Abstract
Free fatty acid receptor 2 (FFAR2) has been reported as a tumor suppressor in colon cancer development. The current study investigated the effects of FFAR2 signaling on energy metabolism and gut microbiota profiling in a colorectal cancer mouse model (ApcMin/+). Ffar2 deficiency promoted colonic polyp development and enhanced fatty acid oxidation and bile acid metabolism. Gut microbiome sequencing analysis showed distinct clustering among wild-type, ApcMin/+, and ApcMin/+-Ffar2-/- mice. The relative abundance of Flavobacteriaceae and Verrucomicrobiaceae was significantly increased in the ApcMin/+-Ffar2-/- mice compared to the ApcMin/+ mice. In addition, knocking-down FFAR2 in the human colon cancer cell lines (SW480 and HT29) resulted in increased expression of several key enzymes in fatty acid oxidation, such as carnitine palmitoyltransferase 2, acyl-CoA dehydrogenase, long-chain acyl-CoA dehydrogenase, C-2 to C-3 short chain, and hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase, alpha subunit. Collectively, these results demonstrated that Ffar2 deficiency significantly altered profiles of fatty acid metabolites and gut microbiome, which might promote colorectal cancer development.
Keywords: Ffar2, ApcMin/+, Colorectal cancer, Metabolomics, Gut microbiota
INTRODUCTION
Colorectal cancer is the second leading cause of cancer death in both sexes in the United States [1]. Many factors are associated with the development of colorectal cancer, such as “unhealthy” diets [2-6], gut inflammation [7-10], and microbial dysbiosis [11-14].
Free fatty acid receptor 2 (FFAR2, also named GPR43) is a member of the G protein-coupled receptor family and is expressed on the adipose tissue, leukocytes [15], and colon [16,17]. FFAR2 receptor can be activated by the short-chain fatty acids (SCFAs), such as acetate, butyrate, and propionate, which are produced during fermentation of the undigested carbohydrates and dietary fiber by gut microbiota [18]. FFAR2 signaling has been reported as a negative regulator of inflammation. For example, dextran sodium sulfate (DSS)-induced ulcerative colitis, a strong risk factor for colorectal cancer in humans, was enhanced in the Ffar2 deficient (Ffar2-/-) mice compared to the wild-type (WT) mice [19-21]. In addition, a potential tumor-suppressive role of Ffar2 in colon cancer has been reported by our group [22,23] and others [24,25]. The decreased FFAR2 expression in colorectal adenocarcinoma tissues compared to the normal tissues has been observed in human patients [22,24]. However, whether loss of FFAR expression is associated with altered biochemical metabolites and microbial dysbiosis in colorectal cancer is unknown. Using black raspberries containing plenty of soluble fibers that can be metabolized into SCFAs by gut bacteria, we have demonstrated that the berries can modulate gut bacterial metabolites in colorectal cancer patients [26] and animals bearing colorectal cancer [27,28]. Interestingly, various components in black raspberries exerted different effects on gut microbiota [29]. Most importantly, we further showed that loss of Ffar2 significantly dampened the anti-colorectal cancer effects of black raspberries [22]. Accordingly, our previous results suggest that functional Ffar2 is vital for high-fiber foods to exert anti-colorectal cancer activities.
Our current study demonstrated that loss of Ffar2 promoted the colon adenomas development in the ApcMin/+ mice. Besides, using a mass spectrometry-based metabolomic analysis, we determined the effects of Ffar2 deficiency on the metabolites. The 16S rRNA gene sequence-based microbial analysis was conducted to determine if loss of Ffar2 could change the gut bacterial composition. Lastly, we knocked-down FFAR2 in the human colon cancer cell lines to determine its effects on the Expression of the key enzymes that are involved in energy metabolism.
MATERIALS AND METHODS
Animals and cell lines
All protocols followed institutional guidelines for animal care dictated by the Medical College of Wisconsin Animal Care and Use Committee (AUA2430). Breeding pairs of the WT and ApcMin/+ mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Breeding pairs of the Ffar2 heterozygous (Ffar2+/-) mice were purchased from Deltagen, Inc. (San Mateo, CA, USA). Four-week-old WT, ApcMin/+, and ApcMin/+-Ffar2-/- mice were fed the synthetic diet AIN-76A from the American Institute of Nutrition (Dyets Inc., Bethlehem, PA, USA) for 8 weeks. Mice were euthanized by CO2 asphyxiation. The number and the burden of polyps were determined. The colonic mucosa and plasma specimens were collected from a subgroup of the WT mice (n = 4), ApcMin/+ mice (n = 5) and ApcMin/+-Ffar2-/- mice (n = 5) for metabolomic profiling. The cecal fecal specimens were collected from a subgroup of the WT mice (n = 5), ApcMin/+ mice (n = 5), and ApcMin/+-Ffar2-/- mice (n = 5) for microbial analysis.
