Abstract
Coronavirus disease 2019 (COVID-19) is a viral pneumonia that is spreading rapidly worldwide. The main feature of this disease is a severe acute respiratory syndrome and caused by coronavirus 2 (SARS-CoV-2). There are several unknowns about the pathogenesis and therapeutically treatment of COVID-19 infection. In addition, available treatment protocols have not been effective in managing COVID-19 infection. It is proposed that natural anti-oxidants such as lemon, green tea, saffron, curcuma longa, etc. with high flavonoids like safranal, crocin, crocetin, catechins, resveratrol, calebin A, curcumin have therapeutic potential against viral infections. In this context, honey and its main components are being investigated as an option for patients with COVID-19. The present study may indicate that honey and its main components inhibit the entry of the virus into the host cell and its replication as well as modulate the inflammatory cascade. This review provides basic information for the possible potential effects of honey and its main components for fighting with SARS-CoV-2.
Keywords: COVID-19, SARS-CoV, SARS-CoV-2, acute respiratory distress syndrome, honey, viral infection
Introduction
Coronaviruses (COVs) are related to the group of RNA viruses that cause mild to severe respiratory diseases. SARS, MERS, and COVID-19 viruses are known as lethal type of coronaviruses. The coronavirus disease 2019 (COVID-19) is characterized by severe acute respiratory syndrome following exposure to coronavirus2 (SARS-CoV-2). This disease was observed in Wuhan, China, during the time of first and spread rapidly throughout the world.1 So far, physicians have been unable to find a suitable treatment regimen for COVID-19 infection, and preclinical and clinical studies on the subject are very limited and have not been able to suggest a more appropriate treatment. Various anti-viral drugs are used for the viral infection, which are directed against the virus or human cells. The protease inhibitors ritonavir and lopinavir (anti-HIV drugs) are used in COVID-19 infected patients. Other anti-viral drugs for human coronaviruses are Remdesivir, Umifenovir (Arbidol), Lamivudine (3TC), nucleoside analogues, neuraminidase inhibitors, Disoproxil and Tenofovir.2 Antibody therapy is also recommended for patients with COVID-19. However, none of these drugs has not yet been confirmed for the treatment of COVID-19-infected patients. Numerous studies have focused on the effect of natural products against infectious diseases.3 The popularity of natural compounds is linked to their positive and effective impacts, low cost and very low toxicity.4 Natural products have long been used for medical purposes, especially in traditional medicine in Asian countries such as China and India.5 In recent years, researchers have focused on the effectiveness of these agents (as anti-oxidant, anti-inflammatory, anti-infective, anti-carcinogenic etc.) in combining various infectious diseases due to resistance of pathogenic microbes to available vaccines and antibiotics. Natural products can modulate the sensitivity of the host to pathogens.6 Some natural products have been investigated to consider them as alternatives drugs for the treatment of infectious diseases based on their experimental results. Furthermore, these compounds may be able to increase the efficacy of chemical drugs and vaccines in infectious patients. It has been shown that most of them act against infectious diseases through similar mechanisms of chemical drugs.7 Medicinal plant extracts and honey are the main sources that can be effective against various infections and inflammatory diseases.8 Honey is a naturally occurring product that has been used as a traditional medicine in many countries since ancient times. Numerous studies reported that honey exerts therapeutics effects against diabetes, cardiovascular diseases and neurological deficits as well as against diseases of the respiratory, urinary and gastrointestinal tract.9-13 It has also been found that honey and its main ingredients can be effective against infectious diseases and also healing in wounds and burn injury.14 There is strong evidence that honey with potential anti-oxidant and anti-inflammatory activities can be effective in various ways against viral infectious diseases. In addition to attenuating oxidative damage induced by pathogens, it also helps to strengthen the immune system. The anti-viral activity of honey and its main constituents is also related to their modulatory effects on various molecular targets involved in cellular signaling pathways such as apoptosis and inflammation. In addition, honey and its main constituents can modulate signaling cascades which are necessary for virus replication and attachment.15 Indeed, it has been found that honey and its main constituents can alter the viral structure of the surface protein and membrane proteins, which leads to an inhibition of virus entry into the cell.16 Although many studies have been conducted on the anti-viral effects of honey, this beneficial effect of honey and its main constituents against coronaviruses is not understood. This review focuses on the potential therapeutic effects of honey and its main constituents against coronaviruses.
Characteristics of the Honey Composition
Honey consists of water, sugars, enzymes, amino acids, flavonoids, organic acids, phenolic acids, minerals, vitamins, and volatile compounds. Several characteristics of honey including color, flavor and aroma are related to the honeybee species, the type and origin of flowers, climate and weather conditions and the processing, packaging and storage of honey.17
Sugar is the main component of honey (90-95%), consisting of 75% monosaccharide (fructose and glucose) and 10 to 15% disaccharides (sucrose, turanose, maltose, maltulose, isomaltose, nigerose, kojibiose, trehalose) and trisaccharides (melezitose and maltotriose), trisaccharides (melezitose and maltotriose) and very small amounts of other sugars.18 Moreover, proteins are found in the honey including mostly free amino acids and enzymes and with the exception of glutamine and asparagine. Most of the amino acid present in honey is proline. In addition, the honey also contains aspartic acid, glutamic acid, histidine, glycine, glutamine, b-alanine, a-alanine, threonine, arginine, tyrosine, c-aminobutyric acid, methionine, valine, cysteine, leucine, isoleucine, phenylalanine, tryptophan, lysine, ornithine, asparagines, serine and alanine are present in honey.19
According to scientific reports, organic acids are present in all honeys at about 0.57% and cause slight acidity and electrical conductivity in the honey and influence the color and taste of the honey. The organic acids are created during the transformation of the nectar into honey or are extracted directly from nectar. Some organic acids in honey include aspartic acid, butyric acid, citric acid, acetic, formic acid, fumaric acid, galacturonic acid, butyric acid, formic acid, gluconic acid, glutamic acid, glyoxylic acid, glutaric acid, 2-hydroxybutyric acid, α-hydroxyglutaric acid, malonic acid, lactic acid, pyruvic acid, isocitric acid, α-ketoglutaric acid, malic acid, methylmalonic acid, quinic acid, propionic acid, shikimic acid, 2-oxopentanoic acid, tar-taric acid, succinic acid, and oxalic acid, however Gluconic acid is the most important acid in honey.20
Honey consists of small amounts of vitamins, including vitamin B[thiamine (B1), riboflavin (B2), pantothenic acid (B5), biotin (B8 or H), nicotinic acid (B3), pyridoxine (B6), and folic acid (B9)] and vitamin C. The low pH-value of honey has no influence on its vitamin substances. Vitamin C is present in various types of honey and is responsible for its anti-oxidant effect. Vitamin B2, B3, B5, B9 and vitamin C are water-soluble vitamins in honey.21
Macro and micro elements including magnesium, potassium, sodium, iron, calcium, phosphorus, iodine, manganese, lithium, zinc, cadmium, cobalt, nickel, barium, copper, chromium, silver, arsenic, selenium, and those observed in honeys. The amount of mineral components in honey varies between 0.04% and 0.2% in light and dark honeys. Some heavy metals such as lead, cadmium, arsenic, and mercury are toxic and they should not exceed the maximum residue levels. The measurement of toxic elements in honeys is necessary for human health.22
Phenolic compounds in honey are divided into phenolic acid (non-flavonoids) and flavonoids (flavones, flavanols, flavanones, isoflavones, chalcones and anthocyanidin). Phenolic acids are present in forms of hydroxybenzoic acid and hydroxycinnamic acid in honey. Hydroxybenzoic acids in honey consistof þ-hydroxybenzoicacid, syringicacid, salicylic (2-hydroxybenzoate), vanillic acid, ellagic acid and gallic acid.23 Various flavonoids such as vanillic acid, syringic acid, caffeic acid, þ-coumaric acid, ferulic acid, kaempferol, quercetin, chrysin, pinobanksin, myricetin, pinocembrin, galangin, ellagic acid, chlorogenic acid, rosmarinic acid, 3- and 4-hydroxybenzoic acid, hesperetin, gallic acid and benzoic acid have been found in honey17 (Table 1).
