Abstract
Chagas disease is a neglected tropical disease caused by the protozoan Trypanosoma cruzi. Currently, only nitroheterocyclic nifurtimox (NFX) and benznidazole (BNZ) are available for the treatment of Chagas disease, with limitations such as variable efficacy, long treatment regimens and toxicity. Different strategies have been used to discover new active molecules for the treatment of Chagas disease. Target-based and phenotypic screening led to thousands of compounds with anti-T. cruzi activity, notably the nitroheterocyclic compounds, fexinidazole and its metabolites. In addition, drug repurposing, drug combinations, re-dosing regimens and the development of new formulations have been evaluated. The CYP51 antifungal azoles, as posaconazole, ravuconazole and its prodrug fosravuconazole presented promising results in experimental Chagas disease. Drug combinations of nitroheterocyclic and azoles were able to induce cure in murine infection. New treatment schemes using BNZ showed efficacy in the experimental chronic stage, including against dormant forms of T. cruzi. And finally, sesquiterpene lactone formulated in nanocarriers displayed outstanding efficacy against different strains of T. cruzi, susceptible or resistant to BNZ, the reference drug. These pre-clinical results are encouraging and provide interesting evidence to improve the treatment of patients with Chagas disease.
Keywords: Trypanosoma cruzi, drug discovery, new chemical entities, repurposing, drug combination, nanomedicine
Introduction
Chagas disease (American trypanosomiasis) was discovered and described by Carlos Chagas in 1909.1 The disease is caused by the protozoan Trypanosoma cruzi and affects 6–7 million people worldwide, with an estimated 75 million people at risk of infection.1,2 It is a neglected disease and related to poverty in tropical and subtropical countries, especially in Latin America.3 The affected population lives in rural and peri-urban areas in inappropriate buildings and in vulnerable socioeconomic conditions.4 Recently, Chagas disease has spread to non-endemic areas in Europe, United States and Japan, and it has become a globalized public health and medical problem.3,4
T. cruzi infection is transmitted mainly via insect vector, where trypomastigotes of T. cruzi in the excreta of contaminated blood-sucking triatomines can penetrate sites of lesioned skin or mucosa in humans.5 Other transmission routes have been also reported, such as congenital infection,6,7 blood transfusions8 or organ transplants, laboratory accidents,9 and oral contamination.10,11 Sustained efforts to control the vector have resulted in a decrease in the incidence of Chagas disease in several countries of Southern Cone in Latin America.12 In recent years, the epidemiological relevance of oral transmission has been increased, especially in countries of the Amazon region.13–15 Several outbreaks associated with orally transmitted acute Chagas disease have been related to the intake of contaminated foods/juices.16
The acute phase of Chagas disease persists for 4–8 weeks after infection and it is asymptomatic in most cases.17 Some symptoms, such as fever, malaise, lymph nodes and subcutaneous edema, hepatosplenomegaly and electrocardiographic or neurological disorders eventually appear.17 The acute severe symptoms, as myocarditis, pericardial effusion, and meningoencephalitis affect 1–5% of patients,18 mainly children and immunosuppressed patients.19–21 In the absence of an effective etiologic treatment, the chronic phase gradually sets in.17,18 Most of chronic patients are asymptomatic, characterized by the undetermined clinical form of the disease, and may remain so for an indefinite period. In these cases, the patients have detectable anti-T. cruzi antibodies, absence of clinical signs and symptoms of cardiac and digestive clinical forms of the disease.22 However, after 10–30 years of asymptomatic period, 30–40% of chronically infected individuals may progress to symptomatic forms of Chagas disease.17,18 About 14–45% of them develop cardiac abnormalities and dysfunctions (cardiac form), and 10–21% present intestinal involvement, as megaviscera, specially megaesophagus and megacolon (digestive form).17,18,22,23
Clinical manifestations of Chagas disease, specially chronic Chagas cardiomyopathy, are responsible for high morbidity and mortality in economically productive young adults, and result in progressive inability to continue working and consequent burden of the health system.24,25 Experimental and clinical studies demonstrate that the presence of the parasites and their DNA in tissues is closely associated with the pathogenesis of the disease which reinforces the hypothesis that the etiological treatment improves patient outcome.26–31
In this review, we attempted to provide an overview of the most impressive experimental preclinical results concerning new chemical entities and therapeutic strategies tested in experimental T. cruzi infection. PubMed, Scopus, and Web of Science databases were accessed concerning chemotherapeutic options that have been tested. As a plethora of new chemical compounds have already been tested in vitro, only in vivo data were selected.
Chagas Disease Chemotherapy
Several new compounds were tested for the treatment of Chagas disease until the 1960s but without promising results.27 Afterwards, a more effective class of anti-T. cruzi agents were introduced - the nitrofurans,32–34 among which nifurtimox (NFX) stood out for its superior efficacy.32 In addition, 2-nitroimidazole derivative benznidazole (BNZ) was discovered and included for the treatment of Chagas disease.35 NFX and BNZ are nitroheterocyclic class of compounds with the nitro groups linked to furan or imidazole rings, respectively.36 Currently, BNZ and NFX are standards of care in clinical chemotherapy of Chagas disease, recommended by WHO.2 Their mechanism of action has not yet been fully elucidated, but both act as prodrugs and must be activated by nitro-reductases present in T. cruzi to exert their cytotoxic effects.36 NFX action on T. cruzi involves redox cycling and radical species that results in damage to the parasites, i.e. causing marked reduction on the level of intracellular thiols, with evidence of DNA damage and lipid peroxidation.39 Differently, the reduction of BNZ occurs through various free radical intermediates and electrophilic metabolites that alkylate macromolecules such as DNA, lipids, and proteins, as recently reviewed by Patterson and Fairlamb.37 There is also the hypothesis that BNZ may act via immune system control producing trypanosome death through interferon-γ that is likely to be increased due to inflammation caused by macromolecule damage.38 Furthermore, it has been shown that the DNA of parasites affected by BNZ could undergo extensive unpacking with overexpression of DNA repair proteins. These findings support the idea that DNA damage could contribute to the mechanism of action of BNZ.39
In 2017, BNZ obtained accelerated first treatment approval for the treatment of Chagas disease in children aged 2 to 12 years by the U.S. Food and Drug Administration (FDA) in the United States.40 The treatment regimen with NFX or BNZ is long, and many adverse effects can occur and compromise the continuity of the treatment. Common adverse events in NFX treatment include gastrointestinal symptoms (nausea, vomiting and anorexia), symptoms of central nervous system toxicity (insomnia, irritability, and disorientation) and occasionally headache, rash, myalgia, arthralgia, dizziness or vertigo and mood changes.17,18 Less commonly, but more severe adverse effects may appear, such as polyneuropathy, paraesthesia and peripheral neuritis.17,18 The common adverse effects of BNZ are allergic dermatitis, nausea, vomiting, anorexia, weight loss, insomnia, loss of taste, onycholysis and dose-dependent peripheral sensitive neuropathy may also appear.17,18 Rare serious events include neuropathy and depression of bone marrow.17,18 Although the treatment of recent stages of infection is efficacious using both nitroheterocyclic drugs, their benefits are limited in the chronic phase, with variation of efficacy following geographical locations.18,41–43 In addition to the decrease in treatment effectiveness with the time of infection, the drug side effects are more frequent in patients of advanced ages.2
In order to search for new treatment alternatives, pre-clinical studies have been identified promising new candidates in specific pharmacological classes. Particularly, the C14-α-demethylase (CYP51) inhibitors were tested in clinical trials, such as posaconazole and fosravuconazole (or E-1224 a prodrug of ravuconazole), but both failed to induce cure and were less effective than the treatment using BNZ.44,45
New Drug Candidates for Chagas Disease
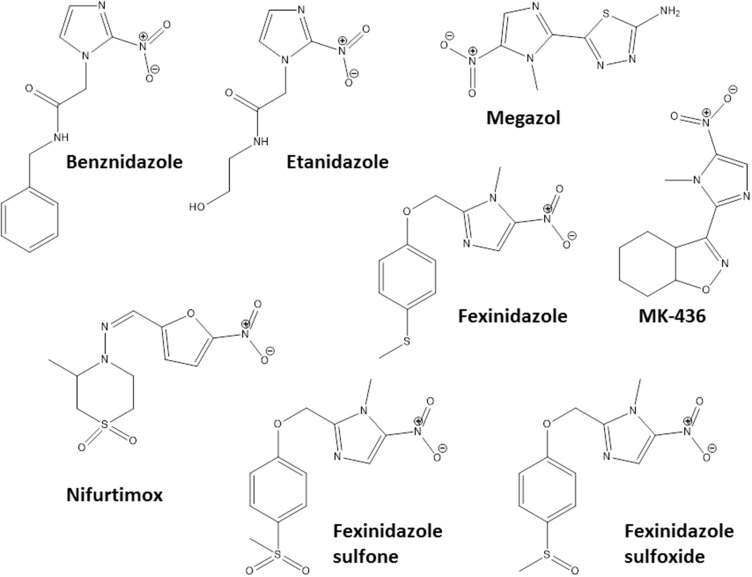
Some pharmacological classes are specially promising to treat Chagas disease: nitroheterocyclic compounds, inhibitors of sterol biosynthesis, cruzipain inhibitors, aromatic amides, trypanothione reductase inhibitors, ruthenium complexes carrying trypanocidal molecules, oxaboroles and nucleoside derivatives. Table 1 summarizes the main data on investigational compounds. In the class of nitroheterocyclic compounds, fexinidazole demonstrated potential against kinetoplastid diseases, and particularly in T. cruzi infections.46–48 Fexinidazole (Figure 1) was more efficacious than BNZ in murine model, promoted the reduction of parasitemia and induced cure in mice infected by BNZ-resistant strains upon treatment with 300 mg/kg/day for 20 days.46 Similarly, the treatment of infected mice (Y strain) with sulfone and sulfoxide metabolites of fexinidazole induced 100% cure in acute phase with 100 mg/kg/day, indicating that these metabolites are more active than BNZ and the parent drug (fexinidazole).49 The mechanism of action of these compounds has not yet been elucidated, but indirect evidence indicates that they are also metabolized by T. cruzi nitro-reductases.37 Other nitroheterocyclic compounds have been extensively investigated for their activity against T. cruzi (see Table 1 and Figure 1),50–58 although toxicity potential of this class of molecules can limit their use. Thereby, a thorough experimental study on the toxic potential of new compounds must be performed in the drug discovery process.
