Abstract
House dust mite (HDM) is one of the significant causes for airway inflammation such as asthma. It induces oxidative stress and an inflammatory response in the lungs through the release of chemokines such as interleukin-8 (IL-8). Reactive oxygen species (ROS) activate inflammatory signaling mediators such as mitogen-activated protein kinases (MAPKs) and redox-sensitive transcription factors including NF-κB and AP-1. Ascorbic acid shows an antioxidant and anti-inflammatory activities in various cells. It ameliorated the symptoms of HDM-induced rhinitis. The present study was aimed to investigate whether HDM could induce IL-8 expression through activation of MAPKs, NF-κB, and AP-1 and whether ascorbic acid could inhibit HDM-stimulated IL-8 expression by reducing ROS and suppressing activation of MAPKs, NF-κB, and AP-1 in respiratory epithelial H292 cells. H292 cells were treated with HDM (5 μg/mL) in the absence or presence of ascorbic acid (100 or 200 μM). HDM treatment increased ROS levels, and activated MAPKs, NF-κB, and AP-1 and thus, induced IL-8 expression in H292 cells. Ascorbic acid reduced ROS levels and inhibited activation of MAPKs, NF-κB and AP-1 and L-8 expression in H292 cells. In conclusion, consumption of ascorbic acid-rich foods may be beneficial for prevention of HDM-mediated respiratory inflammation by suppressing oxidative stress-mediated MAPK signaling pathways and activation of NF-kB and AP-1.
Keywords: House dust mite, Ascorbic acid, Interleukin-8, Respiratory epithelium
INTRODUCTION
House dust mite (HDM), which often instigates airway inflammation and hence incites the development of asthma and allergic symptoms worldwide, has been well known as momentous indoor sensitizing factor. House dust mite is a universal companion in human habitation and feeds on organic detritus like flakes of shed human skin [1]. In Korea, there are about twenty species of house dust mite; Dermatophagoides farinae, Dermatophagoides pterronyssinus, Tyrophagus putrescentiae, Euroglyphus maynei, Glycyphagus destructor, and so on. Among them, Dermatophagoides farinae (D. farinae), so called American house dust mite, is a dominant species [2]. Multiple lines of evidence suggest that exogenous proteases (allergens) from the D. farinae have direct proinflammatory effect in the respiratory tract. Proteolytic activities of these allergens stimulate the release of potent cytokines and chemokines from human airway epithelium [3]. Yi et al. [4] showed that HDM allergen Der f 1 (D. farinae Group 1 allergen), the papain-like cysteine protease, induces interleukin-8 (IL-8) expression in human basophilic cells through generation of reactive oxygen species (ROS) and subsequent activation of extracellular signal-regulated kinase (ERK)/p38 signal pathways. They suggested that Der f 1-induced IL-8 secretion may account for allergic inflammation independent of immunoglobulin E antibodies. HDM-induced airway inflammation is associated with ROS production in human bronchial epithelial cells [5] and in neutrophils [6]. Therefore, reducing ROS may be beneficial to inhibit HDM-induced inflammation by suppressing expression of pro-inflammatory cytokines such as IL-8.
IL-8, a CXC neutrophil chemotactic and activating peptide, is an important mediator of the inflammatory response. The IL-8 secretion is stimulated by various stresses such as bacteria, oxidants, bacterial lipopolysaccharide, and pro-inflammatory cytokines [7-9]. Patients with status asthmaticus exhibit striking increases of IL-8 and neutrophils in the airways. IL-8 is also found in bronco-alveolar lavage fluid and serum from patients with asthma [10]. These findings indicate that IL-8 mediates airway inflammation and innate immunity. In addition, it may be a crucial mediator of human respiratory inflammation such as asthma.
Expression of IL-8 gene, which is stimulus-dependent, is generally controlled at the transcriptional level. There is a promoter element which harbours the NF-κB site in IL-8 gene [11,12]. NF-κB and AP-1 are the pleiotropic transcription factors that regulate the transcription of genes, which responds to immune or inflammatory signals in lung epithelial cells [13]. HDM increased IL-8 levels by activation of NF-κB in nasal epithelial cells [14] and bronchial epithelial cells [15]. Several studies demonstrated that phosphorylation of mitogen-activated protein kinases (MAPKs) including ERK, c-Jun NH2-terminal kinase (JNK), and p38 increased the activity of NF-κB in the promoter region of IL-8 gene [16]. MAPKs have a vital role in the release and expression of IL-8 in response to the stimulation by Mycoplasma pneumoniae [17], German cockroach extract [18], and respiratory syncytial virus in airway epithelial cells [19]. HDM increased IL-8 via activation of ERK in respiratory epithelial cells [20]. ROS are considered as the activators for MAPK signaling and transcription factors NF-κB and AP-1 in lung and airway epithelial cells [21,22]. Therefore, reducing ROS may be a rational strategy to prevent HDM-induced cytokine expression in respiratory system.
