Abstract
Randomized trials of pulmonary vasodilators in pulmonary hypertension due to left heart disease (Group 2) and lung disease (Group 3) have demonstrated potential for harm. Yet these therapies are commonly used in practice. Little is known of the effects of treatment outside of clinical trials. We aimed to establish outcomes of vasodilator treatment for Groups 2/3 pulmonary hypertension in real-world practice. We conducted a retrospective cohort study of 132,552 Medicare-eligible Veterans with incident Groups 2/3 pulmonary hypertension between 2006 and 2016, and a secondary nested case–control study. Our primary outcome was a composite of death by any cause or selected acute organ failures. In our cohort analysis, we calculated adjusted risks of time to our outcome using Cox proportional hazards models with facility-specific random effects. In our case–control analysis, we used logistic mixed-effects models to estimate the effect of any past, recent, and cumulative exposure on our outcome. From our cohort study, 3249 (2.5%) Veterans were exposed to pulmonary vasodilators. Exposure to vasodilators was associated with increased risk of our primary outcome, in both Group 3 (HR: 1.58 (95% CI: 1.37–1.82)) and Group 2 (HR: 1.26 (95% CI: 1.12–1.41)) pulmonary hypertension patients. The case–control study determined odds of our outcome increased by 11% per year of exposure (OR: 1.11 (95% CI: 1.07–1.16)). Treating Groups 2/3 pulmonary hypertension with vasodilators in clinical practice is associated with increased risk of harm. This extension of trial findings to a real-world setting offers further evidence to limit use of vasodilators in Groups 2/3 pulmonary hypertension outside of clinical trials.
Keywords: pulmonary hypertension, comparative effectiveness, evidence-based medicine
Introduction
Pulmonary hypertension (PH) is a common and serious complication of left heart disease and chronic lung disease (known as Group 2 and Group 3 PH, respectively), occurring in more than 50% of at-risk patients.1,2 Patients with Groups 2 and 3 PH suffer from higher morbidity and mortality than those with the underlying heart or lung disease alone,3–8 and treatment of the underlying process is often inadequate to relieve symptoms or prevent disease progression,9,10 driving the search for effective therapies. Pulmonary vasodilators have been viewed as an attractive potential treatment for Groups 2/3 PH given their proven clinical efficacy in Group 1 PH (pulmonary arterial hypertension) and Group 4 PH (due to chronic thromboembolic disease).11–13
Unfortunately, results of randomized controlled trials of pulmonary vasodilators in Groups 2/3 PH have been disappointing, with no consistent benefit observed in mortality, hemodynamics, functional capacity, or quality of life.14–17 A search for a specific patient subgroup that might benefit from vasodilators has also been unsuccessful, with no clear improvements observed in heart failure with reduced18,19 or preserved14,20 ejection fraction, valvular heart disease,21 interstitial lung disease,22,23 or chronic obstructive lung disease,17 among other patient groups. Even more troubling, several clinical trials have raised safety concerns of using vasodilators in Groups 2 and 3 PH, including increased fluid retention, worsened gas exchange, progression of underlying disease, and even higher mortality.19–23 On this basis, clinical practice guidelines recommend against use of pulmonary vasodilators for the treatment of Groups 2/3 PH.24,25
Despite empirical evidence and clinical guidelines, many patients with Groups 2/3 PH are treated with pulmonary vasodilators outside of clinical trials, and use is increasing over time.26–28 Groups 2/3 PH patients in usual clinical practice are older with a higher burden of comorbid illnesses compared to trial participants,29 and may experience different outcomes from use of pulmonary vasodilators. Yet little is known of the effectiveness of treating Groups 2/3 PH with vasodilators outside of clinical trials. The objective of this study was to establish the effect of vasodilator treatment on the morbidity and mortality of patients with Groups 2/3 PH in usual clinical practice by utilizing longitudinal data from the Veterans Health Administration (VA), the largest national integrated healthcare system in the United States. We hypothesized that use of pulmonary vasodilators in Groups 2/3 PH is associated with serious morbidity including increased risk of acute organ failures and death.
Methods
Study designs and data sources
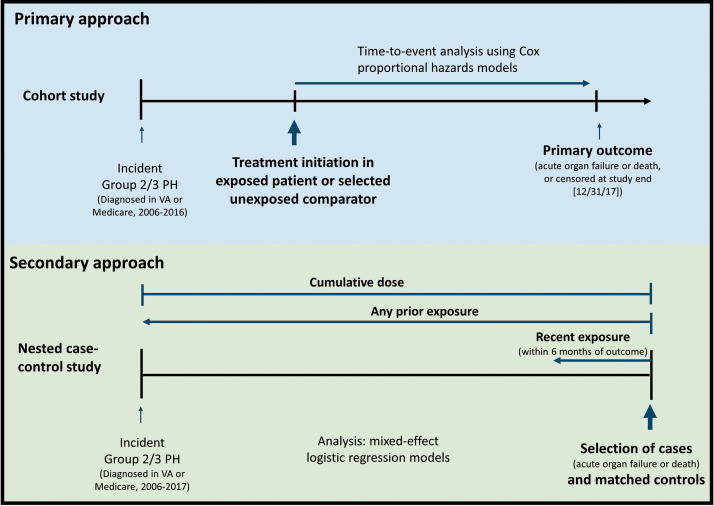
We assessed outcomes of pulmonary vasodilator use in Groups 2 and 3 PH using two distinct yet complementary approaches. In our primary approach, we conducted a cohort study of Medicare-eligible Veterans with Groups 2 and 3 PH, linking national patient-level data from the VA and Centers for Medicare and Medicaid Services. From this cohort, we additionally performed a nested case–control study. The timeline and definitions for our two study designs and analyses are shown in Fig. 1. Our Hospital Institutional Review Board approved this study.
Fig. 1.
Timeline and definitions of cohort and nested case–control study designs and analyses.
PH: pulmonary hypertension; VA: Veterans Health Administration.