Table 1.
Classes of Promising New Chemical Entities for Experimental Treatment of Chagas Disease
| Mechanism/Target | Chemical Class/Compound | Outcome | Ref. |
|---|---|---|---|
| DNA and kinetoplast-DNA targeting and topoisomerase inhibitors and unknow targets |
Amide-containing thiazoles | Safe compounds that had in vitro and in vivo anti-T. cruzi activities. | [93] |
| Arylimidamide derivatives | 18SAB075 exhibited best selective index; and slightly reduced parasitemia and mortality in mice infection. | [92] | |
| Amidines and analogues Bis-arylimidamides |
Bis-arylimidamides displayed in vitro activity. 28SMB032 reduced parasitemia, but without mortality protection. DB1957, DB1959 and DB1890B reduced parasitemia levels and/or mortality. | [91,94] | |
| DB1831 and its mesylate salt derivative (DB1965) | Compounds presented in vitro and in vivo activity. DB1965 produced no parasitological cure, but reduced parasite burden and protected against mortality | [90] | |
| DB766 and analogues | DB766 displayed potent in vitro activity. In murine infection, the drug reduced parasite load in blood and heart; reduced hepatic and cardiac lesions; prevented electrocardiographic alterations and prevented mortality. | [87–89] | |
| Antimicrobial peptides | AS-48 Bacteriocin | AS-48 was active in vitro and in acute stage of mice infection it reduced parasitemia and in chronic stage the parasitic load. | [98,99] |
| Copper complexes | Ternary copper (II) complexes | Compounds with higher selectivity index were able to reduce parasitemia. | [100] |
| Cysteine protease inhibitors and Cruzipain inhibitors |
Fluoromethyl ketone–derivatized pseudopeptides | K777 is an irreversible peptidyl inhibitor of cruzipain and it was able to reduce parasitemia and mortality, and induce cure in murine model. In infected dogs, it reduced cardiac damage, but without cure. Nonpeptidic tetrafluorophenoxymethyl ketone reduce parasitemia in acutely infected mice with no apparent toxicity. |
[77–79] [81] |
| Reversible cruzipain inhibitors containing a nitrile “warhead” | Cz007 and Cz008 presented potent in vitro activity and displayed parasitemia reduction and cure in mice acutely infected. | [80] | |
| Thiazole compounds | Thiazole compounds showed in vitro activity and promoted reduction of parasitemia and mortality in infected mice. These compounds inhibited cruzipain. | [82–85] | |
| Ergosterol biosynthesis inhibitors | 4-aminopyridyl-based lead compounds | Compounds showed anti-T. cruzi effects in acute and chronically infected mice, but without cure. | [67] |
| Fenarimol analogues | Fenarimol analogues were active in vitro and in vivo against T. cruzi infected mice | [57,63,64] | |
| Non-azole LP-10 | LP-10 was able to reduce parasitemia and to induce cure in infected mice. | [65] | |
| Ravuconazole and E1224 (fosravuconazole) | Ravuconazole presented effective control of infection in mice and dogs. E1224 or fosravuconazole, a prodrug of ravuconazole were well tolerated and effective in suppressing parasitemia. Cure was detected in mice infected by Y strain, but no cure was observed in Colombian infection in mice. |
[116,117] [118] |
|
| VFV | VFV was more potent than VNI in reducing parasitemia and presented effects on mortality protection. | [60,119] | |
| VNI | VNI presented promising anti-T. cruzi activity in vitro and in vivo. The drug promoted reduction of parasitemia in mice infected by different strains. Cure was detected in animals acute and chronically infected by Tulahuen strain. | [59–62] | |
| VT-1161 | VT-1161 is a potent T. cruzi CYP51 inhibitor and revealed in vitro and in vivo activity, as well as suppression of parasitemia peak in mice infection by Y strain. | [66] | |
| Nitro-heterocyclic compounds and ROS inducers |
5-Nitroindazole derivatives | Megazol, a nitroimidazole-thiadiazole derivative, demonstrated a curative action in infected mice. The mechanism of action is related to the impairment of protein synthesis. Megazol derivatives showed in vitro activity. In infected mice, S2 led to parasitemia reduction, and S3 to mortality reduction. |
[50,53] [54] |
| Nitrotriazoles | Nitrotriazole-based compounds exhibited in vitro and in vivo anti-T.cruzi activity. Some compounds are potential inhibitors of T. cruzi CYP51 and substrates for the type I nitroreductase. Triazole-based analogues of BNZ showed in vitro activity. In infected mice, the analogues exhibited lower potency and higher toxic than BNZ. |
[68–76] [58] |
|
| Fexinidazole | Fexinidazole was more effective than BNZ, with cure rate greater than 70% in animals infected by different strains and treated during the acute or chronic phases. | [46–48] | |
| Fexinidazole metabolites | Fexinidazole sulfone and fexinidazole sulfoxide induced higher cure rates than fexinidazole. | [47,49] | |
| Imidazole Derivatives | Phthalazine derivatives containing imidazole rings showed in vitro and in vivo anti-T. cruzi activity. | [55] | |
| MK-436 (2,5-nitroimidazole) | MK-436 was highly efficacious against acute and chronic infection by different strains, and induced cure in acute stage. | [51,52] | |
| Ruthenium complexes | The ruthenium compounds exhibited potent in vitro and in vivo trypanocidal activities by acting on active cysteine (cys166) site or releasing nitric oxide. NO donor compound led to parasitological cure. | [101–104] | |
| Purine Salvage Pathway | Nucleoside derivatives | Nucleoside analogues presented in vitro activity and the most potent showed suppression of parasitemia and mortality in acutely infected mice. | [106,107] |
| Xanthine analogs | Xanthine analogs showed in vitro activity and were able to reduce parasitemia in CL-infected mice. | [105] | |
| Oxamates | Benzyl ester of N-propyl oxamate; ethyl esters of N-propyl and N-isopropyl oxamates; and N-isopropyl oxamate | Compounds exhibited trypanocidal activity in vitro and in vivo. They acted as possible inhibitors of T. cruzi α-hydroxy acid dehydrogenase (HADH)-isozyme II | [108–110] |
| Oxaboroles multiple cellular targets |
Oxaboroles | AN4169 showed broad efficacy in vitro against strains of T. cruzi and was a fast-acting trypanocidal. The drug induced cure in mouse model. | [56,57] |
| Proteasome inhibitor | Triazolopyrimidine | GNF6702 cure mice in chronic phase of infection with T. cruzi. | [114,115] |
| Quinolines | Quinolines | Tested quinolines were more active in vitro than BNZ. DB2186 reached 70% reduction of the parasitemia load in mice infected by Y strain. | [111] |
| Sphingosine kinase inhibitor | N,N-dimethylsphingosine (DMS) | DMS blocked sphingosine-1-phosphate production, a cell mediator during inflammatory responses, and exhibited anti-parasitic activity in vitro and in vivo and immunomodulatory actions in chronically infected mice. | [112] |
| Squaramides | Squaramides | New long-chain squaramides displayed in vitro and in vivo activity with low toxicity | [55,113] |
| Terpene and terpenoid derivatives | Terpenoid derivates | Synthesized terpenoid derivates displayed in vitro anti-T. cruzi activity and nontoxic effects upon host cells. In infected mice, these derivatives reduced parasitic load and anti-T. cruzi antibodies during chronic stage. | [55] |
| Polyamine surface transporters and metabolism Trypanothione metabolism inhibitors |
Thiazolidines | LPSF SF29 displayed trypomastigote lysis and amastigote death, probably by interfering with polyamine biosynthesis and consequently trypanothione biosynthesis, leading to increased sensitivity to oxidative metabolism. | [96] |
| Tetradentated polyamine complexes | Tetraamines presented anti-T. cruzi activity in vitro and in vivo with low toxicity. | [97] |
Abbreviations: BNZ, benznidazole; NO, nitric oxide; ROS, reactive oxygen species.
Figure 1.
Chemical structures of the most promising nitro-heterocyclic compounds that induce cure with parasite elimination in mice.
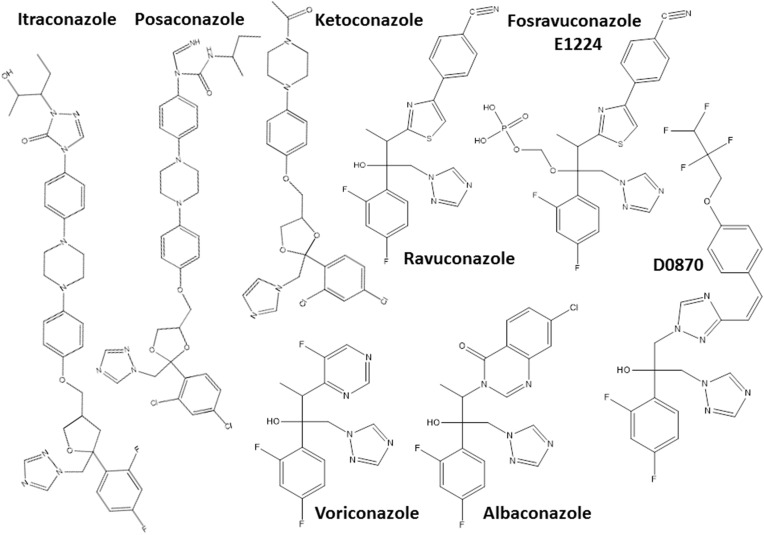
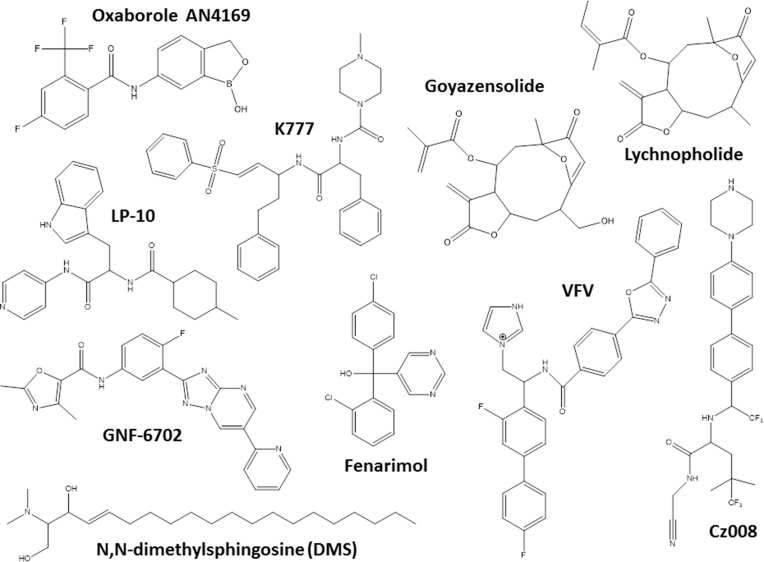
Another hit class for the treatment of T. cruzi infections includes the inhibitors of sterol biosynthesis pathway (Figure 2), in particular C14-α-demethylase (CYP51) inhibitors, used originally as antifungals. In the repurposing strategy, these compounds can block T. cruzi ergosterol biosynthesis, and compromise the parasite survival. For example, treatment with non-azole VNI (Figure 3) led to the cure of animals in acute and chronic phases of infection with Tulahuen strain.59 In addition, VNI promoted suppression of parasitemia and protection against mortality, but did not cure murine infections by Y and Colombian strains.60–62 Similarly, the treatment with VFV, a VNI structure-based fluoro-analog, resulted in complete parasitemia suppression and mortality protection. VFV was more potent than VNI (Figure 3).60 Moreover, fenarimol (Figure 3), a CYP51 inhibitor, showed a potent anti-T. cruzi activity, and two derivatives were able to induce cure in infected mice, with efficacy comparable to posaconazole and superior to BNZ.57,63,64 Other classes of ergosterol biosynthesis inhibitors have been found to impact T. cruzi infections (Figure 3 and 4).65–67
Figure 2.