Ascorbic acid, so called vitamin C, has diverse functions for the maintenance of human health. As an antioxidant, ascorbic acid has protective effects against inflammatory disorders such as common cold [23] and pneumonia [24]. There are relatively few studies on the effect of ascorbic acid on HDM-associated airway inflammation. Anderson et al. [25] demonstrated that 6 month-supplementation with ascorbate (1 g/day) under standard therapy to ten children with bronchial asthma (caused by allergens from HDM) improved cellular immune function, determined by neutrophil chemotaxis and lymphocyte mitogen-induced transformation. Tongtako et al. [26] examined whether exercise training and ascorbic acid supplementation are effective adjuvant treatments in the management of symptoms in HDM-induced allergic rhinitis patients. After 8 weeks, both exercise alone and exercise combined with supplementation of ascorbic acid increased peak aerobic capacity and peak nasal inspiratory flow, and exhibited significantly decreased rhinitis symptoms, nasal blood flow, malondialdehylde levels, and nasal secretion of IL-4.
The purpose of the study is to investigate whether HDM induces expression of IL-8 through regulation of MAPKs, NF-κB, and AP-1, and whether ascorbic acid has an inhibitory effect on HDM-stimulated IL-8 expression by reducing ROS levels and suppressing the activation of MAPKs, NF-κB, and AP-1 in respiratory epithelial H292 cells.
MATERIALS AND METHODS
Cell line, house dust mite, and reagents
Human respiratory epithelial (NCI-H292) cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured as previously described [27]. House dust mite D. farinae extract (CE-DF-101006001) was purchased from Arthropods of Medical Importance Resource Bank (AMIB), Yonsei University College of Medicine, Seoul, Korea. It was dissolved in phosphate-buffered saline (PBS) and then stored at –80°C. Ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sterilized water.
Experimental protocol
For time-course experiments, the cells were treated with HDM (5 μg/mL) and incubated for 60 minutes (for determination of MAPKs), 10 hours (for determination of IL-8 mRNA level), or 24 hours (for determination of IL-8 in the medium). For ascorbic acid experiment, the cells were treated with HDM (5 μg/mL) in the absence or presence of ascorbic acid (100 or 200 μM) for 15 minutes (for determination of MAPKs), 30 minutes (for measuring ROS levels and activities of NF-κB and AP-1), and 2 hours (for determination of IL-8 mRNA level), and 10 hours (for determination of IL-8 in the medium).
For the determination of IL-8 mRNA and protein levels and ROS levels, the cells (3 × 105 cells/well), in 6-well plate, were cultured for indicated time periods. For the determination of MAPKs and activities of NF-κB and AP-1, the cells (2 × 106 cells) were seeded in 10 cm culture dish and cultured for indicated time periods.
RT-PCR analysis of IL-8 mRNA expression
Total RNA were isolated by TRI reagent (RNA/DNA/Protein isolation reagent; Molecular Research Center, Inc., Cincinnati, OH, USA). Total RNA was reverse transcribed into cDNA by reverse transcription process using moloney murine leukemia virus reverse transcriptase and random primer (Promega, Madison, WI, USA) with conditions at 23°C for 10 minutes, 37°C for 60 minutes, and 95°C for 5 minutes. The resulting cDNAs were used for RT-PCR analysis of IL-8 and β-actin. Sequences of IL-8 primers were 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ for forward primer and 5′-TCTCAG CCCTCTTCAAAAACTTCT-3′ for reverse primer, giving a 297 bp PCR product. For β-actin, the forward primer was 5′-ACCAACTGGGACGACATGGAG-3′ and the reverse primer was 5′-GTGAGGATCTTCATGAGGTAGTC-3′, giving a 349 bp PCR product. The cDNA was then amplified by 28 cycles with denaturation at 95°C for 15 seconds, annealing at 60°C 15 seconds, and extension at 72°C 45 seconds. β-actin gene was amplified in the same reaction to serve as the reference gene. After amplication, PCR products were separated on 1.5% agarose gels and visualized by a UV transillumination.
