Abstract
Hydrogen sulfide (H2S), the third gas signal molecule, is associated with the modulation of various physiological and pathological processes. Recent studies have reevealed that endogenous H2S may promote proliferation, induce angiogenesis and inhibit apoptosis, thereby stimulating oncogenesis. Conversely, decreased endogenous H2S release suppresses growth of various tumors including breast cancer. This observation suggests an alternative tumor therapy strategy by inhibiting H2S-producing enzymes to reduce the release of endogenous H2S. Breast cancer is the most common type of cancer in women. Due to the lack of approved targeted therapy, its recurrence and metastasis still affect its clinical treatment. In recent years, significant progress has been made in the control of breast cancer by using inhibitors on H2S-producing enzymes. This review summarized the roles of endogenous H2S-producing enzymes in breast cancer and the effects of the enzyme inhibitors on anticancer and anti-metastasis, with the aim of providing new insights for the treatment of breast cancer.
Keywords: breast cancer, endogenous hydrogen sulfide, anticancer effect
1. Introduction
Hydrogen sulfide (H2S) is an endogenous gasotransmitter produced by mammalian tissues and cells that express three enzymes, namely cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST) (1–3). H2S, together with nitric oxide (NO) and carbon monoxide (CO), constitute a family of endogenous gases that participate in modulating multiple physiological and pathological processes (4–7). In particular, they exhibit pleiotropic and often dose-dependent effects on a variety of diseases, including immunoinflammatory, autoimmune diseases and cancers (8–12). Therefore, their therapeutic potential has recently received increasing attention and some compounds capable of inhibiting or stimulating the synthesis or promoting the release of these gases have been developed in preclinical and clinical setting. Examples include NO-releasing drugs such as NO-aspirin, JS-K and NO-derivatives of antiretroviral protease inhibitors. They are mainly utilized for research on the prevention and treatment of cancer (13–17). In terms of immunoinflammatory and autoimmune diseases (18–20), CO-releasing drugs have exhibited certain efficacy. For numerous years, H2S has been considered a health concern, and it is physiologically beneficial at low concentrations while toxic at high doses (21). However, previous evidence acquired at both preclinical and clinical settings demonstrated that H2S donor compounds such as H2S-naproxene have potential anti-inflammatory effects, highlighting an anti-inflammatory potential of H2S (22). In cancer cells, H2S donors have exhibited a beneficial chemotherapeutic action in a manner that depends on H2S donor doses and cancer status. In other words, the biological response of H2S donors follows a biphasic dose effect, which is characterized by cytoprotective or cytotoxic effects in cancer, that is, low levels of exogenous H2S can induce oncogenesis, while high concentrations of exogenous H2S production can prevent the development of tumors (23–26). Considering that the presence of endogenous H2S can also induce tumorigenesis (22), inhibitors have been developed to prevent the production of endogenous H2S and proven effective in anti-cancer treatment (27–29). This review summarized the biological effects of endogenous H2S related to breast cancer cell biology, to review the experimental evidence on the role of endogenous cancer cell-derived H2S in breast cancer biology, and to outline the therapeutic potential of cystathionine-β-synthase (CSE) or cystathionine-γ-lyase (CBS) inhibition for breast cancer therapy.
2. Endogenous H2S-producing enzymes
In mammalian tissues and cells, three enzymes, two cytosolic enzymes CBS and CSE, and one mitochondrial enzyme 3-mercaptopyruvate sulfurtransferase (3MST) control the synthesis of H2S (30) (Fig. 1). In addition, they participate in the progression of various cancers (31).
Figure 1.
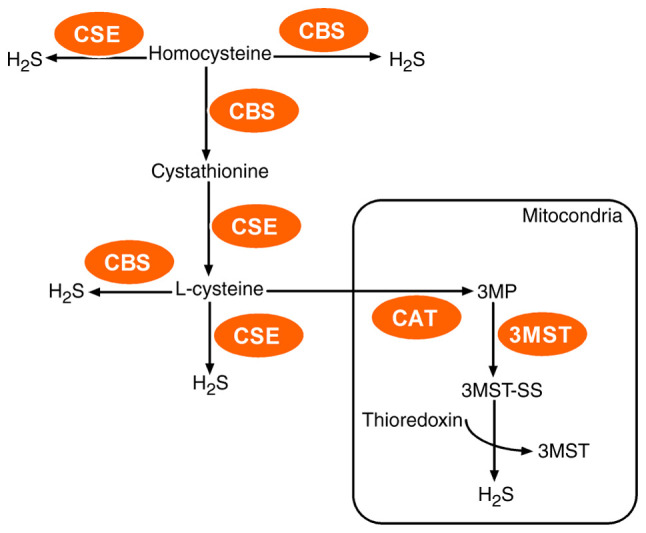
Enzymatic pathways of endogenous H2S production in mammalian systems. CBS and CSE may produce H2S in the cytosol whereas 3MST mainly generates H2S in mitochondria. These pathways utilize l-cysteine as a main precursor of H2S. H2S, hydrogen sulfide; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; 3MST, 3-mercaptopyruvate sulfurtransferase; CAT, cysteine aminotransferase; 3MP, 3-mercaptopyruvate.
Cystathionine-β-synthase (CBS)
CBS is the first enzyme in the trans-sulfuration pathway and produces H2S mainly through catalyzing homocysteine and cysteine. In the presence of homocysteine, CBS catalyzes its condensation with serine to form cystathionine, which is then cleaved by cystathionine γ-lyase (CTH) to form cysteine (32,33); CBS continues to catalyze it to generate H2S through β-replacement reaction accompanied by the generation of serine.
CBS protein consists of four subunits and each subunit contains three domains (34). The N-terminal domain binds to a cofactor heme, which is responsible for successful protein folding. The C-terminal CBS1 and CBS2 domains bind to S-adenosylmethionine (SAM), which are responsible for CBS subunit tetramerization (33). The activity of CBS is mainly regulated by the modification of the heme group at the N-terminus and the binding of SAM at the C-terminus on the protein. The modification of the Fe (II) form of the heme caused by NO inhibits CBS activity and H2S generation (35) while SAM binding activates CBS thereby increasing H2S production (36).
CBS is predominantly expressed in the brain, liver, kidney, and pancreas and consequently exerts multiple biological and pathological functions in the cardiovascular, immune and central nervous systems by regulating the homocysteine and H2S metabolism (37). Notably, compared to adjacent normal tissue or non-transformed cells, CBS expression was increased in tumor tissues and cell lines including breast cancer, suggesting its participation in the process of cancer (38–41), therefore, the overexpression of CBS may be an important factor in the development of tumors.
Cystathionine-γ-lyase (CSE)
Similar to CBS, CSE also utilizes homocysteine and cysteine as substrates to generate H2S along with the production of by-products such as α-ketobutyrate, pyruvate and ammonia (37). The relative concentrations of homocysteine and cysteine determine the primary substrate for CSE to produce H2S in mammalian cells. At physiological concentrations of homocysteine and cysteine, CSE contributes approximately 70% of the total H2S content from cysteine, whereas approximately 90% of H2S is derived from homocysteine when its level increases to a level comparable to hyperhomocysteinemia (42).