Cohort study methods
Study population
We identified a cohort of all Veterans diagnosed with incident PH between 1 January 2006 and 31 December 2017, requiring at least two International Classification of Diseases, 9th or 10th Revision diagnosis codes for PH (416.xx or I27.x). We excluded patients diagnosed with PH in calendar year 2017 so that all patients would have at least one year of follow-up time. To select incident PH, we excluded those with a PH code between 1 October 1999 (the inception of the VA Corporate Data Warehouse) and 31 December 2005. We restricted our sample to Medicare-eligible Veterans (age ≥65 or in the Medicare-denominator file) who were active pharmacy users. Finally, we narrowed our sample to Veterans with Groups 2/3 PH using a previously validated algorithm intended to exclude those with Groups 1, 4, and 5 PH, which showed a high positive predictive value for identifying Groups 2/3 PH.26 In order to maximize the specificity of the algorithm to identify Groups 2/3 PH, we preferentially classified patients as Groups 1, 4, or 5 over Groups 2 or 3. For example, a patient with a diagnosis code for both a Group 1 PH-associated disease (such as scleroderma) and a Group 3 PH-associated disease (such as interstitial lung disease) would be classified as Group 1 PH. The derivation of our study sample is shown in e-Figure 1 in the Supplemental material.
Exposures
From our study population, we identified all patients treated for PH with a pulmonary vasodilator, including phosphodiesterase-5-inhibitor (PDE5i), endothelin receptor antagonist, prostacyclin analogue, or soluble guanylate cyclase stimulator between 1 January 2006 and 31 December 2016 (e-Table 1 in the Supplemental material). Based on prior medical record validation,26 we considered PDE5i prescriptions specifying treatment on ≥ 15 days per month to be for PH rather than erectile dysfunction. We defined the index date as the date PH treatment began (date of first pulmonary vasodilator prescription in VA or Medicare). Those who were treated with a pulmonary vasodilator, other than low-dose PDE5i for treatment of erectile dysfunction, between 1 October 1999 and 31 December 2005 were excluded.
Table 1.
Baseline characteristics of exposed and unexposed patients from cohort study.
| Exposed (n = 3249) | Unexposed (n = 12,991) | |
|---|---|---|
| Patient characteristics | ||
| Age, yr, mean (SD) | 72.8 (9.3) | 73.8 (9.1) |
| Female sex | 52 (1.6) | 317 (2.4) |
| Race/ethnicity | ||
| White | 2568 (79.0) | 10,970 (84.4) |
| Black | 525 (16.2) | 1465 (11.3) |
| Hispanic | 78 (2.4) | 268 (2.1) |
| Other | 78 (2.4) | 288 (2.2) |
| Married | 2085 (64.2) | 8086 (62.2) |
| VA benefits (priority status) | ||
| Highly disabled | 867 (20.9) | 2893 (22.3) |
| Low/moderately disabled | 767 (26.7) | 2744 (21.1) |
| Limited with copayments | 859 (23.6) | 3364 (25.9) |
| Poverty/no copayments | 679 (28.8) | 3990 (30.7) |
| Body mass index | ||
| Underweight | 23 (0.7) | 183 (1.4) |
| Normal | 533 (16.4) | 2387 (18.4) |
| Overweight | 1140 (35.1) | 3918 (30.2) |
| Class I obesity | 906 (27.9) | 3348 (25.8) |
| Class II or III obesity | 647 (19.9) | 3155 (24.3) |
| Elixhauser comorbidity index, mean (SD) | 8.4 (4.2) | 9.6 (3.5) |
| Recent acute organ failurea | ||
| Recent acute right heart failure | 55 (1.7) | 66 (0.5) |
| Recent acute left heart failure | 530 (16.3) | 1601 (12.3) |
| Recent acute respiratory failure | 276 (8.5) | 878 (6.8) |
| Recent acute renal failure | 175 (5.4) | 633 (4.9) |
| Comorbidities | ||
| Major adverse cardiac eventb | 770 (23.7) | 4112 (31.7) |
| Congestive heart failure | 2063 (63.5) | 8876 (68.3) |
| Arrhythmia | 1816 (55.9) | 8339 (64.2) |
| Coronary atherosclerosis | 2109 (64.9) | 9271 (71.4) |
| Stroke | 328 (10.1) | 2136 (16.4) |
| Transient ischemic attack | 143 (4.4) | 944 (7.3) |
| Chronic lung diseasec | 2170 (66.8) | 8736 (67.2) |
| Interstitial lung disease | 1082 (33.3) | 3477 (26.8) |
| Obstructive sleep apnea | 932 (28.7) | 3661 (28.2) |
| Pneumonia | 971 (29.9) | 4481 (34.5) |
| Hypertension | 2979 (88.6) | 12,256 (94.3) |
| Hyperlipidemia | 2563 (78.9) | 10,982 (84.5) |
| Peripheral vascular disease | 971 (29.9) | 4637 (35.7) |
| Venous thromboembolism | 309 (9.5) | 899 (6.9) |
| Diabetes | 1702 (52.4) | 7176 (55.2) |
| Chronic kidney disease | 1277 (39.3) | 5515 (42.5) |
| Hepatitis C | 247 (7.6) | 1326 (10.2) |
| Cirrhosis | 279 (8.6) | 1030 (7.9) |
| Malignancy | 692 (21.3) | 3063 (23.6) |
| Anemia | 1387 (42.7) | 6544 (50.4) |
| Thyroid disease | 647 (19.9) | 2881 (22.2) |
| Arthritis | 812 (25.0) | 3485 (26.8) |
| Dementia | 136 (4.2) | 1286 (9.9) |
| Neurodegenerative disease | 39 (1.2) | 329 (2.5) |
| Depression | 841 (25.9) | 3805 (29.3) |
| Post-traumatic stress disorder | 289 (8.9) | 1190 (9.2) |
| Schizophrenia | 39 (1.2) | 261 (2.0) |
| Other psychiatric condition | 159 (4.9) | 1171 (9.0) |
| Alcohol/substance use disorder | 458 (14.1) | 2118 (16.3) |
| Tobacco use disorder | 1339 (41.2) | 6092 (46.9) |
| Healthcare utilizationd | ||
| Right heart catheterization | 1066 (32.8) | 1464 (11.3) |
| Outpatient visits, mean (SD) | 38.5 (32.7) | 41.7 (26.6) |
| Outpatient pulmonary hypertension visits, mean (SD) | 2.9 (5.4) | 1.1 (2.7) |
| Outpatient visits in VA only | 1087 (33.4) | 2634 (20.3) |
| Outpatient visits in both VA and Medicare | 1672 (51.5) | 8789 (67.7) |
| No outpatient visits | 152 (4.7) | 192 (1.5) |
| Hospitalizations, mean (SD) | 1.7 (2.2) | 1.8 (2.3) |
| Urgent care or emergency room visits, mean (SD) | 2.1 (3.2) | 2.8 (3.9) |
| Long-term VA care days, mean (SD) | 3.3 (19.8) | 4.5 (25.1) |
| Facility characteristics | ||
| Geographical regione | ||
| Northeast | 617 (19.1) | 2454 (19.0) |
| Midwest | 549 (17.0) | 3038 (23.5) |
| South | 1260 (39.1) | 4638 (35.8) |
| West | 797 (24.7) | 2812 (21.7) |
| Facility complexity ratingf | ||
| 1a | 1566 (48.2) | 5317 (40.9) |
| 1b | 650 (20.0) | 2635 (20.3) |
| 1c | 455 (14.0) | 1961 (15.1) |
| 2 | 302 (9.3) | 1691 (13.0) |
| 3 | 253 (7.8) | 1386 (10.7) |
| Rurality | ||
| Urban | 3048 (93.8) | 11,842 (91.2) |
| Rural | 175 (5.4) | 1148 (8.8) |
VA: Veterans Health Administration; SD: standard deviation
Note: Data presented as N (%) unless otherwise noted.
