Abstract
The similarities and differences between trained immunity and other immune processes are the subject of intense interrogation. Therefore, a consensus on the definition of trained immunity in both in vitro and in vivo settings, as well as in experimental models and human subjects, is necessary for advancing this field of research. Here we aim to establish a common framework that describes the experimental standards for defining trained immunity.
Trained immunity has been defined as one form of adaptation of innate host defense mechanisms or a de facto innate immune memory. Following exposure to particular infectious agents or vaccines, trained immunity can mount a faster and greater response against a secondary challenge with homologous or even heterologous pathogens1. Trained immunity has emerged as a focal point in immunology research and has added a layer of complexity to our previous understanding of immune memory, that is, a trait limited to antigen-specific responses of the adaptive immune system. Although more than 95% of species (plants and invertebrates) rely solely on innate immunity for host defense2, immunological memory has been associated mainly with the adaptive arm of the immune response in vertebrates. However, it is highly unlikely that a critical evolutionary trait like immunological memory is restricted to adaptive immunity and has not evolved in the innate arm of immunity in the entire spectrum of living organisms. In fact, systemic acquired resistance (SAR) is a well-defined state corresponding to innate immunological memory in plants3. Similarly, the innate immune system of invertebrates (for example, mosquitoes, the bumble bee Bombus terrestris, snails, and so on) has the capacity to generate memory responses to subsequent reinfection with the same or different pathogens1. There is also compelling evidence in animal models that an initial infection or vaccination with bacteria (for example, Bacille Calmette–Guérin (BCG)), fungi (for example, Candida albicans) or helminth parasites (for example, Nippostrongylus brasiliensis) protects against heterologous infections independently of adaptive immunity1.
Furthermore, while the rationale underlying the use of adjuvants in vaccine formulations is to improve the efficacy of adaptive immunity, little attention has been given to the direct effects of adjuvants on innate immunity and early protection against infection. For instance, β-glucan (mainly encountered as a component of fungal cell walls that activates dectin-1) enhances resistance to acute infection with Staphylococcus aureus4 or chronic infection with Mycobacterium tuberculosis (Mtb)5. Similarly, administration of agonists of NOD-like receptors (for example, NOD2) or Toll-like receptors (for example, TLR9) have been shown to provide protection against Toxoplasma6 and sepsis caused by Escherichia coli7, respectively. Intriguingly, the induction of trained immunity is regulated by a unique set of mediators. For instance, BCG-mediated trained immunity requires type II interferon (IFN)8, Mtb impaired trained immunity via type I IFN9, and the inflammatory cytokines interleukin (IL)-1 and GM-CSF were essential for β-glucan-induced trained immunity10. In addition to what has been seen in experimental animal models, there is now ample evidence that trained immunity is a component of the human host response to pathogens. Epidemiological studies have shown that vaccination with certain live vaccines provides heterologous protection against unrelated pathogens. For example, BCG vaccination in newborn children provides protection not only against tuberculosis but also against respiratory tract infections and neonatal sepsis, and it significantly reduces mortality1. BCG-induced trained immunity has also been shown to provide protection against experimental models of yellow fever11 and malaria12 infection. Interestingly, the anticancer effects of BCG (for example, in bladder cancer) have also been linked to trained immunity13. Thus, trained immunity is an evolutionary trait that increases the fitness of plants, invertebrates and vertebrates against pathogenic microbes.
It is also important to emphasize that, although trained immunity improves the host’s defense against subsequent pathogenic threats, it may also be maladaptive in the context of chronic inflammatory disease, such as atherosclerosis1. Indeed, in addition to microbial products, trained immunity can also be induced by endogenous atherogenic substances, including oxidized low-density lipoprotein particles, lipoprotein (a) and catecholamines1,14. In animal models of atherosclerosis, a Western-type diet induces trained immunity, which persists even after a switch to a healthy chow diet15. Nevertheless, it is remarkable that trained immunity induced by BCG vaccination has also been involved in improved induction of immune regulation and immunological self-tolerance in models of autoimmunity such as type 1 diabetes and multiple sclerosis16. While the precise molecular mechanisms are still not fully understood, this evidence supports the broad rationale that exposure to microbes may help to antagonize conditions sharing chronic inflammation and tissue damage, such as autoimmunity. The beneficial effect of BCG vaccination, at least in some autoimmune disorders, is an interesting facet of host–microbe interplay in the pathophysiology and treatment of immune-mediated diseases.
