Abstract
Background
Ginsenoside Rb1 (G-Rb1), one of the major active compounds in Panax ginseng, has already been shown to reduce inflammation in various diseases. Osteoarthritis (OA) has traditionally been considered a degenerative disease with degradation of joint articular cartilage. However, recent studies have shown the association of inflammation with OA. In the present study, we investigated whether Rb1 had an antiinflammatory effect on monoiodoacetate (MIA)-induced OA in ovariectomized rats as a model of postmenopausal arthritis.
Methods
G-Rb1 at a dosage of 3 and 10 μg/kg body weight was administered every 3 days intraarticularly for a period of 4 weeks to observe antiarthritic effects. Diclofenac (10 mg/kg) served as a positive control.
Results
The administration of Rb1 significantly ameliorated OA inflammatory symptoms and reduced serum levels of inflammatory cytokines. Furthermore, G-Rb1 administration considerably enhanced the expression of bone morphogenetic protein-2 and collagen 2A and reduced the levels of matrix metalloproteinase-13 genes, indicating a chondroprotective effect of G-Rb1. G-Rb1 also significantly reduced the expression of several inflammatory cytokines/chemokines (interferon gamma (IFN-γ), monocyte chemoattractant protein-1 (MCP-1)/CCL-2, interleukin [IL]-1β, and IL-6). Histological analysis demonstrated that G-Rb1 significantly attenuated the pathological changes in MIA-induced OA in ovariectomized rats. Safranin O and toluidine blue staining further demonstrated that G-Rb1 effectively prevented the degradation of cartilage and glycosaminoglycans, respectively.
Conclusion
Overall, our results suggest that G-Rb1 exerts cartilage protective effect on MIA-induced ovariectomized OA rats, by inhibiting inflammatory mediators such as IL-6, IL-1β, MCP-1/CCL-2, cyclooxygenase-2 (COX-2), and prostaglandin E2 (PGE2). These results shed a light on possible therapeutic application of G-Rb1 in OA.
Keywords: Ginsenoside-Rb1, Hstological analysis, Monoiodoacetate, Ovariectomy, Osteoarthritis
1. Introduction
Osteoarthritis (OA) in the knees is the most common degenerative disorder, affecting more than 33.6% of the population over the age of 65 years [1], with women more affected than men [2,3]. The condition is pathologically characterized by changes in the subchondral bone matrix, cartilage degradation, and synovial inflammation.
Chondrocytes respond to degradative consequences of inflammatory cascade by increasing the rate of matrix biosynthesis and releasing antiinflammatory cytokines. However, in OA, the increase synthesis is halted by biochemical alterations take place in articular cartilage. The increased synthesis and antiinflammatory activity of chondrocytes loses the balance with increased degradative activity. At first, higher synthetic activity is confined to deeper layer of cartilage, this creates the imbalance toward degradation of upper layer, and ultimately the chondrocytes will undergo apoptosis and hasten OA progression [4]. The etiology of OA is diverse, including genetic and metabolic, aging, obesity, joint injury, or surgery. In addition, inflammatory cytokines such as interleukin (IL)-1β, IL-6, and IFN-γ play key roles in orchestrating OA progression [5]. The proinflammatory cytokines produced by chondrocytes and synovial cells during OA advancement will also induce chondrocyte apoptosis [6,7].
Several animal models have been extensively studied for OA pathogenesis as well as potential treatment strategies. Although guinea pigs, mice, and nonhuman primates can develop spontaneous OA, it usually takes more than 9 to 12 months for cartilage erosion [8], making the spontaneous model time-consuming to use in arthritis research. Ovariectomy is considered a classical method to induce osteoporosis in animal models [9]. We used monoiodoacetate (MIA) to induce OA in ovariectomized rats as an experimental model.
Current treatment strategies to control OA progression are suboptimal, mainly treating symptoms rather than providing curative treatment. In the search for alternate medicines to cure OA, two important targets are essential: proper structural maintenance of cellular phenotypes and inhibition of enzymatic degradation of extracellular matrix. Ginseng (Panax ginseng) is predominantly used as a crude substance in several Asian countries for both food and medicinal purposes. Ginsenosides are a class of natural products derived from ginseng and identified as the principal active components of ginseng in various pharmacological studies [10]. At present, more than 40 ginsenosides have been characterized to have various biological activities; among them, Rb1 is abundant and widely found to have a number pharmacological functions, including antiapoptotic, antiinflammatory, and neuroprotective activities [11,12]. Earlier studies have shown that Rb1 prevents IL-1β–induced inflammation and apoptosis in human chondrocytes [13], while in vivo studies have proven the effect of Rb1 in attenuating OA in rodent model [[14], [15], [16]]. However, the effect of Rb1 in MIA-induced OA in ovariectomized rats has not been explored.
