Abstract
Objective
Outcome differences between selective abobotulinumtoxin type A (aboBoNT/A) injections into the soleus (SOL) and gastrocnemius (GAS) muscles were investigated in post-stroke patients with spastic foot drop.
Methods
A monocentric observational study was conducted at a university hospital botulinum toxin clinic including 24 free-walking adult, botulinum toxin-naive patients with post-stroke hemiplegia. AboBoNT/A (800 MU in 4 mL saline) was injected into the SOL or GAS muscle under electromyographic guidance. After 30 days post-injection, the effect of aboBoNT/A injection was assessed by patients. The treating physician scored spasticity and measured angles at the knee and ankle joint and gait speed.
Results
After 30 days, significant improvements of subjective and objective outcome measures were observed. No significant difference was observed in the modified Ashworth scale, gait speed, ankle and knee angles, or their angle combinations between the SOL and GAS groups. Tendencies toward greater active range of motion (RoM) improvement in the SOL group and passive RoM improvement in the GAS group were observed. The difference between active and passive ankle extensions plus knee flexions was significantly larger in the SOL group.
Conclusions
Selective 800 MU aboBoNT/A injections into the SOL or GAS muscle were effective but without relevant clinical difference.
Keywords: Spastic foot drop, soleus muscle, gastrocnemius muscle, abobotulinumtoxin type A, active and passive range of motion, muscle fiber decomposition
Introduction
Stroke affects approximately 150 to 200 per 100,000 people per year.1 A large proportion of patients who survive stroke remain severely handicapped because of upper motor neuron syndrome and associated complications or symptoms.2–4 Approximately 19% to 43% of stroke survivors will develop relevant spasticity in the affected limb.5–7
Treatment of spasticity includes oral anti-spastic medication, phenol injections, surgical interventions, and physiotherapy or a combination of these therapies. In addition to these well-established therapies, intramuscular injections of botulinum toxin (BoNT) are effective in the treatment of spasticity.8,9 Following evidence-based evaluations, the Therapeutics and Technology Assessment Committee of the American Academy of Neurology10 and the Royal College of Physicians11 concluded that botulinum toxin type A (BoNT/A) is an effective treatment (level A recommendation) of spasticity in adults. Such recommendations were based on evidence that BoNT/A can reduce muscle tone and improve passive function.
In post-stroke patients, the development of spastic foot drop months after the stroke is severely disabling. Because spastic foot drop leads to a reduction of the foot contact area, it is accompanied by the risk of losing balance, which increases the risk of ankle joint injury and accidental falls.12
Tibial (selective fascicular) neurotomy has successfully been used to reduce spasticity around the ankle joint since 1912.13,14 Subsequently, a new “soleus neurotomy” technique using etidocaine blocks prior to neurotomy was developed.15,16 A clinical study of etidocaine blocks demonstrated “that spasticity of the soleus muscle was exclusively responsible for the spastic equinus of the ankle in 75% of the cases, whereas the gastrocnemius muscle was predominantly involved in only 12.5% of the cases”.16 Despite effective reduction of spasticity of the ankle joint,14 neurotomy has rarely been performed after the introduction of locally-acting treatments such as phenol blocks17,18 and BoNT injections.10
Currently, selective BoNT/A injections either into the soleus (SOL) or gastrocnemius (GAS) muscle of patients with lower leg spasticity have not been compared. Therefore, it is unknown whether the dominant role of SOL function in adult lower limb spasticity, found during neurotomy, can also be observed after BoNT/A treatment of spastic foot drop. Furthermore, it is unclear whether the superiority of SOL treatment over GAS treatment observed after neurotomy can be replicated in BoNT therapy using semiquantitative scores of spasticity or functional outcome measures such as angle measurements or gait speed.
To compare selective SOL and GAS injections of BoNT/A in the present study, 800 MU of abobotulinumtoxin type A (aboBoNT/A, Dysport®; Ipsen Biopharm Limited, Wrexham, UK) was injected either into the SOL or GAS muscle under electromyography (EMG) guidance in 24 BoNT-naive post-stroke patients with spastic foot drop, and the results were observed over an entire treatment cycle.
Methods
Study design and sampling
The present study was approved by the local Ethics Committee of the University of Düsseldorf (Approval number: 4085). Twenty-four adult patients who were free-walking and BoNT treatment-naive, were referred to the BoNT clinic of the university hospital for the treatment of chronic hemiplegia after stroke, and who gave verbal informed consent were consecutively recruited (see Table 1). The 12 patients with odd recruitment numbers (1, 3,…, 23) received injections in the GAS muscle (GAS group), and the 12 patients with even recruitment numbers (2, 4,…, 24) received injections in the SOL muscle (SOL group). The detailed inclusion criteria for the participants are as follows: (i) age ≥18 years; (ii) time since stroke ≥6 months; (iii) clinical evidence of the absence of orthopedic or neurological deficits interfering with walking other than stroke; (iv) no clinical indications of brainstem, cerebellar, or ipsilateral hemispheric lesions; (v) ability to walk without aid for 1 minute without pauses; (vi) no previous BoNT injection; (vii) patients without legal guardians for decision making or impaired judgment. Clinical data of the two patient groups are presented in Table 2.
