were used as positive controls to detect an endogenous SO2 pathway in MCs. The results demonstrated that endogenous SO2 production was detected in the MC culture supernatant, and the SO2 content was 2.75 times and 1.62 times higher than that of VECs and VSMCs, respectively (Fig. 1A). In mammals, the essential enzymes of endogenous SO2 production are AAT1 and AAT2. With VECs and VSMCs as positive controls, the real time RT-PCR results showed that AAT1 and AAT2 mRNA were also present in MCs (Fig. 1B, C). Moreover, western blot analysis showed the presence of AAT1 (Fig. 1D) and AAT2 (Fig. 1E) proteins in MCs. The AAT activity was 10.77 ± 2.03 U/L in MCs, 20.73 ± 2.36 U/L in VECs, and 43.55 ± 18.86 U/L in VSMCs, respectively (Fig. 1F).
Fig. 1.
The presence of endogenous SO2/AAT pathway in MCs. (A) The SO2 content in the supernatant of MCs, VECs and VSMCs detected by HPLC-FD; (B) Relative AAT1 mRNA levels in MC, VEC and VSMC detected by real time RT-PCR; (C) Relative AAT2 mRNA levels in MC, VEC and VSMC detected by real time RT-PCR; (D) Relative AAT1 protein expression in MC, VEC and VSMC detected by western blot; (E) Relative AAT2 protein expression in MC, VEC and VSMC detected by western blot; (F) AAT activity in MC, VEC and VSMC detected by colorimetric analysis. *P < 0.05, **P < 0.01. The data are presented as the mean ± SD. The results in Fig. 1B-1C were normalized with those in MCs. All the research was carried out for three times independently.
AAT1 knockdown promoted mast cell degranulation
To investigate the impact of endogenous SO2 on MC stabilization, MCs were infected with AAT1 knockdown lentivirus. Compared with the scramble group, the SO2 content in the supernatant and the cAMP protein expression in the AAT1-knockdown MCs were significantly reduced (Fig. 2A and B), while MC degranulation, represented by β-hexosaminidase release rate, increased by approximately 2.18 fold (Fig. 2C). Exogenous SO2 donor supplementation successfully rescued the effect of AAT1 knockdown, demonstrated by the upregulation of cAMP protein expression and reduction of MC degranulation (Fig. 2B, C). To further investigate whether the effects of SO2 are dose-dependent, we examined the β-hexosaminidase release rate (Fig. 2D) and cAMP (Fig. 2E) level after supplementing with different dose of SO2 (50–200 μM). The data demonstrate that SO2 upregulated cAMP and downregulated MC degranulation in a dose-dependent manner. Moreover, AAT2 knockdown (Figure Supplementary Fig. S1. A) had no significant effect on the SO2 content in the supernatant (Fig. S1. B), the cAMP level in the MCs (Fig. S1. C) and MC degranulation (Fig. S1. D) compared with the scramble group. These results suggest that SO2 produced by AAT1 mainly upregulates the cAMP level and inhibits mast cell degranulation.
Fig. 2.
Knockdown of AAT1 promoted the degranulation of MCs. (A) The SO2 content in the MC supernatant detected by HPLC-FD. NaHSO3/Na2SO3 mixture (1:3 M/M) at a dose of 100 μM was used as a SO2 donor; (B) cAMP level in MCs detected by immunofluorescence. NaHSO3/Na2SO3 mixture (1:3 M/M) at a dose of 100 μM was used as a SO2 donor; (C) The release rate of β-hexosaminidase in MCs detected by colorimetric analysis. NaHSO3/Na2SO3 mixture (1:3 M/M) at a dose of 100 μM was used as a SO2 donor; (D) The release rate of β-hexosaminidase in MCs treated with different concentration of SO2 donor NaHSO3/Na2SO3 mixture (50–200 μM) detected by colorimetric analysis; (E) The cAMP content in MCs treated with different concentration of SO2 donor NaHSO3/Na2SO3 mixture (50–200 μM) detected by ELISA. *P < 0.05 and the data are presented as the mean ± SD. All the research was carried out for three times independently.