Chemical structures of the most promising azole derivatives that induced cure with parasite elimination in mice.
Figure 3.
Chemical structures of the miscellaneous classes of most promising compounds with different mechanisms of action that induced cure with parasite elimination in mice.
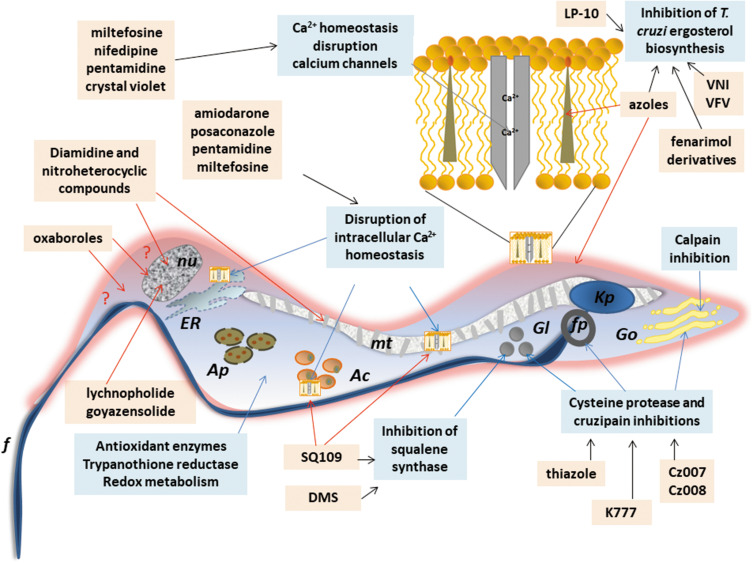
Figure 4.
Schematic representation of a trypomastigote of Trypanosome cruzi and the main cellular targets of the investigational compounds in the pre-clinical phase of development.
Notes: The selected targets are in blue boxes and compounds are in rose boxes. Organelles: Kp: kinetoplast, f: flagellum, fp: flagellar pocket, mt: mitochondrion, nu: nucleus, ER: endoplasmic reticulum, Go: golgi apparatus, Ac: acidocalcisomes, Gl: glycosomes, Ap: autophagosomes. The drawing is based on data from Benaim et al and Vannier-Santos et al.163,228
In view of the excellent activities presented by nitroheterocyclic and azole compounds, new nitrotriazole-based compounds were developed as bifunctional compounds against T. cruzi, acting on CYP51 enzyme and acting as substrates of nitroreductases. Nitro-triazoles displayed in vitro anti-T. cruzi activity and induced the reduction of parasitemia in acute model of infection.68–76
An important drug target for Chagas disease treatment is cruzipain (also named cruzain), an essential cysteine protease of T. cruzi responsible for the proteolytic activity in all stages of parasite life 77. K777, a vinyl sulfone cruzipain inhibitor, exhibited anti-T. cruzi activity and induced the cure in experimental murine models (Figure 3).77,78 In infected dogs, the treatment with K777 reduced the myocardial damage caused by the parasite, though it was not able to induce the parasitological cure.79 In addition, Cz007 and Cz008 cruzipain inhibitors presented potent activity in vitro, reduced parasitemia, and show cure in T. cruzi-infected mice in acute phase.80 Nonpeptidic tetrafluorophenoxymethyl ketone reduced parasitemia of infected mice with no apparent toxicity.81 Thiazole compounds showed in vitro activity and promoted reduction of parasitemia and mortality possibly by acting as cruzipain inhibitors (Figure 3).82–85
Amidine-containing compounds represent a versatile class of drugs, with potential antiprotozoal treatment, acting through multiple mechanisms against T. cruzi.86 Many compounds presented in vitro and in vivo activity against T. cruzi (see Table 1). For example, treatment with DB766 reduced parasite load in blood and heart, and prevented mortality of infected mice; the drug reduced hepatic and cardiac lesions and prevented electrocardiographic abnormalities induced by the parasite infection.87–89 In addition, DB766 was found in DNA-enriched compartments and induced considerable damage to the mitochondria.88 Amide-containing thiazole derivatives, arylimidamide derivatives, and other amidines have been found to be active in vitro and in vivo.90–94
Trypanosomatids, unlike humans, have a unique redox metabolism based on thiol and relying on trypanothione reductase. This trypanothione enzyme acts in defense against oxidative damage, redox homeostasis and replication, significantly supporting infectivity and survival of the parasite in the host system.95 The inhibition of trypanothione reductase metabolism increases the parasite susceptibility to drugs and/or oxidative stress induced by host defense.95 Thiazolidines LPSF and SF29 promoted trypomastigote lysis and amastigote death, probably by interfering with polyamine biosynthesis and consequently trypanothione biosynthesis, leading to increased sensitivity of the parasite to the oxidative metabolism.96 In addition, tetra-amines were able to inhibit iron superoxide dismutase and trypanothione reductase of T. cruzi and presented activity in vitro and in vivo with low toxicity.97
Other classes as antimicrobial peptides;98,99 copper100 or ruthenium101–104 complexes, compounds that impact on purine salvage pathway,105–107 oxaboroles,56,57 oxamates,108–110 quinolines,111 sphingosine kinase inhibitor,112 squaramides,55,113 terpene and terpenoid derivatives55 and proteasome inhibitors,114,115 revealed interesting results, impacting the evolution of T. cruzi infection (Table 1), but the mechanisms are not well known. Figure 4 shows schematic representation of the main drug targets identified in T. cruzi.
Natural Products
In the search for new alternatives for the therapy of Chagas disease, natural diversity can provide a wide range of bioactive agents or lead compounds. Natural products are the source for structural chemical backbone that could be used to inspire synthesis of new active molecules. Natural products isolated from different botanical sources exhibited activity against T. cruzi (see Table 2).120–132 Several approaches have been developed focusing on identification and isolation of plant-based products with anti-T. cruzi activity133,134 (Table 2). In this sense, extracts from different plants as Salvia, Valeriana, Hypericum, Silybum, Arnica, and Curcuma showed activity against T. cruzi.135 Sesquiterpene lactones have been demonstrating outstanding anti-T. cruzi activity.136–145 They are isolated from a variety of species, mainly from the Asteraceae family. Lychnopholide144 and goyazenzolide145 are examples of potent sesquiterpene lactones, which showed high efficacy in T. cruzi-infected mice. Unfortunately, preclinical research involving natural products faces obstacles to translate their findings to clinical phases due to difficulties in isolating the active compound and dimensioning the batches, as well as difficulties in the standardization of the plant extracts following the different seasons.134
Table 2.
Promising Natural Products in Experimental Treatment of Trypanosoma cruzi Infections
| Plant | Natural Product | Outcome | References |
|---|---|---|---|
| Aristeguietia glutinosa | Secondary metabolites isolated from the hydro-ethanolic extract of the aerial parts | The substances displayed low in vitro toxicity and anti- T. cruzi effect in infected mice by inhibiting parasite mitochondrial dehydrogenases and biosynthesis of membrane sterols. | [128] |
| Arrabidaea brachypoda | Dimeric flavonoids from roots | Flavonoids were active against T. cruzi in vitro and in infected mice. | [129] |
| Carica papaya | Crude seed extracts | Extracts were able to reduce parasitemia, but not the cardiac amastigotes nests of infected mice. | [127] |
| Delphinium staphisagria | Flavonoids from aerial parts | Flavonoids were active against T. cruzi in vitro and in infected mice. | [124] |
| Artemisia annua | artemisinin (sesquiterpene lactone) | Artemisinin presented in vitro activity and inhibited calcium-dependent ATPase activity in T. cruzi membranes. | [136] |
| Lychnophora passerina | goyazensolide (Sesquiterpene lactone from the aerial parts) | Goyazensolide was active in vitro and led to parasitemia reduction and negativation in parasitological tests in mice infection by Y and CL strains. | [145] |
| Lychnophora trichocarpha | Lychnopholide (LYC) and LYC-loaded nanocapsules (sesquiterpene lactone) |
Nanocapsules containing lychnopholide reduced parasitemia and mortality in murine infection and induced cure in mice infected with T. cruzi strains resistant and sensitive to BNZ in acute and chronic phases of infection. LYC-nanocapsules formulation prevented cardiotoxicity in long term treatment. | [138–140],[144] |
| Mikania species | Deoxymikanolide and other sesquiterpene lactones | Deoxymikanolide showed the highest selectivity index and induced parasitemia and mortality reduction in infected mice. | [141] |
| Tithonia diversifolia | Tagitinin C a sesquiterpene lactone isolated from leaves | Tagitinin C presented in vitro and in vivo activity alone and combined with BNZ. | [143] |
| Physalis angulata | Physalins (secosteroids) and concentrated ethanolic extract | Physalins reduced the invasion process in vitro, and intracellular parasite load, alone or in combination with BNZ. Concentrated ethanolic extract showed in vitro and in vivo activity alone and associated with BNZ. | [126,130] |
| Piper jericoense | Furofuran lignan | Furofuran lignan was active in vitro and reduced parasitemia levels in infected mice. The compound affected the parasite structure without altering the energetic metabolism. | [132] |
| Salvia gilliesii | Isolated diterpene | Compound reduced parasite load and increased survival of infected mice. | [131] |
| Senna villosa | (8-hydroxymethylen)-trieicosanyl acetate | Isolated compound showed in vitro and in vivo activity against T. cruzi. | [122,125] |
| Zanthoxylum chiloperone | Canthin-6-one alkaloids and leaves extract | Canthin-6-one alkaloids exhibited trypanocidal activity in vitro and in mouse model of acute or chronic infection. Leaves extract reduced parasitaemia in vivo. | [121,123] |
| Zanthoxylum naranjillo | Lignans isolated from the leaves | Lignans displayed in vitro and in vivo activities. | [120] |
Abbreviation: BNZ, benznidazole.
