Abstract
Objective
To determine if plasma exosomal microRNAs (miRNAs) can predict survival in patients with idiopathic pulmonary arterial hypertension (IPAH).
Methods
The study enrolled patients with IPAH that underwent right heart catheterization. Plasma was collected and exosomal miRNAs were extracted. Exosomes were evaluated using transmission electron microscopy, Western blot analysis and particle size distribution analysis. MiRNAs were evaluated using a miRNA microarray and validated using real-time polymerase chain reaction.
Results
This study included 12 patients with IPAH in the study group and 48 patients with IPAH in the validation group. The mean ± SD follow-up duration was 60.3 ± 35.4 months in the overall cohort. The levels of miR-596 were higher in the nonsurvivors compared with the survivors. The levels of miR-596 significantly correlated with survival time, mean right atrial pressure, pulmonary vascular resistance (PVR) and cardiac index. High levels of miR-596 and PVR were significantly associated with poor overall survival. Multivariate analysis demonstrated that exosomal miR-596 (hazard ratio [HR] = 2.119; 95% confidence interval [CI] 1.402, 3.203) and PVR (HR = 1.146; 95% CI 1.010, 1.300) were independent predictors of survival.
Conclusions
High levels of plasma exosomal miR-596 were significantly associated with disease severity and poor prognosis of patients with IPAH.
Keywords: Idiopathic pulmonary arterial hypertension, exosomal, miR-596, survival
Introduction
Idiopathic pulmonary arterial hypertension (IPAH) is a disease of the small muscular pulmonary arteries characterized by a progressive rise in pulmonary artery pressure and pulmonary vascular resistance (PVR) with no apparent cause, which eventually results in death from right heart failure.1 The gold standard of IPAH diagnosis is based on the multiple haemodynamic indices measured by the right heart catheterization (RHC), such as PVR, mean pulmonary artery pressure (mPAP), mean pulmonary arterial wedge pressure (mPAWP) and others.2 In addition to the diagnostic value, some haemodynamic parameters could be used as biomarkers to evaluate the prognosis of IPAH patients.3 However, these parameters are obtained through invasive examination and much better biomarkers should be developed urgently.
Exosomes are a class of small extracellular vesicles with a diameter of 50–150 nm, which are derived from multivesicular endosomes fusing with the plasma membrane.4,5 Secreted exosomes can be taken up by the recipient cells and the contents of exosomes are also delivered into the recipient cells and play important roles in disease development, which represents a novel intercellular communication pathway.6 MicroRNAs (miRNAs) are essential cargo molecules in exosomes.7 Many studies have demonstrated that miRNAs play important roles in the occurrence and development of IPAH.8–11 For example, a previous study demonstrated that exosomes derived from HIV-infected and cocaine-treated macrophages promoted pulmonary smooth muscle proliferation by delivering its pro-survival miRNA cargo, which might play a crucial role in the development of IPAH.10 Although exosomal miRNAs regulate many key drivers of the pathology of pulmonary hypertension,12–14 the plasma exosomal miRNAs that could predict prognosis of IPAH patients remain unknown.
This current study compared plasma exosomal miRNA levels between survivors and nonsurvivors in order to investigate the association between exosomal miRNA and survival with a view to providing optimal prognostic information for clinical practice in IPAH.
Patients and methods
Study population
This study enrolled adult patients with IPAH to the study group between May 2010 and April 2012 and an additional group of adult patients with IPAH were recruited to the validation group between May 2013 and September 2016 in the Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China. The diagnosis of IPAH in the two groups of patients was established according to the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) guidelines from that time.15,16 Specifically, the inclusion criteria for patients in the two groups were as follows: (i) patients with mPAP ≥ 25 mmHg; (ii) patients with mPAWP ≤ 15 mmHg; (iii) patients with PVR > 3 Wood units; (iv) all other causes of PAH had been excluded. Patients with PAH associated with a definite cause such as connective tissue disease and congenital heart disease, and those with pulmonary hypertension due to left heart disease, pulmonary hypertension due to left heart disease and chronic thromboembolic pulmonary hypertension and other pulmonary artery obstructions were excluded. Patients with acute or chronic illnesses that might influence plasma contents (i.e. acute or chronic infections, chronic autoimmune diseases and primary endocrine disorders) and patients receiving any treatment with drugs that markedly inhibit exosome production, either at the time of the study or in the past, were also excluded.
The study protocol was reviewed and approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China (no. K20-195Y). Written informed consent was obtained from each patient prior to the performance of any study-related procedures.
Measurements at baseline and during follow-ups
Demographic data including sex, age, body surface area (BSA), 6-minute walk distance (6MWD), N-terminal pro-brain natriuretic peptide, World health Organization functional class and haemodynamic parameters were recorded. Haemodynamic parameters were collected at baseline by RHC as described in previous study.17 The 6MWD test was performed according to the guidelines of the American Thoracic Society.18
Follow-up intervals were decided by the treating physician based on the individual’s healthcare needs. Patients with PAH were encouraged to attend for follow-up every 3–6 months as where patients with relatively unstable conditions (those with rapid disease progression and poorly controlled disease). Patients with well controlled conditions that had no obvious deterioration were recommended to be followed up every 6–12 months by outpatient or telephone interview according to the ESC/ERS guidelines.16 The primary outcome was all-cause mortality. Survival was estimated from the date of confirmation to 8 November 2020 in the two groups of patients.
