Abstract
Azoospermia is divided into two categories of obstructive azoospermia and non-obstructive azoospermia. Before 1995, couples with a male partner diagnosed with non-obstructive azoospermia had to choose sperm donation or adoption to have a child. Currently, testicular sperm aspiration or micro-dissection testicular sperm extraction combined with intracytoplasmic sperm injection allows patients with non-obstructive azoospermia to have biological offspring. The sperm retrieval rate is significantly higher in micro-dissection testicular sperm extraction compared with testicular sperm aspiration. Additionally, micro-dissection testicular sperm extraction has the advantages of minimal invasion, safety, limited disruption of testicular function, a low risk of postoperative intratesticular bleeding, and low serum testosterone concentrations. Failed micro-dissection testicular sperm extraction has significant emotional and financial implications on the involved couples. Testicular sperm aspiration and micro-dissection testicular sperm extraction have the possibility of failure. Therefore, predicting the sperm retrieval rate before surgery is important. This narrative review summarizes the existing data on testicular sperm aspiration and micro-dissection testicular sperm extraction to identify the possible factor(s) that can predict the presence of sperm to guide clinical practice. The predictors of surgical sperm retrieval in patients with non-obstructive azoospermia have been widely studied, but there is no consensus.
Keywords: Sperm retrieval rate, micro-dissection testicular sperm extraction, testicular sperm aspiration, non-obstructive azoospermia, infertility, intracytoplasmic sperm injection
Introduction
Infertility refers to a couple with a normal sexual life who have not achieved pregnancy without using contraception for 1 year.1 Infertility is a common disorder affecting approximately 20% of couples, and male infertility accounts for approximately 50%.2 Non-obstructive azoospermia (NOA) is the most severe form of male infertility, which affects approximately 1% of all men and 10% to 15% of infertile men.2–4
Before 1995, couples with a male partner who was diagnosed with NOA had to choose sperm donation or adoption to have a child. Currently, with the development of medical technology, couples can have their own biological offspring by testicular sperm aspiration (TESA) or micro-dissection testicular sperm extraction (MD-TESE) combined with subsequent intracytoplasmic sperm injection (ICSI).5 Neither TESA nor MD-TESE guarantees sperm retrieval. The factors that predict the success rate of sperm retrieval to avoid unnecessary procedures, and reduce the economic and psychological burden on patients with NOA have been investigated. Studies have shown that a diagnostic biopsy, hormones levels, volume of the testis, and age are potential predictive factors for sperm retrieval.6 In contrast, other studies have indicated that none of these parameters can precisely predict the sperm retrieval rate (SRR).7,8
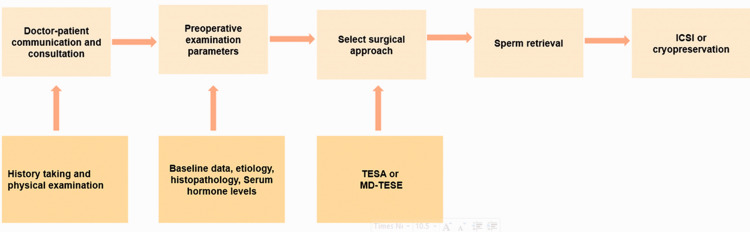
The purpose of this narrative review was to analyze and summarize the existing data on predicting the success rate of TESA and MD-TESE to provide a novel basis for surgical sperm extraction in patients with NOA (Figure 1).
Figure 1.
Preoperative consultation and examination guide for selection of surgical methods.
TESA, testicular sperm aspiration; MD-TESE, micro-dissection testicular sperm extraction; ICSI, intracytoplasmic sperm injection.
Sperm retrieval
Spermatogenesis and its regulation
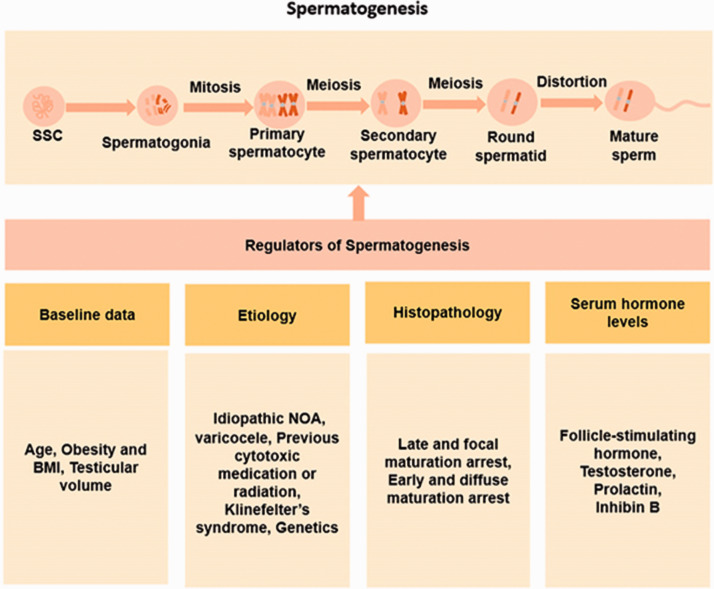
Spermatogenesis originates from spermatogonial stem cells. The process of spermatogonial stem cells changing to mature sperm undergoes the three following stages. (1) In mitosis, type A spermatogonia proliferate to produce two types of spermatogonia. Type A spermatogonia do not enter the differentiation pathway and continue to maintain mitotic capacity and type B spermatogonia enter the differentiation pathway and differentiate into primary spermatocytes. (2) In meiosis, primary spermatocytes undergo chromosomal replication. Homologous chromosomes undergo synapsis, segregation, and the first meiotic division to form two secondary spermatocytes, which enter the second meiotic division. Sister chromosomes separate and form haploid round spermatids. (3) In spermatogenesis, round spermatids undergo complex morphological changes, as well as nucleoprotein transformation and modification to form mature sperm. Spermatogenesis is a complex process of cellular differentiation that is regulated by the neuroendocrine system and spermatogenesis-related genes. The interaction between cells in the testis also has a local regulatory role in spermatogenesis. Problems at any one stage can lead to the inability to obtain sperm (Figure 2).
