Abstract
Objective:
The primary indication for electroconvulsive therapy is medication-resistant major depression. There is some evidence that combining electroconvulsive therapy with an antidepressant, instead of electroconvulsive therapy monotherapy, might improve remission rates. However, data on this topic have not been systematically studied. We undertook a systematic review and meta-analysis to determine the effectiveness of an adjuvant antidepressant during electroconvulsive therapy for major depression.
Methods:
Embase, Medline Ovid, Web of Science, Cochrane Central, PsychINFO Ovid and Google Scholar were searched up to January 2019. Randomized controlled trials and cohort studies reporting on the influence of an adjuvant antidepressant on the efficacy of electroconvulsive therapy for major depression were included. Authors independently screened records, extracted data and assessed study quality. We reported this systematic review and meta-analysis following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Results:
Nine studies were included in the meta-analysis. The meta-analysis revealed a significant advantage of adjuvant antidepressants versus placebo. The overall effect size per category of antidepressant was as follows: tricyclic antidepressants: Hedges’ g 0.32 (95% confidence interval: [0.14, 0.51]) (k = 6) with low heterogeneity (I2: 4%, p = 0.39); selective serotonin reuptake inhibitors/serotonin noradrenaline reuptake inhibitors: Hedges’ g 0.27 (95% confidence interval: [0.03, 0.52]) (k = 2) with a lack of heterogeneity (I2: 0%, p = 0.89); and monoamine oxidase inhibitors: Hedges’ g 0.35 (95% confidence interval: [−0.07, 0.77]) with moderate heterogeneity (I2: 43%, p = 0.17) (k = 3).
Conclusion:
An adjuvant antidepressant enhances the efficacy of electroconvulsive therapy for major depression. Tricyclic antidepressants, selective serotonin reuptake inhibitors/serotonin noradrenaline reuptake inhibitors and monoamine oxidase inhibitors showed the same effect size. However, the effect sizes of tricyclic antidepressants and monoamine oxidase inhibitors are most likely underestimated, due to insufficient doses in most of the included studies. We recommend the routine use of an adequately dosed antidepressant during electroconvulsive therapy for major depression.
Keywords: Review, meta-analysis, electroconvulsive therapy, antidepressant, depression
Introduction
Electroconvulsive therapy (ECT) is considered the most effective treatment for severe major depression (UK ECT Review Group, 2003). The majority of patients receive ECT because they do not respond to antidepressant medication trials (American Psychiatric Association, 2001), although there is evidence that medication resistance can negatively influence the efficacy of ECT. Recent meta-analyses have found remission rates of 48% and 58% for patients with medication-resistant depression (Haq et al., 2015; Heijnen et al., 2010). Improving these remission rates would be of great clinical benefit. Moreover, continuing an antidepressant instead of ceasing the drug prior to ECT prevents withdrawal symptoms, saves time and reduces the risk of full relapse.
There is some evidence to suggest a synergy between ECT and antidepressants. A randomized controlled trial (RCT) by Sackeim et al. (2009) showed a favourable effect of a combination of ECT and nortriptyline on remission rates. A recent case report described a patient with psychotic depression. She responded very slowly to ECT monotherapy and failed to achieve full remission. After a severe relapse of psychotic depression, she very rapidly attained full remission with a combination of ECT and imipramine (Birkenhager and Pluijms, 2016). Further data on the influence of antidepressant medication on the efficacy of ECT are limited and inconclusive. Even guidelines vary in their recommendations regarding adjuvant antidepressant medication during ECT. Some guidelines recommend considering a combination treatment, particularly among patients with medication-resistant depression (American Psychiatric Association, 2010), whereas other guidelines recommend considering ceasing antidepressant medication prior to ECT (State of Queensland, 2018) or weighing the advantages and disadvantages of combination treatment in each individual patient and thus leaving the decision to the clinician (Dutch Psychiatric Association, 2010). The British guidance on the use of ECT does not give specific recommendations, although it states that the combination of ECT and pharmacotherapy is not superior to ECT alone (National Institute for Health and Care Excellence, 2003).
To our knowledge, there are no systematic reviews or meta-analyses on the influence of an adjuvant antidepressant on the efficacy of ECT. Thus, neither the routine use of an adjuvant antidepressant during ECT nor the routine discontinuation of the drug prior to ECT is justified by sound scientific data. We addressed the question of whether ECT should be routinely combined with an antidepressant to improve its efficacy. We conducted this systematic review and meta-analysis to provide well-founded recommendations for clinical practice.
Methods
This systematic review and meta-analysis was registered in the Research Registry (reviewregistry763) (Research Registry, 2019) and it adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009).
Search strategy
One author (E.M.P.) and an experienced biomedical information specialist searched the electronic databases Embase, Medline Ovid, Web of Science, Cochrane Central, PsychINFO Ovid and Google Scholar for relevant English-language studies up to 15 January 2019. Supplementary Table 1 provides the exact search strategies. The electronic database search was supplemented by a manual review of reference lists from eligible articles.
Inclusion criteria
The inclusion criteria were as follows: (1) an RCT or a prospective/retrospective cohort study; (2) a diagnosis of major depressive disorder; (3) a course of ECT; (4) intervention condition: an adjuvant antidepressant during ECT; and (5) control condition: a placebo or an active placebo during ECT or ECT monotherapy in retrospective cohort studies. Both unipolar and bipolar depression were included. For the diagnosis of depression, the diagnostic criteria according to the International Classification of Diseases, tenth edition (ICD-10) (World Health Organization, 2010); Diagnostic and Statistical Manual of Mental Disorders, third edition (DSM-III) (American Psychiatric Association, 1980); Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) (American Psychiatric Association, 1994); Diagnostic and Statistical Manual of Mental Disorders, the text revision of the fourth edition (DSM-IV-TR) (American Psychiatric Association, 2000) and Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM 5) (American Psychiatric Association, 2013) were accepted, as well as diagnoses based on clinical observation. There was no restriction on the type or dose of antidepressant.
Study selection
After removing duplicates, two authors (E.M.P. and W.W.v.d.B.) independently screened all articles on the basis of title and abstract. Articles that were deemed potentially relevant by at least one author were selected. The same authors independently reviewed the full text of the selected articles and assessed their eligibility. We resolved any disagreements by discussion and consensus with a third author (T.K.B.). All eligible articles, both RCTs and cohort studies, were used for qualitative analysis. For quantitative analysis, we only included RCTs.
Data extraction
We used a structured data extraction form to collect the following information from all eligible articles: (1) study characteristics, e.g., study design, study setting, patient population and sample size; (2) ECT method, e.g., electrode placement, waveform, dose strategy, frequency and duration of ECT; (3) details of the intervention condition, e.g., type, dose and monitoring of adjuvant antidepressant; (4) type of control condition, e.g., placebo or type of active placebo or ECT monotherapy; (5) outcome measures; and (6) overall results. Additionally, we extracted data on the study quality of the studies included for quantitative analysis (see Methods, section ‘Quality assessment’).