Table 1.
Demographic data of the entire cohort of 24 patients.
| Age | mean: 54.25 ± 12.93 years | range: 22–77 years |
|---|---|---|
| Duration since stroke | mean: 42.42 ± 39.39 months | range: 6–148 months |
| Sex | 16 males | 8 females |
| Hemorrhage/infarct | 16 infarcts | 8 hemorrhages |
Table 2.
Demographic and baseline data.
| Parameter: mean/S.D. | GAS group mean/S.D. | SOL group mean/S.D. | Significance level |
|---|---|---|---|
| N = | 12 | 12 | n.s. |
| Age at inclusion | 55.4/12.9 years | 53.1/13.5 years | n.s. |
| Range: | 35–77 years | 22–69 years | |
| Female/male ratio | 3/9 | 5/7 | n.s. |
| Hemorrhage/infarct ratio | 5/7 | 3/9 | n.s |
| Duration since stroke mean/S.D. | 36.1/26.6 months | 48.8/49.5 months | n.s. |
| Range: | 7–108 months | 6–148 months | |
| MAS | 2.9/0.57 | 3.0/0.51 | n.s. |
| WD (m) | 19/13.8 | 16/10.1 | n.s. |
| Gait speed (m/s) | 0.32/0.23 | 0.27/0.17 | n.s. |
Mean, mean value; S.D., standard deviation; GAS group, patients with injections into the gastrocnemius muscle; SOL group, patients with injections into the soleus muscle; WD (m), walking distance during 1 minute; MAS, modified Ashworth scale.
Procedure
Baseline investigation
At the baseline visit, patients underwent a detailed clinical neurological examination, detailed angle measurement, scoring of the muscle tone at the ankle joint using the modified Ashworth scale (MAS; for data analysis, a value of 1.5 was assigned for a MAS score of 1+), and gait speed testing. Then, the aboBoNT/A injections were performed.
For angular measurements of the ankle and knee joint, the patients were analyzed in a sitting position with the upper legs supported and the lower legs hanging passively. Starting from this position, the passive range of movement was analyzed by testing how far the foot or lower leg could be moved by the investigator in a selected direction. Passive extensions (ext) and flexions (flex) were determined at the ankle (A) and knee joint (K) (Aext-passive range of motion (pRoM), Aflex-pRoM, Kext-pRoM, and Kflex-pRoM). The active range of motion (aRoM) was analyzed in the same manner by instructing the patient to perform a movement with their foot or lower leg in a selected direction (Aext-aRoM, Aflex-aRoM, Kext-aRoM, and Kflex-aRoM). The active and passive ranges in the inversion (inv) and eversion (ev) directions were measured at the ankle joint (Ainv-pRoM, Aev-pRoM, Ainv-aRoM, and Aev-aRoM). Hip flexion and extension were suppressed, but ankle movements were allowed when knee movements were tested.
The following angle combinations were analyzed to evaluate the clinical response to aboBoNT/A injections: Ainv-pRoM+ Aev-pRoM=AIEV-pRoM; Ainv-aRoM+ Aev-aRoM=AIEV-aRoM; Aext-pRoM+ Aflex-pRoM=AFE-pRoM; Aext-aRoM+ Aflex-aRoM=AFE-aRoM; Kext-pRoM+ Kflex-pRoM=K-pRoM; Kext-aRoM+ Kflex-aRoM= K-aRoM; K-aRoM+AFE-aRoM=K+A-aRoM; K-pRoM+AFE-pRoM=K+A-pRoM (see Table 3).
Table 3.
Active and passive range of motion before and 30 days after the first abobotulinumtoxin type A injection.
| Parameter: mean/S.D. | day 0 | S.D. | day 30 | S.D. | Significance, p< |
|---|---|---|---|---|---|
| PGA | 0 | 0 | 1.13 | 0.68 | 0.05 |
| WD (m) | 17.5 | 12 | 19.1 | 12.5 | 0.036 |
| gait speed (m/s) | 0.29 | 0.12 | 0.32 | 0.13 | 0.036 |
| MAS | 2.95 | .54 | 2.89 | .53 | n.s. |
| K-aRoM | 75 | 35 | 96 | 33 | 0.001 |
| K-pRoM | 123 | 15 | 136 | 15 | 0.001 |
| AFE-aRoM | 21 | 20 | 25 | 21 | 0.16 n.s. |
| AFE-pRoM | 39 | 17 | 49 | 16 | 0.01 |
| AIEV-aRoM | 27 | 26 | 44 | 25 | 0.001 |
| AIEV-pRoM | 69 | 20 | 94 | 10 | 0.001 |
| K+A-aRoM | 96 | 48 | 121 | 46 | 0.001 |
| K+A-pRoM | 161 | 24 | 185 | 20 | 0.001 |
Mean, mean value; S.D., standard deviation; PGA, patient global assessment; WD (m), walking distance during 1 minute; MAS, modified Ashworth scale; K, knee joint; A, ankle joint; a, active; p, passive; RoM, range of motion; AFE, ankle joint flexion/extension direction; AIEV, ankle joint inversion/eversion direction; K+A, knee joint+ankle joint (flexion/extension direction); n.s., not significant.