Drug Repurposing
Drug repurposing is a source of alternative chemotherapies for several diseases, especially those neglected, since the time and cost of the process required for clinical approval can be shortened. Anti-T. cruzi activity of compounds from different pharmacological classes has been tested in experimental infection models (Table 3). Particularly, the antifungals albaconazole, itraconazole, ketoconazole, posaconazole, ravuconazole and its prodrug fosravuconazole, which are CYP51 inhibitors, have demonstrated anti-T. cruzi activity by inhibiting ergosterol biosynthesis of the parasite.116–118,146–157 The effectiveness of this class of compounds has been demonstrated in several experimental models, and high cure rates has been detected in mice or dog infections.116–118,146,147,151,153 In addition, the anticancer drug tipifarnib can also inhibit CYP51 and showed potent suppressive activity on parasitemia in infected mice.158–160
Table 3.
Drugs Repositioned in Experimental Treatment of Chagas Disease
| Compound | Original Use | Outcome | References |
|---|---|---|---|
| Albaconazole | Antifungal | Albaconazole suppressed the parasite proliferation, prevented the death of infected dogs, and induced cure in animals infected by Y strain, but not in those infected by Berenice-78. | [153] |
| Allopurinol | Antihyperuricemic | Allopurinol was effective in reducing parasitemia and/or modifying the evolution of acute and chronic murine infection. Allopurinol derivatives displayed similar in vitro activity than pattern drug and good bioavailability properties for oral absorption. |
[169,170] [171] |
| Amiodarone | Antiarrhythmic | Amiodarone showed in vitro activity against epimastigote and amastigote. In acute treatment of infected mice, it reduced parasitemia and increased survival. | [154] |
| Auranofin | Antirheumatic | Auranofin showed activity against T. cruzi in vitro and reduced parasitemia and mortality in infected mice. | [173] |
| Benidipine | Antihypertensive | Benidipine reduced parasite load and inflammatory process in cardiac and skeletal muscle of chronically infected mice by probably acting as cruzipain inhibitor. | [161,162] |
| Clofazimine | Antibiotic | Clofazimine reduced parasite load and inflammatory process in cardiac and skeletal muscle of chronically infected mice, likely acting as cruzipain inhibitor. | [161,162] |
| Clomipramine | Antidepressant | Clomipramine reduce parasitemia and electrocardiographic changes and preventing myocardial structural damage in murine infection. Clomipramine improved survival by reducing the parasitic tissue load and preventing progression of cardiac damage in chronically infected mice |
[174] [175] |
| Imatinib | Anti-cancer | Imatinib was moderately active against different strains and forms of T. cruzi | [165] |
| Itraconazole | Antifungal | Itraconazole led to parasitemia reduction and mortality protection of infected mice. Itraconazole treatment promoted significant parasitemia reduction in infected dog. |
[146,152,157] [167] |
| Ketoconazole | Antifungal | Ketoconazole showed anti-T. cruzi in vitro activity. The drug displayed in vivo activity in infected mice. |
[148,149] [147,150] |
| Posaconazole | Antifungal | Posaconazole showed potent in vitro activity and in vivo trypanocidal activity, even against multiresistant T. cruzi strains. | [151], [154–156] |
| Ravuconazole | Antifungal | Ravuconazole presented efficacy to control the infection in mice and dogs. | [116,117] |
| Fosravuconazole (E1224) prodrug of ravuconazole | Antifungal | E1224 was well tolerated and effective in suppressing parasitemia with cure in Y strain-infected mice. | [118] |
| Tipifarnib | Anticancer | Tipifarnib showed potent in vitro activity due to CYP51 T. cruzi inhibition. Tipifarnib analog had potent suppressive activity on parasitemia in infected mice. |
[159] [158,160] |
The antihypertensive benidipine and antibiotic clofazimine reduced parasite load and inflammatory process in cardiac and skeletal muscle of chronically infected mice.161,162 The likely mechanism of action of both compounds would be the inhibition of cruzipain161,162 or disruption of calcium homeostasis for benidipine.163,164 Differently, the anti-cancer imatinib was moderately active against different strains and forms of T. cruzi.165 Antidepressants sertraline and fluoxetine showed in vitro anti-T. cruzi activity, while fluoxetine treatment displayed insufficient parasitemia reduction in infected mice.166,167 Antihyperuricemic allopurinol was evaluated for Chagas disease treatment and showed controversial results. This drug is a hypoxanthine analogue and acts as an alternative substrate of the T. cruzi hypoxanthine-guanine phosphoribosyltransferase.168 The enzyme can incorporate allopurinol into parasite RNA, creating a nonfunctional nucleotide, blocking de novo synthesis of purines, affecting protein synthesis, and inducing parasite death.168 Studies have been demonstrated the beneficial results of allopurinol treatment in reducing parasitemia and/or modifying the evolution of acute and chronic murine infection.169–171 Differently, Mazzeti et al demonstrated that this drug did not affect the evolution of Y acute infection in mice.172
Other drug tested using repositioning strategy are the antiarrhythmic amiodarone,154 auranofin173 and clomipramine,174,175 which were also active against T. cruzi in preclinical experimental infections (see Table 3). Amiodarone and miltefosine have mechanisms of action related to disruption of calcium homeostasis in T. cruzi.163 Taken together, the preclinical results of drug repositioning strategy indicate that most of them were active but failed to induce sterile cure in mice.
Drug Combination Therapy
Drug associations concomitantly or sequentially can improve the effectiveness of Chagas disease treatment as well as interfere in the duration of treatment and/or drug dose. Combination therapy using distinct pharmacological classes has been evaluated in experimental T. cruzi infection (Table 4). The use of suboptimal doses or treatment length of BNZ in association with CYP51 inhibitors may maintain or increase the effectiveness of treatment by the synergistic or additive effect of compounds with different mechanisms of action or cellular targets.56,118,119,150,155–157,176–180 Similarly, allopurinol combined with low dose of BNZ had a positive interaction in T. cruzi infection outcome.172,181 Sequential treatment with allopurinol and BNZ was able to reduce parasitemia and attenuate tissue damage in infected mice.182 Generally, drug association allows to decrease the duration of the treatment limiting adverse effects related to time-dependent drug accumulation.155 Table 4 shows other promising drug associations for the treatment of experimental T. cruzi infection.87,90,154,164,183–194
Table 4.
Promising Drug Combinations in Experimental Treatment of Chagas Disease
| Combination | Outcome | References |
|---|---|---|
| BNZ/VFV | Combination presented promising results on parasitic blood load and cure levels in infected mice. | [119] |
| BNZ/itraconazole | Combination improved parasitemia reduction and total survival, reduced heart qPCR positivity and cardiac damage in VL-10-infected dogs. Itraconazole improved plasma concentration of BNZ. Combination led to an improvement in parasitemia reduction, and cardiac damage in infected mice. |
[176,178] [177] [157] |
| BNZ/ketoconazole | Combination induced a synergic effect in mice infected with CL and Y T. cruzi strains, but no differences were observed in Colombian strain-infected animals. | [150] |
| BNZ/fosravuconazole | In vitro interaction was positive and in infected mice. Early treatment induced 100% of cure and beneficial effect on well-established infection. | [118] |
| BNZ/posaconazole | Shorter duration of the treatment with combination induced cure in acutely or chronically Tulahen-infected mice but did not improve the curative effect of posaconazole in Y strain infection. Sequential and combined treatments were beneficial in murine infection, decreasing the time or dose of the BNZ treatment. Combination showed same efficacy of each drug alone in Colombian strain-infected mice and improved efficacy in Brazil strain-infected mice. Treatment of acute murine infection with the combination was more effective in reducing parasitemia and myocardial injury, compared to monotherapies. |
[155] [156] [56] [179] |
| BNZ/voriconazole | In vitro results indicated an additive interaction. In vivo, all treatments were well tolerated, and combination elicited no additional benefits over BNZ alone. | [180] |
| BNZ/arylimidamides | Trypanocidal activity was improved by combination therapies (BNZ plus DB766, DB289 or DB1965) in infected mice. | [87,90] |
| BNZ/allopurinol | Allopurinol combined with low dose of BNZ had a positive interaction on serology and pathology of infected mice. Sequential treatment exhibited beneficial effects on acute and chronic infection in mice. Allopurinol plus NFX or BNZ showed in vitro synergic effect. The combinations increased the cure rate compared to BNZ alone in murine infection. |
[181] [182] [172] |
| BNZ/ascorbic acid | Ascorbic acid combined with a low dose of BNZ improved its trypanocidal activity and attenuated the toxic effects of BNZ. Combination also reduced cardiac inflammation and hepatic damage. | [188] |
| BNZ/acetyl salicylic acid | Aspirin combined with lower dosages of BNZ showed better therapeutic effect in infected mice. | [193] |
| BNZ/clomipramine | Clomipramine improved BNZ activity in vitro and in vivo with no cell toxicity and fewer side effects | [184,189] |
| BNZ/fenofibrate | Fenofibrate plus a low dose of BNZ attenuated cardiac dysfunction and promoted parasite clearance in mice sequentially infected with two strains of different genetic backgrounds. | [185] |
| BNZ/levamisole | Monotherapies of levamisole did not decrease parasitemia nor mortality rates. In combinations with a low dose of BNZ, it led to a slight improvement in the effectiveness of monotherapy | [192] |
| BNZ/NFX | Combining shorter treatments could cure mice, but the association led to the behavioral alterations of treated mice. | [155] |
| BNZ/simvastatin | Simvastatin improved BNZ activity in murine model of Chagas heart disease | [186] |
| BNZ/clofazimine or BNZ/benidipine | Reduced dose of 30mg/kg of BNZ associated with both drugs had the same efficacy as single 75mg/kg of BNZ in chronic model of Chagas disease in mice with no cure. | [164] |
| Itraconazole/amiodarone | The combination was benefic in infected dogs compared to non-treated control. The combinations were more effective in vitro T. cruzi-infection than monotherapy or BNZ; without affecting host cell metabolism and better preserving the integrity of infected cells. |
[190] [191] |
| Posaconazole/amiodarone | Amiodarone has direct in vitro and in vivo activity against T. cruzi and showed synergic effect with posaconazole. In vitro association improved IC50 and lead alterations in the parasite. |
[154] [183] |
| Ravuconazole/amlodipine | In vitro assays showed an additive effect for the combination. Amlodipine improved anti-T. cruzi activity of ravuconazole in infected mice. | [194] |
| NFX/dipyridamole | Dipyridamole potentiated the in vitro effect of NFX and improved efficacy of low dose of NFX in murine model. | [187] |
Abbreviations: BNZ, benznidazole; NFX, nifurtimox.