If studies reported multiple outcome measures, we included the outcome measure that operationalized the clinical psychiatric symptoms the best. We preferred instruments that are validated for the assessment of depressive symptoms, such as the Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960) and the Montgomery Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979). We preferred interviewer-reported questionnaires to self-reported questionnaires. We also accepted outcomes assessed by means of the Clinical Global Impression (CGI) rating scale (Guy, 1976) or clinical observation. If available, we opted for data from an intention-to-treat analysis.
Quality assessment
RCTs
Two authors (E.M.P. and W.W.v.d.B.) independently assessed the risk of bias for each RCT in the quantitative analysis using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins and Green, 2011). We estimated the risk of bias according to the following eight quality criteria: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) use of intention-to-treat analysis and incomplete data; (6) selective reporting; (7) baseline imbalance; and (8) other bias, i.e., intervention or treatment fidelity, which is the extent to which the intervention or treatment is delivered as it should be according to current standards. We judged each potential source of bias as high, low or unclear. If a study had a crossover design, we only considered the part before the crossover. For studies that reported on an acute and a continuation phase, we only included the acute phase of ECT. We resolved any disagreements by discussion and consensus with a third author (A.M.K.).
Cohort studies
Two authors (E.M.P. and A.M.K.) independently rated the strength of each cohort study using the checklist outlined in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (von Elm et al., 2007). We resolved any disagreements by discussion and consensus with a third author (T.K.B.).
Statistical analysis
Per category of adjuvant antidepressant, we calculated pooled effect size estimates between the intervention group and the control group over a minimum of two trials. We distinguished three categories of adjuvant antidepressants, i.e., tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs)/serotonin noradrenaline reuptake inhibitors (SNRIs) and monoamine oxidase inhibitors (MAOIs). SSRIs and SNRIs were pooled, because the mean dose of venlafaxine used in the study by Sackeim et al. (2009) was 187 mg/day. At doses below 225 mg/day, venlafaxine acts as an SSRI (Debonnel et al., 2007). Effect sizes were reported using Hedges’ g and corresponding 95% confidence intervals (CIs; Hedges and Olkin, 1985). The results for each category of adjuvant antidepressant were plotted in a forest plot. In cases of substantial heterogeneity, random-effects analyses were used to estimate an overall treatment effect. Cochran’s Q-test and the I2 and T2 statistics were used to quantify heterogeneity across trials. Heterogeneity was further explored by conducting sensitivity analyses. Specifically, we calculated the overall treatment effect using both fixed- and random-effects modelling and evaluated the impact of the modelling procedure on the overall treatment effect. Additionally, we created subgroups of trials based on type of electrode placement, outcome measures (standardized questionnaires vs other types of rating scales vs clinical observations) and the criteria included in the risk of bias evaluation. We assessed the impact of these moderator variables on the overall effect of adjuvant medication. The effect of the year of publication on the overall treatment effect was assessed using meta-regression analysis. Standardized effect sizes were calculated using comprehensive meta-analysis (CMA). Statistical analyses were performed using the ‘metan’ package in Stata 15 (Stata Corp, 2017). Differences in the mean treatment effect between subgroups were estimated using the ‘metaf’ macro (Wilson, 1999).
Publication bias was assessed visually with a funnel plot. Additionally, we formally assessed whether the effect size decreased in proportion to increasing sample size using Egger’s test (Egger et al., 1997). In case of an asymmetrical funnel plot, missing data were imputed using the trim-and-fill method (Duval and Tweedie, 2000).
Results
Study selection
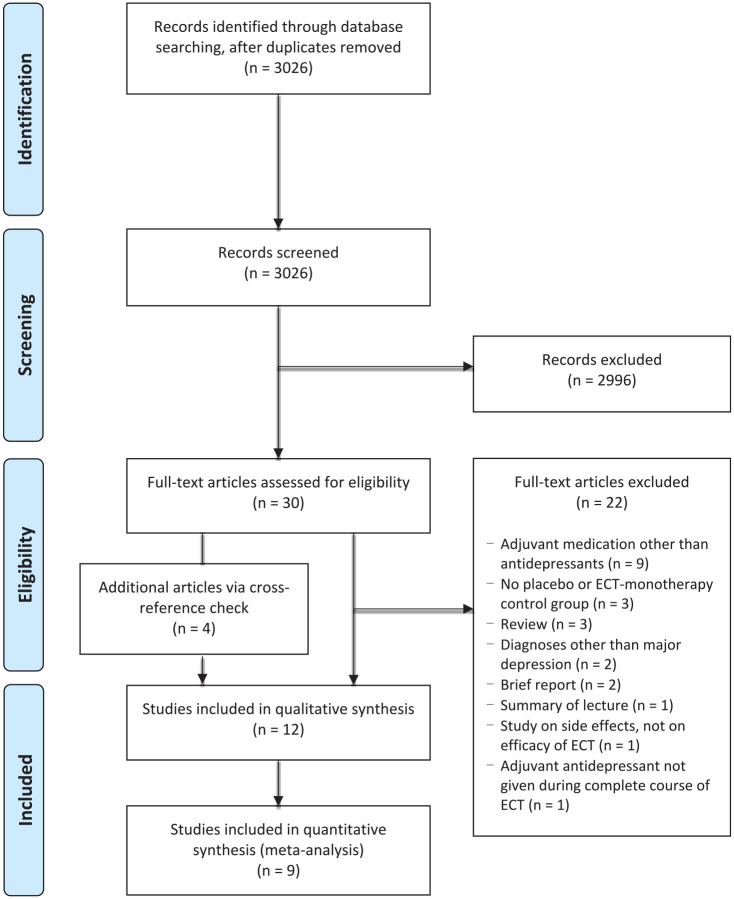
After removing duplicates, the database search identified 3026 abstracts. Further results of the study selection are shown in the PRISMA flow diagram (Figure 1). We excluded 22 out of 30 eligible articles for the reasons as outlined in Figure 1. Eight articles met our inclusion criteria. We identified four additional articles via a cross-reference check. Thus, 12 studies were included in our systematic review for qualitative analysis. Since we only included RCTs in our meta-analysis, the quantitative analysis was based on nine studies. The raw interrater agreement suggested substantial interrater reliability (kappa = 0.78; 95% CI: [0.63, 0.93]).
Figure 1.
Flow diagram of the study selection.
Characteristics of included studies
Twelve studies met our inclusion criteria: nine RCTs (Imlah et al., 1965; Kay et al., 1970; Lauritzen et al., 1996; Mayur et al., 2000; Monaco and Delaplaine, 1964; Muller, 1961; Sackeim et al., 2009; Seager and Bird, 1962; Wilson et al., 1963) and three retrospective cohort studies (Baghai et al., 2006; Kho et al., 2005; Nelson and Benjamin, 1989). We found no prospective cohort studies. Table 1 shows detailed information about the study characteristics. There is a remarkably lack of data from the last decade.
