Abstract
Peptic ulcer disease is an injury of the alimentary tract that leads to a mucosal defect reaching the submucosa. Alpha-lipoic acid (ALA), a natural potent antioxidant, has been known as a gastroprotective drug yet its low bioavailability may restrict its therapeutic efficacy. This study aimed to formulate and optimize ALA using a self-nanoemulsifying drug delivery system (SNEDDS) with a size of nano-range, enhancing its absorption and augmenting its gastric ulcer protection efficacy. Three SNEDDS components were selected as the design factors: the concentrations of the pumpkin oil (X1, 10–30%), the surfactant tween 80 (X2, 20–50%), and the co-surfactant polyethylene glycol 200 (X3, 30–60%). The experimental design for the proposed mixture produced 16 formulations with varying ALA-SNEDDS formulation component percentages. The optimized ALA-SNEDDS formula was investigated for gastric ulcer protective effects by evaluating the ulcer index and by the determination of gastric mucosa oxidative stress parameters. Results revealed that optimized ALA-SNEDDS achieved significant improvement in gastric ulcer index in comparison with raw ALA. Histopathological findings confirmed the protective effect of the formulated optimized ALASNEDDS in comparison with raw ALA. These findings suggest that formulation of ALA in SNEDDS form would be more effective in gastric ulcer protection compared to pure ALA.
Keywords: alpha-lipoic acid, indomethacin, nanotechnology, COX2, stomatitis, gastritis
Introduction
Gastric ulcer is considered one of the most common gastrointestinal tract (GIT) system disorders.1 The etiology of gastric ulcer disease is multifactorial including; smoking, stress, helicobacter pylori and chronic ingestion of non-steroidal anti-inflammatory drugs (NSAIDs).2,3 Ulceration induced by indomethacin (IND), a member of NSAIDs, in rats is regarded as a standard model to better understand the pathophysiological mechanisms and pharmacological studies of acute experimental gastric ulcer.4 Based on several studies, the pathogenesis of IND-induced gastric ulcers include inhibition of protective prostaglandin E2 (PGE2) generation, excess production of inflammatory cytokines such as tumor necrosis tumor factor alpha (TNF-α), increased inducible nitric oxide synthase mediated nitric oxide production as well as augmented formation of reactive oxygen species (ROS).5-7 ROS production interferes with the antioxidant enzyme activities and causes lipid peroxidation that is associated with mucosal damage.8 To date, numerous antiulcer treatment options are available; however, further search is needed for less toxic, efficacious, and inexpensive antiulcerogenic drugs.
Alpha-lipoic acid (ALA) is a natural antioxidant that is present in the cytosol of both prokaryotic and eukaryotic cells and biosynthesized de novo in human cells. It acts as a co-factor for many enzymatic processes in the mitochondria.9 ALA is also present in a number of dietary sources, including; broccoli, yeast extract, spinach, liver and kidney.10 ALA has been known for its remarkable antioxidant and anti-inflammatory effects and has been used for treatment of many disorders.11-13 The antioxidant effects of ALA are attributed to several mechanisms including scavenging of reactive oxygen and nitrogen species, chelation of transitional metals and enhancement of cellular antioxidants including vitamins C and E, and glutathione.14,15 The role of ALA in the protection against gastric ulcer has been elucidated in different models including IND-induced gastric ulcer model.16,17 Despite the beneficial therapeutic effects of ALA, its low bioavailability that is caused by its reduced solubility as well as stomach instability limits its efficacy.18,19
Self-nanoemulsifying systems are chiefly composed of isotopic mixtures of oil, surfactant, and co-surfactant components, which spontaneously form in-situ nanoemulsions under gentle agitation upon contact with GIT fluids. Such formulations have the advantage of presenting the drug in a highly solubilized form when used for drug delivery.20,21 Additionally, the small average droplet size of the produced nanoemulsion offers a large interfacial area for an enhanced absorption of the loaded therapeutics. As compared with traditional emulsions, which are sensitive dispersed forms of low stability characteristics, SNEDDSs are found to be a promising approach as they are thermodynamically stable and easy-to-produce formulations.22 Accordingly, for moieties of lipophilic nature with dissolving-limited absorption, these systems may greatly enhance the rate and extent of absorption, leading to more reproducible plasma concentration-time profiles.
Thus, in the current study, we aimed to develop self-nanoemulsifying drug delivery systems (SNEDDSs) to combat the challenges of low ALA solubility and bioavailability with the provision of high clinical efficacy and possible reduced dosing.23 Here, the ALA-SNEDDs formula was optimized to minimized particle size so as to enhance the solubility and consequently the absorption of ALA. Further, we compared the gastroprotective effects of ALA-SNEDDs with the raw ALA against IND-induced gastric ulcer. Ulcer index, oxidative stress parameters and stomach histopathological changes were used to assess the gastroprotective effects of pure ALA and the proposed ALA formulation.
Materials and Methods
Materials
Alpha-lipoic acid (>99% pure), pumpkin oil, polyethylene glycol 200 and tween 80 were purchased from Sigma Aldrich (St. Louis, MO, USA). All materials were used as supplied.
