Abstract
The aim of this study was to investigate the phytochemicals using reverse-phase high pressure liquid chromatography (RP-HPLC), antioxidant, antifungal and antibacterial activities of Seriphidium oliverianum stem extracts. The extraction was carried out by conventional shaking process (CSP) and ultrasonic assisted process (UAP). The highest total phenolic contents (97.85 ± 0.735 mg gallic acid equivalent (GAE)/g sample) and flavonoid contents (188.15 ± 0.53 mg catechin equivalent (CE)/g sample) were found in methanol extract obtained by CSP. Antioxidant activity was investigated using DPPH° scavenging assay and reducing power assay. Methanol extract using UAP showed the highest DPPH° scavenging activity (79.95% ± 1.80%) followed by methanol and butanol extracts obtained through CSP. Moreover, methanol extracts using CSP showed highest reducing activity (1.032 ± 0.0205 absorbance). In-vitro antimicrobial activity was studied using most common infection causing fungal and bacterial strains. Anti-fungal activity of methanol extract using CSP showed the highest zone of inhibition (10.5 mm) against F. avenaceum fungal strain, while aqueous extracts obtained through showed the highest antibacterial activity (22 ± 1.32 mm zone of inhibition) against S. aureus. The results showed that the methanol stem extract of S. oliverianum is a valued candidate for further screening and could be processed for in-vivo infection induced animal trials.
Keywords: Seriphidium oliverianum, solvent extraction, RP-HPLC, polyphenols, antioxidants, antimicrobial activity
Introduction
Baluchistan is the largest province of Pakistan in term of land area which located southwest of country with coordinates 27.7°N 65.7°E. Its land comprises of variety of variations in land structure and climates. It mainly consists of vast deserts, forests and mountains. Its big area also touches the sea with distinction of Gwadar Port. The environment in all parts of the province shows great variation—hot to quite cool and pleasant. The variation in weather makes it rich in medicinal floras which are known to whole world for its therapeutic potentials. S. oliverianum is medicinally least explored specie which belongs to the Seriphidium genus and Asteraceae family, and is native to Baluchistan region. The plant mainly grows in sandy clay (40-60 cm tall) having suffrutescent shrublet and 5-7 greenish-yellow florets (flower period August to September). The plant also grows in neighbor to Baluchistan such as Iran, Afghanistan and Central Asia (Turkmenia).
Various species of genus Seriphidium are well known for its folk medicine utilization over the globe; however, S. oliverianum first gained the fame due to α-thujone, β-thujone, 1,8-cineole and β-caryophyllene compounds which were identified in its essential oil.1 The leave extract of the plants also a source of pretty good antioxidants such as gallic acid, kaempferol 3-O-β-D-glucopyranoside and 1,2,4,6-O-tetra-galloyl-β-D-glucopyranoside.2 Nusrat et al (2014) reported 11 phytochemicals isolated from S. oliverianum of medicinal interest which had already been isolated from other sources as well.3 In another report the compounds isolated from S. oliverianum leave extract; 3,6-Odimethylquercetagetin-7-O-β-D-glucoside and 5,3′,4′-trihydroxy-6,7-dimethoxyflavone showed mild antioxidant (IC50 = 130 μg/mL) and urease inhibition potential (IC50 = 129 μg/mL), respectively.4
Antioxidant, anti-microbial and number of other biological studies are conducted to test the medicinal potential of natural products.5,6 Approximately 8000 naturally occurring compounds are known as phenolics and all bear at least 1 aromatic ring occupied with 1 or more hydroxyl group/s.7 These compounds are the custodian of variety of biological activities such as antioxidant, antibacterial, anticancer, etc. and could be termed as index of phytomedicine.8,9 The alkaloids (piperine, berberine, dictamnine, kokusagine, masculine, reserpine, sanguinarine, tomatidine, chanoclavine),10,11 organosulfur compounds (allicin, ajoene, isothiocyanates, sulforaphane, phenethyl isothiocyanate, berteroin),12 coumarins,13 terpenes,14 and a variety of phenolics are responsible for antibacterial activities of plants.15 They have also been involved in diverse functions, including protein synthesis, enzyme activity, structural components and allelopathy.16
The aim of this work was to analyze the phenolic and flavonoid compounds, antioxidant and antimicrobial potential of S. oliverianum stem extracts using CSP and UAP. To our best knowledge, few reports have been published on leave extracts, so there is a need to explore the medicinal potential of extracts of other parts of S. oliverianum.
Material and Methods
Material
S. oliverianum was initially collected from deserts of District Quetta, Baluchistan in September 2013, and identified by Prof. Dr. Rasool Bakhsh Tareen, Plant Taxonomist, Department of Botany, University of Baluchistan, Quetta and assigned voucher specimen SO-RBT-06 to save in university herbarium. All the plant parts were separated, washed with distilled water to remove dust particles, and placed in shaded area under aerated condition for 7 days at room temperature. The dried stem part of the plant was pulverized into fine powder using domestic grinder and saved in air-tight bags for further solvent extraction. All the solvents and chemicals that were used in this research work were of analytical grade and obtained from Sigma-Aldrich (Germany) with exception of methanol and butanol that was obtained from Alfa-Acer. The sonicater (VC 130, Sonics & materials, Inc, Newtown, CT) was purchased from USA. The HPLC system was of Perkin-Elmer (USA).
