Abstract
Purpose:
The main objective is to investigate the protective effect of camel milk (CM) on radiation-induced intestinal injury.
Methods:
The C57BL/6 J mice in 2 experiments were assigned into control group (Con), irradiation group (IR), and CM+irradiation group (CM+IR). After receiving the CM via gavage for 14 days, the mice in the first experiment were exposed to 6 Gy X-ray whole body irradiation, and survival rate was compared among the groups. Mice in the second experiment were exposed to 4 Gy irradiation and sacrificed at day 7. The small intestines were collected to examine the histopathological changes and to determine the anti-oxidative index and HMGB1/TLR4 inflammatory pathway. Fasting blood was used to measure serum pro-inflammatory factors.
Results:
Compared with the IR group, the survival time was prolonged, and survival rate was increased in the CM+IR group. CM increased levels of SOD and GSH and decreased MDA in the jejunum. Furthermore, intestinal protein expression of HMGB1/TLR4 pathway (TLR4, NF-κB, and HMGB1) was up-regulated by CM intervention. CM decreased the serum levels of TNF-α and IL-1β and increased IL-10 level.
Conclusions:
CM extended the survival time and had a protective effect against radiation-induced jejunum injury by regulation of antioxidant capacity and HMGB1/TLR4/NF-κB/MyD88 inflammatory signaling pathway.
Keywords: camel milk, radiation, jejunum injury, inflammatory factors, antioxidant
Introduction
With the increasing incidence of cancer, radiotherapy has become one of the most important treatments. Although this technology has made great progress, radiation-related injury restricts its application in clinical practice.1 Small intestine is one of the organs that are most sensitive to radiation.2 The main symptoms of radiation-induced intestine injury include anorexia, vomiting, diarrhea, dehydration, systemic infections, and in extreme cases, septic shock, and death.3,4 These conditions seriously affect the prognosis of patients with abdominal or pelvic tumors and ultimately reduce their quality of life.5 Amifostine is the only radioprotective agent currently approved by the U.S. Food and Drug Administration (FDA) for the prevention of severe radiation-induced toxic reactions. However, it could cause hematotoxicity and gastrointestinal toxicity.6 Therefore, the development of novel radio-protective products with low toxicity from functional food is urgently needed.7
Camel milk (CM), which has a special healthy function, has received recent research attention.8 Compared with traditional cow milk, CM has lower fat, and is richer in protein and lactoferrin content and contains more polyunsaturated fatty acids and linoleic acid, which are necessary for human nutrition.9 In addition, CM has unique antioxidant and anti-inflammatory properties due to its biologically active protein and vitamin components.10,11 It can effectively alleviate the symptoms of rheumatoid arthritis,12 diabetes,13,14 and fatty liver,15 and even promote wound healing.16 Whether CM influences radiation-induced intestine injury is rarely reported. In this study, the protective effect of CM on radiation-induced intestinal injury model was determined. This work provided a novel adjuvant method for patients with abdominal tumors undergoing radiation therapy.
Materials and Methods
Reagents and Antibodies
CM was purchased from Xinjiang Nanshan Pasture (Urumqi, China). The milk was collected from Camelus bactrianus. After traditional pasteurization, it was transported to the laboratory via food cold chain logistics within 48 h. Cytokines TNF-α, IL-1β, IL-6, and IL-10 kits were obtained from Wuhan ColorfulGene Biological Technology Company. SOD, MDA, and GSH kits were acquired from Nanjing Jiancheng Bioengineering Institute. Antibodies for Western blot, namely, HMGB(166525-1-Ig) and TLR4 (19811-1-AP) were purchased from ProteinTech Group (Chicago, IL, USA). MyD88 (YT2928) was purchased from ImmunoWay Biotechnology Company (Plano, TX, USA). IKKβ (D30C6) was obtained from Cell Signaling Technology (Shanghai, China). NF-κB (ARG65677) was acquired from Bio-Platform Technology Company (Shanghai, China). β-actin (BM 0627) was bought from Boster Biological Technology (Wuhan, China). BeyoECL Plus (P0018) and BCA Protein Assay Kit were purchased from Beyotime Company (Shanghai, China).
Animals
Six-week-old C57BL/6 J mice (20 ± 2 g body weight) were obtained from Shanghai Shrek Company Chinese Academy of Sciences and housed under a 12 h/12 h light/dark cycle at a constant temperature of 22 ± 2°C and 60 ± 5% relative humidity. All experimental procedures were performed in accordance with the Guidelines in the Care and Use of Animals and with the approval of Soochow University Animal Welfare Committee.
