Abstract
Background
Alzheimer's disease (AD) is one of the most prevalent neurodegenerative disorders. Enhancing hippocampal neurogenesis by promoting proliferation and differentiation of neural stem cells (NSCs) is a promising therapeutic strategy for AD. 20(S)-protopanaxadiol (PPD) and oleanolic acid (OA) are small, bioactive compounds found in ginseng that can promote NSC proliferation and neural differentiation in vitro. However, it is currently unknown whether PPD or OA can attenuate cognitive deficits by enhancing hippocampal neurogenesis in vivo in a transgenic APP/PS1 AD mouse model. Here, we administered PPD or OA to APP/PS1 mice and monitored the effects on cognition and hippocampal neurogenesis.
Methods
We used the Morris water maze, Y maze, and open field tests to compare the cognitive capacities of treated and untreated APP/PS1 mice. We investigated hippocampal neurogenesis using Nissl staining and BrdU/NeuN double labeling. NSC proliferation was quantified by Sox2 labeling of the hippocampal dentate gyrus. We used western blotting to determine the effects of PPD and OA on Wnt/GSK3β/β-catenin pathway activation in the hippocampus.
Results
Both PPD and OA significantly ameliorated the cognitive impairments observed in untreated APP/PS1 mice. Furthermore, PPD and OA significantly promoted hippocampal neurogenesis and NSC proliferation. At the mechanistic level, PPD and OA treatments resulted in Wnt/GSK-3β/β-catenin pathway activation in the hippocampus.
Conclusion
PPD and OA ameliorate cognitive deficits in APP/PS1 mice by enhancing hippocampal neurogenesis, achieved by stimulating the Wnt/GSK-3β/β-catenin pathway. As such, PPD and OA are promising novel therapeutic agents for the treatment of AD and other neurodegenerative diseases.
Keywords: Alzheimer's disease, 20(S)-protopanaxadiol, oleanolic acid, cognitive deficits, hippocampal neurogenesis
1. Introduction
As a result of the increasingly large aging population, neurodegenerative diseases have become one of the primary threats to human health [1]. Alzheimer's disease (AD) is the most prevalent neurodegenerative disease, currently affecting over 50 million people worldwide. However, by 2050, this number is expected to rise to over 150 million people [2]. AD is characterized by deficits in both cognition and memory. Despite the great advances made in pharmacotherapeutics, there is still no effective strategy to prevent or treat AD.
AD pathogenesis is predominantly driven by neuronal death or dysfunction in the brain. A reasonable therapeutic strategy for AD would, therefore, be to promote hippocampal neurogenesis in situ [3]. Adult hippocampal neurogenesis is a recapitulation of ontogenic processes and involves the generation of new neurons from neural stem cells (NSCs) located in the subgranular zone, which migrate to the hippocampus [4]. However, NSCs have a limited ability to self-renew and differentiate under normal conditions [5] and must be induced by a stimulus to successfully generate neurons. Neurogenesis in the adult hippocampus is closely associated with memory and learning [6] and occurs at a substantial rate to ensure hippocampal plasticity across the entire lifespan [7]. Recent studies have shown that inducing adult hippocampal neurogenesis using exogenous genetic or drug methods can markedly improve cognition in AD models [8].
20(S)-protopanaxadiol (PPD) and oleanolic acid (OA) are two of the active compounds found in ginseng, which has been extensively used to treat AD in Asia. Previous work conducted in our lab found that both PPD and OA can enhance NSC proliferation and neural differentiation in vitro by targeting GSK-3β in the Wnt/GSK-3β/β-catenin signaling pathway [9,10]. These findings indicate that PPD and OA have the potential to promote neurogenesis in vivo. Being small molecules, both PPD and OA can penetrate the blood-brain barrier (BBB) [11,12], rendering them ideal compounds to develop as therapeutic agents for treating AD.
The aim of this study was to determine whether PPD or OA, extracted from ginseng, can reduce AD-associated pathological changes and memory deficits by promoting hippocampal neurogenesis in an AD mouse model. We found that both PPD and OA ameliorated cognitive deficits, enhanced neurogenesis and activated the Wnt/GSK-3β/β-catenin pathway in APP/PS1 mice. Stimulation of hippocampal neurogenesis could, therefore, be a promising therapeutic strategy for AD and other neurodegenerative diseases.
2. Materials and methods
2.1. Reagents and antibodies
PPD (purity assayed by HPLC: 98.59%) and OA (purity assayed by HPLC: 98.33%) were obtained from Must Bio-Technology Co., Ltd (Chengdu, China). Anti-β-actin antibody, dimethyl sulfoxide (DMSO) and 5-bromo-2′-deoxyuridine (BrdU) were obtained from Sigma-Aldrich (St. Louis, MO, USA). 4,6-Diamidino-2-phenylindole (DAPI) was purchased from Roche Ltd. (Basel, Switzerland). Anti-p-GSK-3β(Ser9), anti-GSK-3β, anti-non-p-β-catenin (active), anti-β-catenin, and anti-BrdU antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). The anti-NeuN and anti-Sox2 antibodies were obtained from EMD Millipore Corporation (Temecula, CA, USA).
2.2. Animals and treatments
The experimental animal procedures were officially authorized by the Department of Health of the Government of the Hong Kong Special Administrative Region. The animal studies were conducted in light of the guidelines and security standards of the Committee on the Use of Human and Animal Subjects in Teaching and Research. Male APP/PS1 double-transgenic mice were obtained from the Experimental Animal Center of Guangzhou University of Chinese Medicine (Guangzhou, China). Age- and sex-matched wild-type (WT) littermates were used as controls. The animals were sustained in a humidity- and climate-controlled environment with food and water ad libitum and a 12/12 h light/dark cycle. The genotypes of the APP/PS1 transgenic mice were determined by standard polymerase chain reaction (PCR) analysis (Supplementary Fig. S1).