Exosome purification and identification
Blood samples (5 ml) were collected into ethylenediamine tetra acetic acid anticoagulant tubes and centrifuged at 3000 g for 15 min at 4 °C in an Eppendorf® Centrifuge 5910R (Eppendorf, Hamburg, Germany). The upper aqueous phase (plasma) was then transferred to tubes and stored at –80 °C until used for analysis. The exosomes were extracted from 700 µl of thawed plasma (4 °C) per patient using ExoQuick-TC™ Exosome Precipitation Solution (System Biosciences, Palo Alto, CA, USA).
A NanoSight NS300 instrument (Malvern Panalytical, Malvern, UK) equipped with a sCMOS camera (Applied Nikon, Tokyo, Japan) was used to analyse the size distribution and concentration of exosomes. The NanoSight NS300 instrument uses Nanoparticle Tracking Analysis technology, which combines light scattering and Brownian motion, to measure the size and concentration of particles in the exosomal supernatants. After the exosomes had been isolated, the pellets were resuspended in 100 µl of filtered 0.01 M phosphate-buffered saline (pH 7.4) and then diluted 100 ×. The conditions of the measurements were as follows: temperature of 25 °C; viscosity of 1 cP; 25 s per capture frame; measurement time of 60 s. The results indicate the mean sizes and concentration of at least three individual measurements.
Two marker proteins of exosomes (tumour susceptibility gene 101 protein [TSG101] and CD63) were determined by Western blot analysis as described in a previous study.19 Exosomes were lysed in RIPA lysis and extraction buffer (Thermo Fisher Scientific, Rockford, IL, USA). Protein concentration was determined using the BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA). Proteins from the exosomes were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and then electrophoretically transferred to polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA; Bio-Rad, Hercules, CA, USA). The membrane was then blocked for 1 h in blocking buffer (5% non-fat milk, 0.1% Tween 20 and 0.01 M phosphate-buffered saline [PBS], pH 7.4) at room temperature and then washed twice with 0.01 M PBS (pH 7.4). The membrane was incubated overnight at 4 °C with rabbit or mouse anti-human polyclonal antibodies for TSG101 and CD63 (both 1:1000; Cell Signaling Technology®, Danvers, MA, USA). Membranes were washed three times with Tris-buffered saline-Tween 20 (TBST; pH 7.5; 20 mmol/l Tris–HCl, 150 mmol/l sodium chloride, 0.1% Tween-20) (EpiZyme, Shanghai, China) for 10 min each wash at room temperature, followed by horseradish peroxidase-conjugated secondary anti-rabbit or anti-mouse secondary antibodies (both 1:2000; Cell Signaling Technology®) for 1 h at room temperature. Membranes were washed three times with TBST (pH 7.5) for 10 min each wash at room temperature. Antigen–antibody complexes were visualized using Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific). The immunoblots were determined using Image J software (National Institutes of Health, Bethesda, MD, USA).
MiRNA microarray analysis
Total RNA was extracted using TRIzol® (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Total RNA (5 μg) from each sample was labelled and hybridized on miRNA microarray chips (Exiqon, Vedbaek, Denmark) as previously described.20 A mirVana™ qRT-PCR miRNA Detection Kit (Thermo Fisher Scientific) was used to detect the expression of plasma exosomal miRNAs in the study group. Real-time polymerase chain reaction (PCR) was used to verify the results of the study group and to confirm the results of the validation group. The real time PCR amplification was performed using a standard SYBR® Green PCR protocol on an Applied Biosystems 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The primer sequences for real-time PCR were as follows: miR-411-3p forward, 5ʹ-ACACTCCAGCTGGGTATGTAACACGGTC-3ʹ, reverse 5ʹ-CAGTGCGTGTCGTGGAGT-3ʹ; miR-493-5p forward, 5ʹ-ACACTCCAGCTGGGTTGTACATGGTAGGCT-3ʹ, reverse, 5ʹ-CAGTGCGTGTCGTGGAGT-3ʹ; miR-596 forward, 5ʹ-ACACTCCAGCTGGGAAGCCTGCCCGGCTC-3ʹ, reverse, 5ʹ-CAGTGCGTGTCGTGGAGT-3ʹ; miR-4685-3p forward, 5ʹ-ACACTCCAGCTGGGTCTCCCTTCCTGCCCT-3ʹ, reverse, 5ʹ-CAGTGCGTGTCGTGGAGT-3ʹ; U6 (internal reference control) forward, 5ʹ-CTCGCTTCGGCAGCACA-3ʹ, reverse, 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ. All of the primers were synthesized by Sangon Biotec (Shanghai, China). The cycling programme involved preliminary denaturation at 95 °C for 5 s, followed by 40 cycles of denaturation at 60 °C for 20 s, annealing at 62 °C for 1 s and final elongation at 72 °C for 1 s. Data were managed using the RQ Manager v1.2.1 software (Applied Biosystems). The 2−ΔΔCt method was used to determine the relative quantification of expression levels.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 6 (GraphPad Software Inc., San Diego, CA, USA). Results are presented as mean ± SD or median (interquartile range) for continuous variables and absolute number for categorical variables. Independent-sample t-test or Mann–Whitney U-test was used to compare the difference between mean values for continuous variables and χ2-test was used to assess the distribution difference for categorical variables. Correlations were evaluated using Spearman's rank correlation coefficient. The Kaplan–Meier method was used to generate survival curves and the log-rank test was used to compare the difference of survival curve between different groups. Receiver-operating characteristic (ROC) curves and the areas under the curves were determined to assess the predictive power of factors. Standardization of the coefficient was usually performed to find out which of the independent variables had a greater effect on the dependent variable in a multiple regression analysis. A two-tail P-value of < 0.05 was considered statistically significant.
