Abstract
The treatments of nervous system diseases (NSDs) have long been difficult issues for researchers because of their complexity of pathogenesis. With the advent of aging society, searching for effective treatments of NSDs has become a hot topic. Ginseng polysaccharides (GP), as the main biologically active substance in ginseng, has various biological properties in immune-regulation, anti-oxidant, anti-inflammation and etc. Considering the association between the effects of GP and the pathogenesis of neurological disorders, many related experiments have been conducted in recent years. In this paper, we reviewed previous studies about the effects and mechanisms of GP on diseases related to nervous system. We found GP play an ameliorative role on NSDs through the regulation of immune system, inflammatory response, oxidative damage and signaling pathway. Structure-activity relationship was also discussed and summarized. In addition, we provided new insights into GP as promising neuroprotective agent for its further development and utilization.
Keywords: Ginseng, Molecular mechanism, Nervous system, Polysaccharides
Abbreviations: GP, Ginseng polysaccharides; NSDs, Nervous system diseases; AG, Arabinogalactan; RG, Rhamnogalacturonan; HG, Homogalacturonan; BDNF, Brain-derived neurotrophic factor; TNF-α, tumor necrosis factor-α; IL-17α, Interleukin-17 α; IFN-γ, Interferon-γ; MS, Multiple sclerosis; BBB, Blood–brain barrier
1. Introduction
The data reported by the World Health Organization have shown that nervous system diseases (NSDs) have become another major threat to human life after heart disease and cancer [1]. As the “command center” of human body, the brain consumes 20% of the total glucose but only accounts for 2% of the body volume [2]. Structural complexity underlies its functional diversity, yet also makes it more susceptible to diseases. Common neurological disorders include Alzheimer's disease, depression, epilepsy and Parkinson's disease. As we all know, NSDs are related not only to nervous system, but also to immune system disorders and inflammation. For example, microglial cells, as the macrophages in the brain and spinal cord, are the first line in defense of nervous system against pathogens. They fulfill important functions in immune surveillance, resolution of latent inflammatory reactions and clearance of tissue debris [3,4]. Moreover, this relationship also provides us with a new idea for treatment; that is, we can make the nervous system return to homeostasis by moderately regulating autoimmunity.
To our knowledge, incidences of NSDs increase as people get older. With the advent of aging society, the threat of NSDs to human health is bound to be more serious. In addition to bringing great pain to the patient, they also bring a huge disaster to the social medicine. Hence, there is an urgent need to find drugs that can alleviate or treat such diseases. Through unremitting effort, more and more neural drugs are available and have been put into clinical application in recent years. Yet facts have shown that chemical drugs are often accompanied by a series of side effects while exerting the expected therapeutic effect. Compared to chemical drugs, natural ingredients extracted from plants usually have higher safety. This benefit made researchers turn their research direction to natural components.
Panax ginseng Meyer (Fig. 1) usually grows in cooler areas like Northeast China, Korea, and Russia. Depending on origins of the plant and processing methods, it can be further divided into Chinese ginseng, White ginseng, Korean red ginseng, American ginseng and etc. As a precious traditional Chinese medicine, ginseng has been used as a remedy in clinic for thousands of years. The Greek term “Panax”, which means “cure of all diseases”, implied its important position in the medical field. As recorded in the traditional Chinese work, Jingyue Quanshu (Jing-yue's Complete Works), ginseng has been used in the classic prescription Qifu Yin to treat neurasthenia, senile dementia, brain atrophy and other NSDs. There are also many modern studies showing the neuroprotective effects of ginseng extract [5]. Jia et al found Panax notoginseng polysaccharides exert its neurprotective function against focal cerebral I/R injury through increasing Bal-2/Bax ratio and evoking caspase-3 cascade [6]. Its various therapeutic effects are due to the specific components contained in it. Ginseng has been found to contain a variety of active ingredients, such as saponins, polysaccharides, flavonoids, volatile oil and gintonin [7]. Among them, ginseng polysaccharides (GP), accounting for about 10% of the dry weight of ginseng, is the most abundant active substance in ginseng along with immunoregulatory, anti-oxidant, anti-cancer and anti-inflammatory effects [[8], [9], [10]]. Recently, it is reported that GP can also regulate intestinal metabolism and restore the homeostasis of gut microbiota [11,12]. Considering the existence of "gut-brain axis" and the bidirectional relationship between gastrointestinal and central nervous system [13], this discovery may provide new insights into the mechanism of GP's effects on NSDs. According to whether it contains uronic acid, GP can be divided into neutral and acidic polysaccharides. Their differences in monosaccharide composition and glycoside bond linkage, even the way chains curled, are considered to be the root causes of different therapeutic effects. Ginseng neutral polysaccharides, accounting for over 75% of the total polysaccharides content [14], is mainly composed of Glc (starch-like polysaccharides), Gal and Ara [15,16], and can be used to treat a range of neurological disorders associated with inflammation [17]. Moreover, the acidic portion contains arabinogalactan (AG), type-I rhamnogalacturonan (RG-I)- and homogalacturonan (HG)-rich pectins [18], and can down-regulate immunostimulating by increasing the number of CD25+ immunoregulatory T cells, thus has therapeutic effect on autoimmune diseases. In general, an increasing body of evidence suggested that GP, both neutral and acid polysaccharides, are becoming potential agent to treat NSDs.
Fig. 1.
Plant picture of ginseng.
In this paper, we reviewed previous studies about the therapeutic effects and mechanisms of GP on diseases related to nervous system, in order to provide a basis for further research on the relationship between them.
