Abstract
Background
Cardiovascular disease is the leading cause of mortality and disability worldwide. A noninvasive test that can detect underlying cardiovascular disease has the potential to identify patients at risk prior to the occurrence of adverse cardiovascular events. We sought to determine whether an easily observed imaging finding indicative of retinal ischemia, which we term ‘retinal ischemic perivascular lesions’ (RIPLs), could serve as a biomarker for cardiovascular disease.
Methods
We reviewed optical coherence tomography (OCT) scans of individuals, with no underlying retinal pathology, obtained at UC San Diego Health from July 2014 to July 2019. We identified 84 patients with documented cardiovascular disease and 76 healthy controls. OCT scans were assessed for evidence of RIPLs. In addition, the 10-year atherosclerotic cardiovascular disease (ASCVD) risk calculator was used to risk-stratify the subjects into four different categories.
Findings
Patients with documented cardiovascular disease had higher number of RIPLs compared to healthy controls (2.8 vs 0.8, p < 0.001). After adjusting for age, sex, smoking history, systolic blood pressure and triglycerides, cholesterol and hemoglobin A1C levels, each RIPL was associated with an odds ratio of having cardiovascular disease of 1·60 (1.09–2>37). The number of RIPLs in individuals with intermediate and high 10-year ASCVD risk scores was higher than in those with low ASCVD risk scores (1.7 vs 0.64, p = 0.02 and 2.9 vs 0.64, p 0.002, respectively).
Interpretation
The presence of RIPLs, which are anatomical markers of prior retinal ischemic infarcts, is suggestive of coexisting cardiovascular disease. RIPLs detection, obtained from routine retinal scans, may thus provide an additional biomarker to identify patients at risk of developing adverse cardiovascular events.
Funding
None.
Keywords: RIPLs, Survival, Cardiovascular disease, Retina, Stroke, Optical coherence tomogrpahy, Imaging, ASCVD risk
Research in context.
Evidence before this study
We searched PubMed for articles published in English using the following search terms, ‘retina’ ‘ischemia’, ‘occlusion’, ‘cardiovascular’ and ‘stroke’. Individuals with cardiovascular disease have increased odds of developing retinal vascular occlusions. We have previously demonstrated that the middle layer of the retina is the layer that is most susceptible to ischemia. Ischemic lesions in the middle retinal layers have been shown to occur in different contexts of retinal ischemia, such as retinal artery and vein occlusions, paracentral acute middle maculopathy, and cotton wool spots, as well as in systemic hypertension.
Added value of this study
In this retrospective study we demonstrate that retinal ischemic lesions which we term ‘RIPLs’ are more common in individuals with cardiovascular disease compared to those without. Presence of these lesions was associated with increased odds of cardiovascular disease, and their number positively correlated with increased 10-year ASCVD risk.
Implications of all the available evidence
OCT scans are non-invasive tests that are routinely obtained in ophthalmology clinics. Albeit subclinical, and only detected using OCTs, RIPLs are prevalent in individuals with cardiovascular disease and may provide an additional biomarker to detect the existence of occult cardiovascular disease.
Alt-text: Unlabelled box
1. Introduction
Cardiovascular disease is the leading cause of mortality and disability worldwide [1,2]. It is also prevalent, affecting 9% of the population over 20 years of age [1]. Patients with cardiovascular risk factors can reduce their risk of developing adverse cardiovascular events through lifestyle modification and medications [1,3]. Unfortunately for many, the disease may go undiagnosed until the occurrence of serious events such as myocardial infarction or stroke [4], [5], [6]. Identifying biomarkers of subclinical ischemia can help identify patients with occult cardiovascular disease.
Individuals with cardiovascular disease are prone to developing retinal vascular occlusions [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]. The retina is a multi-layered neural tissue with a complex capillary network [21]. The superficial and deep capillary plexuses provide oxygen to the inner and middle thirds of the retina, respectively, while the outer third of the retina receives its oxygen from the intensely oxygenated choriocapillaris. In severe vascular occlusion, the inner two-thirds of the retina atrophies, whereas in small infarctions, such as paracentral acute middle maculopathy, the middle retina is selectively affected [22], [23], [24], [25]. Noninvasive imaging technology is currently available to directly visualize individual layers of the retina at sub-millimeter resolution in vivo using spectral domain optical coherence tomography (SD-OCT) [26]. These imaging devices are in widespread use in ophthalmology and optometry offices. Using this technology we can identify abnormalities in retinal images indicative of ischemia [22,25,27,28]. On SD-OCT, small retinal infarctions manifest in the acute phase as a hyperreflective perivenular band at the level of the inner nuclear layer (INL) which ultimately atrophies [[22], [23], [24], [25],[28], [29], [30], [31], [32]]. These lesions have been shown to occur in eyes with ischemia resulting from hypoperfusion or microemboli, such as retinal artery and vein occlusions, hypertension, Purtscher retinopathy and sickle cell disease [22,24,27,33]. High-resolution imaging of the retinal microvasculature using OCT angiography demonstrates blood flow signal voids in the acute phase, further supporting an ischemic etiology for these lesions [23]. In this study we sought to determine whether these lesions are prevalent in patients with cardiovascular disease and whether this finding can predict the presence of this disorder.