Results
The study enrolled 12 IPAH patients in the study group and 46 IPAH patients in the validation group that matched the inclusion criteria. The demographic and haemodynamic data are presented in Table 1. The mean ± SD age was 36.3 ± 1.8 years in the study group and 44.6 ± 15.6 years in the validation group. The mean ± SD duration of follow-up was 60.3 ± 35.4 months in all patients. All 58 patients (100%) were fully followed up in the current study. Four patients (two males) and 15 patients (six males) passed away in the study and validation groups, respectively. Patients were further subgrouped into survivors and nonsurvivors in order to undertake further analyses.
Table 1.
Baseline demographic and haemodynamic characteristics of patients with idiopathic pulmonary arterial hypertension (IPAH) that were enrolled in a study to investigate if plasma exosomal miRNA levels can predict prognosis.
| Characteristic | Study group n = 12 | Validation group n = 46 |
|---|---|---|
| Baseline characteristics | ||
| Age, years | 36.3 ± 1.8 | 44.6 ± 15.6 |
| Sex, male/female | 5/7 | 18/28 |
| Nonsurvivors | 4 | 15 |
| Heart rate, beats/min | 83.5 ± 17.3 | 80.6 ± 13.1 |
| SBP, mmHg | 113.0 ± 20.2 | 115.5 ± 19.9 |
| DBP, mmHg | 71.7 ± 11.8 | 70.9 ± 13.8 |
| BSA, m2 | 1.6 ± 0.1 | 1.6 ± 0.2 |
| 6MWD, m | 378.9 ± 84.8 | 362.8 ± 92.5 |
| WHO-FC, n (%) | ||
| I–II | 4 (33.3) | 17 (37.0) |
| III–IV | 8 (66.7) | 29 (63.0) |
| Haemodynamic characteristics | ||
| mRAP, mm Hg | 6.4 ± 5.4 | 5.9 ± 4.5 |
| mPAP, mm Hg | 69.7 ± 22.0 | 63.0 ± 10.6 |
| mPAWP, mm Hg | 9.6 ± 2.5 | 7.2 ± 3.4 |
| PVR, Wood units | 17.0 ± 4.6 | 15.3 ± 4.2 |
| CO, l/min | 4.2 ± 1.5 | 4.4 ± 1.2 |
| CI, l/min/m2 | 2.6 ± 1.0 | 2.8 ± 0.7 |
| Specific medications | ||
| PDE-5 inhibitors | 4 (33.3) | 18 (39.1) |
| ERA | 3 (25.0) | 10 (21.7) |
| Prostacyclin analogues | 2 (16.7) | 5 (10.9) |
| Combination | 2 (16.7) | 11 (23.9) |
| Nonspecific medication | 1 (8.3) | 2 (4.3) |
Data presented as mean ± SD or n of patients (%).
SBP, systolic blood pressure; DBP, diastolic blood pressure; BSA, body surface area; 6MWD, 6-minute walk distance; WHO-FC, World Health Organization Functional Class; mRAP, mean right atrial pressure; mPAP, mean pulmonary arterial pressure; mPAWP, mean pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; CO, cardiac output; CI, cardiac index; PDE-5, phosphodiesterase type 5; ERA, endothelin receptor antagonist.
In the combined cohort from the study and validation groups, there were no significant differences in the demographic characteristics between survivors and nonsurvivors (Table 2). The heart rate was significantly higher in the nonsurvivors compared with the survivors (P = 0.025). The mean right atrial pressure (mRAP), mPAP, mPAWP and PVR were significantly higher in the nonsurvivors compared with the survivors (P < 0.05 for all comparisons). The cardiac output and cardiac index were significantly lower in the nonsurvivors compared with the survivors (P < 0.05 for both comparisons). There were no significant differences in the specific medications administered to nonsurvivors and survivors.