ELISA for IL-8 protein level
The culture mediem were centrifuged at 13,000 ×g for 5 minutes and then collected for measuring IL-8 levels. The level of IL-8 in the medium was determined by using ELISA kit (Biosource International, Camarillo, CA, USA) following manufacturer’s instructions.
Electrophoretic mobility shift assay (EMSA) and Western blotting
Whole cell extracts and nuclear extracts were prepared by the method described previously [28]. NF-κB-DNA binding activity and AP-1-DNA-binding activity were determined by EMSA for nuclear extracts flowed by our previous study [28]. For Western blot analysis of MAPKs, whole cell extracts (30 to 60 μg protein/lane) were subjected to 12% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham Biosciences, Buckinghamshire, UK) by electroblotting. Membranes were blocked and the proteins were detected with antibodies for p38 (1:500, CS-9212; Cell Signaling Technology, Beverly, MA, USA), p-p38 (1:300, SC-7975; Santa Cruz Biotechnology, Dallas, TX, USA), ERK1/2 (p44/p42) (1:500, CS-9102; Cell Signaling Technology), p-ERK1/2 (1:300, SC-7383; Santa Cruz Biotechnology), JNK2/1 (p54/p46) (1:500 to 1,000, CS-9252; Cell Signaling Technology), and p-JNK2/1 (1:500, CS-9252; Cell Signaling Technology), diluted in TBS with Tween 20 (TBS-T) containing 3% dry milk, and incubated overnight at 4°C. After washing with TBS-T, primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (anti-rabbit, anti-mouse), and visualized by the enhanced chemiluminescence detection system (Santa Cruz Biotechnology) [28].
Determination of ROS levels
The cells were treated with 10 μg/mL 2,7-dichlorofluorescein diacetate (DCF-DA; Sigma-Aldrich) and incubated in 5% CO2/95% air at 37°C for 30 minutes. After incubation, the medium was removed, and the cells were washed with PBS. The fluorescence intensity of the de-esterified product, 2,7-dichlorofluorescein in the cells (in 6-well plates) was measured at 522 nm (excitation at 498 nm) with a Victor 5 multi-label counter (PerkinElmer Life and Analytical Sciences, Boston, MA, USA). Intracellular ROS levels were normalized to the cell numbers.
Statistical analysis
The statistical differences were determined using one-way analysis of variance and Newman–Keul’s test. All data are expressed as mean ± standard error (SE) of four different experiments. For each experiment, the number of samples in each group was 4 (n = 4 per each group). A value of P < 0.05 was considered statistically significant.
RESULTS
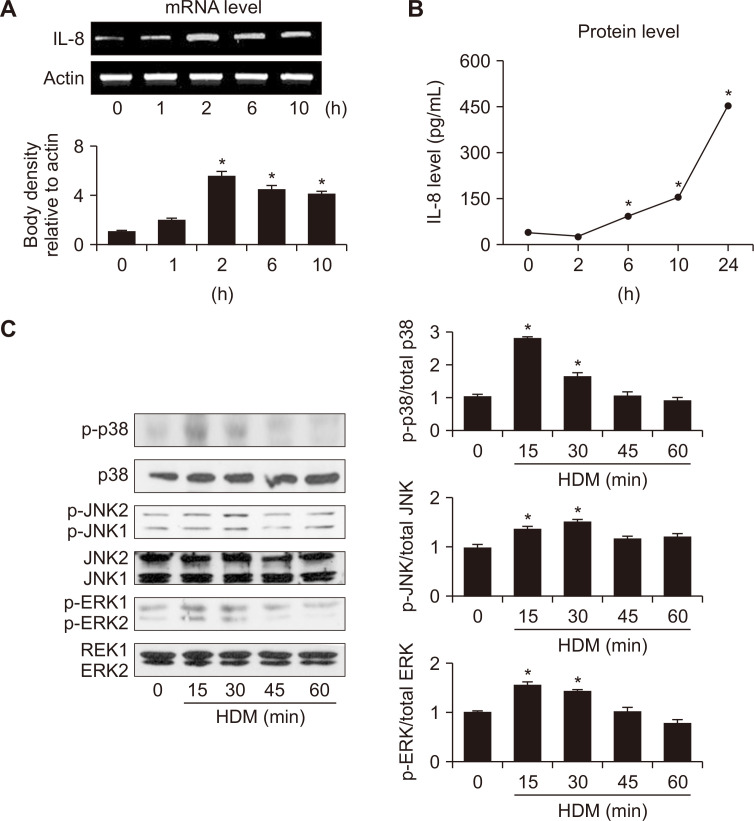
To investigate whether HDM could induce IL-8 expression, cells were incubated with 5 μg/mL HDM for indicated time periods. As shown in Figure 1A and 1B, HDM induced expression of IL-1 mRNA and its protein production, respectively in a time-dependent manner. Figure 1C showed that levels of phosphorylated p38, JNK2/1, and ERK1/2 were increased upon HDM exposure. Total forms of p38, JNK2/1, and ERK1/2 were not changed by HDM treatment. These results indicate that HDM activates MAPKs signaling pathway in H292 cells.