2. Methods
2.1. Study participants
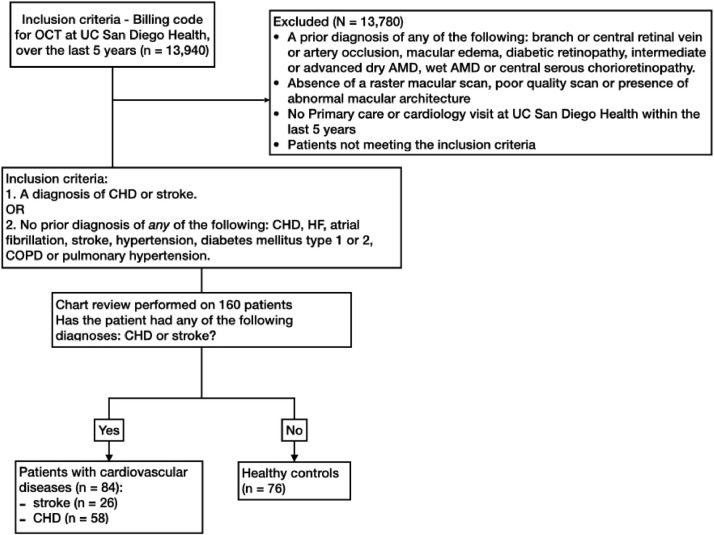
We reviewed the records of 13,940 individuals who had a macular OCT scan at UC San Diego Health for different clinical indications from July 1, 2014 to July 1, 2019. We excluded individuals who had a prior diagnosis of any of the following: branch or central retinal vein or artery occlusion, macular edema, diabetic retinopathy, intermediate or advanced dry age-related macular degeneration (AMD), wet AMD or central serous chorioretinopathy, those with abnormal macular architecture, or poor quality SD-OCT scans and those who had no primary care or cardiology visit at our institution in the last 5 years. We then sought to identify two groups after a thorough medical chart review: one comprised of individuals with documented cardiovascular disease and another consisting of healthy controls. Subjects were classified in the cardiovascular group if they had a diagnosis of CHD or stroke, or in the control group if they did not have any of the following conditions: CHD, stroke, heart failure, atrial fibrillation, hypertension, diabetes mellitus type 1 or 2, chronic obstructive pulmonary disease or pulmonary hypertension. Subjects in both groups had no confounding retinal pathologies (Fig. 1).
Fig. 1.
Flowchart of the method used in identifying healthy controls and patients with cardiovascular disease. Inclusion and exclusion criteria with numbers of participants are included in each step. Initial selection was based on billing records and a thorough chart review was performed on the 160 patients identified to classify them into two groups. Inclusion criteria for the cardiovascular disease group included a diagnosis of coronary heart disease (CHD) or stroke. To identify healthy controls we excluded individuals with any of the following: a history of cardiovascular disease as defined above, atrial fibrillation, heart failure, hypertension, type 1 or 2 diabetes mellitus, chronic obstructive pulmonary disease, pulmonary hypertension or smoking. Additional exclusion criteria for both groups (cardiovascular and healthy controls) included a prior diagnosis of central retinal artery or vein occlusion in either eye, macular edema, diabetic retinopathy, intermediate or advanced age-related macular degeneration, central serous chorioretinopathy, absence of a complete macular scan, poor quality scan, abnormal macular architecture, or absence of a primary care or cardiology visit at the UC San Diego Health System within the last five years. After excluding 13,780 patients, 160 met the inclusion criteria and their medical chart was reviewed. AMD, age-related macular degeneration; COPD, chronic obstructive pulmonary disease.
2.2. Image acquisition
All subjects in this study had a 6 × 6 mm SD-OCT macular raster scan consisting of 49 b-scans (optical cross sections). The OCT scan was performed by an ophthalmic technician using a Spectralis SD-OCT machine (Heidelberg Engineering, Heidelberg, Germany) as part of the routine ophthalmic examination.
2.3. Image analysis
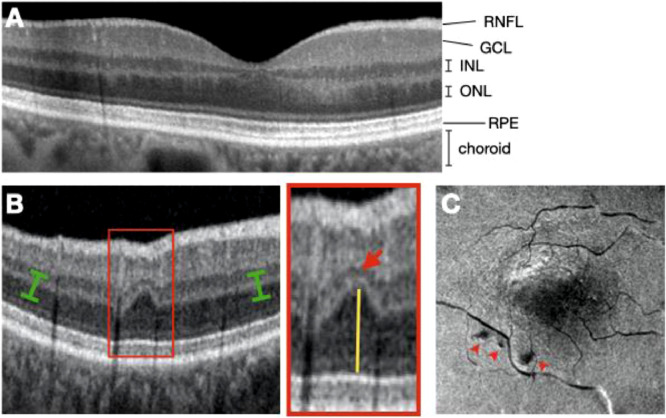
Individual layers of the retina can be visualized using SD-OCT imaging (Fig. 2A). Contraction of the focal infarct in the INL is often accompanied by a compensatory upward expansion of the darker outer nuclear layer into the plane where the outer plexiform layer would be expected, leading to a wavy appearance of the middle retinal layers in OCT cross sections (Fig. 2B) [28]. A horizontal plane at the level of the outer plexiform layer (OPL) extracted from a 3-dimensional volume scan assembled from sequential b-scan slices aids in the identification of these chronic lesions and demonstrates their perivascular location (Fig. 2C). Hence, we term these lesions ‘Retinal Ischemic Perivascular Lesions’ (RIPLs). A ‘RIPL’ was defined by the focal atrophy of the INL with a compensatory expansion of the outer nuclear layer into the plane where the outer plexiform layer would be expected, leading to a wavy appearance of the middle retinal layers in OCT cross sections as shown in Fig. 2B. RIPLs in each B-scan from the most recent SD-OCT scan were counted by two independent and masked observers, and total number of RIPLs from both eyes were reported per subject. For the en face OCT reconstruction shown in Fig. 2C, scans were acquired using the Optovue SD-OCT machine (Optovue, Fremont, California, USA).