Formulation of ALA-SNEDDS
ALA (100 mg) was added to the SNEDDS formula which was prepared as previously described.24 Briefly, the SNEDDS formula was prepared by mixing pumpkin oil, Tween 20 (surfactant), and PEG 200 (co-surfactant).
Experimental Design and Optimization of ALA SNEDDS
The ALA SNEDDS mixture used in this study was based on a 3-component system (oil, surfactant, and co-surfactant). D-optimal design was applied for the optimization of ALA SNEDDS using Design-Expert® Software Version 11 (Stat-Ease Inc, Minneapolis, Minnesota, USA). The ranges of the components’ proportions relative to the total amount was selected based on preliminary phase diagram studies (data not shown) as follows: oil phase; pumpkin oil (X1, 0.1-0.3), surfactant; Tween 20 (X2, 0.2-0.5), and co-surfactant; PEG 200 (X3, 0.3-0.6). The total of the 3 components’ proportions was 1, and the ALA amount was kept constant at 100 mg/g of the developed SNEDDS. The mean droplet size (Y) was selected as a response. The candidate points selected by the software included vertices (high and low level from the constraints on each factor), centers of edges, axial and interior check blends, and an overall centroid). Accordingly, the base design consisted of 16 runs that included 5 replicate points, Table 1. The measured response (droplet size) was fitted to the linear, quadratic, and special cubic models. The best fitting model was chosen by comparing several statistical parameters including adjusted multiple correlation coefficient (adjusted R2), predicted multiple correlation coefficient (predicted R2) and predicted residual sum of square (PRESS). The model maximizing the adjusted and predicted R2 and minimizing PRESS was selected for each response. ANOVA test was utilized to evaluate the influence of the components’ proportions on the droplet size at P < 0.05. Numerical optimization was then applied to select the optimal formulation, and desirability function was computed. The desired goal of the study was to minimize the droplet size of the SNEDDS.
Table 1.
The Composition of ALA SNEDDS Prepared According to D-Optimal Mixture Design and Their Observed Droplet Size.
| Run # | Mixture components proportionsa | Vesicle size (nm) | ||
|---|---|---|---|---|
| Pumpkin oil; X1 | Tween 20; X2 | PEG 200; X3 | ||
| 1 | 0.100 | 0.300 | 0.600 | 98.8 ± 2.3 |
| 2 | 0.100 | 0.300 | 0.600 | 97.5 ± 3.2 |
| 3 | 0.200 | 0.500 | 0.300 | 195.6 ± 5.8 |
| 4 | 0.200 | 0.200 | 0.600 | 160.7 ± 4.7 |
| 5 | 0.300 | 0.400 | 0.300 | 242.5 ± 8.6 |
| 6 | 0.200 | 0.425 | 0.375 | 190.7 ± 6.7 |
| 7 | 0.200 | 0.275 | 0.525 | 175.1 ± 6.5 |
| 8 | 0.300 | 0.200 | 0.500 | 230.9 ± 8.9 |
| 9 | 0.100 | 0.500 | 0.400 | 138.8 ± 4.6 |
| 10 | 0.200 | 0.350 | 0.450 | 183.7 ± 5.1 |
| 11 | 0.200 | 0.500 | 0.300 | 194.9 ± 6.8 |
| 12 | 0.300 | 0.200 | 0.500 | 231.8 ± 9.2 |
| 13 | 0.300 | 0.400 | 0.300 | 243.8 ± 7.4 |
| 14 | 0.100 | 0.500 | 0.400 | 136.9 ± 3.9 |
| 15 | 0.100 | 0.400 | 0.500 | 113.7 ± 4.1 |
| 16 | 0.300 | 0.300 | 0.400 | 246.9 ± 7.1 |
Abbreviations: ALA SNEDDS, alpha-lipoic acid self-nanoemulsifying drug delivery systems.
aThe proportions of each run are summed up to 1.
Stability of the Optimized ALA SNEDDS
The optimized ALA-SNEDDS was diluted with deionized water at a ratio of 1:50 and then subjected to 3 freeze-thaw cycles and centrifugation at 15,000 rpm for 20 min.
In Vivo Assessment of Optimized Alpha-Lipoic Acid Formulation
Preparation of animals
Male Wistar rats (180-200 g) were used in the present study. Rats were obtained from El-Nahda University animal care center, NUB, Beni Suef, Egypt and could acclimatize for one week prior to the experiment. The study protocol was approved by the Research Ethics Committee, Faculty of Pharmacy, Minia University in accordance with the guide for the care and use of laboratory animals (approval number 55/2019).
Protocol and experimental groups
Before induction of ulcer, rats were kept in mesh-bottomed cages and deprived from food with only free access to water for 24 hrs. On the experiment day, rats were randomly divided into 5 groups (n = 8, each); (i) normal control group received only distilled water, (ii) IND group received IND (50 mg/kg, i.p) for induction of ulcer, (iii) ALA-R group received pure ALA (100 mg/kg, p.o), (iv) ALA-F group received ALA formula (which contains an equivalent amount of ALA) and (v) group received plain SNEDDS (the vehicle of ALA formula). Group iii, iv and v were given ALA-R, ALA-F and the vehicle, respectively 30 minutes before IND injection.