To monitor the effects of PPD and OA on neurogenesis, male 6-month-old APP/PS1 and WT mice were randomly divided into the following four groups (n = 6 mice per group): WT, APP/PS1, APP/PS1 + PPD (10 mg/kg), and APP/PS1 + OA (10 mg/kg). The doses of PPD/OA were selected according to our preliminary findings in vivo (Supplementary Fig. S2). PPD and OA were dissolved in normal saline solution supplemented with 2% Tween 80 and then injected intraperitoneally (IP) once daily for 28 consecutive days. The untreated APP/PS1 and WT mice received the equivalent volume of physiological saline. BrdU (50 mg/kg) was administrated as a single injection (IP) once daily for 7 consecutive days prior to the behavioral analyses.
2.3. Morris water maze test
The Morris water maze (MWM) test was carried out as previously described [13]. Briefly, the water maze pool (120 cm in diameter) was filled with water (24 ± 2°C). The pool was equally divided into four imaginary quadrants and starting locations were distributed throughout the pool. The hidden platform (10 cm in diameter) was in the center of one quadrant and hidden 1 cm under the water surface. A video camera, mounted directly above the pool, was used to record the path taken by each mouse. In the acquisition phase, each mouse was trained for 5 days (4 consecutive trials per day with 10 min intertrial intervals). Here, each mouse was released across the four quadrants at random, and allowed to find the hidden platform within a maximum of 60 sec. If the mouse failed to locate the platform within 60 sec, it was guided to it and allowed to stay on the platform for 10 sec.
Spatial memory was then assessed by a probe trial on day 7 whereby the platform was removed, and each mouse was permitted to explore for 60 sec. A Smart 3.0 digital tracking system (Panlab, Barcelona, Spain) recorded the time spent in the target area, the distance traveled, and the number of crossings through the platform location.
2.4. Y maze test
A Y maze test was performed to assess working memory abilities [14]. The Y maze consisted of three identical arms (30 cm long, 7 cm wide, and 15 cm high) that were separated by 120°. During the training phase, one arm was blocked (“novel arm”) but the mouse was allowed to freely explore the other two open arms (“start arm” and “other arm”) for 5 min. The mouse was returned to its cage for 1 h and all three arms were opened in the maze. The mouse was then placed in the start arm again and permitted to explore for a further 3 min. The distance traveled, the time spent, and the number of times the mouse entered the novel arm were recorded with a camera and a Smart 3.0 digital tracking system. The apparatus was cleaned with 75% ethanol after each trial.
2.5. Open field test
The open field test (OFT) assesses locomotor activity and endophenotypes of anxiety [15]. The open field arena (consisting of a 50 cm × 50 cm × 50 cm box) included a central square of 25 cm × 25 cm, which was defined as the central area. In turn, each mouse was placed at the center of the arena and permitted to explore for 5 min to acclimate. Then, the total distance traveled and the time spent in the central area were recorded using the Smart 3.0 software system over the course of the next 5 min. The test arena was cleaned with 75% ethanol after each trial.
2.6. Tissue processing
After behavioral testing, all mice were anesthetized and transcardially perfused with cold saline. The brains were rapidly removed and divided into two parts, the left and right hemispheres. The left hemisphere, used for histopathological examinations, was immersed in fixative for 48 h and then embedded in paraffin and microsectioned at a thickness of 5 μm. The right hemisphere was collected for western blot analyses.
2.7. Nissl staining
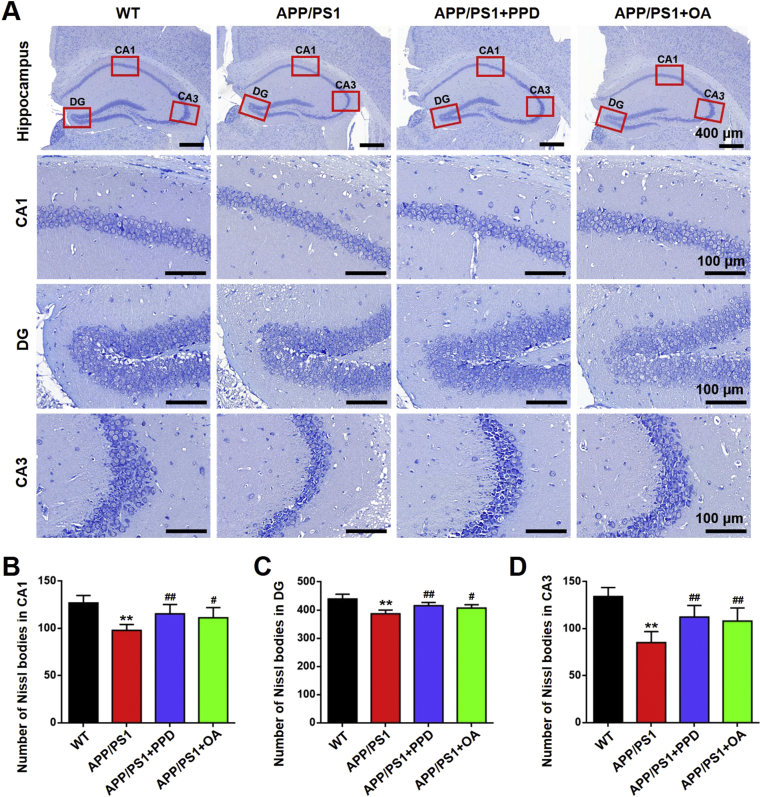
The left hemisphere coronal sections were stained using 0.5% Cresyl Violet Acetate (Beyotime, Beijing, China). The severity of neural damage was assessed by counting the number of surviving neurons in the CA1, CA3, and dentate gyrus (DG) areas of the hippocampus under a microscope (Nikon, Tokyo, Japan). Cells with cytoplasmic Nissl staining, loose chromatin, and prominent nucleoli were considered healthy neurons. The data in Fig. 3B–D represent the number of cells per field.
Fig. 3.