Experiment to Assess Animal Survival
After 1 week of acclimatization, 18 animals were assigned randomly to the control group (Con), 6 Gy group (IR), and CM+6 Gy group (CM+IR). Mice were administered 0.2 ml of CM through gavage twice every day for 14 days before irradiation.8 The mice in the Con and IR groups were administered distilled water. At day 15, mice in the IR and CM+IR groups were exposed 6 Gy of whole-body X-ray irradiation by using a RS-2000 biological X-ray irradiator (Rad source technology, Suwanee, GA, USA) at a dose rate of 1.283 Gy/min.17,18 Animals were observed twice a day until all mice died. Death time and number of mice were recorded. The percentage of surviving animals was used for survival analysis. This experiment ended at 21 days after radiation.
Experiment to Observe Intestinal Injury
For the observation of intestinal injury, a total of 18 animals were divided randomly into the control group (Con), 4 Gy group (IR), and CM+4 Gy group (CM+IR). The same methods involving CM intervention and irradiation exposure (except irradiation dose) were used to assess animal survival. All the mice were observed for any signs of radiation sickness, and body weight was recorded daily.
Collection of Sample
The mice were sacrificed 7 days after 4 Gy irradiation. Portions of the jejunum were fixed immediately in 10% formalin for future histological observation, and the remaining portions were stored at −80°C until further use. The thymus and spleen were immediately dissected and weighed. Blood samples were collected from the retrobulbar vein. Serum was separated by centrifugation and stored at −80°C.
Pathological Observation of Intestinal Tissue
Paraffin-embedded sections of intestinal tissues were dewaxed with 5 µm sections, stained with hematoxylin-eosin (HE) (Sigma-Aldrich, USA), and examined under a microscope for assessment. The number of surviving crypts per circumference of the jejunum was assessed quantitatively by pathologists under blinded conditions. Ten circular transverse sections per mouse were stained with HE and blindly analyzed from the photographs to measure the length of small intestinal villi. The depth of small intestinal crypts were also measured. The ratio of villus length to crypt depth (V/C) was calculated as an index to determine the comprehensive function of the small intestine.18,19
Radiation Injury Score (RIS)
The overall severity of structural radiation injury was assessed using the RIS system.17 RIS is a composite histopathologic scoring system that provides a global measure of the severity of structural radiation injury. Seven histopathologic parameters of radiation injury, namely, mucosal ulceration, epithelial atypia, subserosal thickening, vascular sclerosis, intestinal wall fibrosis, ileitis cystica profunda, and lymph congestion, were assessed and graded from 0 to 3. RIS value is defined as the sum of individual alteration scores.
Determination of Organ Index
To give a quantitative assessment of the immune response, spleen and thymus indexes (Sx) were calculated as follows: Sx = [Weight of experiment organ (mg)]/[Weight of experiment animal (g)].20,21
ELISA Assay for Inflammatory Cytokines
The obtained tissue samples were homogenized with a tissue homogenizer at 4°C for 10 min and then centrifuged at 3000 rpm for 10 min. The supernatants were stored at −80°C. Inflammatory cytokines IL-6 and IL-10 and serum inflammatory factors TNF-α, IL-1β in the supernatants were tested according to kit instructions using quantitative sandwich assay to determine the concentrations. OD value was measured by multimode plate reader (Synergy NEO, BioTek, USA) at 450 nm, and the levels of each factor were calculated.
Measurement of SOD and GSH Activity and MDA Content
SOD activity in jejunum was examined using the xanthine oxidase method provided by the standard assay kit,22 in which the xanthine–xanthine oxidase system was employed to produce superoxide ions that will react with 2-(4-iodophenyl)-3-(4-nitrophenol-5-phenlyltetrazolium chloride) to form a red formazan dye. Absorbance at 550 nm was determined. Protein concentration was determined by a BCA protein assay kit, and the values were expressed as units per mg protein. One unit of SOD was defined as the amount of SOD inhibiting the rate of reaction by 50% at 25°C. GSH activity in jejunum was tested according to the manufacturer’s instructions and recorded using a microplate reader at 405 nm absorbance. Lipid peroxidation was evaluated by measuring the MDA concentrations using the thiobarbituric acid (TBA) method as commercially recommended. This technique was based on the spectrophotometric measurement of the color produced during the reaction of TBA and MDA. MDA content were calculated by the absorbance of TBA reactive substances at 532 nm.
