Abstract
Paramount efforts worldwide are seeking to increase understanding of the basic virology of SARS-CoV-2, characterize the spectrum of complications associated with COVID-19, and develop vaccines that can protect from new and recurrent infections with SARS-CoV-2. While we continue learning about this new virus, it is clear that 1) the virus is spread via the respiratory route, primarily by droplets and contact with contaminated surfaces and fomites, as well as by aerosol formation during invasive respiratory procedures; 2) the airborne route is still controversial; and 3) that those infected can spread the virus without necessarily developing COVID-19 (ie, asymptomatic). With the number of SARS-CoV-2 infections increasing globally, the possibility of co-infections and/or co-morbidities is becoming more concerning. Co-infection with Human Immunodeficiency Virus (HIV) is one such example of polyparasitism of interest. This military-themed comparative review of SARS-CoV-2 and HIV details their virology and describes them figuratively as separate enemy armies. HIV, an old enemy dug into trenches in individuals already infected, and SARS-CoV-2 the new army, attempting to attack and capture territories, tissues and organs, in order to provide resources for their expansion. This analogy serves to aid in discussion of three main areas of focus and draw attention to how these viruses may cooperate to gain the upper hand in securing a host. Here we compare their target, the key receptors found on those tissues, viral lifecycles and tactics for immune response surveillance. The last focus is on the immune response to infection, addressing similarities in cytokines released. While the majority of HIV cases can be successfully managed with antiretroviral therapy nowadays, treatments for SARS-CoV-2 are still undergoing research given the novelty of this army.
Keywords: HIV, AIDS, SARS-CoV-2, COVID-19, ACE-2, remdesivir
Introduction: Briefing
The COronaVIrus Disease (COVID-19) changed the daily routine worldwide since December 2019, when an epidemic was first reported in Wuhan, China.1 This epidemic turned into a pandemic by March 2020.2 Its etiologic agent, the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) belongs to a family of zoonotic coronaviruses that have caused significant epidemics, including SARS in 2003 and Middle East Respiratory Syndrome (MERS) in 2012.3
Comorbidities are of particular interest when there is a new and emerging disease. An estimated 22% of the people in the world have at least one health condition that increases their risk of developing severe COVID-19.4 One such comorbidity of interest is infection with pandemic human immunodeficiency virus (HIV), the etiologic cause of acquired immunodeficiency syndrome (AIDS). Initial reports of AIDS in the United States covered the news of patients with atypical Pneumocystis carinii pneumonia,5 followed by reports of rare and aggressive Kaposi Sarcoma6 and other opportunistic infections, which created the foundation for a concept of a syndrome in which the immune system was completely non-functional, named AIDS, by the CDC in 1982. HIV/AIDS has claimed 33 million lives worldwide since its discovery.7 According to WHO, there are around 38 million People Living with HIV (PLWH) worldwide.8
With SARS-CoV-2 continuing to spread across the globe, clinicians will continue encountering co-infection cases of People Living With HIV and SARS-CoV-2 (PLWH+CoV2). The first published case of SARS-CoV-2/HIV co-infection was a 61-year-old man from Wuhan who was confirmed to have SARS-CoV-2 in January 2020 and had diabetes and HIV infection as comorbidities.9 The Hospital Clinic Barcelona in Barcelona, Spain treated five patients with HIV and SARS-CoV-2 co-infection in March 2020, and a university hospital in Madrid reported 51 cases by April 30, 2020.10,11 An additional 47 cases of HIV/SARS-CoV-2 co-infection were reported as of April 20, 2020 in Italy.12 Further, the Myriam Hospital Infectious Diseases and Immunology Center in Rhode Island, USA reported 27 HIV patients were SARS-CoV-2 positive in July.13
The Italian cohort of PLWH+ SARS-CoV2 presented with dyslipidemia and hypertension as the most common comorbidities, and treatments administered included antiretroviral therapy (ART) to manage HIV infection. In this case, patients with HIV and SARS-CoV-2 coinfection had better clinical outcomes on average than patients at the same hospital with only SARS-CoV-2 infection.12 In contrast, the case report from Madrid reported that patients with HIV and SARS-CoV-2 co-infection were more likely to have additional comorbidities than those with HIV or SARS-CoV-2 alone. Also, patients with co-infection did not differ significantly in clinical symptoms, blood results, and radiological findings from the general SARS-CoV-2 cases, except they had a higher rate for severe illness than SARS-CoV-2 mono-infection patients, 25% compared to 17–21%.11 HIV infection itself is associated with a myriad of non-infectious co-morbidities.14 For instance, HIV infection increases the risk for acute exacerbations of chronic obstructive pulmonary disease (COPD),15,16 chronic kidney disease,17,18 cardiomyopathies and heart failure,19,20 all of which are high priority conditions that may lead to severe COVID-19.21 In addition, the role of HIV antiretroviral therapy in COVID-19 remains under observation.
Key Components: Armaments
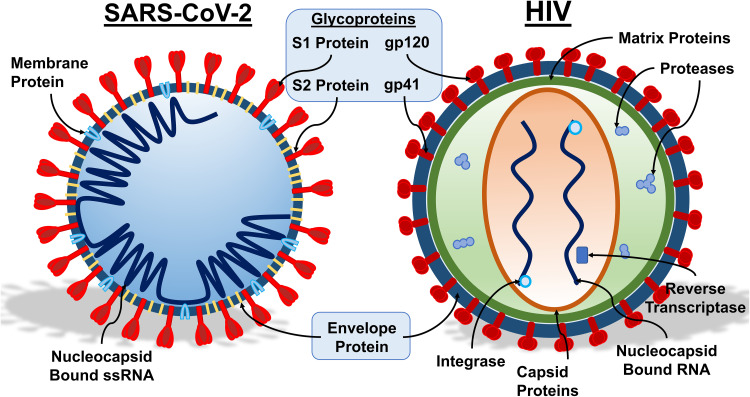
HIV and SARS-CoV-2 viruses are both equipped with different armaments for battle, which are compared in Figure 1. SARS-CoV-2 is a beta coronavirus consisting of four structural proteins genetically coded by positive single-stranded RNA. The viral RNA is bound by the nucleocapsid protein (N) and is required for replication.22 The spike protein (S) consists of S1 and S2 subunits with a furin cleavage site between the S1 and S2 coding sequences.23 The stalk of the spike protein has three hinges, giving the spike protein flexibility when scanning cell surfaces for a receptor, and aids in protecting the spike from antibodies.24 The viral envelope consists of the envelope protein (E) and the membrane protein (M).25 The M protein is responsible for the overall structure of coronaviruses, interacting with the S, E and N proteins, and plays a major role in membrane fusion, an important step in the infection process.26 Besides these structural proteins, the RNA encodes eight open reading frames (ORF) consisting of ORF1a, ORF1b, ORF3, ORF6, ORF7a, ORF7b, ORF8 and ORF10.27 ORF1a and ORF1b encode polyproteins that form non-structural proteins and replicase for replication.28 ORF3a has been shown to have pro-apoptotic activity in vitro by activating caspase-8.29 ORF6 subdues immune response by functioning as an interferon antagonist.30 ORF7a in SARS-CoV was capable of inducing apoptosis, but clinical isolates of SARS-CoV-2 have shown large deletions that likely cause loss of function of ORF7a.31,32 ORF7b was shown to localize in the Golgi, in SARS-CoV, but its function in SARS-CoV-2 has yet to be characterized.33 ORF8 lacks known functional domains and no longer contains the sequence in SARS-CoV ORF8b that triggers stress pathways.27,28 ORF10 does not display homology to known proteins and is unlikely to be expressed.27
Figure 1.
Components of SARS-CoV-2 and HIV. A schematic representation of SARS-CoV-2 (left) and HIV (right) is shown, along with key structural components. For both viruses, the glycoproteins, embedded within the envelope proteins are required for the initial interaction of the virus with susceptible host cells. In SARS-CoV-2, the membrane protein is required for membrane fusion and interacts with other elements of the virus. The envelope protein aids in viral assembly within the host cell, and the single-stranded RNA enclosed in the nucleocapsid comprises the genetic material. In HIV, the capsid proteins are structural proteins arranged to house the viral genetic material. Viral enzymes, coded by the pol gene, include proteases, reverse transcriptase, and integrase. The HIV reverse transcriptase is an RNA-dependent DNA polymerase essential for synthesis of viral cDNA using RNA as template. The viral integrase creates a permanent copy of viral DNA in the infected cell by catalyzing viral DNA integration into host cell DNA. The protease cleaves the newly formed polypeptide into the components of mature virions. The matrix proteins are key in virion packaging. Illustration credit: Nicholas J. Evans.