Isolation of stomach and collection of gastric mucosae
As described in our previous study,21 4 hours after IND injection, rats were sacrificed by decapitation then their stomachs were removed, opened along the greater curvature, washed with ice-cold saline and photographed for scoring of gross mucosal lesions. Gastric mucosae were collected, homogenized in phosphate buffered saline solution and centrifuged at 4 oC to obtain gastric mucosal tissue homogenates that were stored at −80°C until used.
Assessment of gastric mucosal lesions
Quantitative assessment of gastric mucosal lesions was done in accordance with the method described in our previous studies.20,21 Stomachs were cleaned, pinned on board and pictures were taken for the stomach samples then were scored for the degree of mucosal damage using Image J software. Areas of mucosal damage were expressed as a percentage of the total surface area of the examined stomach. The ulcer index (UI) is the mean ulcer score of each animal. The ulcer score was determined by measuring the length of each lesion along its greatest diameter. The ulcer index was used to calculate the preventive index of the drug which is the percentage inhibition of gastric mucosal damage produced by such drug.
Preventive index = (U.I in indomethacin - U.I in treated rats) x 100/U.I in indomethacin.
Determination of Oxidative Stress
Oxidative stress was determined by measurement of gastric mucosal malondialdehyde (MDA) levels and serum total antioxidant activity (TAC).
Measurement of gastric mucosal malondialdehyde (MDA)
Malondialdehyde, a product of lipid peroxidation, was used as an index of oxidative stress. MDA reacts with thiobarbituric acid (TBA) at high temperature and forms MDA-TBA adducts that can be measured calorimetrically based on the method used by Mihara and Uchiyama25 and as described by our previous studies.20
Measurement of serum total antioxidant activity (TAC)
Serum total antioxidant activity was determined colorimetrically using the Total Antioxidant Assay Kit (Biodiagnostic, Giza, Egypt) in accordance with the manufacturer instruction and as previously described.21 The antioxidants in the sample react with the exogenously added hydrogen peroxide then the residual hydrogen peroxide was determined via a colorimetric reaction.
Histological Analysis of the Stomach
Stomach samples obtained from rats in different groups were fixed in 10% formalin for 24 h, and then processed for standard hematoxylin and eosin (H&E) staining. The sections were examined with a light electric microscope for histological changes and photomicrographs were taken using (AmscopeTR Digital Microscope Camera).
Statistical Analysis
The statistical analysis of the in vivo evaluation results was carried out utilizing Prism 5.0 software (Graph Pad Software Inc., San Diego, CA, USA). The comparison of means was performed using analysis of variance (ANOVA), followed by Tukey as a post-hoc test. The data are presented as the mean ± standard error of the mean (S.E.M). The differences were considered significant at P < 0.05.
Results
Optimization of ALA SNEDDS
In order to determine the composition of the optimized ALA SNEDDS with minimized droplet size, D-optimal mixture experimental design was utilized. D-optimality yields a design that best estimates the effect of the variables on the response (droplet size). The prepared SNEDDS exhibited nano-sized droplets ranging from 98.8 ± 2.3 to 246.9 ± 7.1 nm. The data of the droplet size best fitted to the special cubic model based on its highest correlation (R2) and lowest PRESS as shown in Table 2. In addition, the predicted and the adjusted R2 reasonably agreed with each other, and the adequate precision value of 175.17 (greater than 4) confirmed that the model could be applied to navigate the experimental design space.
Table 2.
Analysis of Variance Output for the Effect of Mixture Components on the Droplet Size of ALA SNEDDS.
| Source | Sum of squares | Degree of freedom | Mean square | F-value | P-value |
|---|---|---|---|---|---|
| Model | 40051.78 | 6 | 6675.30 | 4081.71 | <0.0001 |
| Linear mixture | 39509.09 | 2 | 19754.54 | 12079.20 | <0.0001 |
| X1X2 | 50.89 | 1 | 50.89 | 31.12 | 0.0003 |
| X1X3 | 3.67 | 1 | 3.67 | 2.25 | 0.1683 |
| X2X3 | 22.33 | 1 | 22.33 | 13.65 | 0.0050 |
| X1X2X3 | 77.84 | 1 | 77.84 | 47.60 | <0.0001 |
| Residual | 14.72 | 9 | 1.64 | ||
| Lack of fit | 10.57 | 4 | 2.64 | 3.19 | 0.1177 |
| Pure error | 4.15 | 5 | 0.8290 | ||
| Cor total | 40066.50 | 15 |
Abbreviation: ALA SNEDDS, alpha-lipoic acid self-nanoemulsifying drug delivery systems.
Figure 1 is showing diagnostic plots that evaluate the goodness of fit of the selected model. The color points representing the determined droplet size in the externally studentized residuals vs. predicted response plot were randomly distributed within the limits with most of them close to zero-axis indicating no constant error (Figure 1A). In addition, the predicted versus actual size plot showed good linearity and good analogy between the observed and predicted values (Figure 1B).
Figure 1.
Diagnostic plots for droplet size of ALA SNEDS; (A) studentized residuals vs. predicted values plot and (B) predicted vs. actual values plot.