2. Materials and methods
2.1. Chemical reagents
Ginsenoside Rb1 (G-Rb1) was procured from (EMBO Institute, Seoul, South Korea) The purity of the sample is analyzed in high performance liquid chromatography (supplementary fig.1) and monoiodoacetate (MIA), RNAlater, toluidine blue, safranin O, alcian blue, hematoxylin and eosin came from Sigma Aldrich (St. Louis, MO, USA). Phosphate buffered saline (PBS) and antibiotic-antimycotic 100 × was purchased from Gibco (NY, USA). The Biolegendplex rat inflammation marker panel was procured from Biolegend (CA, USA). The primers used for Reverse transcriptase -PCR analysis were synthesized and purchased from Bioneer Inc (Daejeon, South Korea).
2.2. Animals
Sprague-Dawley rats (female, 6 weeks old) were purchased from Koatech (Seoul, South Korea) and fed standard chow food with free access to water. The animals were acclimatized for a week before commencement of experiments. All experiments performed in this study were approved by the Chonbuk National University animal ethical and use committee (CBNU 2018-113).
2.3. Preparation of ovariectomy
Following acclimatization, rats underwent bilateral ovariectomy using a dorsal flank incision [17]. Briefly, the abdominal cavity was entered through a small incision and the ovaries along with fallopian tubes were exteriorized through the incision and then removed by a single cut. The incision was closed with absorbable sutures. Control surgeries were performed without removing the ovaries.
2.4. MIA-induced arthritis
MIA was administered to rats intraarticularly to induce OA [18]. In brief, rats were anesthetized with ether. The hair of the anesthetized rats was shaved near the knee joint area and surface sterilized using 70% ethanol. The rats were injected with 50 μL of sterile PBS containing 3 mg of MIA intraarticularly into the knee joint. In controls, 50 μL of sterile PBS alone was injected. Animals were left for a week to develop OA symptoms.
2.5. Experimental design
The rats were randomly assigned into five different groups (n = 6) after MIA injection.
-
1.
Normal control (NC): ovary exposed but not removed
-
2.
OA (Ovariectomized, MIA injected, received no treatment)
-
3.
OA + 3 μg/kg G-Rb1 (intraarticularly)
-
4.
OA + 10 μg/kg G-Rb1 (intraarticularly)
-
5.
OA + Diclofenac (10 mg/kg, intramuscularly)
2.6. Body weight and paw withdrawal threshold
Rat body weights in every group were measured once per week until the end of the experiment. OA symptoms were assessed in terms of pain sensitivity by measuring withdrawal threshold of the paw in response to mechanical stimuli using von Frey filaments. Before the assessment, the animals were caged with a wire floor net and acclimatized for at least 5 minutes. Each von Frey filament was held for 2 seconds with a frequency interval of 3 minutes between measurements.
2.7. Swelling measurements and X-ray radiography
Leg swelling (knee joint area) following MIA administration was measured precisely using a Vernier caliper, and the other (noninjected) leg served as a control. The degradation of bone structures was evaluated by X-ray radiography in at least three rats per experimental group, with representative images captured.
2.8. Immunohistochemical evaluation
After disarticulation of the knee joint, both the femur and tibia were fixed in 10% buffered formalin for 2 days, after which the bone was decalcified and embedded in paraffin. Spleen samples were embedded in paraffin following formalin treatment. Sections of both spleen and bone (thickness = 5 μm) were analyzed with hematoxylin and eosin stain (H&E stain) to observe pathophysiological changes. Later, bone sections were stained with safranin O, toluidine blue, and Alcian blue to microscopically observe changes in the content of cartilage and glycosaminoglycans. We evaluated pathological changes based on the scoring system of Mankin et al [19].
2.9. Reverse transcriptase PCR
To determine mRNA levels of bone morphogenetic protein 2 (BMP-2), matrix metalloproteinase 13 (MMP-13), COX-2, Col2A, and transforming growth factor beta (TGF-β), total RNA was extracted from cartilage tissue of the experimental groups. Total cDNA was synthesized from 1 μg of total RNA using a power cDNA synthesis kit (iNtRON biotechnology). The primer sequences used in this experiment are given in Table 1.
Table 1.
Nucleic acid sequences of the primers used for RT-PCR.
| Target | Sequence (5′ to 3′) | |
|---|---|---|
| BMP2 | Forward Reverse |
TCCTCAGCGAGTTTGAGTTGAG TGTCCAATAGTCTGGTCACAGG |
| MMP13 | Forward Reverse |
CTTCTGGCACACGCTTTTCC TCTCGGGATGGATGCTCGTA |
| Col2A | Forward Reverse |
GAGCAGCAAGAGCAAGGAGA GGCCCTATGTCCACACCAAA |
| COX-2 | Forward Reverse |
ACAATAGACGCCCAAGAAGTAGA CGGTTTGATGTCACTGTAGCTTG |
| TGF-β | Forward Reverse |
GCTGAACCAAGGAGACGGAA TTGCGACCCACGTAGTAGAC |
| β-Actin | Forward Reverse |
AACCGTGAAAAGATGACCCAGA CCGATAGTGATGACCTGACCG |
2.10. Inflammation assay
The LEGENDplex™ Rat inflammation panel assay is a fluorescence-encoded bead-based assay suitable to use in flow cytometers. This assay allows simultaneous analysis of 13 cytokines from serum samples. Among them, only IFN-γ, IL-1β, IL-6, and monocyte chemoattractant protein-1 (MCP-1)/CCL2 levels were statistically different under OA conditions when compared with the normal control group. The assay was performed according to the manufacturer's instruction. Data were analyzed using LEGENDplex software (version 8.0). The levels of CTX1 and PGE2 in the sera of rats from all the groups were analyzed by enzyme-linked immunosorbent assay according to the manufacturer's instructions.