aWithin 90 days prior to index date.
bIncluding myocardial infarction, coronary revascularization, and cardiac arrest.
cIncluding chronic obstructive lung disease, asthma, bronchiectasis, and pneumoconiosis.
dIn the year prior to index date.
en = 3249 for exposed, 12,942 for unexposed for geography variable.
f1a–1c: high complexity of patient volume and risk, high volume of teaching and research; 2: moderate complexity of patient volume and risk; some teaching and research; 3: low complexity of patient volume and risk, little or no teaching or research.
Four unexposed patients were matched to each exposed patient sequentially. To select unexposed comparators for each exposed patient, we first identified a pool of eligible unexposed (i.e. no prior vasodilator treatment) patients who met the following criteria on the exposed patient’s index date: alive, within the same five-year age group as the exposed patient, Medicare-eligible, active pharmacy user, diagnosed with Group 2/3 PH in the same calendar year as the exposed patient (based on date of first PH diagnosis code or first pulmonary vasodilator treatment, whichever was earlier), and not previously selected for another exposed patient. We then matched four unexposed patients randomly selected from the pool of eligible comparators and assigned the unexposed patients the index date of their exposed counterpart.
Primary and secondary outcomes
We selected outcomes based on harms of vasodilators observed in clinical trials19,21,30–32 and adverse event data collected by the Food and Drug Administration. Our primary outcome was a composite of time to death by any cause or an acute care presentation (hospitalization or emergency department visit) for the primary diagnosis of acute right-sided heart failure, respiratory failure, or renal failure, defined using validated algorithms when available33 (e-Table 2 in the Supplemental material). Our three secondary outcomes evaluated time to each acute organ failure or death individually: right-sided heart failure or death, respiratory failure or death, and renal failure or death. In order to avoid immortal time bias, we chose follow-up to start at the index date.34
Table 2.
Cohort study results.
| Outcome | No. (%) experiencing outcome |
Time to outcome, yrs, mean (SD) |
Adjusteda HR (95% CI) | ||
|---|---|---|---|---|---|
| Exposed | Unexposed | Exposed | Unexposed | ||
| Any organ failure or death | 2701 (83.1) | 9923 (76.4) | 2.5 (2.7) | 2.7 (2.5) | 1.31 (1.25–1.37) |
| Right heart failure or death | 2348 (72.3) | 8352 (64.3) | 3.3 (3.0) | 3.5 (2.6) | 1.39 (1.32–1.46) |
| Respiratory failure or death | 2533 (78.0) | 9308 (71.6) | 2.9 (2.8) | 3.0 (2.6) | 1.30 (1.24–1.36) |
| Renal failure or death | 2570 (79.1) | 9293 (71.5) | 2.8 (2.8) | 3.0 (2.5) | 1.36 (1.29–1.42) |
CI: confidence interval; HR: hazard ratio; SD: standard deviation.
aAdjusted for patient- and facility-level covariates.
Covariates
We identified patient- and facility-level variables we hypothesized would be associated with our outcomes based on clinical experience and prior literature on PH prognosis,35 as listed in Table 1. All variables were derived from both VA and Medicare data and were measured at the index date. Patients were assigned to VA facilities based on the location of the most recent PH-associated visit on or before the index date. We captured patient demographics (age, gender, race and ethnicity, marital status), VA enrollment priority status (a proxy for level of disability and VA benefits36), indicators of general health (body mass index, Elixhauser comorbidity index,37 specific comorbid conditions), and markers of healthcare utilization. Facility-level characteristics included geographic factors and facility complexity rating, which is comprised of patient volume and risk, level of teaching and research, number of specialists, and presence of intensive care units.38 Missing data for body mass index and marital status were imputed using a regression imputation algorithm.39 E-Table 2 in the Supplemental material shows full definitions of variables.
Statistical analyses
For our primary outcome, we assessed organ failure-free survival distributions of exposed and unexposed patients through the comparison of Kaplan–Meier curves and made formal comparisons using a log-rank test. We analyzed adjusted risks of our primary and secondary outcomes using Cox proportional hazards models with normally-distributed facility-specific random effects. Patients were assumed to be censored non-informatively at the end of the study period (31 December 2017). Additionally, unexposed patients who initiated therapy over the course of follow-up were censored from the unexposed group and considered exposed beginning at the date of treatment. To assess the validity of our findings, we performed three sensitivity analyses. First, to increase our confidence that PDE5i prescriptions were intended for PH and not erectile dysfunction, we excluded Veterans with an International Classification of Diseases code for sexual impotence and repeated our Cox proportional hazards models for each of our outcomes. Second, to assess whether our findings were driven by patients with more severe disease, we repeated the analyses for each of our outcomes after excluding those who had had an episode of organ failure within 90 days prior to the index date. Finally, to evaluate outcomes of vasodilator use independently in Group 2 vs Group 3 PH, we repeated our analysis limiting our study sample to Veterans with only Group 2 PH (excluding those with underlying conditions associated with Group 3 PH) and Veterans with only Group 3 PH (excluding those with underlying conditions associated with Group 2 PH). We graphically verified that the proportional hazards assumption was met through log–log survival plots. We assessed the fit of our models using generalized R2 measure based on Schoenfeld residuals.40 All analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc, Cary, NC). All p values were two-sided with a significance level of 0.05.