Figure 1. Interleukin-8 (IL-8) expression and activation of mitogen-activated protein kinases (MAPKs) in house dust mite (HDM)-stimulated H292 cells.
The cells were stimulated with HDM (5 μg/mL) for the indicated time periods. (A, B) Expression of IL-8 mRNA and protein levels in the medium were determined by RT-PCR analysis and ELISA, respectively. (C) Levels of phosphorylated or total form of extracellular signal-regulated kinase (ERK)1/2, c-Jun NH2-terminal kinase (JNK)2/1 and p38 were determined by Western blot analysis. The ratios of phosphorylated form to total form of ERK, JNK and p38 were determined by densitometric analysis of each protein. The density ratio at 0 minute was considered as 1. Data are expressed as mean ± SE. For each experiment, the number of samples in each group was 4. *P < 0.05 vs. 0 minute.
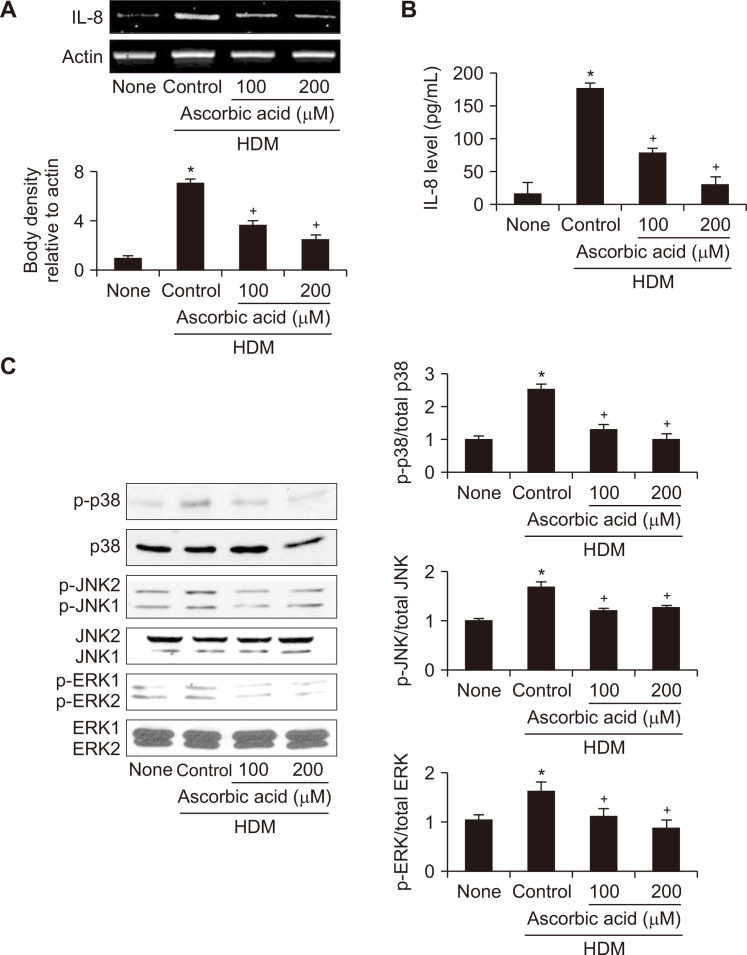
For the effect of ascorbic acid on HDM-induced IL-8 expression in H292 cells, the cells were treated with ascorbic acid and then stimulated with HDM for 2 hours (mRNA level) and 10 hours (protein release in the medium). As shown in Figure 2A and 2B, ascorbic acid concentration-dependently inhibited HDM-induced expression of IL-8 at both mRNA and protein levels, respectively in H292 cells.
Figure 2. Effect of ascorbic acid on interleukin-8 (IL-8) expression and activation of mitogen-activated protein kinases (MAPKs) in house dust mite (HDM)-stimulated H292 cells.
