Abstract
Background
Protopanaxatriol (PPT) is a secondary intestinal metabolite of ginsenoside in ginseng. Although the effects of PPT have been reported in various diseases including cancer, diabetes and inflammatory diseases, the skin protective effects of PPT are poorly understood.
Methods
HaCaT cells were treated with PPT in a dose-dependent manner. mRNA and protein levels which related to skin barrier and hydration were detected compared with retinol. Luciferase assay was performed to explore the relative signaling pathway. Western blot was conducted to confirm these pathways and excavated further signals.
Results
PPT enhanced the expression of filaggrin (FLG), transglutaminase (TGM)-1, claudin, occludin and hyaluronic acid synthase (HAS) −1, −2 and −3. The mRNA expression levels of FLG, TGM-1, HAS-1 and HAS-2 were suppressed under NF-κB inhibition. PPT significantly augmented NF-κB-luc activity and upregulated Src/AKT/NF-κB signaling. In addition, PPT also increased phosphorylation of the mitogen-activated protein kinases (MAPKs) ERK, JNK and p38 and upstream MAPK activators (MEK and MKK). Furthermore, transcriptional activity of AP-1 and CREB, which are downstream signaling targets of MAPK, was enhanced by PPT.
Conclusion
PPT improves skin barrier function and hydration through Src/AKT/NF-κB and MAPK signaling. Therefore, PPT may be a valuable component for cosmetics or treating skin disorders.
Keywords: protopanaxatriol, skin protection, NF-κB, AP-1, CREB
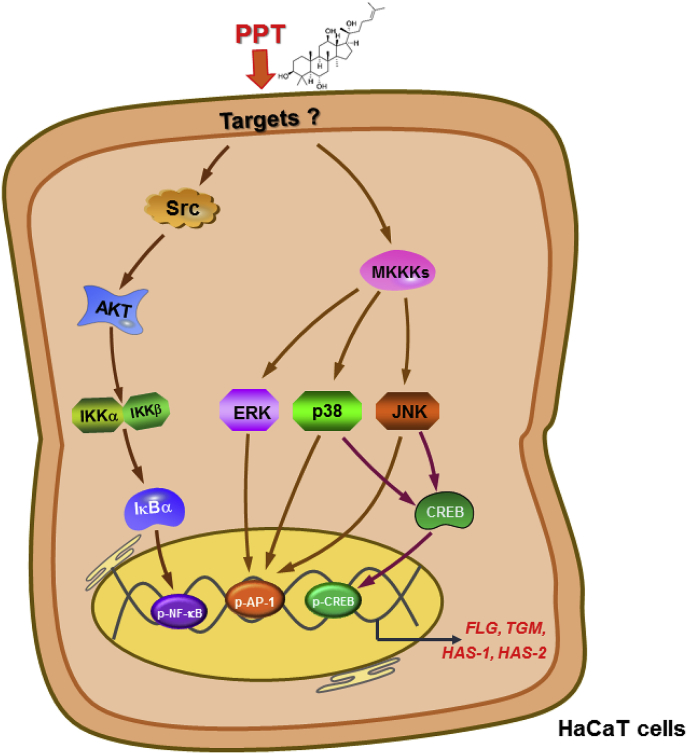
Graphical abstract
1. Introduction
Skin is a complex barrier consisting of tight junctions (TJs) and stratum corneum (SC) that protects our bodies from external invasion and water loss [1]. The TJs, intercellular contacts that form barriers in the stratum granulosum of the skin [2], mainly consist of occludin and claudin [3,4]. The SC is the outermost layer of the skin, and the cytoplasm of dead cells in the SC contains the keratin filament matrix [5]. The periphery of the cells in the SC, called the cornified cell envelope, is composed of e−(g-glutamyl) lysine cross-linked proteins, including involucrin, cornifins/small proline-rich proteins, keratin intermediate filaments, annexin I, and various other proteins [[6], [7], [8]]. Transglutaminases (TGMs) are membrane-associated enzymes that impart integrity to the SCs by catalyzing e−(g-glutamyl) lysine cross-linking reactions [9]. Filaggrin (FLG), a structural protein isolated from SC, also crosslinks with keratin in the SC layer to form a keratin matrix, a parallel structure that is densely packed with intermediate filaments (particularly keratin). The formed keratin matrix which acts as a scaffold to bind cornified-envelope proteins and lipids, thus plays a role in SC formation [10].
The skin maintains viscoelasticity through the moisture present in the SC; 10% or more moisture is essential to keep the elasticity of the SC [22,31]. Proper skin hydration gives the skin flexibility, protects it from damage and enables a process of desquamation by providing an environment in which hydrolytic enzymes can be activated [[11], [12], [13]]. In addition, maintaining the skin’s moisture is necessary to optimize the barrier function of the SC. Furthermore, skin hydration deterioration with aging is closely related to wrinkle formation [14]. Key molecules that affect skin hydration include hyaluronic acid (HA) and aquaporins (AQPs) [[15], [16], [17]]. In the epidermis, HA is mainly located in the extracellular matrix (ECM) in the upper spinous-granular layers and inside the cells in the basal layer [18]. HA is a non-sulfated glycosaminoglycan (GAG) that captures large amounts of water, providing flexibility and moisture to the skin [19]. Most GAGs, which are proteoglycans, are produced in the Golgi apparatus that synthesizes the protein core; HA that is not covalently attached to the protein core is synthesized by hyaluronic acid synthase (HAS), a plasma membrane-bound enzyme [20]. There are three types of HAS (HAS-1, -2, and -3), and each of these has a different enzyme activity and enables synthesis of HA of different lengths [21]. AQPs are membrane proteins that act as channels for transporting water [22]. Among these proteins, AQP3, which is most abundant in the epidermis, is an aquaglyceroporin that is involved in hydration of mammalian skin. AQP3 can transport glycerol as well as water [16].