Fig. 2.
RIPLs, biomarkers of retinal ischemia. A) Spectral domain optical coherence tomography (SD-OCT) b-scan demonstrating an optical vertical cut of a normal eye); RNFL, retinal nerve fiber layer; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. B) SD-OCT b-scan demonstrating a RIPL (red box). There is focal contraction of INL (red arrowhead) accompanied by a compensatory upward expansion of the darker outer nuclear layer (yellow line) into the plane where the outer plexiform layer would be expected. C) An en face view, limited to the OPL, reconstructed from a 3-dimensional volume scan assembled from sequential b-scans. The selected plane follows the curve of the OPL plane (green brackets). Three RIPLs appear as dark spots (red arrowheads).
2.4. Statistical analysis
All statistical analyses were performed using IBM SPSS version 26. Intraclass correlation coefficient between observers was calculated using a two-way mixed model for absolute agreement. When comparing variables between two or three groups, we utilized Student's t-test, and one-way ANOVA with Bonferroni correction, respectively. Differences between groups were analyzed with Pearson-Chi Square tests for categorical variables. Logistic regression models were used to analyze odds ratios for outcomes with 95% confidence intervals.
2.5. Ethic statement
The study adhered to the tenets of the Declaration of Helsinki and was conducted in accordance with the regulations of the Health Insurance Portability and Accountability Act. Internal Review Board (IRB) approval with a waiver of informed consent for retrospective chart review was obtained from the University of California San Diego Health System (IRB # 191,994).
Role of the funding Source: None.
3. Results
We identified 84 and 76 subjects with and without cardiovascular disease, respectively.
Of the 160 subjects who met these criteria, we identified 26 with stroke, 58 with CHD and 76 healthy controls (Fig. 1). Baseline characteristics of the study cohort are shown in Table 1. Subjects with cardiovascular disease were older than healthy controls (mean of 72.0 vs 65.2 years of age, p < 0.001). There were less females in the cardiovascular disease group compared to the control group (51.2% vs 72.4%, p = 0.01). There was no difference in race between both groups (p = 0.61). The percentage of current or former smokers was higher in the cardiovascular group compared to controls (35.7% vs 18.4%, p = 0.02). Compared to controls, subjects with cardiovascular disease had higher levels of hemoglobin A1c (6.0% vs 5.5%, p = 0.001), higher levels of triglycerides (137.7 mg/dL vs 105.3 mg/dL, p = 0.03), lower levels of total cholesterol (164.1 mg/dL vs 197.1 mg/dL, p < 0.001) and higher mean systolic blood pressure (125.5 vs 114.6 mmHg, p < 0.001).
Table 1.
Baseline characteristics of the study cohort. Mean and standard deviation reported for age, hemoglobin A1c, total cholesterol, triglycerides, systolic blood pressure (BP) and diastolic BP. Percentage reported for sex, race and smoking history.
| Overall n = 160 | Control n = 76 | Cardiovascular n = 84 | p-value | ||
|---|---|---|---|---|---|
| Age | 68·8 (10·7) | 65·2 (6·8) | 72·0 (12·5) | < 0·001 | |
| Women,% | 98 (61·3) | 55 (72·4) | 43 (51·2) | 0·01 | |
| Race,% | White | 118 (73·8) | 52 (68·4) | 65 (78·6) | 0·61 |
| Hispanic | 12 (7·5) | 5 (6·6) | 7 (8·3) | ||
| Asian | 17 (10·6) | 6 (7·9) | 11 (13·1) | ||
| African American | 1 (0·6) | 1 (1·3) | – | ||
| Smoking history,% (current or former) | 44 (27·5) N = 160 | 14 (18·4) | 30 (35·7) | 0·02 | |
| Hemoglobin A1C,% | 5·9 (0·8) N = 118 | 5·5 (0·4) N = 41 | 6·0 (0·9) N = 77 | 0·001 | |
| Total cholesterol, mg/dL | 179·2 (43·3) N = 149 | 197·1 (37·9) N = 68 | 164·1 (42·0) N = 81 | < 0·001 | |
| Triglycerides, mg/dL | 123·1 (94·9) N = 151 | 105·3 (57·2) N = 68 | 137·7 (115·4) N = 83 | 0·03 | |
| Systolic BP, mmHg | 120·4 (15·5) N = 160 | 114·6 (10·6) N = 76 | 125·5 (17·4) N = 84 | < 0·001 | |
| Diastolic BP, mmHg | 69·7 (9·4) N = 160 | 69·1 (8·7) N = 76 | 70·3 (10·1) N = 84 | 0·41 |
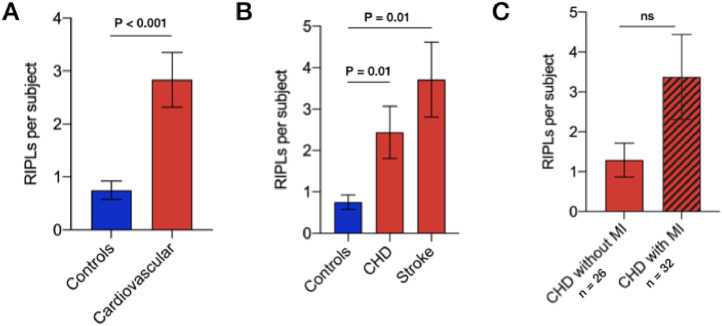
The total number of RIPLs per patient was significantly higher in the cardiovascular group compared to the control group (2.8 vs 0.8, p < 0.001) (Fig. 3A). Intraclass correlation coefficient between both observers was 0.65 (p < 0.001). Number of RIPLs in individuals with CHD and stroke was 2.4 and 3.7 compared to 0.8 in controls (p = 0.01 and p < 0.001, respectively) (Fig. 3B). Out of 58 individuals with CHD, there were 32 (55.2%) with myocardial infarction (Fig. 3C). Individuals with myocardial infarction had 3·4 RIPLs compared to 1·3 RIPLs in those with CHD without myocardial infarction (p = 0.10) (Fig. 3C).