PPD or OA administration alleviates neuron loss in the hippocampus of APP/PS1 mice. (A) Representative images of Nissl staining in the CA1, DG, and CA3 regions of the hippocampus in WT mice, APP/PS1 mice, and APP/PS1 mice treated with PPD or OA. The red rectangles indicate the CA1, DG, and CA3 regions. Scale bars: 400 μm in the hippocampus; 100 μm in CA1, DG, and CA3 regions. (B-D) Quantification of the Nissl bodies in the hippocampal CA1 (B), DG (C), and CA3 (D) regions of WT mice, APP/PS1 mice, and APP/PS1 mice treated with PPD or OA. n = 5–6 mice per group. Error bars represent the mean ± SD. ∗∗p < 0.01, compared with the WT group; #p < 0.05 and ##p < 0.01, compared with the APP/PS1 group. PPD, 20(S)-protopanaxadiol; OA, oleanolic acid; WT, wild type; DG, dentate gyrus; CA, cornu ammonis; SD, standard deviation.
2.8. Immunofluorescent staining
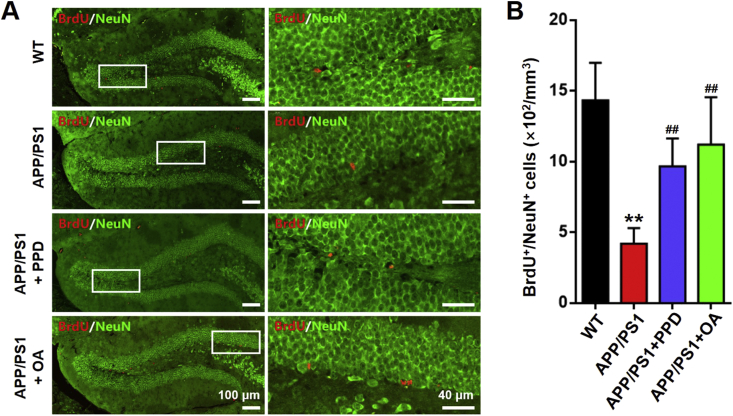
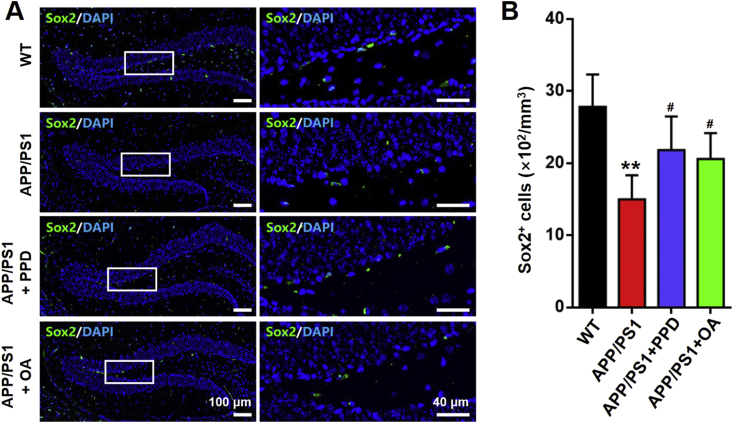
Selected coronal sections were incubated with the appropriate primary antibodies overnight at 4°C, including anti-BrdU to identify proliferating cells, anti-NeuN to identify neurons, and anti-Sox2 to identify NSCs. The sections were then incubated with appropriate secondary antibodies conjugated to fluorescein isothiocyanate (FITC). DAPI (1 μg/mL) was used to counterstain the nuclei. To allow semi-quantitative analysis of the immunostained cells, an image of the hippocampal DG region was captured under a confocal microscope (FluoView FV1000, Olympus, Tokyo, Japan). All stained cells in the image were quantified, including BrdU+/NeuN+ double-stained cells and Sox2+ cells. The data in Fig. 4, Fig. 5B represent the average number of cells per mm3, with 5–6 sections analyzed per mouse.
Fig. 4.
PPD or OA administration enhances neurogenesis in the hippocampus of APP/PS1 mice. (A) Representative NeuN (green) and BrdU (red) staining in the dentate gyrus of brain sections from WT mice, APP/PS1 mice, and APP/PS1 mice treated with PPD or OA. Scale bars: 100 μm in the left panels, 40 μm in the right panels. (B) Quantification of BrdU+/NeuN+ cells in A. n = 5–6 mice per group. Error bars represent the mean ± SD. ∗∗p < 0.01, compared with the WT group; ##p < 0.01, compared with the APP/PS1 group. PPD, 20(S)-protopanaxadiol; OA, oleanolic acid; BrdU, 5-bromo-2′-deoxyuridine; WT, wild type; SD, standard deviation.
Fig. 5.
PPD or OA administration elevates the number of neural stem cells in the hippocampus of APP/PS1 mice. (A) Sox2 (green) and DAPI (blue) staining in the dentate gyrus of brain sections from WT mice, APP/PS1 mice, and APP/PS1 mice treated with PPD or OA. Scale bars: 100 μm in the left panels, 40 μm in the right panels. (B) Quantification of Sox2+ cells as in A. n = 5–6 mice per group. Error bars represent the mean ± SD. ∗∗p < 0.01, compared with the WT group; #p < 0.05, compared with the APP/PS1 group. PPD, 20(S)-protopanaxadiol; OA, oleanolic acid; WT, wild type; DAPI, 4,6-diamidino-2-phenylindole; SD, standard deviation.
2.9. Western blotting
Hippocampal tissue from the right hemisphere was harvested and homogenized. Proteins were then extracted on ice using a protein extraction reagent supplemented with a protease inhibitor (Novagen, Madison, WI, USA). Equal amounts of protein were separated using 10% SDS-polyacrylamide gel electrophoresis and then transferred to a PVDF membrane (Bio-Rad Laboratories). The membranes were blocked in 5% non-fat milk for 1 h and were subsequently incubated with appropriate concentrations of the following primary antibodies at 4°C overnight: p-GSK-3β(Ser9) (1:1000), GSK-3β (1:1000), non-p-β-catenin (active) (1:1000), β-catenin (1:1000), and β-actin (1:5000, internal loading control). The membranes were then incubated with the appropriate secondary antibody conjugated with horseradish peroxidase (HRP) for 1 h. Chemiluminescent detection was performed using a chemiluminescence detection kit (AbFrontier, South Korea). Images were captured using a ChemiDoc Touch imaging system (Bio-Rad Laboratories) and semiquantitative densitometric analysis of the protein bands was carried out using ImageJ (NIH, USA).