2.11. Statistical analysis
All data are expressed as the average and standard deviation of three independent experiments. Comparisons were made using analysis of variance and two-tailed unpaired Student t test. A p values < 0.05 were considered statistically significant.
3. Results
3.1. Body weight and paw withdrawal test
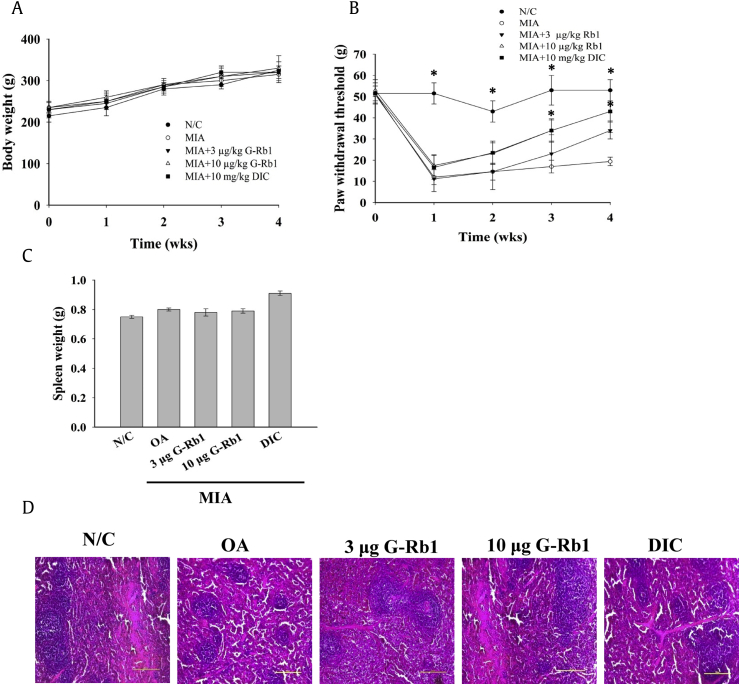
The body weight of all the animals in each group was monitored every week throughout the study period (Fig. 1A). Body weight did not change significantly among the groups other than the normal control. The OA rats showed a significant increase in paw withdrawal threshold to von Frey filament stimuli, whereas G-Rb1 dose-dependently reduced the paw withdrawal threshold. Diclofenac administration resulted in considerable improvement comparable to control levels at the end of the study period (Fig. 1B).
Fig. 1.
Administration of G-Rb1 ameliorated MIA-induced arthritis in ovariectomized rats. (A) Body weight of the rats was measured once per week until the end of the experiment. (B) MIA-induced rats were given G-Rb1 at 3 or 10 μg/kg bw and their paw withdrawal threshold was measured using the von Frey filament test. (C) G-Rb1 administration in OA rats helped regain spleen weight. Diclofenac administration enhanced spleen weight compared to the normal control. (D) H&E staining of spleen sections of various groups indicates normal morphology. Significant differences between each group and the OA group are marked *p < 0.05. G-Rb1, ginsenoside Rb1; OA, osteoarthritis; MIA, monoiodoacetate; H&E, hematoxylin and eosin stain.
3.2. Spleen weight and histology
Spleens were harvested at the end of the experimental period, and the wet organ weight from each group was measured. There were no significant changes among the groups. However there was a minor change in spleen weight in the positive control group compared with normal controls (Fig. 1C). Furthermore, examination of spleen histology revealed that there were no significant morphological changes among groups, despite a greater amount of red pulp in the positive control group (Fig. 1D).
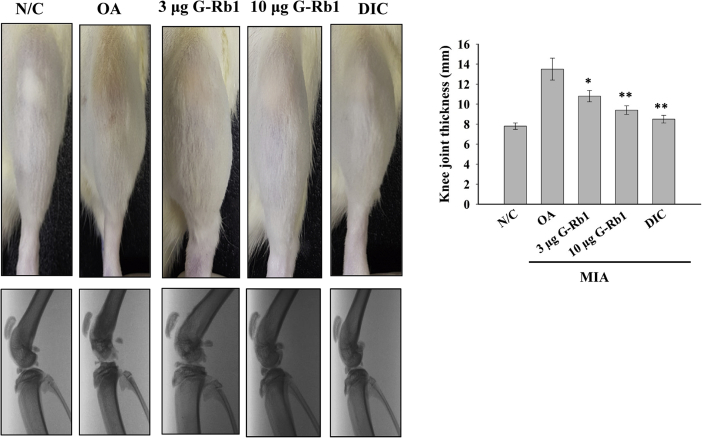
3.3. Knee joint thickness and X-ray radiography
Following MIA administration, the rats were treated with either G-Rb1 or diclofenac. The untreated group served as the OA disease model. At the end of the study period, the knee joint thickness of the animals in each group was measured using a digital Vernier caliper. Administration of G-Rb1 dose-dependently reduced the swelling induced by MIA (Fig. 2, top panel; the thickness of the knee joint was measured in millimeter units and is shown as a bar graph). MIA-induced degradation of articular cartilage was initially assessed using x-ray radiography (Fig. 2, bottom panel). OA rats administered with G-Rb1 and positive controls exhibited inhibited articular cartilage and subchondral bone degradation, while these bone tissues were severely damaged in OA rats. Furthermore, the administration of G-Rb1 protected against bone tissue degradation in a concentration-dependent manner. The results suggest that G-Rb1 significantly ameliorated the symptoms of MIA-induced OA.