Table 1.
Chemical Formula and Structure of the Most Important flavonoids and Phenolic Acids in Honey.
| Main components | Chemical formula | Chemical structure |
|---|---|---|
| Flavonoids | ||
| Chrysin | C15H10O4 |
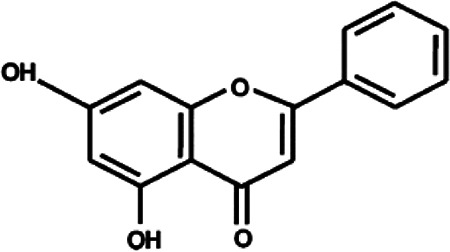
|
| Kaempferol | C15H10O6 |
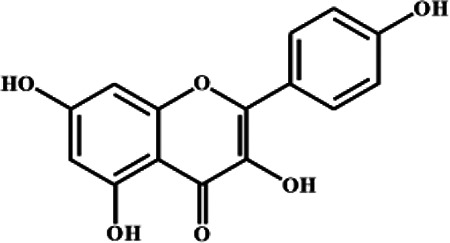
|
| Quercetin | C15H10O7 |
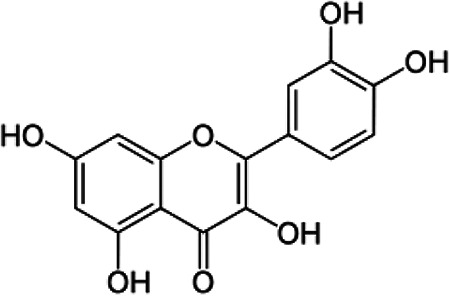
|
| Pinobanksin | C15H12O5 |
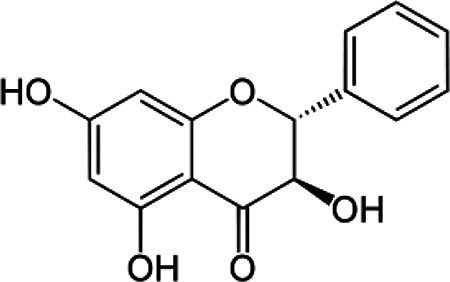
|
| Myricetin | C15H10O8 |
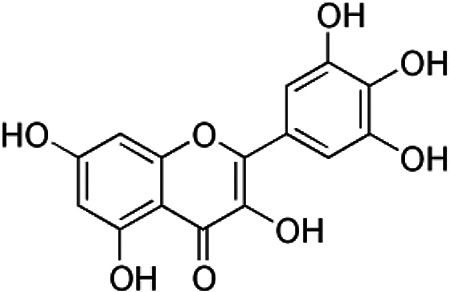
|
| Pinocembrin | C15H12O4 |
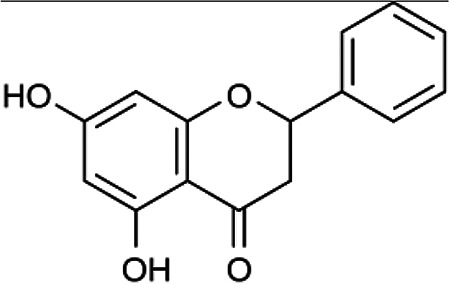
|
| Galangin | C15H10O5 |
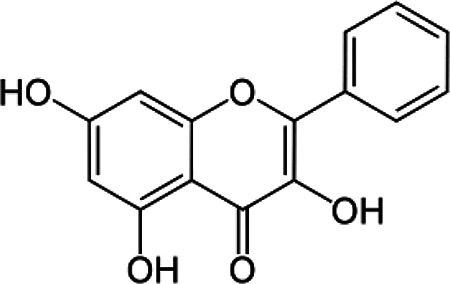
|
| Hesperetin | C16H14O6 |
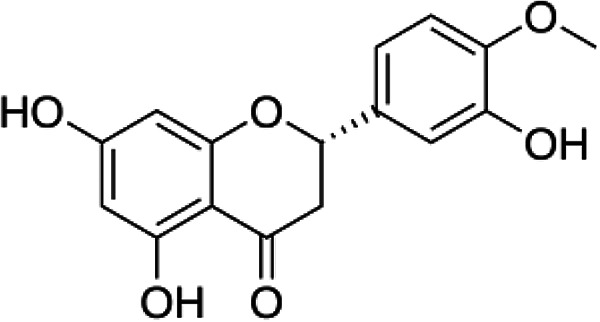
|
| Luteolin | C15H10O6 |
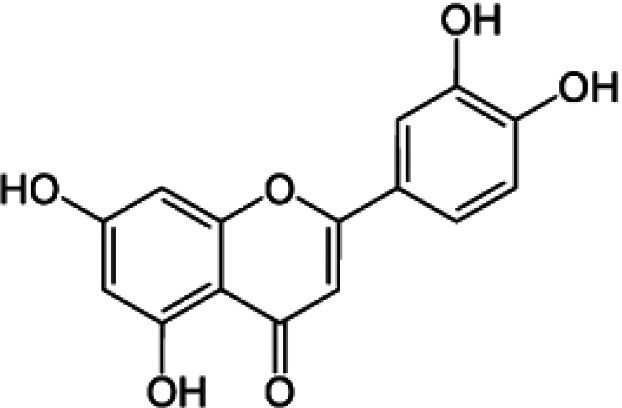
|
| Phenolic acid | ||
| Vanillic acid | C8H8O4 |
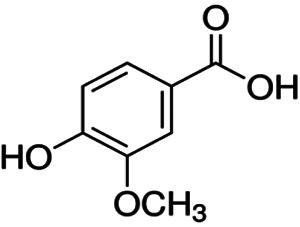
|
| Syringic acid | C9H10O5 |
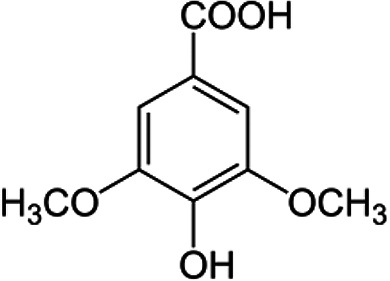
|
| Caffeic acid | C9H8O4 |
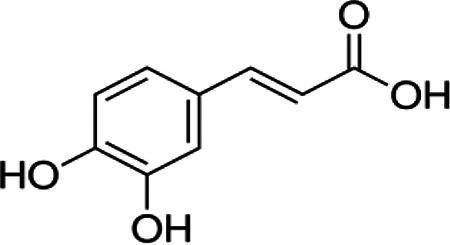
|
| þ-Coumaric acid | C9H8O3 |
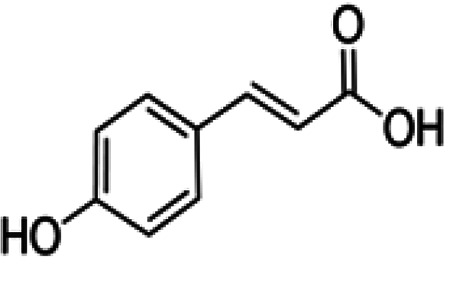
|
| Ferulic acid | C10H10O4 |
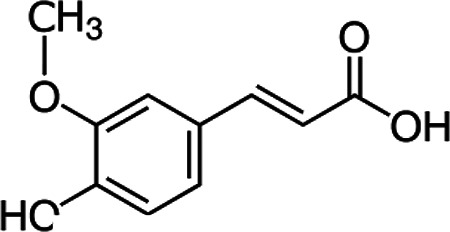
|
| Ellagic acid | C14H6O |
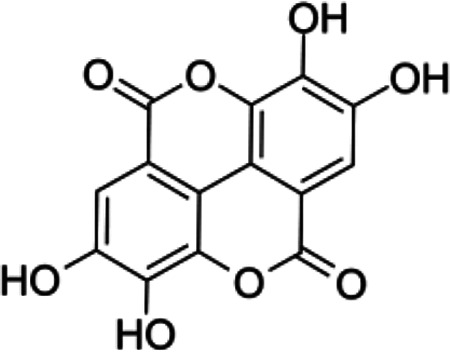
|
| Chlorogenic acid | C16H18O9 |
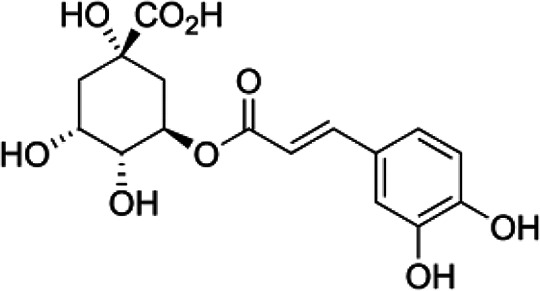
|
| Rosemary acid | C18H16O8 |
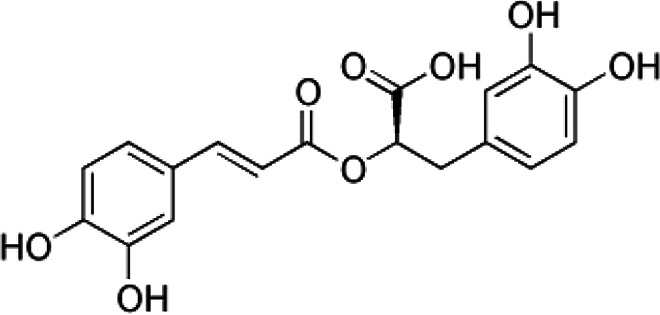
|
| 3- and 4-Hydroxybenzoic acid | C9H8O3 |
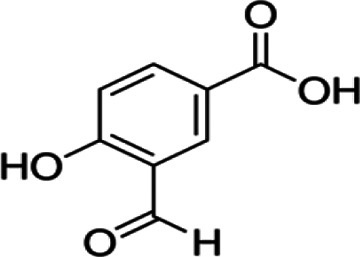
|
| Gallic acid | C7H6O5 |
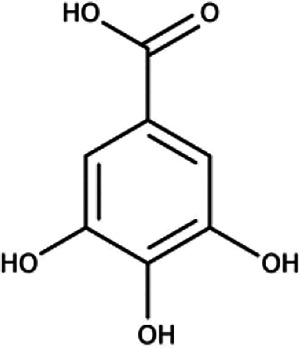
|
| Benzoic acid |
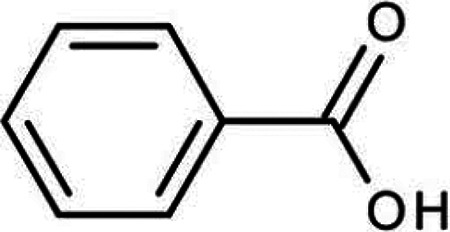
|
|
Volatile compounds are formed which determine the taste of honey and may vary according to nectar, processing conditions, origin and storage. Over the 400 different volatile compounds have been found in honey, belonging to C13-norisoprenoids, monoterpenes, sesquiterpenes, benzene derivatives and to a lower content of alcohols, terpenes, esters, ketones, fatty acids, and aldehydes.24
Virological Characteristic of SARS-CoV-2
Coronaviruses are a single-stranded RNA genome coated with glycoproteins. The coronavirus genera are composed of α, β, γ and δ that the virus related to COVID-19 belongs to β-coronavirus.25 This novel β-coronavirus has 88% similarity to the sequence of 2 bat-derived SARS-like coronaviruses.26 This virus also named “SARS-CoV-2” by the International Virus Classification Commission.
The genome of SARS-CoV-2 is similar to the genome of typical coronaviruses, which contains at least 10 open reading frames (ORFs). Almost 66% of viral RNA is translated into 2 large polyproteins, which are ORFs (ORF1a/b). Other 34% of viral RNA codes for 4 major structural proteins, including spike (S), envelope (E), nucleocapsid (N) and membrane (M) proteins.
SARS-CoV-2 enters the cell via the angiotensin-converting enzyme 2 (ACE2) as a receptor.27 The attachment of the to host cell receptors is necessary for the occurring infectious diseases. SARS-CoV-2 originated in bats28 and then adapted to human ACE2 cell. Further information on receptor binding of coronaviruses may help in the management of coronaviruses infection.29
COVID-19 Pathogenesis
The symptoms of patients with COVID-19 are similar to SARS-CoV and MERS-CoV infections, which include fever and chills, Sore throat, nonproductive cough, smell and taste loss, fatigue, dyspnea, myalgia, normal or reduced leukocyte counts, pneumonia, cardiovascular and neurological disease.30 However, our knowledge of the pathogenesis of COVID-19 is very limited, and the similar mechanisms of other coronaviruses help us to find unknowns about COVID-19.