Figure 2.
Regulation of spermatogenesis.
Surgical sperm extraction
Testicular tissue is obtained by TESA or MD-TESE with the patient in the supine position under spermatic cord anesthesia block. The tissue is placed on a cell culture plate containing sperm culture medium and immediately inspected at high magnification to identify motile sperm for ICSI or cryopreservation.
Factors predicting the SRR in patients with NOA before surgery
Factors predicting the SRR in patients with NOA undergoing TESA
Recent studies on predicting the success of TESA in patients with NOA have mainly focused on age, body mass index (BMI), testicular volume (TV), and serum hormone levels. A model to predict TESA results has also been described.9
Studies have shown that age, BMI, luteinizing hormone (LH), prolactin (PRL), and total testosterone (TT) do not predict the SRR of TESA in patients with NOA.10–12 Other studies have suggested that TV and contrast-enhanced ultrasound can be used to predict the success of TESA.11–13 The predictive significance of follicle-stimulating hormone (FSH) and estradiol remain controversial11,12,14 (Table 1).
Table 1.
Results of studies on factors predicting the sperm retrieval rate in patients with non-obstructive azoospermia who underwent testicular sperm aspiration.
| Factors | Predictable | p value | rs | OR | 95% CI | AUC | Cutoff value | Reference | Year |
|---|---|---|---|---|---|---|---|---|---|
| Age | No | NA | NA | NA | NA | NA | NA | Tang et al.11 | 2018 |
| BMI | No | 0.327 | NA | NA | NA | NA | NA | Li et al.10 | 2019 |
| TV | Yes | <0.001/<0.001 (left/right) |
NA | NA | 0.729–0.863/0.721–0.860 (left/right) | 0.796/0.791 (left/right) |
11 mL | Tang et al.11 | 2018 |
| Yes | <0.001 | 0.844 | NA | NA | 0.984/0.961 (left/right) |
9 mL | Tang et al.12 | 2012 | |
| FSH | No | NA | NA | NA | NA | NA | NA | Tang et al.11 | 2018 |
| Yes | <0.001 | 0.412 | NA | NA | 0.743 | 8.18 mIU/mL | Tang et al.12 | 2012 | |
| Yes | 0.030 | −0.083 | 0.920 | 0.854–0.992 | 0.70 | 18.97 mIU/mL | Liu et al.14 | 2020 | |
| LH | No | NA | NA | NA | NA | NA | NA | Tang et al.11 | 2018 |
| No | > 0.05 | NA | NA | NA | 0.608 | NA | Tang et al.12 | 2012 | |
| E2 | Yes | <0.001/<0.001 (left/right) |
NA | NA | 0.605–0.771/0.641 − 0.792 (left/right) | 0.688/0.716 (left/right) |
144.5 pmol/L/133.5 pmol/L (left/right) | Tang et al.11 | 2018 |
| No | 0.029 | 0.219 | NA | NA | 0.629 | NA | Tang et al.12 | 2012 | |
| PRL | No | NA | NA | NA | NA | NA | NA | Tang et al.11 | 2018 |
| No | >0.05 | NA | NA | NA | 0.538 | NA | Tang et al.12 | 2012 | |
| TT | No | NA | NA | NA | NA | NA | NA | Tang et al.11 | 2018 |
| No | >0.05 | NA | NA | NA | 0.569 | NA | Tang et al.12 | 2012 | |
| CEUS (TTP) | Yes | 0.045 | NA | 4.2 | 1.3–17.5 | NA | NA | Xue et al.13 | 2018 |
| Predictive model (including age, TV, and FSH) | Yes | ≤0.001 | NA | NA | 0.776–0.871 | 0.823 | 64.61% | Ma et al.9 | 2018 |
rs, Spearman’s rank correlation coefficient; OR, odds ratio; CI, confidence interval; AUC, area under the curve; NA, not applicable; BMI, body mass index; TV, testicular volume; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; PRL, prolactin; TT, total testosterone; CEUS, contrast-enhanced ultrasound; TTP, time to peak.
Factors predicting SRR in patients with NOA undergoing MD-TESE
Baseline data
Age
There are few studies on the father’s age and its effect on fertility, and a specific male cutoff age, which negatively affects fertility, has not been identified.7,15 Bernie et al.16 showed no relationship between male age and MD-TESE results, the overall SRR was highest in men aged ≥40 years, and there was no upper age limit for men for the success of MD-TESE. Similarly, Ghalayini et al.17 found that age had no significant effect on sperm recovery in MD-TESE. However, Chen et al.18 studied 38 men who NOA who underwent orchidopexy, and found a correlation between the SRR and age at orchidopexy and TV. Additionally, men who had surgery before the age of 10 years had a higher SRR than those who had surgery after the age of 10 years. However, Wiser et al.19 studied 40 patients who NOA who underwent orchidopexy and found no significant difference in the SRR between patients who underwent surgery before or after the age of 10 years. To determine the factors affecting the SRR of MD-TESE, Althakafi et al.20 performed multiple logistic regression analyses, including age, BMI, serum hormones (FSH, PRL, and TT) and histopathology of patients. They showed that only age (p = 0.044) and histopathology (p < 0.001) were significantly associated with the SRR of MD-TESE.