2. Compositions and structural characterization
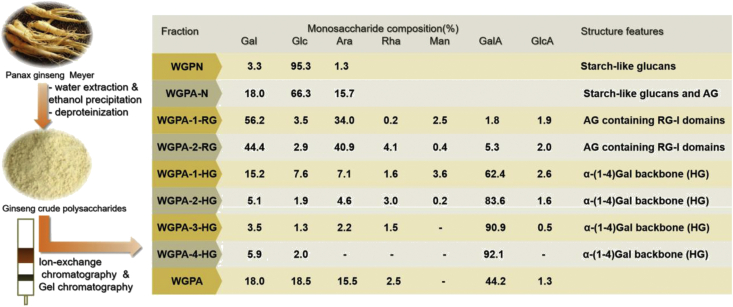
Due to the complexity of traditional Chinese medicine compositions, finding out the active components and clarifying their structure seem to be big challenges. Prior to 1990s, the polysaccharides compositions of ginseng were largely unknown. Since Tomoda et al [19] purified two acidic polysaccharides from ginseng in 1993, more and more research teams have devoted themselves to the purification of GP, as well as the study of structural characterizations. Generally, GP is obtained by solvent extraction and commonly used extraction solvents include hot water, alkali solution [20] and ethylenediamine tetraacetic acid [21]. Among them, hot water extraction is widely used because of its high polysaccharides yield (10.7%). Zhang et al [19] adopted a combination method of ethanol precipitation, ion-exchange and gel permeation chromatography to fraction ginseng polysaccharides into two groups, namely neutral (WGPN and WGPA-N) and acidic polysaccharides (WGPA-1-RG, WGPA-2-RG, WGPA-1-HG, WGPA-2-HG, WGPA-3-HG and WGPA-4-HG).
WGPN and WGPA-N were composed of Glc, Gal and Ara. The proportion of monosaccharide in turn were 96.3%, 3.3%, 1.3% in WGPN and 66.3%, 18.0%, 15.7% in WGPA-N. We knew both of them basically consisted of starch-like glucans by testing with iodine. In addition, WGPA-N also contained about 30% free AG.
Gal and Ara were the main monosaccharide in WGPA-1-RG and WGPA-2-RG, with the ratios of 1.7:1.0 and 1.1:1.0 respectively. The total amount of Gal and Ara accounted for 90.2% in WGPA-1-RG and 84.9% in WGPA-2-RG. Besides this, both of them contained Glc, Rha, Man, GalA and GlcA. Based on NMR analysis, researchers concluded that WGPA-1-RG and WGPA-2-RG consisted of major neutral sugars and minor acidic sugars that belong to the RG-I pectins. Further analysis demonstrated that WGPA-2-RG contained a small backbone of alternating Rha and GalA, attached long AG-II side chains via the C-4 of the Rha residues [22]. About 42.9% of the Ara residues are at the non-reducing end, while the other Ara residues are 1,5- and 1,3,5-linked forms. The AG-II chains are composed of 1,3-linked Gal with substituents of Ara and/or Gal at the O-6 positions.
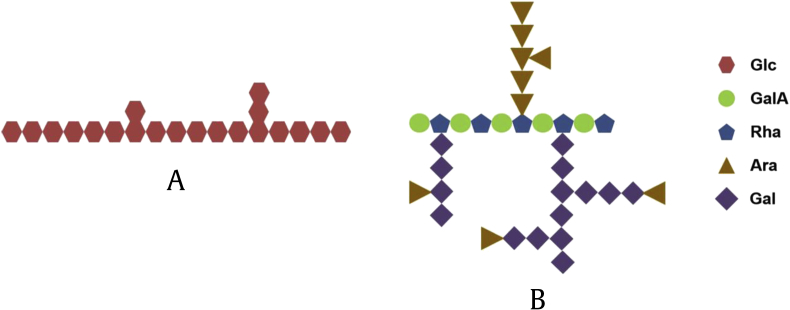
The remaining four acidic polysaccharides (WGPA-1-HG, WGPA-2-HG, WGPA-3-HG and WGPA-4-HG) were mainly composed of GalA. Their order of GalA content was WGPA-1-HG (62.4%) < WGPA-2-HG (83.6%) < WGPA-3-HG (90.9%) < WGPA-4-HG (92.1%). Structure analysis demonstrated that they were HG-rich pectins with different degrees of methyl-esterification, ranging from 0% to 30%. In addition, researchers also tested the molecular weights of six acid polysaccharides which are approximately between 3.5 × 103 and 1.1 × 105. The detailed data for structural characterization of ginseng were summarized as follows (Fig. 2, Fig. 3).
Fig. 2.
The preparation process and composition analysis of GP.
Fig. 3.
Structure characterization of polysaccharide chains. (A) Starch-like glucans. (B) Acidic pectins.
In vivo splenic lymphocyte proliferation assay, WGPN and WGPA induced T and B cell proliferation in a dose-dependent manner. Moreover, RG-I-rich pectins have more potent activities than the HG-rich pectins. The AG side chains are essential structures for stimulating NO secretion and lymphocyte proliferation. Zheng et al found GP extracted by EDTA solution contained the most abundant content of RG-I-type pectin and showed better lymphocyte proliferation property. On this basis, we can conclude that GP do have a regulating effect on the immune system. Although the structure-activity relationship of polysaccharides is unclear, these results have provided a basic support for our following discussion.
3. Bioactivities
With the deepening study of GP's structure, researchers also gained a more comprehensive understanding of its bioactivities over the past few decades. On this basis, we will make a brief introduction.