Benznidazole Re-Dosing Regimens
Current results of clinical trials have highlighted the need to reassess BNZ treatment regimens to achieve efficacy and reduce the incidence of side effects in Chagas disease patients.195 In addition, considering that the treatment protocol with BNZ for experimental murine infection was defined empirically 30 years ago,196 new experimental studies are evaluating other therapeutic regimens. In this sense, intermittent treatment with BNZ was as effective as a continuous scheme in chronically infected mice.197 Similarly, low-doses of BNZ in experimental chronic stage of mice infection promoted absence of parasitism in blood, heart and colon.198 Bustamante et al proposed new once-a-week regimens of BNZ administration at 2.5 to 5 times higher than standard daily dose. This intermittent regimen rapidly eliminated actively replicating parasites and ultimately eradicated the residual, transiently dormant parasite population in mice.199
Molina and co-authors reviewed the outcomes of BNZ treatment regimens in murine infection and found that the dose, either daily or the cumulative dose, had the greatest impact on effectiveness.200 Clearly, the data showed the higher the dose or exposure to the BNZ, the greater the likelihood of cure.200 Mazzeti et al showed time and concentration-dependent trypanocidal effect of BNZ in acute murine model. Furthermore, it was demonstrated that extended treatment for 40 days led to increased levels of cure in mice infected with Y or Colombian strain.201 Efficacy associated with pharmacokinetic (PK/PD) studies can give support in determining the most appropriate therapeutic regimens.
New Drug Delivery Systems
The standard of care and the majority of chemotherapeutic candidates for the treatment of Trypanosoma cruzi infections are poorly water-soluble molecules and their efficacy may be limited by their biopharmaceutical profile (Figures 1-3). Thus, the design of new drug delivery systems can improve drug stability, increase dissolution and absorption rate in the gut, reduce drug efflux and increase intestinal permeability, improving drug bioavailability and biodistribution profile.202 Nanostructured delivery systems can even circumvent multidrug resistance in some cases. In this sense, few attempts were reported concerning the use of classical formulations containing anti-T. cruzi drugs in preclinical studies.203,204 Among them, a solid dispersion and a solution containing co-solvents were used in order to improve BNZ dissolution rate as shown in Table 5.
Table 5.
Drug Delivery and Nanomedicine-Based Strategies Evaluated Against Trypanosoma cruzi in Experimental Pre-Clinical Studies
| Formulation | Drug/Active Molecule | Route | Outcome | Ref. |
|---|---|---|---|---|
| Classical formulations | ||||
| Solid dispersion in poloxamer 407 | BNZ | oral | 15–60 mg/kg/day solid dispersion compared with classical 50 mg mg/kg/day. Same efficacy in infected mice with lower side effects in acute and chronic phases, and reduced hepatotoxicity in mice. | [203] |
| Solutions with co-solvents: PEG400/water and CMC/water |
BNZ | oral | 20–60 mg/kg/day solutions compared with classical BNZ doses produced the same efficacy in infected mice (Tulahuen strain) | [204] |
| Nanostructured lipid-based formulations | ||||
| Nanostructured Lipid formulations |
Amphotericin B | i.v. oral |
Fungizone® deoxycholate, AmBisome® liposome, Amphocil® colloidal dispersion, Abelcet® lipid complex. AmBisome® was the most effective formulation with the lowest host toxicity, but less than BNZ and NFX. Different protocols: BALB/c mice infected T. cruzi Y/Tulahuen strains BNZ or NFX (controls) cleared blood trypomastigote more rapidly than amphotericin B (1 week × 3 weeks). AmBisome was the most effective formulation with the lowest host toxicity. | [205] |
| Liposome (Ambisome®) | Amphotericin B | i.p. | Efficacy in acute and chronic phases of mice infected with T. cruzi Tulahuen strain. The formulation showed efficacy in acute and chronic phases of mice infected with T. cruzi Tulahuen strain. | [206] |
| Liposome (Ambisome®) |
Amphotericin B | i.v. | AmBisome® prolonged survival, without cure. Amastigote nests found in tissues of all mice treated, heart and brain in histopathological analysis in acute and chronic mice models (Y and CL strains). AmBisome® prolonged survival without cure of infection in repeated dose regimen. | [207] |
| pH-sensitive liposomes (DOPE:CHEMS) |
Etanidazole | i.v. | Reduced parasitemia in infected mice with liposome formulation at lower doses versus no effect of free drug. Liposome showed in vitro activity against amastigote and reduced parasitemia of infected mice. | [209] |
| Nanoarchaeosomes | Imiquimod | Infected mice (RA strain) treated with the formulation in acute phase had improved survival and showed parasitemia reduction. However, efficacy was lower than BNZ classical treatment with 100mg/kg/day. | [218] | |
| Self-emulsifying drug delivery system (SEDDS) | BNZ | oral | Toxicity and efficacy similar to free-BNZ. Practical and personalized orally administered liquid dosage form. | [212] |
| Self-emulsifying drug delivery system (SEDDS) | BNZ | oral | Formulation was safe for mice. No additional drug toxicity in infected mice (Y strain) in 20 days at doses of 100 mg/kg/day was observed. Oral BNZ-SEDDS increased BNZ AUC. |
[210] |
| Self-emulsifying drug delivery system (SEDDS) | Ravuconazole | oral | SEDDS demonstrated low in vitro and in vivo toxicity and improved ravuconazole activity in vitro and reduced toxicity in vivo. No formulation and drug toxicity was observed in mice. | [211] |
| Polymeric-based nanomedicines | ||||
| Poly-aggregated Amphotericin B in albumin microspheres | Amphotericin B | oral | T. cruzi-infected mice (Y strain) treated for 10 days (10–15 mg/kg/day) reduced 75% parasitemia and prolonged survival. Unable to cure mice. | [217] |
| Nanospheres of polyethylene glycol-polylactide | Bis-triazole D0870 | i.v. | T. cruzi-infected mice treated for 30 days (3 mg/kg/day) had cure rate of 90% for CL strain and 60% for Y strain in the acute phase of infection | [216] |
| Nanocapsules of polyethylene glycol-polylactide | Lychnopholide (LYC) (sesquiterpene lactone) |
i.v. oral |
Formulation induced high cure levels in acute or chronic mice infection, including drug resistant strains and displayed minimal cardiotoxicity. Potent in vivo anti-T. cruzi activity. LYC-NC reduced cardiotoxicity compared with free-LYC. In vivo LYC-NC effects include: cure rates of 100% by PCR analysis in tissue; it induced cure in mice infected with Colombian strain and in mice in chronic phase of infection; nanocapsules increased AUC of LYC in 12-fold; high cure rates and reduced inflammation in heart of mice infected by Y, CL, VL10 and Colombian strains. |
[138–140],[144] |
| Micelles of polyoxyethylene-polyoxypropylene block copolymer (poloxamer 188) | Benznidazole | oral | Acute treatment of infected mice decreased heart inflammation and anti-T. cruzi specific antibodies levels. Intermittent treatments of mice with chronic infection were as effective as daily treatment. Nicaragua strain-infected mice (C57BL/6) treated (25–75mg/kg/day) during the chronic phase: low doses of BNZ-nanoparticles treatment (25 mg/kg/day) resulted in 40% negative PCR in the immunosuppressed mice. Continuous 30 consecutive days and intermittent regime once time week during 13 weeks for 7 days showed similar reduction of parasitemia compared to classical regime. | [197,213] |
| Nanoparticles of poly-epsilon-caprolactone | Ursolic acid | i.v. | In vivo anti-T. cruzi activity led to reduction of parasitemia levels inT. cruzi-infected mice (C57BL/6), Y strain. Mice treated during the acute phase during 7 days with ursolic acid-nanoparticles showed similar reduction of parasitemia compared to BNZ. Reduced hepatotoxicity for nanoparticles. | [214] |
| Microparticles of poly(D,L-lactide-co-glycolide) | (-)-Hinokinin | s.c. | T. cruzi infected mice (clone CLB5) treated 20 days at 40mg/kg with Hinokinin-nanoparticles and 20 mg/kg/day with free-Hinokinin. Blood parasitemia was slightly reduced with Hinokinin-nanoparticles by fresh blood examination. No mice cure was reported. | [215] |
Abbreviations: i.v, intravenous; i.p., intraperitoneal; s.c., subcutaneous; BNZ, benznidazole; NFX, nifurtimox.
Nanotechnology-Based Formulations
Many formulations based on the nanotechnology were tested in vivo in mice model of experimental infection with T. cruzi (Table 5). Different types of liposomes, nanoemulsions/microemulsions and self-emulsifying delivery systems were developed. Yardley and Croft tested commercially available Amphotericin B lipid nanoformulations by intravenous and oral routes in mice.205 Amphotericin B was active in vitro and reduced parasite burden in T. cruzi-infected mice (Y/Tulahuen strains). Among them, AmBisome® was the most active formulation of amphotericin B, although no formulations cleared parasites from blood as effectively as BNZ. Cencig et al evaluated AmBisome® in Tulahuen infected mice in acute and chronic phases and observed that intraperitoneal treatment with Amphotericin B failed to cure mice from infection and to eliminate parasites.155,206 Similar results were obtained by Clemons et al.207 Taken together these studies demonstrated that amphotericin B was active against T. cruzi infection, but it was unable to produce cure and to eliminate parasites from tissues in pre-clinical models.