cAMP level elevation blocked SO2 reduction-driven MC degranulation
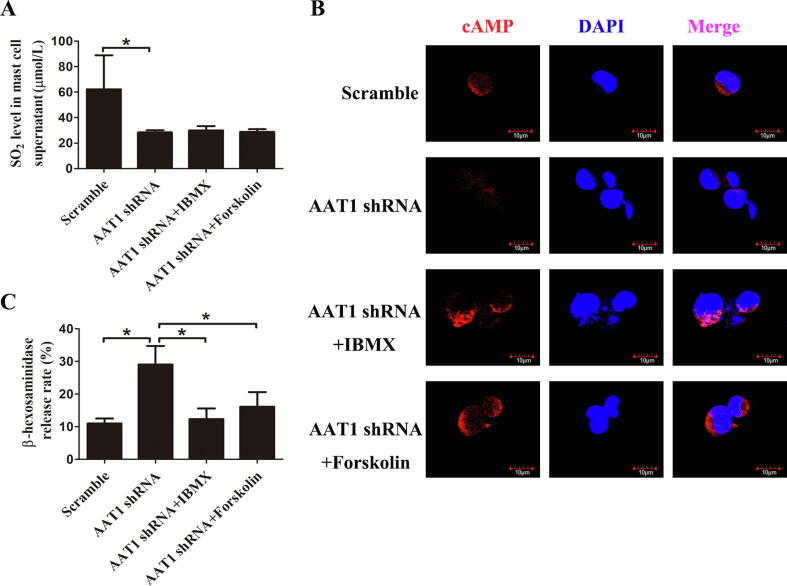
To further investigate whether cAMP mediates the inhibitory effect of endogenous SO2 on MC degranulation, AAT1-depleted MCs were treated with the PDE inhibitor IBMX or adenylate cyclase (AC) activator forskolin. Compared with the scramble group, both the SO2 content and cAMP expression in the AAT1-depleted MCs were significantly decreased (Fig. 3A, B), and MC degranulation was significantly increased (Fig. 3C); however, the treatment of MCs with the AC activator forskolin or the PDE inhibitor IBMX to elevate cAMP content successfully abolished AAT1 knockdown-induced MC degranulation (Fig. 3B, C).
Fig. 3.
The elevation of cAMP levels blocked AAT1 knockdown-induced MC degranulation. (A) The SO2 content in MC supernatant detected by HPLC-FD; (B) The cAMP level in MCs detected by immunofluorescence; (C) The release rate of β-hexosaminidase in MCs detected by colorimetric analysis. *P < 0.05 and the data are presented as the mean ± SD. All the research was carried out for three times independently.
SO2 protected against hypoxia-induced mast cell degranulation in vitro
Hypoxic challenge downregulated AAT1 protein expression but did not affect AAT2 protein expression in the MCs in vitro (Fig. 4A, B). Accordingly, the SO2 content in the MC supernatant was significantly reduced (Fig. 4C). Furthermore, AAT1 overexpression markedly blunted the effect of hypoxia on the intracellular cAMP level (Fig. 4D) and MC degranulation in vitro (Fig. 4E). Importantly, treatment with SQ22536 to inhibit cAMP synthesis abolished AAT1 overexpression-inhibited MC degranulation (Fig. 4E).
Fig. 4.
AAT1 overexpression significantly inhibited the hypoxia-induced degranulation of MCs in vitro. (A-B) Western blot analysis of protein expression of AAT1 (A) and AAT2 (B) in MCs in normoxic and hypoxic groups in vitro; (C) The SO2 content in supernatant of MCs was detected by HPLC in vitro; (D) The cAMP content in MCs detected by ELISA in vitro; (E) Colorimetric analysis of the release rate of β-hexosaminidase in MCs in vitro ; *P < 0.05, **P < 0.01 and data are presented as the mean ± SD. All the research was carried out for three times independently.
SO2 inhibited the hypoxia-driven downregulation of cAMP and increase in MC degranulation in vivo
Chronic hypoxic challenge downregulated the protein and mRNA expression of AAT1 (Fig. 5A, B, C) and the level of cAMP (Fig. 5D), but increased MC accumulation and degranulation in the rat lung tissues in vivo (Fig. 5E). The mRNA expression of AAT2 was also downregulated (Figure Supplementary Fig. S2. A), but hypoxia exposure did not affect AAT2 protein expression (Fig. S2. B, C) in the rat lung tissues in vivo. Importantly, exogenous SO2 donor supplementation successfully rescued the downregulation of cAMP protein expression in vivo (Fig. 5D). Moreover, the supplementation with a SO2 donor decreased the hypoxia-induced perivascular MC accumulation and degranulation in the lung tissues of rats exposed to a hypoxic environment in vivo (Fig. 5E).