Byeon et al [23] discovered the mechanism of immune stimulation by an acidic polysaccharide extracted from Korea red ginseng. GP activate macrophage through transcription factors (NF-κB and AP-1) and their upstream signaling enzymes (ERK and JNK). Activation of MAPK ERK1/2 has been shown to play an important role in Th1/Th2 polarization [24]. ERK has been normally associated with proliferation and growth factors. JNK and p38 are induced by stress responses and cytokines, mediating differentiation and cell death. Several studies have described the involvement of JNK and p38 MAP kinase in inflammation [25]. Transcription factor NK-κB plays a crucial role in acute and chronic inflammation because it binds to the consensus DNA sequences of pro-inflammatory and antiinflammatory genes [26]. Beyond these, GP can reduce immune damage through increasing production of CD25+ Tregs. On this view, we know GP plays a bidirectional role in immune system through immunostimulation and immunosuppression. Furthermore, it is reported that neutral ginseng polysaccharides can reduce inflammation by inhibiting inflammatory-related mediator (NO) and cytokines (TNF-α, IL-6, and IL-1β) release [18]. Oxidative stress and dopamine signaling are also involved in the mechanisms mediated of GP [27]. Noticeably, GP can not only exert directly pharmacological effect on human body, but also exert indirect effect, that is, promoting the absorption of saponins. In intestinal microenvironment, GP played prebiotic-like effect by simultaneously stimulating the growth of two most important probiotics: Lactobacillus spp. and Bacteroides spp [11]. This indicates that GP has great potential in maintaining homeostasis of gut microbiota. However, we believe that GP also have many other unknown activities to be discovered. All of these bioactivities provide the foundation for its eventual use in the treatment of NSDs.
4. Effects of ginseng polysaccharides in nervous system diseases
GP has great effects on NSDs due to its unique structure. It works not only by directly exerting effect on the nervous system, but also indirectly on other systems to exert neuroprotective effect. The information currently available for pharmacological activity of GP in NSDs summarized in detail below (Fig. 4).
Fig. 4.
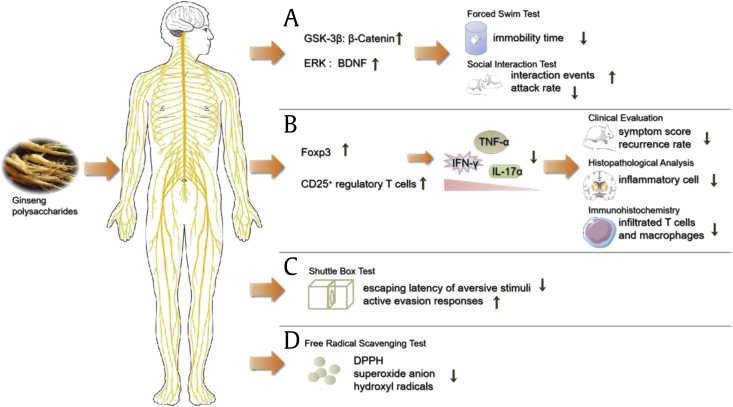
The pharmacological activity and mechanism of GP in nervous system. (A) The Antidepressant-Like Effect of WGPA. (B) Protective effects against MS. (C) Protective effects on learning and memory. (D) Antioxidant activity. (Some figures were produced using Servier Medical Art. https://www.servier.com).
4.1. Antidepressant-like effect
Depression is one of the most common psychiatric disorder [28], leading to increased risk of obesity, diabetes, and mortality [[29], [30], [31]]. With increasing pressure of modern society, number of patients with depression is bound to increase progressively. The World Health Organization has projects that, depression will become the second leading cause of disability worldwide by 2020 [32,33]. It is generally believed that depression is directly related to cellular resilience and impairments of structural plasticity [34]. Effective antidepressant drugs mainly regulate the cell signal transduction pathway to achieve therapeutic goal [35]. Noticeably, preclinical studies have shown that pro-inflammatory factors can even induce major depression in people without a history of mental disorders. This suggests that inflammatory response also plays a significant role in the pathogenesis of depression. In previous studies, ginseng extract has been demonstrated that it can relieve depression in post-menopausal women [36], but not in elderly depressed patients. The reason for this phenomenon may be the different ginseng active ingredients applied in different experiments.
Wang et al [37] have examined the antidepressant-like effect of WGPA. Behavioral tests included: spontaneous activity; elevated plus-maze; social interaction and forced swim test. Structural analysis demonstrated that WGPA contained RG-I-rich pectins and HG-rich pectins, as well as a number of AG [19]. WGPA increased the expression of β-Catenin and brain-derived neurotrophic factor (BDNF) in the hippocampus of mice, which enables us to understand the structural and functional plasticity of the brain [38]. Both β-Catenin and BDNF have been proven to be the definitive mechanism of antidepressants (lithium and valproate) to nourish nerves and treat depression [39]. β-Catenin is affected by GSK-3β signaling pathway, which is closely associated with depression by affecting synaptic plasticity and neurogenesis [40]. BDNF is an important regulatory protein in ERK signaling pathway. It is generally accepted that GSK-3β and ERK signaling pathways are two major signaling pathways that affect depression. Therefore, WGPA reduced immobility time of mice in the forced swim test, which served as a widely accepted test for antidepressant-like effect [41]. The appreciable result was also verified in the social interaction test. Compared with the control group, the number of interaction events increased while the attack rate decreased in the WGPA-treated group [42]. Therefore, researchers concluded that WGPA may increase the plasticity of neurons and cell resilience by regulating ERK and GSK-3β signaling pathways, thus reversing the death of nerve cells, and ultimately achieving the purpose of anti-depression [34].
4.2. Protective effects against multiple sclerosis
Multiple sclerosis (MS) is an inflammatory autoimmune disease characterized by neuronal demyelination and axonal injury in the central nervous system [43]. Generally, in terms of clinical course it can be categorized into four types: relapsing remitting multiple sclerosis, secondary progressive multiple sclerosis, primary progressive multiple sclerosis, and progressive relapsing multiple sclerosis [44,45]. Globally, about 2.5 million people suffer from this disease. Among them approximately 87% can be diagnosed as relapsing remitting multiple sclerosis [46]. It cause a series of symptoms, such as blurred vision, muscle stiffness and cognitive deficits [47,48], and even lead to paralysis [47]. The onset of MS begins with infiltration of macrophages, and activated microglials [49] play an important role in the progression [50]. Considering the involvement of immune and inflammatory responses in its pathology and the effect of GP on the immune system, researchers began to study the effect of GP on MS.