Human colorectal cancer cells HT29 and SW480 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) in April 2016 and were cultured as recommended by ATCC.
Metabolomic profiling
Specimen preparation and extraction, mass spectrometer platforms and setting, and data analysis were conducted by Metabolon, Inc. (Morrisville, NC, USA) [30-32] according to the previous description [26,27]. Briefly, samples were prepared using an automated MicroLab STAR® system (Reno, NV, USA). Homogenized mucosa samples were extracted using 5 µL of methanol per mg tissue, and the plasma samples were extracted using 5 µL of methanol per mL tissue. Samples were characterized using the ultra-high-performance-liquid chromatography/tandem mass spectrometry (UHPLC-MS/MS) in the negative ion mode, the UHPLC-MS/MS in the positive ion mode, and the gas chromatography-mass spectrometry (GC-MS) after sialylation. Chemical entities were identified by comparing them to the metabolomic library of purified standards based on chromatographic properties and mass spectra.
DNA preparation and PCR amplification
Cecal feces were collected from a subgroup of the WT mice (n = 5), ApcMin/+ mice (n = 5), and ApcMin/+-Ffar2-/- mice (n = 5). The fecal DNA samples were isolated using the PowerSoil® DNA Isolation Kit (MO Bio Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions. The 515F-806R region of the 16S rRNA gene was amplified by PCR (94°C for 3 minutes, followed by 35 cycles at 94°C for 45 seconds, 50°C for 60 seconds, and 72°C for 90 seconds and a final extension at 72°C for 10 minutes, hold at 4°C) using primers 515F 5’-GTGCCAGCMGCCGCGGTAA-3’ and 806R 5’-barcode-GGACTACHVGGGTWTCTAAT-3’ [33]. PCR reactions were performed in triplicate with 25 µL of the reaction mixtures containing 10 μL of the five primers hot master mix (2200410; MO Bio Laboratories), 0.5 µL of each primer (10 µM) and 1 µL of the template DNA.
Illumina MiSeq sequencing
The PCR products were quantified by Picogreen (P11496; Thermo Fisher Scientific, Waltham, MA, USA). Two hundred and forty ng of the DNA was pooled for each sample and purified using UltraClean PCR Clean-Up kit (12500; Mo Bio Laboratories) according to the manufacturer’s instructions. Sequencing was conducted using a paired-end, 2 × 250-bp cycle run on an Illumina MiSeq sequencing system and MiSeq Reagent Kit version 2 (500 Cycle) chemistry. Illumina BaseSpace’s 16s Metagenomics App was used to analyze the results.
Sequencing data analysis
To provide an even level of coverage for clustering and statistical comparisons, raw taxonomic counts were subsampled to 13,995 sequences per sample and aggregated at phylum through genus levels using QIIME [34]. Differential abundance analysis comparing the WT, ApcMin/+, and ApcMin/+-Ffar2-/- groups utilized the negative binomial test [35] with P-value adjustment using the False Discovery Rate [36]. Adjusted P-values that were less than 0.05 were considered statistically significant. Hierarchical clustering was performed using Ward’s method with log-normalized proportional values in R.
Immunoblotting analysis
Protein lysates of the human colorectal cancer cell lines were used for immunoblotting analysis. FFAR2-shRNA constructs to knockdown FFAR2 were purchased from OriGene Technologies, Inc. (Rockville, MD, USA), as indicated previously [23]. Antibodies to carnitine palmitoyltransferase 2 (CTP2) (ab181114), acyl-CoA dehydrogenase, long-chain (ACADL) (ab152160), acyl-CoA dehydrogenase, C-2 to C-3 short chain (ACADS) (ab156571), and hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase, alpha subunit (HADHA) (ab203114) were purchased from Abcam (Cambridge, MA, USA) and were used to identify their respective proteins. Antibody to β-actin (691001) was purchased from MP Biomedical (Santa Ana, CA, USA) and was used as a loading control.
Statistical analysis
Data were expressed as mean ± SEM. One-way ANOVA was employed in R version 2.14.2 [37] to identify statistically significant metabolite differences across genotypes. Standard statistical analyses are performed in ArrayStudio on log transformed data. A P-value < 0.05 was considered statistically significant.