2.10. Statistical analyses
The quantitative results are described as mean ± standard deviation (SD). Data were statistically analyzed by one-way analysis of variance (ANOVA), and p < 0.05 was considered as significant. Image analysis and cell counting were performed in ImageJ. All graphs were produced using GraphPad Prism 6.0 software (San Diego, CA, USA).
3. Results
3.1. PPD and OA ameliorate cognitive decline in APP/PS1 mice
APP/PS1 transgenic mice expressing the mutant APPswe and PSEN1dE9 genes were used as a model for AD. These mice develop cognitive impairments in an age-dependent manner [16]. The genotypes of the APP/PS1 transgenic mice in this study were identified using PCR (Supplementary Fig. S1). Preliminary experiments found that both PPD and OA could ameliorate the cognitive decline of APP/PS1 mice in a dose-dependent manner. Specifically, 10 or 20 mg/kg of either PPD or OA significantly improved the cognition in APP/PS1 mice, while 5 mg/kg was not sufficient to achieve these effects (Supplementary Fig. S2). Additionally, as there was no obvious significant difference in therapeutic effect between the 10 and 20 mg/kg groups, a dosage of 10 mg/kg was chosen for subsequent experiments to reduce the likelihood of any adverse effects (Supplementary Fig. S2).
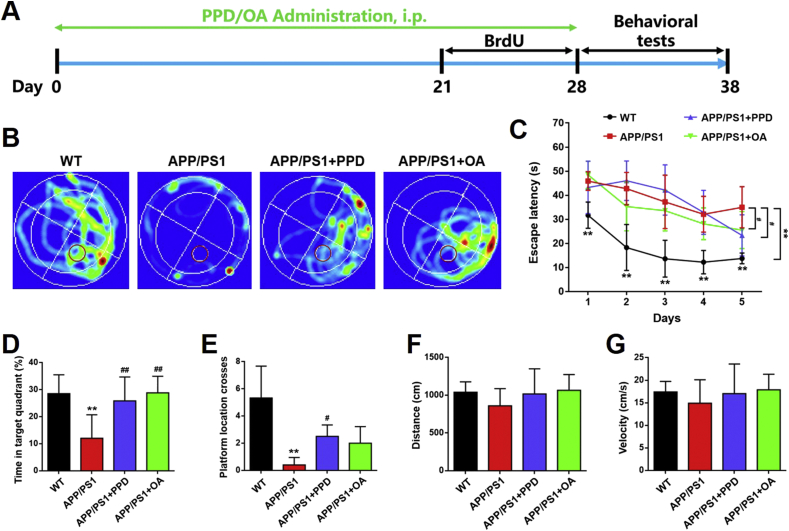
After PPD or OA treatment (10 mg/kg), the mice completed three behavioral tests (the MWM, the Y maze test, and the OFT) to assess whether PPD or OA can ameliorate the cognitive deficits seen in APP/PS1 mice (Fig. 1A). During the MWM tests, the trace of each mouse was recorded using a camera (Fig. 1B). After training for 5 consecutive days, we found that the untreated APP/PS1 mice displayed significantly impaired learning and spatial orientation during the submerged platform phase compared with the WT mice. These deficits were alleviated in PPD- or OA-treated APP/PS1 mice (Fig. 1C). After removing the platform, the APP/PS1 mice treated with PPD or OA spent a significantly longer time in the target quadrant (which originally contained the platform) than the untreated APP/PS1 mice (Fig. 1D). The PPD- or OA-treated mice also crossed the platform location more frequently than the untreated APP/PS1 mice (Fig. 1E). Interestingly, although not statistically significant, the treated APP/PS1 mice showed a tendency to swim further and at a higher velocity than the untreated APP/PS1 mice (Fig. 1F and G).
Fig. 1.
PPD or OA administration enhances performance in the Morris water maze by APP/PS1 mice. (A) The timeline for the experimental procedure. (B-G) The Morris water maze test from WT mice and APP/PS1 mice treated or not treated with PPD or OA. (B) Representative occupancy plots showing the areas in which the mice spent the most time during the test. The red circle represents the platform. (C) The mean escape latency across 5 consecutive test days in mice. (D) The percentage of time spent in the target quadrant by mice. (E) The number of platform location crosses by mice. The total distance traveled (F) and velocity (G) of the mice during the test session. n = 5–6 mice per group. Error bars represent the mean ± SD. ∗∗p < 0.01, compared with the WT group; #p < 0.05 and ##p < 0.01, compared with the APP/PS1 group. PPD, 20(S)-protopanaxadiol; OA, oleanolic acid; IP, intraperitoneal; BrdU, 5-bromo-2′-deoxyuridine; WT, wild type; SD, standard deviation.