Experimental allergy encephalomyelitis, whose immune pathogenesis and lesions is similar to MS, is usually used as the appropriate animal model [51]. Bing et al [52] found WGPA induced the production of CD4+CD25+ regulatory T cells in both spleen and central nervous system through activation of transcription factor Foxp3. Various components produced under pathological conditions, such as vasoactive intestinal peptide, α-galactosylceramide and rapamycin, can be inhibited by CD4+CD25+ regulatory T cells to achieve therapeutic effect. It also induced suppression of autoimmunity mediation and down-regulated pro-inflammatory cytokines [52]. In the experiment group, WGPA decreased IFN-γ, IL-17 α, TNF-α production to 52.6, 57.0 and 52.2% respectively, and consequently relieved the destruction of the myelin sheath and axon. Compared to control group, the WGPA-treated group showed a decline in clinical symptom score (the lower the score, the lighter disease symptoms of the mice). At the same time, the number of relapses dropped by about 40%. Furthermore, histological examinations were consistent with its clinical findings and showed a significant decrease in infiltration of inflammatory cells [52]. The fact that depletion of CD25+ cells results in the disappearance of the beneficial therapeutic effect of WGPA, on the other hand, further verifies CD25+ cells are an important target for therapy [53]. Here, we have sufficient evidence to believe that WGPA play an important role in the treatment of MS. MAPKs (p38, JNK, and ERK 1/2) and NF-kB signaling pathways are involved in the activation of immune system during inflammatory neurodegeneration. Lee et al found Korean red ginseng extract alleviates alleviate demyelination by reducing inflammatory cells infiltration, diminishing the expression levels of proinflammatory mediators (IL-6, IFN-g, and COX-2), enhancing the expression levels of growth factors (IGF-1, TGFb, and VEGF-1), and decreasing the activation of the p38 MAPK/NF-kB signaling pathway in the spinal cords of rats [54].
4.3. Protective effects on learning and memory
Higher organisms can adjust their behavior based on past experience to survive in a complex environment, all of which benefit from the neuroplasticity [55]. Neuroplasticity refers to structural changes in the brain that occurred by strengthening or weakening connection between neuronal cells [56]. Reduced neuroplasticity can lead to a decline in learning and memory. Memory loss is also the major symptom of Alzheimer's disease. Current researches on neuroplasticity mainly focus on nutritional mechanisms (BDNF, IGF-1 and VEGF), synaptic activity, neurogenesis and angiogenesis [55]. Previous studies have demonstrated that ginseng can significantly increase neuroblasts as well as the expression of CDNF and CNTF mRNA in the hippocampus, then improving the learning and memory ability of mice. Jin et al concluded that ginsenoside Rg1 was capable of ameliorating LPS-induced deficit in working and spatial memory, and the beneficial effects were, at least partially, mediated through alterations in ACh levels, AChE activity and α7 nACh receptor expression [57]. As we mentioned in the part of anti-depression, WGPA can enhance neuroplasticity by increasing the number of BDNF [58,59]. Accordingly, GP also have beneficial effect on learning and memory.
Lyubimov et al [60] studied the role of a polysaccharide fraction of Korean red ginseng in learning and memory by shuttle box experiment. In order to ensure objectivity, researchers designed simple and hard models respectively. In test, the learning level of the mice was judged by two criteria: escaping latency of aversive stimuli and the number of conditional active evasion responses [60]. Before administration, the experimental and control groups performed consistently in all tests. By administering GP, both of these indicators in experimental group were significantly improved; in detail, latent period of escape from the aversive stimulus decreased while the number of active escape responses increased. Once the drug was discontinued, the beneficial effects also disappeared. Thus, its protective efficacy on learning and memory was not a random event. Although GP directly induced these beneficial effects, the mechanisms underlying these effects remained unknown. Heo et al [61] found Korean red ginseng showed cognitive benefits for the long-term management of patients with Alzheimer's disease. Unlike several acetylcholinesterase inhibitors, which could not block or reverse the cognitive decline, its efficacy on cognitive deficit can be maintained for at least 2 yr. Xu et al [62] found water-soluble ginseng oligosaccharides protected against scopolamine-induced cognitive impairment by decreasing expression of IL-1β, IL-6 and astrocyte activation in the hippocampus. This suggested that GP's anti-neuroinflammatory effect may be an important mechanism for its protective effects on learning and memory.
4.4. Antioxidant activity
According to the famous “free radical” theory, we know that our body is producing free radicals incessantly during normal metabolism. However, due to the existence of endogenous antioxidant defense machinery, the production and elimination of free radicals are in a dynamic equilibrium state [63]. Noticeably, once free radicals are produced in excess of the body's ability to remove them, they will cause a series of cellular damage and lead to disease [64,65]. The brain's high energy needs, combined with its rich lipid content and low antioxidant capacity, make it a prime target for oxidative damage [66]. Many NSDs (e.g. Alzheimer's disease, Parkinson's disease) [67], have been clearly reported to be associated with oxidative damage to nervous system [68]. For example, Amyloid beta is considered to be one of the main pathological features of Alzheimer's disease. The ROS production induced by it will lead to lipid peroxidation and damage of cell membrane permeability, thus increasing the internal flow of calcium ions and significantly influencing cognitive functions. As a result, more and more attention has been paid to the important role of antioxidants in nervous system.
Chen et al [69] conducted experiments in vitro about the scavenging ability of GP on DPPH free radicals, superoxide anion radicals and hydroxyl radicals. The results showed that it had appreciable scavenging ability to these three free radicals and showed dose dependence in a certain concentration range [27]. Due to the existence of dose-effect relationship, researchers compared the content of GP in above-ground and under-ground and found the latter is higher. At the same time, compared with VC, the scavenging ability of GP is more easily affected by concentration. With the concentration increasing, its clearance rate can even reach more than 60%. Furthermore, when the concentrations remained consistent, the scavenging ability of phosphorylated derivative was increased by about 10% [69]. This indicated that chemical modification can enhance the antioxidant activity significantly. Yang et al [70] also found that, the lower molecular weight of polysaccharides, the stronger scavenging ability it possessed on DPPH and ABTS radicals. This suggested that molecular weight may be an important factor affecting its antioxidant capacity.