Mechanisms of trained immunity
Immunological memory in the two arms of host defense is mediated through different processes. During the induction of adaptive immune memory, two properties are induced at the same time: (1) the specificity of the response, ensured through the rearrangement of immunoglobulin family genes and clonal expansion; and (2) the amplitude and speed of the response, mediated by epigenetic reprogramming that modulates the kinetics of gene transcription1. By contrast, the mechanisms involved in innate memory responses depend solely on epigenetic remodeling, and trained immunity appears to be devoid of specificity. It has been proposed that immune memory in innate and adaptive immunity represents an evolutionary continuum in which a more robust immune response evolved first, mediated by epigenetic mechanisms, while specificity evolved later in a subgroup of species (vertebrates) through gene recombination2.
While some of the mechanisms for epigenetic remodeling and metabolic reprogramming during trained immunity have been recently reviewed1, the duration and maintenance of chromatin-driven innate memory responses are still the subject of intense investigation. In the context of infectious disease, there are three known factors that can influence the epigenetic programming of an immune cell: (1) direct infection, (2) pathogen-associated molecular patterns (PAMPs) from microorganisms, and (3) cytokines released during the induction of the host response. We envision that these key factors impact the duration of trained immunity at a central level, in hematopoietic stem and progenitor cells (HSPCs) in the bone marrow (BM) and in circulating immune cells, and peripherally, at the tissue-specific level. It has been demonstrated that BCG, β-glucan, and a Western-type diet reprogram BM-HSPCs toward myelopoiesis and generate trained immunity8,10,15. These studies provide an explanation for why short-lived innate immune cells can acquire memory with a persistent phenotype in vivo. However, this may also impact the replacement of tissue-resident innate immune cells with new and reprogrammed HSPCs. It has been shown that, following pulmonary insults, a reduction in yolk-sac-derived alveolar macrophages is compensated for through the accumulation of BM-derived macrophages in the lung airways17. For example, infection of mice with gammaherpesviruses provided protection against allergic asthma, as it caused resident alveolar macrophages (AMs) to be replaced with BM-derived AMs18. Thus, a new imprinted AM may have a completely different functional capacity to that of an original fetal-derived AM. It has also been shown that the metabolism of AMs is significantly different from that of bone-marrow-derived macrophages (BMDMs), the latter being more glycolytic and bactericidal19. In addition, a murine model of pulmonary adenoviral infection induces trained immunity in AMs, which is dependent on T cells but independent of BMDMs20. Furthermore, the inflammatory site may also alter the functional capacity of the local stromal cells that induce trained immunity in residential innate immune cells. Strikingly, following skin inflammation, epithelial stem cells maintain prolonged chromatin accessibility at key inflammatory genes. This feature expedites and heightens their response to subsequent stressors and potentially influences stem cell cross-talk with trained immune cells21. Therefore, central and peripheral factors, or a combination of both, can impact the duration and maintenance of trained immunity.
Differentiation, priming, tolerance and training
Adaptations in innate immune compartments are exceptionally diverse, as innate immune cells demonstrate substantial plasticity and adapt to various insults such as trauma, infections and vaccination, and they continue to adapt as they leave the local microenvironment of the bone marrow and travel to the blood and tissues. It is important to note that the magnitude (low versus high dose) and duration (short versus long) of stimulation induces specific adaptations in innate immune cells that reflect their requirement to either enhance immune responses or prevent immunity and excessive immunopathology. Several such adaptive programs have been described, including cell differentiation, priming, tolerance and trained immunity (Fig. 1). Because innate immune cells can undergo any of these functional adaptive programs, it is essential to precisely define the similarities and differences between these cellular adaptations to ensure the field’s focus and avoid confusion in the literature.
Fig. 1 |. Schematic presentation of the behavior of innate immune responses during the different adaptive programs induced in innate immune cells.