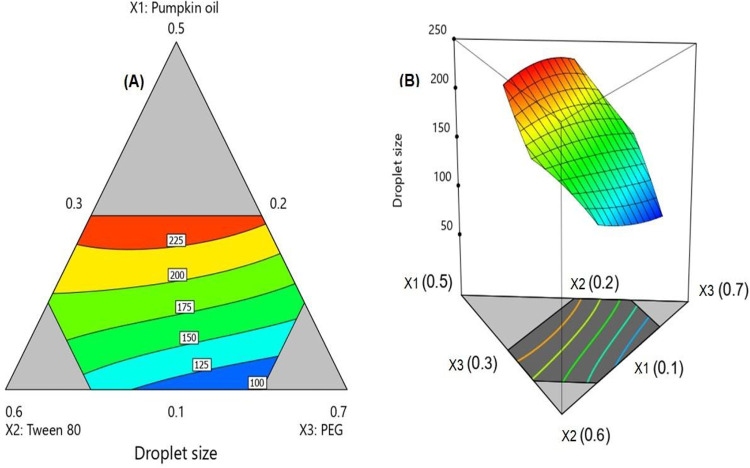
Analysis of variance (ANOVA) for the droplet size affirmed that the special cubic model was significant as depicted by its F-value of 408.71 (P < 0.0001). The non-significant lack of fit relative to pure error with F-value of 3.19 ensured that the data fitted the proposed model. The statistical analysis revealed the significance of oil, surfactant, and co-surfactant proportions on the droplet size. The interactions between surfactant and either oil (X1X2) or co-surfactant (X2X3) and the interaction between the 3 components (X1X2X3) were also significant at the same significance level (Table 3). Further, Figure 2 shows the contour and 3D surface diagrams illustrating the effect of varying proportions of (X1), (X2) and (X3) on the SNEDDS droplet size. In the contour diagram, Figure 2A, the 3 components of the mixture were located at the triangle vertices. The gray regions displayed in the figure represent areas not applied in the regression owing to the constraints of the components. The equation representing the special cubic model was generated in terms of coded factor as follows:
Table 3.
Model Fit Statistics of ALA SNEDDS.
| Model | Sequential P-value | Lack of fit P-value | SD | R2 | Adjusted R2 | Predicted R2 | PRESS |
|---|---|---|---|---|---|---|---|
| Linear | <0.0001 | <0.0001 | 6.55 | 0.9861 | 0.9839 | 0.9780 | 882.46 |
| Quadratic | 0.0001 | <0.0001 | 3.04 | 0.9977 | 0.9965 | 0.9848 | 206.70 |
| Special cubic | <0.0001 | 0.1177 | 1.28 | 0.9996 | 0.9994 | 0.9990 | 41.97 |
Abbreviations: ALA SNEDDS, Alpha-lipoic acid self-nanoemulsifying drug delivery systems; R2, multiple correlation coefficient; PRESS, predicted residual error sum of squares.
Figure 2.
2D contour plot (A) and 3D surface plot (B) for the effect of mixture components on the droplet size of ALA SNEDDS.
The goal of the optimization of pharmaceutical formulations is to detect the levels of the variables from which a product with desired characteristics may be generated. In our study the goal was to minimize the droplet size to enhance the biopharmaceutical performance of the SNEDDS. According to the numerical optimization technique, the SNEDDS with oil, surfactant, and cosurfactant proportions of 0.1, 0.3, and 0.6, respectively, could achieve minimized droplet size with a desirability 0f 0.9991. The percentage relative error between predicted size (98.85 nm) and the observed one (97.12) was 1.75%. This small error percentage confirms the credibility of the optimization process.
Stability of the Optimized ALA SNEDDS
The optimized ALA-SNEDDS stability study was assessed for phase separation. Optimized ALA-SNEDDS stability data indicated non-significant variation (P < 0.05) in the measured SNEDDS globule size before and after the study. These results indicated that optimized ALA-SNEDDS formula showed nano-dispersion stabilization upon storage under various conditions.
In Vivo Assessment of Optimized ALA-SNEDDS Formulation
Effect of pure ALA and ALA-SNEDDS formulation on IND-induced gastric lesions
The degree of gastric mucosal damage was quantified and expressed as ulcer index. As shown in Figure 3A, IND caused a marked gastric mucosal damage with the highest ulcer index among the experimental groups. Pretreatment with both pure ALA and ALA formula resulted in a significant (P < 0.001) reduction in gastric mucosal lesions compared to IND (Figure 3A). In addition, the reduction of mucosal lesions in the ALA formula pretreated rats was more pronounced when compared to raw ALA; however the difference was not statistically significant (Figure 3B). Notably, in the vehicle group, the gastric mucosal damage was significantly lowered when compared to IND (Figure 3A and 3B). Representative photos of the stomachs from the 4 different groups are shown in Figure 3C.
Figure 3.
Bar graphs showing the effect of indomethacin (IND), pure alpha-lipoic acid (ALA), and alpha-lipoic acid formula (ALA-F) on ulcer index (A), preventive index (B), and stomach photos from different groups (C). Data are presented as mean ± S.E.M. # Significantly different from indomethacin at P < 0.05, ### significantly different from indomethacin at P < 0.001.