5. Discussions
In 2001, the world's top scientific journal, science, wrote that the study of active polysaccharides would allow us to understand ourselves thoroughly and finally control our own fate. As one of the four basic substances, polysaccharides play an important role in organisms. Ginseng is rich in neutral and acid polysaccharides. Both of them have been proven to have good therapeutic effects on NSDs. These effects are mostly achieved by regulating the immune system and inflammatory response [71]. Li et al [16] have characterized the core structure of ginseng neutral polysaccharide with the immune-enhancing activity. By the analytical, we know it was composed of t-Araf, 5-Araf, 3, 5-Araf, t-Glcp, 4-Glcp, 4, 6-Glcp, 4-Galp, 6-Galp and 3, 6-Galp, and contained a glucan with →4)-α-D-Glcp-(1→) and →4,6)-α-D-Galp-(1→ residues as the backbone and a AG domain. Dentritic cells are critical antigen-presenting cells in immune responses. Kim et al found GP exerted immunomodulatory activity by inducing maturation of dentritic cells [72]. Wang et al [17] also obtained a novel neutral ginseng polysaccharide (Mw = 3.1kDa), which was composed of Glc and Gal in a molar ratio of 1:1.15 and could inhibit inflammation. Moreover, GV-971, a new drug for Alzheimer's disease, reduced the accumulation of relevant metabolites and inflammation in the brain by restoring the balance of gut microbiota. Therefore, we believe that the regulatory effect of GP on gut microbiota has great research potential in neuroprotection.
However, ginseng obtained from diverse origins and processing methods also differ in composition and function. In addition, different extraction and purified methods make it difficult to analyze for research team. Due to the presence of starch granules in large amounts, some novel and active polysaccharides can not be extracted from ginseng. The analysis of GP mostly focuses on the composition of monosaccharides, repeating units, backbone and the degree of branching. There are few studies on the more detailed structure, which is also owing to the complex structure of polysaccharides. Moreover, most of the experiments were done with crude polysaccharides, rather than purified polysaccharides [73]. As we all know, different components interact with each other, and crude polysaccharides may contain antagonistic components that counteract the effects of polysaccharides, which is detrimental to our research. Therefore, more experiments on purification and structure analysis should be carried out, in order to obtain GP with higher purity and better activity.
In practice, challenges in drug development of NSDs mainly put down to the blood–brain barrier (BBB) [74]. BBB is a highly functionalized vascular border that consists of brain endothelial cells, basement membrane, pericytes and astrocyte end-feet [75]. It is indispensable for maintaining the homeostasis of central nerves system [76], protecting the brain from toxins while ensuring the transport of nutrients to the brain. However, every coin has two sides. Unfortunately, many drugs used to treat NSDs yielded disappointing outcomes because they cannot successfully pass BBB. From the perspective of similar solubility of materials with similar structure, BBB is lipophilic while polysaccharides are hydrophilic. Thankfully, modern studies have shown that the transport of polysaccharides through BBB is associated with glucose transporter 1, not simply homogeneous. This discovery provides a theoretical basis for further research on the feasibility of GP as a potential neuroprotective agent.
This is the first review of the therapeutic effects of GP on nervous system. Deeply researches of structure-activity relationship still need to be carried out. This is just the beginning.
Conflicts of Interest
The author declares no conflicts of interest.
Contributor Information
Na Wang, Email: 17864191105@163.com.
Xianlei Wang, Email: wangxianleihyzx@shandong.cn.
Mengjiao He, Email: 17864190915@163.com.
Wenxiu Zheng, Email: m17862987280@163.com.
Dongmei Qi, Email: dongmeiqi@126.com.
Yongqing Zhang, Email: zyq622003@126.com.
Chun-chao Han, Email: chunchaoh@126.com.
Funding sources
This work was supported by the Project of Shandong Province Key Research and Development Program [Grant No. 2017YYSP030]. Ji'nan Science and Technology Project [Grant No. 201303055]. Major Science and Technology Innovation in Shandong Province [Grant No. 2017CXGC1307]. Key Laboratory of Classical Theory of Traditional Chinese Medicine, Ministry of Education. Shandong Provincial Key Laboratory of Traditional Chinese Medicine for Basic research and Traditional Chinese Medicine Resources and Utilization Innovation Team.