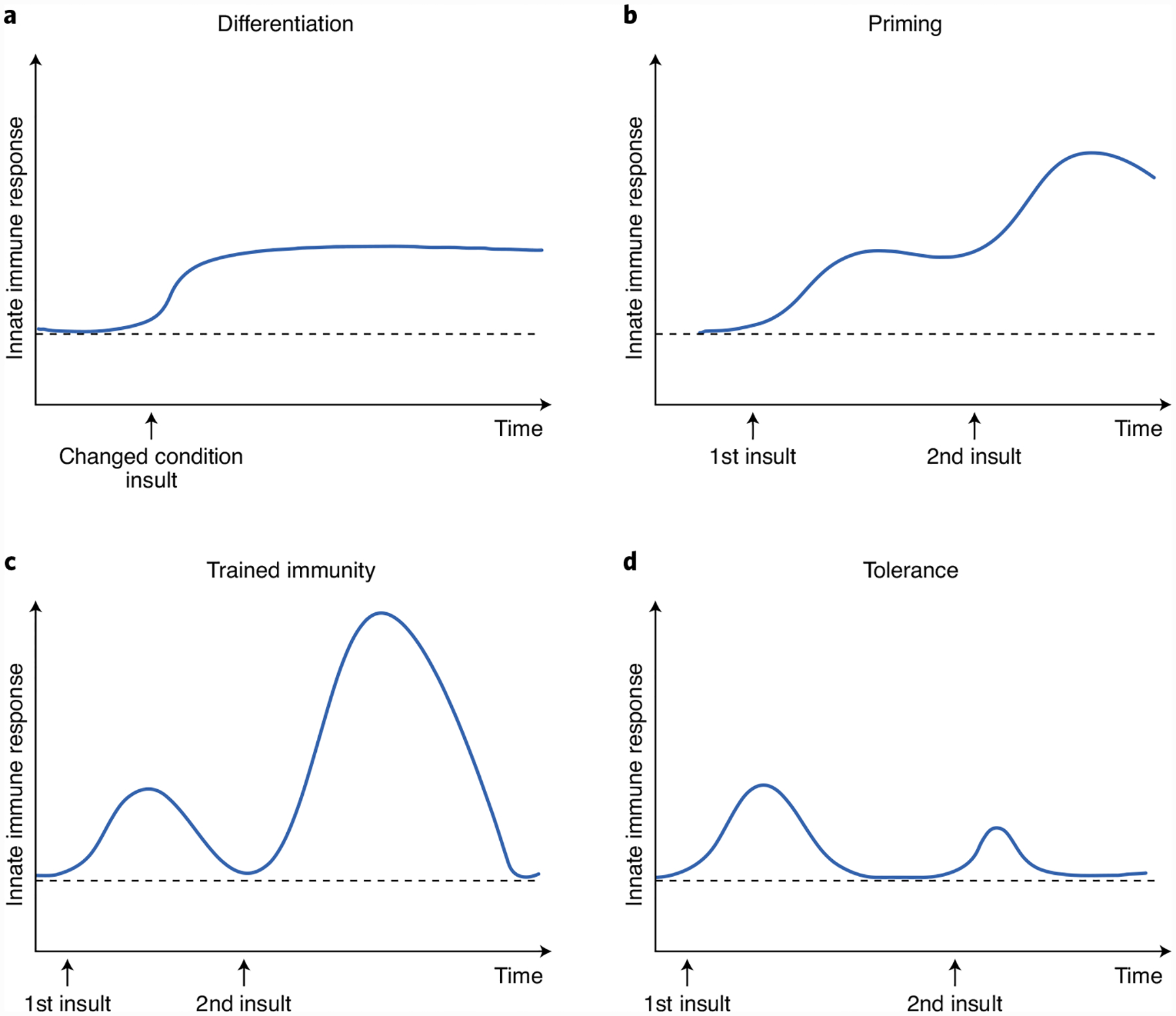
a, Differentiation. b, Priming. c, Trained immunity (innate immune memory). d, Tolerance.