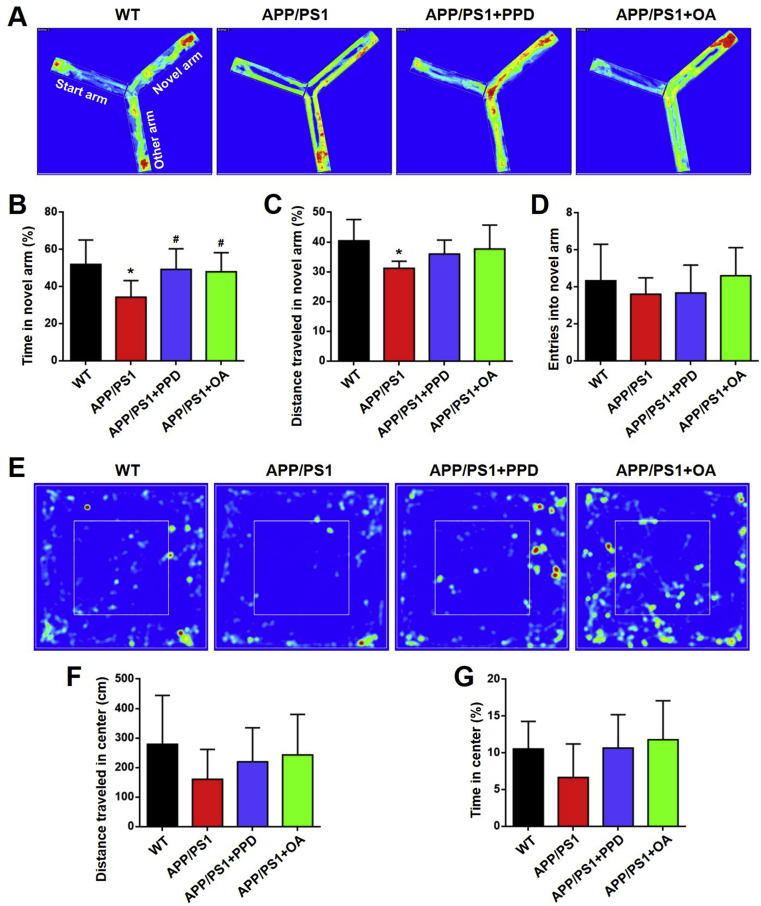
The PPD- or OA-treated APP/PS1 mice spent a significantly longer time in the novel arm of the Y maze than the untreated APP/PS1 mice (Fig. 2A and B). The PPD- or OA-treated mice also showed a tendency to move further in the novel arm and enter the novel arm more times than the untreated mice (Fig. 2C and D). As anxiety is a clinical symptom of AD, we used the OFT to monitor anxiety endophenotypes in APP/PS1 mice. The PPD- or OA-treated APP/PS1 mice displayed a tendency to move further and spend more time in the central area than the untreated APP/PS1 mice (Fig. 2E–G).
Fig. 2.
PPD or OA administration enhances the performance in the Y maze and open field test by APP/PS1 mice. (A-D) The Y maze test for WT mice, APP/PS1 mice, and APP/PS1 mice treated with PPD or OA. (A) Representative occupancy plots showing the areas in which the mice spent the most time during the Y maze test. (B) The percentage of time spent in the novel arm of the Y maze. (C) The total distance traveled in the novel arm of the Y maze. (D) The frequency of entries into the novel arm of the Y maze. (E-G) The open field test for WT mice, APP/PS1 mice, and APP/PS1 mice treated with PPD or OA. (E) Representative occupancy plots showing the areas in which the mice spent the most time during the open field test. (F) The mean distance traveled within the center of the arena during the open field test. (G) The mean time spent within the center of the arena during the open field test. n = 5–6 mice per group. Error bars represent the mean ± SD. ∗p < 0.05, compared with the WT group; #p < 0.05, compared with the APP/PS1 group. PPD, 20(S)-protopanaxadiol; OA, oleanolic acid; WT, wild type; SD, standard deviation.
The improvements observed for the treated mice across all behavioral tests suggest that cognitive impairment and symptoms of anxiety can be ameliorated in APP/PS1 mice after treatment with PPD or OA.
3.2. PPD and OA enhance hippocampal neurogenesis in APP/PS1 mice
We next investigated whether PPD and OA confer their beneficial effects on cognition by enhancing hippocampal neurogenesis. To do so, we performed Nissl staining to highlight any neuropathological changes in the CA1, CA3, and DG regions of the hippocampus in APP/PS1 mice. Compared with the WT mice, the number of Nissl bodies was significantly decreased in the CA1, DG and CA3 regions in the APP/PS1 mice. Following 4 weeks of PPD or OA administration, the PPD- or OA-treated APP/PS1 mice exhibited a significantly increased number of healthy neurons in these regions compared with their untreated counterparts (Fig. 3A–D). These results indicate that PPD and OA might promote hippocampal neurogenesis in APP/PS1 mice. We then examined the expression of the mature neuronal marker NeuN in BrdU-retaining cells. We found that the untreated APP/PS1 mice had a significantly lower percentage of BrdU+/NeuN+ double-positive cells compared with the WT mice (Fig. 4). A significantly higher proportion of BrdU+/NeuN+ cells was identified in the hippocampal regions of the PPD- or OA-treated APP/PS1 mice compared with the untreated APP/PS1 mice (Fig. 4). Together, these results support the hypothesis that both PPD and OA treatment can significantly enhance hippocampal neurogenesis in APP/PS1 mice.
3.3. PPD and OA promote hippocampal NSC proliferation in APP/PS1 mice
Adult hippocampal neurogenesis is the generation of new neurons from NSCs in the adult brain [4]. The subgranular zone of the hippocampal DG is the most important and well-characterized stem cell-containing region in the adult mammalian brain [17]. We next monitored NSC proliferation in the hippocampal DG by quantifying the number of Sox2+ cells. As expected, the number of Sox2+ cells was markedly reduced in the hippocampal DG of the APP/PS1 mice compared with the WT mice. In contrast, the number of Sox2+ cells was significantly increased in the hippocampal DG in both the PPD- and OA-treated APP/PS1 mice compared with their untreated counterparts. This indicates that exposure to PPD or OA can promote hippocampal NSC proliferation (Fig. 5).