Preparation of Extracts
Different solvents such as methanol, butanol and water were used to extract phytochemicals from the stems of S. oliverianum using CSP and UAP. Briefly, CSP was carried out by mixing the dried sample powder (50 g) into 500 mL of solvent followed by subjecting the flask on orbital shaker for 8 h at 200 rpm and room temperature. While the extraction through UAP was carried out by mixing 10 g of dried powder into 100 mL of solvent followed by sonication procedure for 15 min at 50% amplitudes, 20 kHz frequency and 25 mm probe at room temperature using sonicater. Following the extraction process, the mixture was filtered through Whatman filter paper 1, filtrate was evaporated under reduced pressure using Heidolph rotary evaporator and finally the concentrated extract was stored at −4°C until further analysis. Percent yield of all extracts were calculated using following formula:
For every sample triplicates flasks were used for extraction and results were expressed as mean ± standard deviation (SD) (n = 3).
Determination of Total Phenolic Contents
Total phenolic contents (TPC) were determined using Folin-Ciocalteu method.17 Briefly, 1 mL of extract sample (15-120 mg/mL) was mixed with 1 mL of Folin-Ciocalteu reagent and mixture was allowed to stand for 5 min at room temperature followed by the addition of 5 mL of sodium carbonate solution (1 M) and shaken gently. Then the total volume of the mixture was adjusted to 10 mL by adding distilled water. The solution was then incubated at room temperature for 90 min. The absorbance was recorded at 765 nm. Gallic acid (15-120 mg/mL) was used for standard calibration curve. Results were expressed as gallic acid equivalents (GAE)/mg dry weight (DW) of extract.
Determination of Total Flavonoid Contents
Total flavonoid contents (TFC) were determined by using aluminum chloride colorimetric method.18 Briefly, 0.25 mL of extract (31.25-250 mg/mL) was added into 0.75 mL distilled water, followed by the addition of 0.15 mL of sodium nitrite solution (5%). After 5 min incubation period, 0.3 mL aluminum chloride (10%) was added Followed by another round of 5 min incubation, 1 mL of 1 M aqueous sodium hydroxide solution was added. The mixture was shaken gently. The absorbance of the mixture was noted at 510 nm in triplicate. The results were expressed in term of mg of catechin/g DW of extract.
HPLC Analysis of Phenolic Acids
Sample preparation
A method reported by Ying et al, (2009) with slight modification was followed to prepare sample.19 Briefly, 0.5 g of the dried and powdered stem plant was taken in a conical flask with lid along with a 0.5 mL mixture of standard phenolics, then extraction was performed with 50 mL aqueous mixture of methanol (50% V/V) in an ultrasonic bath for 30 min. The mixture was subjected to centrifugation at 3000 rpm for 5 min at 4°C. The supernatant was then filtered with 0.45 µm membrane filter. An aliquot of 20 µL filtrate was injected into HPLC system using micro syringe.
HPLC system and conditions
HPLC analysis was performed using Perkin Elmer series 200 HPLC system incorporated with UV/Visible detector. The system was equipped with reverse phase HPLC analytical C-18 column (4.6 × 250 mm, 5 µm stationary phase particle size), binary LC pump system and temperature control module. The protocol reported by Wen et al, (2005) with slight modification was followed.20 The binary solvent mobile phase system for gradient elution was chosen. Water was set as Solvent A, while methanol was as solvent B. Both solvents were acidified with 0.02% trifluoroacetic acid (TFA). The gradient elution was carried out as follow: 0-3 min, 25% B; 3-7 min, 25%-30% B; 7-12 min, 30%-50% B; 12-15 min, 50% B; 15-18 min, 50%-80% B; 18-22 min, 80% B; 22-25 min, 80%-25% B. The flow rate 1.0 mL/min was adjusted at 25°C column temperature. The detection wavelength 254 nm was selected.
Determination of Antioxidant Activity
Antioxidant activity of S. oliverianum stem extracts in methanol, butanol and aqueous medium was studied using DPPH° scavenging and reducing power assay.
DPPH free radical scavenging assay
DPPH° scavenging activity of plant extract was calculated by recording the potential of extracted sample to reduce DPPH° into DPPH-H (a colorless compound). The antioxidant potential of plant extract was measured by DPPH° scavenging assay with mild modification.21 Briefly, 0.1 mL of plant extract (62.5-500 mg/mL) was added in 2 mL of DPPH° solution (0.1 mM) in methanol and the mixture was allowed to stand for 1 h in dark at room temperature. The absorbance was measured at 517 nm. Free radical scavenging activity of plant extract was calculated by using following formula:
where the initial and final absorbance are the absorbance values of DPPH° at zero time and after 1 h of plant extract addition.
Reducing power assay
The reducing power activity of plant extract measured following pre-reported method.8 Briefly, 1 mL of extract sample (125-1000 µg/mL) mixed with 1 mL of 200 mM sodium phosphate buffer (pH 6.6) and 1 mL of 1% potassium ferricyanide solution. The mixture was shaken gently and incubated at 50°C for 20 min. After incubation period, 1 mL of 10% trichloroacetic acid solution was added followed by centrifugation for 10 min at 3000 rpm. The mixture was converted into supernatant and solid pellet; 2.5 mL supernatant was pipit-out and mixed with 2.5 mL of distilled water followed by the addition of 0.5 mL of 1% aqueous ferric chloride solution and shaken gently. The absorbance of solution was recorded at 700 nm. A typical blank solution contained the same solution mixture without plant extract taken as control while ascorbic acid was used as a reference standard.