The main difference between innate immune cells undergoing these different adaptive programs is their functional status prior to secondary challenges. Innate immune cell ‘differentiation’ (Fig. 1a) is often the change of an immature cell into its mature counterpart, which is defined by a long-term change in the functional program of the cell and is often accompanied by altered morphological characteristics caused by alterations of the tissue environment or chronic exposure to stimuli22. During ‘priming’ (Fig. 1b), the first stimulus changes the functional state of these cells, and their immune status (as defined by active gene transcription) does not return to basal levels before the secondary stimulation or infection. Thus, the impact of a second challenge in primed cells is often additive or synergistic with the original stimuli. In ‘trained immunity’ (Fig. 1c), in sharp contrast to priming, while the first stimulus leads to changes in the functional immune status, the immune activation status returns to the basal level following removal of the stimulus, while the epigenetic alterations persist. However, in response to homologous or heterologous challenges, both gene transcription and cell function are enhanced at much higher levels than those observed during the primary challenge. The opposite of trained immunity is innate immune ‘tolerance,’ wherein the cell is unable to activate gene transcription and does not perform its functions following restimulation (Fig. 1d). For instance, repeated or persistent exposure of macrophages to a high dose of lipopolysaccharide epigenetically enforces tolerance to prevent the expression of inflammatory genes23.
Therefore, studies aiming to investigate trained immunity need to clearly identify the activation state during initial stimulation (represented by effector functions such as cytokine and reactive oxygen species production, phagocytosis, killing, and so on) as well as after the removal of the initial insult. This is challenging when dealing with monocytes and macrophages, as their spectrum of states is far less defined than that of adaptive immune cells. However, improved nomenclature has been proposed for both in vitro and tissue-resident monocytes and macrophages24. To investigate the central effects of trained immunity via HSPCs in the BM, experiments have been conducted predominantly in vivo or ex vivo8,10. However, many in vitro experiments have been performed to study the peripheral impacts of trained immunity. Thus, experimental standards are necessary for expanding our knowledge in this exciting field of immunology.
Defining the adaptive programs of innate immune cells according to their functional state is important because the molecular mechanisms underlying these processes can often overlap. In this respect, the epigenetic and metabolic rewiring that specifically program cell differentiation, trained immunity, immune priming and tolerance can define these processes. Thus, there are unique signatures that define different cellular adaptations. For example, long-term changes in DNA methylation and stable changes in chromatin accessibility can accompany cell differentiation, whereas specific histone marks characterizing ‘latent enhancers,’ such as monomethylated histone H3 K4 (but not solely that), are often ‘tagged’ in trained immunity1. However, while specific pathways and markers differ between the various adaptive programs in innate immunity, they all use the same basic mechanisms (epigenetic, transcriptional and metabolic), but with different flavors.
Experimental models of trained immunity
The models used to study the adaptive programs in innate immunity, including trained immunity, should, therefore, reflect the definitions of these processes (Fig. 2). In vitro and in vivo models have been predominantly used to study peripheral trained immunity. The most common in vitro model system of trained immunity is the training of human peripheral monocytes, in which these cells are exposed to a stimulus (training period) for a short period of time (usually 24 h). Subsequently, cells are incubated for 5–7 days in culture medium without any stimulation. During this resting period, the functional program of the cells returns to steady state. If the primary training stimulus results in epigenetic encoding of these differentiated macrophages, they will show a heightened response to homologous or heterologous secondary stimuli. These models are fundamentally different from models of cell differentiation or priming in which the stimulus is either maintained for a long period of time to induce differentiation/priming or secondary stimulation is performed very quickly after the initial priming. In these cases, in contrast to training, cells are not allowed to return to the functional steady state before secondary stimulation.
Fig. 2 |.