HIV has different equipment as a member of the lentivirus family. HIV also has an RNA genome, consisting of two copies of positive-stranded RNA with nine open reading frames that code for 15 viral proteins.34 The RNA genome is enclosed within the viral core, surrounded by matrix proteins between the lipid envelope and viral capsid.35 The viral capsid also houses enzymes essential for viral replication, eg, reverse transcriptase, integrase and proteases, coded by the viral pol gene.36 The viral reverse transcriptase converts the HIV RNA into DNA when the virus enters the host.37,38 Integrase can then incorporate the viral DNA into the host DNA, creating a permanent copy of the viral DNA in the cell.38,39 After the Gag polyprotein is constructed by the host cell, proteases are used to segregate the viral components so they can be assembled into new viruses.38 Newly formed, infectious virions also contain gp41 glycoproteins anchored in the lipid envelope. Viral glycoproteins of ~120 kD (gp120) are attached to gp41, which form the HIV spike protein40 that is essential for interaction with host cell receptors, leading to infection and reinitiating of the full lifecycle.
Both SARS-CoV-2 and HIV have probable zoonotic origins. SARS-CoV-2 was initially linked to a market selling seafood, snake, bats, poultry, and other farm animals in Wuhan, China, which originated the hypothesis of foodborne infection. Supporting viral sequencing and phylogenetic studies suggested that SARS-CoV-2 is a recombinant virus from bat coronavirus.41–43 Intriguingly, the virus was detected in surfaces in the market but no animals tested positive to the virus.44 In the case of HIV, longstanding evidence points to simian immunodeficiency viruses (SIV) crossing to humans.45,46 The elucidation of the cross-species transmission dynamics has gained attention, as well as opened doors to exploring restriction factors and intrinsic immunity as additional paradigms.47–49
Viral Transmission: Deployment
SARS-CoV-2 is a respiratory virus, primarily spread from person to person through respiratory droplets containing viral particles. A study reported finding viable SARS-CoV-2 virus even after 72 hours on plastic or stainless-steel surfaces, although titer was reduced from 103.7 to 100.6 TCID50 (1259-fold reduction) for both. However, on copper and cardboard surfaces, there was no viable SARS-CoV-2 after 4 and 24 hours, respectively.50 Protection from and prevention of respiratory droplets, in the forms of masks or face coverings, are important deterrents to avoid infecting others.51,52 Masks become essential, considering that asymptomatic individuals can still shed virus and infect those who come in contact with their respiratory droplets.53
HIV is a bloodborne pathogen, also with zoonotic origin. According to the UNAIDS, between 1.2 and 2.2 million people worldwide were identified as newly infected with HIV in 2019.8 Different from SARS-CoV-2, it takes different routes of transmission, relying on mucosal surface contact or percutaneous inoculation. Transmission of HIV occurs primarily via sexual contact, sticks with contaminated needles, and vertical transmission (mother to child). Patel et al reported estimates of HIV transmission risk based on meta-analysis and literature review of publications reporting risk between January 2008 and February 2012. Risk of HIV infection per event was highest for blood transfusion healthcare accidents with a probability of 92.5%, with healthcare-related needle sticks having a probability of 0.23%. The probability of infection due to intravenous drug use was estimated at 0.63%. Risk of contraction from sexual intercourse varies by the act, with penile-vaginal intercourse risk estimates at 0.04–0.08%, and penile-anal intercourse estimates at 0.11–1.38%, with more risk occurring for the receptive person in both cases.54 Pertaining to vertical transmission, strategies to protect unborn children from HIV infection include strict adherence to antiretroviral therapy during pregnancy, avoiding the passage of the baby through the birth canal during labor via scheduled C-sections, and limiting breastfeeding. The use of antiretroviral therapy has successfully decreased HIV transmission from mother to child. A meta-analyses study including studies from 2003 to 2011 documented that mothers enrolled in HIV prevention programs displayed a consistent decrease in HIV transmission over the years.55
Target Tissues: Flanking Maneuver
The differences in transmission between SARS-CoV-2 and HIV lead to different initial and subsequent tissues to be affected. This allows the viruses to essentially flank the tissues, progressing from initial contact to a systemic infection. SARS-CoV-2 works like a paratrooper, dropping in from the air either directly on or near its objective, then potentially deploying a boat to travel by water (blood) to reach objectives that are hard to access. In contrast, HIV would be similar to naval units, most at home in the water (blood) and taking out other boats (T-cells in circulation), but still having the ability to assault ground objectives (organs and tissues).
For SARS-CoV-2, droplets containing the virus may enter the nose and/or mouth, where they can begin infecting tissues in the naso-, oro- and laryngopharynx.56 At this point, the infected individual may experience symptoms such as fever, cough, loss of taste and/or smell, or they may be asymptomatic. From here the virus may continue to spread down into the trachea, bronchi, bronchioles, and alveoli, where it can infect the lung tissues, causing damage and inflammation, and has an opportunity to enter the bloodstream.56–58 Once this occurs, it can spread to other organs including the brain, eyes, heart, blood vessels, liver, kidneys, intestines, testis, thyroid and pancreas.56,57,59,60 Frontal lobe sections from a 74-year-old male, who died after contracting SARS-CoV-2, had viral particles observed through transmission electron microscopy present in and on neural cell bodies and endothelium. SARS-CoV-2 in the brain tissue was confirmed by four parallel RT-PCR assays.60 While originally a concern, the female reproductive system does not appear to be a major target of SARS-CoV-2. This is supported by results from the University of California showing low expression of receptors and proteases for viral entry in uterus, myometrium, ovary, fallopian tube and breast epithelium by single-cell sequencing.61
HIV uses the bloodstream as a means to spread. Integration of the HIV provirus into the host genome and establishment of a reservoir allows for HIV to persist in many organs, even while on ART.62 The cells most permissive to HIV infection are CD4+ T cells, with effector memory and central memory T cells being the most permissive due to their high density of CD4, long lifespan and propensity to proliferate via IL-7.63,64 However, HIV has the ability to enter many others such as macrophages, B cells, granulocytes such as neutrophils, eosinophils, basophils,65 dendritic cells, and Langerhans cells in the epithelium,66,67 summarized in Table 1. Macrophages are of particular interest as these cells can have long lifespan of months to years,68–71 and therefore, may facilitate further infection of CD4+ T cells.72,73
Table 1.
Cellular and Anatomical Targets of SARS-CoV-2 and HIV
| SARS-CoV-2 Targets | Body System | HIV Targets |
|---|---|---|
| Human Epithelial cells in Pharynx, Trachea, and Bronchi, Type II Alveolar cells | Upper and Lower Respiratory Tract | Alveolar Macrophages, T cells, Fibroblasts, Bronchial Epithelial Cells, Smooth Muscle Cells |
| Oral Epithelium, Enterocytes of the Duodenum, Jejunum and Ileum, Epithelium, Hepatocytes, Muscularis Mucosae, Muscularis Propria | Gastrointestinal Tract | GALT, TCM, TEM, TTD, Langerhans cells (Tissue-resident Macrophages), Dendritic cells |
| Lymphocytes, Granulocytes, T cells, B cells, Monocytes | Blood | TNA, TSCM, TCM, TEM, TTD, TMM, γδ T cell, monocyte, NK, TH |
| Basal Epithelium, Sebaceous Gland Smooth Muscle | Skin | Langerhans cells (Tissue-resident Macrophages), CD4+ T cells |
| Olfactory and Gustatory Neurons, Brain | Nervous System | CD4+ T cells, Microglia (Tissue-resident Macrophages), Astrocytes, |
| Endothelial Cells, Vascular Smooth Muscle, Cardiomyocytes | Cardiovascular | Smooth Muscle Cells |
| Adrenal Gland, Hypothalamus and Pituitary Gland, Sertoli and Leydig Cells | Endocrine/Reproductive | Epithelial cells, Testis germ cells, CD4+ T cells, Macrophages |
| Podocytes, Mesangial cells, Epithelium, proximal cells of brush border and collecting ducts | Renal | Tubular and glomerular epithelial cells (podocytes) |
Note: The types of cells shown to harbor latent HIV.
Abbreviations: GALT, gut associated lymphoid tissue; TNA, naïve T cell; TSCM, T stem cell memory; TCM, central memory T cell; TEM, effector memory T cell, TTD, terminally differentiated T cell; TMM, migratory memory T cell; NK, natural killer cell, TH, helper T cell; TFH, follicular helper T cell; TRM, tissue resident memory T cell.56–60,65,154
Key Receptors: Decisive Point
A decisive point is a place, event, system or function that gives an advantage when acquired by a military commander.74 SARS-CoV-2 and HIV both have receptors that could be considered their decisive points, as these receptors are integral to their infection of cells.