Fig. 3.
RIPLs in different cardiovascular diseases.
A) Total number of RIPLs in healthy individuals (n = 76) and those with cardiovascular disease (n = 84). B) Total number of RIPLs in healthy controls (n = 76) and patients with CHD (n = 58) and stroke (n = 26).
C) Total number of RIPLs in subjects patients with CHD, with and without myocardial infarction (MI). Mean and standard error of the mean are shown. Statistical significance tested using unpaired student's t-test with Bonferroni correction.
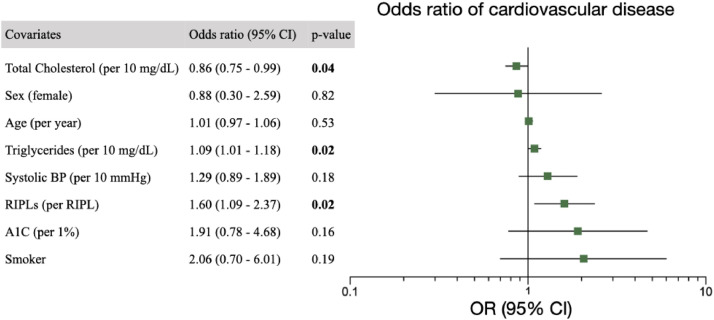
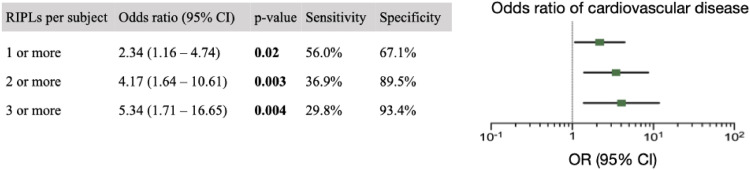
After adjusting for covariates which were significantly different between cardiovascular and control groups, including age, sex, systolic blood pressure, smoking history and triglycerides, cholesterol and hemoglobin A1C levels, each RIPL was associated with an odds ratio of having cardiovascular disease of 1.60 (95% CI, 1.09–2.37), p = 0·02 (Fig. 4). After adjusting for age, sex and smoking status, presence of at least one, two or three RIPLs was associated with an odds ratio of having cardiovascular disease of 2·34 (CI 1.16–4.74, p = 0.02), 4.17 (CI 1.64–10.61, p = 0.003) and 5·34 (CI 1.71–16.65, p = 0.004), respectively (Fig. 5). A minimum of one, two or three RIPLs were present in 56.0%, 36.9% and 29.8% of individuals with cardiovascular disease, respectively. Two or three RIPLs had specificity of 89.5% and 93.4% in detecting coexisting cardiovascular disease, respectively (Fig. 5).
Fig. 4.
Odds ratio of cardiovascular disease. A multivariable logistic regression model for presence of cardiovascular disease. Co-variates include systolic blood pressure (BP), sex, age per years, cholesterol and triglyceride levels, hemoglobin A1C percentage, positive smoking history and RIPLs. Error bars represent 95% confidence intervals. Vertical line indicates odds ratio (OR) of 1.
Fig. 5.
Odds ratio of cardiovascular disease based on number of RIPLs. A multivariable logistic regression model for presence of cardiovascular disease. Co-variates include age, sex and smoking status. Error bars represent 95% confidence intervals. Dotted line indicates odds ratio of 1.
In clinical practice, risk of atherosclerotic cardiovascular disease (ASCVD) risk is estimated based on an individual's age, sex, race, blood pressure, cholesterol levels, smoking history and presence or absence of diabetes mellitus. We stratified subjects based on their 10-year ASCVD risk into four categories that are commonly used in clinical practice, low risk (<5%), borderline risk (5% to 7.5%), intermediate risk (7.5% to 20%) and high risk (>20%). There was no significant difference in number of RIPLs between low and borderline risk groups (0.64 vs 0.6 RIPL, p = 0.89). However, the number of RIPLs in individuals with intermediate and high ASCVD risk was higher than in those with low ASCVD risk (1·7 vs 0.64, p = 0.02 and 2.9 vs 0.64, p = 0.002, respectively) (Fig. 6).
Fig. 6.
