Abstract
Background
Gintonin is a ginseng-derived exogenous G-protein–coupled lysophosphatidic acid (LPA) receptor ligand, which exhibits in vitro and in vivo functions against Alzheimer disease (AD) through lysophosphatidic acid 1/3 receptors. A recent study demonstrated that systemic treatment with gintonin enhances paracellular permeability of the blood–brain barrier (BBB) through the LPA1/3 receptor. However, little is known about whether gintonin can enhance brain delivery of donepezil (DPZ) (Aricept), which is a representative cognition-improving drug used in AD clinics. In the present study, we examined whether systemic administration of gintonin can stimulate brain delivery of DPZ.
Methods
We administered gintonin and DPZ alone or coadministered gintonin with DPZ intravenously or orally to rats. Then we collected the cerebral spinal fluid (CSF) and serum and determined the DPZ concentration through liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
Results
Intravenous, but not oral, coadministration of gintonin with DPZ increased the CSF concentration of DPZ in a concentration- and time-dependent manner. Gintonin-mediated enhancement of brain delivery of DPZ was blocked by Ki16425, a LPA1/3 receptor antagonist. Coadministration of vascular endothelial growth factor (VEGF) + gintonin with DPZ similarly increased CSF DPZ concentration. However, gintonin-mediated enhancement of brain delivery of DPZ was blocked by axitinip, a VEGF receptor antagonist. Mannitol, a BBB disrupting agent that increases the BBB permeability, enhanced gintonin-mediated enhancement of brain delivery of DPZ.
Conclusions
We found that intravenous, but not oral, coadministration of gintonin facilitates brain delivery of DPZ from plasma via LPA1/3 and VEGF receptors. Gintonin is a potential candidate as a ginseng-derived novel agent for the brain delivery of DPZ for treatment of patients with AD.
Keywords: Alzheimer disease, Brain delivery, Donepezil, Ginseng, Gintonin
1. Introduction
Alzheimer disease (AD) is a common and representative neurodegenerative disease observed in the aging population [1]. AD occurs after 65 years of age; however, its occurrence at an earlier age is gradually increasing. The occurrence of AD in women is about two-thirds compared with male patients with AD, which can be attributed to female longevity [2]. The present primary and main clinical treatment of patients with AD is the pharmacological modulator of acetylcholinesterase activity, donepezil (DPZ), which increases brain acetylcholine levels by inhibiting acetylcholine hydrolysis [3]. DPZ treatment can result in symptomatic improvement of cognitive function and is considered a representative medicine for AD [4,5].
Ginseng, the root of Panax ginseng Meyer, has been used as a tonic for human beings to maintain health [6]. Ginseng extract contains diverse components [6]. Recent studies found that ginseng extract also contains novel bioactive phospholipid complexes with ginseng proteins, such as ginseng major latex-like protein151, named gintonin. The functional component of gintonin is lysophosphatidic acids (LPA, 1-acyl-2-hydroxy-sn-glycero-3-phosphate), which is a simple phospholipid that plays a role as a lipid-derived growth factor. The order of amounts of LPAs found in the gintonin is as follows: LPA C18:2 >> LPA C16:0 > LPA C18:1 [7]. Gintonin functions as an exogenous ginseng-derived G-protein–coupled LPA receptor ligand [7,8]. We previously showed that gintonin exhibits various in vitro and in vivo AD-related pharmacological effects [9]. In preclinical tests, gintonin exhibits in vitro and in vivo anti-AD activities. Co-oral administration of gintonin with an AD therapeutic agent, such as DPZ, in patients with AD demonstrated improvement of cognitive functions [10]. However, the previous report did not show whether systemic administration of gintonin could stimulate the brain delivery of DPZ when both are coadministered.
In the present study, we examined the effects of gintonin to determine whether coadministration of gintonin with DPZ facilitates brain delivery of DPZ. We found that gintonin successfully enhanced brain delivery of DPZ, and that gintonin-mediated enhancement of brain delivery of DPZ was blocked by the LPA receptor antagonist. Further, we found that the gintonin-mediated enhancement of brain delivery of DPZ from plasma also involves the vascular endothelial growth factor (VEGF) receptor because the VEGF receptor antagonist and coadministration of VEGF also blocked and enhanced gintonin-mediated brain delivery of DPZ. Finally, we discuss the pharmacological roles of gintonin in the brain delivery of DPZ.
2. Materials and methods
2.1. Materials
Gintonin, devoid of ginseng saponins, was prepared from Panax ginseng according to the previously described methods [11] and dissolved in 0.9% saline. DPZ hydrochloride and dipyridamole were purchased from Sigma-Aldrich (St. Louis, MO, USA). We purchased Ki16425 from Cayman Chemicals (Ann Arbor, MI, USA) and axitinib from Tocris Bioscience (Bristol, UK). The VEGF was obtained from GenScript (Piscataway, NJ, USA), and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Animals
Male Wistar rats (8 weeks old ; OrientBio, Seongnam-si, the South Korea) were purchased from the OrientBio for this study. All experiments were conducted in accordance with the National Institutes of Health Guide of the Care and Use of Laboratory Animals, and all experimental procedures were approved by the Institutional Animal Care and Use Committee of the Biomedical Research Institute at Konkuk University (Permit Number: 17-206).