The S protein of the coronavirus is involved in the enternalization of the virus into host cells.31 The envelope spike glycoprotein of SARS-CoV-2 attaches itself to ACE2 as its cellular receptor.28 The SARS-CoV enters the cells by direct membrane contraction between the virus and cell membrane and by clathrin-dependent and -independent endocytosis.32 After viral entry into the cells, the genomic RNA of this virus is extruded into the cytoplasm to translate the viral proteins, and the virus then replicates. The new form of envelope glycoproteins penetrates organelles such as endoplasmic reticulum or golgi, and forms the nucleocapsid in vesicles; the vesicles then bind to the cell membrane to extrude the virus.33
Although the antigen presentation of SARS-CoV-2 is very effective to understanding the pathogenesis, prevention and treatment of COVID-19, it is not clear, and information on SARS-CoV and MERS-CoV may help us to identify the antigen presentation of SARS-CoV-2. The antigen presentation of SARS-CoV was mainly related to MHC I,31 and somewhat to MHC II and antigen presentation of MERS-CoV infection was related to MHC II. The antigen presentation can activate the humoral and cellular immunity, leading to the production of IgM and IgG. It has been found that for patients with SARS-CoVs serum levels of IgG antibodies increase over a long period of time and play a protective role.34 In patients infected with SARS-CoV-2, the number of CD4+ and CD8+ T cells in peripheral blood significantly decreased while high level of HLA-DR (CD4 3.47%) and CD38 (CD8 39.4%) was found when it was highly activated.35
Acute Respiratory Distress Syndrome (ARDS) is the common cause of death in patients with COVID-19, associated with the release of cytokine cascade, including pro-inflammatory cytokines [tumor necrotic factor (TNF), interferons (IFNs), interleukins (ILs)] as well as CC chemokines (CCLs).36 Serum levels of IFN-α, IL-6, CCL5, CXCL8, and -10 are increased significantly in patients with severe SARS-CoV and Middle East respiratory syndrome (MERS)-CoV infection compared with the mild form of the diseases.37 The pro-inflammatory cytokines can cause ARDS and organ failure which lead to death in patients with severe status.1
Potential Effect of Honey and Its Main Components Against Viral Infectious Diseases
Numerous studies have pointed to the therapeutic effects of honey and its main components against various viral infectious diseases. Honey and its main components could combat against Herpes zoster,38 rubella,39 influenza,40 herpes disease,41 respiratory syncytial virus,42 AIDS,43 immunodeficiency virus,44 viral hepatitis,45 gingivostomatitis,46 rabies,47 rhinoconjunctivitis48 and COVID-19.49 The mechanisms of anti-viral properties of honey and its main components is very vast and unknown.42 The anti-viral activity of honey and its main components is usually associated, similar to other natural products like resveratrol, calebin A or curcumin with anti-oxidant, anti-inflammatory, anti-resistance and anti-apoptotic effects by modulating cellular signaling pathways such as MAPK, NF-κB, Nrf2, etc.50-58 In addition, these agents also have direct effect on the structure of the virus, such as the interaction of honey and its major components with structural and/or non-structural proteins in the virus or binding to target receptors on the virus.58
Effect of Honey and Its Main Components on the Virus Cell Cycle
More information about viral replication in the host cell may help to develop novel anti-viral agents that target viral replication and limit drug resistance in the virus. Viral replication takes place in 3 stages: first step involves the attachment of the virus to host cells, penetration and decoating, the second step involves the replication of the viral genome, transcription and translation and last step is viral assembly.