Obesity and BMI
Patients with NOA and obesity and an elevated BMI have a high sperm DNA fragmentation index, high FSH levels, low TT levels, and low spontaneous fertility.21,22 However, the SRR in MD-TESE appears to be similar in men with obesity compared with men with a normal BMI in the general MD-TESE population, as well as in men with Klinefelter’s syndrome.22 A previous study examined the relationship between obesity and MD-TESE outcomes in patients with NOA, and showed that obesity had no effect on the SRR, but it reduced the clinical pregnancy rate.23
TV
TV is associated with spermatogenesis. However, studies have shown a poor correlation between TV and the SRR.24–26 Although TV is positively correlated with the success rate of MD-TESE,27,28 its role as a good predictor variable remains inconsistent.29 In a meta-analysis of 1764 cases, no threshold of TV associated with the SRR was found,30 which is consistent with findings by Wiser et al.19 Campbell et al.8 concluded that severe testicular atrophy should not be a contraindication for microanatomical testicular sperm retrieval.
Etiology
NOA is caused by multifactorial effects. Etiology is a significant factor predicting the SRR of NOA in men in MD-TESE. The prognosis of patients with idiopathic NOA is poor, with an SRR of 30% to 40%.16,31,32 Men with NOA and a clear etiology, such as cryptorchidism, have a high SRR,16 while idiopathic NOA, which is the most common type of NOA, has the lowest SRR.18,33 Moreover, spermatozoa are significantly less likely to be successfully retrieved by MD-TESE in men with idiopathic azoospermia.34
Among patients with NOA and a clear etiology, the SRR varies among patients with different etiologies. A study compared the clinical data of patients with NOA with or without varicocele, and showed that the success rate of sperm retrieval in patients with NOA and varicocele who underwent varicocelectomy was significantly higher than that in patients without varicocele.35 This finding indicates that varicocele repair in men with NOA might have a predictive role in the success of MD-TESE. Other studies showed that a history of previous cytotoxic medication or radiation was often associated with the SRR in patients with NOA.36,37 Additionally, patients with Klinefelter’s syndrome who underwent micro-TESE had an SRR that was similar to or better than all patients with NOA, and Klinefelter’s syndrome was a good prognostic factor for sperm retrieval.38 This finding is consistent with that by Friedler et al.39 Genetics, particularly microdeletions of the Y chromosome, are helpful in predicting the SRR of MD-TESE in patients with NOA. Studies have shown that the SRR is higher in patients with NOA with AZFc microdeletions compared with those with AZFa or AZFb microdeletions, whereas the SRR is lower in patients with AZFa or AZFb microdeletions.40–42
Histopathology
Histopathology is a powerful factor for predicting the SRR of MD-TESE in patients with NOA.43–45 Bernie et al.46 suggested that acquisition of sperm in men with NOA depended on the heterogeneity of testicular tissue, and the SRR was higher in men with NOA with late and focal maturation arrest than in those with early and diffuse maturation arrest. Yang et al.47 showed that the results of histopathological diagnosis were the best factor for predicting the success of MD-TESE sperm retrieval in patients with NOA, which is consistent with the findings of Althakafi et al.20 In contrast, other studies have indicated that testicular histopathological findings are not related to the success of sperm retrieval in MD-TESE.48,49
Serum hormone levels
FSH
In spermatogenesis, FSH mainly acts on spermatogenic and Sertoli cells of the testis, which directly initiates mitosis of spermatogonia and stimulates the development of primary spermatocytes. Maintaining normal spermatogenesis and sperm maturation are important. Li et al.30 suggested that FSH could not be used as a factor to predict the success of testicular sperm retrieval in patients with NOA, which is similar to previous studies.50–53 Yildirim et al.49 evaluated levels of LH, FSH, and testosterone, testicular biopsy histology, and male age for predicting the SRR of patients with NOA during MD-TESE. These authors found a relationship between the SRR and FSH levels, which is similar to the results of Mitchell et al.54 Another study showed that TV and FSH levels did not predict the SRR in patients with NOA during initial MD-TESE, but had predictive value during the second test.55 Ghalayini et al.17 found that patients with NOA and FSH levels <2N (24 mIU/mL) had a higher SRR compared with those with FSH levels ≥2N. This finding suggests that an increase in FSH levels predicts a decrease in the SRR.
A study in the last decade showed that FSH and a surgical approach were significant variables for predicting the SRR in patients with NOA (p < 0.05).56 Subsequently, another study showed that the mean FSH level was significantly higher in the failed group than in the successful group (p < 0.01), and a plasma FSH level > 19.4 mIU/mL was the best cutoff value for predicting the SRR in patients with NOA.57 A retrospective study of 180 patients who NOA who underwent MD-TESE showed that the mean serum FSH level was significantly higher in the failure group than in the success group (p < 0.001), and a plasma FSH level > 14.6 mIU/mL predicted failure of sperm retrieval.58 In contrast, in our study, we found that the mean serum FSH level was significantly higher in the success group than in the failure group (p = 0.001).14 We also found that a plasma FSH level > 19.01 mIU/mL was the best cutoff value for predicting the SRR in patients with NOA. Similarly, Modarresi et al.59 considered that FSH levels were a reasonable predictor of the success of MD-TESE. Therefore, the predictive significance of FSH for the SRR in patients with NOA remains controversial.