Effect of pure alpha-lipoic acid and alpha-lipoic acid formulations on gastric mucosa lipid peroxidation
Figure 4 shows the effect of pretreatment with ALA and ALA-F on gastric mucosal MDA levels in IND treated rats. IND significantly (P < 0.01) elevated the level of gastric mucosal MDA when compared to control group (Figure 4). The effect of IND on mucosal MDA was significantly (P < 0.01) attenuated with different treatments ALA-R, ALA-F and the vehicle (plain SNEDDS) (Figure 4). There is a non-significant difference between pure ALA and ALA-F effects in this regard.
Figure 4.

Bar graphs showing the effect of indomethacin (IND), pure alpha-lipoic acid (ALA), alpha-lipoic acid formula (ALA-F) and vehicle on mucosal level of MDA. Data are presented as mean ± S.E.M. *** Significantly different from control at P < 0.001, ### significantly different from indomethacin at P < 0.001. Effect of pure alpha-lipoic acid and lipoic acid formulations on serum total antioxidant capacity.
Figure 5 shows the effect of IND on serum total antioxidant capacity. IND resulted in a significant (P < 0.01) increase in serum TAC compared to the control group. Pretreatment with ALA-formula significantly (P < 0.01) attenuated the observed high serum TAC induced by IND when compared to IND or pure ALA. Pure ALA failed to prevent the increased levels of TAC induced by IND treatment (Figure 5).
Figure 5.

Bar graphs showing the effect of indomethacin (IND), pure alpha-lipoic acid (ALA), and alpha-lipoic acid formula (ALA-F) on serum total antioxidant capacity (TAC). Data are presented as mean ± S.E.M. ** significantly different from control at P < 0.01, ## significantly different from indomethacin at P < 0.01, $$ significantly different from ALA-R at P < 0.01.
Effect of Pure Alpha-Lipoic Acid and Alpha-Lipoic Acid Formulations on the Histopathological Features of the Stomach
Figure 6 showed the histopathological examination of H&E-stained stomach sections. Stomach sections of control rats showed normal surface epithelium lining without ulceration nor hemorrhage with acini formed of parietal and chief cells (Figure 6A). Sections examined from IND-treated group revealed scattered areas of superficial mucosal ulceration covered with necrotic and hemorrhagic tissue with underlying congestion and groups of inflammatory cellular infiltrates in the form of neutrophils and lymphocytes (Figure 6B). ALA-R sections examined compact antral glands composed of parietal and chief with area of superficial ulceration, congested mucosa and focal lymphocytic inflammatory infiltrates (Figure 6C). Sections from ALA-F treated rats revealed branching mucous glands of fundic region composed of compact parietal, chief cells with deep glandular structures, few intraepithelial lymphocytic infiltrates with area of superficial mucosal ulceration (Figure 6D). Stomach sections of rats treated with vehicle showed compact branching mucous gland of fundic region with areas of superficial ulceration and intraepithelial lymphocytic infiltrates, eosinophilic and lymphocytic infiltrates in the lamina propria (Figure 6E).
Figure 6.
Representative photomicrographs of H&E-stained stomach sections of: (A) control group, (B) indomethacin treated group, (C) pure ALA + indomethacin, (D) ALA formula + indomethacin, (E) vehicle treated group. Magnification = 200×. H&E stain.
Discussion
There is a growing interest in the gastroprotective effects of ALA in different experimental ulcer models including IND-induced gastric ulcer.15,26,27 However, unfortunately, the physicochemical properties of ALA including its low solubility with consequent reduced absorption obstacle its pharmacological potentials and may lead to the requirement of high doses of ALA to achieve the desired pharmacological actions.17 Thus, in the current study, we reported that formulating ALA in the form of self-nanoemulsifying systems has shown promising improvement in its gastroprotective effects against IND-induced gastric ulcer. ALA was formulated in SNEDDS composed of pumpkin oil, surfactant, and co-surfactant to yield a spontaneous nanoemmulsion of droplet size less than 100 nm (Table 1). The improvement in the pharmacological activity of ALA could be interpreted based on solubility and consequent absorption enhancement.22,27,28 To test the efficacy of formulating ALA in SNEDDS against IND-induced gastric ulcer, firstly, ALA-SNEDDS formula was optimized to minimize the droplet size to reasonably enhance the biopharmaceutical performance of the SNEDDS. According to the numerical optimization technique, the SNEDDS with pumpkin oil, surfactant, and co-surfactant proportions of 0.1, 0.3, and 0.6, respectively, has successfully achieved the targeted minimal droplet size with a desirability of 0.9991. The percentage relative error between predicted size (98.85 nm) and the observed one (97.12) was 1.75%. This small error percentage confirms the reliability of the optimization process. It was evident that the oil proportion plays the most significant effect on the droplet size as evidenced by its highest coefficient. The observed increase in droplet size with increasing oil proportion could be attributed to increased viscosity of the formulation. This result is in accordance with previous studies.29,30 Secondly, we compared the gastroprotective efficacy of ALA formula to raw ALA in IND-induced