One mechanism underling the anti-viral action of honey and its main components is interruption of the proteins necessary for viral attachment and entry into host cells.59
In this regard, it has been pointed out that honey interrupts the disulphide bonds in the HA receptors, which leads to inhibition of the attachment of the influenza virus attachment to the host cell surface.40
Influenza hemagglutinin protein HA and the coronavirus spike protein are 2 major members of the class I fusion protein family.60 SARS-CoV spike protein is necessary for the binding of the virus to the host cell receptor ACE2. Interestingly, it has been reported that chrysin (400 μM) showed a loose inhibition on the interaction of S protein with ACE261 and ACE2 and 3C-like protease (3CL pro) are recognized as the targets for anti-viral drugs Furthermore, Kaempferol and quercetin showed a high affinity to SARS-CoV-2 3CL hydrolase.62 Kaempferol and quercetin were able to attach to ACE2 and modulate signal pathways including prostaglandin-endoperoxide synthase 2 (PTGS2), caspase 3, B-cell lymphoma 2 (Bcl-2), and Kaposi’s sarcoma, which are associated with herpes virus infection, measles, hepatitis C, human cytomegalovirus and Epstein–Barr virus infection.62
Viruses are encoded for the ion-selective channels in the membrane of the infected cell,63,64 and once these channels are activated, they are released in the cell and replicate.65 Ion channel inhibitors can block virus production and allow the infected cell to potentiate its immune system.66,67 The open reading frame 3a (ORF3a) of SARS-CoV encodes for an ion-permeable channel.66,67 Indeed, it has been shown that flavonoids can trigger ion channel and inhibit virus release.66,67 It was suggested that kaempferol is suitable as an agent for 3a-channel proteins of coronaviruses.66,67
Moreover, it has been reported that chrysin inhibited viral replication by blocking viral RNA replication and viral capsid protein formation without cytotoxicity (52). The modulation of molecular signaling pathways is one of the main targets for anti-viral drug. In this context, it was pointed out that kaempferol inhibits the replication of the influenza virus by to inducing the opposite cell-autonomous immune responses by regulating mitogen-activated protein kinase (MAPK) signaling pathways.68 In addition, it was observed that kaempferol and quercetin can inhibit the replication of SARS-CoV-2 by targeting on phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma (PIK3CG) and E2F1 (E2F Transcription Factor 1) via modulating Phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway. Furthermore, quercetin and kaempferol inhibited SARS-CoV-2 replication through modulating of kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway69,70 (Figure 1).
Figure 1.
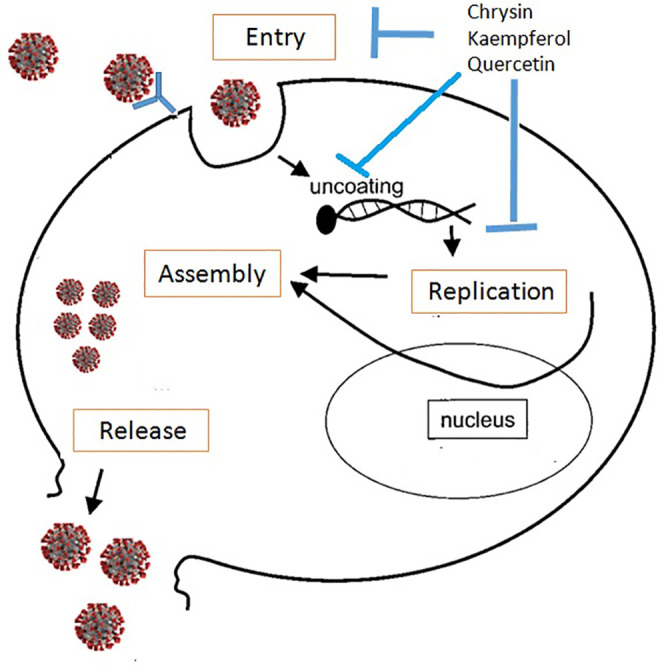
Effect of main components of honey on the cell cycle of corona virus. Main flavonoids present in honey such as chrysin, quercetin and kaempferol may be effective against corona virus by inhibiting virus entry, invasion and replication.
Effect of Honey and Its Main Components on the Viral Proteases
Lysosomal proteases are involved in coronavirus entry by cleaving coronavirus surface spike proteins and inducing the contraction of host and virus membranes.71 Further, quercetin has been found to have protective effects against murine coronavirus by inhibiting H+-ATPase of the lysosomal membrane and preventing removal of the virus coating. In addition, quercetin inhibited the ATPase of multidrug resistance-associated proteins, thereby increasing the bioavailability of anti-viral drugs.72 The structure of main protease of the coronavirus is similar to that of SARS-CoVMpro, its RNA genome is approximately 82% similar to that of SARS-CoV, which belongs to the genus of betacorona virus.73,74
Mpro (3-chymotrypsin-like cysteine enzyme) is necessary for the processing of polyproteins by SARS-CoV-2. 75,76 Furthermore, 6 compounds present in honeybee and propolis have been found to have anti-viral activity against COVID-19 through strong binding affinity to main protease (Mpro) and viral replication.
It has been shown that chrysin could bind to amino acid residues (SER-46, THR-24 and THR-26) of the main protease of COVID-19 through hydrogen bond with 2.4, 2.6, 2.1 Ao, and also caused strong electrostatic interaction of the phenyl ring (HIE-41) with 3.8Ao (78). Galangine could interact with 2 amino acid residues (SER-46, THR-24) and electrostatic interaction with HIE-41 in the main protease in COVID-19. In addition, caffeic acid could interact with the main protease of COVID-19 via its hydroxyl group bound with 2 amino acid residues (GLN-189 and HIE-164) of the receptor by hydrogen bond with 2.0, 2.8Ao, and electrostatic interaction with (HIE-41) with 4.1 Ao..77
Effect of Honey and Its Main Components on the Inflammatory Response in Patients With COVID-19
Alveolar cells in patients with COVID-19 stimulates immune cells to release secrete cytokines and chemokines that are involved in recruiting additional immune cells to the lesion site. The activated immune cells can disrupt the virus by secreting inflammatory cytokines and phagocytosis. However, the excessive immune responses induce cytokine cascade in the lungs of patients with COVID-19. The cytokine cascade blocks the airways and increases vascular permeability, leading to edema and hypoxia. In addition, it has been found that quercetin and kaempferol to suppress the release of cytokines and to reduce immune responses and inflammatory mediators through modulating TNF, NF-κB pathways, PI3K/Akt, MAPK, T-cell, B cell, Ras and apoptosis signaling pathways, leading to inhibition of the coronavirus adsorption and replication.46,59,78-81
Moreover, kaempferol and quercetin also affect PTGS2, heat shock protein 90 alpha family class B member 1 (HSP90AB1), microsomal prostaglandin E synthase-1 (mPGES-1), Leukotriene A4 Hydrolase (LTA4 H), nitric oxide synthase (NOS2), TNF and IL-6, resulting in inhibition of coronavirus invasion and replication.72,82
It has been reported that IFNs have a major role in the control of COVID-19 infection. This virus could inhibit the induction of interferon in humans. In addition, it has been found that the main components of honey can increase the serum levels of interferon-gamma (IFN-gamma). Table 2 contains a summary of articles on the mechanisms of the main components of honey against coronavirus.
Table 2.