2.3. In vivo experiments
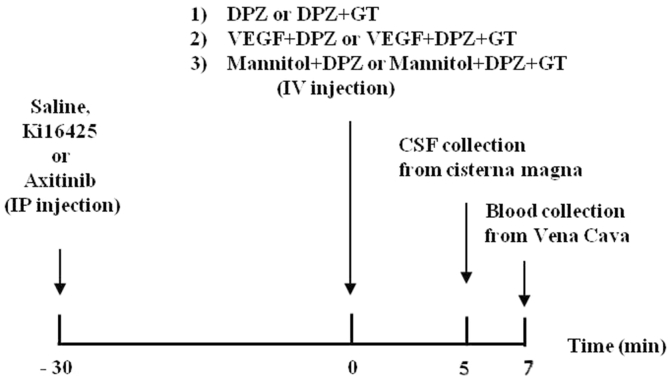
The experiments were performed according to the procedure illustrated by the diagram in Fig. 1. The rats were allocated into four groups according to administered treatments: (1) DPZ (5 mg/kg) alone, DPZ + gintonin (15, 30, 40 mg/kg), VEGF (200 μg/kg) + DPZ, VEGF + DPZ + gintonin (30 mg/kg), mannitol (20% in 0.9% saline) + DPZ (5 mg/kg), mannitol + DPZ + gintonin (30 mg/kg) all via intravenous injection. (2) After other groups were pretreated with Ki16425 (30 mg/kg) or axitinib (25 mg/kg) for 30 min by intraperitoneal injection, DPZ + gintonin were coadministered intravenously. (3) After DPZ (10 mg/kg) was administered orally, gintonin (30 mg/kg) was injected intravenously 50 min later. (4) After gintonin (100 mg/kg) was administered orally using oral gavage after an overnight fast, DPZ (5 mg/kg) was injected intravenously 50 min later. Five minutes after intravenous injection, the cerebral spinal fluid (CSF) samples from all groups were collected from the cisterna magna [12]. Rats were placed under anesthesia with 3% isoflurane and then exposed to 1.5% isoflurane delivered in an 30% O2/70% N2O mixture. Then, the anesthetized rat was placed in a stereotaxic apparatus (Stoelting Co.; Wood Dale, IL ,USA) and a midline incision was made between the ears and over the occipital bone. After the cervicospinal muscle was reflected and the atlanto-occipical membrane was exposed, the membrane was punctured by using a 30-gauge needle and the CSF was drawn into syringe.
Fig. 1.
Schematic diagram of gintonin (GT)-mediated enhancement of delivery of donepezil (DPZ) in the brain. CSF, cerebrospinal fluid; IP, intraperitoneal; IV, intravenous.
2.4. Sample preparation for LPA analysis
The stock standard solution of each acyl-LPAs (LPA C18:2, LPA C18:1, LPA C16:0) and LPA C14:0 internal standard (IS) was prepared in high-pressure liquid chromatography (HPLC) grade methanol. All solutions were stored at 4°C until the time of analysis. Working solutions were prepared from a 100 μg/mL stock solution. The calibration curve of serum samples was prepared by spiking 10 μL of working solution (0.5, 1, 2, 5, 8, 10 μg/mL) into 90 μL of the samples.
A volume of 100 μL of the CSF or serum (real sample) was transferred to a 1 mL tube and spiked with 10 μL IS (5 μg/mL). After vortexing for 30 s, the samples were precipitated with 300 μL methanol. The samples were vortexed for 30 s and then centrifuged for 10 min at 10,000 rpm. The organic layer was filtered through a syringe filter 0.2 μm (MILLEX-LG; Merck Millipore Corporation, Merck KGaA, Darmstadt, Germany). A volume of 2 μL of the solution was injected for LC-MS/MS analysis [13].
Stock solutions of DPZ and dipyridamole (IS) were prepared and diluted in HPLC-grade methanol. The stock solutions of the analytes were stored at 4°C for one month. Working solutions were prepared from a 100 μg/mL stock solution. The calibration curve of CSF samples was prepared by spiking 2.5 μL of working solution (0.1, 0.2, 0.5, 1, 2, 5, 10 μg/mL) into 22.5 μL of the sample. Further, the calibration curves of serum samples were prepared by spiking 5 μL working solution (0.1, 0.2, 0.5, 1, 2, 5, 8, 10 μg/mL) into 45 μL of the sample.
A 25 μL volume of the CSF (real sample) was transferred to a 1 mL tube and spiked with 5 μL IS (50 μg/mL). After vortexing for 30 s, the samples were precipitated with 25 μL of acetonitrile. The samples were vortexed for 30 s and then centrifuged for 10 min at 6,000 rpm. The organic layer was transferred to a insert vial. A volume of 2 μL was injected for LC-MS/MS analysis. A 50 μL volume of the serum (real sample) was transferred to a 1 mL tube and spiked with 10 μL IS (50 μg/mL). After vortexing for 30 s, the samples were precipitated with 300 μL of methanol. The samples were vortexed for 30 s and then centrifuged for 10 min at 10,000 rpm. The organic layer was filtered through a syringe filter (0.2 μm, MILLEX-LG; Merck Millipore Corporation, Merck KGaA, Darmstadt, Germany). A volume of 2 μL of the solution was injected for LC-MS/MS analysis.
2.5. Quantitation of LPAs and DPZ using LC-MS/MS
Analyses were quantified by LC-MS/MS using an Agilent series 1100 HPLC (Agilent Technologies, Santa Clara, CA, USA) instrument, which consisted of a G1311A Quart pump, G1313A autosampler, G1322A degasser, G1316A column oven, and an API 2000™ LC-MS/MS system (Applied Biosystems, Foster City, CA, USA). Tandem mass spectrometric analysis was carried out using an electrospray ion source in positive and negative modes. Analytical data were processed using Analyst 1.4.2 software (AB SCIEX; Seocho-gu, the South Korea) Quantitation was performed by the multiple reaction monitoring mode of the precursor ions and the related product ions using an internal standard method with peak area ratios.