RESULTS
Ffar2 deficiency promoted the development of colonic polyps
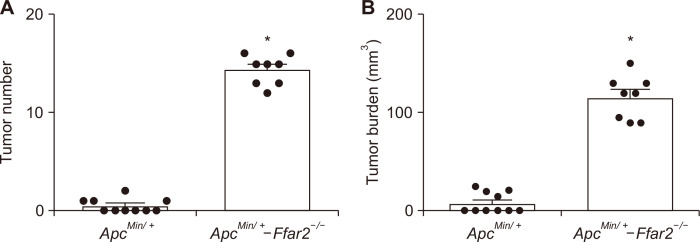
The WT, ApcMin/+, and ApcMin/+-Ffar2-/- mice were given the AIN-76A diet for 8 weeks. Forty % (4/10) of the ApcMin/+ mice developed colonic polyps, whereas the ApcMin/+-Ffar2-/- mice have an 100% (8/8) incidence of colonic polyps development with an increased number (Fig. 1A) and burden (Fig. 1B) of colonic polyps, which were consistent with our previous studies with an increase Ki-67 staining as a proliferation marker and H&E staining [22].
Figure 1. Loss of Ffar2 promotes colon adenoma development.
Colonic polyp number (A) and burden (B) from 12-14 weeks old, the ApcMin/+ and ApcMin/+-Ffar2-/- mice were measured. Ffar2, free fatty acid receptor 2. *P < 0.05.
Ffar2 deficiency enhanced the long-chain fatty acid β-oxidation and bile acid metabolism
To determine the effects of Ffar2 deficiency on the metabolic profiles, we collected the colonic mucosa and plasma specimens and conducted a mass spectrometry-based nontargeted metabolomic analysis. Five hundred and sixteen plasma metabolites and 568 colonic mucosa metabolites were annotated. Of these, 128 plasma metabolites and 75 colonic mucosa metabolites were significantly changed across three genotypes. Similar metabolic alterations, including 59 plasma metabolites (Table S1) and 23 mucosa metabolites (Table S2), have been observed in both the ApcMin/+ and ApcMin/+-Ffar2-/- mice compared to the WT mice. More importantly, Ffar2 deficiency further modulated 31 plasma metabolites (Table S3) and 28 mucosa metabolites (Table S4). Significantly changed metabolites were analyzed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database to identify biochemical pathways.
Fatty acids are oxidized in the mitochondria to generate energy and intermediates for cell proliferation. We observed significantly decreased fatty acid levels, including the medium-chain fatty acids, long-chain fatty acids, and polyunsaturated fatty acids in both the ApcMin/+ and ApcMin/+-Ffar2-/- mice (Table 1). Also, the production of acetylcarnitine, the end-product of fatty acid β-oxidation, was significantly increased in plasma of both the ApcMin/+ and ApcMin/+-Ffar2-/- mice (Table 2). Carnitine-conjugated long-chain fatty acids were markedly increased in the ApcMin/+-Ffar2-/- mice compared to ApcMin/+ mice (Table 1), suggesting the long-chain fatty acid β-oxidation were enhanced by the Ffar2 deficiency. Acetylcarnithine can be converted to acetyl-CoA, which enters into the citric acid cycle to generate 3-hydroxybutyrate (BHBA) through ketogenesis. We observed a significant accumulation of BHBA in plasma of the ApcMin/+-Ffar2-/- mice (Table 1). These results indicate an increased mitochondrial activity and a higher demand for energy by cancer cells.
Table 1.