For further analysis, the following physiological angle combinations were also determined: Aext- aRoM+Kflex-aRoM and Aext-pRoM+Kflex-pRoM.
After the angle measurements, patients were asked to walk without a cane or wheeled walker for 1 minute straight forward at their preferred walking speed. The walking distance in meters during 60 s (WD) was measured to determine the gait speed (m/s).
Injection site and administration
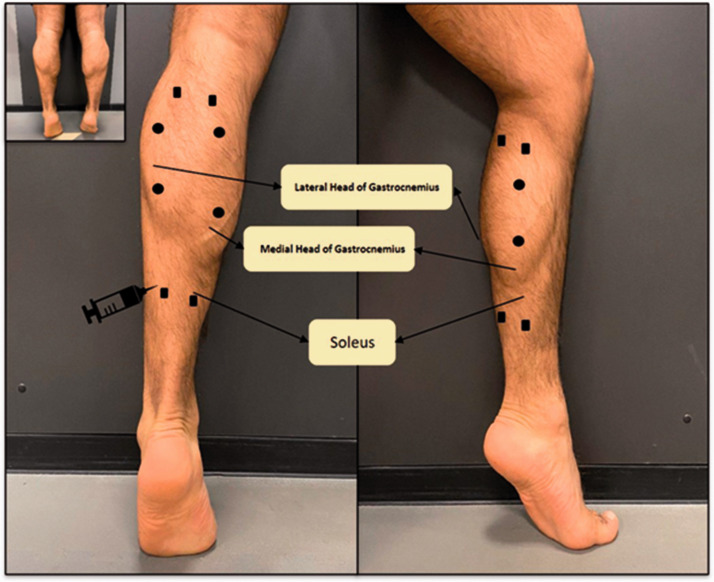
All 24 patients received 800 MU aboBoNT/A (Dysport®), which was diluted in 4 mL of saline. The total dose was distributed to four injection sites with a dose of 200 MU/mL per site. In the GAS group, upper and lower sites were chosen at the lateral and medial belly of the GAS (Figure 1; filled circles). In the SOL group, two upper injection sites were chosen at the upper edge of the lateral and medial belly of the GAS muscle. Two additional sites (Figure 1; filled squares) were chosen at the lower edge of the belly of the GAS muscle.
Figure 1.
The four injection sites for the selective soleus injections are indicated by squares; the four injection sites for the selective gastrocnemius injections are indicated by filled circles.
Injections were administered into the referred sites under EMG guidance. Teflon-coated 27-G 4-cm needles (Neuroline Inoject®; Ambu A/S, Baltorpbakken 13; DK-2750 Ballerup, Denmark) were used for EMG recording and aboBoNT/A injections. The depth of injection into the GAS muscle was approximately 1 cm. The SOL muscle injections were applied by inserting the needle much deeper than in the GAS injections. The discharge rates of the motor units in the GAS were higher (>8 Hz) than those of the SOL motor units ( < 8 Hz). Injections into the SOL muscle were only performed when characteristic slow motor unit discharges could be recorded.
Temporal course of the study and assessment of the clinical effect of BoNT/A injections
The effect of the first aboBoNT/A injection was assessed at 30, 60, and 90 days after the baseline visit. At each of these control visits, the patients rated the clinical effect of the injections using a seven-point patient global assessment (PGA) Likert scale (−3 = much worse, −2 = worse; −1 = slightly worse; 0 = unchanged; 1 = slightly better; 2 = better; 3 = much better).
MAS scoring, angle measurements, and measurement of walking speed were performed at each control visit and clinical investigation.
Statistical analysis
The primary (objective) outcome measure of the study was gait speed, and the secondary (subjective) outcome measure was the PGA score. MAS scores and angle measurements were chosen as further outcome measures.
Two-group repeated measures analysis of variance (RM ANOVA) was performed to analyze the efficacy of the first aboBoNT/A injection at 30, 60, and 90 days and to determine whether a group effect existed (SOL versus GAS group) for gait speed, PGA score, MAS score, and angle measurements and whether particular interactions of active and passive angle measurements with the group effect were present. The angle combination Aext+Kflex was analyzed using a second RM ANOVA (Table 4). The Mann–Whitney U-test was used to compare the differences in ((Aext+Kflex)-aRoM)−((Aext+Kflex)-pRoM) between the SOL and GAS groups. A non-parametric Spearman rank-correlation analysis was performed among the outcome measures. ANOVA, rank-correlation analysis, and the Mann–Whitney U-test are part of the commercially available SPSS® statistical software package (version 25, IBM Corp., Armonk, NY, USA)
Table 4.