Table 1.
Characteristics of the included studies.
| Study | Design, setting and country | Patients and sample size | ECT method | Intervention | Control condition | Outcome measures of interest | Results |
|---|---|---|---|---|---|---|---|
| Randomized controlled trials on adjuvant TCA | |||||||
| Imlah et al. (1965)a | RCT, inpatient clinic, UK | Depressive disorder (clinical observation) Total sample n = 150 ECT + placebo n = 50 ECT + IMI n = 50 ECT + PHE n = 50 |
No information available on electrode placement, waveform and dose strategy; 2 ECT sessions/week until favourable clinical response, maximum of 12 treatments. | ECT + IMI 75 mg | ECT + placebo | Number of ECT sessions until response, based on ‘5-point scale’ | Mean number of ECT sessions until
response: ECT + placebo 7.93 ECT + IMI 7.15 ECT + PHE 6.90 No significant difference between intervention and control group. |
| Kay et al. (1970) | RCT, inpatient clinic, UK | Depressive disorder (clinical observation) Total sample n = 132 ECT + diazepam n = 73 ECT + AMI n = 59 |
No information available on ECT method. | ECT + AMI 50–150 mg | ECT + diazepam 4–12 mg |
HRSD | Mean decrease in HRSD score at
1 month: ECT + diazepam 24.4 ECT + AMI 28.7 No significant difference between intervention and control group. |
| Mayur et al. (2000)a | RCT (discontinuation study), inpatient clinic, India | Major depressive disorder (DSM-IV) Total sample n = 30 ECT + placebo n = 15 ECT + TCA n = 15 |
UL; pulse, square wave; dose titration (stimulus: 2.5× ST); 3 ECT sessions/week for 4 weeks or until remission, whichever was earlier. | ECT + TCA | ECT + placebo | 17-item HRSD, MADRS | Mean HRSD score and mean MADRS score at week 4 not
reported. No significant differences between intervention and control group. |
| Sackeim et al. (2009)b | RCT, inpatient clinic, USA | Major depressive disorder (DSM-IV) Total sample n = 319 ECT + placebo n = 135 ECT + NOR n = 93 ECT + VEN n = 91 |
RUL or BL; pulse, square wave; dose titration (stimulus RUL 6× ST, BL 1.5× ST); 3 ECT sessions/week until remission. | ECT + NOR (mean blood level 82.1 ± 52.2 ng/mL) | ECT + placebo | 24-item HRSD Remission: reduction HRSD score ⩾ 60% and post-ECT HRSD ⩽ 10 |
Remission rate: ECT + placebo 41.4% ECT + NOR 54.8% ECT + VEN 52.8% Difference between intervention and control group shows trend in favour of NOR. Mean post-ECT HRSD score: |
| ECT + placebo 15.9 ± 10.7 ECT + NOR 12.6 ± 9.8 ECT + VEN 13.0 ± 9.7 Significant difference between ECT + NOR and ECT + placebo in favour of NOR; ECT + VEN did not differ from the other conditions. |
|||||||
| Seager and Bird (1962)a | RCT, inpatient clinic, UK | Depressive disorder (clinical observation) Total sample n = 43, analysed sample n = 40 (drop-outs excluded for analysis) ECT + placebo n = 21 ECT + IMI n = 19 |
No information available on electrode placement and dose strategy; sine wave; 2 ECT sessions/week until favourable clinical response. | ECT + IMI 150 mg | ECT + placebo | Number of ECT sessions until response, based on clinical observation | Mean number of ECT sessions until
response: ECT + placebo 7.0 ECT + IMI 6.3 No significant difference between intervention and control group. |
| Wilson et al. (1963)c | RCT, inpatient clinic, USA | Depressive disorder (clinical observation) Total sample n = 10 ECT + atropine n = 6 ECT + IMI n = 4 |
No information available on electrode placement, waveform and dose strategy; 2 ECT sessions/week for six treatments. | ECT + IMI 150 mg | ECT + atropine 0.1 mg | HRSD | Mean decrease in HRSD score at week 5: ECT + atropine 22.3 ± 1.6 ECT + IMI 20.7 ± 1.9 Difference between intervention and control group not statistically analysed. |
| Randomized controlled trials on adjuvant SSRI/SNRI | |||||||
| Lauritzen et al. (1996)a,d | RCT, inpatient clinic, Denmark | Major depressive disorder (DSM-III-R) Total sample n = 87, of which n = 35 in this study arm |
First 3 ECT sessions BL, thereafter UL; pulse, square wave; no information available on dose strategy; 3 ECT sessions/week until remission. | ECT + PAR 30 mg | ECT + placebo | HRSD, number of ECT sessions | Mean post-ECT HRSD score: ECT + placebo 9.2 ± 3.4 ECT + PAR 8.9 ± 4.7 Mean number of ECT sessions until response: ECT + placebo 11.1 ± 3.8 |
| ECT + placebo n = 17 ECT + PAR n = 18 |
ECT + PAR 12.1 ± 6.3 No significant difference between intervention and control group in mean post-ECT HRSD score and number of ECT sessions until response. |
||||||
| Sackeim et al. (2009)b | RCT, inpatient clinic, USA | Major depressive disorder (DSM-IV) Total sample n = 319 ECT + placebo n = 135 ECT + NOR n = 93 ECT + VEN n = 91 |
RUL or BL; pulse, square wave; dose titration (stimulus RUL 6× ST, BL 1.5× ST); 3 ECT sessions/week until remission. | ECT + VEN (mean dose 187 mg/day) | ECT + placebo | 24-item HRSDR emission: reduction HRSD score ⩾ 60% and post-ECT HRSD ⩽ 10 | Remission rate: ECT + placebo 41.4% ECT + NOR 54.8% ECT + VEN 52.8% Difference between intervention and control group shows trend in favour of NOR. Mean post-ECT HRSD score: ECT + placebo 15.9 ± 10.7 ECT + NOR 12.6 ± 9.8 ECT + VEN 13.0 ± 9.7 Significant difference between ECT + NOR and ECT + placebo in favour of NOR; ECT + VEN did not differ from the other conditions. |
| Randomized controlled trials on adjuvant MAOI | |||||||
| Imlah et al. (1965)a | RCT, inpatient clinic, UK | Depressive disorder (clinical observation) Total sample n = 150 ECT + placebo n = 50 ECT + IMI n = 50 ECT + PHE n = 50 |
No information available on electrode placement, waveform and dose strategy; 2 ECT sessions/week until favourable clinical response, maximum of 12 treatments. | ECT + PHE 45 mg | ECT + placebo | Number of ECT sessions until response, based on ‘5-point scale’ | Mean number of ECT sessions until
response: ECT + placebo 7.93 ECT + IMI 7.15 ECT + PHE 6.90 No significant difference between intervention and control group. |
| Monaco and Delaplaine (1964) b | RCT, inpatient clinic, USA | Depressive disorder (clinical observation) Total sample n = 26 ECT + placebo n = 12 ECT + TRA n = 14 |
No information available on ECT method. | ECT + TRA 20 mg | ECT + placebo | Clinical observation | Improvement rate at week 4: ECT + placebo 91.7% ECT + TRA 78.6% Difference between intervention and control group not statistically analysed. |