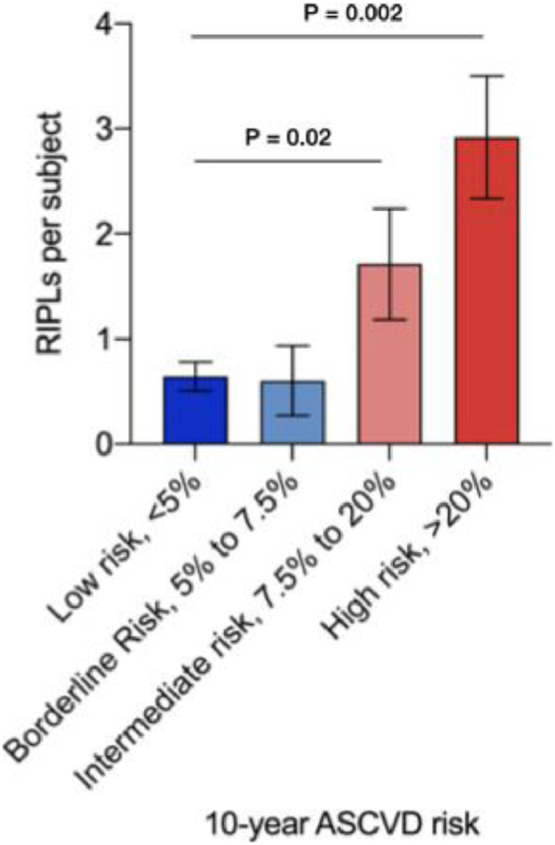
Number of RIPLs per subject in individuals stratified by their 10-year ASCVD risk into 4 categories commonly used in clinical practice; low risk (<5%), borderline risk (5% to 7.5%), intermediate risk (7.5% to 20%) and high risk (>20%). Statistical significance tested using unpaired student t-test.
4. Discussion
We examined the association between the occurrence of RIPLs and the presence of cardiovascular disease in a group of individuals undergoing SD-OCT for various clinical indications. The major findings of our study were 1) the number of RIPLs was larger in subjects with cardiovascular disease than in control subjects, 2) a higher number of RIPLs were associated with a higher odds for cardiovascular disease even after adjusting for confounding covariates, and 3) higher 10-year ASCVD risk scores was associated with a higher number of RIPLs. Our findings support the concept that RIPLs, which are anatomical markers of prior retinal ischemic infarcts, are prevalent in cardiovascular disease patients and may provide an additional biomarker to predict the existence of underlying cardiovascular disease.
In clinical practice, the ASCVD risk calculator is used to estimate the risk of adverse cardiovascular events at 10 years. We found that the number of RIPLs correlated with the severity of the ASCVD risk score. We also found that RIPLs were especially significant in relation to cerebrovascular disease. RIPLs were higher in patients who suffered stroke. Since the retina is a forward extension of the brain, it is more likely that RIPLs would be indicative of cerebral disease than that of coronary vessels. We cannot exclude the possibility that some OCT examinations were prompted by premonitory symptoms of stroke; nevertheless, RIPLs may be an important sign of possible cerebral vascular atherosclerosis. Our results are in line with recent findings which have described retinal microvasculature changes in individuals with cardiovascular disease. For instance, a sparse vascular network pattern that is detected in fundus color photography was associated with an increase in 10-year risk of cardiovascular mortality [34]. Individuals with acute coronary syndrome and those with lower ejection fraction had lower vascular density on OCT angiography scans [35,36].
Some limitations must be considered in interpreting the results of our study. Given the retrospective nature of the study was performed retrospectively, we were not able to perform a consistent workup of the patients. Therefore we cannot relate the value of RIPLs to other risk factors or biomarkers of cardiovascular disease. We also excluded patients with retinal diseases, including those with large-vessel retinal artery or vein occlusion, thereby reducing the number of patients to whom our data is applicable. Excluding eyes with a history of retinal vascular occlusions likely underestimated the number of RIPLs in the cardiovascular group, given the high prevalence of cardiovascular disease in patients with retinal artery and vein occlusion [37], [38], [39], [40], [41]. This would bias the results towards the null hypothesis. About 44% of individuals with cardiovascular disease did not have any RIPLs. Nevertheless, it has high specificity. Using two or three RIPLs as cutoffs led to a specificity of 89.5% and 93.4%, respectively. Given the prevalence of cardiovascular disease, a test with low sensitivity but which has high specificity can be used as a rule-in test to identify patients at risk, and who may benefit from additional, and targeted, diagnostic workup.
Cardiovascular disease poses a heavy economic burden with a total cost expected to reach $1·1 trillion by 2035 [1]. Tests that can help identify patients with cardiovascular disease may lead to earlier interventions, thereby reducing the odds of subsequent adverse cardiovascular events. OCT scans are commonly performed in ophthalmology clinics, noninvasive, inexpensive, do not involve any ionizing radiation and do not require pupillary dilation nor the use of an intravenous dye. The RIPLs reported in this study were all identified by an ophthalmologist. The presence of RIPLs thereby represents additional valuable information that is readily obtainable from a standard retinal examination.
Author's contribution
MFB made the initial observation and conceived the idea. MFB and CL designed the study. MFB, CL, AXC, CBT, SM and AND acquired and/or analyzed the data. MFB and CYB performed the statistical analysis. All authors participated in the writing and revision of the manuscript. CL, AC, CBT, SM, AG, MFB had full access to the dataset.
Data sharing
Individual participant data that underlie the results reported in this article, after de-identification can be obtained from the corresponding author upon reasonable request.
Declaration of Competing Interest
WRF reports personal fees from Nanovision Biosciences, personal fees from Allergan, personal fees from Alcon, personal fees from Genentech, outside the submitted work. CL, AXC, CYB, CBT, SM, MHG and AND have nothing to disclose. A patent application has been filed by UC San Diego on this technology authored by MFB and WRF.
Funding
None.