Determination of Antimicrobial Activity
Anti-microbial activity of different solvent extracts of S. oliverianum stems were tested against infection causing fungal and bacterial strains.
Anti-fungal activity
MTCC 1344 strain of Aspergillus niger (A. niger), ATCC 60644 strain of Fusarium avenaceum (F. avenaceum) and MNCC1206 strain of Fusarium brachygibbosum (F. brachygibbosum) were used to investigate the anti-fungal activities of plant extracts. The anti-fungal activities of different extracted samples of S. oliverianum stems were performed by using well diffusion susceptibility method.22 Briefly, sterilized nutrient agar medium plates were seeded with 18-24 h old cultures of microbial inocula (standard inoculum of 1-2 × 107 CFU mL−1, 0.5 McFarland standard). Six wells (8 mm diameter) were cut into the solidified agar media with sterilized cork borer followed by the addition of an aliquot of 40 µL (4mg/mL) of extract in triplicate wells. Gentamycin and DMSO of equal volume and concentration were also inoculum into triplicate wells as positive and negative control, respectively. Then inoculated plates were incubated for 24 h at 37°C. After completion of incubation period, measured the zone of inhibition in mm.
Antibacterial potential
ATCC 25923 strain of Staphylococcus aureus (S. aureus), ATCC 25922 strain of Escherichia coli (E. coli), ATCC 15380 strain of Klebisella pneumonia (K. pneumonia), ATCC 27853 strain of Pseudomonas aeruginosa (P. aeruginosa), ACTT 17978 strain of Acinetobactor baumannii (A. baumannii), and SSFP(4 s) strain of Salmonella typhi (S. typhi) were obtained from clinical setup. All bacterial strains were cultured at 37°C for 24 h in Mueller Hinton agar (MHA) nutrient. The antibacterial activity of extracts was then carried out using Agar well diffusion method.23 Sterile MHA was used as a media, for preparation of petri plats. The test strains were swabbed on the surface of solidified media and kept for drying at room temperature for 10 min, after that 5 wells (6 mm diameter) were engraved with sterilized cork borer on the surface of solidified petri plate. Four different concentrations of plant extract (0.5-4 mg/mL) were tested with 3 replications. An aliquot of 40 µL of plant extract was inoculum in each well, and ciprofloxacin (5μg/disk) was used as positive control. After 12-15 min of diffusion time at room temperature, the inoculated plates were incubated at 37°C for 48 h. At the end of incubation period the antibacterial activity was determined by measuring the zone of inhibitions in millimeter (mm) unit of length. The diameter of the inhibition zone was measured in 3 directions and mean value was tabulated.
Statistical Analysis
Values are presented as mean ± SEM and scrutinized by 2-way ANOVA, followed by least significant difference (LSD) test using SPSS software and graph paid prism. The results of all extracts compared with standards. P value > 0.05 is considered as non-significant (ns), P value < 0.05 as significant (*) and P value < 0.001 as highly-significant (**).
Results and Discussion
Extraction Yield
The extraction of phytochemicals from S. oliverianum stems in organic and aqueous medium showed good yield. Methanol, butanol and aqueous extracts showed 15.55, 10.16 and 52.30% yield of dry sample using CSP, respectively—while with UAP, it was recorded 28.48, 7.56 and 42%, respectively. It was reported that most of the sandy-clay plants of Baluchistan origin show promising biological activities but poor extract yield.24 Butanol, due to its more lipophilic behavior as compared to methanol and water, showed weak potential to extract phytochemicals; however good yield in aqueous medium might be due to the fact that most of the phytochemicals bear electronegative functional groups which make the compound hydrophilic in nature.
Total Phenolic and Flavonoid Content
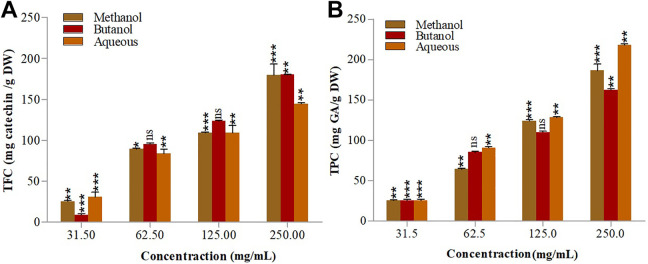
The results showed that methanol extract carry good amount of TPC and TFC followed by extraction in butanol using CSP; while UAP assisted significantly to extract TPC and TFC in aqueous medium. The highest TPC (97.85 ± 0.735 mg (GAE)/g sample) and TFC (188.15 ± 0.53 mg (CE) / g sample) were found in methanol extract using CSP while lowest TPC (42.56 ± 0.775 mg (GAE)/g sample) was recorded in aqueous extract as shown in Figure 1; while 142.11 ± 0.89 mg (CE) / g sample TFC was recorded in aqueous medium as shown in Figure 2. UAP, however assisted the aqueous medium to improve the extraction of TPC and TFC which increased to 64.39 ± 0.895 mg (GAE)/g sample and 219.05 ± 1.58 mg (CE) / g sample, respectively as compared to CSP. The findings are in good agreement with the report published previously.25 It was reported that multiple hydroxyl functional group present in phenolics and flavonoids are responsible of their biological and antioxidant activities. Molluscicidal, anthelmintic, antihepatoxic, anti-inflammatory, antidiarrheal, antiulcer, vasodilatory action, antiallergic and antiviral activities are in the profile of these compounds. They also play role in soil nitrogen mineralization.26 Other unique action of these compounds that has been reported are; inhibition of human immunodeficiency viral replication (HIV), Glucosyl-transferases of Streptococcus mutans, ascorbate autoxidation, human simplex virus (HSV), tumor proliferation, and xanthine/monoamine oxidases.27
Figure 1.