Nested case–control study methods
Study population
We identified a cohort of all Veterans diagnosed with incident Groups 2/3 PH between 1 January 2006 and 31 December 2017, as described above. The derivation of our study sample is shown in e-Figure 2 in the Supplemental material.
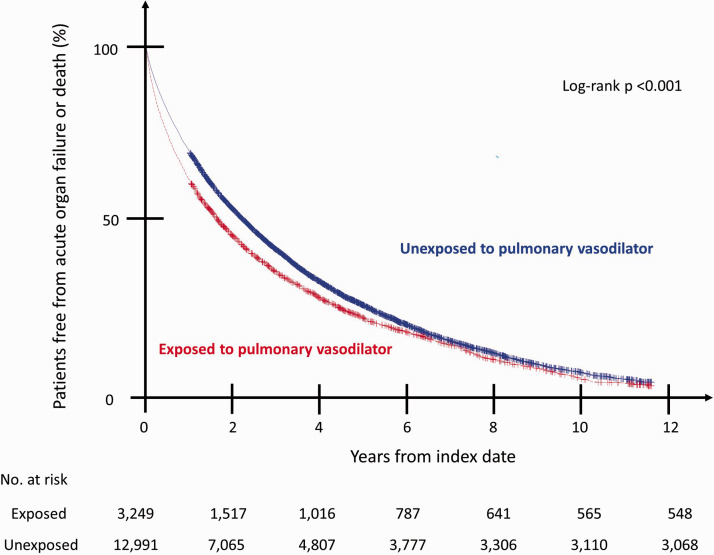
Fig. 2.
Survival curves for primary outcome. The R2 measure from our Cox proportional hazards model was 0.24, indicating a moderate fit. The proportional hazard assumption was verified by examining a log–log plot.
Selection of cases and controls
From our baseline study population, we selected cases who experienced death by any cause or acute right-sided heart failure, respiratory failure, or renal failure, as described above (primary outcome for cohort study). Using risk-set sampling without replacement,41 we matched each case to one control who was alive and previously diagnosed with Groups 2/3 PH. Patients sampled early in the study period as controls could later develop the outcome and become cases. Thus, patients could be represented more than once in the sample as cases or controls. We repeated the selection of cases and controls for our three secondary outcomes. This control sampling method has been shown to produce an odds ratio that approximates the incidence rate ratio from the underlying cohort population without the need for any rare disease assumption.42
Exposures
Among cases and controls, we evaluated for prior exposure to pulmonary vasodilators (e-Table 1). To understand the effects of varying levels of exposure to vasodilators on our outcomes, we assessed three different exposure definitions: any vasodilator use prior to the outcome, recent use within six months prior to the outcome, and cumulative duration of vasodilator exposure, defined as total years dispensed prior to the outcome (total days dispensed divided by 365).
Statistical analyses
We used mixed-effects logistic regression models to estimate the effect of any past vasodilator exposure, recent vasodilator exposure, and cumulative vasodilator exposure on our primary outcome and each of our three secondary outcomes. We controlled for patient- and facility-level variables as described above, measured at the time of PH diagnosis. We included a normally distributed random effect in our models to account for facility-level variation. To account for patients who served as both cases and controls, we included variance components to the responses conditional on the facility random effects. All p values were two-sided with a significance level of 0.05.
Results
Cohort study results
We identified 132,552 Medicare-eligible Veterans diagnosed with incident Groups 2/3 PH between 2006 and 2016. Of these, 3249 (2.5%) were exposed to pulmonary vasodilators and were matched 1:4 to 12,991 unexposed patients to create a study sample of 16,240 (e-Figure 1). Among exposed patients, the most common class of pulmonary vasodilator used was PDE5i (n = 2732), followed by endothelin receptor antagonists (n = 134), prostacyclin analogs (n = 91), and soluble guanylate cyclase stimulators (n = 4). Combination therapy was used in 288 patients.
Table 1 shows the baseline characteristics of exposed and unexposed patients. Overall, the mean age was 73.6 years; 97.7% were male and 83.4% were non-Hispanic white; 22.0% had underlying conditions associated with only Group 2 PH, 8.5% with only Group 3 PH, and 69.5% with both Groups 2 and 3 PH. Exposed patients carried a lower burden of comorbid disease than unexposed patients, with a lower mean Elixhauser comorbidity index (8.4 vs 9.6) and lower prevalence of many comorbidities including congestive heart failure (63.5% vs 68.3%), arrhythmias (55.9% vs 64.2%), stroke (10.1% vs 16.4%), and chronic kidney disease (39.3% vs 42.5%). Exposed patients utilized general healthcare services less frequently than unexposed patients (mean outpatient visits 38.5 vs 41.7; mean urgent care or emergency room visits 2.1 vs 2.8). Only 1066 (32.8%) of exposed patients underwent a right heart catheterization in the year prior to initiation of treatment.
During a mean follow-up of 2.5 years, 2701 (83.1%) exposed patients and 9923 (76.4%) unexposed patients experienced the primary outcome of any organ failure or death. The median organ failure-free survival was 2.0 years among the unexposed and 1.6 years among the exposed (Fig. 2). Exposure to vasodilators was associated with increased risk of our primary outcome (adjusted hazard ratio (HR): 1.31, 95% confidence interval (CI): 1.25–1.37) and all three of our secondary outcomes: right heart failure or death (HR: 1.39, 95% CI: 1.32–1.46), respiratory failure or death (HR: 1.30, 95% CI: 1.24–1.36), and renal failure or death (HR: 1.36, 95% CI: 1.29–1.42) (Table 2). Findings were similar in our sensitivity analysis excluding Veterans potentially treated with PDE5i for erectile dysfunction (HR: 1.39, 95% CI: 1.32–1.47), and in our analysis excluding those with a recent organ failure (HR: 1.31, 95% CI: 1.24–1.39). In disaggregated analyses evaluating Groups 2 and 3 PH independently, our primary outcome was seen among both Veterans with only Group 3 PH (HR: 1.58, 95% CI: 1.37–1.82) and Veterans with only Group 2 PH (HR: 1.26, 95% CI: 1.12–1.41).