The cells were treated with or without ascorbic acid and stimulated with HDM (5 μg/mL) for 2 hours (A, IL-8 mRNA expression), 10 hours (B, IL-8 protein leve in medium), or 15 minutes (C, protein levels of MAPKs). (A, B) Expression of IL-8 mRNA and its protein secretion in the medium were determined by RT-PCR analysis and ELISA, respectively. (C) Levels of phosphorylated or total form of extracellular signal-regulated kinase (ERK)1/2, c-Jun NH2-terminal kinase (JNK)2/1 and p38 were determined by Western blot analysis. The ratios of phosphorylated form to total form of ERK1/2, JNK2/1 and p38 were determined by densitometric analysis of each protein. The density ratio of untreated cells (none) was set as 1. Data are expressed as mean ± SE (n = 4 per each group). *P < 0.05 vs. untreated cells (none); †P < 0.05 vs. HDM control (cell treated with HDM alone).
Figure 2C showed that HDM-induced phosphorylation of p-38, JNK2/1, and ERK1/2 was also suppressed by ascorbic acid. Total forms of MAPKs were not affected by either HDM or ascorbic acid in H292 cells. These results indicate that p38, JNK2/1, and ERK1/2 may be upstream signaling molecules responsible for HDM-induced IL-8 expression, which is reduced by ascorbic acid.
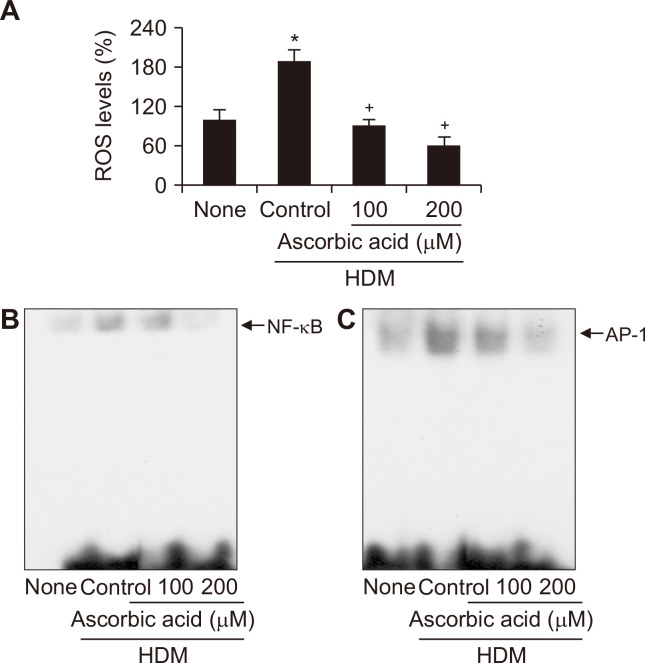
Since ROS mediate activation of transcription factors NF-κB and AP-1 which regulates induction of IL-8 expression, DNA binding activities of NF-κB or AP-1 were determined in HDM-stimulated cells in the absence or presence of ascorbic acid. As shown in Figure 3, exposure of H292 cells to HDM for 30 minutes increased ROS levels and activities of NF-κB and AP-1, which were inhibited by ascorbic acid treatment in a concentration-dependent manner. These results indicate that ascorbic acid may inhibit HDM-induced IL-8 expression by suppressing ROS-mediated activation of these transcription factors.
Figure 3. Effect of ascorbic acid on house dust mite (HDM)-induced increase in ROS and activation of NF-κB and AP-1 in H292 cells.
The cells were treated with ascorbic acid and stimulated with HDM for 30 minutes. (A) reactive oxygen species (ROS) levels were determined dichlorofluorescein (DCF) fluorescence. The level of untreated cells (none) was set as 100. Data are expressed as mean ± SE. For each experiment, the number of samples in each group was 4. (B, C) DNA-binding activities of NF-κB and AP-1 in nuclear extracts were determined by EMSA. *P < 0.05 vs. untreated cells (none); †P < 0.05 vs. HDM control (cell treated with HDM alone).