Structurally, CSE protein is a tetramer composed of two dimers and each monomer binds one pyridoxal phosphate (PLP) (43). Analysis of genetic variations of the CSE-encoding genes has revealed a large number of polymorphisms. CSE activity is influenced by the intracellular Ca2+ concentration: A low level induces H2S production whereas a high level suppresses CSE activity (44). However, the exact mechanism of Ca2+-mediated regulation of CSE activity remains to be further studied.
CSE is broadly expressed in tissues such as the liver, kidney, uterus, pancreatic islets and cardiovascular system as well as the respiratory system (45–47). However, overexpression of CSE genes in cells leads to increased production of H2S, and consequently induces vasorelaxation, and stimulates endothelial cell-related angiogenic properties (48,49). In terms of mechanism, CSE gene expression is enhanced by estradiol (E2) through ER-Sp1 interaction with the binding sites in CSE gene promoter and consequently increases endothelial H2S release (50,51). Moreover, recent studies have also revealed that E2 nongenomically induced vascular endothelial H2S release by promoting CSE phosphorylation (52). Notably, CSE expression in breast cancer tissues and cells was increased when compared with adjacent normal tissue and cells, and this promoted the process of breast cancer (53,54).
3MST
3MST also utilizes cysteine as substrate to generate H2S. Here, cysteine aminotransferase (CAT) firstly converts cysteine into 3-mercaptopyruvate (3MP). Under the action of MST3, MP then transfers a sulfur atom onto 3MST, eventually in the presence of reductant such as thioredoxin, resulting in the formation of persulfide and the release of H2S (55).
3MST activity is intrinsically regulated by its redox state and three redox-sensitive cysteines (Cys154, Cys247 and Cys263) which locate at the catalytic site of the enzyme (56). Studies have shown that oxidative stress could significantly suppress the activity of 3MST (57).
3MST is also expressed in numerous tissues such as myocardial, liver, kidney as well as brain, and consequently exhibits some biological and biochemical features such as its partial mitochondrial localization and its ability to produce polysulfides (58–60). Recent data revealed its potential role in cancer biology since it is upregulated in colon adenocarcinoma, lung adenocarcinoma and various forms of oral carcinomas when compared to the surrounding normal tissues. Emerging data using 3MST silencing approaches or pharmacological inhibitors of 3MST suggest that the 3MST/H2S system plays a role in maintaining cancer cell proliferation and regulating bioenergetic functions (61).
3. Role of the CBS/H2S system in breast cancer
CBS expression in tumor tissues and cell lines has been reported to be increased in colon, ovarian, and prostate (38–41), compared to adjacent normal tissue or non-transformed cells. Similar findings have also been observed in breast cancer, where CBS-derived H2S was revealed to protect breast cancer cells from the attack of microphages. Furthermore, silencing of CBS in the cells significantly attenuated tumor growth in a xenograft model (62) while overexpressing CBS in human breast cancer resulted in cystathionine accumulation, which protected human breast cancer cells against excess reactive oxygen species (ROS) and chemotherapeutic drug-induced apoptosis (63). In addition, the existence of a large number of mutations and polymorphisms modifies the functions of the CBS gene (64). For instance, 844ins68 polymorphism in the CBS gene can not only alter the stability of a domain or residue in the hydrophobic core, leading to protein degradation, but also cause the increase of plasma homocysteine and methionine, leading to genomic DNA hypomethylation (65), ultimately impacting breast cancer oncogenesis (66–68), which further consolidated the pro-cancer effect of CBS in human breast cancer.
Collectively, it has been revealed that CBS overexpression significantly contributes to the pathogenesis of breast cancer cells, and CBS silencing can exert significant antitumor effects in vitro and in vivo. Based on these findings, it has been proposed that the pharmacological inhibitory effect of CBS can confer CBS an antitumor therapeutic potential. The role of the CBS/H2S axis in breast cancer is presented in Fig. 2.
Figure 2.
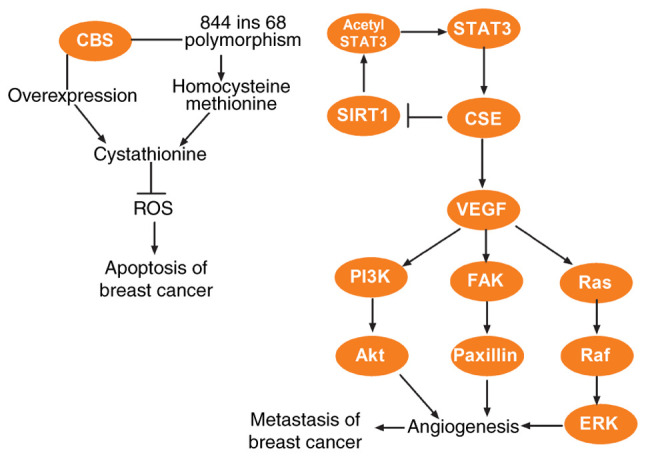
Roles and mechanisms of CBS/H2S and CSE/H2S system in breast cancer. The overexpression of CBS and CBS gene polymorphism in human breast cancer results in the accumulation of cystathionine, which protects human breast cancer cells against excess ROS and chemotherapeutic drug-induced apoptosis. STAT3 may promote CSE expression via activation of the CSE promoter and CSE could reversely regulate STAT3 expression via the SIRT1/acetyl STAT3 pathway and consequently enhance the effect of STAT3 on CSE. CSE also may promote the metastasis of breast cancer via the VEGF signaling pathway. CBS, cystathionine β-synthase; H2S, hydrogen sulfide; CSE, cystathionine γ-lyase; ROS, reactive oxygen species; SIRT1, sirtuin 1; STAT3, signal transducer and activator of transcription 3; VEGF, vascular endothelial growth factor; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; FAK, focal adhesion kinase; Ras, rat sarcoma; Raf, rapidly accelerated fibrosarcoma; ERK, extracellular signal-regulated protein kinase.
4. Role of CSE/H2S axis in breast cancer
The role and functional mechanism of CSE in breast cancer reveal a variety of characteristics. CSE expression has been revealed to be upregulated in both breast cancer tissues and breast cancer cell lines, resulting in proliferation and migration of breast cancer cells (53,54). A previous study has revealed that the role of CSE in breast cancer leading to breast cancer development is associated with the STAT3 signaling pathway, specifically, STAT3 binds to and activates the CSE promoter to stimulate CSE expression (53). Moreover, CSE could reversely regulate STAT3 expression and consequently enhance the effect of STAT3 on CSE (53). Clinically, it was revealed that CSE expression in samples of breast cancer patients with lymph node metastasis was higher than in breast cancer patients without lymph node metastasis (54). Compared with non-metastatic MCF7 breast cancer cells, early metastatic MDA-MB-231 breast cancer cells demonstrated higher mRNA and protein levels of CSE (54). These findings indicate that the metastasis of human breast cancer may be related to the increased expression levels of CSE, and Wang et al revealed that CSE promoted the metastasis of breast cancer through the VEGF signaling pathway (54). Studies in tamoxifen or doxorubicin-resistant MCF-7 cells reveled an additional role for CSE (69,70). Cysteine consumption was revealed to increase with the addition of CSE specific inhibitor propargylglycine, and the consumption resulted in cytotoxicity after sulfur amino acid deprivation, suggesting that inhibition of sulfur amino acid metabolism could affect the viability of tamoxifen-resistant MCF-7 cells, particularly the cysteine synthesis from homocysteine catalyzed by CSE protein (69). The role of the CSE/H2S axis in breast cancer is presented in Fig. 2.