Ginseng is a medicinal plant that is widely used to treat cancer, diabetes, and heart disease in East Asian countries. The pharmacological activity of ginseng is mainly derived from ginsenosides, triterpenoid dammarane structures that belong to the saponin family. To date, over 30 ginsenosides have been isolated from ginseng, and these ginsenosides are classified into three types according to the type of carbohydrate backbone. The type Ⅰ protopanaxadiol (PPD)-type ginsenosides consist of Rb1, Rb2, Rb3, Rc, Rd, F2, Rg3, and Rh2; the type Ⅱ protopanaxatriol (PPT)-type ginsenosides are comprised of Re, Rg1, Rg2, Rg4, Rh1, and Rh4; and Ro is the type Ⅲ oleanonic acid-type saponin [23]. PPT (shown in Fig. 1a) is a secondary metabolite of type Ⅱ ginsenosides metabolized by the intestinal microflora and has been reported to alleviate steatosis [24], fatigue [25], inflammation [26], diabetes [27], and cancer [28] as well as to have photoprotective properties [29]. However, the effects of PPT on the skin environment are not well studied. Thus, we investigated the molecular mechanism of the pharmacological activity of PPT in the skin barrier and in skin hydration.
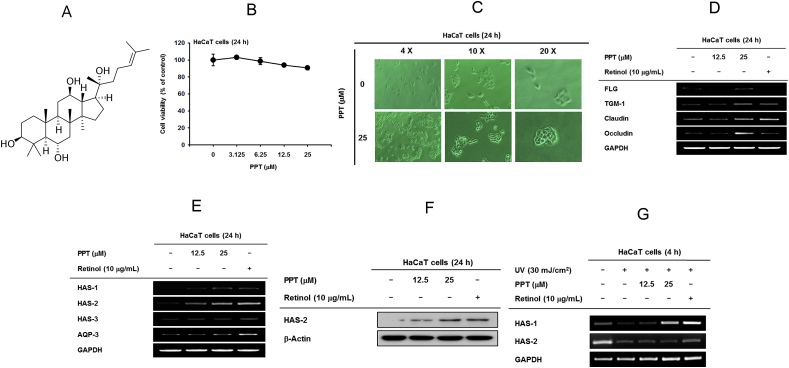
Fig. 1.
The effect of PPT on skin barrier function and hydration. (A) Chemical structure of PPT. (B) Cytotoxicity of PPT was measured using a 3-(4-5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in HaCaT cells. (C) The morphology shooting of HaCaT cells under PPT (25 μM) treatment. (D) The expression levels of skin barrier-related genes [filaggrin (FLG), transglutaminase (TGM)-1, claudin, and occludin] were measured by RT-PCR in HaCaT cells after treatment with PPT (12.5 and 25 μM) or retinol (10 μg/mL) for 24 h. (E) The expression levels of hydration factors [hyaluronic acid synthase (HAS)-1, −2, −3, and aquaporin (AQP)-3] were determined by RT-PCR in HaCaT cells treated with PPT (12.5 and 25 μM) or retinol (10 μg/mL) for 24 h. (F) The levels of HAS-2 protein expression were measured by immunoblot analysis under PPT (12.5 and 25 μM) and retinol (10 μg/mL) treatment conditions in HaCaT cells. (G) The expression levels of hydration factors hyaluronic acid synthase (HAS)-1, −2 were determined by RT-PCR in HaCaT cells treated with PPT (12.5 and 25 μM) or retinol (10 μg/mL) for 12 h under UVB (30 mJ/cm2) irradiation.
2. Materials and methods
2.1. Materials
PPT was obtained from Chengdu Must Bio-Technology Co., Ltd. (Chengdu, Sichuan, China). HaCaT (Human keratinocyte) cells were purchased form CLS Cell Lines Service GmbH (Eppelheim, Germany). HEK293 (Human embryonic kidney cell line) cells were bought from the American Type Culture Collection (Rockville, MD, USA). Fetal bovine serum (FBS), Dulbecco’s Modified Eagle’s Medium (DMEM), RPMI1640, phosphate buffered saline (PBS) and Antibiotics (penicillin-streptomycin) were purchased from HyClone (Logan, UT, USA). 3-(4-5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was bought from Amresco (Brisbane, Australia). The PCR premix was obtained from Bop-D Inc. (Seoul, Korea). Polyethylenimine (PEI) and TRIzol were bought from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). The luciferase assay system kit was obtained from Promega (Madison, WI, USA). The cDNA synthesis kit was received from Thermo Fisher Scientific (Waltham, MA, USA). Forward and reverse primers which used as RT-PCR were synthesized by Macrogen (Seoul, Korea). The polyvinylidene difluoride (PVDF) membrane was purchased from Merck Millipore (Billerica, MA, USA). Several antibodies related phosphorylated or total forms of TAK-1, Src, AKT, PKA, MEKT1/2, MKK4, MKK7, MKK3/6, IKKα/β, IκBα, ERK, JNK, p-38, p50, p65, c-Jun, c-Fos and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). Phosphorylation of CREB and CREB antibodies were obtained from Abcam (Cambridge, UK). HAS-2 antibody was bought from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Cell culture
HaCaT cells with 10 to 30 passage numbers are cultured in DMEM with 10% FBS and 1% antibiotics. HEK293Tcells are cultured in DMEM with 5% FBS and 1% antibiotics. These cell lines were incubated in a 5% humidified CO2 incubator at the temperature of 37°C.
2.3. Cell viability assay
HaCaT cells were seeded in 96-well plates for 5 × 105 cells/well overnight. PPT (3.125, 6.25, 12.5, and 25 μM) was treated in a dose-dependent manner for 24 h. MTT assay was performed to check the cell viability [30]. MTT solution was injected at the dose of 10 μL/well for 3-4 h. MTT stopping solution (10% sodium dodecyl sulfate in 1M HCl) was added to stopping the reaction. After incubating for 16-20 hours, solubilized formazan was measured at the absorbance of 570 nm by multi-plate reader (BioTek, Winooski, VT, USA).