Fig. 5.
SO2 donor inhibited the hypoxia-driven downregulation of cAMP and increase in MC degranulation in vivo. (A) Western blot analysis of AAT1 protein expression in rat lung tissue; (B) Real time RT-PCR analysis of AAT1 mRNA levels in rat lung tissue; (C) Immunohistochemistry analysis of AAT1 protein expression in rat lung tissue; (D) Immunohistochemistry analysis of cAMP protein expression in rat lung tissue; (E) Toluidine blue staining was used to detect MC accumulation and degranulation (red arrows) around rat pulmonary vascular tissue. The rats in the hypoxic + SO2 group were injected with NaHSO3/Na2SO3 mixture (0.18 mmol and 0.54 mmol per kilogram body weight) before hypoxia exposure each day. The rats of the normoxic and hypoxic groups were injected with the same volume of physiological saline. **P < 0.01 and data are presented as the mean ± SD, n = 6 each group.
Discussion
SO2 is regarded as a toxic gas and environmental pollutant; however, SO2 can be endogenously generated in mammals, dissolved in plasma and derived into sulfite and generate bisulfite to maintain the stability of the internal environment [27], [28], [34], [35], [36]. In mammals, sulfur-containing amino acids, such as L-cysteine, are converted to L-cysteine sulfinate by the catalysis of cysteine dioxygenase, which is converted to β-thionylpyruvate through AAT1/2 and eventually spontaneously decomposes into pyruvate and SO2 [27], [34], [36]. In this study, the presence of endogenous SO2 was first detected in the cell culture supernatant of MCs. The mRNA level and the protein expression levels of AAT1 and AAT2, which are key enzymes for the production of endogenous SO2, as well as AAT activity were detected in the MCs. Therefore, we first suggest that there might exist an endogenous SO2/AAT pathway in MCs.
In the present study, VSMCs and ECs, two kinds of endogenous SO2 source cells [27], [28], were used as positive reference to demonstrate the existence of endogenous SO2 pathway in MCs. Interestingly, the AAT expression and activity in the certain cell seemed not parallel to SO2 content released from the cell. For example, VSMC showed the highest AAT1/2 mRNA levels, AAT2 protein and AAT activity, and second highest AAT1 protein level. However, the levels of SO2 in VSMC was lower than that of MC. We speculated that there might be other impactors involved in the regulation of endogenous SO2 content such as non-enzymatic SO2 generation, SO2 consumption and clearance according to a comprehensive review about SO2 metabolism and SO2 donor [37], which merits to be investigated in the future.
Numerous studies have demonstrated that MCs are abundantly present in exposed areas of the body (such as airways, skin and intestines) and are usually located near blood vessels and nerves. In this location, MCs can take on a sentinel role in early host defense and play an important role in many inflammatory diseases such as allergic rhinitis, conjunctivitis, asthma and arthritis [38], [39]. The local microenvironment can directly affect the maturity, phenotype and function of MCs. These mediators released from MC enable MC to initially respond in harmful situations and respond to the altered environment by communicating with cells involved in physiological and immune responses. After myocardial ischemia-reperfusion injury, MCs activate and degranulate. Renin is released, which in turn activates the local renin-angiotensin system and ultimately leads to ventricular fibrillation [40]. MCs are also involved in the pathogenesis of allergic asthma. Activated MCs release histamine and activate smooth muscular contraction, bronchial secretion and airway mucosal edema [41]. However, the endogenous regulatory mechanism underlying degranulation following MC activation is not fully understood. Here, we showed that a spontaneous MC degranulation accompanied with a decrease in SO2 content in AAT1 knockdown MCs, which was concurrently rescued by SO2 donor. Especially, SO2 donor rescued the AAT1 knockdown-induced MC degranulation in a dose-dependent manner. On the contrary, AAT2 knockdown did not affect the SO2 content in the supernatant and MC degranulation. Therefore, these findings suggest that the endogenous SO2/AAT1 pathway might have an important inhibitory effect on MC degranulation.