Chromatographic separations of acyl-LPAs (LPA C18:2, LPA C18:1, LPA C16:0) and LPA C14:0 IS were performed on a Waters XBridgeTM C18 analytical column (Waters; Milford, USA). The mobile phase was composed of acetonitrile, 0.1% formic acid, and 10 mM ammonium formate in water, 85:15 (v/v). The isocratic pump mode was run at a flow rate of 0.25 mL/min, and 2 μL aliquots were injected into the column. The column temperature was maintained at 25°C. The ion spray voltage was set at −4.5 kV in the negative ion mode. The capillary temperature was set at 450°C. The operating conditions, optimized by a flow injection of all analytes, were as follows: nebulizing, auxiliary, and curtain gas flows of 50.0, 70.0, and 15 PSI, respectively. The compound parameters viz. The collision energy and declustering potential were set at −72 V to −106 V and −46 V to −66 V for acyl-LPAs. The mass transitions (precursor ion/product ion) used for LPA C18:2, LPA C18:1, LPA C16:0, and LPA C14:0 (IS) were m/z 433→m/z 79, 435→m/z 79, 409→m/z 79, and m/z 381→m/z 79, respectively.
Chromatographic separations of DPZ and dipyridamole (IS) were performed on a Phenomenex kinetex® EVO C18 analytical column (Phenomenex; Torrance, CA, USA). The mobile phase is made up of acetonitrile and 5-mM ammonium formate in water at a ratio of 70:30 (v/v). The isocratic pump mode was run at a flow rate of 0.15 mL/min, and 2 μL aliquots were injected into the column. The column temperature was maintained at 20°C. The ion spray voltage was set to 5.5 kV in the positive ion mode.
The capillary temperature was set to 450°C. The operating conditions, optimized by a flow injection of all analytes, were as follows: nebulizing, auxiliary, and curtain gas flows of 30.0, 70.0, and 10 PSI, respectively. The compound parameters viz. The collision energy, and declustering potential (DP) were set to 65 V and 96 V for DPZ and 43 V and 121 V for IS. The mass transitions (precursor ion/product ion) used for DPZ and dipyridamole (IS) were m/z 380 → m/z 91 and m/z 505 → m/z 429, respectively.
2.6. Data analysis
Statistical analyses between DPZ alone and the cotreatment of gintonin with DPZ or other drugs were performed using one-way analysis of variance, as indicated, using Sigma Stat 4.0 (Systat Software, Inc; San Jose, CA, USA). All values are presented as the mean ± standard error of mean (S.E.M). The significance of the difference was considered statistically significant when p < 0.05.
3. Results
3.1. Determination of plasma LPA concentrations after intravenous gintonin administration
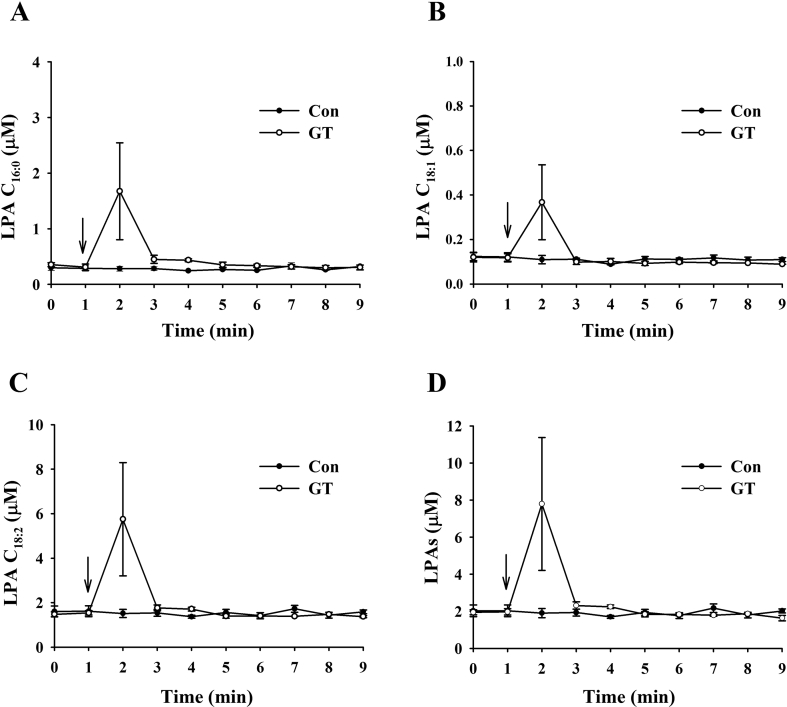
Because gintonin's active ingredients are LPAs that transiently open the blood–brain barrier (BBB) [14], we first determined rat plasma LPA concentration after intravenous administration of gintonin. After administration of gintonin (40 mg/kg) through the rat tail vein, we prepared plasma and determined the LPA concentration with a different time course. As shown in Fig. 2, although the control vehicle group showed only a small and negligible amount of LPA C16:0, LPA C18:1, and LPA C18:2, intravenous gintonin administration increased those LPAs 2 min post-gintonin administration, and these LPAs rapidly returned to a basal level comparable with the control after 1 min. The amount of blood LPA was in the order of LPA C18:2 > LPA C16:0 > LPA C18:1, which is consistent with the ratio contents of LPAs in gintonin [7]. These results show that intravenous gintonin administration transiently increases plasma LPAs.
Fig. 2.
Effects of gintonin on circulating LPA levels in rats. (A–D) Vehicle or gintonin (40 mg/kg) was administered intravenously into catheterized rats into the tail vein, and plasma levels of various LPAs were determined at the indicated time points. Values depict the mean ± standard error of mean (S.E.M.) (n = 4). Experimental procedures for the measurement of LPAs were described in Materials and methods. LPA, lysophosphatidic acid.