References
- 1.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Jordan L.C., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., O'Flaherty M., Pandey A., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Spartano N.L., Stokes A., Tirschwell D.L., Tsao C.W., Turakhia M.P., VanWagner L.B., Wilkins J.T., Wong S.S., Virani S.S. American heart association council on E, prevention statistics c, stroke statistics s. heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. Epub 2019/02/01PubMed PMID: 30700139. [DOI] [PubMed] [Google Scholar]
- 2.DALYs G.B.D. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1260–1344. doi: 10.1016/S0140-6736(17)32130-X. Epub 2017/09/19PubMed PMID: 28919118; PMCID: PMC5605707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu Q., Burt V.L., Dillon C.F., Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the national health and nutrition examination survey, 2001 to 2010. Circulation. 2012;126(17):2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156. Epub 2012/10/24PubMed PMID: 23091084. [DOI] [PubMed] [Google Scholar]
- 4.Cao Q., Pei P., Zhang J., Naylor J., Fan X., Cai B., Dai Q., Sun W., Ye R., Shi R., Liu K., Jiang Y., Liu W., Yang F., Zhu W., Xiong Y., Liu X., Xu G. Hypertension unawareness among Chinese patients with first-ever stroke. BMC Public Health. 2016;16:170. doi: 10.1186/s12889-016-2835-1. Epub 2016/02/20PubMed PMID: 26893185; PMCID: PMC4759941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith E.E., Saposnik G., Biessels G.J., Doubal F.N., Fornage M., Gorelick P.B., Greenberg S.M., Higashida R.T., Kasner S.E., Seshadri S. American Heart association stroke c, council on cardiovascular r, intervention, council on functional g, translational b, council on h. prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the american heart association/American stroke association. Stroke. 2017;48(2):e44–e71. doi: 10.1161/STR.0000000000000116. Epub 2016/12/17PubMed PMID: 27980126. [DOI] [PubMed] [Google Scholar]
- 6.Fan H., Hao X., Yang S., Li Y., Qin W., Yang L., Yuan J., Hu W. Study on the incidence and risk factor of silent cerebrovascular disease in young adults with first-ever stroke. Medicine (Baltimore). 2018;97(48):e13311. Epub 2018/12/05. doi: 10.1097/MD.0000000000013311. PubMed PMID: 30508921; PMCID: PMC6283195. [DOI] [PMC free article] [PubMed]
- 7.Chan Alison X., Bakhoum Christine Y., Bangen Katherine J., Bakhoum Mathieu F. The relationship between retinal vascular occlusions and cognitive dementia in a large cross-sectional cohort. Am J Ophthalmol. 2021 doi: 10.1016/j.ajo.2021.01.026. PMID: 33529587 In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J., Kim S.W., Lee S.C., Kwon O.W., Kim Y.D., Byeon S.H. Co-occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion-weighted magnetic resonance imaging study. Am J Ophthalmol. 2014;157(6):1231–1238. doi: 10.1016/j.ajo.2014.01.033. Epub 2014/02/08PubMed PMID: 24503410. [DOI] [PubMed] [Google Scholar]
- 9.Lavin P., Patrylo M., Hollar M., Espaillat K.B., Kirshner H., Schrag M. Stroke risk and risk factors in patients with central retinal artery occlusion. Am J Ophthalmol. 2018;196:96–100. doi: 10.1016/j.ajo.2018.08.027. Epub 2018/08/29PubMed PMID: 30153430. [DOI] [PubMed] [Google Scholar]
- 10.Wu C.Y., Riangwiwat T., Limpruttidham N., Rattanawong P., Rosen R.B., Deobhakta A. Association of retinal vein occlusion with cardiovascular events and mortality: a systematic review and meta-analysis. Retina. 2019;39(9):1635–1645. doi: 10.1097/IAE.0000000000002472. Epub 2019/03/05PubMed PMID: 30829987. [DOI] [PubMed] [Google Scholar]
- 11.Ponto K.A., Scharrer I., Binder H., Korb C., Rosner A.K., Ehlers T.O., Rieser N., Grubel N.C., Rossmann H., Wild P.S., Feltgen N., Pfeiffer N., Mirshahi A. Hypertension and multiple cardiovascular risk factors increase the risk for retinal vein occlusions: results from the Gutenberg retinal vein occlusion study. J Hypertens. 2019;37(7):1372–1383. doi: 10.1097/HJH.0000000000002057. Epub 2019/05/31PubMed PMID: 31145709. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Y.Y., Wei W.B., Wang Y.X., Lu A.D., Chen S.H., Song L., Wu S.L. Correlation of the history of stroke and the retinal artery occlusion: a nested case-control study. Int J Ophthalmol. 2020;13(3):431–437. doi: 10.18240/ijo.2020.03.10. Epub 2020/04/21PubMed PMID: 32309180; PMCID: PMC7154211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan R.A., Lutsep H.L. Prevalence of retinal emboli and acute retinal artery occlusion in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29(2) doi: 10.1016/j.jstrokecerebrovasdis.2019.104446. Epub 2019/12/16PubMed PMID: 31837921. [DOI] [PubMed] [Google Scholar]