Subsequently, investigating the possible mechanisms by which endogenous SO2 inhibits MC degranulation is an important issue. Previous studies found that the interventions of elevating intracellular cAMP level including cAMP-synthesis agonists Forskolin and isoprenaline, and cAMP-degradation inhibitor IBMX correlated well with the inhibition of histamine, leukotriene C4, and PGD2 release in MCs [42], suggesting that cAMP is an important controller for MC degranulation. It was a coincidence that VSMC-derived SO2 inhibited cell proliferation via increasing the cAMP level and activating PKA pathway [24]. The abovementioned findings give us a hint that cAMP level might bridge endogenous SO2 and MC degranulation. As we expected, the data showed that SO2 donor recovered the AAT1 knockdown-reduced cAMP level in a dose-dependent manner. Furthermore, the AC activator Forskolin and the PDE inhibitor IBMX both increased cellular cAMP levels and simultaneously blocked AAT1 knockdown-induced excessive degranulation of MCs, suggesting that endogenous SO2/AAT1 pathway inhibits the degranulation of MCs by increasing intracellular cAMP levels under physiological conditions.
The potential role of endogenous SO2 in regulating MC degranulation under pathophysiologic conditions, as well as the underlying mechanisms, was unclear. Previous studies showed that hypoxia significantly stimulates MC degranulation, causing the release of cytokines such as angiogenic factors to induce retinal neovascularization, resulting in eye diseases and even blindness [43]. MCs are infiltrated in hypoxic microenvironments caused by tumors, and MC activation promotes angiogenesis and tumor invasion [44]. Hypoxia is a significant feature of inflammatory tissue, including the lung of asthmatic patients, which is closely related to mast cell infiltration and degranulation [45]. In the pathology of fatal asthma, inflammatory cells infiltrate in the airway wall and surrounding parenchyma. MCs can be located in the airway wall and are activated after being stimulated by hypoxia. Inflammatory mediators are then released by MCs, which then recruit more inflammatory cells, ultimately causing intense bronchospasm and sudden death [46]. Moreover, MC degranulation during hypoxic conditions plays an important role in the pathogenesis of pulmonary vascular remodeling [47]. Therefore, hypoxia stimulation was used as a model to activate MC degranulation in the present study. The data showed that AAT1 protein expression in the MCs was decreased while AAT2 protein expression remained unchanged under a hypoxic environment. Simultaneously, the decrease in the supernatant SO2 content, the reduction of cAMP level and the cell degranulation occurred in the hypoxic MCs. However, MCs with AAT1 overexpression no longer responded to hypoxic stimulation representing by the fact that there were no differences in SO2 content, cAMP level and cell degranulation in the AAT1-overexpressing MCs between normoxic and hypoxic groups. While, an AC inhibitor, SQ22536, restored the hypoxia-driven cell degranulation in the AAT1-overexpressing MCs. The abovementioned findings provided in vitro experiment evidence for the hypothesis that the downregulation of endogenous SO2/AAT1 and subsequent decreased cAMP might be involved in the hypoxia-driven MC degranulation.
Furthermore, in a rat model of chronic hypoxic stimulation, we found that hypoxic exposure significantly inhibited AAT1 protein expression but not AAT2 protein expression in the lung tissue, pulmonary vessel and perivascular tissue. Also, the SO2 content in the lung tissue was decreased with perivascular MC accumulation and degranulation and reduction of cAMP expression in the rats with hypoxic exposure. In accordance with the data obtained from in vitro experiments, the supplementation of SO2 donor recovered SO2 content in the lung tissue and blocked the hypoxia-inhibited cAMP expression and hypoxia-induced perivascular MC accumulation and degranulation, which further supported the relationship among endogenous SO2/AAT1 pathway, cAMP level and MC mediator release. Combined with the significance of MC degranulation in the vascular inflammation and the findings that SO2 donor inhibited hypoxic pulmonary vascular inflammation reported in our previous study [29], the abovementioned results in the present study imply that hypoxia-driven endogenous SO2/AAT downregulation might contribute to MC degranulation and be involved in inflammation of the pulmonary blood vessels in vivo.