2.4. Reverse-transcriptase polymerase chain reaction (RT-PCR) analysis
HaCaT cells were seeded in 6-well plates. Injected PPT (12.5 or 25 μM) in the plates for 24 h. Semi-quantitative RT-PCR was performed as described before [31]. Primer sequences are illustrated in Table 1.
Table 1.
List of Primers Used in This Study
| Name | Sequence (5′ to 3′) | |
|---|---|---|
| FLG | F | AGGGAAGATCCAAGAGCCCA |
| R | ACTCTGGATCCCCTACGCTT | |
| TGM-1 | F | GAAATGCGGCAGATGACGAC |
| R | AACTCCCCAGCGTCTGATTG | |
| AQP-3 | F | CAGCCTCCCTTATCGTGTGT |
| R | CAAGGGCTGTAAAAAGGCGG | |
| Claudin | F | AGGAACACATTTATGATGAGCAG |
| R | GAAGTCATCCACAGGCGAA | |
| Occludin | F | GAAGATGAGGATGGCTGTCA |
| R | AAATTCGTACCTGGCATTGA | |
| HAS-1 | F | CCACCCAGTACAGCGTCAAC |
| R | CATGGTGCTTCTGTCGCTCT | |
| HAS-2 | F | TTCTTTATGTGACTCATCTGTCTCACCGG |
| R | ATTGTTGGCTACCAGTTTATCCAAACG | |
| HAS-3 | F | TATACCGCGCGCTCCAA |
| R | GCCACTCCCGGAAGTAAGACT | |
| GAPDH | F | GCACCGTCAAGGCTGAGAAC |
| R | ATGGTGGTGAAGACGCCAGT |
2.5. Luciferase reporter gene assay
Seed HEK293T cells in 24-well plates at 1.2 × 104 cells/well. After incubating 24 h, transfected β-galactosidase and NF-κB, AP-1 or CREB-Luc together with PEI into the cells for 24 h [32]. After incubating 24 hours, HEK293T cells were treated with PPT (12.5 of 25 μM) or retinol (10 μg/mL) for 24 h. The luciferase assay was performed by using the luciferase assay system.
2.6. Immunoblotting analysis
Treat PPT (12.5 or 25 μM), retinol (10 μg/mL), BAY 11-7082 (10 μM), U0126 (10 μM), SP600125 (20 μM) or SB203580 (20 μM) into HaCaT cells for 24 h. Cells were diattached with trypsin, washed with chilled 1x PBS and lysed with lysis buffer (containing 50 mM Tris-HCl, several protease and phosphorylase inhibitors) for 15min on ice. The lysates were used after being clarified by centrifugation at 12,000 rpm for 10 min at 4°C. Then, protein concentration was detected by Bradford assay [33]. The soluble fractions of the lysates were used to perform Western blot, and target proteins were detected as previously reported [34].
2.7. Cell morphology shooting
HaCaT cells were seeded into 6-well plate at the cell density of 1 × 105 cells/mL. PPT treatment was at the dose of 25 mM. After 24 hours, 4X, 10X, and 20X microscope objective photos were taken (Olympus, Tokyo, Japan).
2.8. Statistical analysis
All the data we got are showed as mean ± standard deviation (SDs), and every experiment was consisted of six replications. Results were analyzed by the Mann–Whitney U test to compare the statistical differences. A p value < 0.05 was considered as statistically significant. All the data were calculated by using SPSS software, version 25.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. The effect of PPT on skin barrier function and hydration in HaCaT cells
Before evaluating PPT activity, the cytotoxicity of PPT was assessed by MTT assay. The results confirmed that PPT did not induce cell death in HaCaT cells (Fig. 1B). In addition, PPT did not change the morphology but increased the growth rate of HaCaT cells at low density (Fig. 1C). Then, we examined mRNA expression of molecules associated with the skin barrier, FLG, TGM-1, claudin, and occludin, to verify the skin protective effect of PPT. We observed a significant increase in mRNA expression of FLG, TGM-1, claudin, and occludin in cells treated with 25 μM PPT (Fig. 1D). Retinol was used as a positive control.
To examine the skin hydration efficacy of PPT, mRNA levels of HAS-1, HAS-2, HAS-3 and AQP-3 were investigated. For HAS-1 and HAS-2, the expression levels were significantly increased by treatment with 25 μM PPT (Fig. 1E). Consistent with the mRNA expression results, protein expression of HAS-2 was also elevated in PPT-treated HaCaT cells (Fig. 1F). PPT was found to rescue reduction of HAS-1 in UVB-irradiated HaCaT cells, whereas the mRNA level of HAS-2 was not recovered by this compound (Fig. 1G). These results suggest that PPT has a skin protective capacity through regulation of gene expression involved in skin barrier function and hydration.