Fig. 2.
G-Rb1 ameliorated swelling and bone tissue degradation in MIA-induced arthritis. Thicknesses of rat legs were measured using a Vernier caliper (top panel). The femur and tibia bones of MIA-induced OA rats administered with G-Rb1 (3 and 10 μg/kg) or DIC were analyzed by X-ray radiography (bottom panel). Knee joint thickness of the experimental groups is shown in the right panel. Significant differences between each group and the OA group are marked *p < 0.05 and **p < 0.01. G-Rb1, ginsenoside Rb1; OA, osteoarthritis; MIA, monoiodoacetate.
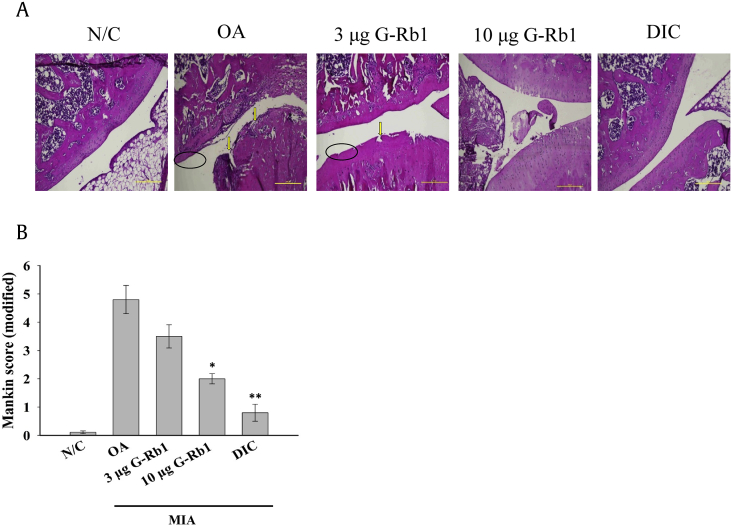
3.4. Histological evaluation
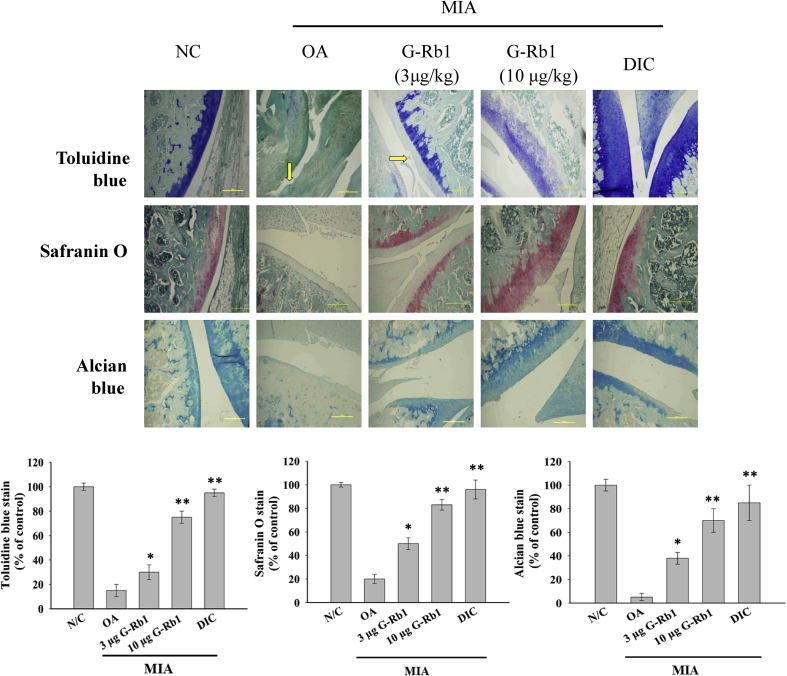
The H&E staining of articular cartilage of the different groups is shown in Fig. 3A; in control group, articular cartilage was observed to be normal. The OA group exhibited a remarkable loss of chondrocytes (hypo-cellularity) and degradation of articular cartilage (clefts in superficial and transitional zones). In contrast, the 3 μg G-Rb1 group showed an improved cartilage structure, and administration of 10 μg G-Rb1 to MIA-induced OA rats yielded a significantly higher number of chondrocytes and reconstructed cartilage. These results revealed a dose-dependent action of G-Rb1 in protecting cartilage degradation. The degradation of cartilage and subchondral bone was considerably reduced in the OA rats treated with diclofenac. Structural changes in the cartilage and subchondral bones were evaluated based on Mankin's scoring system, and the sum of scores is shown in Fig. 3B. Compared with normal controls, cartilage degradation of the OA group was severe and exhibited the highest Mankin's score among all groups. Furthermore, treatment with G-Rb1 at increasing concentrations significantly ameliorated the cartilage lesions. Mankin's score was significantly lower in the positive control group. In addition, toluidine blue, safranin O, and Alcian blue staining are shown in Fig. 4. The cartilage was stained deep violet by toluidine blue and red by safranin O staining, respectively. Alcian blue stained the cartilage a greenish-blue color. All three stains revealed a severe loss of cartilage in the OA group. However, the G-Rb1-treated groups exhibited relatively higher levels of proteoglycans in the bone tissue and cartilage than that of OA group, as determined by toluidine blue, safranin O and Alcian blue staining (Fig. 4). Furthermore, the positive control group showed a significantly higher percentage of cartilage staining.