| Muller (1961) | RCT, outpatient clinic, UK | Depressive disorder (clinical observation) Total sample n = 100 ECT + placebo n = 45 ECT + PHE n = 55 |
No information available on electrode placement, waveform and dose strategy; 2 ECT sessions/week until clinical response. | ECT + PHE 45 mg | ECT + placebo | ‘25-point scale’ | Mean post-ECT score on ‘25-point
scale’: ECT + placebo 6.62 ± 4.04 ECT + PHE 4.82 ± 2.75 Mean decrease on ‘25 point scale’ at end of ECT: ECT + placebo 4.31 ± 5.28 ECT + PHE 7.24 ± 4.37 Significant differences between intervention and control group in mean post-ECT score and mean decrease on ‘25-point scale’. |
| Retrospective cohort studies | |||||||
| Baghai et al. (2006) | Cohort study, inpatient clinic, Germany | Major depression (ICD-10) Total sample n = 358 ECT alone n = 170 ECT + TCA n = 78 ECT + TTCA n = 40 ECT + SSRI n = 30 ECT + other AD n = 40 |
UL or BL; pulse, square wave; dose titration in < 5% of
patients; dose strategy in remaining patients: based on age
in UL, based on half-age in BL; 2.8 ECT sessions/week (mean). |
ECT + AD | ECT alone | CGI | Scores on CGI not reported. Significantly higher severity of illness and less improvement in control group and ECT + SNRI group compared to ECT + TCA, ECT + TTCA and ECT + SSRI groups. |
| Kho et al. (2005) | Cohort study, setting unclear, The Netherlands | Major depressive disorder (DSM-IV) Total sample n = 73 ECT alone n = 26 ECT + TCA n = 19 ECT + SSRI n = 12 ECT + combination treatment and or lithium and or antipsychotic medication n = 49 |
UL, UL > BL or BL; pulse, square wave; dose strategy based on age, adjusted upwards if patients used benzodiazepines and or anticonvulsants; 2 ECT sessions/week until remission. | ECT + psychotropic medication (among which AD) | ECT alone | 17-item HRSD Remission: reduction HRSD score ⩾ 60% and post-ECT HRSD < 8 |
Remission rate: ECT alone 61.5% ECT + psychotropic 68.1% No significant difference between intervention and control group. |
| Nelson and Benjamin (1989) | Cohort study, inpatient clinic, USA | Depressive disorder (clinical observation) Total sample n = 84 ECT alone n = 44 ECT + partial TCA n = 23 ECT + full TCA n = 17 |
UL; pulse, square wave; dose strategy: ‘Initial middle setting’ so that seizures of > 30s were obtained; no information available on frequency of ECT; ECT sessions administered until favourable clinical response. |
ECT + partial TCA (IMI blood level 75–149 ng/mL or daily
dose 50–99 mg) ECT + full TCA (IMI blood level 150–300 ng/mL or daily dose > 100 mg) |
ECT alone | Number of ECT sessions, ‘clinical observation scale’ (no (0), slight (1), moderate (2) and marked (3) improvement) | Mean post-ECT score on ‘clinical observation
scale’ ECT alone 2.0 ECT + partial TCA 2.4 ECT + full TCA 2.6 Significant difference between ECT + full TCA and ECT alone; no significant difference between ECT + partial TCA and ECT alone. Mean number of ECT sessions until response: ECT alone 9.8 ECT + partial TCA 8.0 ECT + full TCA 8.2 Significant difference between control group and both intervention groups; no significant difference between ECT + partial TCA and ECT + full ECT. |
RCT: randomized controlled trial; UK: United Kingdom; USA: United States of America; TCA: tricyclic antidepressant; SSRI: selective serotonin reuptake inhibitor; SNRI: serotonin noradrenaline reuptake inhibitor; MAOI: monoamine oxidase inhibitor; TTCA: tetracyclic antidepressant; AD: antidepressant; IMI: imipramine; AMI: amitriptyline; NOR: nortriptyline; PAR: paroxetine; VEN: venlafaxine; PHE: phenelzine; TRA: tranylcypromine; DSM: Diagnostic and Statistical Manual of Mental Disorders; ECT: electroconvulsive therapy; RUL: right unilateral ECT; UL: unilateral ECT; BL: bilateral ECT; ST: seizure threshold; HRSD: Hamilton Rating Scale for Depression; MADRS: Montgomery Asberg Depression Rating Scale; CGI: Clinical Global Impression; ICD-10: International Classification of Diseases, tenth edition.
These studies consisted of two phases: (1) acute treatment and (2) follow-up. We only included the first phase in our systematic review and meta-analysis.
These studies had a crossover design. We only included the part prior to crossover in our systematic review and meta-analysis.
This study consisted of two phases: (1) ECT + IMI versus ECT + atropine and sham ECT + IMI versus sham ECT + atropine and (2) ECT + atropine versus IMI alone. We only included the ECT + IMI versus ECT + atropine arm of the first phase in our systematic review and meta-analysis.
This study consisted of two study arms: (1) ECT + PAR versus ECT + placebo and (2) ECT + IMI versus ECT + PAR. We only included the first study arm in our systematic review and meta-analysis.
RCTs
Most studies reported on one category of antidepressant. Only two studies reported on two different categories of antidepressants (Imlah et al., 1965; Sackeim et al., 2009). One study specified the type of depression, including both unipolar and bipolar depression (Sackeim et al., 2009). Three studies provided data on electrode placement: in one study, all patients received unilateral ECT (Mayur et al., 2000); in the second study, patients received either unilateral or bilateral ECT (Sackeim et al., 2009); and in the third study, an atypical ECT protocol was used, i.e., all patients were switched from bilateral to unilateral ECT after three sessions (Lauritzen et al., 1996). ECT dose strategies were described in two studies (Mayur et al., 2000; Sackeim et al., 2009). Only one of these studies described an adequate dose strategy of 1.5 times the seizure threshold in bilateral ECT and 6 times the seizure threshold in unilateral ECT (Sackeim et al., 2009). In eight studies, patients were randomized into groups that either received an adjuvant antidepressant or received an adjuvant placebo or active placebo during ECT. In these studies, ECT and the antidepressant or placebo were started simultaneously. One RCT used a different design; patients were randomized into groups that either continued an ongoing treatment with antidepressant medication or withdrew from antidepressant medication with placebo substitution at the start of ECT (Mayur et al., 2000).