Case–control study results
From our base population of 131,765 Medicare-eligible Veterans diagnosed with incident Groups 2/3 PH between 2006 and 2017, we identified 108,629 cases who experienced our primary outcome and matched those to 108,629 controls to create a study sample of 216,538 (e-Figure 2).
Table 3 details the baseline characteristics of cases and their matched controls identified for our primary outcome. Age, sex, and race/ethnicity were similar between cases and controls. Cases had higher prevalence of most comorbid conditions and higher rates of healthcare utilization compared to controls.
Table 3.
Baseline characteristics and exposure status at PH diagnosis date of cases and their matched controls for primary outcome.
| Cases (n = 108,629) | Controls (n = 108,629) | |
|---|---|---|
| Exposed to pulmonary vasodilatora | ||
| Phosphodiesterase-5-inhibitor | 2036 | 1995 |
| Endothelin receptor antagonist | 111 | 98 |
| Prostacyclin analog | 8 | 8 |
| Soluble guanylate cyclase stimulator | 1 | 0 |
| Combination therapy | 135 | 105 |
| Patient characteristics | ||
| Age, yr, mean (SD) | 77.3 (9.6) | 76.8 (9.5) |
| Female sex | 2563 (2.4) | 2879 (2.7) |
| Race/ethnicity | ||
| White | 93,976 (86.5) | 94,362 (86.9) |
| Black | 10,327 (9.5) | 9913 (9.1) |
| Hispanic | 2026 (1.9) | 2027 (1.9) |
| Other | 2300 (2.1) | 2327 (2.1) |
| Married | 68,123 (62.7) | 70,249 (64.7) |
| VA benefits (priority status) | ||
| Highly disabled | 19,824 (18.2) | 19,695 (18.1) |
| Low/moderately disabled | 25,764 (23.7) | 26,785 (24.7) |
| Limited with copayments | 29,859 (27.5) | 30,793 (28.3) |
| Poverty/no copayments | 33,182 (30.5) | 31,356 (28.9) |
| Body mass index | ||
| Underweight | 1657 (1.5) | 1415 (1.3) |
| Normal | 21,638 (19.9) | 20,491 (18.9) |
| Overweight | 38,106 (35.1) | 38,700 (35.6) |
| Class I obesity | 26,173 (24.1) | 27,258 (25.1) |
| Class II or III obesity | 21,055 (19.4) | 20,765 (19.1) |
| Elixhauser comorbidity index, mean (SD) | 9.4 (3.2) | 8.9 (3.2) |
| Comorbidities | ||
| Major adverse cardiac eventb | 38,257 (35.2) | 35,859 (33.0) |
| Congestive heart failure | 75,863 (69.8) | 67,762 (62.4) |
| Arrhythmia | 70,558 (65.0) | 68,163 (62.7) |
| Coronary atherosclerosis | 75,144 (69.2) | 73,506 (67.7) |
| Stroke | 20,424 (18.8) | 18,657 (17.2) |
| Transient ischemic attack | 8266 (7.6) | 8135 (7.5) |
| Chronic lung diseasec | 69,093 (63.6) | 64,682 (59.5) |
| Interstitial lung disease | 26,500 (24.4) | 24,691 (22.7) |
| Obstructive sleep apnea | 20,639 (19.0) | 20,024 (18.4) |
| Pneumonia | 38,383 (35.3) | 31,771 (29.2) |
| Hypertension | 101,416 (93.4) | 100,713 (92.7) |
| Hyperlipidemia | 88,482 (81.5) | 88,997 (81.9) |
| Peripheral vascular disease | 38,549 (35.5) | 35,719 (32.9) |
| Venous thromboembolism | 5224 (4.8) | 4469 (4.1) |
| Diabetes | 55,547 (51.1) | 52,659 (48.5) |
| Chronic kidney disease | 47,522 (43.7) | 39,277 (36.2) |
| Hepatitis C | 10,299 (9.5) | 8859 (8.2) |
| Cirrhosis | 6222 (5.7) | 5846 (5.4) |
| Malignancy | 27,126 (25.0) | 26,553 (24.4) |
| Anemia | 55,826 (51.4) | 50,411 (46.4) |
| Thyroid disease | 23,958 (22.1) | 23,353 (21.5) |
| Arthritis | 27,790 (25.6) | 26,971 (24.8) |
| Dementia | 12,255 (11.3) | 10,977 (10.1) |
| Neurodegenerative disease | 3116 (2.9) | 2946 (2.7) |
| Depression | 27,551 (25.4) | 26,395 (24.3) |
| Post-traumatic stress disorder | 6837 (6.3) | 7142 (6.6) |
| Schizophrenia | 1686 (1.6) | 1524 (1.4) |
| Other psychiatric condition | 9231 (8.5) | 7886 (7.3) |
| Alcohol/substance use disorder | 14,970 (13.8) | 13,828 (12.7) |
| Tobacco use disorder | 46,767 (43.1) | 43,029 (39.6) |
| Healthcare utilizationd | ||
| Outpatient visits, mean (SD) | 35.2 (26.8) | 34.7 (25.9) |
| Outpatient visits in VA only | 20,390 (18.8) | 19,847 (18.3) |
| Outpatient visits in Medicare only | 16,558 (15.2) | 17,078 (15.7) |
| Outpatients visits in both VA and Medicare | 68,617 (63.2) | 69,501 (64.0) |
| No outpatient visits | 3064 (2.8) | 2203 (2.0) |
| Hospitalizations, mean (SD) | 1.8 (1.9) | 1.5 (1.8) |
| Urgent care or emergency room visits, mean (SD) | 2.5 (3.4) | 2.2 (3.3) |
| Long-term VA care days, mean (SD) | 3.6 (22.9) | 3.0 (20.6) |
| Facility characteristics | ||
| Geographical Region | ||
| Northeast | 21,615 (19.9) | 22,053 (20.3) |
| Midwest | 25,368 (23.4) | 24,962 (23.0) |
| South | 37,991 (35.0) | 37,726 (34.7) |
| West | 23,655 (21.8) | 23,888 (22.0) |
| Facility complexity ratinge | ||
| 1a | 44,545 (41.0) | 44,290 (40.8) |
| 1b | 22,421 (20.6) | 22,253 (20.5) |
| 1c | 15,704 (14.5) | 15,776 (14.5) |
| 2 | 14,484 (13.3) | 14,625 (13.5) |
| 3 | 11,468 (10.6) | 11,677 (10.7) |
| Rurality | ||
| Urban | 99,120 (91.2) | 98,966 (91.1) |
| Rural | 9502 (8.7) | 9655 (8.9) |
Note: Data presented as N (%) unless otherwise indicated.