Western Blot Analysis in Jejunum Tissues
Jejunum tissue samples were homogenized in ice-cold lysis buffer and centrifuged, and the supernatants were collected. Protein concentration was measured using the BCA protein assay kit. After denaturation, equal amounts of protein (50 μg) were separated by 10% SDS-PAGE and then transferred onto a PVDF membrane. The membranes were blocked with 5% skim milk in Tris-buffered saline Tween-20 solution for 1 h and then incubated overnight with the following primary antibodies at 4°C: HMGB1 (1:1000), IKKβ (1:1000), TLR4 (1:400), MyD88 (1:1000), NF-κB (1:500), and β-actin (1: 1000). After washing thrice in PBST and incubated with appropriate secondary antibodies, the membranes were developed by chemiluminescence using Superstar ECL Western blot substrate reagents. Band intensities were quantified using Image J software. All experiments were conducted in triplicate, and the average value was obtained.
Statistical Analysis
All data were expressed as the mean ± standard (SD). Differences between groups were tested by 1-way ANOVA (SPSS24), followed by the LSD post hoc test. Differences were considered statistically significant at P < 0.05.
Results
CM Extended the Survival Time of Irradiated Mice
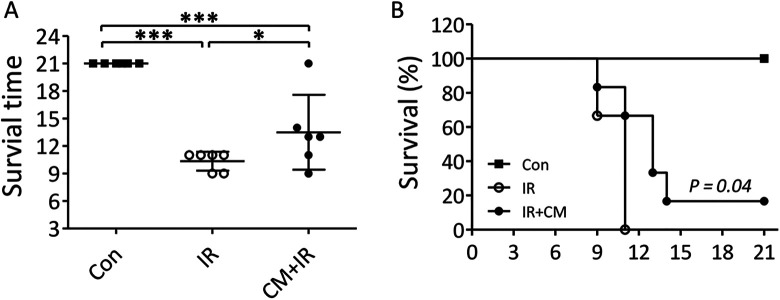
The mice in the IR group started to die at day 9 after irradiation and the last mouse died at day 11. In CM+IR group, the first death also occurred at day 9, and the last mouse died at day 21. The mean survival time was significantly longer in the CM+IR group than in the IR group (14.40 ± 4.09 vs. 10.60 ± 1.03 days, P < 0.05) (Figure 1A). Survival analysis showed that the survival rate was significantly higher in the CM+IR (P = 0.04) than in the IR group (Figure 1B).
Figure 1.
(A) The survival time of the 3 groups during the experiment. *P < 0.05, **P < 0.01, ***P < 0.005. (B) The survival curve of the 3 groups during the experiment. P = 0.04 compared with the IR group.
CM Ameliorated the General Status of Mice
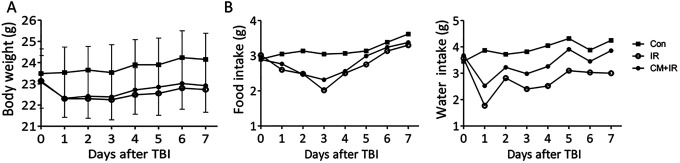
Radiation decreased the food and water intake and the body weight (Figure 2). Although food and water intake recovered within 2-3 days, the body weight did not increase. No significant difference in the body weight and food/water intake was found between the IR and CM+IR groups.
Figure 2.
Body weight (A) and daily food/water intake (B) of mice during the experiment.
CM Attenuated the Radiation-Induced Intestinal Injury in Mice
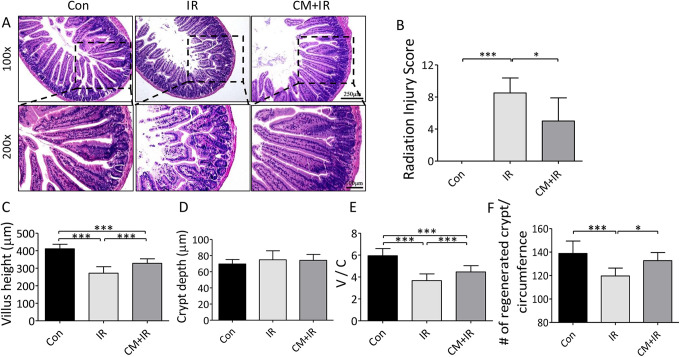
HE staining of the jejunum was performed to evaluate the protective effect of CM on mice at 7 days after radiation. Compared with the Con group, the intestinal villi in the IR group were shorter, thicker, and more irregularly arranged. In the CM+IR group, the villi statue was gradually repaired, and they became regularly organized (Figure 3A). CM had a remarkable improvement effect on radiation-induced intestinal injury. RIS was used to evaluate the extent of intestinal injury. Compared with the IR group, the CM group had significantly decreased RIS (Figure 3B).