gastric ulcer model. IND has been considered a common and reliable model for induction of acute gastric ulcer as reported by many studies.31,32 We demonstrated an increase in ulcer index, a decrease in preventive index as well as macroscopic stomach changes in IND treated group indicating damage of gastric mucosa that was confirmed by loss of normal histopathological architecture manifested as excessive necrosis, hemorrhage and infiltration with neutrophils and lymphocytes. Based on previous studies, the pathological mechanisms underlying IND-induced mucosal damage include; decreased PGE2 levels, increased gastric acid secretion and increased inflammatory and apoptotic mediators.27 Pretreatment with either raw ALA or ALA formula has protected against IND-induced gastric mucosal damage however, the gastroprotective effects of ALA formula was more pronounced than raw ALA. This effect was obvious through the improvement in the stomach macroscopic changes, the significant decrease in the ulcer index and the improvement in histopathological changes compared to IND-treated group. While decreased PGE2 is considered a detrimental influence on IND-induced gastric mucosal damage, other mechanisms have been found to be greatly involved as increased oxidative stress.26,33 In order to fully explore the influence of antioxidant defense system on the ulceration process throughout all gastric tissues, the levels of TAC and MDA were evaluated. In agreement with other studies,31,32,34 we reported a significant increase in gastric mucosal MDA levels in IND-treated groups. ALA loading on SNEDDS exhibited strong antioxidant properties as evidenced by decreased MDA content. This is strengthened by the known role of oxidative stress in the pathogenesis of peptic ulcer.35 MDA, the product of lipid peroxidation, is used as an indicator for oxidative stress since ROS reacts with phospholipid membranes of gastric mucosa leading to damage of gastric mucosa and elevation of MDA levels. Further, IND performs pro-oxidant activity hence forming ROS and interfering with major cellular antioxidants36; hence there was a significant increase in total antioxidant enzyme activity in IND-treated group. It is noteworthy to mention that there is a controversy regarding the effect of IND on the gastric mucosal antioxidant enzyme levels. Importantly, while some studies reported IND-mediated decrease in TAC,37-39 other studies including the current study reported a significant increase in TAC. The effects are likely explained by a compensatory mechanism for IND-induced increase in oxidative stress. For example, following the administration of IND, the antioxidant enzyme, catalase (CAT), activity was increased which was explained by an increased amount of H2O2. 33,37 Here, pretreatment with ALA and ALA formula has shown a significant decrease in MDA levels which parallel a significant prevention of IND-mediated increase in TAC levels. Basically, ALA has been known for its remarkable antioxidant activities, in different models not only in gastric ulcer, via removing heavy metals responsible for increased oxidative stress and restoring the antioxidant defense system.12,16,35\ , 36 Our findings revealed that ALA formula has more beneficial antioxidant effects than raw ALA suggesting that formulated ALA has higher free radical scavenging capacity than raw ALA. It is likely that ALA formula has small particle size that allows high drug solubility, absorption and hence increased pharmacological efficacy. It is important to mention that the vehicle of ALA-formula has shown beneficial effects against IND-induced gastric ulcer, to the extent that it could significantly decrease ulcer index and decrease elevated MDA levels. This finding is consistent with our previous study which reported a gastroprotective effect of pumpkin oil that has been used as a component of SNEDDs in this study.20 This finding may be a potential for using smaller doses of ALA since the vehicle itself of ALA formula could add to the therapeutic activity of ALA against IND-induced ulcer. However, further studies will be needed to confirm this by using different doses of ALA. In conclusion, ALA could protect against IND-induced ulcer via its antioxidant activity, however, using ALA in nanocarrier formulations (ALA-SNEDDS) that allows more drug solubility, bioavailability and stability enhances the gastroprotective effects of ALA against IND-induced ulcer.
Conclusions
In this study, ALA-SNEDDs formula was optimized to minimized particle size in order to enhance the solubility of ALA and consequently its absorption. D-optimal mixture experimental design was utilized to estimate the composition of the optimized ALA SNEDDS with minimized droplet size. Optimized ALA-SNEDDS achieved significant improvement in the gastric ulcer index in comparison with raw ALA. Histopathological findings confirmed the protective effect of the formulated optimized ALA-SNEDDS in comparison with raw ALA. Thus, optimized ALA-SNEDDS formulation significantly enhances the gastroprotective effects of ALA against IND-induced ulcer.
Acknowledgment
The authors acknowledge with thanks the DSR for technical and financial support.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah (grant no. RG-3–166–41). The authors, therefore, acknowledge with thanks the DSR for technical and financial support.