Changes between baseline visit and the control visit at day 30.
| Parameter | GAS group mean/S.D. | SOL group mean/S.D. | Significance level |
|---|---|---|---|
| PGA | 1.08/0.67 | 1.17/0.72 | n.s. |
| MAS | −0.04/0.1 | −0.08/0.1 | n.s. |
| WD (m) | 1.88/2.74 | 1.375/3.4 | n.s. |
| range: | −1 to + 8.0 (m) | −4 to +7.3 (m) | |
| K-pRoM | 13.3/17.7 | 10.3/11.1 | n.s. |
| K-aRoM | 13.8/15.5 | 21.2/28.1 | n.s. |
| AFE-pRoM | 31/28.4 | 20.8/15.3 | n.s. |
| AFE-aRoM | 9.8/13.6 | 11.5/13.3 | n.s. |
| Aext+Kflex-pRoM | 25.3/16.7 | 15.8/9.6 | n.s. |
| Aext+Kflex-aRoM | 18.6/19.6 | 27.3/24.7 | n.s. |
| Aext+Kflex–(aRoM-pRoM) | −6.75/20.9 | 11.5/21.7 | 0.035 |
Mean, mean value; S.D. , standard deviation; GAS group, patients injected in the gastrocnemius muscle; SOL group, patients injected in the soleus muscle; PGA, patient global assessment; MAS, modified Ashworth Scale; WD (m) , walking distance (m) during 1 minute; n.s. , not significant; K-pRoM, knee passive range of motion in the flexion/extension direction; K-aRoM, knee active range of motion in the flexion/extension direction; AFE-pRoM, ankle passive range of motion in the flexion/extension direction; AFE-aRoM, ankle active range of motion in the flexion/extension direction; Aext+Kflex-pRoM, ankle extension+knee flexion passive range of motion; Aext+Kflex-aRoM, ankle extension+knee flexion active range of motion; Aext+Kflex–(aRoM-pRoM) , ankle extension+knee flexion active range of motion–passive range of motion.
Results
Functional improvement after the first treatment of spastic foot drop with 800 MU aboBoNT/A
All 24 patients in the present study received aboBoNT/A for the first time. RM ANOVA was performed to detect significant differences from the baseline values. The mean gait speed (walking distance divided by 60 s), as the primary outcome, was significantly (p = 0.036) improved by 1.63 m/minute (0.027 m/s; Figure 2a and b). Correspondingly, patients´ assessments of the efficacy of the first BoNT/A injection showed a significant (p < 0.05) increase of 1.125 points in the mean score (standard deviation: 0.68). None of the patients reported a worsening of their spasticity or gait. Only four patients reported no change (score = 0) at 30 days after injection, 13 patients reported a mild improvement (score = 1), and 7 patients reported a good improvement (score = 2). The MAS was not significantly different. With the exception of the aRoM at the ankle joint, all angle measurements (presented in Table 3) showed significant improvement.
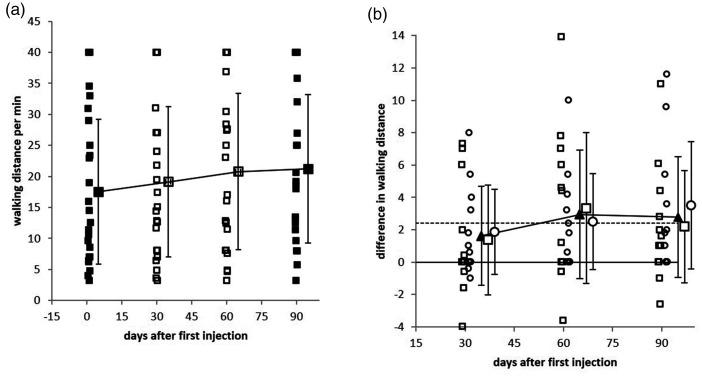
Figure 2.
a: Walking distance during a 1-minute walk for all patients at the baseline investigation (day 0; filled squares), day 30 (open squares), day 60 (open squares), and day 90 (filled squares). b: Comparison of the change from baseline gait speed (of the entire patient cohort, n=24; filled triangles), soleus group (n=12, open squares), and gastrocnemius group (n=12, open circles). The increase in gait speed from day 0 to day 30 was significant in all three patient groups. The gait speed further increased during the next 60 days, but compared with day 30, this further increase was not significant.
RM ANOVA revealed a significant change of gait speed (WD/minute) over time. Gait speed further increased during the next 60 days (Figure 2a and b), but the increase between days 30 and 90 was not statistically significant. Between day 30 and day 90 a mild but not significant decline of the PGA score, MAS score, and all angle measurements could be observed, but none of these parameters declined to baseline levels. None of the demographic parameters (Table 1: age, sex, duration since stroke, or history of stroke (hemorrhage or infarct)) had a significant influence on the outcome measures (gait speed, PGA score, MAS score, or angle measurements). RM ANOVA did not detect a significant interaction between group and time.
A non-parametric correlation analysis (details not presented) among the outcome measures revealed a highly significant correlation between the angle combination K+A-aRoM and the PGA score in the entire cohort (p < 0.001).
Comparison of SOL and GAS injections
At the baseline visit, no significant difference in the demographic data and the outcome measures in Table 1 and 2 was found between the SOL and GAS group.
RM ANOVA did not detect a group effect in comparisons of the SOL and GAS groups in the gait speed, PGA score, MAS score, or any of the standard angle measurements (AFE-pRoM, AFE-aRoM, K-pRoM, K-aRoM, K+A-pRoM, and K+A-aRoM; see Table 4). SOL injections tended to be associated with an improvement of the aRoM, and GAS injections tended to be associated with an improvement of the pRoM. However, no significant difference could be found (Table 4).