List of significantly changed metabolites involved in the fatty acid β-oxidation pathway
| Metabolites | Biochemical pathways | Metabolites | Fold control | |
|---|---|---|---|---|
| A/WT | AF/WT | |||
| Mucosa | Medium chain fatty acid | 5-dodecenoate (12:1n7) | 0.48b | 0.4b |
| Long chain fatty acid | Margarate (17:0) | 0.35b | 0.39b | |
| Eicosenoate (20:1) | 0.25b | 0.29b | ||
| Erucate (22:1n9) | 0.23b | 0.34b | ||
| Polyunsaturated fatty acid (n3 and n6) | Docosadienoate (22:2n6) | 0.34b | 0.44b | |
| Dihomo-linoleate (20:2n6) | 0.31b | 0.32b | ||
| Plasma | Polyunsaturated fatty acid (n3 and n6) | Dihomo-linolenate (20:3n3 or n6) | 0.43b | 0.59b |
| Docosapentaenoate (n6 DPA; 22:5n6) | 0.46b | 0.51b | ||
| Fatty acid metabolism (Acyl Carnitine) | Acetylcarnitine | 1.57a | 1.83a | |
| Decanoylcarnitine | 1.44a | 2a | ||
| AF/WT | AF/A | |||
| Mucosa | Fatty acid metabolism (Acyl Carnitine) | 3-hydroxybutyrylcarnitine | 4.34a | 2.73a |
| Stearoylcarnitine | 1.68a | 1.4a | ||
| Plasma | Fatty acid metabolism (Acyl Carnitine) | Cis-4-decenoyl carnitine | 1.94a | 1.69a |
| Laurylcarnitine | 1.48a | 1.42a | ||
| Myristoylcarnitine | 1.65a | 1.71a | ||
| Palmitoylcarnitine | 1.6a | 1.84a | ||
| Stearoylcarnitine | 1.92a | 1.5a | ||
| Myristoleoylcarnitine | 1.54a | 1.62a | ||
| Suberoylcarnitine | 3.5a | 3.08a | ||
| Adipoylcarnitine | 3.5a | 2.51a | ||
| Ketone bodies | 3-hydroxybutyrate (BHBA) | 5.46a | 3.53a | |
Fold change is calculated as the ratio of the ApcMin/+ (A) vs. WT, ApcMin/+-Ffar2-/- (AF) vs. WT, and ApcMin/+-Ffar2-/- vs. ApcMin/+. Fold change that is labeled a or b presents significantly increased or significantly decreased, respectively. WT, wild-type. P < 0.05.
Table 2.
List of significantly changed metabolites in the bile acid pathway
| Metabolites | Biochemical pathways | Metabolites | Fold control | |||
|---|---|---|---|---|---|---|
| A/WT | AF/WT | |||||
| Mucosa | Primary bile acid metabolism | Cholate sulfate | 0.05b | 0.25b | ||
| Plasma | Secondary bile acid metabolism | Deoxycholate | 3.19a | 2.03a | ||
| A/WT | AF/WT | AF/A | ||||
| Mucosa | Primary bile acid metabolism | Cholate | 1.33 | 4.49a | 3.38a | |
| Chenodeoxycholate | 1.13 | 4.95a | 4.37a | |||
| Beta-muricholate | 0.86 | 2.68a | 3.11a | |||
| Secondary bile acid metabolism | Deoxycholate | 3.02a | 7.65a | 2.53a | ||
| Taurodeoxycholate | 2.75 | 6.29a | 2.28a | |||
| 6-beta-hydroxylithocholate | 2.1a | 4.29a | 2.05a | |||
| 7-ketolithocholate | 5.92a | 11.94a | 2.02a | |||
| Hyocholate | 0.84 | 3.55a | 4.23a | |||
| 3-dehydrocholate | 1.11 | 3.1a | 2.8a | |||
| 7-ketodeoxycholate | 0.9 | 3.83a | 4.24a | |||
Fold change is calculated as the ratio of the ApcMin/+ (A) vs. WT, ApcMin/+-Ffar2-/- (AF) vs. WT, and ApcMin/+-Ffar2-/- vs. ApcMin/+. Fold change that is labeled a or b presents significantly increased or significantly decreased, respectively. WT, wild-type. P < 0.05.
Primary bile acids are synthesized by cholesterol catabolism in the liver and subsequently conjugated [38]. In the intestine, intestinal bacteria could deconjugate a significant portion of the primary bile acids, and structurally modify them into the secondary bile acids, which have been shown to promote colon carcinogenesis [38]. We observed significantly increased levels of both the primary and secondary bile acids in colonic mucosa in the ApcMin/+-Ffar2-/- mice compared to the ApcMin/+ mice, including cholate, chenodeoxycholate, deoxycholate, and taurodeoxycholate (Table 2). Deoxycholate has been demonstrated to promote colon carcinogenesis by 165.1% in the ApcMin/+ mice [39]. Thus, our findings suggest that an increased deoxycholate level could directly contribute to the Ffar2 deficiency-promoted colon cancer development.