Regarding the effect of SO2 on the MC degranulation, Melendez et al. reported an opposite result and described that 0.5 mM and 5 mM of Na2SO3 induced cardiac MC degranulation due to oxidative stress [48]. We speculated that reasons contributing to the discrepancy might include the followings: (1) The aim of the two studies is different. Melendez et al. focused on the toxic effect of SO2 as an air pollutant on the MC, while we aimed to explore the physiological and pathophysiological significance of endogenous SO2 generated from an AAT1-catalyzed transamination reaction in the MC. The difference might affect the experimental design and results of the two studies. (2) The experimental design is different. Based on the findings of the existence of endogenous SO2/AAT pathway in the MCs, we designed to explore the effect of endogenous SO2 on the MCs using AAT1/2 knockdown intervention and SO2 donor rescue. (3) The dose and composition of reagent is different. In the study of Melendez et al, 0.5 mM and 5 mM of Na2SO3 was used, while we mainly used 100 μmol/L NaHSO3/Na2SO3 (1:3 M/M) as a SO2 donor. As described in the material and methods part [27], [28], the dose and composition used in our study is close to physiological status of SO2. The comparison and analysis of the contrary results from the two studies may benefit the researchers to pay attention on the dose range of SO2 and avoid the toxic effect of SO2 at high dose in the future.
SO2 gas and NaHSO3/Na2SO3 (1:3 M/M) are the two common widely used SO2 donors. SO2 gas dissolved in water forms a hydrated SO2 complex (SO2·H2O) that dissociates twice to generate HSO3− and SO32−. The formation of SO2 from a NaHSO3/Na2SO3 (1:3 M/M) solution depends on the complex kinetics of the dynamic equilibrium between gaseous SO2 and HSO3−/SO32− [37]. Therefore, whether a SO2 gas solution or NaHSO3/Na2SO3 (1:3 M/M) solution is administrated to cells or animals, they may exist in the form of SO2 and HSO3−/SO32−. However, there exist the differences in the amount of SO2 releases, release rate, delivery rate to tissues or cells, action efficiency and clearance rate between the SO2 gas solution and NaHSO3/Na2SO3 mixtures [37]. For instance, a previous study demonstrated that gaseous SO2 might have a stronger vasodilator effect compared with a NaHSO3/Na2SO3 mixture [28]. In future studies, we would compare the difference in the usage of SO2 donor between the SO2 gas solution and NaHSO3/Na2SO3 mixture in different animal models and cell types.
SO2, as an endogenous new gas signaling molecule, has a significant inhibitory effect on MC degranulation induced by hypoxia. The findings reported in this work may provide novel ideas to aid in the clinical prevention and treatment of many MC-related diseases, such as allergic rhinitis, asthma, conjunctivitis, psoriasis, mastocytosis, various cancers, cirrhosis and cardiovascular diseases (including idiopathic cardiomyopathy, atherosclerosis, myocarditis, ischemic heart disease and pulmonary hypertension). In addition, this study helps to further clarify the biological and pharmacological roles of SO2 at physiological concentrations, and may attract additional researchers to develop clinically applicable SO2 donors.
Conclusions
In summary, this work demonstrates that an endogenous SO2/AAT pathway exists in MCs and importantly, that SO2 acts as a novel MC stabilizer. Endogenous SO2 deficiency can promote MC degranulation. Conversely, supplementation with SO2 prevents hypoxia-induced MC degranulation. SO2 inhibits MC degranulation by enhancing the cAMP pathway. The present data provide a theoretical basis for exploring the mechanisms for MC-mediated pathological processes, such as inflammatory reactions and allergies. The data presented here also provide basis for novel potential treatment strategies for pulmonary vascular inflammation.
Compliance with Ethics Requirements
All institutional and National Guidelines for the care and use of animals (fisheries) were followed. This study was approved by the Animals Care and Use Committee of Peking University First Hospital, Beijing, China.
Acknowledgments
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81970424, 81770422, 81770278), Beijing Natural Science Foundation (7171010, 7182168 and 7191012); Changjiang Scholars Award Program Young Scholar (Q2017004).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contributions
L. Zhang, H. Jin, Y. Song, J. Du and Y. Huang designed the experiments, performed the experiments, acquired the data, analyzed the data and wrote the manuscript; Y. Wang and Y. Sun performed the experiments; C. Tang designed the experiments; and S. Y. Chen revised the manuscript.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2020.08.017.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Figure S1: Effects of AAT2 knockdown on the cAMP level in MC and MC degranulation. (A) Western blot analysis of AAT2 protein expression; (B) HPLC-FD analysis of SO2 content in the MC supernatant; (C) ELISA analysis of cAMP content in MCs; (D) Colorimetric analysis of the release rate of β-hexosaminidase in MCs. **P < 0.01 and data are presented as the mean ± SD. All the research was carried out for three times independently.
Supplementary Fig. 2.