BNZ lipid formulations were also developed. They failed to improve efficacy compared with classical BNZ treatment.208 Etanidazole, which is more soluble than BNZ was encapsulated in pH-sensitive DOPE/CHEMS liposomes and showed high improvement of the activity toward amastigotes of T. cruzi compared with free-drug. The study demonstrated that pH-sensitive liposomes provide a pathway to reach more efficiently the parasites in the bloodstream and in the macrophages during the acute phase of infection.209 Lipid-based nanocarriers dispersed in aqueous media showed disadvantages due to their susceptibility to oxidation and poor stability, depending on the composition.
In this sense, self-emulsifying drug delivery systems (SEDDS) are simple lipid formulations and more versatile to associate drugs for Chagas disease treatment. These anhydrous systems form nano or microemulsion droplets spontaneously after reaching aqueous fluids in gastrointestinal tract. They are stable under storage with good ability to dissolve drugs with poor water solubility. BNZ was loaded in SEDDS and an improvement of the extent of BNZ absorption and body exposure after oral administration were observed in mice, with an increase of 25% in bioavailability.210 No adverse effects were observed in T. cruzi-infected mice after 20 oral doses of BNZ at 100 mg/kg/day.210 Spósito et al using the same strategy incorporated ravuconazole in SEDDS type IIIA lipid formulation. An increased activity (1.8-fold) of ravuconazole-SEDDS against intracellular amastigotes was observed in host cardiomyocyte cell line compared with free-ravuconazole, without additional drug toxicity.211 Furthermore, an increase in efficacy was achieved with 20 mg/kg ravuconazole-SEDDS in T. cruzi-infected mice (Y strain) compared to free-ravuconazole in 30 day-treatment (unpublished personal results). Thus, SEDDS are promising formulations for use in neglected diseases, including Chagas disease, because of their low cost, high stability, and ease of preparation.212
Polymer-Based Nanomedicines
An interesting work associated BNZ with non-ionic surfactant (poloxamer 188), which produced micelles with mean particle size of 63 nm.197,213 Treated mice had reduced inflammatory cardiomyopathy and fibrosis in a dose-dependent manner, with doses as lower as 25 mg/kg/day, which resulted in 40% negative Polymerase Chain Reaction (PCR) tests in immunosuppressed mice. Thus, BNZ polymeric nanoformulations have potential to be used in experimental therapy. By contrast, polylactides, polyglycolides, polycaprolactone, and their copolymers were used to prepare polymeric nanospheres and nanocapsules (Table 5). Poly-Ɛ-caprolactone nanoparticles containing ursolic acid with sizes lower than 200 nm were prepared by nanoprecipitation method and exhibited no in vitro toxicity toward LLC-MK2 fibroblasts. In T. cruzi-infected mice (Y strain) treated during 7 days with ursolic acid-nanoparticles, the parasitemia levels were reduced similarly to BNZ classical treatment.214 The same group prepared poly(D,L-lactide-co-glycolide) microparticles encapsulating (-)-hinokinin (HNK), which induces only a slight reduction of parasitemia peak compared with HNK-free molecule.215 The nanospheres of polyethylene glycol-block-polylactide were prepared with bis-triazole D0870 and tested in mice infected with CL and Y strains of T. cruzi. Mice were treated daily by the intravenous route with 3 mg/kg/day of D0870-loaded nanospheres and a significant cure rate of 60% for Y and 90% CL strains in acute mice model was observed.216
Lychnopholide (LYC), a sesquiterpene lactone encapsulated in polyethylene glycol-polylactide and in poly-ɛ-caprolactone nanocapsules (LYC-NC) showed one of the most promising nanotechnological approaches investigated up to date. In acute and chronic phases of T. cruzi experimental infection with strains sensitive and resistant to BNZ, LYC-NC administered by oral and intravenous routes showed the highest rates of cure compared with BNZ, free-LYC and other drugs tested in vivo.138–140,144 The treatment promoted complete elimination of amastigote parasites from heart tissue in mice infected with cardiomyotropic VL10 strain, using oral doses of 12 mg/kg/day during 20 days.138–140,144 Nanoencapsulation promoted in this case an outstanding improvement of pharmacokinetic properties of LYC and dramatic reduction of cardiotoxicity.140 This study attests the potential of nanotechnological approaches to the therapy of Chagas disease. Other studies have also shown the potential use of nanotechnology approaches in experimental Chagas disease.197,213,216–218
Final Comments and Future Perspectives
Chagas disease was discovered more than a hundred years ago, but current treatment is based on two old nitroheterocyclic drugs, BNZ and NFX. Knowledge about T. cruzi and the disease has expanded, but the complexity of the parasite, the pathogenesis and the immunology of the infection defy the scientific community and hinder the drug discovery process.200 Notwithstanding, target-based and mainly phenotypic screening approaches have been widely applied. Technological advances have positively influenced the development of new compounds and approaches against T. cruzi infection. In this sense, many challenges on Chagas disease drug discovery pipeline must be overcome. The natural resistance to BNZ and NFX, verified in vitro and in vivo is an intrinsic characteristic of T. cruzi strains. The parasite stocks have shown different susceptibility profiles, a factor that can trigger some treatment failures.196,219–221 The components involved in drug resistance are not yet fully understood,36,39,220–222 but may include alternative activation of enzymes by drugs,223 increased oxidative defense224 or DNA repair pathways,39 induction of drug efflux transporters222 and glutamine metabolism.225 In addition, dormant forms of T. cruzi have been evidenced and increased drug tolerance was demonstrated.226 Thousands of compounds have been experimentally tested in vitro, however, very few achievements in terms of translation were accomplished and among them, very few produced sterile cure in mice. Additionally, we highlight the lack of predictive and harmonized models in vitro and in vivo,227 sensitive and accurate tests to determine therapeutic efficacy, mainly in chronic stage and proper determination of toxicity and pharmacokinetics of compounds.
There are no vaccines available for Chagas disease and, considering the immunological complexity and the long duration of the infection, advances in this area are still incipient. Apart from BNZ standard of care, in front of this plethora of chemical drug classes and the preclinical efficacy results discussed in this review, sesquiterpene lactone class of natural substances associated to nanocapsules seems to be the most promising chemical entities for further investigation for Chagas disease chemotherapy. Lychnopholide and goiazensolide showed outstanding efficacy against different strains of T. cruzi with variable sensitivity to BNZ. Additionally, fexinidazole advanced to new clinical trial with T. cruzi infection (FEX12-NCT03587766). Fexinidazole metabolites seem to be an encouraging approach for further clinical trials. Among new chemical entities and repositioned drugs, nitroheterocyclic compounds and CYP51 inhibitors showed potent activity against T. cruzi. However, a careful evaluation of toxicity and more effective regimens must be established. Additionally, associations of drugs that share or not share the same mechanism of action can lead to superior therapeutic efficacy.
Acknowledgments
This work received support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) - Brazil (#313602/2019-0, BRICS-STI# 442351/2017-8). ALM received support from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (Faperj) - Brazil (E-26/202.367/2019).
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Chagas C. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz. 1909;1(2):159–218. doi: 10.1590/S0074-02761909000200008 [DOI] [Google Scholar]
- 2.WHO Chagas disease (American trypanosomiasis). 2020. Available from: https://www.who.int/westernpacific/health-topics/chagas-disease. Accessed November 20, 2020