Ffar2 deficiency changed the expression of key enzymes in the fatty acid β-oxidation pathway
After observing significant levels of the carnitine-conjugated long-chain fatty acids in the ApcMin/+-Ffar2-/- mice compared to ApcMin/+, we further investigated if loss of Ffar2 could alter the expression of the key enzymes involved in the fatty acid β-oxidation pathway. We first determined the endogenous expression levels of FFAR2 in four human colon cancer cell lines, and found higher levels of FFAR2 expression in SW480 and HT29 cell lines [23]. Furthermore, we knocked-down FFAR2 using shRNA in SW480 and HT29 cells as previously [23]. We observed increased expression levels of several key enzymes in the fatty acid β-oxidation pathway, including CPT2, ACADL, ACADS, and HADHA (Fig. 2A and 2B). This finding suggests the enhanced fatty acid β-oxidation in the FFAR2-deficient colon cancer cells.
Figure 2. FFAR2 deficiency significantly increased the expression of key enzymes in the fatty acid β-oxidation pathway.
Immunoblotting of CPT2, ACADS/L and HADHA in the SW480 (A) and HT29 (B) cells treated with either the vector or the FFAR2-shRNA to knockdown FFAR2. Numbers under each blot indicate the fold differences. FFAR2, free fatty acid receptor 2; ACADL, acyl-CoA dehydrogenase, long chain; ACADS, acyl-CoA dehydrogenase, C-2 to C-3 short chain; CPT2, carnitine palmitoyltransferase 2; HADHA, hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase, alpha subunit.
Ffar2 deficiency changed gut microbiota composition
Evidence has been accumulated to imply the interplay between gut dysbiosis and colorectal cancer [40]. In order to investigate the effects of Ffar2 deficiency on the gut microbiome, we performed 16S rRNA gene sequencing on the cecal fecal samples. Principal coordinate analyses showed clustering according to genotypes (Fig. 3A). The relative abundance of bacteria at the family level (Fig. 3B) showed increased levels of Porphyromonadaceae, Sphingobacteriaceae, Deferribacteraceae, Flavobacteriaceae, and Verrucomicrobiaceae and decreased levels of Ruminococcaceae and Bifidobacteriaceae in both the ApcMin/+ mice and ApcMin/+-Ffar2-/- mice compared to the WT mice. In particular, Flavobacteriaceae and Verrucomicrobiaceae were further increased in the ApcMin/+-Ffar2-/- mice compared to the ApcMin/+ mice, which might contribute to the Ffar2 deficiency-promoted colon cancer development (Fig. 3C).
Figure 3. The ApcMin/+-Ffar2-/- mice show a structurally different microbiota compared to the ApcMin/+ mice.
(A) Assessment of the structure of microbial communities by principal coordinate analyses. Plots were presented for gut bacteria sequenced at species levels. (B) Relative abundance of bacteria presented at the family level. (C) Relative abundance of Flavobacteriaceae and Verrucomicrobiaceae were significantly increased in the ApcMin/+-Ffar2-/- mice compared to the ApcMin/+ mice. FFAR2, free fatty acid receptor 2; WT, wild-type. *P < 0.05.
DISCUSSION
Our previous studies and those of other groups have shown that the expression of FFAR2 was decreased in adenocarcinoma tissues compared to normal tissues of patients with colorectal cancer [22,24]. Our current study, by utilizing ApcMin/+ mice, demonstrated that Ffar2 deficiency promoted the development of colonic polyps. All the ApcMin/+-Ffar2-/- mice developed colonic polyps compared to only 40% of the ApcMin/+ mice. The ApcMin/+-Ffar2-/- mice developed increased tumor burden of colonic polyps. In addition, we investigated if Ffar2 deficiency has effects on the metabolic profiles and the gut bacterial composition. Thirty-one plasma metabolites and 28 colonic mucosa metabolites were changed in the ApcMin/+-Ffar2-/- mice compared to the ApcMin/+ mice. Analysis using KEGG data suggests that loss of Ffar2 enhances the long-chain fatty acid β-oxidation and the bile acid metabolism. Furthermore, Ffar2 deficiency markedly increased the abundance of Flavobacteriaceae and Verrucomicrobiaceae.
Previously we observed significantly decreased fatty acid levels in the colonic mucosa of ApcMin/+ mice [27]. Similarly, the current study detected reduced levels of 11 fatty acids, including medium-chain fatty acids, long-chain fatty acids, and polyunsaturated fatty acids, in the colonic mucosa of ApcMin/+ mice (Table S2). Six of these fatty acids were also significantly decreased in the colonic mucosa of ApcMin/+-Ffar2-/- mice (Table 1). In addition, ApcMin/+-Ffar2-/- mice showed a substantial accumulation of carnitine-conjugated long-chain fatty acids in both colonic mucosa and plasma specimens (Table 1), including stearoylcarnitine, laurylcarnitine, myristoylcarnitine, and palmitoylcarnitine. Increased levels of these carnitine-conjugated long-chain fatty acids have been observed in tumor samples from biofilm-positive colorectal cancer patients [41], suggesting association among the increased fatty acid β-oxidation, loss of Ffar2, and gut microbiota.