3.2. The effect of PPT on the Src/AKT/NF-κB signaling pathway
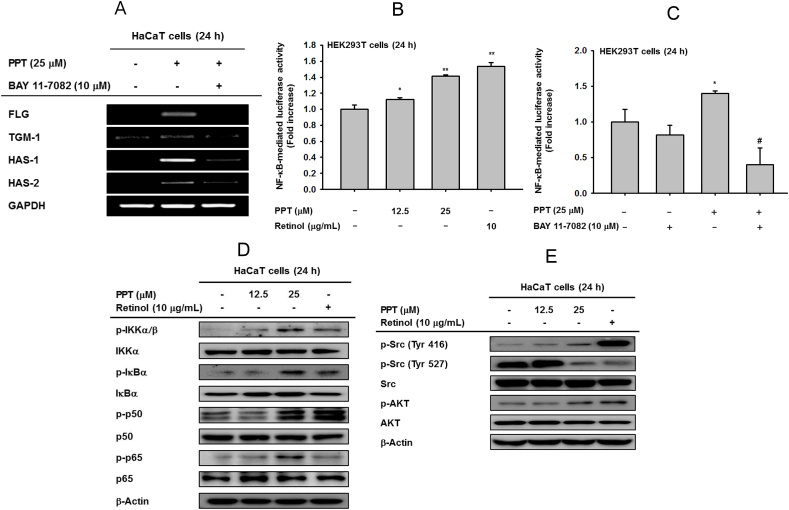
mRNA expression of FLG, TGM-1, HAS-1, and HAS-2 was investigated after treatment with PPT to elucidate the molecular mechanism of increased expression (Fig. 2A). Expression of four genes was completely inhibited by treatment with Bay-11-7082 (Bay), an inhibitor of IKKα and IκBα, suggesting that the NF-κB signal is involved in the pharmaceutical mechanisms of skin barrier and skin hydration. Also, PPT may interact with the NF-κB pathway. Then, a luciferase assay was conducted to determine whether PPT could modulate NF-κB promoter activity. We observed that PPT increased NF-κB-mediated luciferase activity (Fig. 2B) and that PPT-induced NF-κB-luc activity was inhibited by Bay treatment (Fig. 2C). All of these results demonstrated that PPT can regulate the mechanisms of skin barrier and hydration through the NF-κB pathway. Furthermore, PPT augmented the phosphorylation of upstream molecules of NF-κB, including IκBα, IKKα/β, p50, and p65 (Fig. 2D). The tyrosine 416 phospho-Src was also enhanced while Src phosphorylated at tyrosine 527 was suppressed. This indicates that PPT can upregulate the protein-tyrosine enzyme activity by activating the kinase domain (Fig. 2E). Taken together, PPT enhances the expression of genes responsible for skin protection through upregulation of the Src/AKT/NF-κB signaling pathway.
Fig. 2.
The effect of PPT on Src/AKT/NF-κB signal pathway. (A) HaCaT cells were pretreated with BAY11-7082 (10 μM), an inhibitor of IKKα, for 30 min and incubated in the presence of PPT (25 μM) for 24 h. The mRNA levels of FLG, TGM-1, HAS-1, and HAS-2 were measured by RT-PCR. (B and C) HEK293 cells overexpressing NF-kB-luc were treated with PPT (12.5 and 25 μM) and retinol (10 μg/mL) for 24 h (B) or pretreated with BAY11-7082 (10 μM) and incubated in the presence of PPT (25 μM) for 24 h (C). A galactosidase construct was used as a control, and luciferase activity was measured using a luminometer. (D) The effects of PPT on phosphorylation of IKKα/β, IκBα, p50 and p65 were measured by immunoblot analysis after PPT (12.5 and 25 μM) and retinol (10 μg/mL) treatment for 24 h. (E) The effects of PPT on phosphorylation of Src (Tyr416 and Tyr527) and AKT were measured by immunoblot analysis with PPT (12.5 and 25 μM) and retinol (10 μg/mL) treatment for 24 h. ∗p < 0.05 and ∗∗p < 0.01 compared with normal groups. #p < 0.05 and ##p < 0.01 compared with control groups.
3.3. The effect of PPT on MAPK-mediated AP-1 signaling
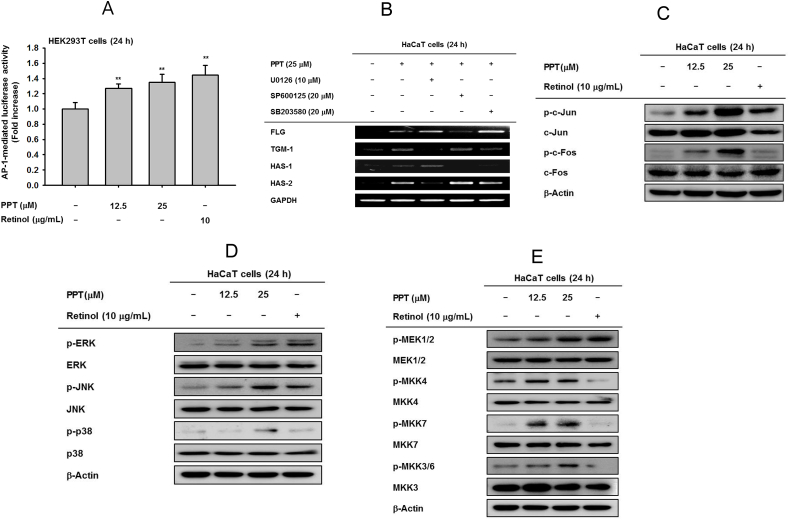
To validate the additional pharmacological activity of PPT, AP-1-mediated luciferase activity was activated by PPT in a dose-dependent manner (Fig. 3A). In addition, the involvement of MAP kinases was evaluated following PPT treatment. PPT-induced gene expression of FLG and HAS-1 was diminished by treatment with SP600126, an inhibitor of JNK (Fig. 3B). Similarly, upregulation of HAS-2 expression after PPT treatment was inhibited by U0126, an ERK inhibitor (Fig. 3B). TGM-1 expression was reduced by both U0126 and SB203580, ERK and p38 inhibitors, respectively, in PPT-treated cells (Fig. 3B). The phosphorylation of c-Jun and c-Fos was increased through PPT treatment (Fig. 3C). The levels of p-ERK, p-JNK, and p-p38 were also increased, with the total form of each MAPK unchanged, following treatment with 12.5 and 25 μM PPT (Fig. 3D). The phosphorylation of MEK1/2, MKK4, MKK7, and MKK3/6, all of which are upstream regulators of MAPK, was simultaneously upregulated after 12.5 and 25 μM PPT treatment(Fig. 3E).
Fig. 3.