3.2. Effects of gintonin on brain delivery of DPZ
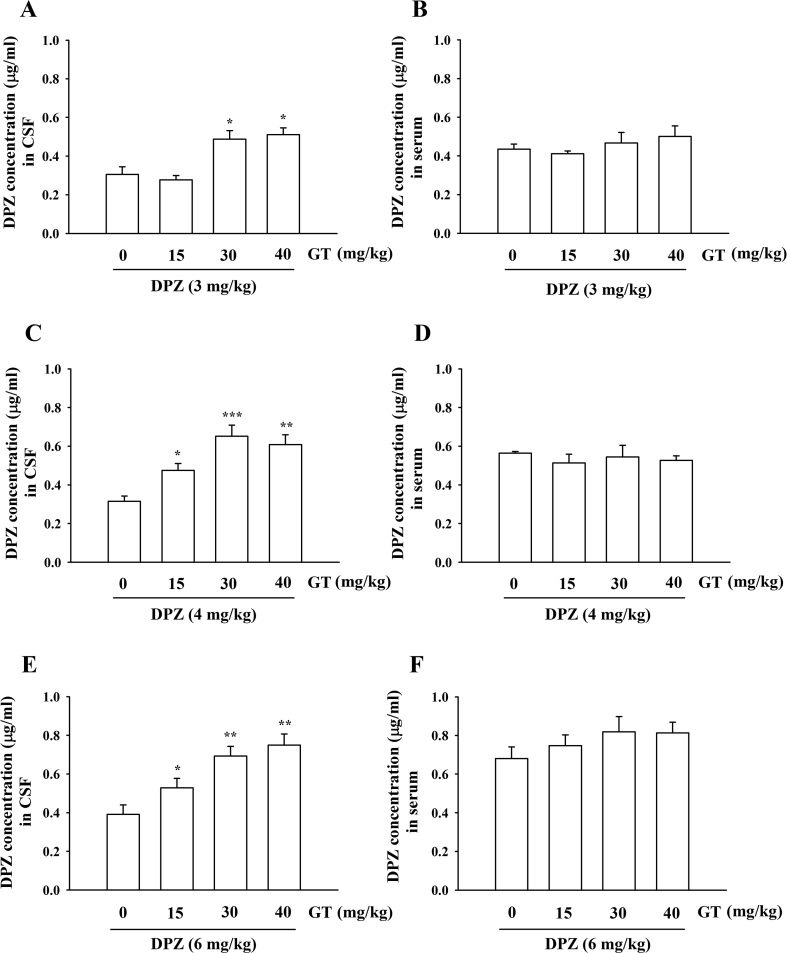
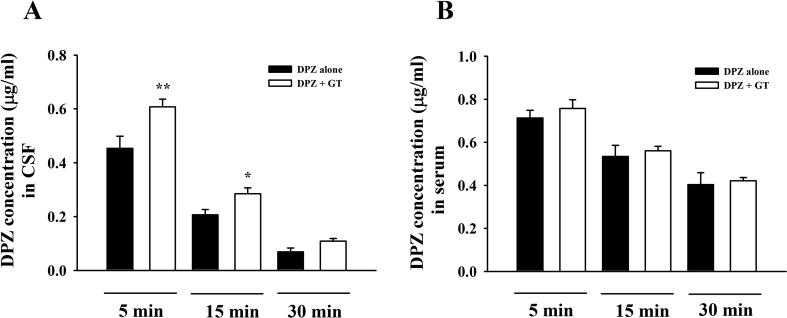
Based on the transient increases of plasma LPAs after gintonin administration (Fig. 2) and the finding that gintonin binds to the LPA1 receptor expressed in human brain microvessel endothelial cells, inducing morphological changes and increasing BBB permeability through the paracellular pathway [14], we next investigated whether gintonin could enhance brain delivery of therapeutic agents for treatment of the neurodegenerative disease, such as AD. DPZ (Aricept) is a primary and well-known anti-AD medicine that is clinically prescribed for patients with AD. In the present study, we first examined the concentration of gintonin or DPZ for the brain delivery of DPZ. As shown in Figs. 3C–3E, coadministration of gintonin (15, 30, or 40 mg/kg) with DPZ (3, 4, or 6 mg/kg) to the rat intravenously increased the DPZ concentration in the rat CSF with a gintonin concentration-dependent manner. Thus, coadministration of gintonin (30 or 40 mg/kg) with DPZ (4 or 6 mg/kg) significantly increased CSF DPZ concentration compared with DPZ alone (Figs. 3C–3E). We could observe that only coadministration of gintonin (30 and 40 mg/kg) with DPZ (3 mg/kg) enhanced CSF DPZ delivery (Fig. 3A). However, gintonin had no significant effects on serum DPZ amount, indicating that gintonin enhances brain delivery of DPZ (Figs. 3B, 3D and 3F). Thus, coadministration of gintonin (30 or 40 mg/kg) with DPZ (>4 mg/kg) exhibits a consistent enhancement of brain delivery of DPZ. The minimally required amount of gintonin and DPZ to enhance brain delivery of DPZ was 30 mg and 3 mg, respectively (Fig. 3A). Furthermore, gintonin-mediated enhancement of the brain delivery of DPZ was time-dependent (Figs. 4A, 4B). Interestingly, coadministration of gintonin (30 mg/kg) with DPZ (4 mg/kg) enhanced brain delivery of DPZ, reaching a maximal level at 5 min after intravenous coadministration of gintonin and DPZ, after which CSF concentration of DPZ decreased, indicating that gintonin rapidly enhances brain delivery of DPZ. Thus, these results demonstrate that gintonin-mediated facilitation of brain delivery of DPZ are concentration- and time-dependent.
Fig. 3.
Determination of mean CSF and plasma concentrations of donepezil for estimation of brain delivery effects of gintonin. Rats were administered 3 mg/kg (A, DPZ concentration in CSF; B, DPZ concentration in serum), 4 mg/kg (C, DPZ concentration in CSF; D, DPZ concentration in serum), and 6 mg/kg (E, DPZ concentration in CSF; F, DPZ concentration in serum) donepezil alone or were coadministered with gintonin (15, 30, or 40 mg/kg) intravenously via the tail vein. CSF and plasma samples were collected within 5 min after each injection. The analysis of donepezil was described in Materials and methods. Values depict the mean ± S.E.M. (n = 10). *P < 0.05, **P < 0.01, ***P < 0.005, compared to with donepezil alone. CSF, cerebrospinal fluid.