- 3.Lidani KCF, Andrade FA, Bavia L, et al. Chagas disease: from discovery to a worldwide health problem. Front Public Health. 2019;7:166. doi: 10.3389/fpubh.2019.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guhl F, JD Ramírez. Poverty, migration, and chagas disease. Curr Trop Med Rep. 2021. doi: 10.1007/s40475-020-00225-y [DOI] [Google Scholar]
- 5.Brener Z. Biology of Trypanosoma Cruzi. Annu Rev Microbiol. 1973;27(1):347–382. doi: 10.1146/annurev.mi.27.100173.002023 [DOI] [PubMed] [Google Scholar]
- 6.Carlier Y, Torrico F. Congenital infection with Trypanosoma cruzi: from mechanisms of transmission to strategies for diagnosis and control. Rev Soc Bras Med Trop. 2003;36(6):767–771. doi: 10.1590/S0037-86822003000600024 [DOI] [PubMed] [Google Scholar]
- 7.Howard JE, Rios C, Ebensperger I, Olivos P. [Congenital Chagas’ disease]. Bol Chil Parasitol. 1957;12(3):42–45. [PubMed] [Google Scholar]
- 8.Wanderley DM, Aranha Camargo LM, de Carvalho ME. [Chagas’ disease: registry of an acute transfusional case]. Rev Inst Med Trop Sao Paulo. 1988;30(6):437–440. doi: 10.1590/s0036-46651988000600009 [DOI] [PubMed] [Google Scholar]
- 9.Dias JCP, Amato Neto V. [Prevention concerning the different alternative routes for transmission of Trypanosoma cruzi in Brazil]. Rev Soc Bras Med Trop. 2011;44(Suppl 2):68–72. doi: 10.1590/s0037-86822011000800011 [DOI] [PubMed] [Google Scholar]
- 10.Dias JCP. Notas sobre o Trypanosoma cruzi e suas características bio-ecológicas, como agente de enfermidades transmitidas por alimentos. Rev Soc Bras Med Trop. 2006;39(4):370–375. doi: 10.1590/s0037-86822006000400010 [DOI] [PubMed] [Google Scholar]
- 11.Pereira KS, Schmidt FL, Guaraldo AMA, Franco RMB, Dias VL, Passos LAC. Chagas’ disease as a foodborne illness. J Food Prot. 2009;72(2):441–446. doi: 10.4315/0362-028x-72.2.441 [DOI] [PubMed] [Google Scholar]
- 12.Coura JR, Viñas PA, Junqueira AC. Ecoepidemiology, short history and control of Chagas disease in the endemic countries and the new challenge for non-endemic countries. Mem Inst Oswaldo Cruz. 2014;109(7):856–862. doi: 10.1590/0074-0276140236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alarcón de Noya B, Díaz‐Bello Z, Colmenares C, et al. Large urban outbreak of orally acquired acute chagas disease at a school in Caracas, Venezuela. J INFECT DIS. 2010;201(9):1308–1315. doi: 10.1086/651608 [DOI] [PubMed] [Google Scholar]
- 14.da Brasil MS. Boletim Epidemiológico - Doença de Chagas aguda no Brasil: série histórica de 2000 a 2013. 2015;46(21):1–9. [Google Scholar]
- 15.Ríos JF, Arboleda M, Montoya AN, Alarcón EP. [Probable outbreak of oral transmission of Chagas disease in Turbo, Antioquia]. Biomedica. 2011;31(2):185–195. doi: 10.1590/S0120-41572011000200005 [DOI] [PubMed] [Google Scholar]
- 16.Shikanai-Yasuda MA, Carvalho NB. Oral transmission of chagas disease. Clin Infect Dis. 2012;54(6):845–852. doi: 10.1093/cid/cir956 [DOI] [PubMed] [Google Scholar]
- 17.Rassi A, Rassi A. Marcondes de Rezende J. American Trypanosomiasis (Chagas Disease). Infect Dis Clin North Am. 2012;26(2):275–291. doi: 10.1016/j.idc.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Molina JA, Molina I. Chagas disease. The Lancet. 2018;391(10115):82–94. doi: 10.1016/S0140-6736(17)31612-4 [DOI] [PubMed] [Google Scholar]
- 19.Barrett MP, Burchmore RJS, Stich A, et al. The trypanosomiases. Lancet. 2003;362(9394):1469–1480. doi: 10.1016/S0140-6736(03)14694-6 [DOI] [PubMed] [Google Scholar]
- 20.Remme JHF, Feenstra P, Lever PR, et al.. Tropical diseases targeted for elimination: chagas disease, lymphatic filariasis, onchocerciasis, and leprosy. In: Jamison DT, Breman JG, Measham AR, et al., editors. Disease Control Priorities in Developing Countries. 2nd ed. World Bank; 2006. Available from: http://www.ncbi.nlm.nih.gov/books/NBK11745/. Accessed November24, 2020. [PubMed] [Google Scholar]
- 21.Salomao K, Figueiredo Sadok Menna-Barreto R, Lisboa de Castro S. Stairway to heaven or hell? Perspectives and limitations of chagas disease chemotherapy. CTMC. 2016;16(20):2266–2289. doi: 10.2174/1568026616666160413125049 [DOI] [PubMed] [Google Scholar]
- 22.Dias JC. The indeterminate form of human chronic Chagas’ disease. A clinical epidemiological review. Rev Soc Bras Med Trop. 1989;22(3):147–156. doi: 10.1590/s0037-86821989000300007 [DOI] [PubMed] [Google Scholar]
- 23.Salvador F, Treviño B, Sulleiro E, et al. Trypanosoma cruzi infection in a non-endemic country: epidemiological and clinical profile. Clin Microbiol Infect. 2014;20(7):706–712. doi: 10.1111/1469-0691.12443 [DOI] [PubMed] [Google Scholar]
- 24.Chapadeiro E. Clinical evolution and morbi-mortality in Chagas disease. Mem Inst Oswaldo Cruz. 1999;94(Suppl 1):309–310. doi: 10.1590/s0074-02761999000700058 [DOI] [PubMed] [Google Scholar]
- 25.Nunes MCP, Do Carmo AAL, Rocha MOC, Ribeiro AL. Mortality prediction in Chagas heart disease. Expert Rev Cardiovasc Ther. 2012;10(9):1173–1184. doi: 10.1586/erc.12.111 [DOI] [PubMed] [Google Scholar]
- 26.Andrade SG, Stocker-Guerret S, Pimentel AS, Grimaud JA. Reversibility of cardiac fibrosis in mice chronically infected with Trypanosoma cruzi, under specific chemotherapy. Mem Inst Oswaldo Cruz. 1991;86(2):187–200. doi: 10.1590/s0074-02761991000200008 [DOI] [PubMed] [Google Scholar]
- 27.Bahia MT, de Diniz LF, Mosqueira VCF. Therapeutical approaches under investigation for treatment of Chagas disease. Expert Opin Investig Drugs. 2014;23(9):1225–1237. doi: 10.1517/13543784.2014.922952 [DOI] [PubMed] [Google Scholar]
- 28.Higuchi M, De Brito T, Martins Reis M, et al. Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic chagasic myocarditis: light microscopy and immunohistochemical findings. Cardiovasc Pathol. 1993;2(2):101–106. doi: 10.1016/1054-8807(93)90021-S [DOI] [PubMed] [Google Scholar]
- 29.Jones EM, Colley DG, Tostes S, Lopes ER, Vnencak-Jones CL, McCurley TL. Amplification of a Trypanosoma cruzi DNA sequence from inflammatory lesions in human chagasic cardiomyopathy. Am J Trop Med Hyg. 1993;48(3):348–357. doi: 10.4269/ajtmh.1993.48.348 [DOI] [PubMed] [Google Scholar]
- 30.Tarleton RL, Zhang L. Chagas disease etiology: autoimmunity or parasite persistence? Parasitol Today. 1999;15(3):94–99. doi: 10.1016/S0169-4758(99)01398-8 [DOI] [PubMed] [Google Scholar]
- 31.Viotti R, Vigliano C, Lococo B, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006;144(10):724–734. doi: 10.7326/0003-4819-144-10-200605160-00006 [DOI] [PubMed] [Google Scholar]
- 32.Bock M, Gönnert R, Haberkorn A. Studies with Bay 2502 on animals. Bol Chil Parasitol. 1969;24(1):13–19. [PubMed] [Google Scholar]
- 33.Brener Z. Atividade terapêutica do 5-nitro-furaldeído - semicarbazona (nitrofurazona) em esquemas de duração prolongada na infecção experimental do camundongo pelo Trypanosoma cruzi. Revista Do Instituto De Medicina Tropical De São Paulo. 1961;3:43–49. [Google Scholar]
- 34.Packchanian A. Chemotherapy of experimental Chagas’ disease with nitrofuran compounds. Antibiot Chemother (Northfield). 1957;7(1):13–23. [PubMed] [Google Scholar]
- 35.de Ferreira HO. Ensaio terapêutico-clínico com benzonidazol na doença de chagas. Rev Inst Med Trop São Paulo. 1976;18(5):357–364. [PubMed] [Google Scholar]
- 36.Wilkinson SR, Kelly JM. Trypanocidal drugs: mechanisms, resistance and new targets. Expert Rev Mol Med. 2009;11:e31. doi: 10.1017/S1462399409001252 [DOI] [PubMed] [Google Scholar]
- 37.Patterson S, Fairlamb AH. Current and future prospects of nitro-compounds as drugs for trypanosomiasis and leishmaniasis. CMC. 2019;26(23):4454–4475. doi: 10.2174/0929867325666180426164352 [DOI] [PubMed] [Google Scholar]
- 38.Maya JD, Cassels BK, Iturriaga-Vásquez P, et al. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp Biochem Physiol a Mol Integr Physiol. 2007;146(4):601–620. doi: 10.1016/j.cbpa.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 39.Rajão MA, Furtado C, Alves CL, et al. Unveiling benznidazole’s mechanism of action through overexpression of DNA repair proteins in Trypanosoma cruzi. Environ Mol Mutagen. 2014;55(4):309–321. doi: 10.1002/em.21839 [DOI] [PubMed] [Google Scholar]
- 40.FDA O of the C. FDA approves first U.S. treatment for Chagas disease. FDA. March 24, 2020. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-us-treatment-chagas-disease Accessed February5, 2021.