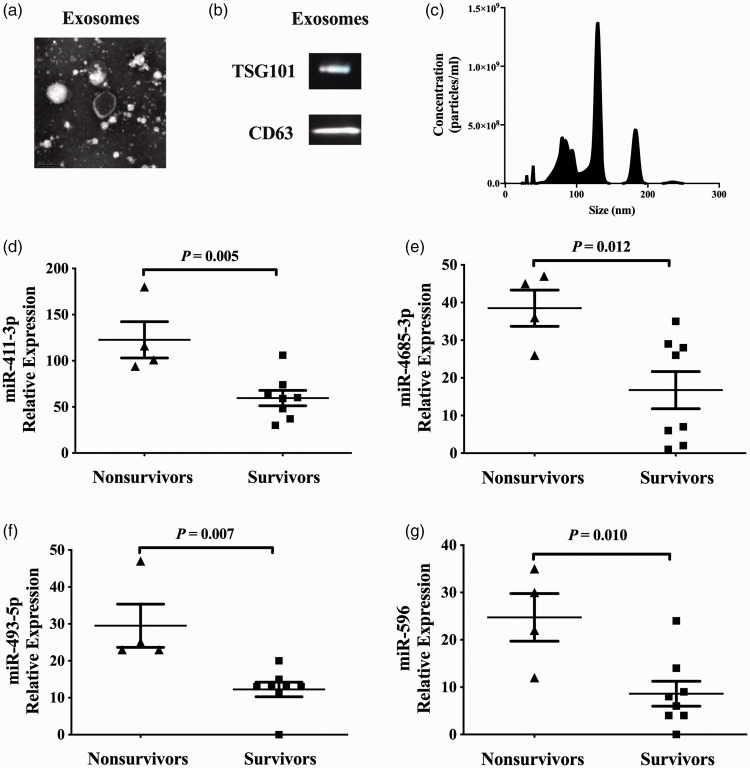
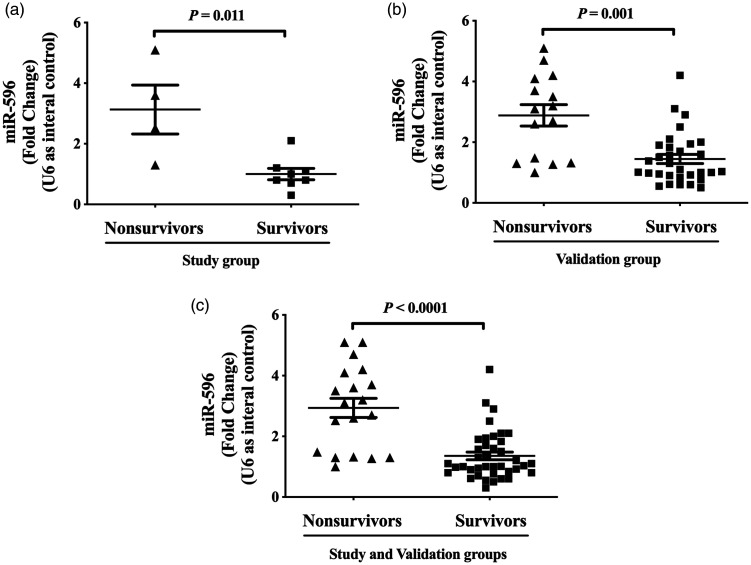
The classical and clear vesicle structure of the exosomes could be observed clearly under transmission electron microscopy (Figure 1a); exosomal markers TSG101 and CD63 were both detected by Western blot analysis (Figure 1b); and the diameter of the majority of the exosomes ranged from 30 to 150 nm (Figure 1c). The levels of plasma exosomal miR-411-3p, miR-4685-3p, miR-493-5p and miR-596 were significantly higher in nonsurvivors compared with survivors in the study group (P = 0.005, P = 0.012, P = 0.007 and P = 0.010, respectively; Figures 1D–1G). Similar results for miR-596 as measured by real-time PCR are shown in both the study and validation groups (P < 0.05 for all comparisons, Figures 2A–2C).
Figure 1.
The characterization of plasma exosomes and several exosomal microRNAs (miRNAs) in patients with idiopathic pulmonary arterial hypertension (IPAH) in the study group (n = 12). (a) Images of plasma exosomes under transmission electron microscopy. (b) The levels of two exosome markers, tumour susceptibility gene 101 protein (TSG101) and CD63, in exosomes as determined by Western blot analysis. (c) Particle size distribution of exosomes. (D–G) The levels of several plasma exosomal miRNAs in patients with IPAH stratified according to survival during follow-up. Data presented as dot plots with the mean shown as the central black horizontal line and the error bars indicating the SD; independent-sample t-test.
Figure 2.
The levels of plasma exosomal microRNA (miR)-596 measured by real-time polymerase chain reaction in patients with idiopathic pulmonary arterial hypertension in the study (n = 12) and validation groups (n = 46). (a) The levels of plasma exosomal miR-596 in the study group. (b) The levels of plasma exosomal miR-596 in the validation group. (c) The levels of plasma exosomal miR-596 in the study and validation groups. Data presented as dot plots with the mean shown as the central black horizontal line and the error bars indicating the SD; Mann–Whitney U-test.
In the combined cohort from the study and validation groups, correlations were identified between several exosomal miRNAs and haemodynamic characteristics (Table 3). Significant negative correlations were found between survival time (–0.647; P < 0.0001), cardiac index (–0.374; P = 0.0005) and miR-596 levels. There were significant positive correlations between mRAP (0.508; P < 0.0001), PVR (0.713; P < 0.0001) and miR-596 levels.
Table 2.