Fig. 3.
(A) G-Rb1 ameliorated OA pathophysiological symptoms in MIA-induced ovariectomized OA rats. Ovariectomized SD rats were injected with MIA intraarticularly, and 1 week later, the rats were subjected to various treatments with G-Rb1 (3 μg and 10 μg/kg) or diclofenac once every 3 days for four weeks. The histological assessment of the protective effect of G-Rb1 against MIA-induced arthritis is shown. MIA-induced clefts in the superficial and transitional zones (arrows) and hypocellularity of chondrocytes (ellipse). Administration of G-Rb1 significantly improved pathophysiological conditions present in OA. The scale bar represents 250 μm. (B) Bar diagram represents the modified Mankin scoring of various groups. *p < 0.05 and **p < 0.01 versus OA group. G-Rb1, ginsenoside Rb1; OA, osteoarthritis; MIA, monoiodoacetate.
Fig. 4.
G-Rb1 ameliorated MIA-induced cartilage degradation. Toluidine blue, safranin O, and Alcian blue staining of articular cartilage in the different groups (magnification × 100). In toluidine blue and safranin O staining, the cartilage was stained deep violet and red, respectively. The cartilage was observed in a greenish blue color in Alcian blue staining. The arrow indicates clefts in the superficial zones. The bar diagram represents the percentage of staining area in the different groups. *p < 0.05 and **p < 0.01 versus OA group. G-Rb1, ginsenoside Rb1; OA, osteoarthritis; MIA, monoiodoacetate.
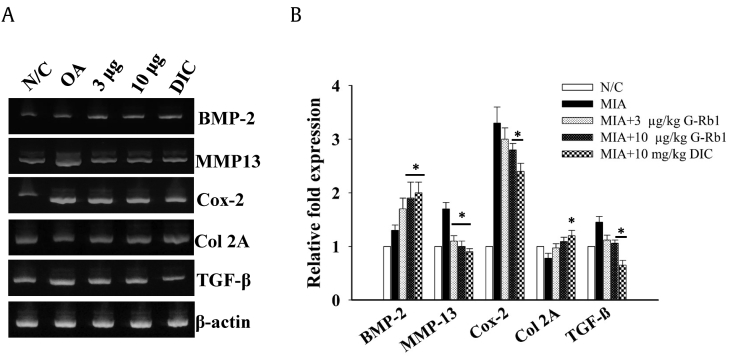
3.5. The effect of G-Rb1 on mRNA expression in chondrocytes
Reverse transcriptase PCR was performed to analyze mRNA expression in chondrocytes. When compared with the control group, the OA group showed a significant increase of MMP13, Cox-2 and TGF-β mRNA expression. However, treatment with G-Rb1 (3, and 10 μg/kg) significantly decreased mRNA expression of MMP13 and upregulate the BMP-2 expression dose dependently. G-Rb1 at 10 μg/kg reduced the expression of Cox-2 and TGF-β (p < 0.05) significantly compared with the OA group (Fig. 5).
Fig. 5.
Effects of G-Rb1 on mRNA expression in chondrocytes. Chondrocytes were isolated from various groups, and RT-PCR was performed with total RNA isolated from chondrocytes. (B) The bar diagram represents relative fold change in expression of various bone growth markers. *p < 0.05 versus OA group. G-Rb1, ginsenoside Rb1; OA, osteoarthritis; MIA, monoiodoacetate.