Retrospective cohort studies
The studies included unipolar depressed patients (Nelson and Benjamin, 1989) or both unipolar and bipolar depressed patients (Baghai et al., 2006; Kho et al., 2005). All studies provided information on electrode placement and their ECT dose strategy. None of the studies used an adequate dose strategy, according to current standards. None of the studies provided information on the way antidepressants were combined with ECT. It is unclear if ECT was added to an ongoing treatment with an antidepressant or if antidepressants and ECT were started simultaneously.
Outcomes of included studies
Table 1 shows detailed information about the study outcomes.
RCTs
Sackeim et al. (2009) found a trend in remission rate in favour of nortriptyline relative to placebo. Muller (1961) showed a significant difference in decrease in symptoms on a ‘25 point scale’ in favour of phenelzine relative to placebo. Six studies failed to demonstrate a significant advantage of an adjuvant TCA (Imlah et al., 1965; Kay et al., 1970; Mayur et al., 2000; Seager and Bird, 1962), SSRI (Lauritzen et al., 1996), SNRI (Sackeim et al., 2009) or MAOI (Imlah et al., 1965) during ECT. Two studies did not perform a statistical analysis (Monaco and Delaplaine, 1964; Wilson et al., 1963).
Retrospective cohort studies
The studies by Nelson and Benjamin (1989) and Baghai et al. (2006) showed an advantage of an adjuvant antidepressant during ECT. Nelson and Benjamin reported a significant difference in improvement on a clinical observation scale in favour of ECT + TCA relative to ECT monotherapy. Baghai et al. found a higher efficacy of ECT + TCA, SSRI or mirtazapine relative to ECT monotherapy. Kho et al. (2005) failed to demonstrate a significant difference in remission rate between patients using TCA or not during ECT.
Quality assessment
RCTs
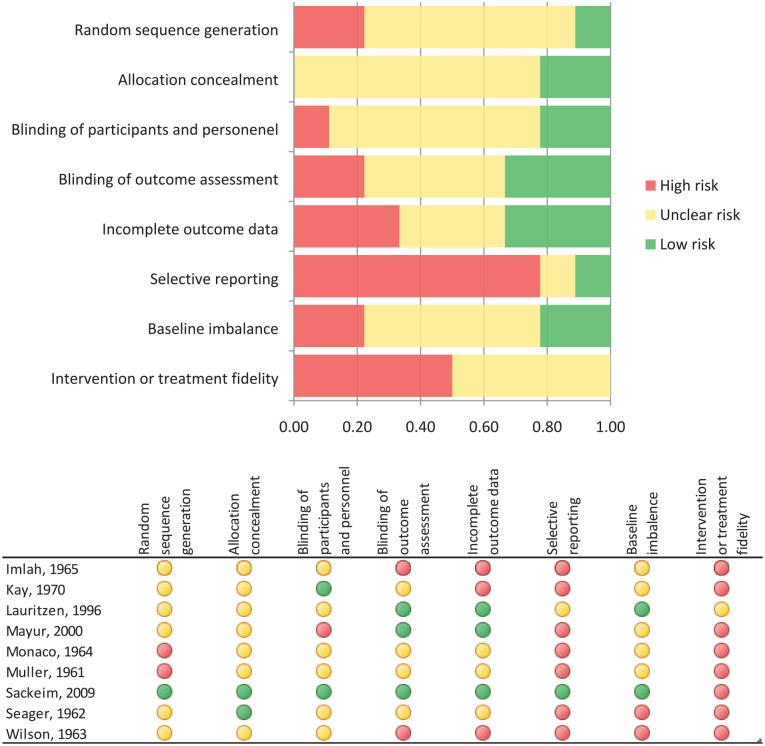
Figure 2 shows the results of the assessment of the risk of bias for each RCT included in this systematic review.
Figure 2.
Risks of bias of studies included in the quantitative analysis.
Regarding the individual RCTs, the study by Sackeim et al. (2009) is the only study that showed a low risk of bias in the majority of quality criteria. In this study, only intervention or treatment fidelity showed bias, since almost 90% of the patients randomized to the unilateral ECT group received a suboptimal stimulus dose due to the maximum settings on the device. The other eight RCTs showed an unclear or a high risk of bias in the majority of quality criteria, since most of these studies did not report on random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, use of intention-to-treat analysis and incomplete data, and baseline imbalance. Moreover, study protocols were frequently lacking, outcome measures were not always prespecified, statistical analyses were not always described, and low doses of TCAs and MAOIs were used.
Regarding the individual quality criteria, the most prevalent risk of bias was found for selective reporting and intervention or treatment fidelity. Selective reporting showed bias in seven out of nine studies (Imlah et al., 1965; Kay et al., 1970; Mayur et al., 2000; Monaco and Delaplaine, 1964; Muller, 1961; Seager and Bird, 1962; Wilson et al., 1963). These studies did not describe a study protocol. In all studies, except for the study by Lauritzen et al. (1996), intervention or treatment fidelity showed bias. In these studies, according to current standards, inadequate doses of antidepressants or ECT were used.
Retrospective cohort studies
The strength of the study by Nelson and Benjamin (1989) was rated as moderate. In this study, the dose of TCA was based on plasma levels in an unspecified number of patients. In the remaining patients, doses of imipramine > 100 mg/day were classified as ‘full TCA’. These doses might have been insufficient (Birkenhager et al., 2005). Moreover, this study relied on the number of ECT sessions and an invalidated clinical outcome scale as outcome measures. The strengths of the studies by Kho et al. (2005) and Baghai et al. (2006) were rated as poor. Kho et al. focused on predictors for the efficacy of ECT. The efficacy of an adjuvant antidepressant was just a small part of this study. The type, dose and plasma levels of TCAs were not reported. An unspecified number of patients received a variety of psychotropic drugs in addition to antidepressants, including benzodiazepines and anticonvulsants. These drugs may interfere with the efficacy of ECT (Boylan et al., 2000; Jha and Stein, 1996; Sienaert and Peuskens, 2007). Baghai et al. used different types of antidepressants. Doses or plasma levels of TCAs were not reported. More than half of the patients received other psychotropic drugs in addition to antidepressants. Almost 30% of the patients received two to six other psychotropic drugs.