5. Effect of CBS inhibition in breast cancer
Aminooxyacetic acid (AOAA) and its methylated derivative
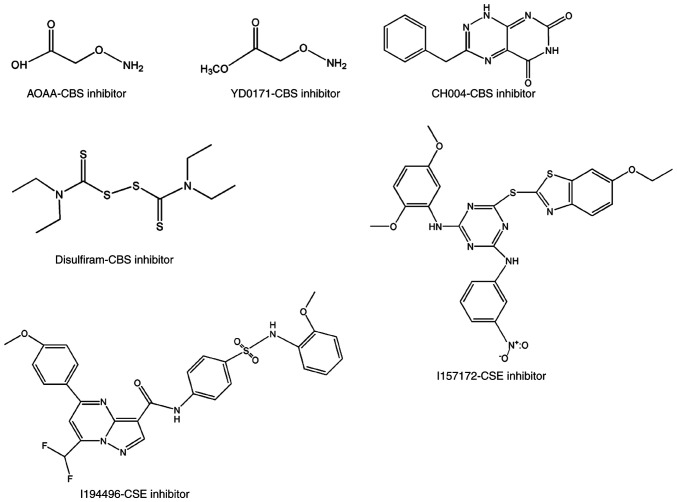
Consistent with the roles of CBS in breast cancer biology aforementioned, CBS inhibitors have exhibited great potential in breast cancer therapy. An example is AOAA (Fig. 3), a classical CBS inhibitor. Its inhibitory effect on breast cancer has been observed in breast cancer cells and BALB/c nude mice bearing MDA-MB-231 human breast cancer subcutaneous xenografts (71). Its mechanism of action has been revealed to be related to the suppression of tumor cell bioenergetics, especially due to AOAA-mediated inhibition of tumor cell aspartate aminotransferase activity (71). In a previous study, a methylated derivative of AOAA, YD0171 (Fig. 3), was also revealed to be active. It exhibited higher potency in inhibiting the growth of cancer cells in vitro and in vivo (72). However, whether AOAA has sufficient therapeutic effect for breast cancer requires further investigation. Not only is its effect on non-cancerous cells not clear, but it also irreversibly binds to the cofactor PLP. Therefore, in addition to inhibiting CBS, it has been revealed to also inhibit other PLP-dependent enzymes such as CTH, 3-MST, and glutamate oxaloacetate transaminase 1 (GOT1) (73,74). All of these pose high challenges to the development of selective CBS inhibitors as therapeutic drugs.
Figure 3.
Pharmacological inhibitors of CBS and CSE with anti-breast cancer activity. AOAA, YD0171, CH004 and disulfiram are CBS pharmacological inhibitors and possess anti-breast cancer activity. I157172 and I194496 are CSE pharmacological inhibitors and possess anti-breast cancer activity. CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; AOAA, aminooxyacetic acid.
Selective CBS inhibitors
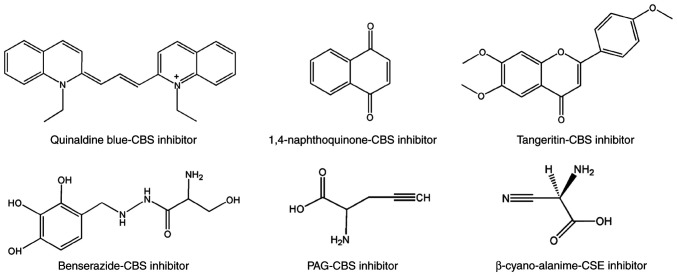
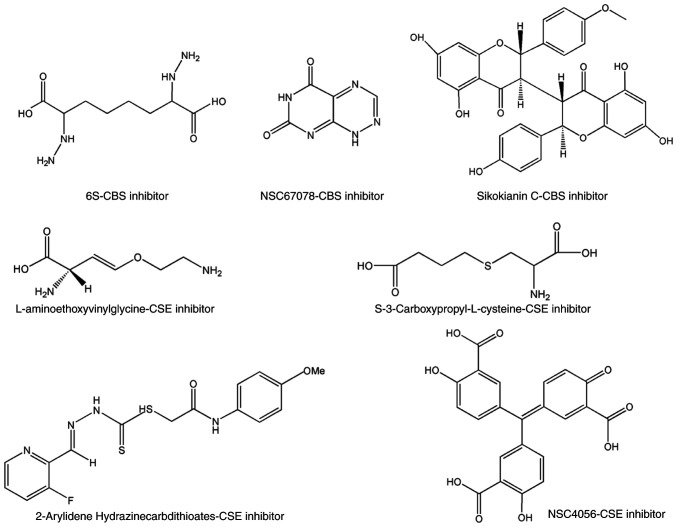
To identify novel selective CBS inhibitors, several groups pursued screening tests and determined that some compounds can inhibit CBS (75–78). Using a tandem-microwell assay, Zhou et al in 2013 (75) revealed a hit, 3-benzyl-1,6-dimethylpyrimido(5,4-e)(1,2,4)triazine-5,7(1H,6H)-dione, naming it CH004 (Fig. 3). which was the focus of a follow-up study published in 2018 (76). The IC50 of CH004 for CBS was <1 µM and its selectivity for CBS was ~30 times higher than that for CSE, thus it may be the most effective CBS inhibitor to date (76). Moreover, CH004 was revealed to possess anti-breast cancer effects in CBS highly-expressed breast cancer cells, with IC50 values in the range of 10–20 µM (77). Using a yeast-based screening model, Marechal et al identified disulfiram (Fig. 3) as a putative inhibitor of cellular CBS activity (78). Despite its pattern of action being unclear, disulfiram has been developed as a potential anticancer drug in multiple tumor types, including breast cancer (79–83). Some therapeutic protocols that include disulfiram as part of combination therapy have also been used in clinical trials (84–86). Quinaldine blue (Fig. 4), an antitumor drug approved by the FDA, may also be a CBS selective inhibitor since it has been revealed to inhibit tumor growth by selectively suppressing the activity of CBS and has a preference to inhibit CBS over CSE (87). Applying an H2S probe-based assay onto a chemical library containing 1,900 compounds (88), Thorson et al revealed that 1,4-naphthoquinone and tangeritin (Fig. 4) could selectively suppress CBS activity without affecting CSE activity. They further demonstrated that both compounds possessed potential anticancer activities. Compound benserazide (Fig. 4) exhibited CBS-inhibitory activity in both a tandem-microwell screening assay and an H2S probe-based screening assay (75,88). Although it is more effective than AOAA in weakening cellular bioenergetics and proliferation rate in colorectal cancer cell lines (89), its role in breast cancer is unclear. Some other selective inhibitors of CBS that have begun to attract attention in cancer biology, include 6S, NSC67078 and Sikokianin C (Fig. 5) (90,91), however, their effects on cancer cell proliferation require further elucidation.