Enhanced fatty acid β-oxidation has been reported in colon cancer patients [42,43]. Our study used human colon cancer cell lines to investigate if the functional FFAR2 could influence the key enzymes of the fatty acid oxidation pathway. Based on relatively higher expression levels of FFAR2 in the SW480 and HT29 cells compared to the Caco-2 and HCT116 cells, we knock-downed FFAR2 in the SW480 and HT29 cells [23]. Using the FFAR2 knocked-down cells, we found that the expression levels of several key enzymes in the fatty acid oxidation pathway have been increased in the FFAR2-deficient cells, including CPT2, ACADL, ACADS, and HADHA.
CPT2 has been shown to be over-expressed in primary prostate cancer [44], and knocking-down of CPT2 inhibited the tumor growth in triple-negative breast cancer [45]. Thus, our findings on increased CPT2 expression in FFAR2-deficient cells could be one of the mechanisms responsible for loss of FFAR2-enhanced colon cancer development.
HADHA has also been reported to be decreased in breast cancer [46] and clear cell renal cell carcinoma [47]. However, we observed increased expression of HADHA in the FFAR2-deficient SW480 and HT29 cells. These results, combined with increased expression of CPT2, ACADL, and ACADS in the FFAR2-deficient cells, suggest an overall accelerated fatty acid oxidation, which may contribute to the FFAR2 deficiency-promoted colon cancer development.
A strong link between microbial dysbiosis and colon cancer has been intensively explored. However, due to the complexity of the gut microbiome, the underlying mechanisms remain unclear. Our current study demonstrated that loss of Ffar2 significantly changed the composition of microbiota in the ApcMin/+ mice. Decreased Bifidobacterium and has been observed in human colon cancer tissues [25]. Also, increased Peptostreptococcaceae has been positively associated with biofilm and an enhanced acetylated polyamines pathway in human colon cancer patients, which promote colon cancer development [41]. In our study, the profile of gut microbiome was found to be significantly changed in the polyp-bearing mice (ApcMin/+ and ApcMin/+-Ffar2-/- mice) compared to WT mice, as revealed by the decreased abundance of Bifidobacterium and increased proportion of Peptostreptococcaceae. More importantly, the abundance of Flavobacteriaceae and Verrucomicrobiaceae was raised in the ApcMin/+ mice compared to WT mice and further increased in ApcMin/+-Ffar2-/- mice, which might contribute to Ffar2 deficiency-enhanced colon cancer development.
We previously reported that the cAMP-protein kinase A (PKA)-cAMP Response Element-Binding Protein (CREB) pathway, downstream of Ffar2, was activated, and this event led to overexpression of histone deacetylases in the Ffar2-deficient mice [23]. Mechanistically, H3K27me3 and H3K4me3 histone marks bind differentially to the promoter regions of inflammation suppressors as verified by ChIP-qPCR analysis. This results in decreased expression of these genes in the Ffar2-deficient mice, thereby promoting colon cancer [23]. We anticipate the changes of histone marks in enzymes regulating fatty acid oxidation, such as CPT2, ACADL, and HADHA, which warrants further investigations.
In summary, we validated Ffar2 as a tumor suppressor Ffar2 in colon carcinogenesis. To the best of our knowledge, this is the first study to link the biochemical metabolites and the gut microbiome profiling to the Ffar2 deficiency-promoted colon cancer development (Fig. 4). Enhanced fatty acid oxidation and bile acid metabolism, as well as the altered gut microbiome, could be, at least in part, constitute, the underlying mechanisms.
Figure 4. Dysregulated free fatty acid receptor 2 exacerbates colonic adenoma formation in ApcMin/+ mice by altering metabolism and gut microbiota composition.
DCA, deoxycholic acid; CDCA, chenodeoxycholic acid; ACADL, acyl-CoA dehydrogenase, long chain; ACADS, acyl-CoA dehydrogenase, C-2 to C-3 short chain; CPT2, carnitine palmitoyltransferase 2; HADHA, hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase, alpha subunit.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.15430/JCP.2021.26.1.32.
ACKNOWLEDGMENTS
This work was supported by NIH grants CA148818 and USDA/NIFA 2020-67017-30843 (to L.-S. Wang), and CA185301, AI129582, and NS106170 (to J. Yu).