These results demonstrated that no significant differences could be detected between selective BoNT/A injections into the SOL and GAS muscles.
Determination of angle combinations favoring SOL injections
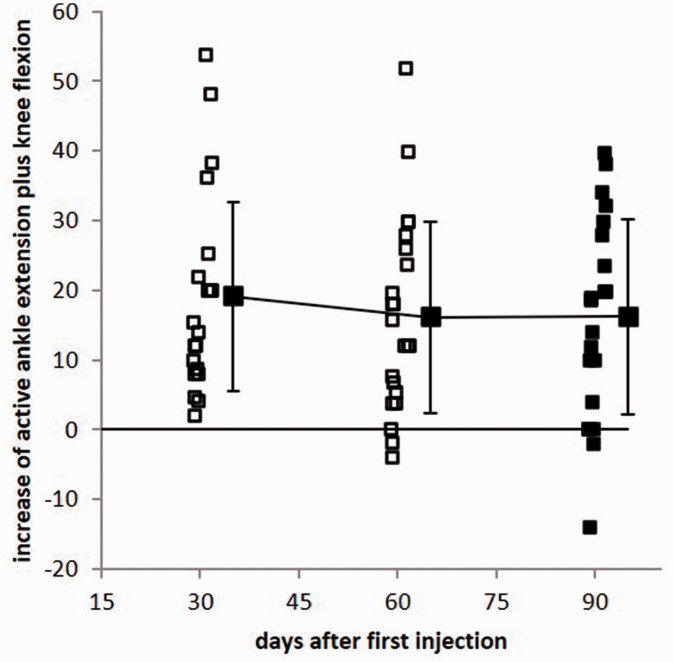
Interestingly, an improvement was observed in angles for which the most relevant muscles had not been injected (including the K-aRoM in the SOL group and AIEV-aRoM and AIEV-pRoM, which are mainly associated with the peroneus longus and tibialis posterior muscles; Table 3). This finding indicated that BoNT/A injections of spastic lower leg muscles may influence functional units rather than single muscles or single angles (for details, see the Discussion). We therefore analyzed the Aext+Kflex combination, which is physiologically relevant during step initiation. In a second RM ANOVA, this combination was found to be highly significantly (p < 0.0001) improved over the entire treatment cycle (Figure 3). All patients showed an improvement at day 30, and this parameter returned to baseline values in only three to four patients after 60 days (Figure 3). The difference in Aext+Kflex-aRoM from baseline was larger in the SOL group than in the GAS group, whereas the difference in Aext+ Kflex-pRoM from baseline was larger in the GAS group than in the SOL group (see the last 3 rows in Table 4 and Figure 4a). The difference in ((Aext+Kflex-aRoM)−((Aext+Kflex)-pRoM) was significantly (Mann–Whitney U-test; p < 0.05) larger in the SOL group than in the GAS group (Figure 4b).
Figure 3.
The sum of active ankle extension and active knee flexion was highly significantly (p < 0.0001) improved in all 24 patients on day 30 (open squares). During the next 60 days, this angle combination decreased to or below the baseline value in only four patients (filled squares).
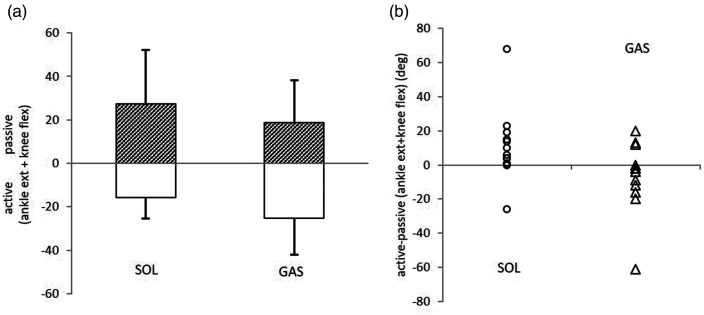
Figure 4.
a: Comparison of the active ankle extension plus knee flexion (upward direction; hatched bars) and the passive ankle extension plus knee flexion (downward direction; open bars) in the soleus (SOL) and gastrocnemius (GAS) groups. b: The difference between active ankle extension plus knee flexion minus the passive ankle extension plus knee flexion was significantly larger in the SOL group (open circles) than in the GAS group (open triangles).
Discussion
Comparison of SOL and GAS muscle fibers and motor unit firing rates
Animal experiments comparing the efficacy of BoNT/A injections into the SOL and GAS muscles do not support the superiority of SOL injections over GAS injections. The atrophy induced by BoNT injection into the mouse GAS muscle was more pronounced than that induced by SOL injection, whereas recovery occurred earlier after SOL injections than after GAS injections.19
In humans, both muscles are different at the macroscopic and microscopic levels. The GAS muscle acts across the ankle and knee joint and consists to a large extent of fast-contracting fibers (type II fibers).20,21 Motor units innervating fast-contracting fibers have high discharge rates (8–15 Hz)22 and high turnover at the motor endplate, which leads to enhanced uptake of BoNT.23 Therefore, an effective response can be expected when BoNT/A is injected into the GAS muscle. However, the SOL muscle acts only across the ankle joint and mainly consists of slow-contracting fibers (up to 70% type I fibers)20,21 with low discharge rates (3–5 Hz).24 Thus, animal experiments and human muscle fiber decomposition and motor unit firing patterns suggest that BoNT uptake after injection into the SOL muscle is less effective than after GAS muscle injection.