Figure 4.
Pharmacological inhibitors of CBS and CSE with anticancer activity in other tumors, but no reports yet in breast cancer. These inhibitors of CBS or CSE possess anticancer activities in gastric, liver and colon cancer as well as other cancer tissues, but their roles in breast cancer have yet to be reported. CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; PAG, propargylglycine.
Figure 5.
Inhibitors of CBS and CSE without reports yet in cancer. These compounds possess the inhibitory activity of CBS or CSE, but their roles in cancer have yet to be reported. CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase.
6. Effect of CSE inhibition in breast cancer
Propargylglycine (PAG), β-cyanoalanine (BCA) and L-aminoethoxyvinylglycine (AVG)
PAG, BCA (Fig. 4) and AVG (Fig. 5) are three classical CSE inhibitors. Despite BCA being more potent than PAG, both BCA and PAG could significantly suppress the proliferation of human gastric cancer AGS cells in a concentration-dependent manner (92). However, the role of PAG and BCA in breast cancer has not been reported. Similar to PAG, AVG has been revealed to selectively inhibit CSE only at a markedly lower concentration (74). Its role in breast cancer remains unknown.
Other selective CSE inhibitors
To identify novel selective CSE inhibitors, several groups screened and confirmed that certain compounds can inhibit CSE. Using a tandem-well-based high-throughput assay, NSC4056 (Fig. 5), also known as aurintricarboxylic acid, was identified as the most potent inhibitor with an IC50 of 0.6 µM for CSE. It was revealed to selectively bind to Arg and Tyr residues in the active site of CSE, and preferably inhibit the CSE activity in cells but did not inhibit CBS (93). Another compound 2-arylidene hydrazinecarbodithioates (Fig. 5) was revealed to be more active than the benchmark inhibitors, and notably, it had higher selectivity for CSE compared to CBS (94). S-3-carboxpropyl-L-cysteine (CPC) (Fig. 5), a new CSE inhibitor, inhibited the γ-elimination reaction of cystathionine and H2S synthesis from cysteine by human CSE (95). However, the inhibition did not depend on the order of substrate/inhibitor addition. Currently, there remains a lack of studies on NSC4056 and 2-arylidene hydrazinecarbodithioates as well as CPC in the context of cancer therapy.
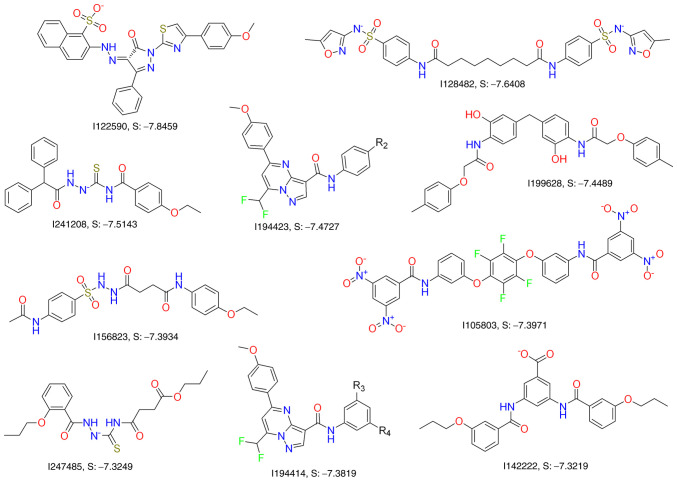
In our previous study (96), a virtual screening method was used to search for CSE inhibitors based on the crystal structure of the CSE protein. MOE Dock (Chemical Computing Group ULC) was further used to simulate the hits and predict their docking affinity with the homology model. Among the final top 12 candidates selected (Fig. 6), I157172 (Fig. 3) had the lowest binding score of-7.9215 and the highest binding affinity. I157172 inhibited the growth, proliferation and migration of breast cancer cells via upregulation of SIRT1, which consequently mediated deacetylation of STAT3 and inactivation of the STAT3 pathway (93). In addition, I157172 inhibited the metastasis of MDA-MB-231 cells via downregulation of the expression of VEGF and numerous of its downstream key proteins, including PI3K, Akt, pAkt, FAK, Paxillin, Raf and pERK1/2, and this may be one of the underlying molecular mechanisms by which CSE inhibition promotes breast cancer metastasis (96). I194496 (Fig. 3) was the second most effective CSE inhibitor with a binding score of-7.9042. Our recent study revealed that I194496 could inhibit the growth of human TNBC cells via dual targeting of the PI3K/Akt and Ras/Raf/ERK pathways and suppress the metastasis of human TNBC cells via downregulation of the Anxa2/STAT3 and VEGF/ FAK/Paxillin signaling pathways (unpublished data).
Figure 6.
Inhibitors of CSE obtained by virtual screening methods. These compounds are obtained by virtual screening methods according to the crystal structure of the CSE protein. Their roles in cancer remain to be further studied. CSE, cystathionine γ-lyase.
7. Conclusions and perspectives
Currently, the treatment of breast cancer is mainly postoperative adjuvant chemotherapy or radiotherapy. Patients not only have to endure the side effects of long-term chemotherapy or radiotherapy, but also have to bear the risk of metastasis and recurrence. Therefore, targeted therapy is particularly important for breast cancer. Recently, endogenous gas transmitter H2S has been revealed to be responsible for breast cancer development. Accordingly, study of the mammalian enzymes responsible for H2S production has become an attractive strategy in breast cancer therapy. Although the overexpression of CBS and CSE significantly promoted the pathogenesis of breast cancers, the exact mechanism of CBS and CSE remains poorly understood, as well as the role of 3-MST expression in breast cancer. In view of the evidence that inhibition of CBS can prevent proliferation of breast cancer and induce apoptosis of breast cancer, inhibiting H2S biosynthesis by targeted H2S-producing enzymes may confer antitumor effects.
Regarding the pharmacological inhibitors CBS and CSE, this review provided a historical background and the latest pharmacological information regarding small molecules called ‘CBS inhibitors’ and ‘CSE inhibitors’. It is emphasized, that currently known compounds can only be used with great caution to study the biological roles of CBS and CSE. Numerous of the compounds are not ideal or remain to be assessed in terms of anti-breast cancer properties. To advance CBS and CSE inhibitors into clinical trials, not only is a comprehensive study required to improve the pharmacological properties of these molecules, but also the mechanism regarding the sensitivity of CBS and CSE inhibitors to breast cancer requires further exploration. It is anticipated that this review on the inhibitory effects of endogenous H2S on breast cancer can stimulate further development in this field.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 82072726), as well as the Natural Science Foundation of Henan Province in China (grant no. 202300410079).