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Aune D, Chan DS, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, et al. Red and processed meat intake and risk of colorectal adenomas: a systematic review and meta-analysis of epidemiological studies. Cancer Causes Control. 2013;24:611–27. doi: 10.1007/s10552-012-0139-z. [DOI] [PubMed] [Google Scholar]
- 3.Murphy N, Norat T, Ferrari P, Jenab M, Bueno-de-Mesquita B, Skeie G, et al. Dietary fibre intake and risks of cancers of the colon and rectum in the European prospective investigation into cancer and nutrition (EPIC) PLoS One. 2012;7:e39361. doi: 10.1371/journal.pone.0039361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan P, Yu J, Wang LS. Colon cancer: what we eat. Surg Oncol Clin N Am. 2018;27:243–67. doi: 10.1016/j.soc.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan P, Yu J, Wang LS. Diet and colon: what matters? Curr Opin Gastroenterol. 2019;35:101–6. doi: 10.1097/MOG.0000000000000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan P, Huang YW, Oshima K, Yearsley M, Zhang J, Yu J, et al. Could aspirin and diets high in fiber act synergistically to reduce the risk of colon cancer in humans? Int J Mol Sci. 2018;19:166. doi: 10.3390/ijms19010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monteleone G, Pallone F, Stolfi C. The dual role of inflammation in colon carcinogenesis. Int J Mol Sci. 2012;13:11071–84. doi: 10.3390/ijms130911071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klampfer L. Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets. 2011;11:451–64. doi: 10.2174/156800911795538066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang YW, Mo YY, Echeveste CE, Oshima K, Zhang J, Yearsley M, et al. Black raspberries attenuate colonic adenoma development in ApcMin mice: relationship to hypomethylation of promoters and gene bodies. Food Front. 2020;1:234–42. doi: 10.1002/fft2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peiffer DS. Modulation of the host microbiome by black raspberries or their components and the therapeutic implications in cancer. Food Front. 2020;1:296–304. doi: 10.1002/fft2.40. [DOI] [Google Scholar]
- 11.Akin H, Tözün N. Diet, microbiota, and colorectal cancer. J Clin Gastroenterol. 2014;48 Suppl 1:S67–9. doi: 10.1097/MCG.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 12.Ohtani N. Microbiome and cancer. Semin Immunopathol. 2015;37:65–72. doi: 10.1007/s00281-014-0457-1. [DOI] [PubMed] [Google Scholar]
- 13.Huang YW, Pan P, Echeveste CE, Wang HT, Oshima K, Lin CW, et al. Transplanting fecal material from wild-type mice fed black raspberries alters the immune system of recipient mice. Food Front. 2020;1:253–9. doi: 10.1002/fft2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan P, Skaer CW, Wang HT, Kreiser MA, Stirdivant SM, Oshima K, et al. Systemic metabolite changes in wild-type C57BL/6 mice fed black raspberries. Nutr Cancer. 2017;69:299–306. doi: 10.1080/01635581.2017.1263748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–52. doi: 10.1016/S0006-291X(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 16.Bindels LB, Dewulf EM, Delzenne NM. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol Sci. 2013;34:226–32. doi: 10.1016/j.tips.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, et al. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol. 2008;39:135–42. doi: 10.1007/s10735-007-9145-y. [DOI] [PubMed] [Google Scholar]
- 18.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104 Suppl 2:S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 19.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 20.Masui R, Sasaki M, Funaki Y, Ogasawara N, Mizuno M, Iida A, et al. G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflamm Bowel Dis. 2013;19:2848–56. doi: 10.1097/01.MIB.0000435444.14860.ea. [DOI] [PubMed] [Google Scholar]
- 21.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan P, W Skaer C, Wang HT, Oshima K, Huang YW, Yu J, et al. Loss of free fatty acid receptor 2 enhances colonic adenoma development and reduces the chemopreventive effects of black raspberries in ApcMin/+ mice. Carcinogenesis. 2017;38:86–93. doi: 10.1093/carcin/bgw122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan P, Oshima K, Huang YW, Agle KA, Drobyski WR, Chen X, et al. Loss of FFAR2 promotes colon cancer by epigenetic dysregulation of inflammation suppressors. Int J Cancer. 2018;143:886–96. doi: 10.1002/ijc.31366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Y, Chen Y, Jiang H, Robbins GT, Nie D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int J Cancer. 2011;128:847–56. doi: 10.1002/ijc.25638. [DOI] [PubMed] [Google Scholar]