Testosterone
Testosterone is the most important paracrine hormone in the testis. Testosterone stimulates the secretion of androgen-binding protein and seminiferous tubule fluid by Sertoli cells. Testosterone might act at some stages of spermatogenesis. Kals et al.60 found that sperm retrieval was possible after a second attempt of MD-TESE in men with NOA who had failed previous attempts or multiple TESA inseminations. This finding suggested a correlation between preoperative serum testosterone levels and the SRR. Subsequently, Mehmood et al.61 found a significant correlation between preoperative testosterone levels and the SRR of MD-TESE. Althakafi et al.20 conducted a retrospective study of 421 patients with NOA. Clinical, biochemical, and histopathological data were collected, and the determinants of MD-TESE in the study population were analyzed with multiple logistic regression. These authors showed no significant correlation between serum testosterone levels and the outcome of MD-TESE in patients with NOA. These results are consistent with previous findings.17,25,62
PRL
PRL affects testicular function by inhibiting secretion of gonadotropin-releasing hormone levels and gonadotropins. Hyperprolactinemia can lead to decreased serum testosterone levels and hypogonadism, resulting in severe spermatogenic failure and sexual dysfunction. To date, no differences have been identified between PRL levels in patients with NOA in the successful sperm retrieval group compared with the failed sperm retrieval group.17,27,63
Inhibin B
Serum inhibin B is a marker of spermatogenesis. Studies have shown that serum inhibin B levels are useful for predicting the presence of testicular sperm in men with NOA, and are a useful non-invasive predictor of the success of MD-TESE.52,64,65 In a retrospective analysis by Boitrelle et al.,66 total TV, FSH levels, and inhibin B levels were associated with the outcome of MD-TESE. Individually, inhibin B (cutoff value of 27.5 pg/mL; area under the curve: 0.683) was the best predictor of outcome in MD-TESE. Tsujimura et al. and Mitchell et al.27,54 found that serum inhibin B levels did not predict the outcome of MD-TESE alone, but showed predictive value when combined with other factors. Tunc et al. and Meachem et al.52,67 showed that that inhibin B was of limited value for predicting the SRR in patients with NOA who underwent MD-TESE. Additionally, these authors found that inhibin B was not a reliable predictor of the presence of sperm in MD-TESE samples.
Imaging examinations
Ultrasound
Non-invasive diagnostic methods before surgery are highly desirable to improve surgical success and to avoid unnecessary surgical interventions. Scrotal ultrasound may be a suitable method. However, Pezzella et al.68 showed that measurement of the epididymal head diameter by ultrasonography did not provide any relevant information clinically for patients with NOA. This measurement also did not predict the success rate of sperm retrieval by TESE in patients with NOA. Altinkilic et al.69 investigated whether color-coded duplex sonography improves the outcome of predicting testicular sperm retrieval in patients with NOA. Their findings suggested that a higher intratesticular peak systolic velocity is helpful as a preoperative diagnostic parameter to predict the success of sperm retrieval. However, after adjusting for other clinical confounders, color-coded duplex sonography did not predict the success of testicular sperm retrieval in patients with NOA.
Magnetic resonance imaging
In recent years, there have been few studies on magnetic resonance imaging (MRI) in predicting the success rate of testicular sperm extraction in patients with NOA. Wang et al.70 showed that functional MRI, including diffusion-weighted MRI and magnetization transfer MRI, appears promising for assessing male infertility with a higher apparent diffusion coefficient (ADC) and a lower magnetization transfer ratio in testicular hypospermatogenesis. Another study suggested that testicular metabolite concentrations measured by 3T proton spectroscopy can be used as non-invasive biomarkers to predict spermatogenesis.71 A study showed that phosphocholine concentrations were significantly higher in normal testes compared with Sertoli cells only.72 This finding indicated that the unique metabolic profile of spermatogenesis may be identified by proton magnetic resonance spectroscopy, which is helpful in non-invasive diagnosis of spermatozoa in men with NOA.
A study of differences in the ADC and fractional anisotropy in the testes showed a higher ADC and fractional anisotropy in the testes of patients with NOA compared with the normal population. The ADC proved to be a more useful diagnostic aid in the population of men with NOA for identifying advanced spermatogenesis lesions. However, diffusion tensor imaging parameters cannot predict the success rate of sperm retrieval in TESE.73 A study including 49 men with NOA and 45 controls showed that TV, the ADC, and the magnetization transfer ratio were useful for predicting the success rate of sperm retrieval in patients with NOA undergoing MD-TESE.74 A recent prospective study showed that proton magnetic resonance spectroscopy can assess metabolic information within the testis of patients with NOA and assess the spermatogenic status of the testis before MD-TESE in these patients.75 In conclusion, MRI has certain clinical value as a non-invasive examination in assessing the spermatogenic status of patients with NOA.
Predictive model
Recently, some researchers have attempted to develop a model to predict the SRR of MD-TESE in patients with NOA compared with single factors. Details of this model are shown in Table 2.
Table 2.