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 82072726), as well as the Natural Science Foundation of Henan Province in China (grant no. 202300410079).
Availability of data and materials
Not applicable.
Authors' contributions
ML, HS and TW conceived the subject of the review. TW designed the review. ML, YL, YD and LP wrote the manuscript, performed the literature research as well as interpreted the relevant literature. HF, XH and YL prepared the figures and analyzed the review critically for important intellectual content. ML and YL edited and revised the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Szabo C. Gaseotransmitters: New frontiers for translational science. Sci Transl Med. 2010;2:59ps54. doi: 10.1126/scitranslmed.3000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal. 2014;20:770–782. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura H. Hydrogen sulfide: Its production, release and functions. Amino Acids. 2011;41:113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 4.Mani S, Untereiner A, Wu L, Wang R. Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid Redox Signal. 2014;20:805–817. doi: 10.1089/ars.2013.5324. [DOI] [PubMed] [Google Scholar]
- 5.Wu D, Wang J, Li H, Xue M, Ji A, Li Y. Role of hydrogen sulfifide in ischemia-reperfusion injury. Oxid Med Cell Longev. 2015;2015:186908. doi: 10.1155/2015/186908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 7.Wang R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 8.Fagone P, Mazzon E, Bramanti P, Bendtzen K, Nicoletti F. Gasotransmitters and the immune system: Mode of action and novel therapeutic targets. Eur J Pharmacol. 2018;834:92–102. doi: 10.1016/j.ejphar.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Mintz J, Vedenko A, Rosete O, Shah K, Goldstein G, Hare JM, Ramasamy R, Arora H. Current advances of nitric oxide in cancer and anticancer therapeutics. Vaccines (Basel) 2021;9:94. doi: 10.3390/vaccines9020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 11.Lazarević M, Battaglia G, Jevtić B, Đedović N, Bruno V, Cavalli E, Miljković Đ, Nicoletti F, Momčilović M, Fagone P. Upregulation of tolerogenic pathways by the hydrogen sulfide donor GYY4137 and impaired expression of H2S-producing enzymes in multiple sclerosis. Antioxidants (Basel) 2020;9:608. doi: 10.3390/antiox9070608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarević M, Mazzon E, Momčilović M, Basile MS, Colletti G, Petralia MC, Bramanti P, Nicoletti F, Miljković Đ. The H2S donor GYY4137 stimulates reactive oxygen species generation in BV2 cells while suppressing the secretion of TNF and nitric oxide. Molecules. 2018;23:2966. doi: 10.3390/molecules23112966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao L, Williams JL. Nitric oxide-donating aspirin induces G2/M phase cell cycle arrest in human cancer cells by regulating phase transition proteins. Int J Oncol. 2012;41:325–330. doi: 10.3892/ijo.2012.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuhashi S, Sugita H, Takamori H, Horino K, Nakahara O, Okabe H, Miyake K, Tanaka H, Beppu T, Baba H. NO donor and MEK inhibitor synergistically inhibit proliferation and invasion of cancer cells. Int J Oncol. 2012;40:807–815. doi: 10.3892/ijo.2011.1243. [DOI] [PubMed] [Google Scholar]
- 15.McMurtry V, Saavedra JE, Nieves-Alicea R, Simeone AM, Keefer LK, Tari AM. JS-K, a nitric oxide-releasing prodrug, induces breast cancer cell death while sparing normal mammary epithelial cells. Int J Oncol. 2011;38:963–971. doi: 10.3892/ijo.2011.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maksimovic-Ivanic D, Fagone P, McCubrey J, Bendtzen K, Mijatovic S, Nicoletti F. HIV-protease inhibitors for the treatment of cancer: Repositioning HIV protease inhibitors while developing more potent NO-hybridized derivatives? Int J Cancer. 2017;140:1713–1726. doi: 10.1002/ijc.30529. [DOI] [PubMed] [Google Scholar]
- 17.Rothweiler F, Michaelis M, Brauer P, Otte J, Weber K, Fehse B, Doerr HW, Wiese M, Kreuter J, Al-Abed Y, et al. Anticancer effects of the nitric oxide-modified saquinavir derivative saquinavir-NO against multidrug-resistant cancer cells. Neoplasia. 2010;12:1023–1030. doi: 10.1593/neo.10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolic I, Saksida T, Mangano K, Vujicic M, Stojanovic I, Nicoletti F, Stosic-Grujicic S. Pharmacological application of carbon monoxide ameliorates islet-directed autoimmunity in mice via anti-inflammatory and anti-apoptotic effects. Diabetologia. 2014;57:980–990. doi: 10.1007/s00125-014-3170-7. [DOI] [PubMed] [Google Scholar]
- 19.Fagone P, Mangano K, Coco M, Perciavalle V, Garotta G, Romao CC, Nicoletti F. Therapeutic potential of carbon monoxide in multiple sclerosis. Clin Exp Immunol. 2012;167:179–187. doi: 10.1111/j.1365-2249.2011.04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagone P, Mangano K, Quattrocchi C, Motterlini R, Di Marco R, Magro G, Penacho N, Romao CC, Nicoletti F. Prevention of clinical and histological signs of proteolipid protein (PLP)-induced experimental allergic encephalomyelitis (EAE) in mice by the water-soluble carbon monoxide-releasing molecule (CORM)-A1. Clin Exp Immunol. 2011;163:368–374. doi: 10.1111/j.1365-2249.2010.04303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Módis K, Wolanska K, Vozdek R. Hydrogen sulfide in cell signaling, signal transduction, cellular bioenergetics and physiology in C. elegans. Gen Physiol Biophys. 2013;32:1–22. doi: 10.4149/gpb_2013001. [DOI] [PubMed] [Google Scholar]
- 22.Wallace JL, Ferraz JG, Muscara MN. Hydrogen sulfide: An endogenous mediator of resolution of inflammation and injury. Antioxid Redox Signal. 2012;17:58–67. doi: 10.1089/ars.2011.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D, Si W, Wang M, Lv S, Ji A, Li Y. Hydrogen sulfide in cancer: Friend or foe? Nitric Oxide. 2015;50:38–45. doi: 10.1016/j.niox.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Ngowi EE, Afzal A, Sarfraz M, Khattak S, Zaman SU, Khan NH, Li T, Jiang QY, Zhang X, Duan SF, et al. Role of hydrogen sulfide donors in cancer development and progression. Int J Biol Sci. 2021;17:73–88. doi: 10.7150/ijbs.47850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, Deng LW. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One. 2011;6:e21077. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu S, Gao Y, Huang X, Wang X. GYY4137, a hydrogen sulfide (H2S) donor, shows potent anti-hepatocellular carcinoma activity through blocking the STAT3 pathway. Int J Oncol. 2014;44:1259–1267. doi: 10.3892/ijo.2014.2305. [DOI] [PubMed] [Google Scholar]