Figure 3.
Effects of CM on radiation-induced intestinal injury in mice. (A) Representative images of the HE stained sections of the jejunum; (B) radiation injury score; (C) villus length of small intestine; (D) crypt depth of small intestine; (E) V/C ratio of small intestine; (F) regenerated crypt/circumference of small intestine. *P < 0.05, ***P < 0.005.
The intestinal villus length, crypt depth and number, and V/C ratio were measured (Figure 3C-F). The average villus height was shorter in the IR group than in the Con group (271.90 ± 37.09 µm vs. 411.14 ± 25.701 µm), and the V/C ratio was markedly reduced from 5.94 ± 0.65 in the Con group to 3.67 ± 0.61 in the IR group. All these data indicated that radiation seriously damaged the intestine. CM effectively improved the villus statue by increasing the length to 327.95 ± 26.08 µm and the V/C ratio to 4.66 ± 0.57 in the CM+IR group, indicating its protective effect on radiation-induced intestinal damage. Although no significant differences were found in crypt depth among the 3 groups, CM significantly increased the number of crypts.
CM Promoted Anti-Oxidative Activity in Jejunum Exposed to Radiation
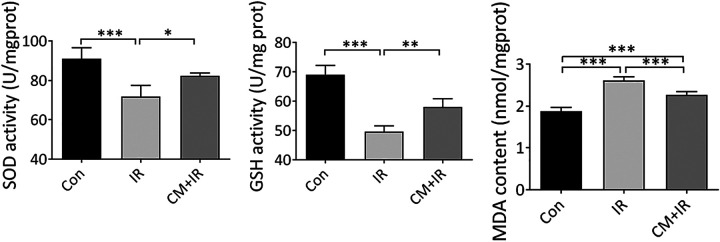
SOD, GSH, and MDA levels were detected to evaluate the oxidative stress level of jejunum tissues. Compared with the Con group, SOD and GSH levels significantly decreased, and the MDA level significantly increased in the IR group. This finding indicated that radiation induced oxidative damage to mice jejunum. Pre-treatment with CM before radiation effectively alleviated this phenomenon. Compared with the IR group, the mice in the CM+IR group showed an increase in SOD and GSH levels and a decrease in MDA level (Figure 4).
Figure 4.
The effect of CM on anti-oxidative activity in jejunum of mice. (A) SOD activity; (B) GSH activity; (C) MDA content. *P < 0.05, **P < 0.01, ***P < 0.005.
CM Inhibited the Expression of HMGB1/TLR4 Pathway Inflammation-Related Proteins in Jejunum Exposed to Radiation
The expressions of inflammation-related proteins were examined by using Western blot analysis to address the underlying mechanism of CM protective effect on radiation-induced intestinal injury. TLR4, IKKβ, NF-κB, MyD88, and HMGB1 levels increased significantly after irradiation but decreased after the pre-treatment with CM. These results showed that CM could mediate HMGB1/TLR4 pathway and inhibit radiation-induced inflammation in the jejunum (Figure 5).
Figure 5.
CM reduced inflammation response via HMGB1/TLR4 pathway. (A) Representative protein expression of Western blot. (B) Average band intensity of TLR4, IKKβ, NF-κB, MyD88, and HMGB1 in jejunum homogenate. The expression was normalized with respect to the Con group. *P < 0.05, **P < 0.01, ***P < 0.005.
CM Improved the Organ Index and Inhibited the Inflammatory Cytokine Levels of Irradiated Mice
Radiation significantly decreased the thymus and spleen indexes. Compared with the IR group, the thymus and spleen indexes significantly increased in the CM+IR group. CM protected the immune organs that were damaged by radiation (Figure 6A-C).
Figure 6.