3.4. PPD and OA activate the Wnt/GSK-3β/β-catenin pathway in the hippocampus of APP/PS1 mice
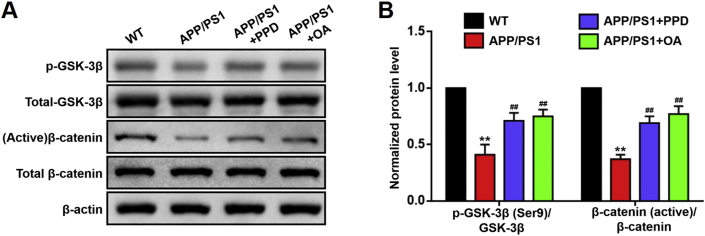
The Wnt/GSK-3β/β-catenin pathway regulates the proliferation and differentiation of NSCs [18]. Inactivation of GSK-3β by phosphorylation at serine 9 [p-GSK-3β(Ser9)] leads to an accumulation of unphosphorylated active β-catenin; this accumulation activates the Wnt pathway [19]. We have previously confirmed that both PPD and OA can activate the Wnt/GSK-3β/β-catenin pathway in NSCs in vitro [9,10]. In the present study, we monitored the activity of this pathway in vivo, in the hippocampus of APP/PS1 mice. Consistent with the previous study [20], we confirmed that the activation of the Wnt/GSK-3β/β-catenin pathway was suppressed in the hippocampus of APP/PS1 mice compared with that of WT mice (Fig. 6). PPD and OA administration significantly upregulated the p-GSK-3β(Ser9)/GSK-3β and β-catenin (active)/β-catenin expression ratios in the hippocampus of the APP/PS1 mice (Fig. 6). This significant increase in the activity of the Wnt/GSK-3β/β-catenin pathway in the PPD- and OA-treated APP/PS1 mice, together with enhanced NSC proliferation (Section 3.3), suggests that PPD- and OA-mediated hippocampal neurogenesis in APP/PS1 mice is dependent on Wnt/GSK-3β/β-catenin pathway activation.
Fig. 6.
PPD or OA administration activates Wnt/GSK-3β/β-catenin pathway in the hippocampus of APP/PS1 mice. (A, B) Western blot analysis (A) and normalized protein levels (B) of the indicated protein markers of the Wnt/GSK-3β/β-catenin pathway in the hippocampus of WT mice, APP/PS1 mice, and APP/PS1 mice treated with PPD or OA. PPD and OA significantly increased the p-GSK-3β(Ser9)/GSK-3β and β-catenin (active)/β-catenin ratios in the hippocampus of APP/PS1 mice. n = 5–6 mice per group. Error bars represent the mean ± SD. ∗∗p < 0.01, compared with the WT group; ##p < 0.05, compared with the APP/PS1 group. PPD, 20(S)-protopanaxadiol; OA, oleanolic acid; WT, wild type; SD, standard deviation.
4. Discussion
Alzheimer's disease, the most prevalent neurodegenerative disorder worldwide, is considered to pose a significant threat to human health. The incidence of AD is expected to dramatically increase in the coming decades, in line with the rapidly increasing aging population [21]. Current pharmacotherapeutics for AD can only alleviate the symptoms and do not offer a permanent cure [22], and there are no effective strategies to prevent or treat AD.
Many factors are associated with the onset and the progression of AD. Dysregulation of amyloid-β (Aβ) levels leading to the formation of senile plaques, which contain deposits of Aβ, has long been considered the most core pathological cause of AD. Most of the highly anticipated clinical trials of drugs that target Aβ have, however, failed, suggesting that the pathogenesis of AD is more complex and is not limited to Aβ deposition alone. The clinical manifestation of AD is predominantly driven by neuronal loss in the hippocampus [23]. It has been reported that Aβ induces neuronal cell death, inhibits the proliferation of NSCs and suppresses the formation of new neurons in the hippocampus [24]. Therefore, Aβ deposition is thought to be one of the main causes of impaired hippocampal neurogenesis, making it an important neuropathological hallmark in patients with AD [25].
Another important pathological cause of AD is hyperphosphorylated tau. Autopsies and neuroimaging studies in AD patients indicate that Aβ deposition precedes tau pathology [26]. In AD, tau becomes abnormally phosphorylated and forms inclusions in the brain, leading to synapse loss and neuronal cell death [26]. Tau plays a critical role in regulation of hippocampal neurogenesis in the adult brain [27].
Lastly, in recent decades neuroinflammation has been increasingly regarded as the third core neuropathological feature of AD. Neuroinflammation plays an important role in the pathogenesis of AD. The neuroinflammatory process is responsive to neuronal loss and declining hippocampal neurogenesis, leading to dysregulation of hippocampus-dependent behaviors [28].
In addition to the pathologic factors discussed above, age is the greatest risk factor for developing AD, with most cases of AD occurring in those over 65 years of age [29]. Hippocampal neurogenesis is thought to persist throughout the course of life; however, a remarkable decline in neurogenesis occurs with age [30]. Enhancing adult hippocampal neurogenesis may, thus, be able to prevent or limit age-related neurodegenerative diseases, such as AD.
The above studies suggest that a decrease in hippocampal neurogenesis is a pathogenic mechanism brought about by various contributing factors (Aβ plaques, tau hyperphosphorylation, neuroinflammation, age etc.) in AD. As a result, enhancing adult hippocampal neurogenesis itself directly, rather than targeting one of these contributing factors alone, may be a promising therapeutic strategy to treat AD. In this study, we demonstrated that promoting hippocampal neurogenesis with PPD or OA treatment significantly ameliorates cognitive deficits in an AD mouse model. However, as the underlying molecular pathologies of AD, such as Aβ deposition, are not fully eliminated with this approach, further research is needed to explore whether the cognitive deficits associated with AD will recur after cessation of the medication (PPD/OA).
Vascular dementia is the second most common cause of dementia, behind AD. Both AD and vascular dementia have emerged as the leading causes of age-related cognitive impairment [31]. Vascular dementia occurs as a result of an inability of cerebral blood vessels to dilate and match blood flow with the metabolic needs of the brain, causing hypoperfusion, neuronal death, and dementia [32].