The effect of PPT on mitogen activated protein kinase (MAPK)- mediated AP-1 signaling. (A) HEK293T cells transfected with activator protein (AP)-1-luc were incubated with PPT (12.5 and 25 μM) and retinol (10 μg/mL) for 24 h. A galactosidase construct was used as a control, and luciferase activity was measured using a luminometer. (B) HaCaT cells were pretreated with MAPK inhibitors (U0126, SP600125 and SB203580) for 30 min and incubated in the presence of PPT (25 μM) for 24 h. The mRNA expression levels of FLG, TGM-1, HAS-1, and HAS-2 were determined by RT-PCR. (C) HaCaT cells were incubated with PPT (12.5 and 25 μM) and retinol (10 μg/mL) for 24 h. Phosphorylation levels of c-Jun and c-Fos were determined by immunoblot analysis. (D) HaCaT cells were incubated with PPT (12.5 and 25 μM) and retinol (10 μg/mL) for 24 h. Phosphorylation levels of ERK, JNK and p38 were determined by immunoblot analysis. (E) Phosphorylation levels of MAPK activators including TAK1, MEK1/2, MKK4, MKK7, and MKK3/6 were measured by immunoblotting in HaCaT cells-treated with PPT (12.5 and 25 μM) or retinol (10 μg/mL) for 24 h.
3.4. The effect of PPT on MAPK-mediated CREB signaling
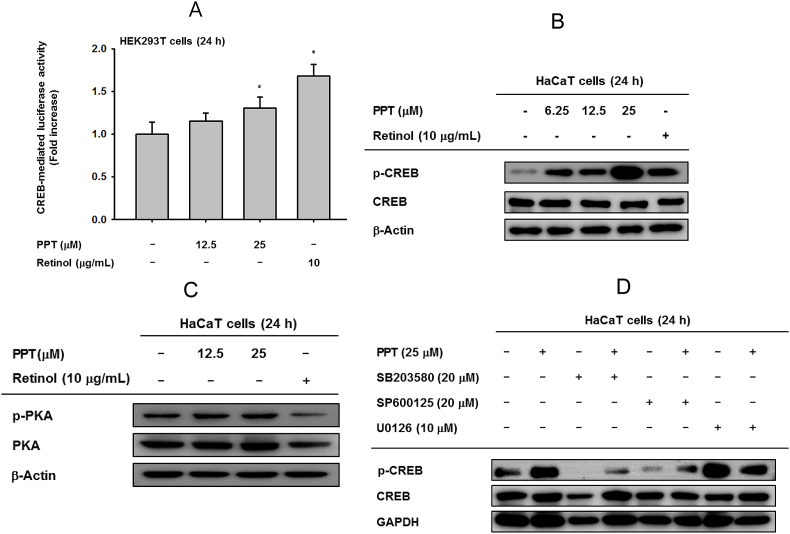
Previously, some papers reported that CREB is regulated by ERK [35,36], and HAS-2 expression is dependent on ERK and CREB [37]. Therefore, the effect of PPT on the transcriptional activity of CREB was studied. PPT gradually increased CREB-luc activity (Fig. 4A) and phosphorylation of CREB (Fig. 4B). However, the phosphorylation of PKA, upstream enzyme of CREB, was not reduced (Fig. 4C), while retinol suppressed the phosphorylation of PKA. Therefore, other upstream signaling mechanisms needed to be further detected. To this end, several MAPK inhibitors were treated singly or co-treated with PPT. The result demonstrated that SP600126 and SB203580 can dramatically downregulate p-CREB and that PPT can recover these depletions. However, U0126 had no significant effects (Fig. 4D). These data show that the phosphorylation of CREB is related to JNK and p38. Taken together, these results imply that PPT exhibits skin-protective activity through the regulation of MAPK-mediated AP-1 activity and crosstalk with CREB signaling.
Fig. 4.
The effect of PPT in CREB signaling. (A) HEK293T cells-transfected with cyclic adenosine monophosphate response element-binding protein (CREB)-Luc were incubated with PPT (12.5 and 25 μM) and retinol (10 μg/mL) for 24 h. A galactosidase construct was used as a control, and luciferase activity was measured by using a luminometer. (B, C) The phosphor- and total forms of CREB and PKA were determined by immunoblot analysis under PPT (12.5 and 25 μM) and retinol (10 μg/mL) treatment conditions. (D) HaCaT cells were pretreated with MAPK inhibitors (U0126, SP600125 and SB203580) for 30 min and incubated singly or in the presence of PPT (25 μM) for 24 h.
4. Discussion
In this study, we assessed the effects of PPT on the skin barrier including skin hydration in HaCaT cells. To this end, the effect of PPT on alteration of genes that encode representative skin barrier proteins such as FLG, TGM-1, claudin, and occludin was examined. In addition, we investigated whether PPT could regulate the expression of the HAS and AQP3 moisturizing factors. Additionally, the action mechanism of PPT was dissected at a molecular level. In particular, since SC acts as the major barrier in the skin, we focused on understanding how PPT regulates the expression of four molecules present in the SC (FLG, TGM-1, HAS-1 and HAS-2).
Our results demonstrated that PPT strengthens the skin barrier by increasing the expression of the main elements of SC, FLG, and TGM-1, as well as claudin and occludin, important components of TJs. Also, PPT simultaneously enhances skin hydration by elevating the expression of HAS-1, -2, and -3. FLG is a constituent of the skin barrier. However, FLG monomers isolated from keratin by deamidation are degraded by proteases such as caspase 14 to form natural moisturizing factors (NMFs) [38,39]. Thus, increased expression of FLG may be associated with moisturizing. In addition, exogenous HA is rapidly degraded in the dermis, so HA synthesis or HA degradation should be targeted to improve skin hydration [40]. Thus, PPT, which can increase the expression of HAS-1, HAS-2, and FLG, is expected to be an effective skin moisturizer. Moreover, collapsed skin barriers and dry skin are known symptoms of various skin–related diseases such as atopic dermatitis (AD), irritant contact dermatitis and psoriasis [13,41,42]. The expression of FLG is significantly reduced in AD patients [43]. Decreasing expression of occludin in flaky tail mice, an experimental animal model of AD, has also been reported [44]. Interestingly, our study showed that PPT treatment led to higher expression of FLG, TGM-1, and occludin than retinol treatment. Therefore, we expect that PPT can be utilized as a powerful active ingredient for the treatment or symptom relief of skin diseases such as atopic dermatitis [45].