- 14.Fallico M., Lotery A.J., Longo A., Avitabile T., Bonfiglio V., Russo A., Murabito P., Palmucci S., Pulvirenti A., Reibaldi M. Risk of acute stroke in patients with retinal artery occlusion: a systematic review and meta-analysis. Eye (Lond) 2020;34(4):683–689. doi: 10.1038/s41433-019-0576-y. Epub 2019/09/19PubMed PMID: 31527762; PMCID: PMC7093449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayreh S.S., Podhajsky P.A., Zimmerman M.B. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009;116(10):1928–1936. doi: 10.1016/j.ophtha.2009.03.006. Epub 2009/07/07PubMed PMID: 19577305; PMCID: PMC2757505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helenius J., Arsava E.M., Goldstein J.N., Cestari D.M., Buonanno F.S., Rosen B.R., Ay H. Concurrent acute brain infarcts in patients with monocular visual loss. Ann Neurol. 2012;72(2):286–293. doi: 10.1002/ana.23597. Epub 2012/08/29PubMed PMID: 22926859; PMCID: PMC3430972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avery M.B., Magal I., Kherani A., Mitha A.P. Risk of stroke in patients with ocular arterial occlusive disorders: a retrospective Canadian study. J Am Heart Assoc. 2019;8(3) doi: 10.1161/JAHA.118.010509. Epub 2019/02/05PubMed PMID: 30712440; PMCID: PMC6405587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mir T.A., Arham A.Z., Fang W., Alqahtani F., Alkhouli M., Gallo J., Hinkle D.M. Acute Vascular ischemic events in patients with central retinal artery occlusion in the United States: a nationwide study 2003-2014. Am J Ophthalmol. 2019;200:179–186. doi: 10.1016/j.ajo.2019.01.009. Epub 2019/01/29PubMed PMID: 30689989; PMCID: PMC6542256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laczynski D.J., Gallop J., Lyden S.P., Bena J., Yuan A., Smolock C.J., Caputo F.J. Retinal artery occlusion does not portend an increased risk of stroke. J Vasc Surg. 2020;72(1):198–203. doi: 10.1016/j.jvs.2019.08.279. Epub 2019/12/18PubMed PMID: 31843299. [DOI] [PubMed] [Google Scholar]
- 20.Leisser C., Findl O. Rate of strokes 1 year after retinal artery occlusion with analysis of risk groups. Eur J Ophthalmol. 2020;30(2):360–362. doi: 10.1177/1120672119830925. Epub 2019/02/21PubMed PMID: 30782009. [DOI] [PubMed] [Google Scholar]
- 21.Spaide R.F., Klancnik J.M., Jr., Cooney M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133(1):45–50. doi: 10.1001/jamaophthalmol.2014.3616. Epub 2014/10/16PubMed PMID: 25317632. [DOI] [PubMed] [Google Scholar]
- 22.Bakhoum M.F., Freund K.B., Dolz-Marco R., Leong B.C.S., Baumal C.R., Duker J.S., Sarraf D. Paracentral acute middle maculopathy and the ischemic cascade associated with retinal vascular occlusion. Am J Ophthalmol. 2018;195:143–153. doi: 10.1016/j.ajo.2018.07.031. Epub 2018/08/07PubMed PMID: 30081014. [DOI] [PubMed] [Google Scholar]
- 23.Nemiroff J., Kuehlewein L., Rahimy E., Tsui I., Doshi R., Gaudric A., Gorin M.B., Sadda S., Sarraf D. Assessing deep retinal capillary ischemia in paracentral acute middle maculopathy by optical coherence tomography angiography. Am J Ophthalmol. 2016;162:121–132. doi: 10.1016/j.ajo.2015.10.026. e1Epub 2015/11/13PubMed PMID: 26562176. [DOI] [PubMed] [Google Scholar]
- 24.Chen X., Rahimy E., Sergott R.C., Nunes R.P., Souza E.C., Choudhry N., Cutler N.E., Houston S.K., Munk M.R., Fawzi A.A., Mehta S., Hubschman J.P., Ho A.C., Sarraf D. Spectrum of retinal vascular diseases associated with paracentral acute middle maculopathy. Am J Ophthalmol. 2015;160(1):26–34. doi: 10.1016/j.ajo.2015.04.004. e1. Epub 2015/04/08PubMed PMID: 25849522. [DOI] [PubMed] [Google Scholar]
- 25.Sarraf D., Rahimy E., Fawzi A.A., Sohn E., Barbazetto I., Zacks D.N., Mittra R.A., Klancnik J.M., Jr., Mrejen S., Goldberg N.R., Beardsley R., Sorenson J.A., Freund K.B. Paracentral acute middle maculopathy: a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013;131(10):1275–1287. doi: 10.1001/jamaophthalmol.2013.4056. Epub 2013/08/10PubMed PMID: 23929382. [DOI] [PubMed] [Google Scholar]
- 26.Hee M.R., Izatt J.A., Swanson E.A., Huang D., Schuman J.S., Lin C.P., Puliafito C.A., Fujimoto J.G. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113(3):325–332. doi: 10.1001/archopht.1995.01100030081025. Epub 1995/03/01PubMed PMID: 7887846. [DOI] [PubMed] [Google Scholar]
- 27.Maltsev D.S., Kulikov A.N., Burnasheva M.A., Chhablani J. Prevalence of resolved paracentral acute middle maculopathy lesions in fellow eyes of patients with unilateral retinal vein occlusion. Acta Ophthalmol. 2020;98(1):e22–ee8. doi: 10.1111/aos.14196. Epub 2019/07/28PubMed PMID: 31347293. [DOI] [PubMed] [Google Scholar]
- 28.Yu S., Wang F., Pang C.E., Yannuzzi L.A., Freund K.B. Multimodal imaging findings in retinal deep capillary ischemia. Retina. 2014;34(4):636–646. doi: 10.1097/IAE.0000000000000048. Epub 2013/11/19PubMed PMID: 24240565. [DOI] [PubMed] [Google Scholar]