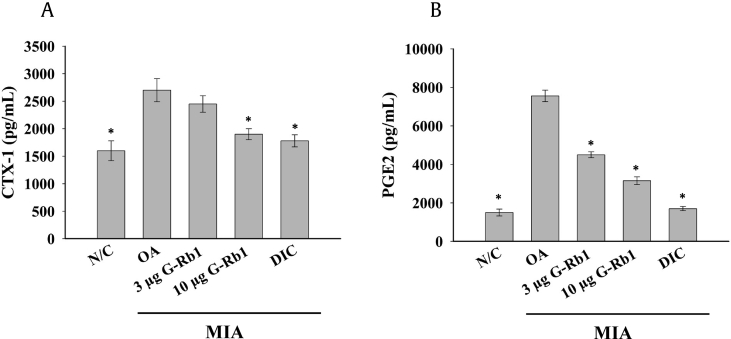
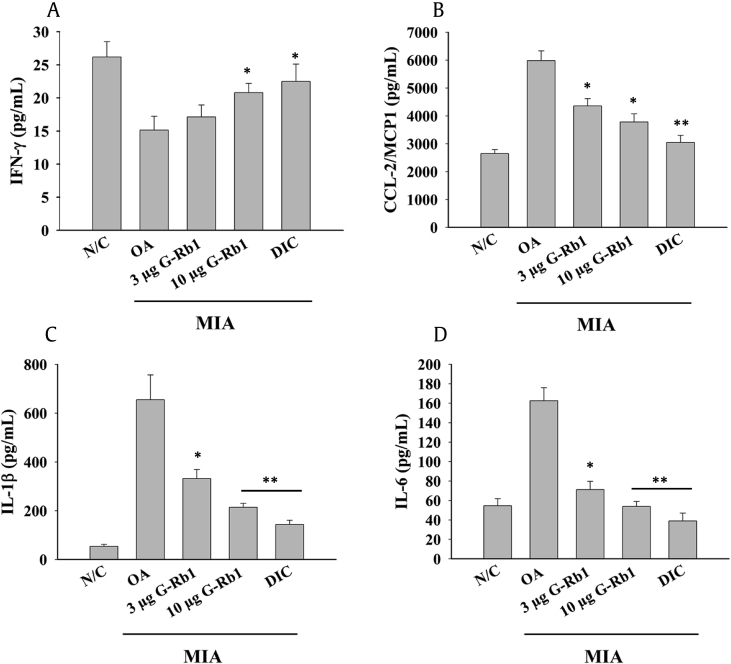
3.6. G-Rb1 ameliorates inflammatory mediators
Serum levels of CTX-1 were analyzed in all groups according to the manufacturer's instruction. There was reduced bone resorption in the G-Rb1 groups, with G-Rb1 at 10 μg/kg concentration significantly reducing the serum CTX-1 level to levels observed in the diclofenac group (Fig. 6A). In addition, PGE2 levels were significantly higher in the OA group (Fig. 6B) compared to the control group, signifying the inflammation and fever associated with OA. However, the administration of G-Rb1 dose-dependently reduced serum PGE-2 levels. Similarly, bead-based assay results (Fig. 7) revealed the downregulation of IFN-γ and upregulation of MCP1/CCL-2, IL-1β, and IL-6 in the OA group, implicating a pathological condition in the MIA-induced arthritis model. G-Rb1 administration dose-dependently ameliorated inflammatory cytokine levels.
Fig. 6.
G-Rb1 reduced serum levels of inflammatory mediators. (A) CTX1 and (B) PGE2 levels were measured using ELISA. Significant differences between each group and the OA group are marked *p < 0.05. G-Rb1, ginsenoside Rb1; OA, osteoarthritis; ELISA, enzyme-linked immunosorbent assay.
Fig. 7.
Effect of G-Rb1 on inflammatory cytokines in MIA-induced ovariectomized rats. Serum levels of various inflammatory cytokines were analyzed using a LEGENDplex rat inflammation panel that detects 13 proinflammatory cytokines in a single assay. Only four inflammatory cytokines were significantly different between the control and OA groups. (A) IFN-γ, (B) CCL-2/MCP1, (C) IL-1β, and (D) IL-6. *p < 0.05 and **p < 0.01 versus OA group. G-Rb1, ginsenoside Rb1; OA, osteoarthritis; MIA, monoiodoacetate; IL, interleukin.
4. Discussion
Many studies have described the pharmacological importance of G-Rb1 [[20], [21]]. However, the role of G-Rb1 in an MIA-induced ovariectomized OA rat model has not been elucidated. To address this lack in knowledge, we examined the pharmacological effects of G-Rb1 on OA in MIA-administered ovariectomized rats as a disease model. MIA is an inhibitor of glyceraldehyde-3-phosphate dehydrogenase, intervening with glycolysis activity and leading to chondrocyte death [22] when administered in the intraarticular region. Rats in all groups tolerated the treatment and did not show any significant change in body weight during the treatment period with either G-Rb1 or the positive control. This result revealed that G-Rb1 did not exhibit any toxicity or side-effects. A week after MIA injection, the knee joint was swollen excessively, along with degradation of the subchondral bone and synovium, as observed in human OA [[23], [24]]. The most vital factor in generating OA is pain; therefore, weight load bearing ability was assessed during free walking. Paw withdrawal pressure was also significantly increased after G-Rb1 treatment in a way comparable to the positive control group, suggesting that G-Rb1 could serve as an antiinflammatory agent. Histological analysis of spleen tissue from various groups did not reveal any significant differences, despite a higher splenic weight in the positive control group. The H&E staining of articular cartilage tissue revealed a protective effect of G-Rb1. The higher percentage of the cartilage area stained with safranin O, toluidine blue and Alcian blue indicated the effectiveness of G-Rb1 in protecting chondrocytes from MIA-induced OA in ovariectomized rats. A significant increase in CTX-1 levels in the serum of OA rats indicated bone resorption in comparison with the control group. These findings are in accordance with Høegh-Anderson et al., who have shown that ovariectomy can induce bone turnover that is similar to the menopause transition [9]. Gong et al., further showed that administration of ginsenoside recovered the bone mineral density of ovariectomized rats suffering from osteoporosis [25]. Our results also revealed that G-Rb1 inhibited or delayed bone loss by decreasing bone turnover. Several studies have unveiled a link between inflammation and OA pathogenesis [[26], [27], [28]] PGE2, an important inflammatory mediator, is the major product of COX activity that functions pathologically in several inflammatory diseases [29]. PGE2 is capable of upregulating MMPs and other inflammatory cytokines [30]. Therefore, we examined the effects of G-Rb1 on PGE2 levels in the sera of the various groups. As expected, PGE2 levels were significantly higher in the OA group, but G-Rb1 dose-dependently reduced the levels. The mRNA expression levels of MMP-13 were also decreased markedly by