Next, we sought to understand how PPT can regulate the expression of target genes. Gene expression of FLG, TGM-1, HAS-1, and HAS-2 was blocked by the inhibitor of IKKα and IκBα. In addition, PPT significantly enhanced NF-kB-luc activity and phosphorylation levels of IκBα, IKKα/β and AKT, indicating that the skin protective activity of PPT is dependent on the NF-kB signaling pathway. FLG, TGM-1, HAS-1, and HAS-2 expression levels were also blocked by ERK, JNK, and p38 inhibitors (U0126, SP600125, and SB203580, respectively). Therefore, the influence of PPT on AP-1, a downstream transcriptional factor of MAPK, was studied. PPT upregulated AP-1-mediated Luc activity as well as phosphorylation of ERK, JNK, and p38. Additionally, phosphorylation of MEK1/2, MKK4/7, and MKK3/6, the upstream regulators of ERK, JNK and p38, respectively, was increased by PPT. In contrast, retinol did not enhance the phosphorylation of MKK4/7, MKK3/6, and p38, implying that PPT has some different inhibitory characteristics compared to those of retinol in AP-1 signaling pathway. Furthermore, luc activity and phospho-CREB level, were upregulated by JNK and p38. Collectively, our results indicate that PPT increases gene expression of skin-barrier and moisturizing proteins via the regulation of NF-κB, MAPK/AP-1, and JNK/p38/CREB signal transduction. Interestingly, this study newly revealed that multifunctional transcription factors such as NF-κB and AP-1, which act as key regulators in inflammatory responses and cancer development [[46], [47], [48]], were also closely involved in the expression of FLG, TGM-1 and HAS. This suggests that substances with known anti-inflammatory or anti-cancer effects may also function to improve the skin barrier. Indeed, PPT shows anti-inflammatory and antiallergic effects in macrophages and mast cells through the inhibition of NF-κB and AP-1 [49].
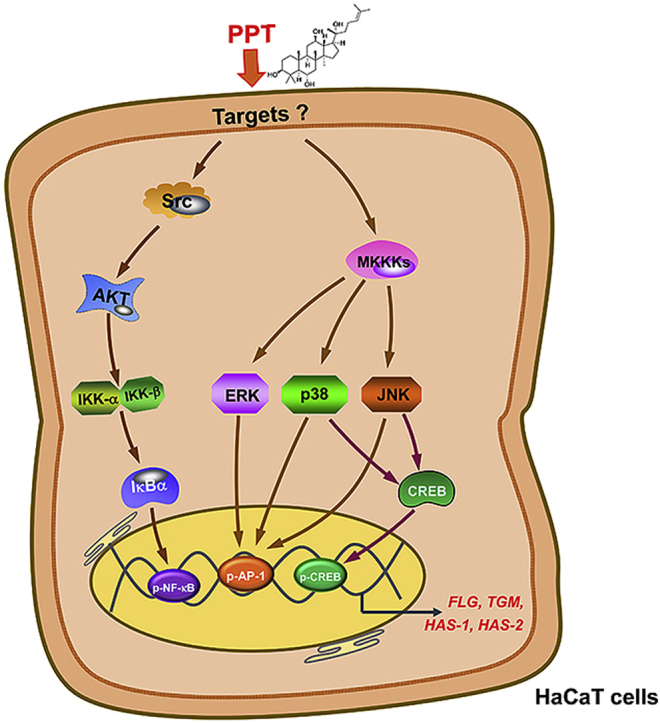
Taken together, our results demonstrate that PPT reinforces the skin barrier and skin hydration through the NF-κB, AP-1, and CREB pathways, as summarized in Fig. 5. Furthermore, we propose that PPT can be used as an active ingredient for skin moisturizing cosmetics as well as for the treatment of AD.
Fig. 5.
Action mechanism of PPT in skin barrier function and skin hydration. PPT was found to significantly increase the expression of FLG, TGM-1, HAS-1 and HAS-2 mRNA. Finally, PPT is able to activate the Src-AKT–NF–κB signal as well as stimulate ERK, JNK, and p38 activities linked to AP-1 and CREB activation.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgments
This paper was supported by Konkuk University, South Korea, in 2020.
Contributor Information
Jeong-Oog Lee, Email: ljo7@konkuk.ac.kr.
So-Hyeon Hwang, Email: sohyun031195@naver.com.
Ting Shen, Email: shenting1019@163.com.
Ji Hye Kim, Email: kjhkjhmlml@skku.edu.
Long You, Email: youlonghc@gmail.com.
Weicheng Hu, Email: hu_weicheng@163.com.
Jae Youl Cho, Email: jaecho@skku.edu.