Moreover, BoNT diffuses around the injection site with at least a 1-cm radius25 and spreads out along the fibers. Therefore BoNT/A injections cannot be applied as selectively as selective denervation combined with specific nerve stimulation.
Functional improvement in the entire cohort after the first treatment of spastic foot drop with 800 MU aboBoNT/A
Gait speed, as an objective functional outcome measure, and patients' subjective assessment of the effect of the first BoNT/A injection both revealed a significant improvement after 30 days (Table 3). This result is consistent with a larger study on 234 patients26 who were treated with 500, 1000, or 1500 MU aboBoNT/A, which also showed a significant change in gait speed over 12 weeks after the injection. However, in another study on 23 adult post-stroke patients, no significant change in gait speed after a single injection of 1000 MU aboBoNT/A could be observed.12 In the present study gait speed significantly (p = 0.036) changed from the baseline visit to day 30 by approximately 0.027 m/s, although this value was well below 0.04 m/s (corresponding to 2.4 m/minute; see hatched line in Figure 4), which has been reported to be a critical value for a relevant improvement of gait speed in BoNT/A treatment of lower leg spasticity.27 In addition to gait speed, the PGA score also significantly improved after the first BoNT/A injection. With the exception of the aRoM at the ankle joint, all standard angle measurements also significantly increased (Table 3).
In the present study, spastic foot drop was the focus of patient spasticity. Correspondingly, the MAS value was high, with a mean close to 3. The MAS value did not change significantly after the first injection of 800 MU aboBoNT/A. In a recent study on improvement of body sway by BoNT injections in post-stroke patients, the MAS score improved after 300 U of onaBoNT/A (Allergan®, Allergan Pharmaceuticals, Ireland) was injected,28 corresponding to 750 to 900 U of aboBoNT/A, depending on a conversion ratio of 2.5 to 3 between ona- and aboBoNT/A. In a previous study, the MAS score significantly decreased after injection of 1000 MU of aboBoNT/A into the calf muscles.12 Compared with this study, the duration since stroke was longer in the present cohort, which may offer a possible explanation for the lack of effect on muscle tone, together with the fact that a relatively low dose of aboBoNT/A was used.
Comparison of the efficacy of selective SOL and GAS aboBoNT/A injections
Based on the result demonstrated by application of etidocaine blocks,16 i.e., “that spasticity of the soleus muscle was exclusively responsible for the spastic equinus of the ankle in 75% of the cases, whereas the gastrocnemius muscle was predominantly involved in only 12.5% of the cases,” it could be expected that the clinical effect of selective BoNT/A injections in the SOL would be superior to selective GAS injections (see the Introduction). However, this superiority of SOL injections could not be confirmed. RM ANOVA did not reveal a significant between-group effect for any objective or subjective outcome measures (WD, PGA score, MAS scores, various angle measurements) (Table 4).
The present study is more in agreement with the analysis of human muscle fiber decomposition,20,21 analysis of motor unit firing rates in rats,22,24 and comparison of selective BoNT injections into the SOL or GAS muscles of mice19 than with the results of selective nerve blocks.16 Even the mild decline of gait speed between day 60 and day 90 in the SOL group (squares in Figure 2b), which was not observed in the GAS group (circles in Figure 2b), is compatible with the observation of an earlier recovery of the SOL muscle than the GAS muscle after BoNT/A injections in mice.19
This finding does not imply that, in clinical practice, no differences can be detected between SOL and GAS injections. Although not statistically significant, a consistently greater improvement of the aRoM in the SOL group and a greater improvement of the pRoM in the GAS group were observed (Table 4). Because patient assessment of the first BoNT/A injection was significantly (p < 0.001) correlated with the (K+A)-aRoM, this fits the tendency (p = 0.16) of a higher PGA score in the SOL group after 30 days than in the GAS group.
Determination of further sensitive outcome measures in the treatment of lower leg spasticity
We therefore aimed to determine further parameters that may be more sensitive to monitor the efficacy of BoNT/A injections of lower leg spasticity than single joint angles and their combinations and possibly reveal differences between selective SOL and GAS treatment.
“The gastrocnemius muscle contributes to total force production relatively less than the soleus muscle during continuous voluntary plantar flexion at 40% of the maximum voluntary contraction”.29 This is the force range used during walking. Furthermore, the functions of the SOL and GAS muscles seem to be different during the control of standing,30 walking, and running.31 Di Guilio et al. found that “the main difference between soleus and gastrocnemius is that soleus was almost always modulated in activity as an active agonist, while gastrocnemius also had periods of un-modulated activity where changes in muscle length were passively driven by ankle rotation”.30 This finding may imply that the SOL muscle in particular continuously supplies the spinal cord and the central pattern generators of walking in the brainstem32,33 with information on ankle position via the muscle spindle activity. However, muscle spindle activity is disturbed in patients with spasticity. The reduction of muscle spindle activity after injection of BoNT/A34 may improve the information for sensory-motor integration needed for walking in patients with hemiplegia.