SARS-CoV-2 gains entry to tissues through binding of the S1 portion of the spike protein, with the angiotensin-converting enzyme 2 (ACE2).75,76 ACE2 was also utilized by SARS-CoV, but SARS-CoV-2 receptor binding domain has differences in multiple key amino acids, enabling increased binding affinity than the previous virus.75 The C-terminal domain of the S1 protein is responsible for binding ACE2.23 Upon binding, host proteases such as TransMembrane Protease Serine-2 (TMPRSS2), prime the virus for entry into cells through endocytosis.77,78 Once inside, fusion of the endosome with the virus releases the viral RNA into the cell.79
Binding of S1 protein to ACE2 activates the renin-angiotensin system (RAS), which in turn will activate ADAM17, a metalloproteinase normally responsible for cleaving and activating tumor necrosis factor (TNF).80,81 ACE2 shedding is an event where activated ADAM17 cleaves ACE2, removing it from the cell.81 In the absence of ACE2, angiotensin II conversion into angiotensin1-7 is limited, preventing vasodilation from occurring.81,82 In ACE2 knockout mice, elevated angiotensin II led to myocardial fibrosis and hypertrophy, and caused diastolic heart failure. The mice also had elevated levels of superoxide radicals, but these effects could be remedied by administering recombinant human ACE2 (rhACE2) to the mice, suppressing angiotensin II pathology.83 Similar treatments are being explored for COVID-19, with recruitment for clinical trials underway.
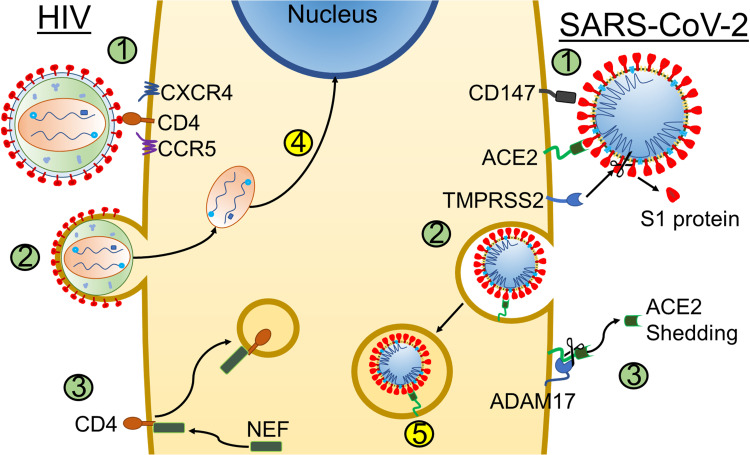
Originally, ACE2 was the only identified receptor for SARS-CoV-2 S1 binding. However, Wang et al determined the spike protein is capable of binding CD147, a glycoprotein often related to tumor development, for entry into the cell (Figure 2). Interaction of S1 and CD147 was confirmed using Co-IP and Surface Plasmon Resonance assays and immune-electron microscopy showed co-localization of the proteins.84 They also explored the infectivity of Vero E6 cells after CD147 was blocked with Meplazumab, an anti-CD147 antibody, and found Meplazumab had an inhibitory effect on SARS-CoV-2 infection.84 A trial conducted by Bial et al investigated using Meplazumab for treatment of COVID-19, looking for changes in the time until viral clearance, determined by negative qRT-PCR, and the time until recovery of vital signs. They observed significant changes in recovery and clearance, with the Meplazumab group having a negative RT-PCR for SARS-CoV-2, and recovery vital signs earlier than the control group.85 Current evidence supports that CD147 is found primarily in human bronchial epithelial cells (HBECs), immune cells in bronchoalveolar lavages (BAL), skin and in the circulation, including granulocytes, T and B cells, monocytes, innate lymphoid cells, and whole blood.86 This may explain the spread of SARS-CoV-2 to infect tissues that have lower ACE2 expression.
Figure 2.
Comparative overview of viral entry and receptor downregulation. Schematic representation of a host cell potentially co-infected with HIV (left side) and SARS-CoV-2 (right side). [1] Binding of HIV or SARS-CoV-2 to key receptors. Both SARS-CoV-2 require initial interaction with specific receptors at the cell membrane: HIV binds to CD4 and co-receptors CCR5 and CXCR4 (left) and SARS-CoV-2 binds to host cell receptors CD147 or ACE2 (right). Binding of SARS-CoV-2 to ACE2 receptor requires initial cleavage of the viral S1 glycoprotein assisted by human protease TMPRSS2 so that viral S2 protein can be internalized along with the rest of the virion (right). [2] Entry of virus into cell. HIV fusion with the cell membrane leads to disassembly of the virion in the cytoplasm, leading to the release of viral genetic material, reverse transcription and [4] eventual integration of viral DNA into the host cell DNA, whereas SARS-CoV-2 enters the cell through endocytosis after binding to ACE2 or CD147. [3] Downregulation of CD4 by NEF or ACE2 by ADAM17. Viral internalization (infection) leads to downregulation of the initial entry receptor for HIV (CD4, mediated by the viral Nef polypeptide) and ACE2 for SARS-CoV-2, which is cleaved by the host ADAM17. [5] Fusion of the endosome and the virus. The viral membrane and endosome membrane fuse, releasing SARS-CoV-2’s genome into the host cell. Illustration credit: Nicholas J. Evans.
The primary human receptor targeted by HIV, using gp120, is CD4 and the binding of HIV (HTLV-III/LAV) to CD4 (T4 molecule), was first identified in 1986 by McDougal et al.87 Further investigation by the scientific community confirmed that HIV may utilize additional surface proteins on the host cell as co-receptors for entry along with CD4 (Figure 2). These co-receptors are C-X-C chemokine receptor type 4 (CXCR4) and C-C chemokine receptor 5 (CCR5).88,89 The CCR5 is a receptor for RANTES/CCL5, macrophage inflammatory protein-α (MIP-α/CCL3), and MIP-β/CCL4 in primary macrophages.90 The CXCR4 is the unique receptor for stromal-derived factor-1 (SDF-1/CXCL12).91 Conventionally, HIV virions that use CCR5 as portals of entry are designated as “R5”, while virions using CXCR4 are referred to as “X4”. HIV is thus termed to be “X4” or “R5” viruses if it uses CXCR4 or CCR5, respectively. The HIV preference for CCR5 co-receptor switches to a preference for CXCR4 over the course of HIV infection; this co-receptor switch predicts progression to AIDS in ~50% of HIV+ individuals.92 Studies have shown that blockage of CCR5 signaling leads to decreased neutrophilic infiltrations and decreased recruitment of CD8+ T cells which may have protective roles in lung inflammation,93–95 CXCR4 and its natural ligand SDF-1/CXCL12 activates downstream pathways such as ras and PI3 kinase, which play a role in lymphocyte chemotaxis, as well as in progression and metastasis of cancer96–101 including non-small cell lung cancer.102–104 The difference in tropism ultimately dictates what cells are susceptible to HIV as the presence of CD4 with either co-receptor on the surface of any cell will, in theory, be permissive to HIV. Macrophages, an early target in HIV infection, were also identified as HIV reservoirs105,106 and have been extensively studied in the context of HIV infection. Despite their lower density of CD4, macrophages are still be susceptible to HIV infection.107 Moreover, there are strains of HIV that are extremely effective at targeting macrophages. It has been demonstrated that these strains of HIV, R5-tropic HIV,108 have adapted modifications in the CD4 binding site of gp120, giving it a higher affinity to CD4.89 This allows for infection of other cells with low expression of CD4, such as microglia in the brain.109 However, other minor cofactors have been shown to also be involved in the entry of macrophages and essentially replace CCR5 in the fusion step.110 Such cofactors include CCR1, CCR2b, CCR3, CCR8, CX3CR1, CXCR6, formyl peptide receptor 1, G protein-coupled receptor 1 (GPCR1), GPCR15, apelin receptor, and CCBP2.65 Furthermore, because HIV is a budding virus, one must also consider the possibility of other host cell surface molecules being incorporated into the HIV envelope during budding events that will potentially increase infectivity.111 Important molecules gained during budding events that have been demonstrated to increase infectivity and replication of HIV in T cells include ICAM-1 and MHC class II molecules, which bind to LFA-1 and CD4, respectively.112,113
Once inside a cell, HIV uses a number of mechanisms to promote transmission of virions to other cells. An important mechanism that has been described to aid in transmission of HIV is downregulation of viral and host immune receptors (MHC class I and II, CD4, CXCR4, and CCR5) in the host cell. This has been shown to be mediated by the HIV accessory proteins Vpu and Nef.114–116 It is thought that downregulating CD4, and the co-receptors CXCR4 and CCR5, in the host cell will prevent superinfection and aid in incorporation of Env protein into the virions at the time of budding by preventing Env–CD4 interactions.115,116 Downregulation of surface MHC class I is namely mediated by HIV protein Nef via endocytic vesicles and sequestration of molecules being transported to the surface. The extent of MHC class I downregulation is linked to the progression of AIDS and is thus hypothesized to be primarily for evasion of immune surveillance.116