TPC of S. oliverianum stem extracts in different solvents using CSP (A) and UAP (B).
Figure 2.
TFC of S. oliverianum stem extracts in different solvents using CSP (A) and UAP (B).
HPLC Analysis
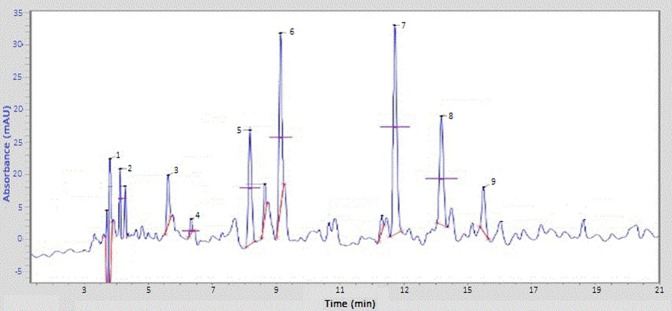
RP-HPLC with C18 columns is the most popular technique for the analysis of polyphenols of different foods and plant extracts. A typical HPLC chromatogram of phenolic constituents of S. oliverianum stem extract is shown in Figure 3. The amount of phenolic acids detected in the analyzed sample is shown in Table 1. Four phenolic acids; p-coumeric acid, sinapic acid, ferulic acid and caffeic acid were found in good amount while quercetin was detected in least quantity. It was reported that the ferulic acid, p-coumeric acid sinapic acid are actively involved in antimicrobial, antiviral, anti-mutagenesis, anti-cancer and diabetes mitigation, anti-inflammatory, anti-neurodegeneration activities,28,29 Caffeic acid play good role in therapy of Alzheimer’s Brain disease.30 Other detected phenolics has also been reported for their promising biological activities.31,32 Presence of these phenolic acids in S. oliverianum stem extract reflects its possible utilization in phytomedicines and food preservation processes.
Figure 3.
RP-HPLC analysis of S. oliverianum stem extract for phenolics.
Table 1.
Determination of Phenolic Contents Using RP-HPLC.
| Peak number | Compounds | Retention time (min) | Area (%age) |
|---|---|---|---|
| 1 | Ellagic acid | 3.741 | 0.412 |
| 2 | Gallic acid | 4.096 | 0.21 |
| 3 | Quercetin | 5.517 | 0.021 |
| 4 | HB acid | 6.312 | 0.33 |
| 5 | Caffeic acid | 8.141 | 1.73 |
| 6 | p-coumeric acid | 9.152 | 3.62 |
| 7 | Sinapic acid | 11.698 | 3.418 |
| 8 | Ferulic acid | 13.201 | 3.355 |
| 9 | Catechin | 15.511 | 0.78 |
Analysis of DPPH° Scavenging Activity
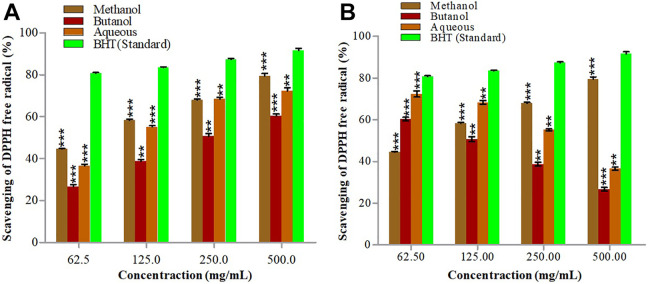
DPPH° scavenging assay is known to natural product researchers a most valued procedure to assess the antioxidant activity of the plant extracts. DPPH° (2,2-dipheny-l-picrylhydrazyl) is dark purple colored stable free radical that converts into colorless 2,2-dipheny-l-picrylhydrazine (DPPH-H) by accepting a hydrogen atom from phenolic compound. The quenching intensity of the DPPH° directly relates the concentration of phenolic. DPPH° scavenging potential of S. oliverianum stem extracts is shown in Figure 4. All of the assessed stem sample extracts revealed a gradual fading in purple-colored radical DPPH° into the yellow-colored DPPH-H by increasing the extract concentration. Methanol extract using UAP showed highest scavenging ability (79.95% ± 1.80%) followed by methanol and butanol extracts using CSP method, 76.66 ± 1.52 and 75.83% ± 1.60% respectively. Butylated hydroxyl toluene (BHT), which was used as standard DPPH° scavenging molecule showed 91.59% ± 1.52% scavenging activity. Phenolics and flavonoids, among the plant constituents are considered strong free radical scavenging compounds—hence can be termed as the index of free radical scavenging activity.33
Figure 4.
DPPH° scavenging activity of S. oliverianum stem extract in different solvents using CSP (A) and UAP (B).