Predictive model for the sperm retrieval rate of micro-dissection testicular sperm extraction in patients with non-obstructive azoospermia.
| Reference | Number of patients | Variables to be included | Variables included in the final model | Sensitivity of the prediction model (%) | Specificity of the prediction model (%) | AUC | Remarks |
|---|---|---|---|---|---|---|---|
| Tsujimura et al. (2004)27 | 100 | Age, TV, FSH, LH, E2, PRL, TT, FT, and inhibin B | FSH, TT, and inhibin B | 71.0 | 71.4 | 0.76 | NA |
| Samli et al. (2004)63 | 303 | Age, duration of infertility, FSH, LH, TT, PRL, and left and right testicular volume | Age, duration of infertility, FSH, LH, TT, PRL, and left and right testicular volume | 68.0 | 80.8 | NA | NA |
| Ramasamy et al. (2013)76 | 1026 | Age, FSH, TV, history of cryptorchidism and Klinefelter’s syndrome, and presence of varicocele | Age and history of Klinefelter’s syndrome and cryptorchidism | 67.0 | 49.5 | 0.59 | NA |
| Tsujimura et al. (2005)77 | 100 | Age, TV, JS, FSH, LH, TT, FT, PRL, E2, and inhibin B | Age, FSH, and JS | 78.0 | 76.3 | 0.83 | Excluding chromosomal abnormalities |
TV, testicular volume; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; PRL, prolactin; TT, total testosterone; FT, free testosterone; AUC, area under the curve; NA, not applicable; JS, Johnsen’s score.
Discussion
NOA is diagnosed in 10% of men with infertility.60 NOA is an untreatable testicular failure due to various causes, and is characterized by impaired testicular endocrine (producing testosterone) and/or exocrine (producing sperm) function.62,66 Spermatogenesis involves multifactorial regulation, and is related to many factors, such as age, TV, histopathological diagnosis, genetic diagnosis, and serum hormone levels. Previous studies have shown that FSH and histopathological diagnosis are the most potentially useful predictors of NOA. However, the predictive value of FSH remains inconsistent. Preoperative counseling should be provided to patients with NOA to predict the success rate of surgery, and reduce the psychological and physical burden on patients. Predictive factors of spermatogenesis in patients with NOA need to be examined to improve the success rate of surgery and reduce the burden on patients.
Conclusions
The predictors of surgical sperm retrieval in patients with NOA have been extensively studied, with inconsistent results. More studies are required to further examine the relevant predictors to improve the diagnosis and treatment of patients with NOA.
Footnotes
Ethics statement: The protocol for this study was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University (Project number: 2019-KY-101).
Availability of data and materials: Not applicable.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Youth Fund from the Natural Science Foundation of China (81701505).
Author contributions: All authors contributed to the concepts in the manuscript. YPL wrote the first draft of the manuscript. LQ, NNZ, and YCS contributed to writing the manuscript. All authors approved the final version of the manuscript.
ORCID iDs: Lin Qi https://orcid.org/0000-0002-8927-7131
Ying C Su https://orcid.org/0000-0001-5552-7414
References
- 1. WHO. WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction. 5th ed. Cambridge: Cambridge Press, 2009. [Google Scholar]
- 2.Stephen EH, Chandra A. Declining estimates of infertility in the United States: 1982-2002. Fertil Steril 2006; 86: 516–523. [DOI] [PubMed] [Google Scholar]
- 3.Klami R, Mankonen H, Perheentupa A. Successful microdissection testicular sperm extraction for men with non-obstructive azoospermia. Reprod Biol 2018; 18: 137–142. [DOI] [PubMed] [Google Scholar]
- 4.Cerilli LA, Kuang W, Rogers D. A practical approach to testicular biopsy interpretation for male infertility. Arch Pathol Lab Med 2010; 134: 1197–1204. [DOI] [PubMed] [Google Scholar]
- 5.Su LM, Palermo GD, Goldstein M, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: testicular histology can predict success of sperm retrieval. J Urol 1999; 161: 112–116. [PubMed] [Google Scholar]
- 6.Ramasamy R, Schlegel PN. Microdissection testicular sperm extraction: effect of prior biopsy on success of sperm retrieval. J Urol 2007; 177: 1447–1449. [DOI] [PubMed] [Google Scholar]
- 7.Humm KC, Sakkas D. Role of increased male age in IVF and egg donation: is sperm DNA fragmentation responsible? Fertil Steril 2013; 99: 30–36. [DOI] [PubMed] [Google Scholar]
- 8.Bryson CF, Ramasamy R, Sheehan M, et al. Severe testicular atrophy does not affect the success of microdissection testicular sperm extraction. J Urol 2014; 191: 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Li FP, Wang L, et al . A risk prediction model of sperm retrieval failure with fine needle aspiration in males with non-obstructive azoospermia. Hum Reprod 2018; 34: 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li F, Yang Q, Shi H, et al. Effects of obesity on sperm retrieval, early embryo quality and clinical outcomes in men with nonobstructive azoospermia undergoing testicular sperm aspiration-intracytoplasmic sperm injection cycles. Andrologia 2019; 51: e13265. [DOI] [PubMed] [Google Scholar]