References
- 1.Nery T.G.M., Silva E.M., Tavares R., Passetti F. The challenge to search for new nervous system disease biomarker candidates: the opportunity to use the proteogenomics approach. J Mol Neurosci. 2019;67(1):150–164. doi: 10.1007/s12031-018-1220-1. [DOI] [PubMed] [Google Scholar]
- 2.Magistretti P.J., Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86(4):883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Cragnolini A.B., Lampitella G., Virtuoso A., Viscovo I., Panetsos F., Papa M., Cirillo G. Regional brain susceptibility to neurodegeneration: what is the role of glial cells? Neural Regen Res. 2020;15(5):838–842. doi: 10.4103/1673-5374.268897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco R., Fernández-Suárez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:65–86. doi: 10.1016/j.pneurobio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Rokot N.T., Kairupan T.S., Cheng K.C., Runtuwene J., Kapantow N.H., Amitani M., Morinaga A., Amitani H., Asakawa A., Inui A. A role of ginseng and its constituents in the treatment of central nervous system disorders. Evid Based Complement Alternat Med. 2016;2016:2614742. doi: 10.1155/2016/2614742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia D., Deng Y., Gao J., Liu X., Chu J., Shu Y. Neuroprotective effect of Panax notoginseng plysaccharides against focal cerebral ischemia reperfusion injury in rats. Int J Biol Macromol. 2014;63:177–180. doi: 10.1016/j.ijbiomac.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates g protein-coupled lysophosphatidic acid receptors with high affinity. Molecules & Cells. 2012;33(2):151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira S.S., Passos C.P., Madureira P., Vilanova M., Coimbra M.A. Structure-function relationships of immunostimulatory polysaccharides: a review [published correction appears in Carbohydr Polym. 2016 Aug 20;147:557-558] Carbohydr Polym. 2015;132:378–396. doi: 10.1016/j.carbpol.2015.05.079. [DOI] [PubMed] [Google Scholar]
- 9.Sun L., Wu D., Ning X., Yang G., Lin Z.H., Tian M.H., Zhou Y.F. α-Amylase-assisted extraction of polysaccharides from Panax ginseng. Int J Biol Macromol. 2015;75:152–157. doi: 10.1016/j.ijbiomac.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Song Y.R., Sung S.K., Jang M., Lim T.G., Cho C.W., Han C.J., Hong H.D. Enzyme-assisted extraction, chemical characteristics, and immunostimulatory activity of polysaccharides from Korean ginseng (Panax ginseng Meyer) Int J Biol Macromol. 2018;116:1089–1097. doi: 10.1016/j.ijbiomac.2018.05.132. [DOI] [PubMed] [Google Scholar]
- 11.Shen H., Gao X.J., Li T., Jing W.H., Han B.L., Jia Y.M., Hu N., Yan Z.X., Li S.L., Yan R. Ginseng polysaccharides enhanced ginsenoside Rb1 and microbial metabolites exposure through enhancing intestinal absorption and affecting gut microbial metabolism. J Ethnopharmacol. 2018;216:47–56. doi: 10.1016/j.jep.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Zhou S.S., Xu J., Zhu H., Wu J., Xu J.D., Yan R., Li X.Y., Liu H.H., Duan S.M., Wang Z. Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci Rep. 2016;6:22474. doi: 10.1038/srep22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arzani M., Jahromi S.R., Ghorbani Z., Vahabizad F., Martelletti P., Ghaemi A., Sacco S., Togha M. Gut-brain Axis and migraine headache: a comprehensive review. J Headache Pain. 2020;21(1):15. doi: 10.1186/s10194-020-1078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y. Structure and biological activities of the polysaccharides from the leaves, roots and fruits of Panax ginseng C.A. Meyer: an overview. Carbohydr Polym. 2011;85(3):490–499. [Google Scholar]
- 15.Qi Y.L., Li S.S., Qu D., Chen L.X., Gong R.Z., Gao K., Sun Y.S. Effects of ginseng neutral polysaccharide on gut microbiota in antibiotic-associated diarrhea mice. Zhongguo Zhong Yao Za Zhi. 2019;44(4):811–818. doi: 10.19540/j.cnki.cjcmm.20181129.002. [DOI] [PubMed] [Google Scholar]
- 16.Li B., Zhang N., Feng Q., Li H., Wang D., Ma L., Liu S., Chen C., Wu W., Jiao L. The core structure characterization and of ginseng neutral polysaccharide with the immune-enhancing activity. Int J Biol Macromol. 2019;123:713–722. doi: 10.1016/j.ijbiomac.2018.11.140. [DOI] [PubMed] [Google Scholar]
- 17.Wang L., Yu X., Yang X., Li Y., Yao Y., Lui E.M., Ren G. Structural and anti-inflammatory characterization of a novel neutral polysaccharide from North American ginseng (Panax quinquefolius) Int J Biol Macromol. 2015;74:12–17. doi: 10.1016/j.ijbiomac.2014.10.062. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X., Yu L., Bi H., Li X., Ni W., Han H. Total fractionation and characterization of the water-soluble polysaccharides isolated from panax ginseng C. A. Meyer. Carbohydr Polym. 2009;77(3):544–552. [Google Scholar]
- 19.Tomoda M., Takeda K., Shimizu N., Gonda R., Ohara N., Takada K., Hirabayashi K. Characterization of two acidic polysaccharides having immunological activities from the root of Panax ginseng. Biol Pharm Bull. 1993;16(1):22–25. doi: 10.1248/bpb.16.22. [DOI] [PubMed] [Google Scholar]
- 20.Ji L., Jie Z., Ying X., Yue Q., Zhou Y., Sun L. Structural characterization of alkali-soluble polysaccharides from Panax ginseng C. A. Meyer. R Soc Open Sci. 2018;5(3):171644. doi: 10.1098/rsos.171644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Y., Yang G., Zhao Z., Guo T., Shi H., Zhou Y. Structural analysis of ginseng polysaccharides extracted by edta solution. Rsc Advances. 2015;6(4):2724–2730. [Google Scholar]
- 22.Zhang X., Li S., Sun L., Ji L., Zhu J., Fan Y., Tai G., Zhou Y. Further analysis of the structure and immunological activity of an RG-I type pectin from Panax ginseng. Carbohydr Polym. 2012;89(2):519–525. doi: 10.1016/j.carbpol.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Byeon S.E., Lee J., Kim J.H., Yang W.S., Kwak Y.S., Kim S.Y., Choung E.S., Rhee M.H., Cho J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agrawal A., Dillon S., Denning T.L., Pulendran B. ERK1-/- mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2006;176(10):5788–5796. doi: 10.4049/jimmunol.176.10.5788. [DOI] [PubMed] [Google Scholar]