Effect of CM on the organ indexes and inflammatory cytokine levels in mice. (A) Thymus index. (B) Spleen index. (C) Spleen anatomy. (D) Serum TNF-α level. (E) Serum IL-1β level. (F) Jejunum IL-6 level. (G) Jejunum IL-10 level. *P < 0.05, **P < 0.01, ***P < 0.005.
ELISA was used to detect the levels of pro-inflammatory factors TNF-α and IL-1β in the serum and the levels of inflammatory cytokines IL-6 and IL-10 in jejunum tissues (Figure 6D-G). The serum levels of TNF-α and IL-1β in the IR group were significantly higher than those in the Con group. After CM intervention, the levels of TNF-α and IL-1β were significantly restored (P < 0.005). The level of anti-inflammatory factor IL-10 significantly decreased in the IR group (P < 0.05) but significantly increased after CM intervention (P < 0.05).
Discussion
Radiation is commonly used in cancer therapy, especially in the treatment of gynecological and colorectal cancers. The high proliferative rate of intestinal epithelial cells and stem cells makes the bowel one of the organs that are most sensitive to radiation injury. Thus, methods to alleviate radiation-induced small intestine injury are needed to extend survival.19,23
In this study, CM was first examined to explore its protective effect on radiation-induced intestinal injury. CM intervention extended the survival time and improved the survival rate of irradiated mice. Survival extension was attributed to the alleviation of the injury of the small intestines, which are susceptible to radiation because they contain rapidly dividing transit cells.24 Crypt areas were visualized by HE staining, and the findings showed that CM histopathologically attenuated the intestinal injury and quantitatively increased the villus length and V/C ratio. The level of oxidative stress in jejunum was also tested to further understand the underlying mechanism. Radiation substantially reduced SOD and GSH activities in jejunum tissues and increased MDA level. These adverse reactions were effectively improved by CM intervention. Arab also revealed that CM can inhibit renal oxidative stress and enhance antioxidant capacity.25 The distinctive anti-oxidant properties of CM is likely due to its high content of vitamins C and E, zinc, and selenium.12
CM protected the immune organs destroyed by radiation, decreased inflammatory factors TNF-α and IL-1β in the serum, and increased anti-inflammatory factor IL-10 in the jejunum. Inflammatory factors play an important role in the occurrence and development of radiation-induced intestinal injury.26 CM has a potential anti-inflammatory effect that contributes to its immune-protective influence on radiation-induced intestinal injury. Zhu et al found that CM reduces the LPS-induced increase in neutrophil infiltration and TNF-α and IL-1β levels in rats.27 By constructing a rheumatoid arthritis mouse model, Salama et al found that CM lowers TNF-α and augments the anti-inflammatory IL-10 levels in the sera and exudates of arthritic rats.12 The antioxidant and immune regulation capacities of CM played important roles in its radio-protective effect.
Several studies have shown that CM has antioxidant capacity. Since HMGB1 plays pivotal roles in the regulation of the cellular response to stress. We further investigated whether the antioxidant effect of CM is mediated by HMGB1 and downstream signaling pathways, which included TLR4, IKKβ, NF-κB, and MyD88.28 HMGB1 is a late-stage inflammatory factor that can be passively released by necrotic and damaged cells or activated through immune cell activation. Once released in the extracellular space and upon its interaction with a large panel of cell surface receptors, HMGB1 exerts a plethora of cell regulatory functions from maturation, proliferation, and motility to inflammation, survival, and cell death.28-31 HMGB1 can also directly bind to TLR4 and induce the secretion of pro-inflammatory cytokines.32 CM significantly reduced the irradiation-induced upregulation of HMGB1/TLR4 protein in intestinal tissues.
CM contains many nutrients, and the protective effect on radiation-induced intestinal injury is due to the combined actions of various proteins, vitamins, and nutritional factors. Lactoferrin, which exhibits various beneficial properties,33 is used as an example. CM has higher lactoferrin content than milk from cows, goats, and buffaloes.34 A study revealed that lactoferrin could increase the survival rate of mice exposed to X-ray irradiation by decreasing the levels of serum IL-6 and TNF-α and reducing the expression of radiation-induced IKKα/β and NF-κB.19
In conclusion, our findings demonstrated that CM reduces the level of inflammatory cytokines by regulating the HMGB/TLR4/NF-κB/MyD88 pathway and the antioxidant capacity to decrease radiation-induced intestinal damage (Figure 7). To elucidate the exact and comprehensive mechanism in the future, it is necessary to conduct local irradiation that is limited to the gastrointestinal tract.