Main Mechanisms of Controlling Coronavirus by the Main Honey Components.
| Honey or its main components | Target | Effect | Reference |
|---|---|---|---|
| Chrysin | -S protein with ACE2 -3-chymotrypsin-like cysteine enzyme |
Inhibition of virus entry in to host cells and virus replication | 52,61,77 |
| Kaempferol | - SARS-CoV-2 3CL hydrolase -ORF3a of SARS-CoV - PI3K-Akt, JAK/STAT, MAPK signaling pathway - COX-2, TNF, mPGES-1, AKT1, MAPK1, JUN, IL-6, CASP3, EGFR, IL1B, NOS2, PTGS2, HSP90AB1, mPGES-1, LTA4 H |
Inhibition of the virus adsorption, invasion and replication |
62,66-70,72,78-82
|
| Quercetin | - SARS-CoV-2 3CL hydrolase -H+-ATPase of the lysosomal membrane -PI3K-Akt and JAK/STAT signaling pathway -PI3K-Akt, JAK/STAT, MAPK signaling pathway -COX-2, TNF, mPGES-1, AKT1, MAPK1, JUN, IL-6, CASP3, EGFR, IL1B, NOS2, PTGS2, HSP90AB1, mPGES-1, LTA4H |
Inhibition of the virus coating, adsorption, invasion and replication |
69,70,72,78-82
|
| Galangin | 3-chymotrypsin-like cysteine enzyme | Inhibition of the virus adsorption, invasion and replication | 7 7 |
| Caffeic acid |
Potential Effect of Honey and Its Main Components on Vital Organs Complications of Coronavirus
Coronaviruses are able to stimulate several inflammatory mediators, which leads to various organ dysfunctions including ARDS. Honey and its main components have anti-fibrotic activity by reducing the expression of inflammatory mediators involved in lung infection. It has been found that honey lowers levels of prostaglandins (PG) E2,8 PG2a,83 thromboxane B284 and increases nitric oxide end products. These properties could help explain some biological and therapeutic properties of honey, particularly as an anti-bacterial agent or wound healing.46 Moreover, it is suggested that honey could be effective against human respiratory syncytial virus (RSV) by inhibition viral replication.42 Pulmonary fibrosis is a serious consequence of COVID-19 infection associated with ARDS. Furthermore, COVID-19 may affect the respiratory system and cause ARDS to secrete inflammatory mediators related to pulmonary fibrosis such as transforming growth factor beta (TGF-β) and IL-1β. Indeed, chrysin could inhibit the cellular inflammatory response by improving the NF-кB signaling pathway and fibrotic response in a rat model of viral-induced acute lung injury.85 In addition, chrysin reduced inflammation, collagen deposition, malondialdehyde (MDA) levels in the lung in an experimental model of bleomycin-induced pulmonary fibrosis.86 Furthermore, it has been shown that kaempferol reduced pulmonary inflammation and fibrosis in the experimental model of silicosis.87
In some patients with COVID-19, pulmonary edema was observed accompanied by a decrease in the activity of the epithelial sodium channels and the ion channel of the E-protein on the pulmonary epithelial cells.88 In addition, chrysin inhibited alpha-naphthylthiourea (ANTU)-induced pulmonary edema in the animal model through regulating inflammatory responses and oxidative/nitrosative stress.89 Moreover, cardiovascular disturbances may occur in patients with COVID-19 due to the systemic inflammation. It has been shown that honey reduced the degree of infiltration of inflammatory cells and to preserve the morphology of myocardial fiber in heart attack model.90 In a rat model, chrysin has been found to have a protective effect on myocardial fiber structure in isoproterenol-induced acute myocardial infarction.91
Chrysin modulated the hemodynamic and ventricular functions in isoproterenol-induced acute myocardial infarction in a rat model through decreasing oxidative stress and also by reversing arterial ligation and peroxisome proliferator-activated receptor gamma (PPAR-γ) inhibition. Treatment with chrysin also led to an improvement in receptor for advanced glycation end-products (RAGE), inhibitor of nuclear factor kappa B (IKK-β) and nuclear factor kappa B (NF-κB) expressions and TNF-α levels. More interestingly, chrysin exerts cardiovascular protection by reducing apoptosis indices.91-95
The connection between COVID-19 and kidney damage is not clear. However, it was found that some patients with COVID-19 infection showed signs of kidney damage without previous history. There is some evidence linking COVID-19 infection to kidney damage: 1) the ability of coronavirus to attach to kidney cells and enter to the cell, 2) the decrease in blood oxygen, 3) the induction of systemic inflammation, 4) the formation of clots in the bloodstream that can block the blood vessels in the kidney. In addition, honey and its main components may be effective for kidney inflammation associated with COVID-19 treatment. It has been reported that rosmarinic acid improved blood pressure by inhibiting angiotensin-converting enzyme activity in 2-kidneys 1-clip model in rats.96 Further, chrysin showed an inhibited therapeutic effect against adenine-induced CKD in a mouse model of focal cerebral ischemia/reperfusion injury by suppressing the NF-κB signaling pathway.97 It was suggested that clot formation in small and large blood vessels may be major factor in organ failures and death from COVID-19 and that inhibition of clot formation may be effective against organ failures and death from COVID-19.98 In this context, in vitro and in vivo assays confirmed the inhibitory effects of honey and its main components on platelet aggregation and blood coagulation.99-106 For example, the in vitro, in vivo, and ex vivo models showed the anti-thrombotic and anti-coagulant effect of quercetin.103 Indeed, several studies have reported that quercetin decreased, similar to other natural polyphenols (resveratrol, curcumin, ginkgo biloba and bilberry) diastolic pressure by potentiating eNOS activation, nitric oxide production107,108 and the activity of thrombin, formation of fibrin clots and blood clotting through modulating the coagulation cascade.103
Quercetin and apigenin were found to decrease collagen- and Adenosine diphosphate (ADP)-induced aggregation in platelet-rich plasma for 2 weeks in healthy volunteers.104 Moreover, rosmarinic acid exerted a mild anti-thrombotic effect though inhibiting platelet aggregation and fibrinolytic activity in anesthetized rats with tight ligature in the inferior vena cava below the left renal vein.105
The anti-thrombotic effects of caffeic acid was investigated on cerebral arterioles and venules of mice by intravital microscopy and also in vitro. Furthermore, caffeic acid was able to inhibit platelet-mediated thrombosis by the activating of p38, extracellular signal-regulated kinases (ERKs) and c-Jun N-terminal kinases (JNKs) and led to an increase in cyclic adenosine monophosphate (cAMP) levels and a decrease in the expression of P-selectin and integrin αIIbβ3 activation.106
Potential Effect of Honey and Its Main Components on Interaction Coronavirus With Nrf2 Signaling Pathways
Nuclear factor erythroid 2-related factor 2 (Nrf2) dependent antioxidant genes expression is markedly reduced in COVID-19 patients. Nrf2 stimulators may inhibit the replication of SARS-CoV2 and also related inflammatory genes expression.109 Previous studies indicated the Nrf2-mediated antioxidative effect of honey and its main components in various diseases. In this regard, it was found that honey stimulated the Keap1/Nrf2 signaling in the epidermis and induced epidermal barrier recovery.110 Honey significantly improved hypertension via stimulation of Nrf2 in the kidney of hypertensive rats.111 In murine macrophages exposed to the lipopolysaccharides (LPS), Carthamus tinctorius L. honey induced the expressions of Nrf-2/Heme Oxygenase-1 (HO-1), leading to inhibition of inflammation.112 Chrysin ameliorated the neutrophils infiltration and other lung pathological damages through modulation of oxidative stress dependent Nrf2 pathway in lungs of rats exposed to carrageenan.113 Chrysin, luteolin and apigenin protected rat primary hepatocytes against oxidative stress through modulating Nrf2 signaling pathway.114
Regarding to stimulatory effects of honey on the Nrf2 signaling, this natural agent can potential effect to combat against SARS-CoV2.
Conclusion and Remarks
The current study provided several pieces of evidence based on the potential effects of honey and its main ingredients against the corona virus infection. We focused on the modulating effects of honey and its main components on the molecular targets suitable for the treatment of coronavirus infections. The present study indicated that honey and its major components could be considered against COVID-19 infection because of their ability to regulate the molecular targets involved in the attachment and entry of this virus into the host cell and its RNA replication. Honey and its major components may also regulate cellular signaling pathways including oxidative stress, inflammation and apoptosis.
Honey and its main components may also be effective against pulmonary edema and fibrosis in COVID-19 infection due to their anti-fibrotic and immunomodulatory effects. In addition, systemic inflammation is one of the major threats in patients with COVID-19, which can be suppressed with honey and its main components. The inhibition of systemic inflammation by honey and its main components is attributed to their therapeutic effect on kidney, lung and cardiovascular damage in COVID-19 Infection. One of the main potential benefits of honey and its main components is its anti-thrombotic effects. In addition, it has been suggested that clot formation in patients with COVID-19 infection causes organ damage and eventually death. Since honey and its main components can inhibit the stimulation of molecular signaling pathways underlying coagulation and inflammation, they may be helpful in severe patients with COVID-19 as an adjunct to improve the cytokine cascade.