Reducing Power Assay
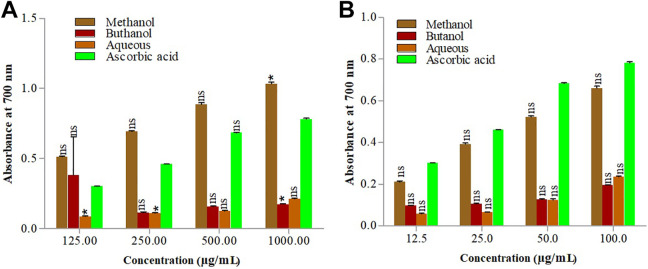
The chemistry of reducing power assay based on the reduction of potassium ferricyanide (Fe3+) to form potassium ferrocyanide (Fe2+) by components of plant extracts, followed by reacting with ferric chloride (FeCl3) which resulted in the formation of ferric–ferrous complex that has an absorption maximum at 700 nm. The concentration of ferric–ferrous complex formation depends upon the potassium ferrocyanide formation. It was reported as concentration dependent activity, which increases with the increase of natural product contents especially the polyphenolic compounds and flavonoids. Figure 5 shows the dose-dependent reducing power of S. oliverianum stem extracts. The highest absorbance was recorded (1.032 ± 0.0205) with 1 mg /mL methanol extract obtained using CSP. Moreover, as compared to other extracts obtained through UAP method methanol extract also showed highest absorbance 0.660 ± 0.0769. Previously reported studies describe that the free radical scavenging activity of plant extracts is correlated with their TPC, and our results are in agreement with their findings.34 The reducing potential of standard antioxidant, ascorbic acid showed comparatively low absorbance value (0.780 ± 0.110) at similar concentration level.
Figure 5.
Reducing power activity of S. oliverianum stem extract in different solvents using CSP (A) and UAP (B).
Antifungal Study
Methanol, butanol and aqueous extracts of S. oliverianum stems, obtained by CSP and UAP, were also evaluated for their anti-fungal activity. The results obtained against the tested microorganisms are shown in Table 2. Among the UAP, the aqueous extract was the most potent which showed the 9.5 ± 0.25 mm ZOI against A. niger while among the CSP process, the methanol and butanol extracts showed 10.5 ± 0.11 and 10 ± 0.14 mm ZOI against F. avenaceum and F. brachygibbosum, respectively appeared most potent. The comparison of anti-fungal activities between the 2 extraction procedures, indicated CSP process assisted more, than UAP process in the extraction of anti-fungal components. The results of all extracts were also compared with standard anti-fungal agent, gentamycin which showed 17.5 ± 1.675, 15.2 ± 1.13 and 14.9 ± 1.18 mm ZOI against F. brachygibbosum, A. niger and F. avenaceum, respectively. It was reported that some tannins, flavonoids, saponins, steroids, alkaloids mainly and α-bisabolol, a natural monocyclic sesquiterpene alcohol to some extent, are responsible for anti-fungal activity.35 This indicates the S. oliverianum stems possess good amount of these compounds as well and could be tested for further evaluation.
Table 2.
Anti-Fungal Activity of Stem Extracts of S. Oliverianum Plant Against Different Fungal Strains.
| Extraction solvent | Extraction technique | Zone of inhibition value (mm) | |||
|---|---|---|---|---|---|
| Concentration mg/mL | A. niger | F. avenaceum | F. brachygibbosum | ||
| Aqueous | CSP | 4 | 8.5 ± 0.5 | 8.5 ± 0.5 | 9 ± 0.5 |
| Methanol | 4 | 9.5 ± 0.5 | 10.5 ± 0.5 | 10 ± 0.5 | |
| Butanol | 4 | 9.5 ± 0.5 | 10 ± 0.5 | 10 ± 0.5 | |
| Aqueous | UAP | 4 | 9.5 ± 0.5 | 8.5 ± 1 | 8.5 ± 1 |
| Methanol | 4 | 8.5 ± 0.5 | 10. ± 0.5 | 9.5 ± 0.5 | |
| Butanol | 4 | 8 ± 0.5 | Negative | 8 ± 1 | |
| Gentamycin (standard drug) | 5µg/disk | 15 ± 1 | 15 ± 1 | 17.5 ± 0.32 | |
Values are expressed as mean ± standard deviation.
Antibacterial Activity
Antibacterial activity was tested against 1 Gram positive (S. aureus) and 5 Gram negative bacteria (P. aeruginosa, E. coli, K. pneumonia, A. baumannii & S. typhi) using agar well diffusion method. Antibacterial activity of all extracts is shown in Table 3. The results showed that all the extract showed good dose-dependent antibacterial activity. The highest growth inhibition potential attributed to 4 mg/mL extract sample. At this concentration in case of CSP; the aqueous extract was the most effective extract which showed the highest 22 ± 1.32 mm ZOI against S. aureus bacteria followed by butanol and methanol extracts (21 ± 1.04 and 18.0 ± 0.28 mm, respectively) against P. aeruginosa—while in case of UAP, butanol extract showed 20.5 ± 0.28 and 19 ± 0.100 mm ZOI against P. aeruginosa and A. baumannii, respectively followed by methanol extract which showed 18 ± 1.32 mm ZOI against P. aeruginosa. Moreover, K. pneumonia, A. baumannii and S. typhi in general, showed resistance to almost all extracts. Butanol and aqueous UAP extracts, however faced strong resistance from S. typhi with zero growth inhibition results. It is worth understanding that all these bacteria are referred to as food poising and food borne diseases; which need to address carefully.36 Most of the published food poisoning reports and bacterial infections indicated the involvement of members of Gram negative bacteria, especially P. aeruginosa, E. coli, K. pheumoniae, A. baumannii & S. typhi. The Gram negative bacteria also offer more resistance to antibacterial agents as compared to the Gram positive bacterial strains such as S. typhi is one that cause severe food borne illness known as typhoid fever and is treated with a set of antibiotics instead of single antibiotic.37,38 The results of the study were compared with the ciprofloxacin taken as standard antibacterial agent, it showed strong growth inhibition of P. aeruginosa with 25.5 ± 1.21 mm ZOI followed by 20.2 ± 1.10 mm ZOI against K. pneumonia, however some results were missed due to COVID-19 epidemic lockdown.