Figure S2: The effects of hypoxia on AAT2 mRNA and protein expression in rat lung tissue. (A) Real time RT-PCR analysis of AAT2 mRNA levels in rat lung tissue; (B) Western blot analysis of AAT2 protein expression in rat lung tissue; (C) Immunohistochemistry analysis of AAT2 protein expression in rat lung tissue. The rats in the hypoxic + SO2 group were injected with NaHSO3/Na2SO3 mixture (0.18 mmol and 0.54 mmol per kilogram body weight) before hypoxia exposure each day. The rats of the normoxic and hypoxic groups were injected with the same volume of physiological saline. **P < 0.01 and data are presented as the mean ± SD. All the research was carried out for three times independently.
References
- 1.Arthur G., Bradding P. New developments in mast cell biology: clinical implications. Chest. 2016;150:680–693. doi: 10.1016/j.chest.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Borriello F., Iannone R., Marone G. Histamine release from mast cells and basophils. Handb Exp Pharmacol. 2017;241:121–139. doi: 10.1007/164_2017_18. [DOI] [PubMed] [Google Scholar]
- 3.Galli S.J., Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovanen P.T., Kaartinen M., Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92(5):1084–1088. doi: 10.1161/01.cir.92.5.1084. [DOI] [PubMed] [Google Scholar]
- 5.Patella V., Marinò I., Arbustini E., Lamparter-Schummert B., Verga L., Adt M. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97(10):971–978. doi: 10.1161/01.cir.97.10.971. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura A., Yamazaki K., Suzuki K., Sato S. Increased portal tract infiltration of mast cells and eosinophils in primary biliary cirrhosis. Am J Gastroenterol. 1997;92:2245–2249. [PubMed] [Google Scholar]
- 7.Matsunaga Y., Kawasaki H., Terada T. Stromal mast cells and nerve fibers in various chronic liver diseases: relevance to hepatic fibrosis. Am J Gastroenterol. 1999;94:1923–1932. doi: 10.1111/j.1572-0241.1999.01232.x. [DOI] [PubMed] [Google Scholar]
- 8.Cildir G., Pant H., Lopez A.F., Tergaonkar V. The transcriptional program, functional heterogeneity, and clinical targeting of mast cells. J Exp Med. 2017;214:2491–2506. doi: 10.1084/jem.20170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jönsson F., de Chaisemartin L., Granger V., Gouel-Chéron A., Gillis C.M., Zhu Q. An IgG-induced neutrophil activation pathway contributes to human drug-induced anaphylaxis. Sci Transl Med. 2019;11(500):aat1479. doi: 10.1126/scitranslmed. [DOI] [PubMed] [Google Scholar]
- 10.Turner H., Kinet J.P. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402(6760 Suppl):B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 11.Gwack Y., Feske S., Srikanth S., Hogan P.G., Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Klemm S., Ruland J. Inflammatory signal transduction from the Fc epsilon RI to NF-kappa B. Immunobiology. 2006;211:815–820. doi: 10.1016/j.imbio.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Kanagy N.L., Szabo C., Papapetropoulos A. Vascular biology of hydrogen sulfide. Am J Physiol Cell Physiol. 2017;312(5):C537–C549. doi: 10.1152/ajpcell.00329.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maher A., Abdel Rahman M.F., Gad M.Z. The role of nitric oxide from neurological disease to cancer. Adv Exp Med Biol. 2017;1007:71–88. doi: 10.1007/978-3-319-60733-7_5. [DOI] [PubMed] [Google Scholar]
- 15.Wang W., Wang B. SO2 donors and prodrugs, and their possible applications: a review. Front Chem. 2018;6:559. doi: 10.3389/fchem.2018.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian Y., Mainali XQ Liu, Tang C.S., Du J.B. Effect of sulfur dioxide on small pulmonary artery endothelial cell inflammation reaction of hypoxic pulmonary hypertensive rats. J Appl Clin Pediat. 2008;23:985–987. [Google Scholar]
- 17.Huang X.L., Zhou J.L., Zhou X.H., Xian X.H., Ding C.H. Ameliorative effects of exogenous sulfur dioxide on lipopolysaccharide-induced acute lung injury in rats. Sheng Li Xue Bao. 2009;61:499–503. [PubMed] [Google Scholar]