Baseline demographic and haemodynamic characteristics of patients with idiopathic pulmonary arterial hypertension (IPAH) in the study and verification groups stratified according to survival during follow-up.
| Characteristic | Nonsurvivors n = 19 | Survivors n = 39 | Statistical significancea |
|---|---|---|---|
| Baseline characteristics | |||
| Age, years | 36.1 ± 14.6 | 43.0 ± 16.0 | NS |
| Sex, male/female | 8/11 | 15/24 | NS |
| Heart rate, beats/min | 86.6 ± 10.5 | 77.2 ± 13.5 | P = 0.025 |
| SBP, mmHg | 111.2 ± 20.6 | 115.7 ± 20.6 | NS |
| DBP, mmHg | 70.7 ± 13.2 | 71.0 ± 14.5 | NS |
| BSA, m2 | 1.6 ± 0.1 | 1.6 ± 0.1 | NS |
| Survival time, months | 18.3 ± 12.6 | 80.8 ± 22.2 | P < 0.0001 |
| 6MWD, m | 337.7 ± 98.6 | 377.9 ± 85.4 | NS |
| WHO-FC | NS | ||
| I–II | 10 (52.6) | 18 (46.2) | |
| III–IV | 9 (47.4) | 21 (53.8) | |
| Haemodynamics | |||
| mRAP, mm Hg | 7.1 ± 5.4 | 4.0 ± 3.8 | P = 0.036 |
| mPAP, mm Hg | 62.7 ± 15.7 | 52.9 ± 13.2 | P = 0.018 |
| mPAWP, mm Hg | 9.3 ± 4.2 | 6.7 ± 2.7 | P = 0.030 |
| PVR, Wood units | 14.8 ± 5.7 | 10.9 ± 4.1 | P = 0.006 |
| CO, l/min | 3.8 ± 1.7 | 4.7 ± 1.3 | P = 0.013 |
| CI, l/min/m2 | 2.4 ± 0.6 | 2.9 ± 0.8 | P = 0.012 |
| Specific medications | NS | ||
| PDE-5 inhibitors | 9 (47.4) | 18 (46.2) | |
| ERA | 5 (26.3) | 8 (20.5) | |
| Prostacyclin analogues | 2 (10.5) | 5 (12.8) | |
| Combination | 3 (15.8) | 5 (12.8) | |
| Nonspecific medication | 0 (0.0) | 3 (7.7) | |
Data presented as mean ± SD or n of patients (%).
aIndependent-sample t-test or Mann–Whitney U-test was used to compare continuous variables and χ2-test was used to compare categorical variables.
SBP, systolic blood pressure; DBP, diastolic blood pressure; BSA, body surface area; 6MWD, 6-minute walk distance; WHO-FC, World Health Organization Functional Class; mRAP, mean right atrial pressure; mPAP, mean pulmonary arterial pressure; mPAWP, mean pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; CO, cardiac output; CI, cardiac index; PDE-5, phosphodiesterase type 5; ERA, endothelin receptor antagonist; NS, no significant between-group difference (P ≥ 0.05).
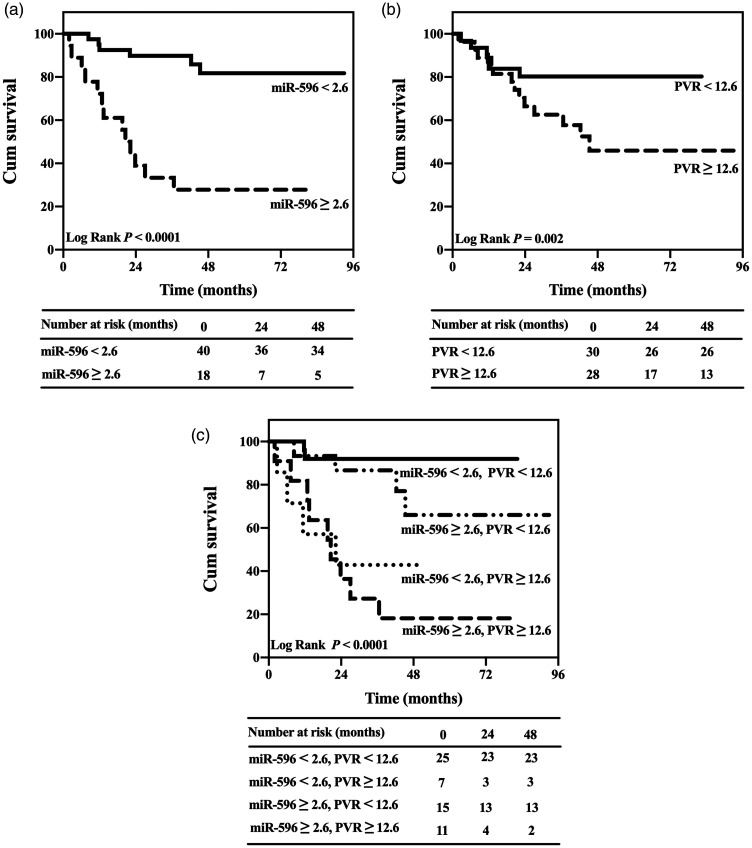
Univariate and multivariate analyses showed that the levels of plasma exosomal miR-596 and PVR were independent predictors of survival in the study group (P < 0.05 for both; Table 4). To assess the predictive power of plasma exosomal miR-596 levels and PVR in patients with IPAH, ROC analysis was undertaken in the combined cohort from the study and validation groups (Table 5). ROC analysis demonstrated that the cut-off values of plasma exosomal miR-596 (cut-off 2.6) and PVR (cut-off 12.6 Wood units) discriminated survivors from nonsurvivors with a sensitivity of 70.6% and 76.5% and specificity of 92.3% and 66.7% in all patients, respectively (P < 0.0001 and P = 0.007, respectively).