Figure 7.
Mechanism of CM protective role in radiation-induced intestinal injury.
Footnotes
Author Contributions: Yu-Zhong Chen and Chao Li contributed equally to the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the National Natural Science Foundation of China (No.81673101, 81703159, 81973024, 82073482), China Postdoctoral Science Foundation (2015M571889), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJA310006), the postdoctoral research funding plan in Jiangsu province (1601121C), and cultivation and scientific research project of Suzhou Kowloon Hospital (JL201808), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
ORCID iD: Yu-Zhong Chen  https://orcid.org/0000-0002-6920-9967
https://orcid.org/0000-0002-6920-9967
References
- 1. Pan J, Li D, Xu Y, et al. Inhibition of Bcl-2/xl With ABT-263 selectively kills senescent type II pneumocytes and reverses persistent pulmonary fibrosis induced by ionizing radiation in mice. Int J Radiat Oncol Biol Phys. 2017;99(2):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo J, Liu Z, Zhang D, et al. TLR4 agonist monophosphoryl lipid a alleviated radiation-induced intestinal injury. J Immunol Res. 2019;2019:2121095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng Y, Dong Y, Hou Q, et al. The protective effects of XH-105 against radiation-induced intestinal injury. J Cell Mol Med. 2019;23(3):2238–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu L, Jiang M, Zhu C, He J, Fan S. Amelioration of whole abdominal irradiation-induced intestinal injury in mice with 3,3’-Diindolylmethane (DIM). Free Radic Biol Med. 2019;130:244–255. [DOI] [PubMed] [Google Scholar]
- 5. Bhanja P, Norris A, Gupta-Saraf P, Hoover A, Saha S. BCN057 induces intestinal stem cell repair and mitigates radiation-induced intestinal injury. Stem Cell Res Ther. 2018;9(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li L, Zhang K, Zhang J, et al. Protective effect of polydatin on radiation-induced injury of intestinal epithelial and endothelial cells. Biosci Rep. 2018;38(6):BSR20180868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kontou N, Psaltopoulou T, Panagiotakos D, Dimopoulos MA, Linos A. The Mediterranean diet in cancer prevention: a review. J Med Food. 2011;14(10):1065–1078. [DOI] [PubMed] [Google Scholar]
- 8. Arab HH, Salama SA, Eid AH, Omar HA, Arafa ES, Maghrabi IA. Camel’s milk ameliorates TNBS-induced colitis in rats via downregulation of inflammatory cytokines and oxidative stress. Food Chem Toxicol. 2014;69:294–302. [DOI] [PubMed] [Google Scholar]
- 9. Salwa MQ, Lina AF. Antigenotoxic and anticytotoxic effect of camel milk in mice treated with cisplatin. Saudi J Biol Sci. 2010;17(2):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shori AB. Camel milk as a potential therapy for controlling diabetes and its complications: a review of in vivo studies. J Food Drug Anal. 2015;23(4):609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arab HH, Salama SA, Maghrabi IA. Camel milk ameliorates 5-fluorouracil-induced renal injury in rats: targeting MAPKs, NF-κB and PI3K/Akt/eNOS pathways. Cell Physiol Biochem. 2018;46(4):1628–1642. [DOI] [PubMed] [Google Scholar]
- 12. Arab HH, Salama SA, Abdelghany TM, et al. Camel milk attenuates rheumatoid arthritis via inhibition of mitogen activated protein kinase pathway. Cell Physiol Biochem. 2017;43(2):540–552. [DOI] [PubMed] [Google Scholar]
- 13. Agrawal RP, Jain S, Shah S, Chopra A, Agarwal V, Agarwal V. Effect of camel milk on glycemic control and insulin requirement in patients with type 1 diabetes: 2-years randomized controlled trial. Eur J Clin Nutr. 2011;65(9):1048–1052. [DOI] [PubMed] [Google Scholar]