Table 3.
Antibacterial Activities of Stem Extracts of S. Oliverianum Plant Against Different Bacterial Strains.
| Extraction solvent | Extraction technique | Conc.mg/mL | Zones of inhibition value (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| S. aureus | P. aeruginosa | E. coli | K. pneumonia | A. baumannii | S. typhi | |||
| Methanol | CSP | 0.5 | 8.5 ± 0.28 | 8 ± 0.28 | 7.5 ± 0.84 | - | 7 ± 0.57 | 8.5 ± 0.50 |
| 1 | 9.5 ± 0.50 | 9.5 ± 0.50 | 8.5 ± 0.40 | 10 ± 1 | 9 ± 1.04 | 10 ± 0.76 | ||
| 2 | 9 ± 0.76 | 10 ± 0.86 | 12 ± 0.40 | 10.5 ± 0.57 | 10 ± 0.76 | 9 ± 0.76 | ||
| 4 | 11 ± 0.76 | 18. ± 0.28 | 14 ± 0.84 | 11 ± 0.76 | 9.5 ± 10.4 | 10 ± 1.0 | ||
| Butanol | 0.5 | - | 14 ± 0.25 | 8 ± 0.28 | 8 ± 1 | 14 ± 1.00 | 10 ± 0.57 | |
| 1 | - | 15 ± 0.28 | 9 ± 0.11 | 8.5 ± 0.50 | 8 ± 0.76 | 11 ± 0.76 | ||
| 2 | - | 19 ± 0.76 | 9. ± 50.86 | 9 ± 1 | 8. ± 50.57 | 12 ± 1.04 | ||
| 4 | 8 ± 0.57 | 21 ± 1.04 | 10.5 ± 0.57 | 15.5 ± 0.5 | 8.5 ± 0.57 | 9 ± 1.00 | ||
| Water | 0.5 | 7 ± 0.57 | 7 ± 0.50 | 7 ± 0.28 | - | - | 9 ± 1.00 | |
| 1 | 8 ± 0.50 | 7.5 ± 0.76 | 8 ± 0.76 | 8.5 ± 1.00 | - | 9 ± 1.00 | ||
| 2 | 9.5 ± 0.57 | 14 ± 0.28 | 9.5 ± 0.28 | 9 ± 1.00 | - | 8 ± 0.76 | ||
| 4 | 22 ± 1.32 | 16 ± 0.28 | 17 ± 1.04 | 10 ± 1.00 | - | 8 ± 0.57 | ||
| Methanol | UAP | 0.5 | 9 ± 0.57 | 9.5 ± 0.76 | 7 ± 0.28 | - | 9 ± 1.00 | 13 ± 1.04 |
| 1 | 10 ± 0.76 | 12 ± 0.50 | 7 ± 0.57 | 9.5 ± 0.50 | 9 ± 1.00 | 12.5 ± 0.50 | ||
| 2 | 12 ± 0.57 | 10 ± 0.50 | 9 ± 0.28 | 15 ± 0.76 | 8 ± 1.00 | 9 ± 1.00 | ||
| 4 | 10 ± 0.76 | 18 ± 1.32 | 12.5 ± 0.76 | 10.5 ± 0.57 | 9.5 ± 0.76 | 10 ± 1.00 | ||
| Butanol | 0.5 | 10 ± 1.52 | 10.5 ± 0.76 | 7.5 ± 0.28 | 7.5 ± 0.28 | 16 ± 1.73 | - | |
| 1 | 13 ± 0.28 | 14.5 ± 0.57 | 9.5 ± 0.76 | 8.5 ± 0.57 | 19.5 ± 132 | - | ||
| 2 | 14 ± 0.76 | 16 ± 0.50 | 9 ± 0.28 | 8 ± 0.76 | 17.5 ± 1.32 | - | ||
| 4 | 20.5 ± 0.5 | 20.5 ± 0.28 | 12 ± .1.04 | 9 ± 0.76 | 19 ± 0.100 | - | ||
| Water | 0.5 | 7.5 ± 0.57 | 8.5 ± 0.5 | 7.5 ± 0.50 | - | - | - | |
| 1 | 9.0 ± .76 | 9 ± 0.5 | 11 ± 0.50 | - | - | - | ||
| 2 | 9.5 ± 0.76 | 11 ± 0.76 | 8.5 ± 0.52 | 8 ± 1.00 | 8 | - | ||
| 4 | 13 ± 0.11 | 14.5 ± 0.57 | 7.5 ± 0.76 | 9 ± 1.00 | 9 | - | ||
| Ciprofloxacin | 5µg | 25 ± 0.15 | - | 25 ± | 11 ± | 20 | ? | |
| standard drug | ||||||||
Values are expressed as mean ± standard deviation; (-) No Result.