Table 3.
The correlations between exosomal microRNA (miR)-596 and haemodynamic parameters in patients with idiopathic pulmonary arterial hypertension in the study and verification groups.
| miR-596 | Survival time | mRAP | PVR | CI | |
|---|---|---|---|---|---|
| miR-596 | –0.647 | 0.508 | 0.713 | –0.374 | |
| Survival time | P < 0.0001 | –0.191 | –0.366 | 0.316 | |
| mRAP | P < 0.0001 | NS | 0.581 | –0.075 | |
| PVR | P < 0.0001 | P = 0.0006 | P < 0.0001 | –0.497 | |
| CI | P = 0.0005 | P = 0.019 | NS | P = 0.0001 |
The upper right half (dark grey) are the Spearman's rank correlation coefficients and the lower left half (light grey) are the associated P-values.
mRAP, mean right atrial pressure; PVR, pulmonary vascular resistance; CI, cardiac index; NS, no significant correlation (P ≥ 0.05).
Table 4.
Results of univariate and multivariate analyses of survival in relation to selected miRNAs levels in patients with idiopathic pulmonary arterial hypertension in the study group.
| Characteristic |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 0.974 (0.943, 1.006) | NS | ||
| Sex | 0.844 (0.339, 2.099) | NS | ||
| BSA | 1.123 (0.952, 1.326) | NS | ||
| 6MWD | 0.995 (0.990, 1.001) | NS | ||
| WHO-FC | 0.834 (0.413, 1.687) | NS | ||
| mRAP | 1.121 (1.027, 1.224) | P = 0.010 | ||
| mPAP | 1.036 (1.008, 1.064) | P = 0.011 | ||
| mPAWP | 1.213 (1.050, 1.403) | P = 0.009 | ||
| PVR | 1.155 (1.050, 1.270) | P = 0.003 | 1.146 (1.010, 1.300) | P = 0.034 |
| CI | 0.038 (0.138, 0.800) | P = 0.011 | ||
| miR-596 | 1.164 (1.011, 1.340) | P = 0.034 | 2.119 (1.402, 3.203) | P < 0.0001 |
HR, hazard ratio; CI, confidence interval; BSA, body surface area; 6MWD, 6-minute walk distance; WHO-FC, World Health Organization Functional Class; mRAP, mean right atrial pressure; mPAP, mean pulmonary arterial pressure; mPAWP, mean pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; CI, cardiac index; miR, microRNA; NS, no significant correlation (P ≥ 0.05).
Table 5.
Receiver operating characteristic analysis of microRNA (miR)-596 and pulmonary vascular resistance (PVR) in patients with idiopathic pulmonary arterial hypertension in the study and verification groups.
| Variables | Cut-off value | Sensitivity | Specificity | AUC | 95% CI | P-value |
|---|---|---|---|---|---|---|
| miR-596 | 2.6 | 70.6% | 92.3% | 0.855 | 0.749, 0.962 | P < 0.0001 |
| PVR | 12.6 | 76.5% | 66.7% | 0.727 | 0.567, 0.887 | P = 0.007 |
AUC, area under the curve; CI, confidence interval.
Kaplan–Meier curves that were generated according to the cut-off values of exosomal miR-596 (cut-off 2.6) and PVR (cut-off 12.6 Wood units) showed that patients with lower levels of exosomal miR-596 and PVR presented with a significantly better prognosis than those with higher levels of exosomal miR-596 and PVR in the combined cohort from the study and validation groups (Log rank P < 0.0001 and P = 0.002, respectively; Figures 3A and 3B). The combination of the two independent predictors identified subgroups with significantly different probabilities of survival (Log rank P < 0.0001; Figure 3c). The subgroup with miR-596 < 2.6 and PVR < 12.6 Wood units had better survival than the other three subgroups. While the subgroup with miR-596 ≥ 2.6 and PVR ≥ 12.6 Wood units had the worst survival among these four subgroups.
Figure 3.
Kaplan–Meier survival curve analyses in patients with idiopathic pulmonary arterial hypertension in the study (n = 12) and validation groups (n = 46). (a) Analysis based on microRNA (miR)-596 levels (cut-off 2.6). (b) Analysis based on pulmonary vascular resistance (PVR) levels (cut-off 12.6 Wood units). (c) Combined analysis with both miR-596 and PVR levels.