Immune Response: Elastic Defense
The Elastic Defense was a layered defensive tactic used by the Germans in World War II that allowed them to minimize casualties when faced with overwhelming forces. The modern equivalent is the Resilient Defense that builds upon the same pillars. These strategies utilize mobile forces and importantly connected defensive positions with artillery to repel attacking armies. In our scenario, artillery fire is replaced by cytokines and antibodies released into the system, and our troops are white blood cells fighting on fronts as they change, connected by the bloodstream in a similar fashion to the German defenses being connected by tunnels.117
Development of antibodies for SARS-CoV-2 seems to progress similar to other viral infections. A puzzling report from China reported one patient developed symptoms on January 23rd and was admitted to a hospital on February 6th after chest computed tomography (CT) confirmed pneumonia. The patient tested positive for IgG and IgM antibodies against SARS-CoV-2 on February 29th, although four previous tests of pharyngeal samples with RT-PCR were negative for SARS-CoV-2 and other common pathogens.118
SARS-CoV-2 is able to evade the immune system through several mechanisms. The M protein of SARS-CoV-2 has been found to target the Golgi complex, suppressing Interferon type I (IFN-a/-B) gene expression.119 By blocking the production of IFN-a and IFN-B, SARS-CoV-2 delays the response of leukocytes and fibroblasts, which have a pro-inflammatory role in response to viral infection.120 SARS-CoV-2 also has the virulence factor nonstructural protein 1 (Nsp1) which aids in immune evasion by binding with the 40s ribosomal subunit and preventing entry of mRNA. This interaction was confirmed via cryo-electron microscopy and both Nsp1-40S and Nsp1-80S complexes were observed.121 ORF8 in SARS-CoV-2 gives the virus additional immune evasion by associating with MHC-I, with colocalization confirmed with confocal microscopy. MHC-I is bound by ORF8, removed from the membrane by autophagy, and then targeted for lysosome degradation.122
The “cytokine storm” that has been observed with SARS-CoV-2, is similar to the large release of cytokines seen with SARS-CoV infection previously.123 Cytokines found to be elevated in patients are IL-1B, IL-1RA, IL-7, IL-8, IL-9, IL-10, basic FGF, GCSF, GMCSF, IFN-y, IP-10, MCP-1, MIP 1-a, MIP 1-B, PDGF, TNF-a and VEGF, with higher levels of IL-2, IL-7, IL-10, GCSF, IP-10, MCP-1, MIP 1-a and TNF-a in ICU patients relative to non-ICU patients.124 This cytokine storm, along with the loss of ACE2, contributes to multiple system organ failure in severe cases.118,125 Interestingly, while infants are susceptible to SARS-CoV-2 and may experience symptoms, they often have milder cases of COVID-19, with less cases of cytokine storms reported. One study described four infants under twenty-eight days old who all had mild symptoms and one of the four was asymptomatic.126 Another study highlighted nine infants aged one month to one year who also only experienced mild symptoms, one-third of the infants showed no symptoms.127 In both cases, none of the children were recorded as needing intensive care.126,127
Lymphopenia has been observed with SARS-CoV-2 infection. Further analysis showed that patients characterized with severe COVID-19 had lower B cell, T cell and natural killer cell (NK) counts than those with a milder illness.128,129 Lymphopenia can reduce the immune response to the virus, allowing the infection to progress further. This also occurs in untreated HIV infection, in which HIV will severely deplete the host CD4+ T cell population. CD4+ T cells may die via a number of mechanisms as a result of HIV infection. For instance, apoptosis may occur in CD4+ T cells as a result of direct viral cytotoxicity triggered by HIV viral proteins and abnormally activated immune signals.130 Additionally, Doitsh et al demonstrated in ex vivo cultures of HIV-infected human tonsil tissue that >95% of dying CD4+ T cells were abortively infected.131 Abortively infected CD4+ T cells triggers caspase-1 and caspase-3 signaling pathways, eventually leading to cell death by pyroptosis, an inflammatory form of apoptosis. This is followed by recruitment of additional immune cells to the site of pyroptosis. Inevitably, this may lead to more frequent abortive infections, causing CD4+ T cell death via pyroptosis in a cascade-like fashion.132
This is further complicated as the early stages of HIV infection may go largely unnoticed, often being dismissed as a cold or flu. A research group following a cohort based in San Diego, California identified the most common symptoms of acute HIV infection as fever, myalgia, fatigue, headache, night sweats, pharyngitis, and gastrointestinal symptoms; whereas rash, weight loss, and arthralgia are less common, but prevalent nonetheless.133 As the disease progresses, the host innate response is detected just before or when viral RNA reaches detectable levels, which is 100 copies per mL as determined by Koff et al.134,135 This is then followed by an increase in the levels of cytokines and chemokines in the plasma of the host. IL-15, type I IFNs, and CXCL10 will increase rapidly, but only briefly. IL-18, TNF, IFNy, and IL-22 increase rapidly; however, their levels are maintained high.136 Induction of type I IFNs places the secreting cell and neighboring cells in an antiviral state, activating IFN stimulating genes in the process.137 This IFN cascade triggers upregulation of the constitutively expressed genes TRIM5a, APOBEC3, and tetherin, which are host intrinsic restriction factors that function as an innate defense against viruses. Tetherin and APOBEC3 prevent virion particles from escaping and TRIM5a prevents productive infection of HIV. However, HIV has evolved a mechanism of escape from these proteins. In particular, activity from APOBEC3G and BST-2/tetherin can be counteracted by HIV Vif and Vpu, respectively.138,139
As the viremia in the host begins to decline 1 to 2 weeks after reaching peak levels, the T cell response will spike.140,141 During the first few weeks of infection, the population of HIV has not diverged from the founder virus, remaining largely a homogenous population up to this point in time. This occurs because the host adaptive immunity takes approximately 21 days to respond to the initial inoculation of HIV.142 Within 50 days, HIV-specific T cells targeting HIV env and nef, appear. As this occurs, viremia in the host begins to decline and the host will enter the next stage of infection. As the population of HIV declines, mutant strains are selected for and are able to escape the immune system. The first mutants will have changes in epitopes targeted by T cells, namely env and nef. As selection continues to occur, first by T cells and later by HIV-specific antibodies, HIV will begin to develop clusters of mutations throughout its genome as one of the mechanisms to escape the host immune system. Though most of the initial strain of HIV will be gone, the few remaining will be mutant strains that are able to efficiently evade the immune system.143
A study in KwaZulu-Natal, South Africa, focused on the immune response of PLWH+CoV2 and included 124 participants. Immune response to SARS-CoV-2 was altered in PLWH, with higher levels of CD8 T cells being observed. Despite elevated CD8 T cells, PLWH who were on an ART regiment experienced milder symptoms of COVID-19, even if their HIV loads were undetectable.144
Antiretroviral Therapy: Refusing the Flank
When an army is flanked and fighting on two fronts, they may “refuse the flank” by concentrating their forces and effort on one side of the flank. This may allow them to defeat one threat, and then focus their remaining forces on the other threat. In the case of SARS-CoV-2 and HIV co-infection (Figure 2), ART can be given to patients to keep HIV in check, allowing the immune system to focus on SARS-CoV-2.9,10 Noteworthy, the lifelong persistence of the virus, particularly in anatomical reservoirs in patients receiving suppressive antiretroviral therapy, provides a latent source of virus that can easily increase viremia and re-gain territory upon discontinuation of antiretroviral drugs. Therefore, although HIV infections may be considered a chronic, epidemic disease, PLWH still live with a virus waiting for an opportunity to attack.