- 25.Sivaprakasam S, Gurav A, Paschall AV, Coe GL, Chaudhary K, Cai Y, et al. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis. 2016;5:e238. doi: 10.1038/oncsis.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan P, Skaer CW, Stirdivant SM, Young MR, Stoner GD, Lechner JF, et al. Beneficial regulation of metabolic profiles by black raspberries in human colorectal cancer patients. Cancer Prev Res (Phila) 2015;8:743–50. doi: 10.1158/1940-6207.CAPR-15-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan P, Skaer CW, Wang HT, Stirdivant SM, Young MR, Oshima K, et al. Black raspberries suppress colonic adenoma development in ApcMin/+ mice: relation to metabolite profiles. Carcinogenesis. 2015;36:1245–53. doi: 10.1093/carcin/bgv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan P, Oshima K, Huang YW, Yearsley M, Zhang J, Arnold M, et al. Gut bacteria are required for the benefits of black raspberries in ApcMin/+ mice. J Berry Res. 2018;8:239–49. doi: 10.3233/JBR-180337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan P, Lam V, Salzman N, Huang YW, Yu J, Zhang J, et al. Black raspberries and their anthocyanin and fiber fractions alter the composition and diversity of gut microbiota in F-344 rats. Nutr Cancer. 2017;69:943–51. doi: 10.1080/01635581.2017.1340491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81:6656–67. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 31.DeHaven CD, Evans AM, Dai H, Lawton KA. Software techniques for enabling high-throughput analysis of metabolomic datasets. In: Roessner U, editor. Metabolomics. IntechOpen; London: 2012. pp. 167–92. [Google Scholar]
- 32.Dehaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform. 2010;2:9. doi: 10.1186/1758-2946-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–4. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. doi: 10.1016/S0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 37.R Core Team, author. [Accessed December 10, 2020];R: a language and environment for statistical computing. https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing#citation .
- 38.Degirolamo C, Modica S, Palasciano G, Moschetta A. Bile acids and colon cancer: solving the puzzle with nuclear receptors. Trends Mol Med. 2011;17:564–72. doi: 10.1016/j.molmed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Cao H, Luo S, Xu M, Zhang Y, Song S, Wang S, et al. The secondary bile acid, deoxycholate accelerates intestinal adenoma-adenocarcinoma sequence in Apc (min/+) mice through enhancing Wnt signaling. Fam Cancer. 2014;13:563–71. doi: 10.1007/s10689-014-9742-3. [DOI] [PubMed] [Google Scholar]
- 40.Tsuei J, Chau T, Mills D, Wan YJ. Bile acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp Biol Med (Maywood) 2014;239:1489–504. doi: 10.1177/1535370214538743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson CH, Dejea CM, Edler D, Hoang LT, Santidrian AF, Felding BH, et al. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015;21:891–7. doi: 10.1016/j.cmet.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ong ES, Zou L, Li S, Cheah PY, Eu KW, Ong CN. Metabolic profiling in colorectal cancer reveals signature metabolic shifts during tumorigenesis [published online ahead of print February 10, 2010] Mol Cell Proteomics. doi: 10.1074/mcp.M900551-MCP200. doi: 10.1074/mcp.M900551-MCP200. [DOI] [PubMed] [Google Scholar]
- 43.Tan B, Qiu Y, Zou X, Chen T, Xie G, Cheng Y, et al. Metabonomics identifies serum metabolite markers of colorectal cancer. J Proteome Res. 2013;12:3000–9. doi: 10.1021/pr400337b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iglesias-Gato D, Wikström P, Tyanova S, Lavallee C, Thysell E, Carlsson J, et al. The proteome of primary prostate cancer. Eur Urol. 2016;69:942–52. doi: 10.1016/j.eururo.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 45.Park JH, Vithayathil S, Kumar S, Sung PL, Dobrolecki LE, Putluri V, et al. Fatty acid oxidation-driven Src links mitochondrial energy reprogramming and oncogenic properties in triple-negative breast cancer. Cell Rep. 2016;14:2154–65. doi: 10.1016/j.celrep.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mamtani M, Kulkarni H. Association of HADHA expression with the risk of breast cancer: targeted subset analysis and meta-analysis of microarray data. BMC Res Notes. 2012;5:25. doi: 10.1186/1756-0500-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Z, Lu J, Han L, Wang X, Man Q, Liu S. Prognostic significance of two lipid metabolism enzymes, HADHA and ACAT2, in clear cell renal cell carcinoma. Tumour Biol. 2016;37:8121–30. doi: 10.1007/s13277-015-4720-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.