Conclusion and Future Perspective
It is appealing that methanol, butanol and aqueous S. oliverianum stem extracts showed considerable amount of ferulic acid, p-coumeric acid sinapic acid as detected by RP-HPLC which are indicator that extract of stem could be refined as functional food and phytomedicine. The results of antioxidant such as 79.95% ± 1.80% DPPH° scavenging activity and 1.032 ± 0.0205 (absorbance) reducing activity are also promising phytomedicine indicators. Further, 10.5 mm ZOI using F. avenaceum fungal strain and 22 ± 1.32 mm ZOI against S. aureus reflect plant’s medicinal impact. In conclusion, S. oliverianum stem extracts could be subjected for further investigation using preclinical in-vitro and in-vivo models.
Acknowledgments
The authors are grateful to Higher Education Commission (HEC), Islamabad, Pakistan and Government College University, Faisalabad, Pakistan for supporting in research facilities. The authors are also thankful to Dr. Nasir Rasool for his assistance to collect plant material.
Authors’ Note: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Syed Ali Raza Naqvi  https://orcid.org/0000-0002-2172-9066
https://orcid.org/0000-0002-2172-9066
References
- 1. Syed AG, Yoshiharu F, Mami S, Kazuo NW. Chemotypic variations and phytotoxic studies of essential oils of endemic medicinal plant, seriphidium kurramense, from Pakistan. J Med Plants Res. 2010;4(4):309–315. [Google Scholar]
- 2. Ho S-T, Tung Y-T, Chen Y-L, Zhao Y-Y, Chung M-J, Wu J-H. Antioxidant activities and phytochemical study of leaf extracts from 18 indigenous tree species in Taiwan. Evid-Based Complement Alternat Med. 2012;2012:215959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shafiq N, Ali L, Riaz N, et al. Isolation, characterization of flavonoids from seriphidium oliverianum and their antioxidant and anti-urease activities. J Chem Soc Pak. 2014;36(3):517–523. [Google Scholar]
- 4. Nusrat S, Naila R, Muhammad S, Maryam R. Phytochemical screening and biological evaluation of some potential constituents of Seriphidium oliverianum. RJLBPCS. 2017;3(2):37–42. [Google Scholar]
- 5. Naqvi SAR, Mahmood N, Naz S, et al. Antioxidant and antibacterial evaluation of honey bee hive extracts using in vitro models. Med J Nutrition Metab. 2013;6(3):247–253. [Google Scholar]
- 6. Ding H, Yang X, Xu L, et al. Analysis and comparison of tribological performance of fatty acid-based lubricant additives with phosphorus and sulfur. J Bioresour Bioproducts. 2020;5(2):134–142. [Google Scholar]
- 7. Stalikas CD. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Separat Sci. 2007;30(18):3268–3295. [DOI] [PubMed] [Google Scholar]
- 8. Sahar A, Naqvi SAR, Khan ZA, et al. Screening of phytoconstituents, investigation of antioxidant and antibacterial activity of methanolic and aqueous extracts of Cucumis sativus. J Chem Soc Pak. 2013;35(2):457–463. [Google Scholar]
- 9. Asghar N, Naqvi SAR, Hussain Z, et al. Compositional difference in antioxidant and antibacterial activity of all parts of the Carica papaya using different solvents. Chem Cent J. 2016;10:5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dwivedi GR, Maurya A, Yadav DK, et al. Synergy of clavine alkaloid ‘chanoclavine’ with tetracycline against multi-drug-resistant E. coli. J Biomol Struct Dyn. 2019;37(5):1307–1325. [DOI] [PubMed] [Google Scholar]
- 11. Alhanout K, Malesinki S, Vidal N, Peyrot V, Rolain JM, Brunel JM. New insights into the antibacterial mechanism of action of squalamine. J Antimicrob Chemother. 2010;65(8):1688–1693. [DOI] [PubMed] [Google Scholar]
- 12. Sofrata A, Santangelo EM, Azeem M, Borg-Karlson AK, Gustafsson A, Pütsep K. Benzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against Gram-negative bacteria. PloS One. 2011;6(8):e23045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Majedy YK, Kadhum AAH, Al-Amiery AA, Mohamad AB. Coumarins: the antimicrobial agents. Syst Rev Phar. 2017;8(1):62. [Google Scholar]
- 14. Paduch R, Kandefer-Szerszeń M, Trytek M, Fiedurek J. Terpenes: substances useful in human healthcare. Arch Immunol Ther Exp. 2007;55(5):315. [DOI] [PubMed] [Google Scholar]
- 15. Khameneh B, Iranshahy M, Soheili V, Fazly Bazzaz BS. Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control. 2019;8(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nour V, Trandafir I, Cosmulescu S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J Chromatogr Sci. 2013;51(9):883–890. [DOI] [PubMed] [Google Scholar]
- 17. Naqvi SAR, Ali S, Sherazi TA, et al. Antioxidant, antibacterial, and anticancer activities of bitter gourd fruit extracts at three different cultivation stages. J Chem. 2020;2020:7394751. [Google Scholar]