PH: pulmonary hypertension; SD: standard deviation; VA: Veterans Health Administration.
aAny exposure during the study period.
bIncluding myocardial infarction, coronary revascularization, and cardiac arrest.
cIncludes chronic obstructive lung disease, asthma, bronchiectasis, and pneumoconiosis.
dIn the year prior to index date.
e1a–1c: high complexity of patient volume and risk, high volume of teaching, and research; 2: moderate complexity of patient volume and risk; some teaching and research; 3: low complexity of patient volume and risk, little or no teaching or research.
Among cases who experienced the primary outcome, 2291 (2.1%) were exposed to a pulmonary vasodilator at any prior time point, and 414 (0.4%) were exposed in the six months prior to the outcome. Exposure to any pulmonary vasodilator in the past was associated with increased odds of acute organ failure or death (adjusted odds ratio [OR]: 1.10; 95% CI: 1.04–1.17) (Table 4). The odds of organ failure or death increased by 11% per year of vasodilator exposure (OR: 1.11; 95% CI: 1.07–1.16). Any past exposure, recent exposure, and cumulative exposure were all associated with increased odds of all three of our secondary outcomes.
Table 4.
Case–control study results.
| Multivariable-adjusted OR (95%
CI) a |
||||
|---|---|---|---|---|
| Exposure level | Any organ failure or death (n = 108,629)b | Right heart failure or death (n = 96,973)b | Respiratory failure or death (n = 106,397)b | Renal failure or death (n = 110,329)b |
| Any vasodilator use | 1.10 (1.04–1.17) | 1.31 (1.24–1.39) | 1.17 (1.10–1.23) | 1.16 (1.10–1.23) |
| Recent vasodilator usec | 1.04 (0.91–1.19) | 1.31 (1.15–1.50) | 1.15 (1.01–1.31) | 1.15 (1.01–1.31) |
| Cumulative dosed | 1.11 (1.07–1.16) | 1.20 (1.16–1.24) | 1.14 (1.10–1.19) | 1.14 (1.10–1.18) |
OR: odds ratio; CI: confidence interval.
aIncluding patient- and facility-level covariates.
bSample size for cases and controls, each.
cUse in the six months prior to outcome.
dOR signifies effect of one year of therapy.
Discussion
In this national study of patients in usual practice, we found increased risk of harm associated with using pulmonary vasodilators to treat Groups 2 and 3 PH. In our primary time-to-event approach, use of vasodilators was associated with 31% increased risk of any acute organ failure or death, with similar results seen in our secondary outcomes evaluating each organ failure independently. These findings were confirmed in our nested case–control study, in which any past exposure to vasodilators was associated with increased odds of developing our primary outcome and all three of our secondary outcomes, with odds of harm increasing with longer duration of therapy. Notably, fewer than a third of patients treated with pulmonary vasodilators underwent a right heart catheterization in the year prior to initiation of therapy, a concerning pattern discordant with clinical guidelines.43 This pattern of incomplete diagnostic evaluations of PH patients has previously been seen in both VA44 and non-VA settings.45
Our results are consistent with both the direction and magnitude of outcomes observed in pulmonary vasodilator trials in Group 2 PH, with no multicenter trial showing benefit.25 In a trial of the endothelin receptor antagonist macitentan in Group 2 PH patients, treatment was associated with a 10% increased risk of significant fluid retention.20 Likewise, in a trial of the PDE5i sildenafil in patients with PH secondary to repaired valvular heart disease, patients randomized to receive sildenafil had 61% increased odds of experiencing the composite outcome (i.e. major clinical event (any-cause mortality or heart failure admission requiring diuretics), worsened functional class, or worsened global self-assessment).21
Clinical trial evidence of pulmonary vasodilators in Group 3 PH is more limited. Few multi-center trials have been conducted, and these have produced mixed results. While some Group 3 PH trials have shown improvements in hemodynamics or exercise tolerance with use of pulmonary vasodilators,46,47 others have shown either no benefit16,17 or a signal of harm.22,23 A trial of the soluble guanylate cyclase stimulator riociguat in PH associated with idiopathic interstitial pneumonia showed increased rates of mortality and serious adverse events (worsening underlying lung disease, pneumonia) in the riociguat group, leading to early termination of the study.22 While data on the role of inhaled formulations appears promising,48 our results showing increased risk of harm associated with pulmonary vasodilator use in Group 3 PH should caution against indiscriminate use outside of clinical trials.
We found harm associated with prolonged exposure to pulmonary vasodilators for each of our outcomes, with more than 10% increased odds of developing organ failure or death per year of vasodilator exposure. These results extend those of clinical trials, as most trials of vasodilators in Groups 2/3 PH have evaluated shorter durations of therapy, ranging from 12 to 16 weeks.14,16–18,20 Notably, two clinical trials attempting to evaluate longer durations of therapy in Group 3 PH were stopped early due to increased harm in the treated group.22,49 Several clinical trials evaluating longer duration of therapy in Group 2 PH are currently underway.25
Our findings broaden the results from clinical trials to a non-trial setting and provide important evidence for clinicians facing the decision of whether or not to offer treatment with pulmonary vasodilators to their patients with Groups 2/3 PH. Despite clear guideline recommendations against use in Groups 2/3 PH,24,25 vasodilator use continues to rise in this population.26,27 The decision to prescribe these medications is often complex, influenced by many factors at the patient, provider, and system level.50 In prior qualitative work exploring drivers of guideline-discordant prescribing of vasodilators in Groups 2/3 PH, we found that clinicians often interpret the evidence and the guidelines differently, with high prescribers citing a belief that their patients may not be reflected in the aggregate data from clinical trials.51 These beliefs are not unfounded. Indeed, we have shown that Groups 2/3 PH patients in usual clinical practice differ from clinical trial participants, with the former being older with more comorbid conditions.29 We now show that Group 2/3 PH patients in non-trial settings may experience significant harm associated with use of vasodilators, lending further support to the call from clinical guidelines to limit their use outside of clinical trials.