- 27.Sonke E, Verrydt M, Postenka CO, Pardhan S, Willie CJ, Mazzola CR, Hammers MD, Pluth MD, Lobb I, Power NE, et al. Inhibition of endogenous hydrogen sulfide production in clear-cell renal cell carcinoma cell lines and xenografts restricts their growth, survival and angiogenic potential. Nitric Oxide. 2015;49:26–39. doi: 10.1016/j.niox.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oláh G, Módis K, Törö G, Hellmich MR, Szczesny B, Szabo C. Role of endogenous and exogenous nitric oxide, carbon monoxide and hydrogen sulfide in HCT116 colon cancer cell proliferation. Biochem Pharmacol. 2018;149:186–204. doi: 10.1016/j.bcp.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellmich MR, Szabo C. Hydrogen sulfide and cancer. Handb Exp Pharmacol. 2015;230:233–241. doi: 10.1007/978-3-319-18144-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dilek N, Papapetropoulos A, Toliver-Kinsky T, Szabo C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol Res. 2020;161:105119. doi: 10.1016/j.phrs.2020.105119. [DOI] [PubMed] [Google Scholar]
- 31.Szabo C. Hydrogen sulfide, an endogenous stimulator of mitochondrial function in cancer cells cells. Cells. 2021;10:220. doi: 10.3390/cells10020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jhee KH, Kruger WD. The role of cystathionine beta-synthase in homocysteine metabolism. Antioxid Redox Signal. 2005;7:813–822. doi: 10.1089/ars.2005.7.813. [DOI] [PubMed] [Google Scholar]
- 33.Zhu H, Blake S, Chan KT, Pearson RB, Kang J. Cystathionine β-synthase in physiology and cancer. Biomed Res Int. 2018;2018:3205125. doi: 10.1155/2018/3205125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ereño-Orbea J, Majtan T, Oyenarte I, Kraus JP, Martínez-Cruz LA. Structural basis of regulation and oligomerization of human cystathionine β-synthase, the central enzyme of transsulfuration. Proc Natl Acad Sci USA. 2013;110:E3790–E3799. doi: 10.1073/pnas.1313683110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taoka S, Banerjee R. Characterization of NO binding to human cystathionine beta-synthase: Possible implications of the effects of CO and NO binding to the human enzyme. J Inorg Biochem. 2001;87:245–251. doi: 10.1016/S0162-0134(01)00335-X. [DOI] [PubMed] [Google Scholar]
- 36.Niu WN, Yadav PK, Adamec J, Banerjee R. S-glutathionylation enhances human cystathionine β-synthase activity under oxidative stress conditions. Antioxid Redox Signal. 2015;22:350–361. doi: 10.1089/ars.2014.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renga B. Hydrogen sulfide generation in mammals: The molecular biology of cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE) Inflamm Allergy Drug Targets. 2011;10:85–91. doi: 10.2174/187152811794776286. [DOI] [PubMed] [Google Scholar]
- 38.Szabo C, Coletta C, Chao C, Módis K, Szczesny B, Papapetropoulos A, Hellmich MR. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci USA. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips CM, Zatarain JR, Nicholls ME, Porter C, Widen SG, Thanki K, Johnson P, Jawad MU, Moyer MP, Randall JW, et al. Upregulation of cystathionine-β-synthase in colonic epithelia reprograms metabolism and promotes carcinogenesis. Cancer Res. 2017;77:5741–5754. doi: 10.1158/0008-5472.CAN-16-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhattacharyya S, Saha S, Giri K, Lanza IR, Nair KS, Jennings NB, Rodriguez-Aguayo C, Lopez-Berestein G, Basal E, Weaver AL, et al. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS One. 2013;8:e79167. doi: 10.1371/journal.pone.0079167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo H, Gai JW, Wang Y, Jin HF, Du JB, Jin J. Characterization of hydrogen sulfide and its synthases, cystathionine β-synthase and cystathionine γ-lyase, in human prostatic tissue and cells. Urology. 2012;79:483.e1–e5. doi: 10.1016/j.urology.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009;284:11601–11612. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messerschmidt A, Worbs M, Steegborn C, Wahl MC, Huber R, Laber B, Clausen T. Determinants of enzymatic specificity in the Cys-Met-metabolism PLP-dependent enzymes family: Crystal structure of cystathionine gamma-lyase from yeast and intrafamiliar structure comparison. Biol Chem. 2003;384:373–386. doi: 10.1515/BC.2003.043. [DOI] [PubMed] [Google Scholar]
- 44.Mikami Y, Shibuya N, Ogasawara Y, Kimura H. Hydrogen sulfide is produced by cystathionine γ-lyase at the steady-state low intracellular Ca(2+) concentrations. Biochem Biophys Res Commun. 2013;431:131–135. doi: 10.1016/j.bbrc.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Cheung SH, Kwok WK, To KF, Lau JY. Anti-atherogenic effect of hydrogen sulfide by over-expression of cystathionine gamma-lyase (CSE) gene. PLoS One. 2014;9:e113038. doi: 10.1371/journal.pone.0113038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan S, Yurdagul A, Jr, Peretik JM, Alfaidi M, Al Yafeai Z, Pardue S, Kevil CG, Orr AW. Cystathionine γ-lyase modulates flow-dependent vascular remodeling. Arterioscler Thromb Vasc Biol. 2018;38:2126–2136. doi: 10.1161/ATVBAHA.118.311402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivanciuc T, Sbrana E, Casola A, Garofalo RP. Cystathionine γ-lyase deficiency enhances airway reactivity and viral-induced disease in mice exposed to side-stream tobacco smoke. Pediatr Res. 2019;86:39–46. doi: 10.1038/s41390-019-0396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhoták Š, Meng QH, Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 49.Pan LL, Liu XH, Gong QH, Yang HB, Zhu YZ. Role of cystathionine γ-lyase/hydrogen sulfide pathway in cardiovascular disease: A novel therapeutic strategy? Antioxid Redox Signal. 2012;17:106–118. doi: 10.1089/ars.2011.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu X, Zhou K, Gao Q, Zheng S, Chen H, Li P, Zhang Y, Suo K, Simoncini T, Wang T. 17β-estradiol attenuates atherosclerosis development: The possible role of hydrogen sulfide. Int J Cardiol. 2013;167:1061–1063. doi: 10.1016/j.ijcard.2012.10.071. [DOI] [PubMed] [Google Scholar]