The central pattern generators do not code activity of single muscles and movements around a single joint but control the activity of functional units.32,33 We therefore analyzed the physiological angle combination of ankle extensions and knee flexions, which are relevant for lifting the leg during the initial swing phase of a step to prepare for forward transport.
Among all parameters analyzed, the sum of ankle extensions and knee flexions was the only parameter showing an improvement after 30 days in all patients. This improvement persisted for 90 days in 20 of 24 patients (>80%). Furthermore, this angle combination even showed greater improvement of aRoM after SOL injection than GAS injection (Table 4, lower part and Figure 4a). The difference in (Aext-Kflex)-aRoM−(Aext+ Kflex)-pRoM was significantly (p < 0.035) larger in the SOL group than in the GAS group. The mean value was positive in the SOL group and negative in the GAS group (Table 4 lower part and Figure 4b).
This difference between SOL and GAS injections was found after re-analysis of the data and searching for sensitive parameters. Future studies should be performed to assess the value of such an angle combination as a primary outcome measure. In clinical practice, this difference seems to be of less importance as long as the dose injected into the SOL muscle is comparable to the dose injected into the GAS muscle.
Strengths and limitations of the study
The strength of the study is the demonstration of a functional improvement of walking in adult post-stroke patients with a spastic foot drop after treatment with a single injection of 800 MU aboBoNT/A into the calf muscles. The limitations of the study were that the muscle tone and angle measurements were performed in a sitting position, and the use of an instrumental gait analysis could have more clearly demonstrated the improvement of physiological angle combinations.
Conclusions
Treatment of spastic foot drop in adult post-stroke patients with 800 MU of aboBoNT/A is effective and may even result in functional improvement. No significant differences in efficacy were observed between selective aboBoNT/A injections into the SOL and GAS muscles of adult post-stroke patients. Therefore, aboBoNT/A treatment of lower leg spasticity should be equal in the GAS and SOL muscles. Physiological angle combinations may be more sensitive outcome measures than single joint angles to monitor the clinical effect of BoNT/A injections on lower leg spasticity. However, this should be tested in future studies.
Footnotes
Author contributions: All four authors were involved in the development and design of the study. W. Nickels and H. Hefter were the treating physicians of the patients and performed angle measurements and BoNT injections. S. Samadzadeh and D. Rosenthal performed the statistical analysis. H. Hefter wrote the first draft of the manuscript; all four authors contributed to and read the final version of the manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: H. Hefter received a general grant from the German Federal State North-Rhine Westphalia (NRW) and the European Union (European Regional Development Fund: Investing in Your Future) to investigate the effect of and optimize the rehabilitation process in stroke patients by application of botulinum toxin. This grant did not interfere with the design and the performance of the present study.
Grant number: EFRE-0800962/LS-1-2-015d
ORCID iD: Harald Hefter https://orcid.org/0000-0003-1069-7684
References
- 1.Bakhai A. The burden of coronary, cerebrovascular and peripheral arterial disease. Pharmacoeconomics 2004; 22: 11–18. [DOI] [PubMed] [Google Scholar]
- 2.Rathore SS, Hinn AR, Cooper LS, et al. Characterization of incident stroke signs and symptoms: findings from the atherosclerosis risk in communities study. Stroke 2002; 33: 2718–2721. [DOI] [PubMed] [Google Scholar]
- 3.Sheean G. The pathophysiology of spasticity. Eur J Neurol 2002; 9: 3–9. [DOI] [PubMed] [Google Scholar]
- 4.Hefter H, Jost WH, Reissig A, et al. Classification of posture in poststroke upper limb spasticity: a potential decision tool for botulinum toxin A treatment? Int J Rehabil Res 2012; 35: 227–233. [DOI] [PubMed] [Google Scholar]
- 5.Watkins CL, Leathley MJ, Gregson JM, et al. Prevalence of spasticity post stroke. Clin Rehabil 2002; 16: 515–522. [DOI] [PubMed] [Google Scholar]
- 6.Sommerfeld DK, Eek EU, Svensson AK, et al. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke 2004; 35: 134–139. [DOI] [PubMed] [Google Scholar]
- 7.Urban PP, Wolf T, Uebele M, et al. Occurence and clinical predictors of spasticity after ischemic stroke. Stroke 2010; 41: 2016–2020. [DOI] [PubMed] [Google Scholar]
- 8.Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil 2015; 94: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for lower limb spasticity. Medicine (Baltimore) 2016; 95: e2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson DM, Hallett M, Ashman EJ, et al. Practise guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016; 86: 1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royal College of Physicians BSoRM. Spasticity in adults: management using botulinum toxin. National guidelines. London: Royal College of Physicians, 2018, pp.1–72. [Google Scholar]