- 18. Naqvi SAR, Waseem R, Mahmood N, et al. Phenolic acid content, antioxidant properties, and antibacterial potential of flowers and fruits from selected Pakistani indigenous medicinal plants. Sci Asia. 2013;39(5):340–345. [Google Scholar]
- 19. Ying X, Wang R, Xu J, et al. HPLC determination of eight polyphenols in the leaves of crataegus pinnatifida Bge. var. major. J Chromatogr Sci. 2009;47(3):201–205. [DOI] [PubMed] [Google Scholar]
- 20. Wen D, Li C, Di H, Liao Y, Liu H. A universal HPLC method for the determination of phenolic acids in compound herbal medicines. J Agric Food Chem. 2005;53(17):6624–6629. [DOI] [PubMed] [Google Scholar]
- 21. Asif M, Raza Naqvi SA, Sherazi TA, et al. Antioxidant, antibacterial and antiproliferative activities of pumpkin (cucurbit) peel and puree extracts-an in vitro study. Pak J Pharm Sci. 2017;30(4):1327–1334. [PubMed] [Google Scholar]
- 22. Jehan B, Amjad I, Mohammad S. Antimicrobial potentials of Eclipta alba by well diffusion method. Pak J Bot. 2011;43(Special Issue):169–174. [Google Scholar]
- 23. Oke F, Aslim B, Ozturk S, Altundag S. Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia Ten. Food Chem. 2009;112(4):874–879. [Google Scholar]
- 24. Khalil AW. Nutritional, Phytochemical and Pharmacological Investigation of Boerhavia Procumbens. HEC-Islamabad: Department of Agricultural Chemistry, The University of Agriculture, Peshawar-Pakistan; 2018. [Google Scholar]
- 25. Um M, Han T-H, Lee J-W. Ultrasound-assisted extraction and antioxidant activity of phenolic and flavonoid compounds and ascorbic acid from rugosa rose (Rosa rugosa Thunb.) fruit. Food Sci Biotechnol. 2017;27(2):375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen L-C, Guan X, Wang Q-K, Yang Q-P, Zhang W-D, Wang S-L. Effects of phenolic acids on soil nitrogen mineralization over successive rotations in Chinese fir plantations. J Forest Res. 2020;31(1):303–311. [Google Scholar]
- 27. Proestos C, Chorianopoulos N, Nychas GJE, Komaitis M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. JAgric Food Chem. 2005;53(4):1190–1195. [DOI] [PubMed] [Google Scholar]
- 28. Chen C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid Med Cell Long. 2016;2016:3571614–3571614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaudhary A, Jaswal VS, Choudhary S, et al. Ferulic acid: a promising therapeutic phytochemical and recent patents advances. Recent Pat Inflamm Allergy Drug Discov. 2019;13(2):115–123. [DOI] [PubMed] [Google Scholar]
- 30. Habtemariam S. Protective effects of caffeic acid and the Alzheimer’s brain: an update. Mini Rev Med Chem. 2017;17(8):667–674. [DOI] [PubMed] [Google Scholar]
- 31. Iqbal T, Hussain AI, Chatha SAS, Naqvi SAR, Bokhari TH. Antioxidant activity and volatile and phenolic profiles of essential oil and different extracts of wild mint (<i>Mentha longifolia</i>) from the Pakistani flora. J Anal Methods Chem. 2013;2013:536490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He S, Lin L, Wu Z, Chen Z. Application of finite element analysis in properties test of finger-jointed lumber. J Bioresour Bioproducts. 2020;5(2):124–133. [Google Scholar]
- 33. Farah N, Bukhari SA, Ali M, Naqvi SAR, Mahmood S. Phenolic acid profiling and antiglycation studies of leaf and fruit extracts of tyrosine primed Momordica charantia seeds for possible treatment of diabetes mellitus. Pak J Phar Sci. 2018;31(6):2667–2672. [PubMed] [Google Scholar]
- 34. Chen T-S, Liou S-Y, Wu H-C, Tsai F-J, Tsai C-H, Huang C-Y, Chang Y-L. New analytical method for investigating the antioxidant power of food extracts on the basis of their electron-donating ability: comparison to the ferric reducing/antioxidant power (FRAP) assay. J Agric Food Chem. 2010;58(15):8477–8480. [DOI] [PubMed] [Google Scholar]
- 35. Tabanca N, Demirci B, Crockett SL, Başer KHC, Wedge DE. Chemical composition and antifungal activity of arnica longifolia, aster hesperius, and chrysothamnus nauseosus essential oils. J Agric Food Chem. 2007;55(21):8430–8435. [DOI] [PubMed] [Google Scholar]
- 36. Pandey A, Singh P. Antibacterial activity of Syzygium aromaticum (clove) with metal ion effect against food borne pathogens. Asian J Plant Sci Res. 2011;1(2):69–80. [Google Scholar]
- 37. Naqvi SAR, Shah SMA, Kanwal L, et al. Antimicrobial and antihypercholesterolemic activities of pulicaria gnaphalodes. Dose-Res. 2020;18(1):1559325820904858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith PA, Koehler MFT, Girgis HS, et al. Optimized arylomycins are a new class of Gram-negative antibiotics. Nature. 2018;561(7722):189–194. [DOI] [PubMed] [Google Scholar]