- 51.Lambertini E, Penolazzi L, Angelozzi M, Grassi F, Gambari L, Lisignoli G, De Bonis P, Cavallo M, Piva R. The expression of cystathionine gamma-lyase is regulated by estrogen receptor alpha in human osteoblasts. Oncotarget. 2017;8:101686–101696. doi: 10.18632/oncotarget.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu X, Yan Q, Liu X, Li P, Li X, Chen Y, Simoncini T, Liu J, Zhu D, Fu X. 17β-Estradiol nongenomically induces vascular endothelial H2S release by promoting phosphorylation of cystathionine γ-lyase. J Biol Chem. 2019;294:15577–15592. doi: 10.1074/jbc.RA119.008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.You J, Shi X, Liang H, Ye J, Wang L, Han H, Fang H, Kang W, Wang T. Cystathionine-γ-lyase promotes process of breast cancer in association with STAT3 signaling pathway. Oncotarget. 2017;8:65677–65686. doi: 10.18632/oncotarget.20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Shi H, Liu Y, Zhang W, Duan X, Li M, Shi X, Wang T. Cystathionine-γ-lyase promotes the metastasis of breast cancer via the VEGF signaling pathway. Int J Oncol. 2019;55:473–487. doi: 10.3892/ijo.2019.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanaoka K, Sasakura K, Suwanai Y, Toma-Fukai S, Shimamoto K, Takano Y, Shibuya N, Terai T, Komatsu T, Ueno T, et al. Discovery and mechanistic characterization of selective inhibitors of H2S-producing enzyme: 3-Mercaptopyruvate sulfurtransferase (3MST) targeting active-site cysteine persulfide. Sci Rep. 2017;7:40227. doi: 10.1038/srep40227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagahara N. Regulation of mercaptopyruvate sulfurtransferase activity via intrasubunit and intersubunit redox-sensing switches. Antioxid Redox Signal. 2013;19:1792–1802. doi: 10.1089/ars.2012.5031. [DOI] [PubMed] [Google Scholar]
- 57.Módis K, Asimakopoulou A, Coletta C, Papapetropoulos A, Szabo C. Oxidative stress suppresses the cellular bioenergetic effect of the 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Biochem Biophys Res Commun. 2013;433:401–407. doi: 10.1016/j.bbrc.2013.02.131. [DOI] [PubMed] [Google Scholar]
- 58.Jeddi S, Gholami H, Gheibi S, Kashfi K, Ghasemi A. Altered gene expression of hydrogen sulfide-producing enzymes in the liver and muscles tissues of hyperthyroid rats. J Cell Physiol. 2019;234:17937–17945. doi: 10.1002/jcp.28426. [DOI] [PubMed] [Google Scholar]
- 59.Huang CW, Moore PK. H2S synthesizing enzymes: Biochemistry and molecular aspects. Handb Exp Pharmacol. 2015;230:3–25. doi: 10.1007/978-3-319-18144-8_1. [DOI] [PubMed] [Google Scholar]
- 60.Li M, Xu C, Shi J, Ding J, Wan X, Chen D, Gao J, Li C, Zhang J, Lin Y, et al. Fatty acids promote fatty liver disease via the dysregulation of 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Gut. 2018;67:2169–2180. doi: 10.1136/gutjnl-2017-313778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Augsburger F, Szabo C. Potential role of the 3-mercaptopyruvate sulfurtransferase (3-MST)-hydrogen sulfide (H2S) pathway in cancer cells. Pharmacol Res. 2020;154:104083. doi: 10.1016/j.phrs.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 62.Sen S, Kawahara B, Gupta D, Tsai R, Khachatryan M, Roy-Chowdhuri S, Bose S, Yoon A, Faull K, Farias-Eisner R, Chaudhuri G. Role of cystathionine β-synthase in human breast cancer. Free Radic Biol Med. 2015;86:228–238. doi: 10.1016/j.freeradbiomed.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 63.Sen S, Kawahara B, Mahata SK, Tsai R, Yoon A, Hwang L, Hu-Moore K, Villanueva C, Vajihuddin A, Parameshwar P, et al. Cystathionine: A novel oncometabolite in human breast cancer. Arch Biochem Biophys. 2016;604:95–102. doi: 10.1016/j.abb.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 64.Kraus JP, Oliveriusová J, Sokolová J, Kraus E, Vlcek C, de Franchis R, Maclean KN, Bao L, Bukovsk, Patterson D, Paces V, et al. The human cystathionine beta-synthase (CBS) gene: Complete sequence, alternative splicing, and polymorphisms. Genomics. 1998;52:312–324. doi: 10.1006/geno.1998.5437. [DOI] [PubMed] [Google Scholar]
- 65.Tsai MY, Bignell M, Yang F, Welge BG, Graham KJ, Hanson NQ. Polygenic influence on plasma homocysteine: Association of two prevalent mutations, the 844ins68 of cystathionine beta-synthase and A(2756)G of methionine synthase, with lowered plasma homocysteine levels. Atherosclerosis. 2000;149:131–137. doi: 10.1016/S0021-9150(99)00297-X. [DOI] [PubMed] [Google Scholar]
- 66.Weiner AS, Boyarskikh UA, Voronina EN, Selezneva IA, Sinkina TV, Lazarev AF, Petrova VD, Filipenko ML. Polymorphisms in the folate-metabolizing genes MTR, MTRR, and CBS and breast cancer risk. Cancer Epidemiol. 2012;36:e95–e100. doi: 10.1016/j.canep.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 67.Stevens VL, McCullough ML, Pavluck AL, Talbot JT, Feigelson HS, Thun MJ, Calle EE. Association of polymorphisms in one-carbon metabolism genes and postmenopausal breast cancer incidence. Cancer Epidemiol Biomarkers Prev. 2007;16:1140–1147. doi: 10.1158/1055-9965.EPI-06-1037. [DOI] [PubMed] [Google Scholar]
- 68.Gallegos-Arreola MP, Figuera-Villanueva LE, Ramos-Silva A, Salas-González E, Puebla-Pérez AM, Peralta-Leal V, García-Ortiz JE, Dávalos-Rodríguez IP, Zúñiga-González GM. The association between the 844ins68 polymorphism in the CBS gene and breast cancer. Arch Med Sci. 2014;10:1214–1224. doi: 10.5114/aoms.2014.47830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryu CS, Kwak HC, Lee JY, Oh SJ, Phuong NT, Kang KW, Kim SK. Elevation of cysteine consumption in tamoxifen-resistant MCF-7 cells. Biochem Pharmacol. 2013;85:197–206. doi: 10.1016/j.bcp.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 70.Ryu CS, Kwak HC, Lee KS, Kang KW, Oh SJ, Lee KH, Kim HM, Ma JY, Kim SK. Sulfur amino acid metabolism in doxorubicin-resistant breast cancer cells. Toxicol Appl Pharmacol. 2011;255:94–102. doi: 10.1016/j.taap.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Thornburg JM, Nelson KK, Clem BF, Lane AN, Arumugam S, Simmons A, Eaton JW, Telang S, Chesney J. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008;10:R84. doi: 10.1186/bcr2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chao C, Zatarain JR, Ding Y, Coletta C, Mrazek AA, Druzhyna N, Johnson P, Chen H, Hellmich JL, Asimakopoulou A, et al. Cystathionine-beta-synthase inhibition for colon cancer: Enhancement of the efficacy of aminooxyacetic acid via the prodrug approach. Mol Med. 2016;22:361–379. doi: 10.2119/molmed.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hellmich MR, Coletta C, Chao C, Szabo C. The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer. Antioxid Redox Signal. 2015;22:424–448. doi: 10.1089/ars.2014.