Although the safety of these compounds is approved in both animal models and human, but the low bioavailability of these compounds may reduce their efficacy. It is therefore, necessary to develop a new formula with high oral bioavailability.
Overall, the current study indicates that honey and its main components have potential implications for the preventing and treatment of coronavirus infection, including COVID-19. Till now, no clinical trial or case study have published on the protective effects of honey against COVID-19. According to our knowledge, there are some registered clinical trials including NCT04345549, NCT04323345, NCT04468139, NCT04347382 that are doing study to evaluate the effect of honey in patients with COVID-19 .
Therefore, clinical trials should be done to confirm or reject the efficacy of honey and its main components in patients with COVID-19 infection.
Abbreviations
- 3CL pro
3C-like protease
- ACE2
angiotensin-converting enzyme 2
- ADP
adenosine diphosphate
- AKT
protein kinase B
- ANTU
alpha-naphthylthiourea
- ARDS
acute respiratory distress syndrome
- Bcl-2
B-cell lymphoma 2
- cAMP
Cyclic adenosine monophosphate
- CCLs
chemokines
- COVID-19
coronavirus disease 2019
- COVs
coronaviruses
- E2F1
E2F Transcription Factor 1
- ERKs
extracellular signal-regulated kinases
- HO-1
heme Oxygenase-1
- HSP90AB1
heat shock protein 90 alpha family class B member 1
- IFNs
interferons
- IKK-β
nuclear factor kappa B
- ILs
interleukins
- JAK/STAT
Janus kinase/signal transducer and activator of transcription
- JNKs
c-Jun N-terminal kinases
- LPS
lipopolysaccharides
- LTA4H
leukotriene A4 hydrolase
- MDA
malondialdehyde
- MERS
middle east respiratory syndrome
- mPGES-1
microsomal prostaglandin E synthase-1
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NOS2
nitric oxide synthase
- Nrf2
nuclear factor erythroid 2-related factor 2
- ORF3a
open reading frame 3a
- PG
prostaglandins
- PI3K
phosphatidylinositol 3-kinase
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- PTGS2
prostaglandin-endoperoxide synthase 2
- RAGE
receptor for advanced glycation end-products
- TGF-β
transforming growth factor beta
- TNF
tumor necrotic factor
Footnotes
Authors’ Note: F.A., M.S., and S.S. designed study. All authors contributed to the collection of data, to the writing of the manuscript, and to designing tables and figures. S.S. and M.S. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Saeed Samarghandian  https://orcid.org/0000-0001-5461-9579
https://orcid.org/0000-0001-5461-9579
References
- 1. Gralinski LE, Bankhead A III, Jeng S, et al. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. MBio. 2013;4(4):e00271–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaur J, Kaur R, Kaur A. Dietary Antioxidants and Infectious Diseases, in Infectious Diseases and Your Health. Springer; 2018:307–316. [Google Scholar]
- 4. Young I, Woodside J. Antioxidants in health and disease. J Clin Pathol. 2001;54(3):176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. WHO Guidelines for Indoor Air Quality: Household Fuel Combustion. World Health Organization; 2014. [PubMed] [Google Scholar]
- 6. Pugh-Clarke K, Donlon S, McCann M. Ce: Continuing education article prevention of infection in patients with chronic kidney disease part 1: application of infection control principles to the renal care environment. J Ren Care. 2010;36(4):191–198. [DOI] [PubMed] [Google Scholar]
- 7. Butler MS, Buss AD. Natural products—the future scaffolds for novel antibiotics? Biochem Pharmacol. 2006;71(7):919–929. [DOI] [PubMed] [Google Scholar]
- 8. Molan PC. Honey: Antimicrobial actions and role in disease management. 2009.
- 9. Farkhondeh T, Jalali S, Ashrafizadeh M, Samarghandian S, Samini F. Effects of chrysin on serum corticosterone levels and brain oxidative damages induced by immobilization in rat. Cardiovasc Hematol Disord Drug Targets. 2020;20(1):47–53. [DOI] [PubMed] [Google Scholar]
- 10. Samini M, Farkhondeh T, Azimi-Nezhad M, Samarghandian S. Chrysin impact on oxidative and inflammation damages in the liver of aged male rats. Endocr Metab Immune Disord Drug Targets. 2020. [DOI] [PubMed] [Google Scholar]
- 11. Farkhondeh T, Abedi F, Samarghandian S. Chrysin attenuates inflammatory and metabolic disorder indices in aged male rat. Biomed Pharmacother. 2019;109:1120–1125. [DOI] [PubMed] [Google Scholar]
- 12. Samarghandian S, Farkhondeh T, Samini F. Honey and health: a review of recent clinical research. Pharmacognosy Res. 2017;9(2):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Samarghandian S, Azimi-Nezhad M, Borji A, et al. Inhibitory and cytotoxic activities of chrysin on human breast adenocarcinoma cells by induction of apoptosis. Pharmacognosy Mag. 2016;12(Suppl 4):S436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alvarez-Suarez JM, Tulipani S, Díaz D, et al. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem Toxicol. 2010;48(8-9):2490–2499. [DOI] [PubMed] [Google Scholar]
- 15. Ahmed S, Othman NH. Honey as a potential natural anticancer agent: a review of its mechanisms. Evid Based Complement Alternat Med. 2013;2013:829070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmed S, Sulaiman SA, Baig AA, et al. Honey as a potential natural antioxidant medicine: an insight into its molecular mechanisms of action. Oxid Med Cell Longev. 2018;2018:8367846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. da Silva PM, Gauche C, Gonzaga LV, Costa ACO, Fett R. Honey: chemical composition, stability and authenticity. Food Chem. 2016;196(1):309–323. [DOI] [PubMed] [Google Scholar]
- 18. Belitz H-D, Grosch W, Schieberle P. Sugars, sugar alcohols and honey. In: Belitz H-D, Grosch W, Schieberle P, eds. Food Chemistry. Springer; 2004:862–891. [Google Scholar]
- 19. Lloyd AM. Analysis of Amino Acids in Mānuka Honey. University of Waikato; 2015. [Google Scholar]
- 20. Cianciosi D, Forbes-Hernández TY, Afrin S, et al. Phenolic compounds in honey and their associated health benefits: a review. Molecules. 2018;23(9):2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ciulu M, Solinas S, Floris I, et al. RP-HPLC determination of water-soluble vitamins in honey. Talanta. 2011;83(3):924–929. [DOI] [PubMed] [Google Scholar]
- 22. Tutun H, Kahraman HA, Aluc Y, Avci T, Ekici H. Investigation of some metals in honey samples from West Mediterranean region of Turkey. In: Veterinary Research Forum. Faculty of Veterinary Medicine, Urmia University, Urmia, Iran; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Biesaga M, Pyrzyńska K. Stability of bioactive polyphenols from honey during different extraction methods. Food Chem. 2013;136(1):46–54. [DOI] [PubMed] [Google Scholar]
- 24. Manyi-Loh CE, Ndip RN, Clarke AM. Volatile compounds in honey: a review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int J Mol Sci. 2011;12(12):9514–9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ren L-L, Wang Y-M, Wu Z-Q, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J. 2020;133(9):1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92(4):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wan Y, Shang J, Graham R, Baric RS, Li F. An analysis based on decade-long structural studies of SARS 3. J Virol. 2020. JVI Accepted Manuscript Posted Online 29 January 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo W, Yu H, Gou J, et al. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19). Preprints. 2020;2020:2020020407. [Google Scholar]
- 33. Tahvildari A, Arbabi M, Farsi Y, et al. Clinical features, diagnosis, and treatment of COVID-19 in hospitalized patients: a systematic review of case reports and case series. Front Med. 2020;7:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 2020;14(1):69–71. [DOI] [PubMed] [Google Scholar]