Several factors contribute to the development of vascular dementia, including Aβ deposition and cerebral amyloid angiopathy (CAA). Elevated Aβ is known to increase vulnerability to ischemic brain injury [33]. CAA itself is also characterized by Aβ deposition in the leptomeningeal and cortical blood vessels, and is an age-dependent risk factor for ischemic injury and contributes to cerebrovascular dysfunction leading to cognitive impairment [34]. The process of Aβ accumulation in CAA is still not fully understood. A recent study reported that overexpressing Aβ in neurons in the brain can lead to the development of CAA, suggesting that the Aβ is likely derived from the brain during the development of CAA, rather than from the blood [35]. Although the pathological mechanisms underlying the development of CAA and vascular dementia are not fully understood, the associated neuronal death and impaired neurogenesis play a crucial role in the development and progression of dementia. The ability to enhance neurogenesis may thus have important implications for the treatment of CAA and vascular dementia. It has been reported that neuronal progenitors can migrate to areas of infarction and differentiate into neurons in patients with vascular dementia, indicating that enhancing neurogenesis might be a novel treatment approach [36]. Unlike the hippocampal neurogenesis generated from NSCs located in the subgranular zone seen in AD, the NSCs contributing to neurogenesis during vascular dementia mostly originate from the subventricular zone [36]. Previous studies in our lab show that that both PPD and OA can promote NSC proliferation and neuronal differentiation by targeting GSK-3β in vitro [9,10], suggesting that these compounds might also have the ability to promote neurogenesis originating from the subventricular zone in the treatment of vascular dementia. However, extensive research evidence relating to neurogenesis in vascular dementia is still lacking.
In conclusion, we have demonstrated that both PPD and OA administration can significantly ameliorate cognitive decline in APP/PS1 mice. We propose that these improvements in cognition might be due, at least in part, to an elevated level of hippocampal neurogenesis driven by Wnt/GSK-3β/β-catenin pathway activation. Additionally, the dose of PPD and OA (10 mg/kg) used in our study is lower than the doses of ginsenosides required for anti-AD effects reported in previous studies [37,38]. The reason for this greater therapeutic efficiency might be because PPD and OA, which are both aglycones of ginsenosides, are small molecules and can cross the BBB more efficiency than larger ginsenosides. These findings can inform future work to identify novel therapeutics based on PPD and OA that can enhance hippocampal neurogenesis in patients with AD and other hippocampus-dependent neurodegenerative behavioral disorders.
Declaration of competing interest
The authors declare that they have no conflicts of interests.
Acknowledgements
This study was financially supported by the grants of National Natural Science Foundation of China (No. 81703728).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jgr.2020.07.003.
Contributor Information
Ken Kin-Lam Yung, Email: kklyung@hkbu.edu.hk.
Shiqing Zhang, Email: shiqingzhang@hkbu.edu.hk.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Stayte S., Vissel B. New hope for devastating neurodegenerative disease. Brain. 2017;140:1177–1179. doi: 10.1093/brain/awx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Disease International . Alzheimer’s Disease International; London: 2019. World Alzheimer Report 2019: attitudes to dementia. [Google Scholar]
- 3.Bray N. Neurodegenerative disease: towards transplant therapy. Nat Rev Neurosci. 2017;18:572. doi: 10.1038/nrn.2017.120. [DOI] [PubMed] [Google Scholar]
- 4.Semënov M.V. Adult hippocampal neurogenesis is a developmental process involved in cognitive development. Front Neurosci. 2019;13:159. doi: 10.3389/fnins.2019.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bizen N., Inoue T., Shimizu T., Tabu K., Kagawa T., Taga T. A Growth-promoting signaling component Cyclin D1 in neural stem cells has antiastrogliogenic function to execute self-renewal. Stem Cells. 2014;32:1602–1615. doi: 10.1002/stem.1613. [DOI] [PubMed] [Google Scholar]
- 6.Deng W., Aimone J.B., Gage F.H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boldrini M., Fulmore C.A., Tartt A.N., Simeon L.R., Pavlova I., Poposka V., Rosoklija G.B., Stankov A., Arango V., Dwork A.J. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi S.H., Tanzi R.E. Is Alzheimer's disease a neurogenesis disorder? Cell Stem Cell. 2019;25:7–8. doi: 10.1016/j.stem.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Lin K.L., Liu B., Lim S.L., Fu X.Q., Sze S.C.W., Yung K.K.L., Zhang S.Q. 20(S)-protopanaxadiol promotes the migration, proliferation and differentiation of neural stem cells by targeting GSK-3β in the Wnt/GSK-3β/β-catenin pathway. J Ginseng Res. 2020;44:475–482. doi: 10.1016/j.jgr.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S.Q., Lin K.L., Law C.Y., Liu B., Fu X.Q., Tse W.S., Wong S.S.M., Sze S.C.W., Yung K.K.L. Oleanolic acid enhances neural stem cell migration, proliferation, and differentiation in vitro by inhibiting GSK3β activity. Cell Death Discov. 2018;5:48. doi: 10.1038/s41420-018-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musende A.G., Eberding A., Wood C.A., Adomat H., Fazli L., Hurtado-Coll A., Jia W., Bally M.B., Guns E.S.T. A novel oral dosage formulation of the ginsenoside aglycone protopanaxadiol exhibits therapeutic activity against a hormone-insensitive model of prostate cancer. Anticancer Drugs. 2012;23:543–552. doi: 10.1097/CAD.0b013e32835006f5. [DOI] [PubMed] [Google Scholar]