- 11.Tang WH, Zhou SJ, Song SD, et al. A clinical trial on the consistency of bilateral testicular tissue histopathology and Johnsen score: single side or bilateral side biopsy?. Oncotarget 2018; 9: 23848–23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang WH, Jiang H, Ma LL, et al. Correlation of testicular volume and reproductive hormone level with the results of testicular sperm aspiration in non-obstructive azoospermia patients. Zhonghua Nan Ke Xue 2012; 18: 48–51. [PubMed] [Google Scholar]
- 13.Xue H, Wang SY, Cui LG, et al. Can Contrast-Enhanced Ultrasound Increase or Predict the Success Rate of Testicular Sperm Aspiration in Patients With Azoospermia? AJR Am J Roentgenol 2018; 212: 1054–1059. [DOI] [PubMed] [Google Scholar]
- 14.Liu YP, Qi L, Zhang NN, et al. Follicle-stimulating hormone may predict sperm retrieval rate and guide surgical approach in patients with non-obstructive azoospermia. Reprod Biol 2020; 20: 573–579. [DOI] [PubMed] [Google Scholar]
- 15.Okada H, Goda K, Yamamoto Y, et al. Age as a limiting factor for successful sperm retrieval in patients with nonmosaic Klinefelter's syndrome. Fertil Steril 2005; 84: 1662–1664. [DOI] [PubMed] [Google Scholar]
- 16.Bernie AM, Ramasamy R, Schlegel PN. Predictive factors of successful microdissection testicular sperm extraction. Basic Clin Androl 2013; 23: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghalayini IF, Al-Ghazo MA, Hani OB, et al. Clinical comparison of conventional testicular sperm extraction and microdissection techniques for non-obstructive azoospermia. J Clin Med Res 2011; 3: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen XF, Ma Y, Zou SS, et al. Comparison and outcomes of nonobstructive azoospermia patients with different etiology undergoing MicroTESE and ICSI treatments. Transl Androl Urol 2019; 8: 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiser A, Raviv G, Weissenberg R, et al. Does age at orchidopexy impact on the results of testicular sperm extraction? Reprod Biomed Online 2009; 19: 778–783. [DOI] [PubMed] [Google Scholar]
- 20.Althakafi SA, Mustafa OM, Seyam RM, et al. Serum testosterone levels and other determinants of sperm retrieval in microdissection testicular sperm extraction. Transl Androl Urol 2017; 6: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kort HI, Massey JB, Elsner CW, et al. Impact of body mass index values on sperm quantity and quality. J Androl 2006; 27: 450–452. [DOI] [PubMed] [Google Scholar]
- 22.Iwatsuki S, Sasaki S, Taguchi K, et al. Effect of obesity on sperm retrieval outcome and reproductive hormone levels in Japanese azoospermic men with and without Klinefelter syndrome. Andrology 2016; 5: 82–86. [DOI] [PubMed] [Google Scholar]
- 23.Ramasamy R, Bryson C, Reifsnyder JE, et al. Overweight men with nonobstructive azoospermia have worse pregnancy outcomes after microdissection testicular sperm extraction. Fertil Steril 2013; 99: 372–376. [DOI] [PubMed] [Google Scholar]
- 24.Tsujimura A. Microdissection testicular sperm extraction: prediction, outcome, and complications. Int J Urol 2007; 14: 883–889. [DOI] [PubMed] [Google Scholar]
- 25.Gul U, Turunc T, Haydardedeoglu B, et al. Sperm retrieval and live birth rates in presumed Sertoli-cell-only syndrome in testis biopsy: a single centre experience. Andrology 2013; 1: 47–51. [DOI] [PubMed] [Google Scholar]
- 26.Ravizzini P, Carizza C, Abdelmassih V, et al. Microdissection testicular sperm extraction and IVF-ICSI outcome in nonobstructive azoospermia. Andrologia 2008; 40: 219–226. [DOI] [PubMed] [Google Scholar]
- 27.Tsujimura A, Matsumiya K, Miyagawa Y, et al. Prediction of Successful Outcome of Microdissection Testicular Sperm Extraction in Men with Idiopathic Nonobstructive Azoospermia. J Urol 2004; 172: 1944–1947. [DOI] [PubMed] [Google Scholar]
- 28.Kizilkan Y, Toksoz S, Turunc T, et al. Parameters predicting sperm retrieval rates during microscopic testicular sperm extraction in nonobstructive azoospermia. Andrologia 2019; 51: e13441. [DOI] [PubMed] [Google Scholar]
- 29.Devroey P, Liu J, Nagy Z, et al. Pregnancies after testicular sperm extraction and intracytoplasmic sperm injection in non-obstructive azoospermia. Hum Reprod 1995; 10: 1457–1460. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Chen LP, Yang J, et al. Predictive value of FSH, testicular volume, and histopathological findings for the sperm retrieval rate of microdissection TESE in nonobstructive azoospermia: a meta-analysis. Asian J Androl 2018; 20: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klami R, Mankonen H, Perheentupa A. Microdissection testicular sperm extraction in Finland - results of the first 100 patients. Acta Obstet Gynecol Scand 2018; 97: 53–58. [DOI] [PubMed] [Google Scholar]
- 32.Cissen M, Meijerink AM, D'Hauwers KW, et al. Prediction model for obtaining spermatozoa with testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod 2016; 31: 1934–1941. [DOI] [PubMed] [Google Scholar]
- 33.Skakkebaek NE, Rajpert-De Meyts E, Main KM. . Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001; 16: 972–978. [DOI] [PubMed] [Google Scholar]