DISCUSSION
The human respiratory epithelium constitutes the lining of the airway, extending from the nasal cavity through the branching respiratory tree to the terminal air sacs of the lungs. It acts as a physical barrier by filtering and conditioning the air we breathe. Together, respiratory epithelium is a critical controller of local airway inflammation because of its capacity to synthesize a variety of mediators including cytokines [29]. HDM is an environmental cause of inflammation diseases which are originated from respiratory epithelium such as asthma. Allergens from HDM have been shown to incite the expression of several cytokines such as IL-8 in alveolar epithelial cells [30]. IL-8 expression is mediated by transcription factors such as NF-κB [11,12] and AP-1 [31,32]. It has been shown that HDM induces NF-κB-mediated IL-8 expression in lung alveolar A549 cells [33] and eosinophils [34]. Osterlund et al. [35] demonstrated that non-proteolytic HDM allergen Der p 2 (D. pteronyssinus Group 2 allergen) induced IL-8 expression in A549 cells which was dependent on NF-κB and MAPK activation. Therefore, inhibiting activation of, MAPKs, NF-κB and AP-1 may be helpful to prevent HDM-associated respirator inflammation.
Pan et al. [36] showed that HDM-induced expression of IL-6 and IL-8 cytokines was mediated via epidermal growth factor receptor (EGFR) signaling in normal bronchial epithelial BEAS-2B cells. HDM allergen Der p 1 (D. pteronyssinus Group 1 allergen) induced expression of IL-8 through protease activated receptor-2 (PAR-2) in nasal epithelial cells [14]. HDM allergen Der p 5 stimulates airway epithelial cells through a toll-like receptor 2 (TLR2) pathway [37]. These results indicate that inflammatory signaling may be dependent on HDM allergens. Recently, Zhang et al. [38] showed that HDM elevated the levels of TGF-beta3 (TGF-β3) which was accompanied by increased ROS production in airway epithelial cells. They also found that NADPH oxidase 4 (NOX4) expression was induced in TGF-β3-stimulated epithelial cells. Moreover, HDM increased ROS in a NOX4-dependent manner with concomitant enhancement of mucin secretion in epithelial cells. Therefore, inhibition of NOX4 may be one of the treatment strategies for HDM-induced respiratory inflammation. Further study should be performed to determine whether ascorbic acid suppresses NOX4, EGFR, PAR-2, or TLR2 signaling in respiratory epithelial cells exposed to allergens other than HDM.
In summary, our present study demonstrates that HDM-induced IL-8 expression is mediated with MAPKs (p-38, JNK, and ERK), NF-κB and AP-1 in human respiratory epithelial H292 cells. Ascorbic acid suppressed HDM-induced expression of IL-8 in H292 cells by inhibiting phosphorylation of MAPKs, and suppressing NF-κB and AP-1 activation in H292 cells. Reducing ROS by ascorbic acid may inhibit the activation of MAPKs and transcription factors NF-κB and AP-1 to induce IL-8 expression in respiratory epithelial cells. Therefore, consumption of ascorbic acid-rich foods may be beneficial for preventing HDM-associated respiratory inflammation.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100(6 Pt 1):S2–24. doi: 10.1016/S0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- 2.Ree HI, Jeon SH, Lee IY, Hong CS, Lee DK. Fauna and geographical distribution of house dust mites in Korea. Korean J Parasitol. 1997;35:9–17. doi: 10.3347/kjp.1997.35.1.9. [DOI] [PubMed] [Google Scholar]
- 3.Robinson C, Kalsheker NA, inivasan N, Sr, King CM, Garrod DR, Thompson PJ, et al. On the potential significance of the enzymatic activity of mite allergens to immunogenicity. Clues to structure and function revealed by molecular characterization. Clin Exp Allergy. 1997;27:10–21. doi: 10.1111/j.1365-2222.1997.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 4.Yi MH, Kim HP, Jeong KY, Kim CR, Kim TY, Yong TS. House dust mite allergen Der f 1 induces IL-8 in human basophilic cells via ROS-ERK and p38 signal pathways. Cytokine. 2015;75:356–64. doi: 10.1016/j.cyto.