Discussion
Circulating miRNAs can be used as biomarkers for various diseases. For example, previous research has demonstrated that plasma miRNAs in patients with pulmonary hypertension are of value in their diagnosis and prognosis.21–23 Although several studies suggest that plasma miRNAs could predict the prognosis of PAH,21–24 evidence for plasma exosomal miRNAs in PAH is limited.13,14 To the best of our knowledge, this is the first study to demonstrate a significant predictive value for exosomal miR-596 levels in predicting survival in patients with IPAH.
The main finding in the present study was significantly higher levels of exosomal miR-596 in nonsurviving patients with IPAH compared with surviving patients with IPAH, which suggests a role for exosomal miR-596 in the course and progression of IPAH. Studies on miR-596 in PAH are very limited, but some studies demonstrated a relationship between miR-596 and malignant diseases.25–29 Previous studies found that circulating miR-596 levels were a predictor of overall survival in patients with ependymoma and a predictor of recurrence in patients with early stage colon cancer.25,26 In oral squamous cell carcinoma with the pattern of DNA hypermethylation, miR-596 levels were downregulated.27 Interestingly, another study concluded that miR-596 levels were upregulated in craniopharyngiomas.28 This previous study was consistent with the current findings,28 which suggest that the upregulation of exosomal miR-596 might be a regulator in IPAH. However, this needs further verification.
Several studies have focused on the mechanism of action and targets of miR-596 as a cancer suppressor.30–32 A previous study found that miR-596 regulated the promotion and development of bladder cancer by modulating the expression of matrix metalloproteinase 9.32 Overexpressed miR-596 downregulated cell proliferation, migration and invasion but upregulated cell apoptosis in melanoma cells through inhibiting MAPK/ERK signalling.33 Another study confirmed that overexpressed miR-596 induced an increase of p53-mediated apoptosis in HeLa and HCT116 cells.34 However, more research into the role of exosomal miR-596 in IPAH is required. For example, investigations into miR-596 expression in pulmonary tissue and pulmonary arterial muscle cells; and the mechanism of action, the targets and signal pathways involved in the progression of IPAH should be undertaken.
The current study demonstrated significant correlations between exosomal miR-596 levels and haemodynamic parameters in the combined cohort of patients with IPAH from the study and validation groups, which suggested that exosomal miR-596 could be a valuable predictor for IPAH prognosis. The levels of exosomal miR-596 and PVR predicted survival in patients with IPAH in the current study. An area under the curve of 0.855 measured in the ROC analysis demonstrated the prognostic value of miR-596, with high sensitivity (70.6%) and specificity (92.3%). The optimal cut-off value ROC analysis suggested that both exosomal miR-596 and PVR had good sensitivity and specificity in the prognosis of IPAH. This might be an indicator of earlier changes in miR-596 that are similar to PVR. This result indicated that plasma exosomal miR-596 levels could predict the survival rate of patients with IPAH.
This current study had several limitations. First, due to the small sample size, the generalizability of these current findings should be further confirmed in a larger study population. Secondly, more clinical outcomes in addition to survival should be considered during follow-up. Thirdly, these clinical findings need in vitro studies to identify the potential pathophysiological mechanisms involved.
In conclusion, this current study found, for the first time, a significant difference in the levels of plasma exosomal miR-596 between surviving and nonsurviving patients with IPAH. Plasma exosomal miR-596 levels appear to be an independent predictor of survival, which would provide a reference for clinical prognosis and the assessment of disease severity in patients with IPAH.
Acknowledgements
All work in this study was completed in the Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China. We would like to thank Professors Jinming Liu and Yuan Li for their help with the English language. We also thank the patients that participated in the study.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This study was funded by the Programme of National Natural Science Foundation of China (no. 81870042 and no. 81900050), the Youth Project of Shanghai Municipal Commission of Health and Family Planning (no. 20174Y0143) and the National Science and Technology Information System of the People’s Republic of China (no. 2018YFC1313603).