- 34.Enatsu N, Miyake H, Chiba K, et al. Predictive factors of successful sperm retrieval on microdissection testicular sperm extraction in Japanese men. Reprod Med Biol 2015; 15: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haydardedeoglu B, Turunc T, Kilicdag EB, et al. The effect of prior varicocelectomy in patients with nonobstructive azoospermia on intracytoplasmic sperm injection outcomes: a retrospective pilot study. Urology 2010; 75: 83–86. [DOI] [PubMed] [Google Scholar]
- 36.Dabaja AA, Schlegel PN. Microdissection testicular sperm extraction: an update. Asian J Androl 2013; 15: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsiao W, Stahl PJ, Osterberg EC, et al. Successful treatment of postchemotherapy azoospermia with microsurgical testicular sperm extraction: the Weill Cornell experience. J Clin Oncol 2011; 29: 1607–1611. [DOI] [PubMed] [Google Scholar]
- 38.Ramasamy R, Ricci JA, Palermo GD, et al. Successful fertility treatment for Klinefelter's syndrome. J Urol 2009; 182: 1108–1113. [DOI] [PubMed] [Google Scholar]
- 39.Friedler S, Raziel A, Strassburger D, et al. Outcome of ICSI using fresh and cryopreserved-thawed testicular spermatozoa in patients with non-mosaic Klinefelter's syndrome. Hum Reprod 2001; 16: 2616–2620. [DOI] [PubMed] [Google Scholar]
- 40.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod 1999; 14: 131–135. [DOI] [PubMed] [Google Scholar]
- 41.Hopps CV, Mielnik A, Goldstein M, et al. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod 2003; 18: 1660–1665. [DOI] [PubMed] [Google Scholar]
- 42.Stahl PJ, Masson P, Mielnik A, et al. A decade of experience emphasizes that testing for Y microdeletions is essential in American men with azoospermia and severe oligozoospermia. Fertil Steril 2010; 94: 1753–1756. [DOI] [PubMed] [Google Scholar]
- 43.Schoor RA, Elhanbly S, Niederberger CS, et al. The role of testicular biopsy in the modern management of male infertility. J Urol 2002; 167: 197–200. [PubMed] [Google Scholar]
- 44.Abdel Raheem A, Garaffa G, Rushwan N, et al. Testicular histopathology as a predictor of a positive sperm retrieval in men with non-obstructive azoospermia. BJU Int 2013; 111: 492–499. [DOI] [PubMed] [Google Scholar]
- 45.Toksoz S, Kizilkan Y. Comparison of the Histopathological Findings of Testis Tissues of Non-Obstructive Azoospermia with the Findings after Microscopic Testicular Sperm Extraction. Urol J 2019; 16: 212–215. [DOI] [PubMed] [Google Scholar]
- 46.Bernie AM, Shah K, Halpern JA, et al. Outcomes of microdissection testicular sperm extraction in men with nonobstructive azoospermia due to maturation arrest. Fertil Steril 2015; 104: 569–573.e1. [DOI] [PubMed] [Google Scholar]
- 47.Yu Y, Xi Q, Wang R, et al. Heterogenicity of testicular histopathology and tubules as a predictor of successful microdissection testicular sperm extraction in men with nonobstructive azoospermia. Medicine (Baltimore) 2018; 97: e10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalsi J, Thum MY, Muneer A, et al. In the era of micro-dissection sperm retrieval (m-TESE) is an isolated testicular biopsy necessary in the management of men with non-obstructive azoospermia?. BJU Int 2011; 109: 418–424. [DOI] [PubMed] [Google Scholar]
- 49.Yildirim ME, Koc A, Kaygusuz IC, et al. The Association between Serum Follicle-Stimulating Hormone Levels and the Success of Microdissection Testicular Sperm Extraction in Patients with Azoospermia. Urol J 2014; 11: 1825–1828. [PubMed] [Google Scholar]
- 50.Seo JT, Ko WJ. . Predictive factors of successful testicular sperm recovery in non-obstructive azoospermia patients. Int J Androl 2001; 24: 306–310. [DOI] [PubMed] [Google Scholar]
- 51.Turunc T, Gul U, Haydardedeoglu B, et al. Conventional testicular sperm extraction combined with the microdissection technique in nonobstructive azoospermic patients: a prospective comparative study. Fertil Steril 2010; 94: 2157–2160. [DOI] [PubMed] [Google Scholar]
- 52.Tunc L, Kirac M, Gurocak S, et al. Can serum Inhibin B and FSH levels, testicular histology and volume predict the outcome of testicular sperm extraction in patients with non-obstructive azoospermia? Int Urol Nephrol 2006; 38: 629–635. [DOI] [PubMed] [Google Scholar]
- 53.Guler I, Erdem M, Erdem A, et al. Impact of testicular histopathology as a predictor of sperm retrieval and pregnancy outcome in patients with nonobstructive azoospermia: correlation with clinical and hormonal factors. Andrologia 2016; 48: 765–773. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell V, Robin G, Boitrelle F, et al. Correlation between testicular sperm extraction outcomes and clinical, endocrine and testicular histology parameters in 120 azoospermic men with normal serum FSH levels. Int J Androl 2011; 34: 299–305. [DOI] [PubMed] [Google Scholar]
- 55.Ramasamy R, Ricci JA, Leung RA, et al. Successful repeat microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol 2011; 185: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 56.Colpi GM, Colpi EM, Piediferro G, et al. Microsurgical TESE versus conventional TESE for ICSI in non-obstructive azoospermia: a randomized controlled study. Reprod Biomed Online 2009; 18: 315–319. [DOI] [PubMed] [Google Scholar]