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Lee HY, Lee GH, Kim HK, Chae HJ. Platycodi Radix and its active compounds ameliorate against house dust mite-induced allergic airway inflammation and ER stress and ROS by enhancing anti-oxidation. Food Chem Toxicol. 2019;123:412–23. doi: 10.1016/j.fct.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Fukunaga M, Gon Y, Nunomura S, Inoue T, Yoshioka M, Hashimoto S, et al. Protease-mediated house dust mite allergen-induced reactive oxygen species production by neutrophils. Int Arch Allergy Immunol. 2011;155 Suppl 1:104–9. doi: 10.1159/000327492. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura T, Matsushima K, Oppenheim JJ, Leonard EJ. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1) J Immunol. 1987;139:788–93. [PubMed] [Google Scholar]
- 8.Marini M, Vittori E, Hollemborg J, Mattoli S. Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J Allergy Clin Immunol. 1992;89:1001–9. doi: 10.1016/0091-6749(92)90223-O. [DOI] [PubMed] [Google Scholar]
- 9.Bellini A, Yoshimura H, Vittori E, Marini M, Mattoli S. Bronchial epithelial cells of patients with asthma release chemoattractant factors for T lymphocytes. J Allergy Clin Immunol. 1993;92:412–24. doi: 10.1016/0091-6749(93)90120-5. [DOI] [PubMed] [Google Scholar]
- 10.Hollander C, Sitkauskiene B, Sakalauskas R, Westin U, Janciauskiene SM. Serum and bronchial lavage fluid concentrations of IL-8, SLPI, sCD14 and sICAM-1 in patients with COPD and asthma. Respir Med. 2007;101:1947–53. doi: 10.1016/j.rmed.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990;265:21128–33. doi: 10.1016/S0021-9258(17)45336-1. [DOI] [PubMed] [Google Scholar]
- 12.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol. 1994;56:554–8. doi: 10.1002/jlb.56.5.554. [DOI] [PubMed] [Google Scholar]
- 13.Barnes PJ, Karin M. Nuclear factor-kB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 14.Shi J, Luo Q, Chen F, Chen D, Xu G, Li H. Induction of IL-6 and IL-8 by house dust mite allergen Der p1 in cultured human nasal epithelial cells is associated with PAR/PI3K/NFkB signaling. ORL J Otorhinolaryngol Relat Spec. 2010;72:256–65. doi: 10.1159/000312687. [DOI] [PubMed] [Google Scholar]
- 15.Bossios A, Gourgiotis D, Skevaki CL, Saxoni-Papageorgiou P, Lötvall J, Psarras S, et al. Rhinovirus infection and house dust mite exposure synergize in inducing bronchial epithelial cell interleukin-8 release. Clin Exp Allergy. 2008;38:1615–26. doi: 10.1111/j.1365-2222.2008.03058.x. [DOI] [PubMed] [Google Scholar]
- 16.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–80. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 17.Sohn MH, Lee KE, Choi SY, Kwon BC, Chang MW, Kim KE. Effect of Mycoplasma pneumoniae lysate on interleukin-8 gene expression in human respiratory epithelial cells. Chest. 2005;128:322–6. doi: 10.1016/S0012-3692(15)37964-2. [DOI] [PubMed] [Google Scholar]
- 18.Lee KE, Kim JW, Jeong KY, Kim KE, Yong TS, Sohn MH. Regulation of German cockroach extract-induced IL-8 expression in human airway epithelial cells. Clin Exp Allergy. 2007;37:1364–73. doi: 10.1111/j.1365-2222.2007.02797.x. [DOI] [PubMed] [Google Scholar]
- 19.Monick M, Staber J, Thomas K, Hunninghake G. Respiratory syncytial virus infection results in activation of multiple protein kinase C isoforms leading to activation of mitogen-activated protein kinase. J Immunol. 2001;166:2681–7. doi: 10.4049/jimmunol.166.4.2681. [DOI] [PubMed] [Google Scholar]
- 20.Sohn MH, Lee KE, Kim KW, Kim ES, Park JY, Kim KE. Calcium-calmodulin mediates house dust mite-induced ERK activation and IL-8 production in human respiratory epithelial cells. Respiration. 2007;74:447–53. doi: 10.1159/000099264. [DOI] [PubMed] [Google Scholar]
- 21.Shen GN, Wang C, Luo YH, Wang JR, Wang R, Xu WT, et al. 2-(6-Hydroxyhexylthio)-5,8-dimethoxy-1,4-naphthoquinone induces apoptosis through ROS-mediated MAPK, STAT3, and NF-κB signalling pathways in lung cancer A549 cells. Evid Based Complement Alternat Med. 2020;2020:7375862. doi: 10.1155/2020/7375862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mata M, Pallardo F, Morcillo EJ, Cortijo J. Piclamilast inhibits the pro-apoptotic and anti-proliferative responses of A549 cells exposed to H2O2 via mechanisms involving AP-1 activation. Free Radic Res. 2012;46:690–9. doi: 10.3109/10715762.2012.669040. [DOI] [PubMed] [Google Scholar]