- 25.Rincón M., Flavell R.A., Davis R.A. The JNK and P38 MAP kinase signaling pathways in T cell-mediated immune responses. Free Radic Biol Med. 2000;28(9):1328–1337. doi: 10.1016/s0891-5849(00)00219-7. [DOI] [PubMed] [Google Scholar]
- 26.Ivanovska N., Saso L., Dimitrov P. Kinase inhibitors with redox and anti-inflammatory activities. Curr Top Med Chem. 2015;15(9):872–885. doi: 10.2174/1568026615666150220115838. [DOI] [PubMed] [Google Scholar]
- 27.Wang J., Li Y., Luo P., Chen Y., Xi Q., Wu H., Zhao W., Shu G., Wang S., Gao P. Oral supplementation with ginseng polysaccharide promotes food intake in mice. Brain Behav. 2019;9(9) doi: 10.1002/brb3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu W., Ma S., Qu R., Kang D. Antidepressant-like effect of saponins extracted from Chaihu-jia-longgu-muli-tang and its possible mechanism. Life Sci. 2006;79(8):749–756. doi: 10.1016/j.lfs.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Penninx B.W. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2017;74(Pt B):277–286. doi: 10.1016/j.neubiorev.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Buigues C., Padilla-Sánchez C., Garrido J.F., Navarro-Martínez R., Ruiz-Ros V., Cauli O. The relationship between depression and frailty syndrome: a systematic review. Aging Ment Health. 2015;19(9):762–772. doi: 10.1080/13607863.2014.967174. [DOI] [PubMed] [Google Scholar]
- 31.Zhang E., Liao P. Brain-derived neurotrophic factor and post-stroke depression. J Neurosci Res. 2020;98(3):537–548. doi: 10.1002/jnr.24510. [DOI] [PubMed] [Google Scholar]
- 32.Patel A. Review: the role of inflammation in depression. Psychiatr Danub. 2013;25(Suppl 2):S216–S223. [PubMed] [Google Scholar]
- 33.Kessler R.C., Bromet E.J. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanfumey L., Hamon M. Approche neurobiologique de la depression: nouvelles données [Neurobiology of depression: new data] Therapie. 2005;60(5):431–440. doi: 10.2515/therapie:2005064. [DOI] [PubMed] [Google Scholar]
- 35.Coyle J.T., Duman R.S. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38(2):157–160. doi: 10.1016/s0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 36.Wiklund I.K., Mattsson L.A., Lindgren R., Limoni C. Effects of a standardized ginseng extract on quality of life and physiological parameters in symptomatic postmenopausal women: a double-blind, placebo-controlled trial. Swedish Alternative Medicine Group. Int J Clin Pharmacol Res. 1999;19(3):89–99. [PubMed] [Google Scholar]
- 37.Wang J., Flaisher-Grinberg S., Li S., Liu H., Sun L., Zhou Y., Einat H. Antidepressant-like effects of the active acidic polysaccharide portion of ginseng in mice. J Ethnopharmacol. 2010;132(1):65–69. doi: 10.1016/j.jep.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 38.McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muneer A. Wnt and GSK3 signaling pathways in bipolar disorder: clinical and therapeutic implications. Clin Psychopharmacol Neurosci. 2017;15(2):100–114. doi: 10.9758/cpn.2017.15.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cryan J.F., Page M.E., Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182(3):335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 41.Takashima A. GSK-3β and memory formation. Front Mol Neurosci. 2012;5:47. doi: 10.3389/fnmol.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porsolt R.D., Bertin A., Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–336. [PubMed] [Google Scholar]
- 43.Ghorbani M.M., Farazmandfar T., Nasirikenari M., Abediankenari S., Meamarian A., Shahbazi M. Evaluation of IL-17 serum level, brain inflammation and demyelination in experimental autoimmune encephalomyelitis C57BL/6 mice model with different doses of myelin oligodendrocyte glycoprotein. Iran J Allergy Asthma Immunol. 2019;18(3):300–309. doi: 10.18502/ijaai.v18i3.1123. [DOI] [PubMed] [Google Scholar]
- 44.Buc M. Role of regulatory T cells in pathogenesis and biological therapy of multiple sclerosis. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/963748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lublin F.D., Reingold S.C., Cohen J.A., Cutter G.R., Sørensen P.S., Thompson A.J., Wolinsky J.S., Balcer L.J., Banwell B., Barkhof F. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Kaer L., Postoak J.L., Wang C., Yang G., Wu L. Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cell Mol Immunol. 2019;16(6):531–539. doi: 10.1038/s41423-019-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garg N., Smith T.W. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 2015;5(9):e00362. doi: 10.1002/brb3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu F., Shi M., Zheng C., Shen D., Zhu J., Zheng X., Cui L. The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neuroimmunol. 2018;318:1–7. doi: 10.1016/j.jneuroim.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Hatch M.N., Schaumburg C.S., Lane T.E., Keirstead H.S. Endogenous remyelination is induced by transplant rejection in a viral model of multiple sclerosis. J Neuroimmunol. 2009;212(1–2):74–81. doi: 10.1016/j.jneuroim.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Kasper L.H., Shoemaker J. Multiple sclerosis immunology: the healthy immune system vs the MS immune system. Neurology. 2010;74(Suppl 1):S2–S8. doi: 10.1212/WNL.0b013e3181c97c8f. [DOI] [PubMed] [Google Scholar]
- 51.Hwang I., Ahn G., Park E., Ha D., Song J.Y., Jee Y. An acidic polysaccharide of Panax ginseng ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Immunol Lett. 2011;138(2):169–178. doi: 10.1016/j.imlet.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Bing S.J., Ha D., Hwang I., Park E., Ahn G., Song J.Y., Jee Y. Protective effects on central nervous system by acidic polysaccharide of panax ginseng in relapse-remitting experimental autoimmune encephalomyelitis-induced SJL/J mice. Am J Chin Med. 2016;44(6):1099–1110. doi: 10.1142/S0192415X16500610. [DOI] [PubMed] [Google Scholar]