Several studies have used the antiretroviral drug Lopinavir/Ritonavir (LPV/r), a type 1 aspartate protease inhibitor shown to have in vitro efficacy against SARS-CoV and SARS-CoV-2, to test their efficacy in treating SARS-CoV-2, but the treatment was not significantly efficacious in some studies, and many reported patients having adverse digestive events, such as nausea, diarrhea and vomiting, due to the LPV/r.145–147 Cao et al reported no differences in time to clinical improvement between the LPV/r and control groups, with improvement averaging 16 days for both. Cao et al also noted that adverse events of nausea, vomiting and diarrhea were more common with LPV/r treatment.145 Li et al conducted a trial having LPV/r, Arbidol, and control groups. Arbidol, generic name umifenovir, is an anti-influenza medication not currently approved in the US There were no significant differences in the rates of viral clearance for any of the groups, with each group averaging approximately 9 days for patients to test negative for SARS-CoV-2 nucleic acid. Additionally, 35.2% of the LPV/r patients experienced adverse events, as well as 14.3% of the Arbidol group, with no apparent adverse events in the control group.146 Another study investigating LPV/r explored monotherapy against LPV/r and Arbidol combination therapy and was conducted by Deng et al. By day seven of the trial, 75% of the combination group had negative pharyngeal samples for SARS-CoV-2, whereas the monotherapy group had a rate of 35%. Similarly, 69% of the combination group had improved chest CT, with only 29% showing improvements with monotherapy.147 A small study of 10 patients in China used LPV for treatment of SARS-CoV-2 upon initial hospitalization, but three had to stop treatment due to adverse events, while 5 of the remaining patients reported digestive adverse events. In this report, 80% of the patients reported increases in eosinophil counts with LPV treatment; however, the conclusions of that study suffered from the absence of a control group for comparison.148
Remdesivir is an adenosine nucleoside analog which inhibits replication of viral RNA genomes by inhibiting RNA-dependent RNA polymerase and previously showed efficacy in vitro against Ebola, MERS, and SARS.149–151 A large double-blind, randomized, placebo-controlled trial, dubbed ACTT-1, for treatment of COVID-19 with remdesivir concluded on April 19, 2020 and had 1063 participants that were randomized into a control or remdesivir group.152 Patients taking remdesivir had a median of 11 days until recovery versus a median of 15 days for the control group. While not significant, the remdesivir group had a lower mortality rate than the control group, with Kaplan-Meier estimates of 7.1% and 11.9%, respectively. Fewer patients in the remdesivir group experienced adverse events than the control group, 21% and 27%, respectively.152 A smaller trial was conducted in Hubei, China, finished March 12, 2020 and 237 patients participated, 158 patients in the remdesivir group and 79 in the placebo group. Clinical Improvement rates at day 14 were not significantly different between the remdesivir group and the control with 27% and 23% recovering, respectively.153 By day 28, mortality rates did not differ significantly either with the remdesivir group having 15% mortality and the control having 13%. Adverse events were also not significantly different between the remdesivir group and control group, with 66% and 64% of the patients having some adverse events, respectively.153 More research is being conducted on the use of remdesivir to treat COVID-19. Recruitment for Adaptive COVID-19 Treatment Trial 3 (ACTT-3) is ongoing and will explore the use of a remdesivir plus interferon beta-1a combination.
Closing Remarks: Debrief
The prognosis of polyparasitic infections with HIV and SARS-CoV-2 remains as enigmatic as SARS-CoV-2 infections and COVID-19 itself. In this comparative review, we describe the clinical observations derived from small and large cohorts of patients co-infected with HIV and SARS-CoV-2 in several countries, as well as virological similarities and differences between these two pandemic viruses.
With HIV known to deplete immune cells in the host, and successful antiretroviral therapy known to rescue immune competency, chronic HIV-infected individuals still face co-morbidities that can sometimes be life-threatening, like severe pulmonary vascular disease. Now, with SARS-CoV-2 as a co-pathogen, it is conceivable that co-infections may lead to severe COVID-19. Current reports in the literature document a wide spectrum of symptoms and severities for COVID-19, and future research focusing on co-morbidities may elucidate the key factors that determine severity.
An important distinction between HIV and SARS-CoV-2 is that the former directly attacks and kills the immune system’s soldiers on the front lines, utilizing direct and indirect cytopathic effects to macrophages and lymphocytes. In stark contrast, SARS-CoV-2 while it causes some lymphopenia, it has the capacity to cause friendly fire with the body, invoking severe cytokine storms that end up hurting the host, with varying degrees of COVID-19 onset, development, and recovery.
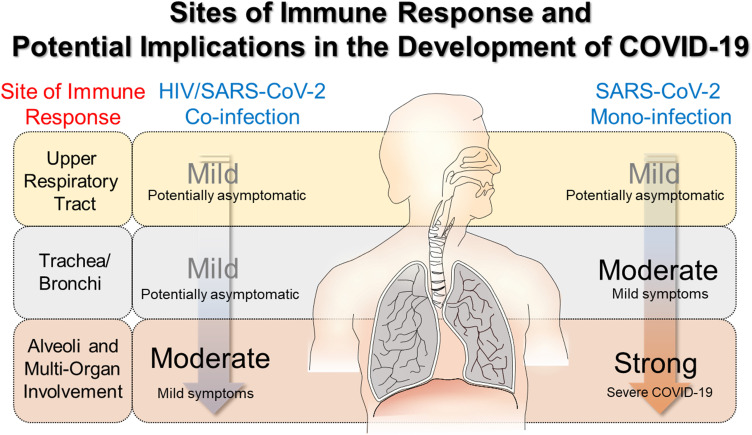
Severe COVID-19 has been postulated to result from damaging, elevated immune responses leading to multi-organ failure. It remains unknown whether HIV contributes to the progression to COVID-19 or if current antiretroviral interventions, originally tailored for HIV, protect the patients from developing severe COVID-19. It is likely that PLWH+CoV-2 are spared from a severe cytokine storm when the virus reaches the lungs and spreads throughout the body via the blood-air exchange at the alveolar-capillary barrier. Severity of symptoms in this case would vary depending on the individual and the progression of the viral infection. This is illustrated in Figure 3. This may be due to chronic impact of HIV in lowering the overall immunocompetency of the patients, even when receiving antiretroviral therapy. While immunosuppression would make them more susceptible to infection, it also may decrease the immune response to infection, reducing the damage that occurs from the inflammation resulting from SARS-CoV-2 infection.
Figure 3.
Hypothetical differences in immune response in HIV/SARS CoV-2 co-infection and SARS-CoV-2 mono-infection and potential implications in the development of COVID-19. A milder immune response is observed in HIV/SARS CoV-2 co-infection, potentially due to a chronically lower immunocompetency in patients living with managed HIV. While the patients are not immunodeficient, they are immunosuppressed. This may reduce the severity of the inflammation that occurs with infection, and lead to a better prognosis for PLWH who become infected with SARS-CoV-2. In turn this would mean a weakened cytokine storm would occur when the virus has reached the lungs and entered the blood, spreading to other organs. The weakened cytokine storm would entail lower systemic inflammation and cause less damage to other organs.
Based on the clinical profiles discussed above, the immune system largely determines the progression of COVID-19 symptoms and severity of symptoms. Following airborne exposure, effective barriers and deployment of the immune system troops can prevent spreading of the SARS-CoV-2 infection to the lower respiratory tract and eventual systemic infection. If the virus remains in the upper respiratory tract, the yellow box in Figure 3, only milder symptoms such as anosmia can occur. In some subjects, SARS-CoV-2 may evade the immune response at the upper respiratory level but meet resistance in the bronchioles and alveoli, leading to severe and sometimes fatal COVID-19.
The novelty of SARS-CoV-2, along with the unknowns associated with the impact of pathogen co-infections, challenges the design of therapeutic approaches that might help patients with increased susceptibility for severe COVID-19 illness. For COVID-19, an ideal strategy would block viral entry to susceptible cells, disrupt viral replication in cells, and boost the immune system while avoiding an increased release of cytokines on top of the cytokine storm. Nevertheless, the most important defensive measure to take is still prevention of transmission. The fact that enveloped viruses like SARS-CoV-2 are susceptible to detergents and alcohols make it an easily killable target; therefore, basic hand washing and use of face coverings to contain secretions should prevail as first lines of defense.