- 35. Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Invest. 2020;50(3):e13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):1. [DOI] [PubMed] [Google Scholar]
- 37. Wygrecka M, Jablonska E, Guenther A, Preissner KT, Markart P. Current view on alveolar coagulation and fibrinolysis in acute inflammatory and chronic interstitial lung diseases. Thromb Haemost. 2008;99(03):494–501. [DOI] [PubMed] [Google Scholar]
- 38. Hashemipour MA, Tavakolineghad Z, Arabzadeh SAM, Iranmanesh Z, Nassab SAHG. Antiviral activities of honey, royal jelly, and acyclovir against HSV-1. Wounds. 2014;26(2):47. [PubMed] [Google Scholar]
- 39. Zeina B, Othman O, Al-Assad S. Effect of honey versus thyme on rubella virus survival in vitro. J Altern Complement Med. 1996;2(3):345–348. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe K, Rahmasari R, Matsunaga A, Haruyama T, Kobayashi N. Anti-influenza viral effects of honey in vitro: potent high activity of manuka honey. Arch Med Res. 2014;45(5):359–365. [DOI] [PubMed] [Google Scholar]
- 41. Semprini A, Singer J, Shortt N, Braithwaite I, Beasley R, Pharmacy Research Network. Protocol for a randomised controlled trial of 90% kanuka honey versus 5% aciclovir for the treatment of herpes simplex labialis in the community setting. BMJ Open. 2017;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zareie PP. Honey as an Antiviral Agent Against Respiratory Syncytial Virus. University of Waikato; 2011. [Google Scholar]
- 43. Behbahani M. Anti-HIV-1 activity of eight monofloral Iranian honey types. PLoS One. 2014;9(10):e108195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Al-Waili NS, Al-Waili TN, Al-Waili AN, Saloom KS. Influence of natural honey on biochemical and hematological variables in AIDS: a case study. Scientific World J. 2006;61985–61989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abdulrhman M, Shatla R, Mohamed S. The effects of honey supplementation on Egyptian children with hepatitis A: a randomized double blinded placebo-controlled pilot study. J Apitherapy. 2016;1(1):23. [Google Scholar]
- 46. Awad OGA-N, Hamad A-MH. Honey can help in herpes simplex gingivostomatitis in children: prospective randomized double blind placebo controlled clinical trial. Am J Otolaryngol. 2018;39(6):759–763. [DOI] [PubMed] [Google Scholar]
- 47. Igado O, Omobowale T, Nottidge H. The effect of honey and vitamin C on the response of dogs to anti-rabies vaccination. Sahel J Vet Sci. 2010;9(2). [Google Scholar]
- 48. Rajan T, Tennen H, Lindquist RL, Cohen L, Clive J. Effect of ingestion of honey on symptoms of rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2002;88(2):198–203. [DOI] [PubMed] [Google Scholar]
- 49. Filipe J. Epidemics and pandemics: Covid-19 and the “The Drop of Honey Effect.” IJEBA. 2020;VIII(2):240–249. [Google Scholar]
- 50. Vickers NJ. Animal communication: when I’m calling you, will you answer too? Curr Bio. 2017;27(14):R713–R715. [DOI] [PubMed] [Google Scholar]
- 51. Buhrmann C, Popper B, Kunnumakkara AB, Aggarwal BB, Shakibaei M. Evidence that Calebin A, a component of curcuma longa suppresses NF-κB mediated proliferation, invasion and metastasis of human colorectal cancer induced by TNF-β (Lymphotoxin). Nutrients. 2019;11(12):2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Buhrmann C, Shayan P, Banik K, et al. Targeting NF-κB signaling by calebin A, a compound of turmeric, in multicellular tumor microenvironment: potential role of apoptosis induction in CRC cells. Biomed. 2020;8(8):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Docherty JJ, Smith JS, Fu MM, Stoner T, Booth T. Effect of topically applied resveratrol on cutaneous herpes simplex virus infections in hairless mice. Antiviral Res. 2004;61(1):19–26. [DOI] [PubMed] [Google Scholar]
- 54. Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-κB ligand (RANKL) activation of NF-κB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem. 2011;286(13):11492–11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shakibaei M, Csaki C, Nebrich S, Mobasheri A. Resveratrol suppresses interleukin-1β-induced inflammatory signaling and apoptosis in human articular chondrocytes: potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem Pharmacol. 2008;76(11):1426–1439. [DOI] [PubMed] [Google Scholar]
- 56. Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-κB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73(9):1434–1445. [DOI] [PubMed] [Google Scholar]
- 57. Shakibaei M, Mobasheri A Buhrmann C. Curcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitro. Genes Nutr. 2011;6(2):171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yaghoobi R, Kazerouni A. Evidence for clinical use of honey in wound healing as an anti-bacterial, anti-inflammatory anti-oxidant and anti-viral agent: a review. Jundishapur J Nat Pharm Prod. 2013;8(3):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Münstedt K. Bee products and the treatment of blister-like lesions around the mouth, skin and genitalia caused by herpes viruses—a systematic review. Complement Ther Med. 2019;43:81–84. [DOI] [PubMed] [Google Scholar]
- 60. Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ho T-Y, Wu S-L, Chen J-C, Li C-C, Hsiang C-Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74(2):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen C, Jiang Z-Y, Yu B, et al. Study on the anti-H1N1 virus effects of quercetin and oseltamivir and their mechanism related to TLR7 pathway. J Asian Nat Prod Res. 2012;14(9):877–885. [DOI] [PubMed] [Google Scholar]
- 63. Fischer WB, Thiel G, Fink RH. Viral membrane proteins. Eur Biophy J. 2010;39(7):1041–1042. [DOI] [PubMed] [Google Scholar]
- 64. Fischer WB, Wang Y-T, Schindler C, Chen C-P. Mechanism of function of viral channel proteins and implications for drug development. In: Kwang J, ed. International Review of Cell and Molecular Biology. Elsevier; 2012:259–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schwarz S, Sauter DRP, Lu W, Wang K. Coronaviral ion channels as target for Chinese herbal medicine. In: Bonavida B, Chouaib S, Kashfi K, eds. Forum on Immunopathological Diseases and Therapeutics. Begel House Inc; 2012. [Google Scholar]
- 66. Schwarz S, Sauter D, Wang K, et al. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014;80(02-03):177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang J, Zhang T, Du J, Cui S, Yang F, Jin Q. Anti-enterovirus 71 effects of chrysin and its phosphate ester. PLoS One. 2014;9(3):e89668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dong W, Wei X, Zhang F, et al. A dual character of flavonoids in influenza A virus replication and spread through modulating cell-autonomous immunity by MAPK signaling pathways. Sci Rep. 2014;4:7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ye C, Gao M, Lin W, Yu K, Li P, Chen G. Theoretical study of the anti-NCP molecular mechanism of traditional Chinese medicine Lianhua-Qingwen Formula (LQF). Polar. 2020;2(21.52):10.68–18.03. [Google Scholar]
- 70. Huang Y-F, Bai C, He F, Xie Y, Zhou H. Review on the potential action mechanisms of Chinese medicines in treating coronavirus disease 2019 (COVID-19). Pharmacol Res. 2020;158:104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zheng Y, Shang J, Yang Y, et al. Lysosomal proteases are a determinant of coronavirus tropism. J Virol. 2018;92(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Choi H-J, Kim J-H, Lee C-H, et al. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009;81(1):77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhou P, Yang X-L, Shi Z-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. [DOI] [PubMed] [Google Scholar]
- 76. Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281(18):4085–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hashem H. IN silico approach of some selected honey constituents as SARS-CoV-2 main protease (COVID-19) inhibitors. 2020.