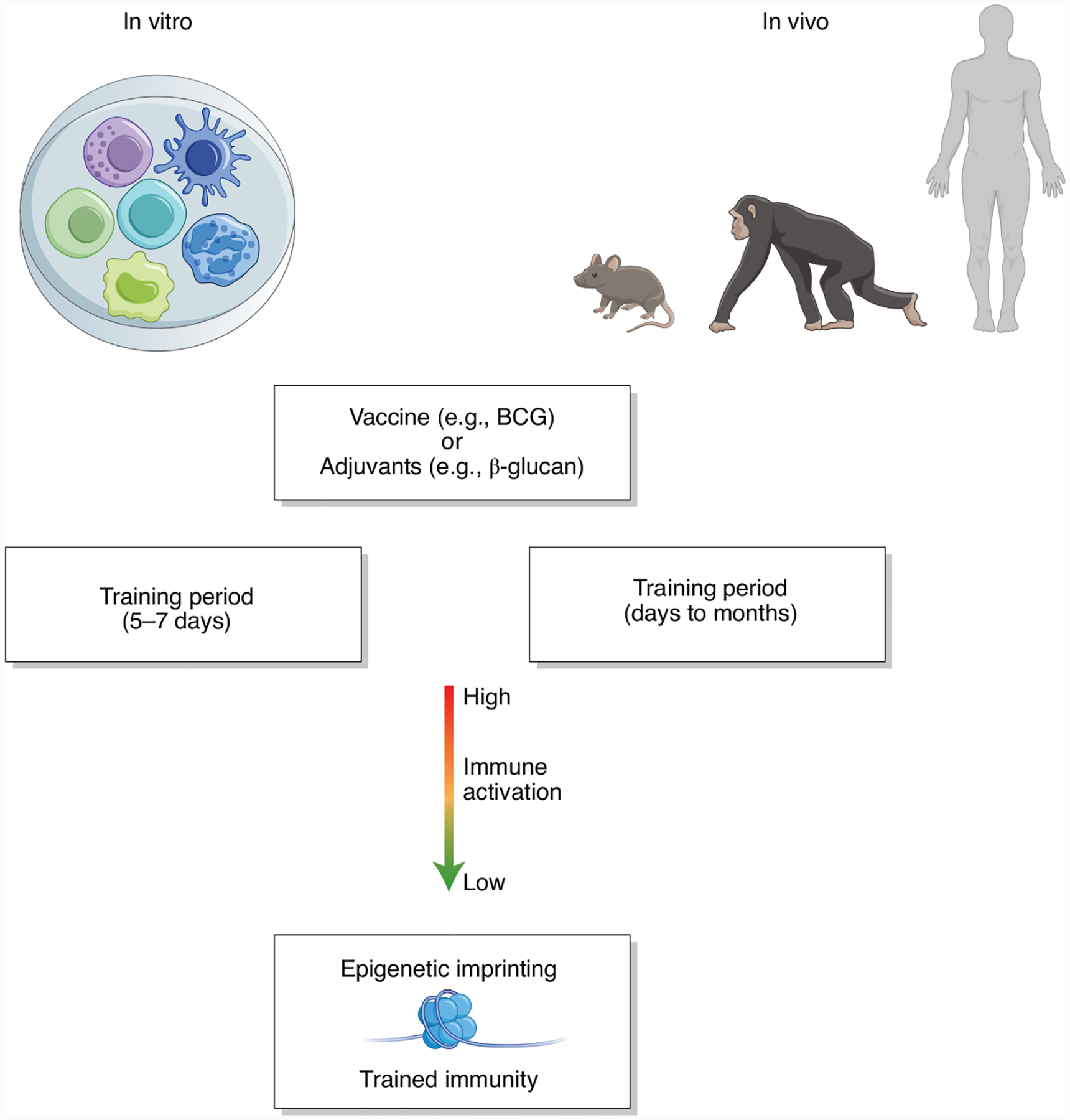
Experimental models of in vitro and in vivo trained immunity.
Similar considerations are valid for models of the induction of trained immunity in vivo, although different transcriptional and epigenetic changes can be seen in mature innate immune cells in the periphery or in bone marrow progenitors of innate immune cells. To reprogram BM-HSPCs for central trained immunity, administration of training agents (for example, β-glucan) or a vaccine (for example, BCG) leads initially to proliferation and expansion of HSPCs. Although the kinetics and dynamics of this expansion differ between model systems, HSPCs phenotypically return to a basal status before a secondary challenge experiment (Fig. 2). Murine models of trained immunity allow us to study the direct impact of BM-HSC training on the immune response to a new homologous or heterologous challenge. One current possibility to study this in vivo is to generate a chimeric or serial engraftment mouse model wherein the HSC compartment is reconstituted with trained HSCs8–10. As the reconstitution of hematopoiesis in the recipient is mediated by donor hematopoietic progenitors at earlier time points and entirely by the donor HSC compartment at later time points (16 weeks) post-transplantation, this system is an excellent model to study the long-term effects of training on HSC-mediated innate memory responses to subsequent homologous or heterologous challenges9.