- 29.Sarda V., Nakashima K., Wolff B., Sahel J.A., Paques M. Topography of patchy retinal whitening during acute perfused retinal vein occlusion by optical coherence tomography and adaptive optics fundus imaging. Eur J Ophthalmol. 2011;21(5):653–656. doi: 10.5301/EJO.2011.6374. Epub 2011/03/02PubMed PMID: 21360483. [DOI] [PubMed] [Google Scholar]
- 30.Gomez M.L., Mojana F., Bartsch D.U., Freeman W.R. Imaging of long-term retinal damage after resolved cotton wool spots. Ophthalmology. 2009;116(12):2407–2414. doi: 10.1016/j.ophtha.2009.05.012. Epub 2009/10/10PubMed PMID: 19815278; PMCID: PMC4172325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLeod D. Misery perfusion, diffusive oxygen shunting and interarterial watershed infarction underlie oxygenation-based hypoperfusion maculopathy. Am J Ophthalmol. 2019;205:153–164. doi: 10.1016/j.ajo.2019.03.015. Epub 2019/03/25PubMed PMID: 30905727. [DOI] [PubMed] [Google Scholar]
- 32.Ghasemi Falavarjani K., Phasukkijwatana N., Freund K.B., Cunningham E.T., Jr., Kalevar A., McDonald H.R., Dolz-Marco R., Roberts P.K., Tsui I., Rosen R., Jampol L.M., Sadda S.R., Sarraf D. En face optical coherence tomography analysis to assess the spectrum of perivenular ischemia and paracentral acute middle maculopathy in retinal vein occlusion. Am J Ophthalmol. 2017;177:131–138. doi: 10.1016/j.ajo.2017.02.015. Epub 2017/02/27PubMed PMID: 28237415. [DOI] [PubMed] [Google Scholar]
- 33.Burnasheva M.A., Maltsev D.S., Kulikov A.N., Sherbakova K.A., Barsukov A.V. Association of chronic paracentral acute middle maculopathy lesions with hypertension. Ophthalmol Retina. 2020;4(5):504–509. doi: 10.1016/j.oret.2019.12.001. Epub 2020/01/18PubMed PMID: 31948908. [DOI] [PubMed] [Google Scholar]
- 34.Arnould L., Binquet C., Guenancia C., Alassane S., Kawasaki R., Daien V., Tzourio C., Kawasaki Y., Bourredjem A., Bron A., Creuzot-Garcher C. Association between the retinal vascular network with Singapore "I" vessel assessment (SIVA) software, cardiovascular history and risk factors in the elderly: the Montrachet study, population-based study. PLoS ONE. 2018;13(4) doi: 10.1371/journal.pone.0194694. Epub 2018/04/04PubMed PMID: 29614075; PMCID: PMC5882094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnould L., Guenancia C., Azemar A., Alan G., Pitois S., Bichat F., Zeller M., Gabrielle P.H., Bron A.M., Creuzot-Garcher C., Cottin Y. The EYE-MI pilot study: a prospective acute coronary syndrome cohort evaluated with retinal optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2018;59(10):4299–4306. doi: 10.1167/iovs.18-24090. Epub 2018/10/30PubMed PMID: 30372758. [DOI] [PubMed] [Google Scholar]
- 36.Hannappe M.A., Arnould L., Meloux A., Mouhat B., Bichat F., Zeller M., Cottin Y., Binquet C., Vergely C., Creuzot-Garcher C., Guenancia C. Vascular density with optical coherence tomography angiography and systemic biomarkers in low and high cardiovascular risk patients. Sci Rep. 2020;10(1):16718. doi: 10.1038/s41598-020-73861-z. Epub 2020/10/09PubMed PMID: 33028913; PMCID: PMC7542456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein R., Klein B.E., Moss S.E., Meuer S.M. Retinal emboli and cardiovascular disease: the beaver dam eye study. Arch Ophthalmol. 2003;121(10):1446–1451. doi: 10.1001/archopht.121.10.1446. Epub 2003/10/15PubMed PMID: 14557181. [DOI] [PubMed] [Google Scholar]
- 38.Fallico M., Lotery A.J., Longo A., Avitabile T., Bonfiglio V., Russo A., Murabito P., Palmucci S., Pulvirenti A., Reibaldi M. Risk of acute stroke in patients with retinal artery occlusion: a systematic review and meta-analysis. Eye (Lond) 2019 doi: 10.1038/s41433-019-0576-y. Epub 2019/09/19PubMed PMID: 31527762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rim T.H., Han J., Choi Y.S., Hwang S.S., Lee C.S., Lee S.C., Kim S.S. Retinal artery occlusion and the risk of stroke development: twelve-year nationwide cohort study. Stroke. 2016;47(2):376–382. doi: 10.1161/STROKEAHA.115.010828. Epub 2016/01/09PubMed PMID: 26742801. [DOI] [PubMed] [Google Scholar]
- 40.Ho J.D., Liou S.W., Lin H.C. Retinal vein occlusion and the risk of stroke development: a five-year follow-up study. Am J Ophthalmol. 2009;147(2):283–290. doi: 10.1016/j.ajo.2008.08.006. e2. Epub 2008/10/07PubMed PMID: 18835470. [DOI] [PubMed] [Google Scholar]
- 41.Rim T.H., Kim D.W., Han J.S., Chung E.J. Retinal vein occlusion and the risk of stroke development: a 9-year nationwide population-based study. Ophthalmology. 2015;122(6):1187–1194. doi: 10.1016/j.ophtha.2015.01.020. Epub 2015/03/03PubMed PMID: 25726093. [DOI] [PubMed] [Google Scholar]