Fig. 4.
Determination of mean CSF and plasma concentrations time profile of donepezil on brain delivery effect of gintonin. CSF (A, DPZ concentration in CSF) and plasma (B, DPZ concentration in serum) samples were collected within 5, 15, and 30 min after intravenous injection of DPZ alone (6 mg/kg) or cotreatment with gintonin (30 mg/kg). Values depict the mean ± standard error of mean (S.E.M.) (n = 8). *p < 0.05, **p < 0.01, compared with donepezil alone. CSF, cerebrospinal fluid; DPZ, donepezil.
3.3. Involvement of LPA and VEGF receptors in gintonin-mediated enhancement of brain delivery of DPZ
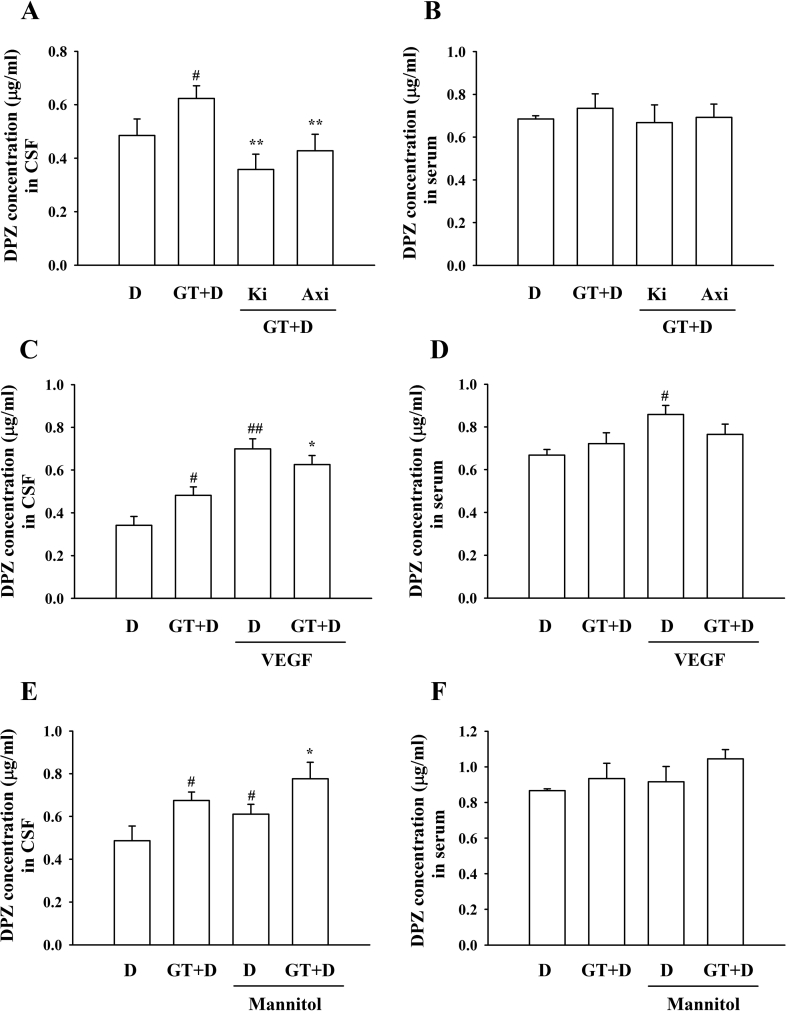
In a previous report, we showed that gintonin activates six LPA receptor subtypes to elicit [Ca2+]i transient in mammalian cells [7]. However, there is no selective antagonist on each LPA receptor subtype except Ki16425, which is a selective LPA1/3 receptor antagonist [15]. Subsequently, we examined whether gintonin-mediated enhancement of brain delivery of DPZ is attenuated by the LPA1/3 receptor antagonist, Ki16425, because the gintonin-mediated increase of the BBB permeability was blocked by Ki16425 [14]. As shown in Fig. 5A, coadministration of Ki16425 with gintonin + DPZ abolished gintonin-mediated enhancement of brain delivery of DPZ, indicating that gintonin-mediated enhancement of brain delivery of DPZ is achieved via LPA1/3 receptors. In previous reports, we showed that gintonin stimulates VEGF release in human umbilical vessel endothelial cells and astrocytes [16,17]. Furthermore, it was reported that VEGF is a kind of BBB opening agent [18]. Therefore, we examined the effects of the VEGF receptor antagonist on gintonin-mediated enhancement of the brain delivery of DPZ. Fig. 5A shows that the VEGF receptor antagonist, axitinib, attenuates gintonin-mediated enhancement of brain delivery of DPZ, raising the possibility that gintonin-mediated enhancement of brain delivery of DPZ might also involve VEGF receptor activation.
Fig. 5.
Effects of pretreatment of LPA1/3 receptor (Ki16425), VEGF, VEGF antagonist (Axitinib), or mannitol on gintonin-mediated enhancement of brain delivery of donepezil. (A–B) After Ki16425 (30 mg/kg) or axitinib (25 mg/kg) was pretreated for 30 min by intraperitoneal injection, rats received administration of DPZ (6 mg/kg) or coadministration with gintonin (30 mg/kg) intravenously via the tail vein. #p < 0.01, compared with donepezil alone. *p < 0.01, **p < 0.001, compared with gintonin + donepezil. (C–D) Rats were treated with DPZ (6 mg/kg) + VEGF (200 μg/kg) or DPZ + VEGF + gintonin (30 mg/kg) via intravenous injection. #p < 0.01, compared with donepezil alone; ##p < 0.01, compared with donepezil + gintonin. *p < 0.01, compared with gintonin + donepezil. (E–F) After mannitol (5 mg/kg; 20% in 0.9% saline) + DPZ (6 mg/kg) or mannitol + DPZ + gintonin (30 mg/kg) treatment to the rats, CSF and plasma samples were collected within 5 min. #p < 0.01, compared with donepezil alone; *p < 0.01, compared with mannitol + donepezil. Values depict the mean ± standard error of mean (S.E.M.) (n = 8). CSF, cerebrospinal fluid; DPZ, donepezil; VEGF, vascular endothelial growth factor.