Meta-analysis
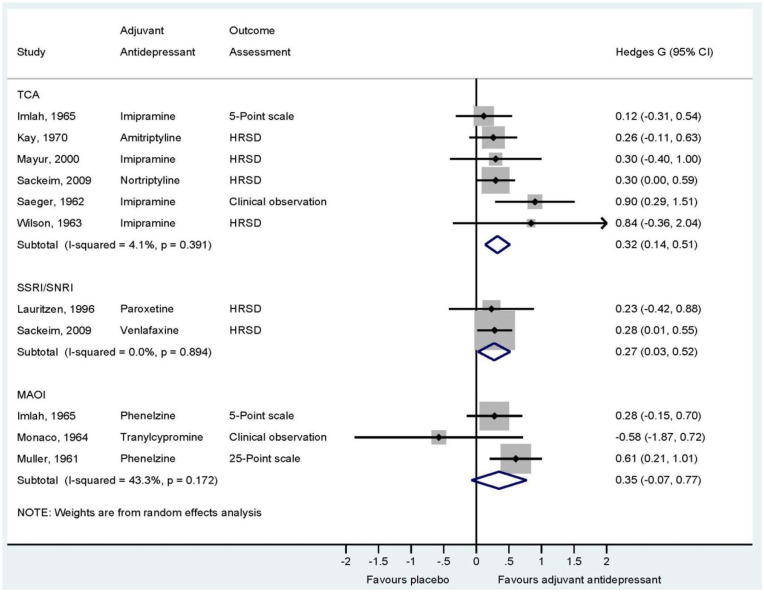
A total of 9 RCTs, estimating 11 effect sizes, were included in the meta-analysis. Figure 3 shows the effect of an adjuvant TCA, SSRI/SNRI and MAOI. The overall effect size of TCAs was Hedges’ g 0.32 (95% CI: [0.14, 0.51]) (k = 6) using random-effects estimation. Heterogeneity was low (I2: 4%, p = 0.39). Fixed- and random-effects estimations resulted in identical effect size estimates. The overall effect size of SSRI/SNRI was Hedges’ g 0.27 (95% CI: [0.03, 0.52]) with a lack of heterogeneity (I2: 0%, p = 0.89) (k = 2). Fixed- and random-effects estimations resulted in identical effect size estimates. Finally, MAOI showed an overall effect size of Hedges’ g 0.35 (95% CI: [−0.07, 0.77]) using random-effects estimation (k = 3). Heterogeneity was moderate (I2: 43%, p = 0.17). Fixed-effects estimation resulted in a higher effect size with a smaller CI (Hedges’ g: 0.40; 95% CI: [0.12, 0.69]).
Figure 3.
Forest plot showing meta-analytic results of efficacy of an adjuvant TCA, SSRI/SNRI and MAOI versus placebo or active placebo on ECT.
The funnel plot including the effect sizes extracted from the RCTs was symmetrically shaped (Figure 4), suggesting no indication of publication bias. The Egger’s test supported this finding (β = 0.15; 95% CI: [−1.69, 1.99]; p = 0.85). Results remained unchanged using the trim-and-fill method.
Figure 4.
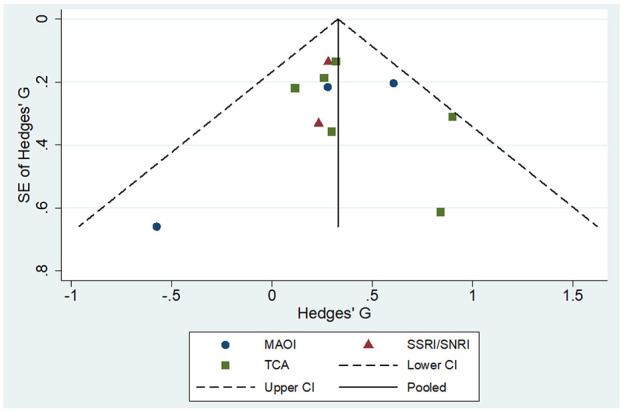
Funnel plot of included studies.
A sensitivity analysis was performed regarding design characteristics, study quality and year of publication. We were not able to estimate the impact of the type of electrode placement, due to insufficient data. More recent studies showed more homogeneity compared to early studies. However, we found no indication of a linear association between year of publication and reported add-on medication effect (β = −0.002; 95% CI: [−0.009, 0.005]; p = 0.498). We found no indication of an impact of the instrument used for outcome assessment on the overall effect (Q = 0.89, df = 9, p = 0.99). The randomization procedure did not significantly impact the overall effect (Q = 0.99, df = 8, p = 0.99), nor did the allocation procedure (Q = 0.80, df = 10, p = 0.99), blinding of personnel (Q = 0.73, df = 9, p = 0.99), blinding of the assessor (Q = 0.80, df = 10, p = 0.99), incomplete data (Q = 0.80, df = 10, p = 0.99) or baseline imbalance (Q = 0.89, df = 9, p = 0.99). We were not able to estimate the impact of intervention or treatment fidelity due to insufficient variation between studies.
Discussion
Main findings
The results of our meta-analysis indicate that an adjuvant antidepressant, compared to placebo or active placebo, enhances the efficacy of ECT in patients with major depression. Although effect sizes were small to moderate, they are clinically relevant, since they reflect an add-on effect to ECT, which is considered the most effective treatment for major depression (UK ECT Review Group, 2003).
Different categories of antidepressants, i.e., TCAs, SSRIs/SNRIs and MAOIs, showed approximately the same effect size. Given the previously established evidence of the superior efficacy of TCAs compared to SSRIs in severely depressed inpatients (Anderson, 2000), we expected to find TCAs to be more effective than SSRIs and venlafaxine at doses below 225 mg/day. Insufficient doses of TCAs and MAOIs in most of the included studies versus adequate doses of SSRIs and SNRI most likely resulted in underestimated effect sizes for adjuvant treatment with TCAs and MAOIs. This is supported by the study by Sackeim et al. (2009), the only study in our meta-analysis in which a TCA was given at doses that were aimed to achieve therapeutic plasma levels. This study showed a positive effect of adding nortriptyline to unilateral or bilateral ECT.
Limitations
The prime limitation of our meta-analysis is that most included studies are relatively old. Five out of nine studies were conducted in the sixties before the introduction of modern techniques for administering ECT (Imlah et al., 1965; Monaco and Delaplaine, 1964; Muller, 1961; Seager and Bird, 1962; Wilson et al., 1963). ECT dosage and waveform have changed over the last decades, and anaesthetics have been introduced. In early studies, the administration of ECT might have been suboptimal, at least in unilateral ECT. Additionally, the patient population receiving ECT has changed a great deal. ECT used to be a first-line antidepressant treatment, but medication resistance is currently an important indication for its inclusion. This may have resulted in an overestimation of the effect of an adjuvant antidepressant during ECT. On the contrary, in early studies, the doses of antidepressants were often subtherapeutic. The inclusion of patients with bipolar depression in at least one large study (Sackeim et al., 2009) may have decreased the efficacy of the adjuvant antidepressants. Six studies investigated adjuvant treatment with a TCA (Imlah et al., 1965; Kay et al., 1970; Mayur et al., 2000; Sackeim et al., 2009; Seager and Bird, 1962; Wilson et al., 1963). Of those, five did not use plasma-level targeted dosing (Imlah et al., 1965; Kay et al., 1970; Mayur et al., 2000; Seager and Bird, 1962; Wilson et al., 1963). In three studies, imipramine was given at a dose of 75–150 mg/day (Imlah et al., 1965; Seager and Bird, 1962; Wilson et al., 1963), which is insufficient for the large majority of patients (Birkenhager et al., 2005). Three studies investigated adjuvant treatment with an MAOI (Imlah et al., 1965; Monaco and Delaplaine, 1964; Muller, 1961). These studies used low doses of phenelzine (45 mg/day) or a very low dose of tranylcypromine (20 mg/day). These subtherapeutic antidepressant doses may have resulted in an underestimation of the effect of an adjuvant antidepressant during ECT. In eight out of nine RCTs, antidepressants and ECT were started simultaneously. In one study, ECT was added to an ongoing treatment with an antidepressant (Mayur et al., 2000). Since there is hardly any variation regarding this methodological aspect, it probably has negligible influence on the result.