- 57.Chen SC, Hsieh JT, Yu HJ, et al . Appropriate cut-off value for follicle-stimulating hormone in azoospermia to predict spermatogenesis. Reprod Biol Endocrinol 2010; 8: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jahromi BN, Zeyghami S, Parsanezhad ME, et al. Determining an optimal cut-off value for follicle-stimulating hormone to predict microsurgical testicular sperm extraction outcome in patients with non-obstructive azoospermia. Arch Endocrinol Metab 2020; 64: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Modarresi T, Hosseinifar H, Daliri HA, et al. Predictive factors of successful microdissection testicular sperm extraction in patients with presumed sertoli cell-only syndrome. Int J Fertil Steril 2015; 9: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalsi JS, Shah P, Thum Y, et al. Salvage micro-dissection testicular sperm extraction; outcome in men with non-obstructive azoospermia with previous failed sperm retrievals. BJU Int 2015; 116: 460–465. [DOI] [PubMed] [Google Scholar]
- 61.Mehmood S, Aldaweesh S, Junejo NN, et al. Microdissection testicular sperm extraction: Overall results and impact of preoperative testosterone level on sperm retrieval rate in patients with nonobstructive azoospermia. Urol Ann 2019; 11: 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reifsnyder JE, Ramasamy R, Husseini J, et al. Role of optimizing testosterone before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol 2012; 188: 532–536. [DOI] [PubMed] [Google Scholar]
- 63.Samli MM, Dogan I. An artificial neural network for predicting the presence of spermatozoa in the testes of men with nonobstructive azoospermia. J Urol 2004; 171: 2354–2357. [DOI] [PubMed] [Google Scholar]
- 64.Nagata Y, Fujita K, Banzai J, et al. Seminal plasma inhibin-B level is a useful predictor of the success of conventional testicular sperm extraction in patients with non-obstructive azoospermia. J Obstet Gynaecol Res 2005; 31: 384–388. [DOI] [PubMed] [Google Scholar]
- 65.Bailly M, Guthauser B, Bergere M, et al. Effects of low concentrations of inhibin B on the outcomes of testicular sperm extraction and intracytoplasmic sperm injection. Fertil Steril 2003; 79: 905–908. [DOI] [PubMed] [Google Scholar]
- 66.Boitrelle F, Robin G, Marcelli F, et al. A predictive score for testicular sperm extraction quality and surgical ICSI outcome in non-obstructive azoospermia: a retrospective study. Hum Reprod 2011; 26: 3215–3221. [DOI] [PubMed] [Google Scholar]
- 67.Meachem SJ, Nieschlag E, Simoni M. Inhibin B in male reproduction: pathophysiology and clinical relevance. Eur J Endocrinol 2011; 145: 561–571. [DOI] [PubMed] [Google Scholar]
- 68.Pezzella A, Barbonetti A, D'Andrea S, et al. Ultrasonographic caput epididymis diameter is reduced in non-obstructive azoospermia compared with normozoospermia but is not predictive for successful sperm retrieval after TESE. Hum Reprod 2014; 29: 1368–1374. [DOI] [PubMed] [Google Scholar]
- 69.Altinkilic B, Pilatz A, Diemer T, et al. Prospective evaluation of scrotal ultrasound and intratesticular perfusion by color-coded duplex sonography (CCDS) in TESE patients with azoospermia. World J Urol 2018; 36: 125–133. [DOI] [PubMed] [Google Scholar]
- 70.Wang H, Guan J, Lin J, et al. Diffusion-Weighted and Magnetization Transfer Imaging in Testicular Spermatogenic Function Evaluation: Preliminary Results. J Magn Reson Imaging 2017; 47: 186–190. [DOI] [PubMed] [Google Scholar]
- 71.Storey P, Gonen O, Rosenkrantz AB, et al. Quantitative Proton Spectroscopy of the Testes at 3 T: Toward a Noninvasive Biomarker of Spermatogenesis. Invest Radiol 2018; 53:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aaronson DS, Iman R, Walsh TJ, et al. A novel application of 1H magnetic resonance spectroscopy: non-invasive identification of spermatogenesis in men with non-obstructive azoospermia. Hum Reprod 2010; 25: 847–852. [DOI] [PubMed] [Google Scholar]
- 73.Tsili AC, Ntorkou A, Goussia A, et al. Diffusion tensor imaging parameters in testes with nonobstructive azoospermia. J Magn Reson Imaging 2018; 48: 1318–1325. [DOI] [PubMed] [Google Scholar]
- 74.Ntorkou A, Tsili AC, Goussia A, et al. Testicular Apparent Diffusion Coefficient and Magnetization Transfer Ratio: Can These MRI Parameters Be Used to Predict Successful Sperm Retrieval in Nonobstructive Azoospermia? AJR Am J Roentgenol 2019; 213: 610–618. [DOI] [PubMed] [Google Scholar]
- 75.Ntorkou A, Tsili AC, Astrakas L, et al. In vivo biochemical investigation of spermatogenic status: 1H-MR spectroscopy of testes with nonobstructive azoospermia. Eur Radiol 2020; 30: 4284–4294. [DOI] [PubMed] [Google Scholar]
- 76.Ramasamy R, Padilla WO, Osterberg EC, et al. A comparison of models for predicting sperm retrieval before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol 2013; 189: 638–642. [DOI] [PubMed] [Google Scholar]
- 77.Tsujimura A, Miyagawa Y, Takao T, et al. Impact of age, follicle stimulating hormone and Johnsen's score on successful sperm retrieval by microdissection testicular sperm extraction. Reprod Med Biol 2005; 4: 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]