Disclosure
Dr Nicola Petrosillo report personal fees from Pfizer, Speakers’ bureau from MSD, Shionogi, Becton & Dickinson, Johnson & Johnson, Cepheid, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Costa VG, Moreli ML, Saivish MV. The emergence of SARS, MERS and novel SARS-2 coronaviruses in the 21st century. Arch Virol. 2020;165(7):1517–1526. doi: 10.1007/s00705-020-04628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark A, Jit M, Warren-Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003–e1017. doi: 10.1016/s2214-109x(20)30264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottlieb MS, Fan PT, Saxon A, Weisman JD. Pneumocystis Pneumonia—Los Angeles. Morbidity and Mortality Weekly Rep. 1981;30(21):1–3. [PubMed] [Google Scholar]
- 6.Altman L. Rare Cancer Seen in 41 Homosexuals. The New York Times; 1981. [Google Scholar]
- 7.World Health Organization. HIV/AIDS Fact Sheets. World Health Organization; 2020. [Google Scholar]
- 8.UNAIDS. Global HIV & AIDS Statistics — 2020 Fact Sheet. UNAIDS; 2020. [Google Scholar]
- 9.Zhu F, Cao Y, Xu S, Zhou M. Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020;92(6):529–530. doi: 10.1002/jmv.25732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco JL, Ambrosioni J, Garcia F, et al. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7(5):e314–e316. doi: 10.1016/s2352-3018(20)30111-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vizcarra P, Pérez-Elías MJ, Quereda C, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7(8):e554–e564. doi: 10.1016/s2352-3018(20)30164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gervasoni C, Meraviglia P, Riva A, et al. Clinical Features and Outcomes of Patients With Human Immunodeficiency Virus With COVID-19. Clin Infect Dis. 2020;71(16):2276–2278. doi: 10.1093/cid/ciaa579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrd KM, Beckwith CG, Garland JM, et al. SARS-CoV-2 and HIV coinfection: clinical experience from Rhode Island, United States. J Int AIDS Soc. 2020;23(7):e25573. doi: 10.1002/jia2.25573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel P, Rose CE, Collins PY, et al. Noncommunicable diseases among HIV-infected persons in low-income and middle-income countries: a systematic review and meta-analysis. Aids. 2018;32 Suppl 1(Suppl1):S5–s20. doi: 10.1097/qad.0000000000001888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert AA, Kirk GD, Astemborski J, Mehta SH, Wise RA, Drummond MB. HIV Infection Is Associated With Increased Risk for Acute Exacerbation of COPD. J Acquir Immune Defic Syndr. 2015;69(1):68–74. doi: 10.1097/QAI.0000000000000552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antoniou T, Yao Z, Raboud J, Gershon AS. Incidence of chronic obstructive pulmonary disease in people with HIV in Ontario, 1996–2015: a retrospective population-based cohort study. CMAJ Open. 2020;8(1):E83–E89. doi: 10.9778/cmajo.20190028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaboré NF, Poda A, Zoungrana J, et al. Chronic kidney disease and HIV in the era of antiretroviral treatment: findings from a 10-year cohort study in a west African setting. BMC Nephrol. 2019;20(1):155. doi: 10.1186/s12882-019-1335-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halle MP, Essomba N, Djantio H, et al. Clinical characteristics and outcome of HIV infected patients with chronic kidney disease in Sub Saharan Africa: an example from Cameroon. BMC Nephrol. 2019;20(1):253. doi: 10.1186/s12882-019-1446-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shuldiner SR, Wong L-Y, Peterson TE, et al. Myocardial fibrosis among antiretroviral therapy-treated persons with human immunodeficiency virus in South Africa. Open Forum Infect Dis. 2020;8(1). doi: 10.1093/ofid/ofaa600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remick J, Georgiopoulou V, Marti C, et al. Heart failure in patients with human immunodeficiency virus infection: epidemiology, pathophysiology, treatment, and future research. Circulation. 2014;129(17):1781–1789. doi: 10.1161/CIRCULATIONAHA.113.004574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC. Scientific Evidence for Conditions That Increase Risk of Severe Illness. CDC; 2020. [Google Scholar]
- 22.Sarma P, Shekhar N, Prajapat M, et al. In-silico homology assisted identification of inhibitor of RNA binding against 2019-nCoV N-protein (N terminal domain). J Biomol Struct Dyn. 2020:1–9. doi: 10.1080/07391102.2020.1753580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Zhang Y, Wu L, et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020;181(4):894–904.e9. doi: 10.1016/j.cell.2020.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turoňová B, Sikora M, Schürmann C, et al. In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges. Science. 2020;370(6513):eabd5223. doi: 10.1126/science.abd5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortola E, Roy P. Efficient assembly and release of SARS coronavirus-like particles by a heterologous expression system. FEBS Lett. 2004;576(1–2):174–178. doi: 10.1016/j.febslet.2004.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuman BW, Kiss G, Kunding AH, et al. A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol. 2011;174(1):11–22. doi: 10.1016/j.jsb.2010.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D, Lee J-Y, Yang J-S, Kim JW, Kim VN, Chang H. The Architecture of SARS-CoV-2 Transcriptome. Cell. 2020;181(4):914–921.e10. doi: 10.1016/j.cell.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan JF-W, Kok K-H, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Em Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren Y, Shu T, Wu D, et al. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell Mol Immunol. 2020;17(8):881–883. doi: 10.1038/s41423-020-0485-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuen C-K, Lam J-Y, Wong W-M, et al. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Em Microbes Infect. 2020;9(1):1418–1428. doi: 10.1080/22221751.2020.1780953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopecky-Bromberg SA, Martinez-Sobrido L, Palese P. 7a protein of severe acute respiratory syndrome coronavirus inhibits cellular protein synthesis and activates p38 mitogen-activated protein kinase. J Virol. 2006;80(2):785–793. doi: 10.1128/JVI.80.2.785-793.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Addetia A, Xie H, Roychoudhury P, et al. Identification of multiple large deletions in ORF7a resulting in in-frame gene fusions in clinical SARS-CoV-2 isolates. J Clin Virol. 2020;129:104523. doi: 10.1016/j.jcv.2020.104523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaecher SR, Mackenzie JM, Pekosz A. The ORF7b Protein of Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Is Expressed in Virus-Infected Cells and Incorporated into SARS-CoV Particles. Journal of Virology. 2007;81(2):718–731. doi: 10.1128/jvi.01691-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frankel AD, Young JA. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem. 1998;67:1–25. doi: 10.1146/annurev.biochem.67.1.1 [DOI] [PubMed] [Google Scholar]
- 35.Momany C, Kovari LC, Prongay AJ, et al. Crystal structure of dimeric HIV-1 capsid protein. Nature Structural Biology. 1996;3(9):763–770. doi: 10.1038/nsb0996-763 [DOI] [PubMed] [Google Scholar]
- 36.Aquaro S, Borrajo A, Pellegrino M, Svicher V. Mechanisms underlying of antiretroviral drugs in different cellular reservoirs with a focus on macrophages. Virulence. 2020;11(1):400–413. doi: 10.1080/21505594.2020.1760443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu WS, Hughes SH. HIV-1 Reverse Transcription. Cold Spring Harb Perspect Med. 2012;2(10):a006882. doi: 10.1101/cshperspect.a006882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray JM, Kelleher AD, Cooper DA. Timing of the Components of the HIV Life Cycle in Productively Infected CD4+ T Cells in a Population of HIV-Infected Individuals. J Virol. 2011;85(20):10798–10805. doi: 10.1128/jvi.05095-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craigie R. The molecular biology of HIV integrase. Future Virol. 2012;7(7):679–686. doi: 10.2217/fvl.12.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolbert WD, Sherburn R, Gohain N, et al. Defining rules governing recognition and Fc-mediated effector functions to the HIV-1 co-receptor binding site. BMC Biol. 2020;18(1). doi: 10.1186/s12915-020-00819-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji W, Wang W, Zhao X, Zai J, Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020;92(4):433–440. doi: 10.1002/jmv.25682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banerjee A, Doxey AC, Mossman K, Irving AT. Unraveling the Zoonotic Origin and Transmission of SARS-CoV-2. Trends Ecol Evol. 2021;36(3):180–184. doi: 10.1016/j.tree.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel A, Jernigan DB. Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak - United States, December 31, 2019-February 4, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(5):140–146. doi: 10.15585/mmwr.mm6905e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haider N, Rothman-Ostrow P, Osman AY, et al. COVID-19-Zoonosis or Emerging Infectious Disease? Front Public Health. 2020;8:596944. doi: 10.3389/fpubh.2020.596944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med. 2011;1(1):a006841. doi: 10.1101/cshperspect.a006841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmes EC. On the origin and evolution of the human immunodeficiency virus (HIV). Biol Rev Camb Philos Soc. 2001;76(2):239–254. doi: 10.1017/s1464793101005668 [DOI] [PubMed] [Google Scholar]