All studies, except for the study by Sackeim et al. (2009), were deemed to be of poor to moderate quality. Six out of nine RCTs were conducted in the sixties and seventies (Imlah et al., 1965; Kay et al., 1970; Monaco and Delaplaine, 1964; Muller, 1961; Seager and Bird, 1962; Wilson et al., 1963). At that time, reports on studies were less transparent. Among other things, these studies lack information on their study protocol, randomization procedure and allocation concealment. This makes it difficult to appraise the quality of these studies and their subsequent results. Despite these flaws, we found no statistical association between year of publication and reported add-on effect. Moreover, the same six RCTs did not report on electrode placement. Due to insufficient data, it is impossible to make a statement on the impact of the type of electrode placement on the results.
Another limitation of our meta-analysis is the small number of included studies and the fact that two of studies (Imlah et al., 1965; Sackeim et al., 2009) reported on two categories of adjuvant antidepressants. A larger number of studies might have provided better evidence. Despite a lack of significance in almost all individual studies, our meta-analysis shows a homogeneous and positive effect in favour of an adjuvant antidepressant in all but one study. This indicates an underlying effect of an adjuvant antidepressant on the efficacy of ECT. Additionally, we found no indication of publication bias. For future research, a comparison trial of different types of adjuvant antidepressants during ECT would be very relevant.
Conclusion
Our results suggest that an adjuvant antidepressant enhances the efficacy of ECT in patients with major depression. Although the included studies had some methodological limitations, effect sizes were consistently small to moderate. We speculate that modern-day controlled trials using adequately dosed TCAs and MAOIs will most likely result in larger effect sizes. From a clinical point of view, we prefer an adjuvant TCA to an adjuvant MAOI, since TCAs are generally safe to use with ECT (American Psychiatric Association, 2001; Baghai et al., 2006; Naguib and Koorn, 2002; Sackeim et al., 2009), whereas MAOIs warrant precautions during anaesthesia for ECT (Dolenc et al., 2004; Naguib and Koorn, 2002). Moreover, MAOIs are prescribed far less commonly than TCAs and the use of an MAOI requires dietary restrictions.
Thus, if ECT is indicated for a patient with major depression, we recommend the routine use of an adequately dosed adjuvant antidepressant to improve the efficacy of ECT. We leave the choice between a TCA, an SSRI/SNRI and an MAOI up to the clinician. Our findings warrant renewed interest in adjuvant pharmacotherapy during ECT for major depression.
Supplemental Material
Supplemental material, Supplementary_Table_1_Search_strategy_per_database for Influence of an adjuvant antidepressant on the efficacy of electroconvulsive therapy: A systematic review and meta-analysis by Esther M Pluijms, Astrid M Kamperman, Witte JG Hoogendijk, Tom K Birkenhäger and Walter W van den Broek in Australian & New Zealand Journal of Psychiatry
Acknowledgments
The authors wish to thank Wichor Bramer, biomedical information specialist, Medical Library, Erasmus MC, University Medical Centre, Rotterdam, The Netherlands, for his assistance in the literature search.
Footnotes
Author Contributions: E.M.P., T.K.B. and W.W.v.d.B. formulated the research question. E.M.P. searched the electronic databases. E.M.P. and W.W.v.d.B. screened all articles, assessed their eligibility and assessed the risk of bias of the included RCTs. E.M.P. and A.M.K. assessed the strengths of the included retrospective cohort studies. Disagreement was resolved by discussion and consensus with A.M.K. or T.K.B. A.M.K. was responsible for statistical analyses. E.M.P. wrote the manuscript and integrated the comments of all other authors. All authors participated in critical revision of the manuscript drafts and approved the final version.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Esther M Pluijms  https://orcid.org/0000-0001-7514-0946
https://orcid.org/0000-0001-7514-0946
Data Availability: All authors had full access to all data. Since this study reports on a systematic review and meta-analysis, all data are available in the public domain.
Supplemental Material: Supplemental material for this article is available online.
References
- American Psychiatric Association (1980) Diagnostic and Statistical Manual of Mental Disorders (DSM-3), 3rd Edition. Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), 4th Edition. Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders: Text Revision (DSM-IV-TR), 4th Text Revision Edition. Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association (2001) The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training and Privileging, 2nd Edition. Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association (2010) Practice Guideline for the Treatment of Patients with Major Depressive Disorder. Available at: http://psychiatryonline.org/guidelines.aspx (accessed 27 October 2019). [PubMed]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th Edition. Washington, DC: American Psychiatric Association. [Google Scholar]
- Anderson IM. (2000) Selective serotonin reuptake inhibitors versus tricyclic antidepressants: A meta-analysis of efficacy and tolerability. Journal of Affective Disorders 58: 19–36. [DOI] [PubMed] [Google Scholar]
- Baghai TC, Marcuse A, Brosch M, et al. (2006) The influence of concomitant antidepressant medication on safety, tolerability and clinical effectiveness of electroconvulsive therapy. The World Journal of Biological Psychiatry 7: 82–90. [DOI] [PubMed] [Google Scholar]
- Birkenhager TK, Pluijms EM. (2016) Possible synergy between electroconvulsive therapy and imipramine: A case report. Journal of Psychiatric Practice 22: 478–480. [DOI] [PubMed] [Google Scholar]
- Birkenhager TK, van den Broek WW, Moleman P, et al. (2005) Imipramine dose in relation to therapeutic plasma level: Are clinical trials using imipramine as a positive control flawed? Psychopharmacology 181: 595–599. [DOI] [PubMed] [Google Scholar]
- Boylan LS, Haskett RF, Mulsant BH, et al. (2000) Determinants of seizure threshold in ECT: Benzodiazepine use, anesthetic dosage, and other factors. Journal of ECT 16: 3–18. [DOI] [PubMed] [Google Scholar]
- Debonnel G, Saint-Andre E, Hebert C, et al. (2007) Differential physiological effects of a low dose and high doses of venlafaxine in major depression. International Journal of Neuropsychopharmacology 10: 51–61. [DOI] [PubMed] [Google Scholar]
- Dolenc TJ, Habl SS, Barnes RD, et al. (2004) Electroconvulsive therapy in patients taking monoamine oxidase inhibitors. Journal of ECT 20: 258–261. [DOI] [PubMed] [Google Scholar]
- Dutch Psychiatric Association (2010) Guideline on electroconvulsive therapy [Richtlijn elektroconvulsietherapie], 2nd Revised Edition. Utrecht: De Tijdstroom. [Google Scholar]
- Duval S, Tweedie R. (2000) A nonparametric ‘trim and fill’ method of accounting for publication bias in meta-analysis. Journal of the Acoustical Society of America 95: 89–98. [Google Scholar]
- Egger M, Davey Smith G, Schneider M, et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. (1976) Clinical Global Impression Scale. In: Guy W. (ed.) ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health, pp. 218–222. [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq AU, Sitzmann AF, Goldman ML, et al. (2015) Response of depression to electroconvulsive therapy: A meta-analysis of clinical predictors. Journal of Clinical Psychiatry 76: 1374–1384. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. (1985) Statistical Methods for Meta-Analysis. Orlando, FL: Academic Press. [Google Scholar]
- Heijnen WT, Birkenhager TK, Wierdsma AI, et al. (2010) Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: A meta-analysis. Journal of Clinical Psychopharmacology 30: 616–619. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. (eds) (2011) Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). Available at: www.handbook.cochrane.org (accessed 02 September 2019).