- 18.Rodrigues L., Ekundi-Valentim E., Florenzano J., Cerqueira A.R., Soares A.G., Schmidt T.P. Protective effects of exogenous and endogenous hydrogen sulfide in mast cell-mediated pruritus and cutaneous acute inflammation in mice. Pharmacol Res. 2017;115:255–266. doi: 10.1016/j.phrs.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Wang X.B., Jin H.F., Tang C.S., Du J.B. Significance of endogenous sulphur-containing gases in the cardiovascular system. Clin Exp Pharmacol Physiol. 2010;37:745–752. doi: 10.1111/j.1440-1681.2009.05249.x. [DOI] [PubMed] [Google Scholar]
- 20.Oláh G., Módis K., Törö G., Hellmich M.R., Szczesny B., Szabo C. Role of endogenous and exogenous nitric oxide, carbon monoxide and hydrogen sulfide in HCT116 colon cancer cell proliferation. Biochem Pharmacol. 2018;149:186–204. doi: 10.1016/j.bcp.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barreiro-Costa O., Tobío A., Alfonso A., Botana L.M. Different role of cAMP pathway on the human mast cells HMC-1(560) and HMC-1(560,816) activation. J Cell Biochem. 2014;115:896–909. doi: 10.1002/jcb.24732. [DOI] [PubMed] [Google Scholar]
- 22.Serra-Pages M., Olivera A., Torres R., Picado C., de Mora F., Rivera J. E-prostanoid 2 receptors dampen mast cell degranulation via cAMP/PKA-mediated suppression of IgE-dependent signaling. J Leukoc Biol. 2012;92:1155–1165. doi: 10.1189/jlb.0212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gri G., Piconese S., Frossi B., Manfroi V., Merluzzi S., Tripodo C. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu D., Huang Y., Bu D., Liu A.D., Holmberg L., Jia Y. Sulfur dioxide inhibits vascular smooth muscle cell proliferation via suppressing the Erk/MAP kinase pathway mediated by cAMP/PKA signaling. Cell Death Dis. 2014;5:e1251. doi: 10.1038/cddis.2014.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann J., Bartel S., Eschenhagen T., Haverich A., Hirt S., Karczewski P. Dissociation of the effects of forskolin and dibutyryl cAMP on force of contraction and phospholamban phosphorylation in human heart failure. J Cardiovasc Pharmacol. 1999;33(1):157–162. doi: 10.1097/00005344-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 26.Nunes A.R., Batuca J.R., Monteiro E.C. Acute hypoxia modifies cAMP levels induced by inhibitors of phosphodiesterase-4 in rat carotid bodies, carotid arteries and superior cervical ganglia. Br J Pharmacol. 2010;159(2):353–361. doi: 10.1111/j.1476-5381.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du S.X., Jin H.F., Bu D.F., Zhao X., Geng B., Tang C.S. Endogenously generated sulfur dioxide and its vasorelaxant effect in rats. Acta Pharmacol Sin. 2008;29(8):923–930. doi: 10.1111/j.1745-7254.2008.00845.x. [DOI] [PubMed] [Google Scholar]
- 28.Meng Z.Q., Li J.L., Zhang Q.X., Bai W.M., Yang Z.H., Zhao Y. Vasodilator effect of gaseous sulfur dioxide and regulation of its level by Ach in rat vascular tissues. Inhal Toxicol. 2009;21(14):1223–1228. doi: 10.3109/08958370902798463. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y., Tian Y., Prabha M., Liu D., Chen S., Zhang R. Effects of sulfur dioxide on hypoxic pulmonary vascular structural remodeling. Lab Invest. 2010;90(1):68–82. doi: 10.1038/labinvest.2009.102. [DOI] [PubMed] [Google Scholar]
- 30.He Y., Zuo C., Jia D., Bai P., Kong D., Chen D. Loss of DP1 aggravates vascular remodeling in pulmonary arterial hypertension via mTORC1 signaling. Am J Respir Crit Care Med. 2020;201(10):1263–1276. doi: 10.1164/rccm.201911-2137OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambert M., Boet A., Rucker-Martin C., Mendes-Ferreira P., Capuano V., Hatem S. Loss of KCNK3 is a hallmark of RV hypertrophy/dysfunction associated with pulmonary hypertension. Cardiovasc Res. 2018;114(6):880–893. doi: 10.1093/cvr/cvy016. [DOI] [PubMed] [Google Scholar]