3.4. Effects of VEGF or mannitol on gintonin-mediated enhancement of brain delivery of DPZ
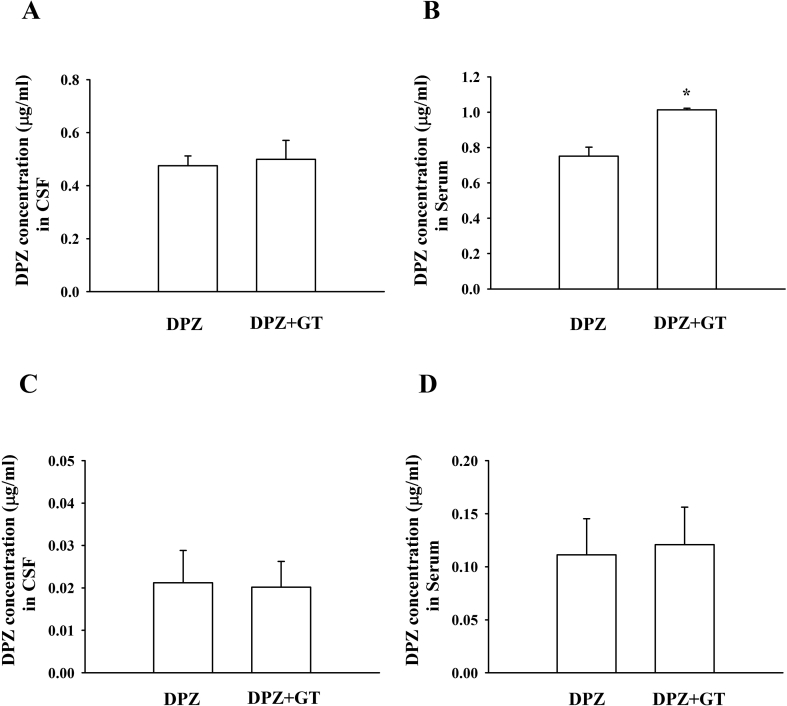
Because the VEGF receptor antagonist attenuated gintonin-mediated enhancement of the brain delivery of DPZ, we also examined whether coadministration of VEGF with gintonin + DPZ further enhances gintonin-mediated brain delivery of DPZ. Fig. 5C shows that VEGF coadministration with gintonin + DPZ further enhanced brain delivery of DPZ compared with DPZ alone or gintonin + DPZ. Interestingly, coadministration of VEGF and DPZ enhanced the serum DPZ concentration (Fig. 5D). We also examined the effects of mannitol, a BBB disrupting agent indicated to increase BBB permeability through osmolarity changes, on gintonin-mediated enhancement of brain delivery of DPZ. Mannitol itself not only enhanced brain delivery of DPZ but also showed a slightly additive effect on gintonin-mediated enhancement of brain delivery of DPZ (Fig. 5E). This result is consistent with a previous report [17] and with the claim that mannitol might aid in the gintonin-mediated enhancement of brain delivery of DPZ. When both gintonin and DPZ were coadministered via the oral route, gintonin did not exhibit any changes in CSF and serum concentrations of DPZ (Fig. 6). These results indicate that gintonin-mediated enhancement of brain delivery of DPZ is mainly effective by coadministration of both gintonin and DPZ through the intravenous, but not the oral route, of either gintonin or DPZ.
Fig. 6.
Effects of orally administered gintonin on donepezil brain delivery system. (A) Donepezil (10 mg/kg) was administered intravenously 60 min after oral treatment of gintonin (100 mg/kg). (C) Gintonin (40 mg/kg) was administered intravenously 60 min after oral treatment of donepezil (10 mg/kg). CSF (A and C) and plasma samples (B and D) were collected within 5 min. The analysis of donepezil was described in Materials and methods. Values depict the mean ± standard error of mean (S.E.M.) *p < 0.05, compared with donepezil alone (n = 8).
4. Discussion
AD is a representative neurodegenerative disease that is dramatically increased in aging population. The BBB remains the main obstacle for efficient drug delivery to treat the disease [19,20]. Because most of the newly developed drugs do not permeate into the brain because of the BBB, efficient drug delivery into the brain without cause of brain damage is one of the biggest challenges for AD treatment, as well as other brain diseases. In the present study, we investigated whether gintonin could enhance brain delivery of DPZ. We found that intravenous administration of gintonin rapidly and transiently increased plasma LPA concentration (Fig. 2) and that intravenous but not oral coadministration of gintonin with DPZ enhances brain delivery of DPZ in a concentration- and time-dependent manner (Fig. 3, Fig. 4). Gintonin-mediated enhancement of brain delivery of DPZ was blocked by Ki16425, a LPA1/3 receptor antagonist (Fig. 5A). We further demonstrated that gintonin-mediated enhancement of brain delivery of gintonin might involve the VEGF receptor because coadministration of the VEGF receptor antagonist with gintonin and DPZ blocked the enhanced brain delivery of DPZ (Fig. 5A). Moreover, coadministration of the VEGF with DPZ or gintonin + DPZ also further enhanced brain delivery of DPZ (Fig. 5C).