ORCID iD: Rana Bakhaidar  https://orcid.org/0000-0002-8761-1538
https://orcid.org/0000-0002-8761-1538
References
- 1. Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract. The ‘undrained abscess’ of multiple organ failure. Ann Surg. 1993;218(2):111–119. doi:10.1097/00000658-199308000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shiotani A, Graham DY. Pathogenesis and therapy of gastric and duodenal ulcer disease. Med Clin North Am. 2002;86(6):1447–1466, viii. doi:10.1016/s0025-7125(02)00083-4 [DOI] [PubMed] [Google Scholar]
- 3. Lee A. Animal models of gastroduodenal ulcer disease. Baillieres Best Pract Res Clin Gastroenterol. 2000;14(1):75–96. doi:10.1053/bega.2000.0060 [DOI] [PubMed] [Google Scholar]
- 4. Lucas S. The pharmacology of indomethacin. Headache. 2016;56(2):436–446. doi:10.1111/head.12769 [DOI] [PubMed] [Google Scholar]
- 5. Albayrak F, Odabasoglu F, Halici Z, et al. The role of erythropoietin in the protection of gastric mucosa from indometacin-induced gastric injury and its relationship with oxidant and antioxidant parameters in rats. J Pharm Pharmacol. 2010;62(1):85–90. doi:10.1211/jpp.62.01.0009 [DOI] [PubMed] [Google Scholar]
- 6. Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation. 2010;33(4):224–234. doi:10.1007/s10753-009-9176-5 [DOI] [PubMed] [Google Scholar]
- 7. AlKreathy HM, Alghamdi MK, Esmat A. Tetramethylpyrazine ameliorates indomethacin-induced gastric ulcer in rats: impact on oxidative, inflammatory, and angiogenic machineries. Saudi Pharm J. 2020;28(8):916–926. doi:10.1016/j.jsps.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jabri MA, Rtibi K, Tounsi H, et al. Fatty acid composition and mechanisms of the protective effects of myrtle berry seed aqueous extract in alcohol-induced peptic ulcer in rat. Can J Physiol Pharmacol. 2017;95(5):510–521. doi:10.1139/cjpp-2016-0094. [DOI] [PubMed] [Google Scholar]
- 9. Reed LJ, DeBusk BG, Gunsalus IC, Hornberger CS, Jr. Crystalline alpha-lipoic acid; a catalytic agent associated with pyruvate dehydrogenase. Science. 1951;114(2952):93–94. doi:10.1126/science.114.2952.93 [DOI] [PubMed] [Google Scholar]
- 10. Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997;29(3):315–331. doi:10.1016/s0306-3623(96)00474-0 [DOI] [PubMed] [Google Scholar]
- 11. Xiang W, Wang L, Cheng S, Zhou Y, Ma L. Protective effects of α-lipoic acid on vascular oxidative stress in rats with hyperuricemia. Curr Med Sci. 2019;39(6):920–928. doi:10.1007/s11596-019-2124-1 [DOI] [PubMed] [Google Scholar]
- 12. Piechota-Polanczyk A, Zielińska M, Piekielny D, Fichna J. The influence of lipoic acid on caveolin-1-regulated antioxidative enzymes in the mouse model of acute ulcerative colitis. Biomed Pharmacother. 2016;84:470–475. doi:10.1016/j.biopha.2016.09.066 [DOI] [PubMed] [Google Scholar]
- 13. Kabel AM, Salama SA, Alghorabi AA, Estfanous RS. Amelioration of cyclosporine-induced testicular toxicity by carvedilol and/or alpha-lipoic acid: Role of TGF-β1, the proinflammatory cytokines, Nrf2/HO-1 pathway and apoptosis. Clin Exp Pharmacol Physiol. 2020;47(7):1169–1181. doi:10.1111/1440-1681.13281 [DOI] [PubMed] [Google Scholar]
- 14. Ghibu S, Richard C, Vergely C, Zeller M, Cottin Y, Rochette L. Antioxidant properties of an endogenous thiol: alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J Cardiovasc Pharmacol. 2009;54(5):391–398. doi:10.1097/fjc.0b013e3181be7554 [DOI] [PubMed] [Google Scholar]
- 15. Tibullo D, Li Volti G, Giallongo C, et al. Biochemical and clinical relevance of alpha lipoic acid: antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm Res. 2017;66(11):947–959. doi:10.1007/s00011-017-1079-6 [DOI] [PubMed] [Google Scholar]
- 16. Kaplan KA, Odabasoglu F, Halici Z, et al. Alpha-lipoic acid protects against indomethacin-induced gastric oxidative toxicity by modulating antioxidant system. J Food Sci. 2012;77(11):H224–H230. doi:10.1111/j.1750-3841.2012.02920.x [DOI] [PubMed] [Google Scholar]
- 17. Li XM, Miao Y, Su QY, Yao JC, Li HH, Zhang GM. Gastroprotective effects of arctigenin of Arctium lappa L. on a rat model of gastric ulcers. Biomed Rep. 2016;5(5):589–594. doi:10.3892/br.2016.770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salehi B, Berkay Y, Antika G, et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules. 2019;9(8):356. Published 2019 August 9. doi:10.3390/biom9080356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gorąca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid - biological activity and therapeutic potential. Pharmacol Rep. 2011;63(4):849–858. doi:10.1016/s1734-1140(11)70600-4 [DOI] [PubMed] [Google Scholar]