- 53.Hossain M.J., Morandi E., Tanasescu R., Frakich N., Caldano M., Onion D., Faraj T.A., Erridge C., Gran B. The soluble form of toll-like receptor 2 is elevated in serum of multiple sclerosis patients: a novel potential disease biomarker. Front Immunol. 2018;9:457. doi: 10.3389/fimmu.2018.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee M.J., Chang B.J., Oh S., Nah S.Y., Cho I.H. Korean Red Ginseng mitigates spinal demyelination in a model of acute multiple sclerosis by downregulating p38 mitogen-activated protein kinase and nuclear factor-κB signaling pathways. J Ginseng Res. 2018;42:436–446. doi: 10.1016/j.jgr.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cassilhas R.C., Tufik S., de Mello M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol Life Sci. 2016;73(5):975–983. doi: 10.1007/s00018-015-2102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnston M.V. Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev. 2009;15(2):94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- 57.Gulyaeva N.V. Molecular mechanisms of neuroplasticity: an expanding universe. Biochemistry (Mosc) 2017;82(3):237–242. doi: 10.1134/S0006297917030014. [DOI] [PubMed] [Google Scholar]
- 58.Jin Y., Peng J., Wang X., Zhang D., Wang T. Ameliorative effect of ginsenoside Rg1 on lipopolysaccharide-induced cognitive impairment: role of cholinergic system. Neurochem Res. 2017;42(5):1299–1307. doi: 10.1007/s11064-016-2171-y. [DOI] [PubMed] [Google Scholar]
- 59.Shaw C.A., Lanius R.A., van den Doel K. The origin of synaptic neuroplasticity: crucial molecules or a dynamical cascade? Brain Res Brain Res Rev. 1994;19(3):241–263. doi: 10.1016/0165-0173(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 60.Lyubimov I.I., Borzenkov V.M., Chepurnova N.E., Chepurnov S.A. Effect of a polysaccharide fraction of ginseng root on learning and memory in rats (using an active escape response as an example) Neurosci Behav Physiol. 1997;27(5):555–558. doi: 10.1007/BF02463901. [DOI] [PubMed] [Google Scholar]
- 61.Heo J.H., Lee S.T., Oh M.J., Park H.J., Shim J.Y., Chu K., Kim M. Improvement of cognitive deficit in Alzheimer’s disease patients by long term treatment with Korean red ginseng. J Ginseng Res. 2011;35(4):457–461. doi: 10.5142/jgr.2011.35.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu T., Shen X., Yu H., Sun L., Lin W., Zhang C. Water-soluble ginseng oligosaccharides protect against scopolamine-induced cognitive impairment by functioning as an antineuroinflammatory agent. J Ginseng Res. 2016;40(3):211–219. doi: 10.1016/j.jgr.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halliwell B. Free radicals and antioxidants: a personal view. Nutr Rev. 1994;52(8 Pt 1):253–265. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 64.Huang J., Brumell J.H. NADPH oxidases contribute to autophagy regulation. Autophagy. 2009;5(6):887–889. doi: 10.4161/auto.9125. [DOI] [PubMed] [Google Scholar]
- 65.Patel M. Targeting oxidative stress in central nervous system disorders. Trends Pharmacol Sci. 2016;37(9):768–778. doi: 10.1016/j.tips.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dias V., Junn E., Mouradian M.M. The role of oxidative stress in Parkinson's disease. J Parkinsons Dis. 2013;3(4):461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. 2017;360(1):201–205. doi: 10.1124/jpet.116.237503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiong X., Huang G., Huang H. The antioxidant activities of phosphorylated polysaccharide from native ginseng. Int J Biol Macromol. 2019;126:842–845. doi: 10.1016/j.ijbiomac.2018.12.266. [DOI] [PubMed] [Google Scholar]
- 69.Chen F., Huang G. Antioxidant activity of polysaccharides from different sources of ginseng. Int J Biol Macromol. 2019;125:906–908. doi: 10.1016/j.ijbiomac.2018.12.134. [DOI] [PubMed] [Google Scholar]
- 70.Yang X., Wang R., Zhang S., Zhu W., Tang J., Liu J., Chen P., Zhang D., Ye W., Zheng Y. Polysaccharides from Panax japonicus C.A. Meyer and their antioxidant activities. Carbohydr Polym. 2014;101:386–391. doi: 10.1016/j.carbpol.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 71.Ahn J.Y., Song J.Y., Yun Y.S., Jeong G., Choi I.S. Protection of Staphylococcus aureus-infected septic mice by suppression of early acute inflammation and enhanced antimicrobial activity by ginsan. FEMS Immunol Med Microbiol. 2006;46(2):187–197. doi: 10.1111/j.1574-695X.2005.00021.x. [DOI] [PubMed] [Google Scholar]
- 72.Kim M.H., Byon Y.Y., Ko E.J., Song J.Y., Yun Y.S., Shin T., Joo H.G. Immunomodulatory activity of ginsan, a polysaccharide of panax ginseng, on dendritic cells. Korean J Physiol Pharmacol. 2009;13(3):169–173. doi: 10.4196/kjpp.2009.13.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xing X., Cui S.W., Nie S., Phillips G.O., Douglas Goff H., Wang Q. A review of isolation process, structural characteristics, and bioactivities of water-soluble polysaccharides from Dendrobium plants. Bioactive Carbohydrates & Dietary Fibre. 2013;1(2):131–147. [Google Scholar]
- 74.Bramini M., Ye D., Hallerbach A., Nic Raghnaill M., Salvati A., Aberg C., Dawson K.A. Imaging approach to mechanistic study of nanoparticle interactions with the blood-brain barrier. ACS Nano. 2014;8(5):4304–4312. doi: 10.1021/nn5018523. [DOI] [PubMed] [Google Scholar]
- 75.Engelhardt B., Liebner S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. 2014;355(3):687–699. doi: 10.1007/s00441-014-1811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deli M.A., Veszelka S., Csiszár B., Tóth A., Kittel A., Csete M., Sipos A., Szalai A., Fülöp L., Penke B. Protection of the blood-brain barrier by pentosan against amyloid-β-induced toxicity. J Alzheimers Dis. 2010;22(3):777–794. doi: 10.3233/JAD-2010-100759. [DOI] [PubMed] [Google Scholar]