It can be argued that if gintonin allows other molecules from the plasma besides DPZ to enter the brain at the same time, it could impair normal brain function and affect brain homeostasis. However, this is unlikely, because plasma LPAs after gintonin administration reached a peak 1 min post-gintonin administration and rapidly decreased to the basal levels (Fig. 2). With transient time course of plasma LPA concentrations, gintonin administration induces a rapid enhancement of DPZ delivery to brain 5 min after administration, and the gintonin effect was not observed at a later time point (Fig. 4). These results suggest that a transient increase of plasma LPAs by gintonin administration is in sequence coupled to the transient opening of the BBB for the DPZ entrance into the brain (Fig. 4).
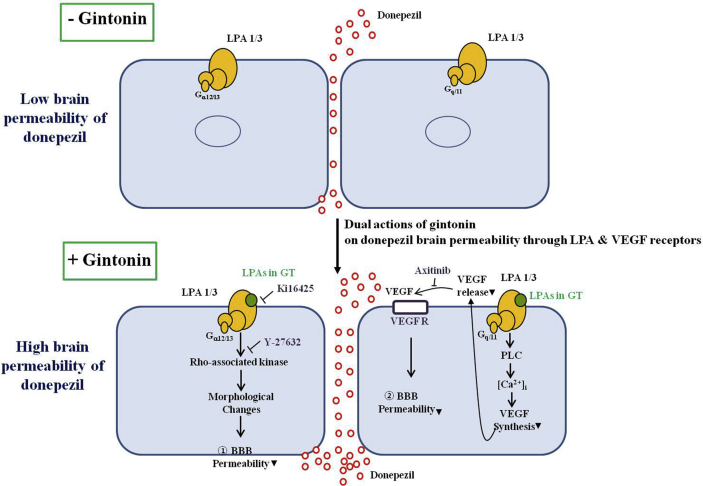
It would also be interesting to speculate the underlying mechanism of how intravenous coadministration of gintonin with DPZ enhances brain delivery of DPZ compared with DPZ administration alone. In the previous report, we showed that gintonin opens the BBB transiently through LPA1/3 receptor signaling pathways [14]. The dual direct and indirect mechanisms might be involved in the gintonin-mediated enhancement of brain delivery of DPZ. The first contributing element is the direct role of the LPA1/3 receptor in gintonin-mediated enhancement of DPZ through a paracellular pathway. In a previous report, gintonin was shown to bind to LPA1/3 receptor on human brain microvessel endothelial cells and induce morphological changes through Rho-associated kinase activation for transient opening of the BBB for its entrance to brain, via the paracellular pathway [14]. The second contributing element for the enhancement of brain delivery of DPZ is an indirect role of gintonin via the VEGF and VEGF receptor for gintonin-mediated enhancement of brain delivery of DPZ. Previous reports showed that the main role of tha VEGF is as a cytokine growth factor for the formation of blood vessels [21]. In addition to its angiogenic effects, recent studies also showed that the VEGF increases the permeability of the BBB, as indicated in Fig. 3 [18,19]. We recently reported that gintonin stimulates VEGF release in blood vessel cells and cortical astrocytes [16,17], which are key cells for BBB formation in the brain. Thus, the VEGF release from the blood microvessel endothelial cells by gintonin administration might be coupled to transient BBB opening via VEGF receptor activation. Acting jointly, gintonin-induced activations of LPA receptors on brain microvessel endothelial cells and gintonin-mediated VEGF release from brain microvessel endothelial cells might also contribute to gintonin-mediated enhancement of DPZ (Fig. 7).
Fig. 7.
Schematic illustration of gintonin-mediated stimulations of donepezil permeability into brain. Gintonin-mediated stimulations of donepezil permeability into brain might include two signaling pathways. One possibility is that activation of LPA1/3 receptors by gintonin induces morphological changes of brain microvessel endothelial cells through Gα12/13-Rho-associated kinase morphological changes signaling pathway that is coupled to increases in brain permeability to donepezil, as shown in a previous report [14]. The other is through the VEGF. The activations of LPA1/3 receptors by gintonin is coupled to an increase in VEGF synthesis and release from brain microvessel endothelial cells through the Gαq/11-phospholipase C (PLC)-Ca2+ signaling pathway. Released VEGFs increase brain permeability, as indicated in previous reports [18,19]. VEGF, vascular endothelial growth factor.
Interestingly, when we added gintonin to VEGF + DPZ, we could observe a slight decrease of DPZ concentration levels in the brain compared with VEGF + DPZ treatment (Fig. 5C). Thus, the addition of gintonin in the presence of the VEGF did not exhibit additive or synergic effects on the brain delivery of DPZ. Currently, we cannot explain the exact cause. One reason might be because of a saturation of VEGF treatment for brain delivery of DPZ, although the receptors for VEGF and gintonin are different. In case of mannitol effects with gintonin on the brain delivery of DPZ, the molecular mechanisms on the mannitol-induced increase of DPZ delivery to the brain is different from that of the VEGF and gintonin because mannitol simply increases brain permeability by disturbance of the osmolarity, whereas gintonin- and VEGF-induced increase of brain permeability is achieved through their respective LPA and VEGF receptors. Therefore, we could observe a slight increase in the gintonin + mannitol + DPZ compared with the gintonin + DPZ for the brain delivery of DPZ (Fig. 5E).
In a previous clinical study, we showed that long-term oral coadministration of gintonin-enriched fraction to patients with mild AD administered anti-AD drugs, such as DPZ, improved cognitive functions without adverse effects [10]. In the present study, we further extended the role of gintonin by showing that acute intravenous coadministration of gintonin with DPZ enhanced brain delivery of DPZ. If acute coadministration of gintonin with DPZ enhances brain delivery of DPZ in clinical applications, it could be anticipated that intravenous coadministration of gintonin with DPZ can reduce the dosage of DPZ administration. This can be associated with beneficial effects of gintonin through attenuations of DPZ-induced adverse effects [22]. However, further clinical studies should be required to confirm whether a lower amount of DPZ exhibits the same efficacy in improving cognitive functions in patients with AD when it is coadministered with gintonin.