- 47.Gürtler LG, Eberle J. Aspects on the history of transmission and favor of distribution of viruses by iatrogenic action: perhaps an example of a paradigm of the worldwide spread of HIV. Med Microbiol Immunol. 2017;206(4):287–293. doi: 10.1007/s00430-017-0505-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirmaier A, Krupp A, Johnson WE. Understanding restriction factors and intrinsic immunity: insights and lessons from the primate lentiviruses. Future Virol. 2014;9(5):483–497. doi: 10.2217/fvl.14.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marx PA, Apetrei C, Drucker E. AIDS as a zoonosis? Confusion over the origin of the virus and the origin of the epidemics. J Med Primatol. 2004;33(5–6):220–226. doi: 10.1111/j.1600-0684.2004.00078.x [DOI] [PubMed] [Google Scholar]
- 50.Van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Eng J Med. 2020;382(16):1564–1567. doi: 10.1056/nejmc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hendrix MJWC, Findley K, Trotman R. Absence of Apparent Transmission of SARS-Cov-2 from Two Stylists After Exposure at a Hair Salon with a Universal Face Covering Policy — Springfield, Missouri. Vol. 69. Morbidity and Mortality Weekly Report; 2020:930–932. [DOI] [PubMed] [Google Scholar]
- 52.Mahase E. Covid-19: what is the evidence for cloth masks? BMJ. 2020;m1422. doi: 10.1136/bmj.m1422 [DOI] [PubMed] [Google Scholar]
- 53.Gao M, Yang L, Chen X, et al. A study on infectivity of asymptomatic SARS-CoV-2 carriers. Respir Med. 2020;169:106026. doi: 10.1016/j.rmed.2020.106026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28(10):1509–1519. doi: 10.1097/QAD.0000000000000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng H, Chow EP, Zhao Y, et al. Prevention of mother-to-child HIV transmission cascade in China: a systematic review and meta-analysis. Sex Transm Infect. 2016;92(2):116–123. doi: 10.1136/sextrans-2014-051877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamming I, Timens W, Bulthuis M, Lely A, Navis G, Van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo J, Wei X, Li Q, et al. Single-cell RNA Analysis on ACE2 Expression Provides Insight into SARS-CoV-2 Blood Entry and Heart Injury. medRxiv. 2020:20047621. doi: 10.1101/2020.03.31.20047621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1). doi: 10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92(7):699–702. doi: 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goad J, Rudolph J, Rajkovic A. Female reproductive tract has low concentration of SARS-CoV2 receptors. bioRxiv. 2020;163097. doi: 10.1101/2020.06.20.163097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. doi: 10.1038/nm.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vandergeeten C, Fromentin R, DaFonseca S, et al. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood. 2013;121(21):4321–4329. doi: 10.1182/blood-2012-11-465625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kovacs JA, Lempicki RA, Sidorov IA, et al. Induction of prolonged survival of CD4+ T lymphocytes by intermittent IL-2 therapy in HIV-infected patients. The Journal of Clinical Investigation. 2005;115(8):2139–2148. doi: 10.1172/jci23196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woodham AW, Skeate JG, Sanna AM, et al. Human immunodeficiency virus immune cell receptors, coreceptors, and cofactors: implications for prevention and treatment. AIDS Patient Care STDS. 2016;30(7):291–306. doi: 10.1089/apc.2016.0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ballweber L, Robinson B, Kreger A, et al. Vaginal Langerhans Cells Nonproductively Transporting HIV-1 Mediate Infection of T Cells. J Virol. 2011;85(24):13443–13447. doi: 10.1128/jvi.05615-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geijtenbeek TBH, Kwon DS, Torensma R, et al. DC-SIGN, a Dendritic Cell–Specific HIV-1-Binding Protein that Enhances trans-Infection of T Cells. Cell. 2000;100(5):587–597. doi: 10.1016/s0092-8674(00)80694-7 [DOI] [PubMed] [Google Scholar]
- 68.Gendelman HE, Orenstein JM, Martin MA, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167(4):1428–1441. doi: 10.1084/jem.167.4.1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu T, Muthui D, Holte S, et al. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002;76(2):707–716. doi: 10.1128/jvi.76.2.707-716.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abreu CM, Veenhuis RT, Avalos CR, et al. Myeloid and CD4 T Cells Comprise the Latent Reservoir in Antiretroviral Therapy-Suppressed SIVmac251-Infected Macaques. mBio. 2019;10(4). doi: 10.1128/mBio.01659-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128(3):415–435. doi: 10.1084/jem.128.3.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swingler S, Brichacek B, Jacque JM, Ulich C, Zhou J, Stevenson M. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature. 2003;424(6945):213–219. doi: 10.1038/nature01749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelly J, Beddall MH, Yu D, Iyer SR, Marsh JW, Wu Y. Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology. 2008;372(2):300–312. doi: 10.1016/j.virol.2007.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dictionary D. DOD Dictionary of Military and Associated Terms. Washington DC: The Joint Staff; 2020. [Google Scholar]
- 75.Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Millet JK, Whittaker GR. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, Yang P, Liu K, et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18(2):290–301. doi: 10.1038/cr.2008.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xia S, Liu M, Wang C, et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30(4):343–355. doi: 10.1038/s41422-020-0305-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Black RA, White JM. ADAMs: focus on the protease domain. Current Opinion in Cell Biology. 1998;10(5):654–659. doi: 10.1016/s0955-0674(98)80042-2 [DOI] [PubMed] [Google Scholar]
- 81.Patel VB, Clarke N, Wang Z, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017 [DOI] [PubMed] [Google Scholar]
- 82.Shen M, Hu M, Fedak PWM, Oudit GY, Kassiri Z. Cell-Specific Functions of ADAM17 Regulate the Progression of Thoracic Aortic Aneurysm. Circ Res. 2018;123(3):372–388. doi: 10.1161/circresaha.118.313181 [DOI] [PubMed] [Google Scholar]
- 83.Zhong J, Basu R, Guo D, et al. Angiotensin-Converting Enzyme 2 Suppresses Pathological Hypertrophy, Myocardial Fibrosis, and Cardiac Dysfunction. Circulation. 2010;122(7):717–728. doi: 10.1161/circulationaha.110.955369 [DOI] [PubMed] [Google Scholar]
- 84.Wang K, Chen W, Zhou Y-S, et al. SARS-Cov-2 Invades Host Cells via a Novel Route: CD147-Spike Protein. Cold Spring Harbor Laboratory; 2020. [Google Scholar]
- 85.Bian H, Zheng Z-H, Wei D, et al. Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. medRxiv. 2020. doi: 10.1101/2020.03.21.20040691 [DOI] [Google Scholar]
- 86.Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26 and other SARS‐CoV‐2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID‐19 risk factors. Allergy. 2020;75(11):2829–2845. doi: 10.1111/all.14429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McDougal JS, Kennedy MS, Sligh JM, Cort SP, Mawle A, Nicholson JK. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986;231(4736):382–385. doi: 10.1126/science.3001934 [DOI] [PubMed] [Google Scholar]
- 88.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272(5263):872–877. doi: 10.1126/science.272.5263.872 [DOI] [PubMed] [Google Scholar]
- 89.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997;94(5):1925–1930. doi: 10.1073/pnas.94.5.1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Nature. 1996;381(6584):661–666. doi: 10.1038/381661a0 [DOI] [PubMed] [Google Scholar]
- 91.Bleul CC, Farzan M, Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Nature. 1996;382(6594):829–833. doi: 10.1038/382829a0 [DOI] [PubMed] [Google Scholar]
- 92.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1–infected individuals. Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. J Exp Med. 1997;185(4):621–628. doi: 10.1084/jem.185.4.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park EJ, Roh J, Kim SN, Kim Y, Han SB, Hong JT. CCR5 plays an important role in resolving an inflammatory response to single-walled carbon nanotubes. Research Support, Non-U.S. Gov’t. J Appl Toxicol. 2013;33(8):845–853. doi: 10.1002/jat.2744 [DOI] [PubMed] [Google Scholar]
- 94.Grommes J, Drechsler M, Soehnlein O. CCR5 and FPR1 mediate neutrophil recruitment in endotoxin-induced lung injury. J Innate Immun. 2013;6(1):111–116. doi: 10.1159/000353229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kohlmeier JE, Miller SC, Smith J, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. Immunity. 2008;29(1):101–113. doi: 10.1016/j.immuni.2008.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liao YX, Zhou CH, Zeng H, et al. The role of the CXCL12-CXCR4/CXCR7 axis in the progression and metastasis of bone sarcomas (Review). Int J Mol Med. 2013;32(6):1239–1246. doi: 10.3892/ijmm.2013.1521 [DOI] [PubMed] [Google Scholar]
- 97.Zhang P, Dong L, Yan K, et al. CXCR4-mediated osteosarcoma growth and pulmonary metastasis is promoted by mesenchymal stem cells through VEGF. Research Support, Non-U.S. Gov’t. Oncol Rep. 2013;30(4):1753–1761. doi: 10.3892/or.2013.2619 [DOI] [PubMed] [Google Scholar]
- 98.Mukherjee D, Zhao J. The Role of chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer Res. 2013;3(1):46–57. [PMC free article] [PubMed] [Google Scholar]
- 99.Murakami T, Kawada K, Iwamoto M, et al. The role of CXCR3 and CXCR4 in colorectal cancer metastasis. Research Support, Non-U.S. Gov’t. Int J Cancer. 2013;132(2):276–287. doi: 10.1002/ijc.27670 [DOI] [PubMed] [Google Scholar]