- 12.Burbaud P, Wiart L, Dubos JL, et al. A randomised, double blind, placebo controlled trial of botulinum toxin in the treatment of spastic foot in hemiparetic patients. J Neurol Neurosurg Psychiatry 1996; 61: 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoffel A. The treatment of spastic contractures. Am J Orthop Surg 1912; 10: 611–644. [Google Scholar]
- 14.Buffenoir K, Roujeau T, Lapierre F, et al. Spastic equinus foot: multicenter study of the long-term results of tibial neurotomy. Neurosurgery 2004; 55: 1130–1137. [DOI] [PubMed] [Google Scholar]
- 15.Decq P, Filipetti P, Cubillos A, et al. Soleus neurotomy for treatment of the spastic equinus foot. Groupe d'Evaluation et de Traitement de la Spasticite et de la Dystonie. Neurosurgery 2000; 47: 1154–1160; discussion 60–1. [DOI] [PubMed] [Google Scholar]
- 16.Decq P, Cuny E, Filipetti P, et al. Role of soleus muscle in spastic equinus foot. Lancet 1998; 352: 118. [DOI] [PubMed] [Google Scholar]
- 17.Khalili AA, Harmel MH, Forster S, et al. Management of spasticity by selective peripheral nerve block with dilute phenol solutions in clinical rehabilitation. Arch Phys Med Rehabil 1964; 45: 513–519. [PubMed] [Google Scholar]
- 18.Awad EA. Phenol block for control of hip flexor and adductor spasticity. Arch Phys Med Rehabil 1972; 53: 554–557. [PubMed] [Google Scholar]
- 19.Duchen LW. Changes in motor innervation and cholinesterase localization induced by botulinum toxin in skeletal muscle of the mouse: differences between fast and slow muscles. J Neurol Neurosurg Psychiatry 1970; 33: 40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson MA, Polgar J, Weightman D, et al. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 1973; 18: 111–129. [DOI] [PubMed] [Google Scholar]
- 21.Edgerton VR, Smith JL, Simpson DR. Muscle fiber populations of human leg muscles. Histochem J 1975; 7: 259–266. [DOI] [PubMed] [Google Scholar]
- 22.Burke RE, Levine DN, Tsairis P, et al . Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 1973; 234: 723–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore P, Naumann M. General and clinical aspects of treatment with botulinum toxin. In: Moore P, Naumann M. (eds) Handbook of botulinum toxin treatment. 2nd ed. Malden, Oxford, Carlton, Berlin: Blackwell Science, 2003, pp.28–75. [Google Scholar]
- 24.Burke RE, Levine DN, Salcman M, et al. Motor units in cat soleus muscle: physiological, histochemical and morphological characteristics. J Physiol 1974; 238: 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez-Castaneda J, Jankovic J, Comella C, et al. Diffusion, spread, and migration of botulinum toxin. Mov Disord 2013; 28: 1775–1783. [DOI] [PubMed] [Google Scholar]
- 26.Pittock SJ, Moore AP, Hardiman O, et al. A double-blind randomised placebo-controlled evaluation of three doses of botulinum toxin type A (Dysport) in the treatment of spastic equinovarus deformity after stroke. Cerebrovasc Dis 2003; 15: 289–300. [DOI] [PubMed] [Google Scholar]
- 27.Foley N, Murie-Fernandez M, Speechley M, et al. Does the treatment of spastic equinovarus deformity following stroke with botulinum toxin increase gait velocity? A systematic review and meta-analysis. Eur J Neurol 2010; 17: 1419–1427. [DOI] [PubMed] [Google Scholar]
- 28.Kerzoncuf M, Viton JM, Pellas F, et al. Poststroke postural sway improved by botulinum toxin: a multicenter randomized double-blind controlled trial. Arch Phys Med Rehabil 2020; 101: 242–248. [DOI] [PubMed] [Google Scholar]
- 29.Ratkevicius A, Mizuno M, Povilonis E, et al. Energy metabolism of the gastrocnemius and soleus muscles during isometric voluntary and electrically induced contractions in man. J Physiol 1998; 507: 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Giulio I, Maganaris CN, Baltzopoulos V, et al. The proprioceptive and agonist roles of gastrocnemius, soleus and tibialis anterior muscles in maintaining human upright posture. J Physiol 2009; 587: 2399–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duysens J, Tax AA, Van Der Doelen B, et al. Selective activation of human soleus or gastrocnemius in reflex responses during walking and running. Exp Brain Res 1991; 87: 193–204. [DOI] [PubMed] [Google Scholar]
- 32.Grillner S. Control of locomotion in bipeds, tetrapods, and fish: In: Brookhart JM, Mountcastle VB (eds) Handbook of physiology – the nervous system II. Bethesda: American Physiological Society, 1981, pp.1179–1236. [Google Scholar]
- 33.Duysens J, Van De Crommert HW. Neural control of locomotion: the central pattern generator from cats to humans. Gait Posture 1998; 7: 131–141. [DOI] [PubMed] [Google Scholar]
- 34.Filippi GM, Errico P, Santarelli R, et al. Botulinum A toxin effects on rat jaw muscle spindles. Acta Otolaryngol 1993; 113: 400–404. [DOI] [PubMed] [Google Scholar]