Human models of trained immunity have also been established as proof of concept to study innate immune memory. BCG vaccination in healthy individuals can induce trained immunity in HSCs and circulating monocytes, which gain an enhanced protective capacity against a range of infectious agents1. The function of innate immune cells, as well as their epigenetic and transcriptional programs, can be studied before and after BCG vaccination (or after any other vaccine or experimental infection, for that matter). However, to ensure that the initial vaccine has been cleared from the organism, it is important that there is a sufficient interval between vaccination and the subsequent assessment of the innate immune system by ex vivo restimulation with a non-specific stimulus. As an example, BCG may be present at the site of vaccination for up to one month25, thus trained immunity assessments should be performed at later time points. During the first month after vaccination, the ex vivo–stimulation assay can actually be a model for studying innate immune priming. Furthermore, an ex vivo system could also be used to study (1) the bias of hematopoiesis (for example, myelopoiesis versus lymphopoiesis) in HSPCs using colony-forming unit assays and (2) the functional capacity of myeloid immune cells (for example, macrophages or neutrophils).
Perspectives and conclusions
Understanding innate immune memory is critical for deciphering new approaches to vaccine development. By dissecting the cellular and molecular mechanisms of trained immunity, we hope to develop new vaccine strategies with cross-protective efficacy against a range of infections. In addition, we can envisage more effective vaccines that combine the induction of trained immunity with adaptive immune memory. While we have made enormous progress in our fundamental understanding of trained immunity in health and diseases, accurate nomenclature and experimental standardization are important and would encourage progress in the field. By doing this work, we hope scientists who are new to trained immunity will establish accurate experimental models to close the knowledge gap in this field. Furthermore, as a more precise mechanistic description of trained immunity is developed, we will need to formulate updated recommendations on trained immunity.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Netea MG et al. Nat. Rev. Immunol 20, 375–388 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Netea MG, Schlitzer A, Placek K, Joosten LAB & Schultze JL Cell Host Microbe 25, 13–26 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Kachroo A & Robin GP Curr. Opin. Plant Biol 16, 527–533 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Di Luzio NR & Williams DL Infect. Immun 20, 804–810 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moorlag SJCFM et al. Cell Rep 31, 107634 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krahenbuhl JL, Sharma SD, Ferraresi RW & Remington JS Infect. Immun 31, 716–722 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz N et al. Infect. Immun 78, 4226–4233 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufmann E et al. Cell 172, 176–190.e19 (2018).29328912 [Google Scholar]
- 9.Khan N et al. Cell 183, 752–770.e22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitroulis I et al. Cell 172, 147–161.e12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arts RJW et al. Cell Host Microbe 23, 89–100.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Walk J et al. Nat. Commun 10, 874 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redelman-Sidi G, Glickman MS & Bochner BH Nat. Rev. Urol 11, 153–162 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Bekkering S et al. Arterioscler. Thromb. Vasc. Biol 34, 1731–1738 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Christ A et al. Cell 172, 162–175.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ristori G, Faustman D, Matarese G, Romano S & Salvetti M Curr. Opin. Immunol 55, 89–96 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Guilliams M et al. J. Exp. Med 210, 1977–1992 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machiels B et al. Nat. Immunol 18, 1310–1320 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Russell DG, Huang L & VanderVen BC Nat. Rev. Immunol 19, 291–304 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao Y et al. Cell 175, 1634–1650.e17 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Naik S et al. Nature 550, 475–480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavin Y et al. Cell 159, 1312–1326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster SL, Hargreaves DC & Medzhitov R Nature 447, 972–978 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Murray PJ et al. Immunity 41, 14–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minassian AM et al. J. Infect. Dis 205, 1035–1042 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]