- 20. Ahmed OAA, Fahmy UA, Bakhaidar R, et al. Pumpkin oil-based nanostructured lipid carrier system for antiulcer effect in NSAID-induced gastric ulcer model in rats. Int J Nanomedicine. 2020;15:2529–2539. Published 2020 April 15. doi:10.2147/IJN.S247252 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Ahmed OAA, Fahmy UA, Bakhaidar R, et al. Omega-3 self-nanoemulsion role in gastroprotection against indomethacin-induced gastric injury in rats. Pharmaceutics. 2020;12(2):140. Published 2020 February 7. doi:10.3390/pharmaceutics12020140 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Shahba AA, Mohsin K, Alanazi FK. Novel self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of cinnarizine: design, optimization, and in-vitro assessment. AAPS PharmSciTech. 2012;13(3):967–977. doi:10.1208/s12249-012-9821-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheong AM, Tan ZW, Patrick NO, Tan CP, Lim YM, Nyam KL. Improvement of gastroprotective and anti-ulcer effect of kenaf seed oil-in-water nanoemulsions in rats. Food Sci Biotechnol. 2018;27(4):1175–1184. doi:10.1007/s10068-018-0342-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fahmy UA, Ahmed OA, Hosny KM. Development and evaluation of avanafil self-nanoemulsifying drug delivery system with rapid onset of action and enhanced bioavailability. AAPS PharmSciTech. 2015;16(1):53–58. doi:10.1208/s12249-014-0199-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mihara M, Uchiyama M. Properties of thiobarbituric acid-reactive materials obtained from lipid peroxide and tissue homogenate. Chem Pharm Bull (Tokyo). 1983;31(2):605–611. doi:10.1248/cpb.31.605 [DOI] [PubMed] [Google Scholar]
- 26. Hosny KM, Bahmdan RH, Alhakamy NA, Alfaleh MA, Ahmed OA, Elkomy MH. Physically optimized nano-lipid carriers augment raloxifene and vitamin d oral bioavailability in healthy humans for management of osteoporosis. J Pharm Sci. 2020;109(7):2145–2155. doi: 10.1016/j.xphs.2020.03.009. Epub 2020 Mar 17. PMID: 32194094. [DOI] [PubMed] [Google Scholar]
- 27. Gomes MB, Negrato CA. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndr. 2014;6(1):80. doi:10.1186/1758-5996-6-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malfait AM, Gallily R, Sumariwalla PF, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97(17):9561–9566. doi:10.1073/pnas.160105897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Odabasoglu F, Cakir A, Suleyman H, et al. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J Ethnopharmacol. 2006;103(1):59–65. doi:10.1016/j.jep.2005.06.043 [DOI] [PubMed] [Google Scholar]
- 30. Morsy MA, El-Moselhy MA. Mechanisms of the protective effects of curcumin against indomethacin-induced gastric ulcer in rats. Pharmacology. 2013;91(5-6):267–274. doi:10.1159/000350190 [DOI] [PubMed] [Google Scholar]
- 31. Shahin NN, Abdelkader NF, Safar MM. A Novel role of irbesartan in gastroprotection against indomethacin-induced gastric injury in rats: targeting DDAH/ADMA and EGFR/ERK signaling. Sci Rep. 2018;8(1):4280. Published 2018 March 9. doi:10.1038/s41598-018-22727-631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Şen LS, Özdemir Kumral ZN, Memi G, Ercan F, Yeğen BC, Yeğen C. The gastroprotective effect of obestatin on indomethacin-induced acute ulcer is mediated by a vagovagal mechanism [published online ahead of print, 2020 Jul 17]. Physiol Int. 2020. doi:10.1556/2060.2020.00025 [DOI] [PubMed] [Google Scholar]
- 33. Ou P, Tritschler HJ, Wolff SP. Thioctic (lipoic) acid: a therapeutic metal-chelating antioxidant?. Biochem Pharmacol. 1995;50(1):123–126. doi:10.1016/0006-2952(95)00116-h33. [DOI] [PubMed] [Google Scholar]
- 34. Fahmy UA, Aljaeid BM. Combined strategy for suppressing breast carcinoma MCF-7 cell lines by loading simvastatin on alpha lipoic acid nanoparticles. Expert Opin Drug Deliv. 2016;13(12):1653–1660. doi:10.1080/17425247.2016.1236788 [DOI] [PubMed] [Google Scholar]
- 35. Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94(2):329–354. doi:10.1152/physrev.00040.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park CH, Youn HR, Lee J, et al. Improved efficacy of appetite suppression by lipoic acid particles prepared by nanocomminution. Drug Dev Ind Pharm. 2009;35(11):1305–1311. doi:10.3109/03639040902902385. [DOI] [PubMed] [Google Scholar]
- 37. Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Potentials and challenges in self-nanoemulsifying drug delivery systems. Expert Opin Drug Deliv. 2012;9(10):1305–1317. doi:10.1517/17425247.2012.719870 [DOI] [PubMed] [Google Scholar]
- 38. Li JH, Ju GX, Jiang JL, Li NS, Peng J, Luo XJ. Lipoic acid protects gastric mucosa from ethanol-induced injury in rat through a mechanism involving aldehyde dehydrogenase 2 activation. Alcohol. 2016;56:21–28. doi:10.1016/j.alcohol.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 39. Patel J, Patel A, Raval M, Sheth N. Formulation and development of a self-nanoemulsifying drug delivery system of irbesartan. J Adv Pharm Technol Res. 2011;2(1):9–16. doi:10.4103/2231-4040.79799 [DOI] [PMC free article] [PubMed] [Google Scholar]