- 12.Martín R., Carvalho-Tavares J., Hernández M., Arnés M., Ruiz-Gutiérrez V., Nieto M.L. Beneficial actions of oleanolic acid in an experimental model of multiple sclerosis: a potential therapeutic role. Biochem Pharmacol. 2010;79:198–208. doi: 10.1016/j.bcp.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Park J.H., Ju Y.H., Choi J.W., Song H.J., Jang B.K., Woo J., Chun H., Kim H.J., Shin S.J., Yarishkin O. Newly developed reversible MAO-B inhibitor circumvents the shortcomings of irreversible inhibitors in Alzheimer's disease. Sci Adv. 2019;5 doi: 10.1126/sciadv.aav0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Audrain M., Fol R., Dutar P., Potier B., Billard J.M., Flament J., Alves S., Burlot M.A., Dufayet-Chaffaud G., Bemelmans A.P. Alzheimer's disease-like APP processing in wild-type mice identifies synaptic defects as initial steps of disease progression. Mol Neurodegener. 2016;11:5. doi: 10.1186/s13024-016-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Zhu T., Wang M., Zhang F., Zhang G., Zhao J., Zhang Y., Wu E., Li X. Icariin attenuates M1 activation of microglia and Aβ plaque accumulation in the hippocampus and prefrontal cortex by up-regulating PPARγ in restraint/isolation-stressed APP/PS1 mice. Front Neurosci. 2019;13:291. doi: 10.3389/fnins.2019.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han J., Oh J.P., Yoo M., Cui C.H., Jeon B.M., Kim S.C., Han J.H. Minor ginsenoside F1 improves memory in APP/PS1 mice. Mol Brain. 2019;12:77. doi: 10.1186/s13041-019-0495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonçalves J.T., Schafer S.T., Gage F.H. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Selvaraj P., Xiao L., Lee C., Murthy S.R.K., Cawley N.X., Lane M., Merchenthaler I., Ahn S., Loh Y.P. Neurotrophic factor-α1: a key Wnt-β-catenin dependent anti-proliferation factor and ERK-Sox9 activated inducer of embryonic neural stem cell differentiation to astrocytes in neurodevelopment. Stem Cells. 2017;35:557–571. doi: 10.1002/stem.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H.Y. Instructive role of Wnt/β-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- 20.Zhu L., Chi T., Zhao X., Yang L., Song S., Lu Q., Ji X., Liu P., Wang L., Zou L. Xanthoceraside modulates neurogenesis to ameliorate cognitive impairment in APP/PS1 transgenic mice. J Physiol Sci. 2018;68:555–565. doi: 10.1007/s12576-017-0561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu C., Fratiglioni L. Aging without dementia is achievable: current evidence from epidemiological research. J Alzheimers Dis. 2018;62:933–942. doi: 10.3233/JAD-171037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lleó A., Greenberg S.M., Growdon J.H. Current pharmacotherapy for Alzheimer’s disease. Annu Rev Med. 2006;57:513–533. doi: 10.1146/annurev.med.57.121304.131442. [DOI] [PubMed] [Google Scholar]
- 23.Lazarov O., Hollands C. Hippocampal neurogenesis: learning to remember. Prog Neurobiol. 2016:138–140. doi: 10.1016/j.pneurobio.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartolome F., de la Cueva M., Pascual C., Antequera D., Fernandez T., Gil C., Martinez A., Carro E. Amyloid β-induced impairments on mitochondrial dynamics, hippocampal neurogenesis, and memory are restored by phosphodiesterase 7 inhibition. Alzheimers Res Ther. 2018;10(1):24. doi: 10.1186/s13195-018-0352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno-Jiménez E.P., Flor-García M., Terreros-Roncal J., Rábano A., Cafini F., Pallas-Bazarra N., Ávila J., Llorens-Martín M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat Med. 2019;25(4):554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 26.van der Kant R., Goldstein L.S.B., Ossenkoppele R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci. 2020;21:21–35. doi: 10.1038/s41583-019-0240-3. [DOI] [PubMed] [Google Scholar]
- 27.Dioli C., Patrício P., Sousa N., Kokras N., Dalla C., Guerreiro S., Santos-Silva M.A., Rego A.C., Pinto L., Ferreiro E. Tau-dependent suppression of adult neurogenesis in the stressed hippocampus. Mol Psychiatry. 2017;22(8):1110–1118. doi: 10.1038/mp.2017.103. [DOI] [PubMed] [Google Scholar]
- 28.Sung P.S., Lin P.Y., Liu C.H., Su H.C., Tsai K.J. Neuroinflammation and neurogenesis in Alzheimer's disease and potential therapeutic approaches. Int J Mol Sci. 2020;21:701. doi: 10.3390/ijms21030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Metwally A., Toivola P., Al-Rashidi M., Nooruddin S., Jawed M., AlKanhal R., Razzak H.A., Albawardi N. Epidemiology of Alzheimer's disease and dementia in Arab countries: a systematic review. Behav Neurol. 2019;2019:3935943. doi: 10.1155/2019/3935943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozareva D.A., Cryan J.F., Nolan Y.M. Born this way: hippocampal neurogenesis across the lifespan. Aging Cell. 2019;18 doi: 10.1111/acel.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844-66. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.I Iadecola C., Duering M., Hachinski V., Joutel A., Pendlebury S.T., Schneider J.A., Dichgans M. Vascular cognitive impairment and dementia: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73(25):3326–3344. doi: 10.1016/j.jacc.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pluta R., Ułamek-Kozioł M., Januszewski S., Czuczwar S.J. Shared genomic and proteomic contribution of amyloid and tau protein characteristic of Alzheimer's disease to brain ischemia. Int J Mol Sci. 2020;21(9):E3186. doi: 10.3390/ijms21093186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi X.M., Ma J.F. The role of amyloid beta clearance in cerebral amyloid angiopathy: more potential therapeutic targets. Transl Neurodegener. 2017;6:22. doi: 10.1186/s40035-017-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weller R.O., Preston S.D., Subash M., Carare R.O. Cerebral amyloid angiopathy in the aetiology and immunotherapy of Alzheimer disease. Alzheimers Res Ther. 2009;1(2):6. doi: 10.1186/alzrt6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekonomou A., Ballard C.G., Pathmanaban O.N., Perry R.H., Perry E.K., Kalaria R.N., Minger S.L. Increased neural progenitors in vascular dementia. Neurobiol Aging. 2011;32(12):2152–2161. doi: 10.1016/j.neurobiolaging.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Li N., Liu Y., Li W., Zhou L., Li Q., Wang X., He P. A UPLC/MS-based metabolomics investigation of the protective effect of ginsenosides Rg1 and Rg2 in mice with Alzheimer's disease. J Ginseng Res. 2016;40(1):9–17. doi: 10.1016/j.jgr.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nie L., Xia J., Li H., Zhang Z., Yang Y., Huang X., He Z., Liu J., Yang X. Ginsenoside Rg1 ameliorates behavioral abnormalities and modulates the hippocampal proteomic change in triple transgenic mice of Alzheimer's disease. Oxid Med Cell Longev. 2017;2017:6473506. doi: 10.1155/2017/6473506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.