- 100.Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99(3):539–542. doi: 10.1111/j.1349-7006.2007.00712.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barbolina MV, Kim M, Liu Y, et al. Microenvironmental regulation of chemokine (C-X-C-motif) receptor 4 in ovarian carcinoma. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. Mol Cancer Res. 2010;8(5):653–664. doi: 10.1158/1541-7786.MCR-09-0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choi YH, Burdick MD, Strieter BA, Mehrad B, Strieter RM. CXCR4, but not CXCR7, discriminates metastatic behavior in non-small cell lung cancer cells. Mol Cancer Res. 2013;12(1):38–47. doi: 10.1158/1541-7786.MCR-12-0334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dai X, Mao Z, Huang J, Xie S, Zhang H. The CXCL12/CXCR4 autocrine loop increases the metastatic potential of non-small cell lung cancer in vitro. Oncol Lett. 2013;5(1):277–282. doi: 10.3892/ol.2012.960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan L, Cai Q, Xu Y. The ubiquitin-CXCR4 axis plays an important role in acute lung infection-enhanced lung tumor metastasis. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. Clin Cancer Res. 2013;19(17):4706–4716. doi: 10.1158/1078-0432.CCR-13-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herbein G, Coaquette A, Perez-Bercoff D, Pancino G. Macrophage activation and HIV infection: can the Trojan horse turn into a fortress? Curr Mol Med. 2002;2(8):723–738. doi: 10.2174/1566524023361844 [DOI] [PubMed] [Google Scholar]
- 106.Shen R, Richter HE, Smith PD. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am J Reprod Immunol. 2011;65(3):261–267. doi: 10.1111/j.1600-0897.2010.00939.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lorey S, Stöckel-Maschek A, Faust J, et al. Different modes of dipeptidyl peptidase IV (CD26) inhibition by oligopeptides derived from the N-terminus of HIV-1 Tat indicate at least two inhibitor binding sites. Eur J Biochem. 2003;270(10):2147–2156. doi: 10.1046/j.1432-1033.2003.03568.x [DOI] [PubMed] [Google Scholar]
- 108.Ugolini S, Mondor I, Sattentau QJ. HIV-1 attachment: another look. Trends Microbiol. 1999;7(4):144–149. doi: 10.1016/s0966-842x(99)01474-2 [DOI] [PubMed] [Google Scholar]
- 109.Peters PJ, Dueñas-Decamp MJ, Sullivan WM, Clapham PR. Variation of macrophage tropism among HIV-1 R5 envelopes in brain and other tissues. J Neuroimmune Pharmacol. 2007;2(1):32–41. doi: 10.1007/s11481-006-9042-2 [DOI] [PubMed] [Google Scholar]
- 110.Gorry PR, Francella N, Lewin SR, Collman RG. HIV-1 envelope-receptor interactions required for macrophage infection and implications for current HIV-1 cure strategies. J Leukoc Biol. 2014;95(1):71–81. doi: 10.1189/jlb.0713368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aloia RC, Tian H, Jensen FC. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc National Acad Sci. 1993;90(11):5181–5185. doi: 10.1073/pnas.90.11.5181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cantin R, Fortin JF, Lamontagne G, Tremblay M. The acquisition of host-derived major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T-lymphoid cells. Blood. 1997;90(3):1091–1100. doi: 10.1182/blood.V90.3.1091.1091_1091_1100 [DOI] [PubMed] [Google Scholar]
- 113.Hioe CE, Chien PC Jr, Lu C, et al. LFA-1 expression on target cells promotes human immunodeficiency virus type 1 infection and transmission. J Virol. 2001;75(2):1077–1082. doi: 10.1128/jvi.75.2.1077-1082.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Umviligihozo G, Cobarrubias KD, Chandrarathna S, et al. Differential Vpu-Mediated CD4 and tetherin downregulation functions among major HIV-1 Group M Subtypes. J Virol. 2020;94(14):e00293. doi: 10.1128/jvi.00293-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Doria M. Role of the CD4 down-modulation activity of Nef in HIV-1 infectivity. Curr HIV Res. 2011;9(7):490–495. doi: 10.2174/157016211798842125 [DOI] [PubMed] [Google Scholar]
- 116.Pawlak EN, Dikeakos JD. HIV-1 Nef: a master manipulator of the membrane trafficking machinery mediating immune evasion. Biochimica et Biophysica Acta. 2015;1850(4):733–741. doi: 10.1016/j.bbagen.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 117.Millen RA. The Resilient Defense. Land Warfare Papers. 2014;2014:100. [Google Scholar]
- 118.Dong X, Cao YY, Lu XX, et al. Eleven faces of coronavirus disease 2019. Allergy. 2020;75(7):1699–1709. doi: 10.1111/all.14289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Siu K-L, Kok K-H, Ng M-HJ, et al. Severe acute respiratory syndrome coronavirus M protein inhibits type i interferon production by impeding the formation of TRAF3·TANK·TBK1/IKKϵ Complex. J Biol Chem. 2009;284(24):16202–16209. doi: 10.1074/jbc.m109.008227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14(4):778–809. doi: 10.1128/cmr.14.4.778-809.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thoms M, Buschauer R, Ameismeier M, et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science. 2020;369(6508):1249–1255. doi: 10.1126/science.abc8665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y, Zhang J, Chen Y, et al. The ORF8 Protein of SARS-CoV-2 Mediates Immune Evasion through Potently Downregulating MHC-I. bioRxiv. 2020. doi: 10.1101/2020.05.24.111823 [DOI] [Google Scholar]
- 123.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang ZJ, Yu XJ, Fu T, et al. Novel coronavirus infection in newborn babies aged <28 days in China. Eur Respir J. 2020;55(6). doi: 10.1183/13993003.00697-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang Z-J. Novel Coronavirus Infection in Hospitalized Infants Under 1 Year of Age in China. JAMA. 2020;323(13):1313–1314. doi: 10.1001/jama.2020.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao Q, Meng M, Kumar R, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis. 2020;96:131–135. doi: 10.1016/j.ijid.2020.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cummins NW, Badley AD. Mechanisms of HIV-associated lymphocyte apoptosis: 2010. Cell Death Dis. 2010;1(11):e99–e99. doi: 10.1038/cddis.2010.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Doitsh G, Cavrois M, Lassen KG, et al. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143(5):789–801. doi: 10.1016/j.cell.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Doitsh G, Galloway NLK, Geng X, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505(7484):509–514. doi: 10.1038/nature12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hoenigl M, Green N, Camacho M, et al. Signs or symptoms of acute HIV infection in a cohort undergoing community-based screening. Emerg Infect Dis. 2016;22(3):532–534. doi: 10.3201/eid2203.151607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10(1):11–23. doi: 10.1038/nri2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koff WC. HIV vaccine development: challenges and opportunities towards solving the HIV vaccine-neutralizing antibody problem. Vaccine. 2012;30(29):4310–4315. doi: 10.1016/j.vaccine.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 136.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83(8):3719–3733. doi: 10.1128/jvi.01844-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baum A, García-Sastre A. Induction of type I interferon by RNA viruses: cellular receptors and their substrates. Amino Acids. 2010;38(5):1283–1299. doi: 10.1007/s00726-009-0374-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ross SR. Are Viruses Inhibited by APOBEC3 Molecules from Their Host Species? PLoS Pathogens. 2009;5(4):e1000347. doi: 10.1371/journal.ppat.1000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Neil S, Bieniasz P. Human immunodeficiency virus, restriction factors, and interferon. J Interferon Cytokine Res. 2009;29(9):569–580. doi: 10.1089/jir.2009.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68(9):6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wilson JDK, Ogg GS, Allen RL, et al. Direct visualization of HIV-1-specific cytotoxic T lymphocytes during primary infection. Aids. 2000;14(3):225–233. doi: 10.1097/00002030-200002180-00003 [DOI] [PubMed] [Google Scholar]
- 142.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17(13):1871–1879. doi: 10.1097/00002030-200309050-00005 [DOI] [PubMed] [Google Scholar]
- 143.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009;206(6):1253–1272. doi: 10.1084/jem.20090365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Karim F, Gazy I, Cele S, et al. HIV infection alters SARS-CoV-2 responsive immune parameters but not clinical outcomes in COVID-19 disease. medRxiv. 2020. doi: 10.1101/2020.11.23.20236828 [DOI] [Google Scholar]
- 145.Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Eng J Med. 2020;382(19):1787–1799. doi: 10.1056/nejmoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li Y, Xie Z, Lin W, et al. An exploratory randomized controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI). medRxiv. 2020. doi: 10.1101/2020.03.19.20038984 [DOI] [Google Scholar]
- 147.Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. 2020;81(1):e1–e5. doi: 10.1016/j.jinf.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu F, Xu A, Zhang Y, et al. Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Agostini ML, Andres EL, Sims AC, et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is mediated by the viral polymerase and the Proofreading Exoribonuclease. mBio. 2018;9(2). doi: 10.1128/mBio.00221-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Eastman RT, Roth JS, Brimacombe KR, et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6(5):672–683. doi: 10.1021/acscentsci.0c00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Madelain V, Baize S, Jacquot F, et al. Ebola viral dynamics in nonhuman primates provides insights into virus immuno-pathogenesis and antiviral strategies. Nat Commun. 2018;9(1):4013. doi: 10.1038/s41467-018-06215-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 — preliminary Report. N Eng J Med. 2020;383(19):1813–1826. doi: 10.1056/nejmoa2007764 [DOI] [PubMed] [Google Scholar]
- 153.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/s0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Barton K, Winckelmann A, Palmer S. HIV-1 Reservoirs During Suppressive Therapy. Trends Microbiol. 2016;24(5):345–355. doi: 10.1016/j.tim.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]