ORCID iDs: Yuan-Yuan Sun https://orcid.org/0000-0002-4577-3854
Ping Yuan https://orcid.org/0000-0002-1198-6042
References
- 1.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 2004; 351: 1655–1665. [DOI] [PubMed] [Google Scholar]
- 2.Forfia PR, Trow TK. Diagnosis of pulmonary arterial hypertension. Clin Chest Med 2013; 34: 665–681. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y, Li Y, Meng X, et al. Acute vasoreactivity testing predicts outcome of idiopathic pulmonary arterial hypertension patients with a negative acute response. Ann Transl Med 2020; 8: 1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chivet M, Javalet C, Hemming F, et al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans 2013; 41: 241–244. [DOI] [PubMed] [Google Scholar]
- 5.Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 2016; 113: E968–E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 2004; 16: 415–421. [DOI] [PubMed] [Google Scholar]
- 7.Zheng D, Huo M, Li B, et al. The Role of Exosomes and Exosomal MicroRNA in Cardiovascular Disease. Front Cell Dev Biol 2021; 8: 616161. [DOI] [PMC free article] [PubMed]
- 8.Bienertova-Vasku J, Novak J, Vasku A. MicroRNAs in pulmonary arterial hypertension: pathogenesis, diagnosis and treatment. J Am Soc Hypertens 2015; 9: 221–234. [DOI] [PubMed] [Google Scholar]
- 9.Lipps C, Northe P, Figueiredo R, et al. Non-invasive approach for evaluation of pulmonary hypertension using extracellular vesicle-associated small non-coding RNA. Biomolecules 2019; 9: E666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma H, Chinnappan M, Agarwal S, et al. Macrophage-derived extracellular vesicles mediate smooth muscle hyperplasia: role of altered miRNA cargo in response to HIV infection and substance abuse. FASEB J 2018; 32: 5174–5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen KH, Dasgupta A, Lin J, et al. Epigenetic dysregulation of the dynamin-related protein 1 binding partners MiD49 and MiD51 increases mitotic mitochondrial fission and promotes pulmonary arterial hypertension: mechanistic and therapeutic implications. Circulation 2018; 138: 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Liu J, Zheng K, et al . Exosomal miR-211 contributes to pulmonary hypertension via attenuating CaMK1/PPAR-γ axis. Vascul Pharmacol 2021; 136: 106820. [DOI] [PubMed] [Google Scholar]
- 13.Aliotta JM, Pereira M, Wen S, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res 2016; 110: 319–330. [DOI] [PMC free article] [PubMed]
- 14.Deng L, Blanco FJ, Stevens H, et al . MicroRNA-143 Activation Regulates Smooth Muscle and Endothelial Cell Crosstalk in Pulmonary Arterial Hypertension. Cardiovasc Res 2016; 110: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 16.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 17.Jing ZC, Xu XQ, Han ZY, et al. Registry and survival study in Chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest 2007; 132: 373–379. [DOI] [PubMed] [Google Scholar]
- 18.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: Guidelines for the six-minute walk test. Am J Resp Crit Care 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 19.Andriolo G, Provasi E, Lo Cicero V, et al. Exosomes from human cardiac progenitor cells for therapeutic applications: development of a GMP-grade manufacturing method. Front Physiol 2018; 9: 1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo L, Qiu ZP, Wei LP, et al. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-α1C. Hypertension 2012; 59: 1006–1013. [DOI] [PubMed] [Google Scholar]
- 21.Baptista R, Marques C, Catarino S, et al. MicroRNA-424(322) as a new marker of disease progression in pulmonary arterial hypertension and its role in right ventricular hypertrophy by targeting SMURF1. Cardiovasc Res 2018; 114: 53–64. [DOI] [PubMed] [Google Scholar]
- 22.Kheyfets VO, Sucharov CC, Truong U, et al. Circulating miRNAs in pediatric pulmonary hypertension show promise as biomarkers of vascular function. Oxid Med Cell Longev 2017; 2017: 4957147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes CJ, Wharton J, Boon RA, et al. Reduced microRNA-150 is associated with poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 187: 294–302. [DOI] [PubMed] [Google Scholar]
- 24.Sarrion I, Milian L, Juan G, et al. Role of circulating miRNAs as biomarkers in idiopathic pulmonary arterial hypertension: possible relevance of miR-23a. Oxid Med Cell Longev 2015; 2015: 792846. [DOI] [PMC free article] [PubMed]
- 25.Codta FF, Bischof JM, Vanin EF, et al. Identification of microRNAs as potential prognostic markers in ependymoma. PLoS One 2011; 6: e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shivapurkar N, Weiner LM, Marshall JL, et al. Recurrence of early stage colon cancer predicted by expression pattern of circulating microRNAs. PLoS One 2014; 9: e84686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endo H, Muramatsu T, Furuta M, et al. Potential of tumor-suppressive miR-596 targeting LGALS3BP as a therapeutic agent in oral cancer. Carcinogenesis 2013; 34: 560–569. [DOI] [PubMed] [Google Scholar]
- 28.Samis J, Vanin EF, Sredni ST, et al. Extensive miRNA expression analysis in craniopharyngiomas. Childs Nerv Syst 2016; 32: 1617–1624. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Dai DQ. MicroRNA-596 acts as a tumor suppressor in gastric cancer and is upregulated by promotor demethylation. World J Gastroenterol 2019; 25: 1224–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei M, Cao Y, Jia D, et al. CREPT promotes glioma cell proliferation and invasion by activating Wnt/β-catenin pathway and is a novel target of microRNA-596. Biochimie 2019; 162: 116–124. [DOI] [PubMed] [Google Scholar]
- 31.Song YX, Sun JX, Zhao JH, et al. Non-coding RNAs participate in the regulatory network of CLDN4 via ceRNA mediated miRNA evasion. Nat Commun 2017; 8: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olivieri M, Ferro M, Terreri S, et al. Long non-coding RNA containing ultraconserved genomic region 8 promotes bladder cancer tumorigenesis. Oncotarget 2016; 7: 20636–20654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu SM, Lin CH, Lu J, et al. miR-596 modulates melanoma growth by regulating cell survival and death. J Invest Dermatol 2018; 138: 911–921. [DOI] [PubMed] [Google Scholar]
- 34.Ma M, Yang J, Wang B, et al. High-throughput identification of miR-596 inducing p53-mediated apoptosis in HeLa and HCT116 cells using cell microarray. SLAS Technol 2017; 22: 636–645. [DOI] [PubMed] [Google Scholar]