- 78. Mehla R, Bivalkar-Mehla S, Chauhan A. A flavonoid, luteolin, cripples HIV-1 by abrogation of tat function. PLoS One. 2011;6(11):e27915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Telles S, Puthige R, Visweswaraiah NK. An Ayurvedic basis for using honey to treat herpes comment to: topical honey application vs. acyclovir for the treatment of the recurrent herpes simplex lesions. Med Sci Monit. 2007;13(11):LE17–LE17. [PubMed] [Google Scholar]
- 80. Semprini A, Singer J, Braithwaite I, et al. Kanuka honey versus aciclovir for the topical treatment of herpes simplex labialis: a randomised controlled trial. BMJ open. 2019;9(5):e026201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Littlejohn ESV. The Sensitivity of Adenovirus and Herpes simplex virus to Honey. The University of Waikato; 2009. [Google Scholar]
- 82. Johari J, Kianmehr A, Mustafa MS, Abubakar S, Zandi K. Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. Int J Mol Sci. 2012;13(12):16785–16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kassim M, Achoui M, Mansora M, Yusoff KM. The inhibitory effects of Gelam honey and its extracts on nitric oxide and prostaglandin E2 in inflammatory tissues. Fitoterapia. 2010;81(8):1196–1201. [DOI] [PubMed] [Google Scholar]
- 84. Al-Waili NS, Boni NS. Natural honey lowers plasma prostaglandin concentrations in normal individuals. J Med Food. 2003;6(2):129–133. [DOI] [PubMed] [Google Scholar]
- 85. Bai J, Luo Y, Zhanchun S, Fan W, et al. Effects and the mechanisms of chrysin on sepsis-associated acute lung injury of rats chrysin inhibits acute lung injury. Life Sci J. 2013;10(10):1052–1058. [Google Scholar]
- 86. Kilic T, Ciftci O, Cetin A, Kahraman H. Preventive effect of chrysin on bleomycin-induced lung fibrosis in rats. Inflammation. 2014;37(6):2116–2124. [DOI] [PubMed] [Google Scholar]
- 87. Liu H, Yu H1, Cao Z, et al. Kaempferol modulates autophagy and alleviates silica-induced pulmonary fibrosis. DNA Cell Biol. 2019;38(12):1418–1426. [DOI] [PubMed] [Google Scholar]
- 88. DeDiego ML, Nieto-Torres JL, Jimenez-Guardeño JM, et al. Coronavirus virulence genes with main focus on SARS-CoV envelope gene. Virus Res. 2014;194:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang L, Wang Y, Lei Z. Chrysin ameliorates ANTU-induced pulmonary edema and pulmonary arterial hypertension via modulation of VEGF and eNOs. J Biochem Mol Toxicol. 2019:33(7):e22332. [DOI] [PubMed] [Google Scholar]
- 90. Eteraf-Oskouei T, Shaseb E, Ghaffary S, Najafi M. Prolonged preconditioning with natural honey against myocardial infarction injuries. Pak J Pharm Sci. 2013;26(4):681–686. [PubMed] [Google Scholar]
- 91. Yang M, Xiong J, Zou Q, Wang D-D, Huang C-X. Chrysin attenuates interstitial fibrosis and improves cardiac function in a rat model of acute myocardial infarction. J Mol Histol. 2018;49(6):555–565. [DOI] [PubMed] [Google Scholar]
- 92. Wu J, Xun N, Yang Y, et al. Chrysin attenuates myocardial ischemia–reperfusion injury by inhibiting myocardial inflammation. RSC Adv. 2018;8(25):13739–13746. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93. Rani N, Bharti S, Bhatia J, Nag TC, Ray R, Arya DS. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem-bio Interact. 2016;250:59–67. [DOI] [PubMed] [Google Scholar]
- 94. Dong F, Zhang J, Zhu S, Lan T, Yang J, Li L. Chrysin alleviates chronic hypoxia–induced pulmonary hypertension by reducing intracellular calcium concentration in pulmonary arterial smooth muscle cells. J Cardiovasc Pharmacol. 2019;74(5):426–435. [DOI] [PubMed] [Google Scholar]
- 95. Veerappan R, Malarvili T. Chrysin pretreatment improves angiotensin system, cGMP concentration in L-NAME induced hypertensive rats. Indian J Clin Biochem. 2019;34(3):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ferreira LG, Evora PRB, Capellini VK. Effect of rosmarinic acid on the arterial blood pressure in normotensive and hypertensive rats: role of ACE. Phytomedicine. 2018;38:158–165. [DOI] [PubMed] [Google Scholar]
- 97. Yao Y, Chen L, Xiao J, et al. Chrysin protects against focal cerebral ischemia/reperfusion injury in mice through attenuation of oxidative stress and inflammation. Int J Mol Sci. 2014;15(11):20913–20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Becker RC. COVID-19 update: Covid-19-associated coagulopathy [published online May 15, 2020]. J Thromb Thrombolysis. 2020:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ravishankar D, Salamah M, Attina A, et al. Ruthenium-conjugated chrysin analogues modulate platelet activity, thrombus formation and haemostasis with enhanced efficacy. Sci Rep. 2017;7(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ahmed A, Khan RA, Azim MK, et al. Effect of natural honey on human platelets and blood coagulation proteins. Pak J Pharm Sci. 2011;24(3):389–397. [PubMed] [Google Scholar]
- 101. Lungkaphin A, Pongchaidecha A, Palee S, Arjinajarn P, Pompimon W, Chattipakorn N. Pinocembrin reduces cardiac arrhythmia and infarct size in rats subjected to acute myocardial ischemia/reperfusion. Appl Physiol Nutr Metab. 2015;40(10):1031–1037. [DOI] [PubMed] [Google Scholar]
- 102. Yang C-C, Lin C-C, Hsiao L-D, Yang C-M. Galangin inhibits thrombin-induced MMP-9 expression in SK-N-SH cells via protein kinase-dependent NF-κB phosphorylation. Int J Mol Sci. 2018;19(12):4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Choi JH, Kim KJ, Kim S. Comparative effect of quercetin and quercetin-3-O-β-d-glucoside on fibrin polymers, blood clots, and in rodent models. J Biochem Mol Toxicol. 2016;30(11):548–558. [DOI] [PubMed] [Google Scholar]
- 104. Janssen K, Mensink RP, Cox FJ, et al. Effects of the flavonoids quercetin and apigenin on hemostasis in healthy volunteers: results from an in vitro and a dietary supplement study. Am J Clin Nutr. 1998;67(2):255–262. [DOI] [PubMed] [Google Scholar]
- 105. Zou Z, Xu L, Tian J. Antithrombotic and antiplatelet effects of rosmarinic acid, a water-soluble component isolated from radix Salviae miltiorrhizae (Danshen). Yao xue xue bao; Acta pharmaceutica Sinica. 1993;28(4):241–245. [PubMed] [Google Scholar]
- 106. Lu Y, Li Q, Liu Y-Y, et al. Inhibitory effect of caffeic acid on ADP-induced thrombus formation and platelet activation involves mitogen-activated protein kinases. Sci Rep. 2015;5:13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Biesinger S, Michaels HA, Quadros AS, et al. A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. Eur J Clin Nutr. 2016;70(1):10–16. [DOI] [PubMed] [Google Scholar]
- 108. Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009;53(1):115–128. [DOI] [PubMed] [Google Scholar]
- 109. Olagnier D, Farahani E, Thyrsted J, et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Commun. 2020;11(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ogawa T, Ishitsuka Y, Nakamura Y, et al. Honey and chamomile activate keratinocyte antioxidative responses via the KEAP1/NRF2 system. Clin Cosmet Investig Dermatol. 2020;13:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Erejuwa OO, Sulaiman SA, Ab Wahab MS, Sirajudeen KNS, Salleh S, Gurtu S. Honey supplementation in spontaneously hypertensive rats elicits antihypertensive effect via amelioration of renal oxidative stress. Oxid Med Cell Longev. 2012;2012:374037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sun LP, Shi FF, Zhang WW, et al. Antioxidant and anti-inflammatory activities of safflower (Carthamus tinctorius L.) honey extract. Foods. 2020;9(8):1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yang Z, Guan Y, Li J, Li L, Li Z. Chrysin attenuates carrageenan-induced pleurisy and lung injury via activation of SIRT1/NRF2 pathway in rats. Eur J Pharmacol. 2018;836:83–88. [DOI] [PubMed] [Google Scholar]
- 114. Huang CS, Lii CK, Lin AH, et al. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch Toxicol. 2013;87(1):167–178. [DOI] [PubMed] [Google Scholar]