- 14. Mansour AA, Nassan MA, Saleh OM, Soliman M. Protective effect of Camel milk as anti-diabetic supplement: biochemicalmolecular and immunohistochemical study. Afr J Tradit Complement Altern Med. 2017;14(4):108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Korish AA, Arafah MM. Camel milk ameliorates steatohepatitis, insulin resistance and lipid peroxidation in experimental non-alcoholic fatty liver disease. BMC Complement Altern Med. 2013;13:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ebaid H, Abdel-Salam B, Hassan I, Al-Tamimi J, Metwalli A, Alhazza I. Camel milk peptide improves wound healing in diabetic rats by orchestrating the redox status and immune response. Lipids Health Dis. 2015;14:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gu J, Chen YZ, Zhang ZX, et al. At What dose can total body and whole abdominal irradiation cause lethal intestinal injury among C57BL/6 J mice? Dose Response. 2020;18(3):1559325820956783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei YL, Xu JY, Zhang R, Zhang Z, Zhao L, Qin LQ. Effects of lactoferrin on X-ray-induced intestinal injury in Balb/C mice. Appl Radiat Isot. 2019;146:72–77. [DOI] [PubMed] [Google Scholar]
- 19. Zuo T, Li X, Chang Y, et al. Dietary fucoidan of Acaudina molpadioides and its enzymatically degraded fragments could prevent intestinal mucositis induced by chemotherapy in mice. Food Funct. 2015;6(2):415–422. [DOI] [PubMed] [Google Scholar]
- 20. Piao J, Meng F, Fang H, et al. Effect of taurine on thymus differentiation of Dex-induced immunosuppressive Mice. Adv Exp Med Biol. 2019;1155:381–390. [DOI] [PubMed] [Google Scholar]
- 21. Wu F, Ding XY, Li XH, Gong MJ, An JQ, Huang SL. Correlation between elevated inflammatory cytokines of spleen and spleen index in acute spinal cord injury. J Neuroimmunol. 2020;344:577264. [DOI] [PubMed] [Google Scholar]
- 22. Mao G-X, Zheng L-D, Cao Y-B, et al. Antiaging effect of pine pollen in human diploid fibroblasts and in a mouse model induced by D-galactose. Oxid Med Cell Longev. 2012;2012:750963–750963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumagai T, Rahman F, Smith AM. The microbiome and radiation induced-bowel injury: evidence for potential mechanistic role in disease pathogenesis. Nutrients. 2018;10(10):1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leibowitz BJ, Wei L, Zhang L, et al. Ionizing irradiation induces acute haematopoietic syndrome and gastrointestinal syndrome independently in mice. Nat Commun. 2014;5:3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arab HH, Salama SA, Maghrabi IA. Camel milk attenuates methotrexate-induced kidney injury via activation of PI3K/Akt/eNOS signaling and intervention with oxidative aberrations. Food Funct. 2018;9(5):2661–2672. [DOI] [PubMed] [Google Scholar]
- 26. Gecse KB, Vermeire S. Differential diagnosis of inflammatory bowel disease: imitations and complications. Lancet Gastroenterol Hepatol. 2018;3(9):644–653. [DOI] [PubMed] [Google Scholar]
- 27. Zhu WW, Kong GQ, Ma MM, et al. Short communication: camel milk ameliorates inflammatory responses and oxidative stress and downregulates mitogen-activated protein kinase signaling pathways in lipopolysaccharide-induced acute respiratory distress syndrome in rats. J Dairy Sci. 2016;99(1):53–56. [DOI] [PubMed] [Google Scholar]
- 28. Yu Y, Tang D, Kang R. Oxidative stress-mediated HMGB1 biology. Front Physiol. 2045;6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta. 2010;1799(1-2):101–113. [DOI] [PubMed] [Google Scholar]
- 30. Bertheloot D, Latz E. HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol. 2017;14(1):43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li LC, Gao J, Li J. Emerging role of HMGB1 in fibrotic diseases. J Cell Mol Med. 2014;18(12):2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang H, Wang H, Ju Z, et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J Exp Med. 2015;212(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ibrahim HM, Mohammed-Geba K, Tawfic AA, El-Magd MA. Camel milk exosomes modulate cyclophosphamide-induced oxidative stress and immuno-toxicity in rats. Food Funct 2019;10(11):7523–7532. [DOI] [PubMed] [Google Scholar]
- 34. Li X, Li Z, Xu E, et al. Determination of lactoferrin in camel milk by ultrahigh-performance liquid chromatography-tandem mass spectrometry using an isotope-labeled winged peptide as internal standard. Molecules. 2019;24(22):4199. [DOI] [PMC free article] [PubMed] [Google Scholar]