G-Rb1 in a dose-dependent manner. This study suggests that G-Rb1 exerts antiinflammatory activity by partially inhibiting both production and mRNA expression of inflammatory mediators of chondrocytes in rat knee joints. Also, it was well known that proinflammatory cytokines can induce apoptotic cell death in chondrocytes by increasing IL-6, TNF-α, and IL-1β, resulting in cartilage degradation [[26], [31], [32]]. The earlier study showed that IFN-γ plays a vital role in protecting cartilage degradation in early OA by modulating expression of IL-1β and attenuating MMPs production [33]. Interestingly, in line with previous reports, our results show that level of inflammatory cytokines such as IL-6 and IL-1β was decreased by G-Rb1 treatment, further IFN-γ levels were dose-dependently increased, resulting in the inhibition of apoptosis in cartilage chondrocyte.
Finally, our findings clearly indicate that the reduction of these inflammatory cytokines or chemokines could be a key factor in controlling OA progression by G-Rb1. In conclusion, G-Rb1 protected against cartilage degradation, possibly by inhibiting levels of MMP13 and COX-2 mRNA and suppressing proinflammatory cytokines like PGE2, IL-6, IFN-γ, and IL-β, contributing to antiinflammatory effects. The results may provide evidence to support the antiarthritic effects of G-Rb1 in MIA-induced OA in ovariectomized rats.
Conflicts of interest
None of the authors have conflicts of interest to declare.
Acknowledgment
“This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C0661).”
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2020.01.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hame S.L., Alexander R.A. Knee osteoarthritis in women. Curr Rev Musculoskel Med. 2013;6:182–187. doi: 10.1007/s12178-013-9164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Connor M.I. Osteoarthritis of the hip and knee: sex and gender differences. Orthopedic Clinics. 2006;37:559–568. doi: 10.1016/j.ocl.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Srikanth V.K., Fryer J.L., Zhai G., Winzenberg T.M., Hosmer D., Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Xia B., Chen D., Zhang J., Hu S., Jin H., Tong P. Osteoarthritis pathogeneis: a review of molecular mechanisms. Calcif Tissue Int. 2014;95:495–505. doi: 10.1007/s00223-014-9917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wojdasiewicz P., Poniatowski Ł.A., Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat Inflam. 2014:2014. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlier E., Relic B., Deroyer C., Malaise O., Neuville S., Collée J., Malaise M.G., De Seny D. Insights on molecular mechanisms of chondrocytes death in osteoarthritis. Int J Mol Sci. 2016;17:2146. doi: 10.3390/ijms17122146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuerwegh A., Dombrecht E., Stevens W., Van Offel J., Bridts C., De Clerck L. Influence of pro-inflammatory (IL-1α, IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthritis Cartilage. 2003;11:681–687. doi: 10.1016/s1063-4584(03)00156-0. [DOI] [PubMed] [Google Scholar]
- 8.Bendele A. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact. 2001;1:363–376. [PubMed] [Google Scholar]
- 9.Høegh-Andersen P., Tankó L.B., Andersen T.L., Lundberg C.V., Mo J.A., Heegaard A.-M. Ovariectomized rats as a model of postmenopausal osteoarthritis: validation and application. Arthritis Res Ther. 2004;6:R169. doi: 10.1186/ar1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S.-K., Park J.H. Trends in ginseng research in 2010. J Ginseng Res. 2011;35:389. doi: 10.5142/jgr.2011.35.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto R., Yu J., Koizumi H., Ouchi Y., Okabe T. Ginsenoside Rb1 prevents MPP+-induced apoptosis in PC12 cells by stimulating estrogen receptors with consequent activation of ERK1/2, Akt and inhibition of SAPK/JNK, p38 MAPK. Evidence-based complementary and alternative medicine. eCAM. 2012:2012. doi: 10.1155/2012/693717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radad K., Gille G., Moldzio R., Saito H., Rausch W.-D. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004;1021:41–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Cheng W., Wu D., Zuo Q., Wang Z., Fan W. Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation and apoptosis in human articular chondrocytes. Int Orthopaedics. 2013;37:2065–2070. doi: 10.1007/s00264-013-1990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H.A., Kim S., Chang S.H., Hwang H.J., Choi Y-n. Anti-arthritic effect of ginsenoside Rb1 on collagen induced arthritis in mice. Int Immunopharmacology. 