- Imlah NW, Ryan E, Harrington JA. (1965) The influence of antidepressant drugs on the response to electroconvulsive therapy and on subsequent relapse rates. Neuropsychopharmacology 4: 438–442. [Google Scholar]
- Jha A, Stein G. (1996) Decreased efficacy of combined benzodiazepines and unilateral ECT in treatment of depression. Acta Psychiatrica Scandinavica 94: 101–104. [DOI] [PubMed] [Google Scholar]
- Kay DW, Fahy T, Garside RF. (1970) A seven-month double-blind trial of amitriptyline and diazepam in ECT-treated depressed patients. British Journal of Psychiatry 117: 667–671. [DOI] [PubMed] [Google Scholar]
- Kho KH, Zwinderman AH, Blansjaar BA. (2005) Predictors for the efficacy of electroconvulsive therapy: Chart review of a naturalistic study. Journal of Clinical Psychiatry 66: 894–899. [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Odgaard K, Clemmesen L, et al. (1996) Relapse prevention by means of paroxetine in ECT-treated patients with major depression: A comparison with imipramine and placebo in medium-term continuation therapy. Acta Psychiatrica Scandinavica 94: 241–251. [DOI] [PubMed] [Google Scholar]
- Mayur PM, Gangadhar BN, Subbakrishna DK, et al. (2000) Discontinuation of antidepressant drugs during electroconvulsive therapy: A controlled study. Journal of Affective Disorders 58: 37–41. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Journal of Clinical Epidemiology 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- Monaco JT, Delaplaine P. (1964) Tranylcypromine with ECT. American Journal of Psychiatry 120: 1003. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. (1979) A new depression scale designed to be sensitive to change. British Journal of Psychiatry 134: 382–389. [DOI] [PubMed] [Google Scholar]
- Muller D. (1961) 1. Nardil (phenelzine) as a potentiator of electroconvulsive therapy (ECT) – 2: A survey of outpatient ECT. Journal of Mental Science 107: 994–996. [DOI] [PubMed] [Google Scholar]
- Naguib M, Koorn R. (2002) Interactions between psychotropics, anaesthetics and electroconvulsive therapy: Implications for drug choice and patient management. CNS Drugs 16: 229–247. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (2003) Guidance on the use of electroconvulsive therapy. Available at: www.nice.org.uk/guidance/ta59 (accessed 27 October 2019).
- Nelson JP, Benjamin L. (1989) Efficacy and safety of combined ECT and tricyclic antidepressant drugs in the treatment of depressed geriatric patients. Convulsive therapy 5: 321–329. [PubMed] [Google Scholar]
- Research Registry (2019) Influence of an adjuvant antidepressant on the efficacy of electroconvulsive therapy: A systematic review and meta-analysis (Reviewregistry763). Available at: www.researchregistry.com/browse-the-registry#registryofsystematicreviewsmeta-analyses/registryofsystematicreviewsmeta-analysesdetails/5dc695a75b2ef10019a7acf1/ (accessed 09 November 2019). [DOI] [PMC free article] [PubMed]
- Sackeim HA, Dillingham EM, Prudic J, et al. (2009) Effect of concomitant pharmacotherapy on electroconvulsive therapy outcomes: Short-term efficacy and adverse effects. Archives of General Psychiatry 66: 729–737. [DOI] [PubMed] [Google Scholar]
- Seager CP, Bird RL. (1962) Imipramine with electrical treatment in depression: A controlled trial. Journal of Mental Science 108: 704–707. [DOI] [PubMed] [Google Scholar]
- Sienaert P, Peuskens J. (2007) Anticonvulsants during electroconvulsive therapy: Review and recommendations. Journal of ECT 23: 120–123. [DOI] [PubMed] [Google Scholar]
- Stata Corp (2017) Stata: Release 15. College Station, TX: Stata Corp. [Google Scholar]
- State of Queensland (2018) Guideline for the administration of electroconvulsive therapy. Available at: www.health.qld.gov.au/__data/assets/pdf_file/0028/444763/2018_Guideline-for-the-administration-of-Electroconvulsive-Therapy-v0.7.pdf (accessed 27 October 2019).
- UK ECT Review Group (2003) Efficacy and safety of electroconvulsive therapy in depressive disorders: A systematic review and meta-analysis. The Lancet 361: 799–808. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, et al. (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. The Lancet 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- Wilson DB. (1999) Metaf Version 1.0: 06-01-1999: Meta-analysis Macros for SAS, SPSS, and Stata. Available at: http://mason.gmu.edu/~dwilsonb/ma.html (accessed 10 September 2019).
- Wilson IC, Vernon JT, Guin T, et al. (1963) A controlled study of treatments of depression. Journal of Neuropsychiatry 4: 331–337. [PubMed] [Google Scholar]
- World Health Organization (2010) ICD-10: International Classification of Diseases and Related Health Problems, 10th Edition. Geneva: World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table_1_Search_strategy_per_database for Influence of an adjuvant antidepressant on the efficacy of electroconvulsive therapy: A systematic review and meta-analysis by Esther M Pluijms, Astrid M Kamperman, Witte JG Hoogendijk, Tom K Birkenhäger and Walter W van den Broek in Australian & New Zealand Journal of Psychiatry