- 23.Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980. doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;8:CD005532. doi: 10.1002/14651858.CD005532.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Anderson R, Hay I, van Wyk H, Oosthuizen R, Theron A. The effect of ascorbate on cellular humoral immunity in asthmatic children. S Afr Med J. 1980;58:974–7. [PubMed] [Google Scholar]
- 26.Tongtako W, Klaewsongkram J, Mickleborough TD, Suksom D. Effects of aerobic exercise and vitamin C supplementation on rhinitis symptoms in allergic rhinitis patients. Asian Pac J Allergy Immunol. 2018;36:222–31. doi: 10.12932/AP-040417-0066. [DOI] [PubMed] [Google Scholar]
- 27.Matsukura S, Kokubu F, Noda H, Tokunaga H, Adachi M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J Allergy Clin Immunol. 1996;98(6 Pt 1):1080–7. doi: 10.1016/S0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- 28.Park Y, Lee H, Lim JW, Kim H. Inhibitory effect of β-carotene on Helicobacter pylori-Induced TRAF expression and hyper-proliferation in gastric epithelial cells. Antioxidants (Basel) 2019;8:637. doi: 10.3390/antiox8120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holgate ST, Lackie PM, Davies DE, Roche WR, Walls AF. The bronchial epithelium as a key regulator of airway inflammation and remodelling in asthma. Clin Exp Allergy. 1999;29 Suppl 2:90–5. doi: 10.1046/j.1365-2222.1999.00016.x. [DOI] [PubMed] [Google Scholar]
- 30.Tomee JF, van Weissenbruch R, de Monchy JG, Kauffman HF. Interactions between inhalant allergen extracts and airway epithelial cells: effect on cytokine production and cell detachment. J Allergy Clin Immunol. 1998;102:75–85. doi: 10.1016/S0091-6749(98)70057-0. [DOI] [PubMed] [Google Scholar]
- 31.Trevino JG, Gray MJ, Nawrocki ST, Summy JM, Lesslie DP, Evans DB, et al. Src activation of Stat3 is an independent requirement from NF-kB activation for constitutive IL-8 expression in human pancreatic adenocarcinoma cells. Angiogenesis. 2006;9:101–10. doi: 10.1007/s10456-006-9038-9. [DOI] [PubMed] [Google Scholar]
- 32.Kim YM, Reed W, Wu W, Bromberg PA, Graves LM, Samet JM. Zn2+-induced IL-8 expression involves AP-1, JNK, and ERK activities in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1028–35. doi: 10.1152/ajplung.00479.2005. [DOI] [PubMed] [Google Scholar]
- 33.Liu R, Bai J, Xu G, Xuan L, Zhang T, Meng A, et al. Multi-allergen challenge stimulates steriod-resistant airway inflammation via NF-κB-mediated IL-8 expression. Inflammation. 2013;36:845–54. doi: 10.1007/s10753-013-9611-5. [DOI] [PubMed] [Google Scholar]
- 34.Coward WR, Sagara H, Wilson SJ, Holgate ST, Church MK. Allergen activates peripheral blood eosinophil nuclear factor-kappaB to generate granulocyte macrophage-colony stimulating factor, tumour necrosis factor-alpha and interleukin-8. Clin Exp Allergy. 2004;34:1071–8. doi: 10.1111/j.1365-2222.2004.02003.x. [DOI] [PubMed] [Google Scholar]
- 35.Osterlund C, Grönlund H, Polovic N, Sundström S, Gafvelin G, Bucht A. The non-proteolytic house dust mite allergen Der p 2 induce NF-kB and MAPK dependent activation of bronchial epithelial cells. Clin Exp Allergy. 2009;39:1199–208. doi: 10.1111/j.1365-2222.2009.03284.x. [DOI] [PubMed] [Google Scholar]
- 36.Pan HH, Hsiao YP, Chen PJ, Kang YT, Chao YH, Sheu JN, et al. Epithelial growth factor receptor tyrosine kinase inhibitors alleviate house dust mite allergen Der p2-induced IL-6 and IL-8. Environ Toxicol. 2019;34:476–85. doi: 10.1002/tox.22701. [DOI] [PubMed] [Google Scholar]
- 37.Pulsawat P, Soongrung T, Satitsuksanoa P, Le Mignon M, Khemili S, Gilis D, et al. The house dust mite allergen Der p 5 binds lipid ligands and stimulates airway epithelial cells through a TLR2-dependent pathway. Clin Exp Allergy. 2019;49:378–90. doi: 10.1111/cea.13278. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Tang HM, Liu CF, Yuan XF, Wang XY, Ma N, et al. TGF-β3 induces autophagic activity by increasing ROS generation in a NOX4-dependent pathway. Mediators Inflamm. 2019;2019:3153240. doi: 10.1155/2019/3153240. [DOI] [PMC free article] [PubMed] [Google Scholar]