- 32.Ban Y., Liu Y., Li Y., Zhang Y., Xiao L., Gu Y. S-nitrosation impairs KLF4 activity and instigates endothelial dysfunction in pulmonary arterial hypertension. Redox Biol. 2019;21:101099. doi: 10.1016/j.redox.2019.101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang D., Wang X., Tian X., Zhang L., Yang G., Tao Y. The increased endogenous sulfur dioxide acts as a compensatory mechanism for the downregulated endogenous hydrogen sulfide pathway in the endothelial cell inflammation. Front Immunol. 2018;9:882. doi: 10.3389/fimmu.2018.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stipanuk M.H., Benevenga N.J. Effect of cystine on the metabolism on methionine in rats. J Nutr. 1977;107:1455–1467. doi: 10.1093/jn/107.8.1455. [DOI] [PubMed] [Google Scholar]
- 35.Han Y., Yi W., Qin J., Zhao Y., Zhang J., Chang X. Dose-dependent effect of sulfur dioxide on brain damage induced by recurrent febrile seizures in rats. Neurosci Lett. 2014;563:149–154. doi: 10.1016/j.neulet.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y., Tang C., Du J., Jin H. Endogenous sulfur dioxide: a new member of gasotransmitter family in the cardiovascular system. Oxid Med Cell Longev. 2016;2016:8961951. doi: 10.1155/2016/8961951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W.Y., Wang B.H. SO2 donors and prodrugs, and their possible applications: a review. Front Chem. 2018;16(6):559. doi: 10.3389/fchem.2018.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall J.S. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 39.Maurer M., Pucillo C. What we know (and don't know) about the biology and functions of mast cells and basophils. Immunol Rev. 2018;282:5–7. doi: 10.1111/imr.12645. [DOI] [PubMed] [Google Scholar]
- 40.Aldi S., Marino A., Tomita K., Corti F., Anand R., Olson K.E. E-NTPDase1/CD39 modulates renin release from heart mast cells during ischemia/reperfusion: a novel cardioprotective role. FASEB J. 2015;29:61–69. doi: 10.1096/fj.14-261867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamauchi K., Ogasawara M. The role of histamine in the pathophysiology of asthma and the clinical efficacy of antihistamines in asthma therapy. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peachell P.T., MacGlashan D.W., Jr, Lichtenstein L.M., Schleimer R.P. Regulation of human basophil and lung mast cell function by cyclic adenosine monophosphate. J Immunol. 1988;140:571–579. [PubMed] [Google Scholar]
- 43.Matsuda K., Okamoto N., Kondo M., Arkwright P.D., Karasawa K., Ishizaka S. Mast cell hyperactivity underpins the development of oxygen-induced retinopathy. J Clin Invest. 2017;127:3987–4000. doi: 10.1172/JCI89893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang X., Yin G., Ma Y., Xu K., Liu J., Li J. The critical role of mast cell-derived hypoxia-inducible factor-1α in regulating mast cell function. J Pharm Pharmacol. 2016;68:1409–1416. doi: 10.1111/jphp.12622. [DOI] [PubMed] [Google Scholar]
- 45.Rorke Steuart, Holgate Stephen T. Targeting adenosine receptors: novel therapeutic targets in asthma and chronic obstructive pulmonary disease. Am J Respir Med. 2002;1(2):99–105. doi: 10.1007/BF03256599. [DOI] [PubMed] [Google Scholar]
- 46.Elliot J.G., Abramson M.J., Drummer O.H., Walters E.H., James A.L. Time to death and mast cell degranulation in fatal asthma. Respirology. 2009;14(6):808–813. doi: 10.1111/j.1440-1843.2009.01551.x. [DOI] [PubMed] [Google Scholar]
- 47.Banasová A., Maxová H., Hampl V., Vízek M., Povýsilová V., Novotná J. Prevention of mast cell degranulation by disodium cromoglycate attenuates the development of hypoxic pulmonary hypertension in rats exposed to chronic hypoxia. Respiration. 2008;76(1):102–107. doi: 10.1159/000121410. [DOI] [PubMed] [Google Scholar]
- 48.Melendez G.C., Voloshenyuk T.G., McLarty J.L., Levick S.P., Brower G.L. Oxidative stress-mediated cardiac mast cell degranulation. Toxicol Environ Chem. 2010;92:1293–1301. doi: 10.1080/02772240903306409. [DOI] [Google Scholar]