Abbreviations
- Stratum corneum
(SC)
- Transglutaminases
(TGMs)
- Filaggrin
(FLG)
- Natural moisturizing factors
(NMFs)
- Hyaluronic acid
(HA)
- Glycosaminoglycan
(GAGs)
- Extracellular matrix
(ECM)
- Protopanaxadiol
(PPD)
- Protopanaxatriol
(PPT)
- Aquaporins
(AQPs)
- Tight junction
(TJ)
- Atopic dermatitis
(AD)
- Mitogen-activated protein kinase
(MAPK)
- Activator protein-1
(AP-1)
- Cyclic adenosine monophosphate response element-binding protein
(CREB)
References
- 1.Basler K., Bergmann S., Heisig M., Naegel A., Zorn-Kruppa M., Brandner J.M. The role of tight junctions in skin barrier function and dermal absorption. J Control Release. 2016;242:105–118. doi: 10.1016/j.jconrel.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Brandner J.M. Importance of tight junctions in relation to skin barrier function. Curr Probl Dermatol. 2016;49:27–37. doi: 10.1159/000441541. [DOI] [PubMed] [Google Scholar]
- 3.Tsukita S., Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- 4.Feldman G.J., Mullin J.M., Ryan M.P. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005;57:883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Dale B.A., Ling S.Y. Immunologic cross-reaction of stratum corneum basic protein and a keratohyalin granule protein. J Invest Dermatol. 1979;72:257–261. doi: 10.1111/1523-1747.ep12531715. [DOI] [PubMed] [Google Scholar]
- 6.Matoltsy A.G., Matoltsy M.N. The membrane protein of horny cells. J Invest Dermatol. 1966;46:127–129. [PubMed] [Google Scholar]
- 7.Steinert P.M., Marekov L.N. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995;270:17702–17711. doi: 10.1074/jbc.270.30.17702. [DOI] [PubMed] [Google Scholar]
- 8.Robinson N.A., Lapic S., Welter J.F., Eckert R.L. S100A11, S100A10, annexin I, desmosomal proteins, small proline-rich proteins, plasminogen activator inhibitor-2, and involucrin are components of the cornified envelope of cultured human epidermal keratinocytes. J Biol Chem. 1997;272:12035–12046. doi: 10.1074/jbc.272.18.12035. [DOI] [PubMed] [Google Scholar]
- 9.Thacher S.M., Rice R.H. Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell. 1985;40:685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- 10.Sandilands A., Sutherland C., Irvine A.D., McLean W.H. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009;122:1285–1294. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding C.R., Watkinson A., Rawlings A.V., Scott I.R. Dry skin, moisturization and corneodesmolysis. Int J Cosmet Sci. 2000;22:21–52. doi: 10.1046/j.1467-2494.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 12.Rawlings A.V., Scott I.R., Harding C.R., Bowser P.A. Stratum corneum moisturization at the molecular level. J Invest Dermatol. 1994;103:731–741. doi: 10.1111/1523-1747.ep12398620. [DOI] [PubMed] [Google Scholar]
- 13.Blank I.H. Factors which influence the water content of the stratum corneum. J Invest Dermatol. 1952;18:433–440. doi: 10.1038/jid.1952.52. [DOI] [PubMed] [Google Scholar]
- 14.Choi J.W., Kwon S.H., Huh C.H., Park K.C., Youn S.W. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: a comprehensive and objective approach. Skin Res Technol. 2013;19 doi: 10.1111/j.1600-0846.2012.00650.x. e349–355. [DOI] [PubMed] [Google Scholar]
- 15.Papakonstantinou E., Roth M., Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4:253–258. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boury-Jamot M., Daraspe J., Bonte F., Perrier E., Schnebert S., Dumas M., Verbavatz J.M. Skin aquaporins: function in hydration, wound healing, and skin epidermis homeostasis. Handb Exp Pharmacol. 2009:205–217. doi: 10.1007/978-3-540-79885-9_10. [DOI] [PubMed] [Google Scholar]
- 17.Juhlin L. Hyaluronan in skin. J Intern Med. 1997;242:61–66. doi: 10.1046/j.1365-2796.1997.00175.x. [DOI] [PubMed] [Google Scholar]
- 18.Tammi R., Ripellino J.A., Margolis R.U., Tammi M. Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J Invest Dermatol. 1988;90:412–414. doi: 10.1111/1523-1747.ep12456530. [DOI] [PubMed] [Google Scholar]
- 19.Turino G.M., Cantor J.O. Hyaluronan in respiratory injury and repair. Am J Respir Crit Care Med. 2003;167:1169–1175. doi: 10.1164/rccm.200205-449PP. [DOI] [PubMed] [Google Scholar]
- 20.Prehm P. Hyaluronate is synthesized at plasma membranes. Biochem J. 1984;220:597–600. doi: 10.1042/bj2200597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itano N., Kimata K. Mammalian hyaluronan synthases. IUBMB Life. 2002;54:195–199. doi: 10.1080/15216540214929. [DOI] [PubMed] [Google Scholar]
- 22.Agre P. The aquaporin water channels. Proc Am Thorac Soc. 2006;3:5–13. doi: 10.1513/pats.200510-109JH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Yu L., Cai W., Fan S., Feng L., Ji G., Huang C. Protopanaxatriol, a novel PPARgamma antagonist from Panax ginseng, alleviates steatosis in mice. Sci Rep. 2014;4:7375. doi: 10.1038/srep07375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh H.A., Kim D.E., Choi H.J., Kim N.J., Kim D.H. Anti-fatigue effects of 20(S)-Protopanaxadiol and 20(S)-Protopanaxatriol in mice. Biol Pharm Bull. 2015;38:1415–1419. doi: 10.1248/bpb.b15-00230. [DOI] [PubMed] [Google Scholar]