Our study has limitations. First, while we controlled for both patient- and facility-level confounding variables likely to be associated with our outcome, our retrospective analyses using enriched administrative data may not have captured all possible confounders. For example, we lacked clinically relevant data such as hemodynamics from right heart catheterizations, echocardiography or pulmonary function testing results, or World Health Organization functional class. While exposed patients were generally healthier with a lower comorbidity index than unexposed patients, we cannot exclude the possibility that exposed patients had more severe PH at time of treatment compared to unexposed patients. Additionally, as fewer than a third of treated patients had received a right heart catheterization in the year prior to initiation of therapy, we cannot exclude the possibility that the poorer outcomes seen in exposed patients were partly due to lower quality of care in general. However, the consistent findings from our two distinct approaches, which are in line with clinical trials results, increase our confidence in the results. Additionally, as cumulative exposure further increased odds of harm, our findings are unlikely due to unmeasured confounding alone. Our data source also did not allow us to determine the specialty of the provider prescribing the pulmonary vasodilator and we therefore could not account for differences in outcomes among specialties. Second, while our validated algorithm26 was conservatively designed to increase specificity for selecting Groups 2/3 PH over other groups, the possibility that Groups 1 or 4 PH patients are included in our cohort remains. As Groups 1 and 4 PH patients have improved outcomes when treated with pulmonary vasodilators,52 their presence in our sample would bias our findings toward the null. In contrast, while we found the overall rate of pulmonary vasodilator prescribing to be low, our conservative algorithm likely resulted in an underestimation of the true rate of prescribing. Finally, our analysis of Medicare-eligible Veterans may not be generalizable to all usual clinical practice settings.
Consistent with clinical trial results, use of pulmonary vasodilators to treat Groups 2 and 3 PH in a usual clinical practice setting is associated with increased risk of acute organ failure and death. Given the rising use of these therapies, efforts are needed to develop and implement targeted strategies to ensure appropriate patient selection for pulmonary vasodilator therapy in PH.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_20458940211001714 for Outcomes of pulmonary vasodilator use in Veterans with pulmonary hypertension associated with left heart disease and lung disease by Kari R. Gillmeyer, Donald R. Miller, Mark E. Glickman, Shirley X. Qian, Elizabeth S. Klings, Bradley A. Maron, Joseph T. Hanlon, Seppo T. Rinne and Renda S. Wiener in Pulmonary Circulation
Acknowledgements
The views expressed in this article do not necessarily represent the views of the Department of Veterans Affairs or the United States Government. The sponsors had no role in the design of the study, the acquisition and analysis of the data, or the drafting of the manuscript.
Contributorship: Study concept and design: K.R.G., D.R.M., M.E.G., E.S.K., B.A.M., J.T.H., S.T.R., and R.S.W.; acquisition of data: D.R.M. and S.X.Q.; analysis and interpretation of data: all authors; drafting of the manuscript: K.R.G. and R.S.W.; and critical revision of the manuscript for important intellectual content: all authors.
Conflict of interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Elizabeth S. Klings received research support from Actelion Pharmaceuticals, Pfizer, Bayer, Reata, Micelle, Novartis and Arena. The other authors have no conflicts of interest to report.
Ethical approval: The Edith Nourse Rogers Memorial VA Hospital Institutional Review Board approved this study.
Guarantor: K.R.G. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by VA HSR&D IIR 15-115 and by resources from the Edith Nourse Rogers Memorial Veterans Hospital and VA Boston Healthcare System. In addition, Dr. Gillmeyer receives support through an NIH NRSA Grant, 1F32HL149236-01; Dr Rinne is funded by a VA HSR&D Career Development Award. Support for VA/CMS data provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). Dr Elizabeth S. Klings received support from 1U01HL128566-01. Dr Hanlon also received support from VA HSR&D IIR 18-228.
ORCID iD: Kari R. Gillmeyer https://orcid.org/0000-0002-5333-259X
Supplemental material: Supplemental material for this article is available online.
References
- 1.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 2005; 127: 1531–1536. [DOI] [PubMed] [Google Scholar]
- 2.Thenappan T, Shah SJ, Gomberg-Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2011; 4: 257–265. [DOI] [PubMed] [Google Scholar]
- 3.Caminati A, Cassandro R, Harari S. Pulmonary hypertension in chronic interstitial lung diseases. Eur Respir Rev 2013; 22: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weitzenblum E, Chaouat A, Canuet M, et al. Pulmonary hypertension in chronic obstructive pulmonary disease and interstitial lung diseases. Semin Respir Crit Care Med 2009; 30: 458–470. [DOI] [PubMed] [Google Scholar]
- 5.Burgess MI, Mogulkoc N, Bright-Thomas RJ, et al. Comparison of echocardiographic markers of right ventricular function in determining prognosis in chronic pulmonary disease. J Am Soc Echocardiogr 2002; 15: 633–639. [DOI] [PubMed] [Google Scholar]
- 6.Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest 1995; 107: 1193–1198. [DOI] [PubMed] [Google Scholar]
- 7.Melenovsky V, Hwang SJ, Lin G, et al. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014; 35: 3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 2001; 37: 183–188. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 10.Elinoff JM, Agarwal R, Barnett CF, et al. Challenges in pulmonary hypertension: controversies in treating the tip of the iceberg. A Joint National Institutes of Health Clinical Center and Pulmonary Hypertension Association symposium report. Am J Respir Crit Care Med 2018; 198: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu HL, Chen XY, Li JR, et al. Efficacy and safety of pulmonary arterial hypertension-specific therapy in pulmonary arterial hypertension: a meta-analysis of randomized controlled trials. Chest 2016; 150: 353–366. [DOI] [PubMed] [Google Scholar]
- 12.Shlobin OA, Brown AW, Nathan SD. Pulmonary hypertension in diffuse parenchymal lung diseases. Chest 2017; 151: 204–214. [DOI] [PubMed] [Google Scholar]
- 13.Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: the role of the endothelium in pathophysiology and management. Circulation 2000; 102: 1718–1723. [DOI] [PubMed] [Google Scholar]