2007;7:1286–1291. doi: 10.1016/j.intimp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Endale M., Im E.J., Lee J.Y., Kim S.D., Yayeh T., Song Y.-B. Korean red ginseng saponin fraction rich in ginsenoside-Rb1, Rc and Rb2 attenuates the severity of mouse collagen-induced arthritis. Mediat Inflam. 2014;2014 doi: 10.1155/2014/748964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W., Zeng L., Wang Z.-M., Zhang S., Rong X.-F., Li R.-H. Ginsenoside Rb1 inhibits matrix metalloproteinase 13 through down-regulating Notch signaling pathway in osteoarthritis. Exp Biol Med. 2015;240:1614–1621. doi: 10.1177/1535370215587918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang P.Y., Tang C.C., Chang Y.C., Huang S.Y., Hsieh S.P., Fan S.S. Effects of tibolone on osteoarthritis in ovariectomized rats: association with nociceptive pain behaviour. Eur J Pain. 2014;18:680–690. doi: 10.1002/j.1532-2149.2013.00406.x. [DOI] [PubMed] [Google Scholar]
- 18.Park J.G., Yi Y.-S., Hong Y.H., Yoo S., Han S.Y., Kim E. Tabetri™(Tabebuia avellanedae ethanol extract) ameliorates osteoarthritis symptoms induced by monoiodoacetate through its Anti-Inflammatory and Chondroprotective Activities. Mediat Inflamm. 2017;2017 doi: 10.1155/2017/3619879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mankin H., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. The Journal of bone and joint surgery. American. 1971;53:523–537. [PubMed] [Google Scholar]
- 20.Kim J.H., Yi Y.-S., Kim M.-Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teitelbaum S.L. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guzman R.E., Evans M.G., Bove S., Morenko B., Kilgore K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicologic Pathol. 2003;31:619–624. doi: 10.1080/01926230390241800. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y., Kim E-h, Lee K.S., Lee K., Park S.H., Na S.H., Ko C., Kim J., Yooon Y.W. The effects of intra-articular resiniferatoxin on monosodium iodoacetate-induced osteoarthritic pain in rats. Korean J Physiol Pharmacol. 2016;20:129–136. doi: 10.4196/kjpp.2016.20.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felson D.T., Chaisson C.E., Hill C.L., Totterman S.M., Gale M.E., Skinner K.M. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 25.Gong Y.-S., Chen J., Zhang Q.-Z., Zhang J.-T. Effect of 17β-oestradiol and ginsenoside on osteoporosis in ovariectomised rats. J Asian Nat Prod Res. 2006;8:649–656. doi: 10.1080/10286020500246063. [DOI] [PubMed] [Google Scholar]
- 26.Kong P., Chen G., Jiang A., Wang Y., Song C., Zhuang J. Sesamin inhibits IL-1β-stimulated inflammatory response in human osteoarthritis chondrocytes by activating Nrf2 signaling pathway. Oncotarget. 2016;7:83720. doi: 10.18632/oncotarget.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchev A.S., Dimitrova P.A., Burns A.J., Kostov R.V., Dinkova-Kostova A.T., Georgiev M.I. Oxidative stress and chronic inflammation in osteoarthritis: can NRF2 counteract these partners in crime? Ann N Y Acad Sci. 2017;1401:114–135. doi: 10.1111/nyas.13407. [DOI] [PubMed] [Google Scholar]
- 28.Sellam J., Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 29.Abramson S.B., Attur M., Amin A.R., Clancy R. Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Current Rheumatology Reports. 2001;3:535–541. doi: 10.1007/s11926-001-0069-3. [DOI] [PubMed] [Google Scholar]
- 30.Tung J.T., Arnold C.E., Alexander L.H., Yuzbasiyan-Gurkan V., Venta P.J., Richardson D.W. Evaluation of the influence of prostaglandin E2 on recombinant equine interleukin-1β-stimulated matrix metalloproteinases 1, 3, and 13 and tissue inhibitor of matrix metalloproteinase 1 expression in equine chondrocyte cultures. Am J Vet Res. 2002;63:987–993. doi: 10.2460/ajvr.2002.63.987. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L., Zhu M., Li M., Du Y., Duan S., Huang Y. Ginsenoside Rg1 attenuates adjuvant-induced arthritis in rats via modulation of PPAR-γ/NF-κB signal pathway. Oncotarget. 2017;8:55384. doi: 10.18632/oncotarget.19526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan R., Dai Y., Gao X., Xia Y. Scopolin isolated from Erycibe obtusifolia Benth stems suppresses adjuvant-induced rat arthritis by inhibiting inflammation and angiogenesis. Inter Immunopharmacol. 2009;9:859–869. doi: 10.1016/j.intimp.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Page C., Smale S., Carty S., Amos N., Lauder S., Goodfellow R. Interferon-γ inhibits interleukin-1β-induced matrix metalloproteinase production by synovial fibroblasts and protects articular cartilage in early arthritis. Arthritis Res Ther. 2010;12(2010):R49. doi: 10.1186/ar2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.