- 26.Jang S., Lim Y., Valacchi G., Sorn S., Park H., Park N.Y., Lee M. Preventive effects of protopanaxadiol and protopanaxatriol ginsenosides on liver inflammation and apoptosis in hyperlipidemic apoE KO mice. Genes Nutr. 2012;7:319–329. doi: 10.1007/s12263-011-0245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng J., Liu Y., Duan Z., Zhu C., Hui J., Mi Y., Ma P., Ma X., Fan D., Yang H. Protopanaxadiol and protopanaxatriol-type saponins ameliorate glucose and lipid metabolism in type 2 diabetes mellitus in high-fat diet/streptozocin-induced mice. Front Pharmacol. 2017;8:506. doi: 10.3389/fphar.2017.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X.J., Zhang X.J., Shui Y.M., Wan J.B., Gao J.L. Anticancer activities of protopanaxadiol- and protopanaxatriol-type ginsenosides and their metabolites. Evid Based Complement Alternat Med. 2016;2016:5738694. doi: 10.1155/2016/5738694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh S.J., Kim K., Lim C.J. Photoprotective properties of 20(S)-protopanaxatriol, an aglycone of ginseng saponins: protection from ultraviolet-B radiation-induced oxidative stress in human epidermal keratinocytes. Mol Med Rep. 2016;14:2839–2845. doi: 10.3892/mmr.2016.5581. [DOI] [PubMed] [Google Scholar]
- 30.Baek K.S., Yi Y.S., Son Y.J., Jeong D., Sung N.Y., Aravinthan A., Kim J.H., Cho J.Y. Comparison of anticancer activities of Korean Red Ginseng-derived fractions. J Ginseng Res. 2017;41:386–391. doi: 10.1016/j.jgr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong D., Lee J., Park S.H., Kim Y.A., Park B.J., Oh J., Sung G.H., Aravinthan A., Kim J.H., Kang H. Antiphotoaging and antimelanogenic effects of penthorum chinense pursh ethanol extract due to antioxidant- and autophagy-inducing properties. Oxid Med Cell Longev. 2019;2019:9679731. doi: 10.1155/2019/9679731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang W.S., Yi Y.S., Kim D., Kim M.H., Park J.G., Kim E., Lee S.Y., Yoon K., Kim J.H., Park J. Nuclear factor kappa-B- and activator protein-1-mediated immunostimulatory activity of compound K in monocytes and macrophages. J Ginseng Res. 2017;41:298–306. doi: 10.1016/j.jgr.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 34.Baek K.S., Yi Y.S., Son Y.J., Yoo S., Sung N.Y., Kim Y., Hong S., Aravinthan A., Kim J.H., Cho J.Y. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J Ginseng Res. 2016;40:437–444. doi: 10.1016/j.jgr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaywitz A.J., Greenberg M.E. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 36.Peng S., Zhang Y., Zhang J., Wang H., Ren B. ERK in learning and memory: a review of recent research. Int J Mol Sci. 2010;11:222–232. doi: 10.3390/ijms11010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rauhala L., Jokela T., Karna R., Bart G., Takabe P., Oikari S., Tammi M.I., Pasonen-Seppanen S., Tammi R.H. Extracellular ATP activates hyaluronan synthase 2 (HAS2) in epidermal keratinocytes via P2Y2, Ca(2+) signaling, and MAPK pathways. Biochem J. 2018;475:1755–1772. doi: 10.1042/BCJ20180054. [DOI] [PubMed] [Google Scholar]
- 38.Mechin M.C., Enji M., Nachat R., Chavanas S., Charveron M., Ishida-Yamamoto A., Serre G., Takahara H., Simon M. The peptidylarginine deiminases expressed in human epidermis differ in their substrate specificities and subcellular locations. Cell Mol Life Sci. 2005;62:1984–1995. doi: 10.1007/s00018-005-5196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishida-Yamamoto A., Senshu T., Eady R.A., Takahashi H., Shimizu H., Akiyama M., Iizuka H. Sequential reorganization of cornified cell keratin filaments involving filaggrin-mediated compaction and keratin 1 deimination. J Invest Dermatol. 2002;118:282–287. doi: 10.1046/j.0022-202x.2001.01671.x. [DOI] [PubMed] [Google Scholar]
- 40.Reed R.K., Laurent U.B., Fraser J.R., Laurent T.C. Removal rate of [3H]hyaluronan injected subcutaneously in rabbits. Am J Physiol. 1990;259:H532–H535. doi: 10.1152/ajpheart.1990.259.2.H532. [DOI] [PubMed] [Google Scholar]
- 41.Proksch E., Lachapelle J.M. The management of dry skin with topical emollients--recent perspectives. J Dtsch Dermatol Ges. 2005;3:768–774. doi: 10.1111/j.1610-0387.2005.05068.x. [DOI] [PubMed] [Google Scholar]
- 42.Kubo A., Nagao K., Amagai M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest. 2012;122:440–447. doi: 10.1172/JCI57416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seguchi T., Cui C.Y., Kusuda S., Takahashi M., Aisu K., Tezuka T. Decreased expression of filaggrin in atopic skin. Arch Dermatol Res. 1996;288:442–446. doi: 10.1007/BF02505232. [DOI] [PubMed] [Google Scholar]
- 44.Nakai K., Yoneda K., Hosokawa Y., Moriue T., Presland R.B., Fallon P.G., Kabashima K., Kosaka H., Kubota Y. Reduced expression of epidermal growth factor receptor, E-cadherin, and occludin in the skin of flaky tail mice is due to filaggrin and loricrin deficiencies. Am J Pathol. 2012;181:969–977. doi: 10.1016/j.ajpath.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Otsuka A., Doi H., Egawa G., Maekawa A., Fujita T., Nakamizo S., Nakashima C., Nakajima S., Watanabe T., Miyachi Y. Possible new therapeutic strategy to regulate atopic dermatitis through upregulating filaggrin expression. J Allergy Clin Immunol. 2014;133:139–146 e131-110. doi: 10.1016/j.jaci.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Neriah Y., Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 47.Schonthaler H.B., Guinea-Viniegra J., Wagner E.F. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann Rheum Dis. 2011;70(Suppl 1) doi: 10.1136/ard.2010.140533. i109–112. [DOI] [PubMed] [Google Scholar]
- 48.Matthews C.P., Colburn N.H., Young M.R. AP-1 a target for cancer prevention. Curr Cancer Drug Targets. 2007;7:317–324. doi: 10.2174/156800907780809723. [DOI] [PubMed] [Google Scholar]
- 49.Oh G.S., Pae H.O., Choi B.M., Seo E.A., Kim D.H., Shin M.K., Kim J.D., Kim J.B., Chung H.T. 20(S)-Protopanaxatriol, one of ginsenoside metabolites, inhibits inducible nitric oxide synthase and cyclooxygenase-2 expressions through inactivation of nuclear factor-kappaB in RAW 264.7 macrophages stimulated with lipopolysaccharide. Cancer Lett. 2004;205:23–29. doi: 10.1016/j.canlet.2003.09.037. [DOI] [PubMed] [Google Scholar]