- 14.Hoendermis ES, Liu LC, Hummel YM, et al. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J 2015; 36: 2565–2573. [DOI] [PubMed] [Google Scholar]
- 15.Corte TJ, Keir GJ, Dimopoulos K, et al. Bosentan in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2014; 190: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco I, Santos S, Gea J, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J 2013; 42: 982–992. [DOI] [PubMed] [Google Scholar]
- 17.Goudie AR, Lipworth BJ, Hopkinson PJ, et al. Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med 2014; 2: 293–300. [DOI] [PubMed] [Google Scholar]
- 18.Bonderman D, Ghio S, Felix SB, et al. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation 2013; 128: 502–511. [DOI] [PubMed] [Google Scholar]
- 19.Kaluski E, Cotter G, Leitman M, et al. Clinical and hemodynamic effects of Bosentan dose optimization in symptomatic heart failure patients with severe systolic dysfunction, associated with secondary pulmonary hypertension – a multi-center randomized study. Cardiology 2008; 109: 273–280. [DOI] [PubMed] [Google Scholar]
- 20.Vachiéry JL, Delcroix M, Al-Hiti H, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J 2018; 51: 1701886. [DOI] [PubMed] [Google Scholar]
- 21.Bermejo J, Yotti R, García-Orta R, et al. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur Heart J 2018; 39: 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan SD, Behr J, Collard HR, et al. Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP): a randomised, placebo-controlled phase 2b study. Lancet Respir Med 2019; 7: 780–790. [DOI] [PubMed] [Google Scholar]
- 23.Raghu G, Nathan SD, Behr J, et al. Pulmonary hypertension in idiopathic pulmonary fibrosis with mild-to-moderate restriction. Eur Respir J 2015; 46: 1370–1377. [DOI] [PubMed] [Google Scholar]
- 24.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. Epub ahead of print 24 January 2019. DOI: 10.1183/13993003.01914-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. Epub ahead of print 24 January 2019. DOI: 10.1183/13993003.01897-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim D, Lee KM, Freiman MR, et al. Phosphodiesterase-5 inhibitor therapy for pulmonary hypertension in the United States. Actual versus recommended use. Ann Am Thorac Soc 2018; 15: 693–701. [DOI] [PubMed] [Google Scholar]
- 27.Wijeratne DT, Lajkosz K, Brogly SB, et al. Increasing incidence and prevalence of World Health Organization Groups 1 to 4 pulmonary hypertension: a population-based cohort study in Ontario, Canada. Circ Cardiovas Qual Outcomes 2018; 11: 13. [DOI] [PMC free article] [PubMed]
- 28.Maron BA, Ryan JJ. A concerning trend for patients with pulmonary hypertension in the era of evidence-based medicine. Circulation 2019; 139: 1861–1864. [DOI] [PubMed] [Google Scholar]
- 29.Gillmeyer KR, Rinne ST, Walkey AJ, et al. How closely do clinical trial participants resemble “Real-World” Groups 2 and 3 pulmonary hypertension patients? A structured review. Ann Am Thorac Soc 2020; 17: 779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Califf RM, Adams KF, McKenna WJ, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the Flolan International Randomized Survival Trial (FIRST). Am Heart J 1997; 134: 44–54. [DOI] [PubMed] [Google Scholar]
- 31.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309: 1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lederer DJ, Bartels MN, Schluger NW, et al. Sildenafil for chronic obstructive pulmonary disease: a randomized crossover trial. COPD 2012; 9: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 34.Lévesque LE, Hanley JA, Kezouh A, et al. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010; 340: b5087. [DOI] [PubMed] [Google Scholar]
- 35.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 36.Veterans Health Administration. VHA enrollment determination. VHA handbook 1601A.01. Washington, DC: VHA, 2020.
- 37.Elixhauser A, Steiner C, Harris DR, et al . Comorbidity measures for use with administrative data. Med Care 1998; 36: 8–27. [DOI] [PubMed] [Google Scholar]
- 38.Office of Quality and Safety. 2010 VHA facility quality and safety report. Washington, DC: Department of Veterans Affairs, Veterans Health Administration, 2010.
- 39.Little R, Rubin D. Statistical analysis with missing data. 3rd edition. Hoboken, NJ: Wiley, 2019.
- 40.Gillespie B, Mccullough K. Use of generalized R‐squared in Cox regression. In: APHA scientific session and event listing, Boston, MA, November 4-8, 2006.
- 41.Vandenbroucke JP, Pearce N. Case-control studies: basic concepts. Int J Epidemiol 2012; 41: 1480–1489. [DOI] [PubMed] [Google Scholar]
- 42.Greenland S, Thomas DC. On the need for the rare disease assumption in case-control studies. Am J Epidemiol 1982; 116: 547–553. [DOI] [PubMed] [Google Scholar]
- 43.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 44.Maron BA, Choudhary G, Khan UA, et al. Clinical profile and underdiagnosis of pulmonary hypertension in US veteran patients. Circ Heart Fail 2013; 6: 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deano RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med 2013; 173: 887–893. [DOI] [PubMed] [Google Scholar]
- 46.Vitulo P, Stanziola A, Confalonieri M, et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Heart Lung Transplant 2017; 36: 166–174. [DOI] [PubMed] [Google Scholar]
- 47.Rao RS, Singh S, Sharma BB, et al. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci 2011; 53: 81–85. [PubMed] [Google Scholar]
- 48.King CS, Shlobin OA. The trouble with group 3 pulmonary hypertension in interstitial lung disease: dilemmas in diagnosis and the conundrum of treatment. Chest 2020; 158: 1651–1664. [DOI] [PubMed] [Google Scholar]
- 49.Raghu G, Behr J, Brown KK, et al. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med 2013; 158: 641–649. [DOI] [PubMed] [Google Scholar]
- 50.Gillmeyer KR, Rinne ST, Glickman ME, et al. Factors associated with potentially inappropriate phosphodiesterase-5 inhibitor use for pulmonary hypertension in the United States, 2006 to 2015. Circ Cardiovasc Qual Outcomes 2020; 13: e005993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCullough M, Bolton R, Solch A, et al. Understanding clinical decision-making to de-implement low value care for pulmonary hypertension. In: HSR&D/QUERI national conference, Washington, DC, www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4041 (2019, accessed 15 December 2020).
- 52.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. Epub ahead of print 24 January 2019. DOI: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_20458940211001714 for Outcomes of pulmonary vasodilator use in Veterans with pulmonary hypertension associated with left heart disease and lung disease by Kari R. Gillmeyer, Donald R. Miller, Mark E. Glickman, Shirley X. Qian, Elizabeth S. Klings, Bradley A. Maron, Joseph T. Hanlon, Seppo T. Rinne and Renda S. Wiener in Pulmonary Circulation