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE) Br J Pharmacol. 2013;169:922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y, Yu J, Lei X, Wu J, Niu Q, Zhang Y, Liu H, Christen P, Gehring H, Wu F. High-throughput tandem-microwell assay identifies inhibitors of the hydrogen sulfide signaling pathway. Chem Commun. 2013;49:11782–11784. doi: 10.1039/c3cc46719h. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Cai H, Hu Y, Liu F, Huang S, Zhou Y, Yu J, Xu J, Wu F. A pharmacological probe identifies cystathionine β-synthase as a new negative regulator for ferroptosis. Cell Death Dis. 2018;9:1005. doi: 10.1038/s41419-018-1063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zuhra K, Augsburger F, Majtan T, Szabo C. Cystathionine-β-synthase: Molecular regulation and pharmacological inhibition. Biomolecules. 2020;10:697. doi: 10.3390/biom10050697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marechal D, Brault V, Leon A, Martin D, Lopes Pereira P, Loaëc N, Birling MC, Friocourt G, Blondel M, Herault Y. Cbs overdosage is necessary and sufficient to induce cognitive phenotypes in mouse models of down syndrome and interacts genetically with Dyrk1a. Hum Mol Genet. 2019;28:1561–1577. doi: 10.1093/hmg/ddy447. [DOI] [PubMed] [Google Scholar]
- 79.Chen D, Cui QC, Yang H, Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 80.Safi R, Nelson ER, Chitneni SK, Franz KJ, George DJ, Zalutsky MR, McDonnell DP. Copper signaling axis as a target for prostate cancer therapeutics. Cancer Res. 2014;74:5819–5831. doi: 10.1158/0008-5472.CAN-13-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zha J, Chen F, Dong H, Shi P, Yao Y, Zhang Y, Li R, Wang S, Li P, Wang W, Xu B. Disulfiram targeting lymphoid malignant cell lines via ROS-JNK activation as well as Nrf2 and NF-κB pathway inhibition. J Transl Med. 2014;12:163. doi: 10.1186/1479-5876-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Papaioannou M, Mylonas I, Kast RE, Brüning A. Disulfiram/copper causes redox-related proteotoxicity and concomitant heat shock response in ovarian cancer cells that is augmented by auranofin-mediated thioredoxin inhibition. Oncoscience. 2013;1:21–29. doi: 10.18632/oncoscience.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cong J, Wang Y, Zhang X, Zhang N, Liu L, Soukup K, Michelakos T, Hong T, DeLeo A, Cai L, et al. A novel chemoradiation targeting stem and nonstem pancreatic cancer cells by repurposing disulfiram. Cancer Lett. 2017;409:9–19. doi: 10.1016/j.canlet.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 84.Malcolm R, Olive MF, Lechner W. The safety of disulfiram for the treatment of alcohol and cocaine dependence in randomized clinical trials: Guidance for clinical practice. Expert Opin Drug Saf. 2008;7:459–472. doi: 10.1517/14740338.7.4.459. [DOI] [PubMed] [Google Scholar]
- 85.Jiao Y, Hannafon BN, Ding WQ. Disulfiram's anticancer activity: Evidence and mechanisms. Anticancer Agents Med Chem. 2016;16:1378–1384. doi: 10.2174/1871520615666160504095040. [DOI] [PubMed] [Google Scholar]
- 86.DeVito EE, Babuscio TA, Nich C, Ball SA, Carroll KM. Gender differences in clinical outcomes for cocaine dependence: Randomized clinical trials of behavioral therapy and disulfiram. Drug Alcohol Depend. 2014;145:156–167. doi: 10.1016/j.drugalcdep.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng J, Weitner M, Shi W, Zhang S, Sullivan D, Zhang Y. Identification of additional anti-persister activity against Borrelia burgdorferi from an FDA drug library. Antibiotics (Basel) 2015;4:397–410. doi: 10.3390/antibiotics4030397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thorson MK, Majtan T, Kraus JP, Barrios AM. Identification of cystathionine β-synthase inhibitors using a hydrogen sulfide selective probe. Angew Chem Int Ed Engl. 2013;52:4641–4644. doi: 10.1002/anie.201300841. [DOI] [PubMed] [Google Scholar]
- 89.Druzhyna N, Szczesny B, Olah G, Módis K, Asimakopoulou A, Pavlidou A, Szoleczky P, Gerö D, Yanagi K, Törö G, et al. Screening of a composite library of clinically used drugs and well-characterized pharmacological compounds for cystathionine β-synthase inhibition identifies benserazide as a drug potentially suitable for repurposing for the experimental therapy of colon cancer. Pharmacol. Res. 2016;113:18–37. doi: 10.1016/j.phrs.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niu W, Wu P, Chen F, Wang J, Shang X, Xu C. Discovery of selective cystathionine β-synthase inhibitors by high-throughput screening with a fluorescent thiol probe. Medchemcomm. 2016;8:198–201. doi: 10.1039/C6MD00493H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McCune CD, Chan SJ, Beio ML, Shen W, Chung WJ, Szczesniak LM, Chai C, Koh SQ, Wong PT, Berkowitz DB. ‘Zipped synthesis’ by cross-metathesis provides a cystathionine β-synthase inhibitor that attenuates cellular H2S levels and reduces neuronal infarction in a rat ischemic stroke model. ACS Central Sci. 2016;2:242–252. doi: 10.1021/acscentsci.6b00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sekiguchi F, Sekimoto T, Ogura A, Kawabata A. Endogenous hydrogen sulfide enhances cell proliferation of human gastric cancer AGS cells. Biol Pharm Bull. 2016;39:887–890. doi: 10.1248/bpb.b15-01015. [DOI] [PubMed] [Google Scholar]
- 93.Hu Y, Wang L, Han X, Zhou Y, Zhang T, Wang L, Hong T, Zhang W, Guo XX, Sun J, et al. Discovery of a bioactive inhibitor with a new scaffold for cystathionine γ-lyase. J Med Chem. 2019;62:1677–1683. doi: 10.1021/acs.jmedchem.8b01720. [DOI] [PubMed] [Google Scholar]
- 94.Bhattacharjee A, Sinha A, Ratia K, Yin L, Delgado-Rivera L, Petukhov PA, Thatcher GRJ, Wardrop DJ. 2-Arylidene hydrazinecarbodithioates as potent, selective inhibitors of cystathionine γ-lyase (CSE) ACS Med Chem Lett. 2017;8:1241–1245. doi: 10.1021/acsmedchemlett.7b00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yadav PK, Vitvitsky V, Kim H, White A, Cho US, Banerjee R. S-3-Carboxypropyl-l-cysteine specifically inhibits cystathionine γ-lyase-dependent hydrogen sulfide synthesis. J Biol Chem. 2019;294:11011–11022. doi: 10.1074/jbc.RA119.009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang L, Shi H, Zhang X, Zhang X, Liu Y, Kang W, Shi X, Wang T. I157172, a novel inhibitor of cystathionine γ-lyase, inhibits growth and migration of breast cancer cells via SIRT1-mediated deacetylation of STAT3. Oncol Rep. 2019;41:427–436. doi: 10.3892/or.2018.6798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.