In summary, we showed that intravenous gintonin administration transiently increased plasma LPAs. Coadministration of gintonin with DPZ induces rapid enhancement of the brain delivery of DPZ, and the gintonin-mediated enhancement of brain delivery of DPZ involves LPA and VEGF receptors. Finally, the present study indicates the possibility that a systemic coadministration of gintonin with DPZ might increase treatment efficiency of AD by increasing the concentration of brain DPZ.
Conflicts of interest
There is no conflict of interest.
Acknowledgments
This study was supported by the Brain Research Program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Science, Information & Communication Technology (ICT), and Future Planning (NRF-2016M3C7A1913845, NRF-2016M3C7A1913933). S-.Y.N. received funding from the Basic Science Research Program (NRF-2017R1D1A1A09000520).
References
- 1.Meng G., Zhong X., Mei H. A systematic investigation into aging related genes in brain and their relationship with Alzheimer's disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–88. doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colović M.B., Krstić D.Z., Lazarević-Pašti T.D., Bondžić A.M., Vasić V.M. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns A., Rossor M., Hecker J., Gauthier S., Petit H., Moller H.J. The effects of donepezil in Alzheimer’s disease – results from a multinational trial. Dement Geriatr Cogn Disord. 1999;10:237–244. doi: 10.1159/000017126. [DOI] [PubMed] [Google Scholar]
- 5.Jelic V., Darreh-Shori T. Donepezil: a review of pharmacological characteristics and role in the management of Alzheimer disease. Clin Med Insights Ther. 2010;2:771–788. [Google Scholar]
- 6.Nuri T.H., Yee J.C., Gupta M., Khan M., Ming L.C. A review of Panax ginseng as an herbal medicine. Arch Pharma Pract. 2016;7:61–65. [Google Scholar]
- 7.Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K., Lee J.H., Kang J., Kim H.J., Park C.W. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi S.H., Jung S.W., Lee B.H., Kim H.J., Hwang S.H., Kim H.K., Hwang S.H., Kim H.K., Nah S.Y. Ginseng pharmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front Pharmacol. 2015;6:245. doi: 10.3389/fphar.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nah S.Y. Gintonin: a novel ginseng-derived ligand that targets G protein-coupled lysophosphatidic acid receptors. Curr Drug Targets. 2012;13:1659–1664. doi: 10.2174/138945012803529947. [DOI] [PubMed] [Google Scholar]
- 10.Moon J., Choi S.H., Shim J.Y., Park H.J., Oh M.J., Kim M., Nah S.Y. Gintonin administration is safe and potentially beneficial in cognitively impaired elderly. Alzheimer Dis Assoc Disord. 2018;32:85–87. doi: 10.1097/WAD.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 11.Pyo M.K., Choi S.H., Hwang S.H., Shin T.J., Lee B.H., Lee S.M., Lim Y.H., Kim D.H., Nah S.Y. Novel glycolipoproteins from ginseng. J Ginseng Res. 2011;35:92–103. [Google Scholar]
- 12.Liu L., Duff K. A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J Vis Exp. 2008;10:960. doi: 10.3791/960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho H.J., Choi S.H., Kim H.J., Lee B.H., Rhim H., Kim H.C., Hwang S.H., Nah S.Y. Bioactive lipids in gintonin-enriched fraction from ginseng. J Ginseng Res. 2019;43:209–217. doi: 10.1016/j.jgr.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim D.G., Jang M., Choi S.H., Kim H.J., Jhun H., Kim H.C., Rhim H., Cho I.H., Nah S.Y. Gintonin, a ginseng-derived exogenous lysophosphatidic acid receptor ligand, enhances blood-brain barrier permeability and brain delivery. Int J Biol Macromol. 2018;15:1325–1337. doi: 10.1016/j.ijbiomac.2018.03.158. [DOI] [PubMed] [Google Scholar]
- 15.Ohta H., Sato K., Murata N., Damirin A., Malchinkhuu E., Kon J. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- 16.Hwang S.H., Lee B.H., Choi S.H., Kim H.J., Won K.J., Lee H.M., Rhim H., Kim H.C., Nah S.Y. Effects of gintonin on the proliferation, migration, and tube formation of human umbilical-vein endothelial cells: involvement of lysophosphatidic-acid receptors and vascular-endothelial-growth-factor signaling. J Ginseng Res. 2016;40:325–333. doi: 10.1016/j.jgr.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi S.H., Kim H.J., Cho H.J., Park S.D., Lee N.E., Hwang S.H., Rhim H., Kim H.C., Cho I.H., Nah S.Y. Gintonin-mediated release of astrocytic vascular endothelial growth factor protects cortical astrocytes from hypoxia-induced cell damages. J Ginseng Res. 2019;43:305–311. doi: 10.1016/j.jgr.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang S., Xia R., Jiang Y., Wang L., Gao F. Vascular endothelial growth factors enhance the permeability of the mouse blood-brain barrier. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkins B.T., Sykes D.B., Miller D.S. Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci. 2010;30:1417–1425. doi: 10.1523/JNEUROSCI.5103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonda-Turo C., Origlia N., Mattu C. Current limitations in the treatment Parkinson's and Alzheimer's diseases: state-of-the-art and future perspective of polymeric carriers. Curr Med Chem. 2018;25:5755–5771. doi: 10.2174/0929867325666180221125759. [DOI] [PubMed] [Google Scholar]
- 21.Johnson K.E., Wilgus T.A. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care. 2014;3:647–661. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson S., Ham R.J., Wilkinson D. The safety and tolerability of donepezil in patients with Alzheimer's disease. Br J Clin Pharmacol. 2004;58:1–8. doi: 10.1111/j.1365-2125.2004.01848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]