- 41.Ribeiro V, Dias N, Paiva T, et al. Current trends in the pharmacological management of Chagas disease. Int J Parasitol Drugs Drug Resist. 2020;12:7–17. doi: 10.1016/j.ijpddr.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urbina JA. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 2010;115(1–2):55–68. doi: 10.1016/j.actatropica.2009.10.023 [DOI] [PubMed] [Google Scholar]
- 43.Yun O, Lima MA, Ellman T, et al. Feasibility, drug safety, and effectiveness of etiological treatment programs for Chagas disease in Honduras, Guatemala, and Bolivia: 10-year experience of Médecins Sans Frontières. PLoS Negl Trop Dis. 2009;3(7):e488. doi: 10.1371/journal.pntd.0000488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molina I, Gómez I, Prat J, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;370(20):1899–1908. doi: 10.1056/NEJMoa1313122 [DOI] [PubMed] [Google Scholar]
- 45.Torrico F, Gascon J, Ortiz L, et al. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: a proof-of-concept, randomised, placebo-controlled trial. Lancet Infect Dis. 2018;18(4):419–430. doi: 10.1016/S1473-3099(17)30538-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bahia MT, De andrade IM, Martins TAF, et al. Fexinidazole: a potential new drug candidate for chagas disease. Pollastri MP, ed. PLoS Negl Trop Dis. 2012;6(11):e1870. doi: 10.1371/journal.pntd.0001870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francisco AF, Jayawardhana S, Lewis MD, et al. Nitroheterocyclic drugs cure experimental Trypanosoma cruzi infections more effectively in the chronic stage than in the acute stage. Sci Rep. 2016;6(1):35351. doi: 10.1038/srep35351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raether W, Seidenath H. The activity of fexinidazole (HOE 239) against experimental infections with Trypanosoma cruzi, trichomonads and Entamoeba histolytica. Ann Trop Med Parasitol. 1983;77(1):13–26. doi: 10.1080/00034983.1983.11811668 [DOI] [PubMed] [Google Scholar]
- 49.Bahia MT, Nascimento AFS, Mazzeti AL, et al. Antitrypanosomal activity of fexinidazole metabolites, potential new drug candidates for chagas disease. Antimicrob Agents Chemother. 2014;58(8):4362–4370. doi: 10.1128/AAC.02754-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Filardi LS, Brener Z. A nitroimidazole-thiadiazole derivative with curative action in experimental Trypanosoma cruzi infections. Ann Trop Med Parasitol. 1982;76(3):293–297. doi: 10.1080/00034983.1982.11687544 [DOI] [PubMed] [Google Scholar]
- 51.Andrade SG, Silva RC, Santiago CMG. Freitas L a. R. Therapeutic action of MK-436 (2,5-nitroimidazole) on Trypanosoma cruzi infections in mice: a parasitological, serological, histopathological, and ultrastructural study. Bull World Health Organ. 1987;65(5):625. [PMC free article] [PubMed] [Google Scholar]
- 52.Andrade SG, Silva RC, Santiago CM. Treatment of chronic experimental Trypanosoma cruzi infections in mice with MK-436, a 2-substituted 5-nitroimidazole. Bull World Health Organ. 1989;67(5):509. [PMC free article] [PubMed] [Google Scholar]
- 53.de Castro SL, de Meirelles MN. Mechanism of action of a nitroimidazole-thiadiazole derivate upon Trypanosoma cruzi tissue culture amastigotes. Mem Inst Oswaldo Cruz. 1990;85(1):95–99. doi: 10.1590/s0074-02761990000100016 [DOI] [PubMed] [Google Scholar]
- 54.Salomão K, de Souza EM, Carvalho SA, et al. In vitro and in vivo activities of 1,3,4-thiadiazole-2-arylhydrazone derivatives of megazol against Trypanosoma cruz i. Antimicrob Agents Chemother. 2010;54(5):2023–2031. doi: 10.1128/AAC.01241-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sánchez-Moreno M, Gómez-Contreras F, Navarro P, et al. Phthalazine derivatives containing imidazole rings behave as Fe-SOD inhibitors and show remarkable anti-T. cruzi activity in immunodeficient-mouse mode of infection. J Med Chem. 2012;55(22):9900–9913. doi: 10.1021/jm3011004 [DOI] [PubMed] [Google Scholar]
- 56.Bustamante JM, Craft JM, Crowe BD, Ketchie SA, Tarleton RL. New, combined, and reduced dosing treatment protocols cure trypanosoma cruzi infection in mice. J Infect Dis. 2014;209(1):150–162. doi: 10.1093/infdis/jit420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moraes CB, Giardini MA, Kim H, et al. Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: implications for Chagas disease drug discovery and development. Sci Rep. 2015;4(1):4703. doi: 10.1038/srep04703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leite DI, de Fontes FV, Bastos MM, et al. New 1,2,3-triazole-based analogues of benznidazole for use against Trypanosoma cruzi infection: in vitro and in vivo evaluations. Chem Biol Drug Des. 2018;92(3):1670–1682. doi: 10.1111/cbdd.13333 [DOI] [PubMed] [Google Scholar]
- 59.Villalta F, Dobish MC, Nde PN, et al. VNI cures acute and chronic experimental chagas disease. J Infect Dis. 2013;208(3):504–511. doi: 10.1093/infdis/jit042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guedes-da-silva FH, Batista DGJ, Da Silva CF, et al. Antitrypanosomal activity of sterol 14α-demethylase (CYP51) inhibitors VNI and VFV in the Swiss mouse models of chagas disease induced by the trypanosoma cruzi Y strain. Antimicrob Agents Chemother. 2017;61(4):e02098-16, e02098-16. doi: 10.1128/AAC.02098-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guedes-da-silva FH, Batista DGJ, da Silva CF, et al. Different therapeutic outcomes of benznidazole and VNI treatments in different genders in mouse experimental models of trypanosoma cruzi infection. Antimicrob Agents Chemother. 2015;59(12):7564–7570. doi: 10.1128/AAC.01294-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Soeiro NC, de Souza EM, da Silva CF, et al. In vitro and in vivo studies of the antiparasitic activity of Sterol 14α-Demethylase (CYP51) Inhibitor VNI against drug-resistant strains of Trypanosoma cruzi. Antimicrob Agents Chemother. 2013;57(9):4151–4163. doi: 10.1128/AAC.00070-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keenan M, Alexander PW, Diao H, et al. Design, structure-activity relationship and in vivo efficacy of piperazine analogues of fenarimol as inhibitors of Trypanosoma cruzi. Bioorg Med Chem. 2013;21(7):1756–1763. doi: 10.1016/j.bmc.2013.01.050 [DOI] [PubMed] [Google Scholar]
- 64.Keenan M, Abbott MJ, Alexander PW, et al. Analogues of fenarimol are potent inhibitors of Trypanosoma cruzi and are efficacious in a murine model of Chagas disease. J Med Chem. 2012;55(9):4189–4204. doi: 10.1021/jm2015809 [DOI] [PubMed] [Google Scholar]
- 65.Doyle PS, Chen C-K, Johnston JB, et al. A nonazole CYP51 inhibitor cures Chagas’ disease in a mouse model of acute infection. Antimicrob Agents Chemother. 2010;54(6):2480–2488. doi: 10.1128/AAC.00281-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoekstra WJ, Hargrove TY, Wawrzak Z, et al. Clinical Candidate VT-1161’s antiparasitic effect in vitro, activity in a murine model of chagas disease, and structural characterization in complex with the target enzyme CYP51 from Trypanosoma cruzi. Antimicrob Agents Chemother. 2016;60(2):1058–1066. doi: 10.1128/AAC.02287-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calvet CM, Choi JY, Thomas D, et al. 4-aminopyridyl-based lead compounds targeting CYP51 prevent spontaneous parasite relapse in a chronic model and improve cardiac pathology in an acute model of Trypanosoma cruzi infection. PLoS Negl Trop Dis. 2017;11(12):e0006132. doi: 10.1371/journal.pntd.0006132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Papadopoulou MV, Bloomer WD, Rosenzweig HS, et al. Nitrotriazole-based compounds as antichagasic agents in a long-treatment in vivo assay. Antimicrob Agents Chemother. 2017;61(5):e02717-16, e02717-16. doi: 10.1128/AAC.02717-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papadopoulou MV, Bloomer WD, Rosenzweig HS, Wilkinson SR, Szular J, Kaiser M. Nitrotriazole-based acetamides and propanamides with broad spectrum antitrypanosomal activity. Eur J Med Chem. 2016;123:895–904. doi: 10.1016/j.ejmech.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papadopoulou MV, Bloomer WD, Rosenzweig HS, et al. Discovery of potent nitrotriazole-based antitrypanosomal agents: in vitro and in vivo evaluation. Bioorg Med Chem. 2015;23(19):6467–6476. doi: 10.1016/j.bmc.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 71.Papadopoulou MV, Bloomer WD, Rosenzweig HS, O’Shea IP, Wilkinson SR, Kaiser M. 3-Nitrotriazole-based piperazides as potent antitrypanosomal agents. Eur J Med Chem. 2015;103:325–334. doi: 10.1016/j.ejmech.2015.08.042 [DOI] [PubMed] [Google Scholar]
- 72.Papadopoulou MV, Bloomer WD, Rosenzweig HS, Wilkinson SR, Kaiser M. Novel nitro(triazole/imidazole)-based heteroarylamides/sulfonamides as potential antitrypanosomal agents. Eur J Med Chem. 2014;87:79–88. doi: 10.1016/j.ejmech.2014.09.045 [DOI] [PubMed] [Google Scholar]
- 73.Papadopoulou MV, Bloomer WD, Rosenzweig HS, et al. Novel 3-nitro-1H-1,2,4-triazole-based compounds as potential anti-Chagasic drugs: in vivo studies. Future Med Chem. 2013;5(15):1763–1776. doi: 10.4155/fmc.13.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papadopoulou MV, Bloomer WD, Rosenzweig HS, Kaiser M, Chatelain E, Ioset J-R. Novel 3-nitro-1H-1,2,4-triazole-based piperazines and 2-amino-1,3-benzothiazoles as antichagasic agents. Bioorg Med Chem. 2013;21(21):6600–6607. doi: 10.1016/j.bmc.2013.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papadopoulou MV, Bloomer WD, Rosenzweig HS, et al. Novel 3-nitro-1H-1,2,4-triazole-based amides and sulfonamides as potential antitrypanosomal agents. J Med Chem. 2012;55(11):5554–5565. doi: 10.1021/jm300508n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Papadopoulou MV, Trunz BB, Bloomer WD, et al. Novel 3-nitro-1H-1,2,4-triazole-based aliphatic and aromatic amines as anti-chagasic agents. J Med Chem. 2011;54(23):8214–8223. doi: 10.1021/jm201215n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Engel JC, Doyle PS, Hsieh I, McKerrow JH. Cysteine protease inhibitors cure an experimental trypanosoma cruzi infection. J Exp Med. 1998;188(4):725–734. doi: 10.1084/jem.188.4.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doyle PS, Zhou YM, Engel JC, McKerrow JH. A cysteine protease inhibitor cures Chagas’ disease in an immunodeficient-mouse model of infection. Antimicrob Agents Chemother. 2007;51(11):3932–3939. doi: 10.1128/AAC.00436-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barr SC, Warner KL, Kornreic BG, et al. A cysteine protease inhibitor protects dogs from cardiac damage during infection by Trypanosoma cruzi. Antimicrob Agents Chemother. 2005;49(12):5160–5161. doi: 10.1128/AAC.49.12.5160-5161.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ndao M, Beaulieu C, Black WC, et al. Reversible cysteine protease inhibitors show promise for a chagas disease cure. Antimicrob Agents Chemother. 2014;58(2):1167–1178. doi: 10.1128/AAC.01855-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brak K, Kerr ID, Barrett KT, et al. Nonpeptidic tetrafluorophenoxymethyl ketone cruzain inhibitors as promising new leads for Chagas disease chemotherapy. J Med Chem. 2010;53(4):1763–1773. doi: 10.1021/jm901633v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Cardoso MV, de Siqueira LRP, da Silva EB, et al. 2-Pyridyl thiazoles as novel anti-Trypanosoma cruzi agents: structural design, synthesis and pharmacological evaluation. Eur J Med Chem. 2014;86:48–59. doi: 10.1016/j.ejmech.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 83.da Silva EB, Oliveira E, DA S, et al. . Desing and synthesis of potent anti-Trypanosoma cruzi agents new thiazoles derivatives which induce apoptotic parasite death. Eur J Med Chem. 2017;130:39–50. doi: 10.1016/j.ejmech.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 84.De oliveira Filho GB, de Cardoso MVO, Espíndola JWP, et al. Structural design, synthesis and pharmacological evaluation of thiazoles against Trypanosoma cruzi. Eur J Med Chem. 2017;141:346–361. doi: 10.1016/j.ejmech.2017.09.047 [DOI] [PubMed] [Google Scholar]
- 85.de Oliveira Filho GB, de Oliveira Cardoso MV, Espíndola JWP, et al. Structural design, synthesis and pharmacological evaluation of 4-thiazolidinones against Trypanosoma cruzi. Bioorg Med Chem. 2015;23(23):7478–7486. doi: 10.1016/j.bmc.2015.10.048 [DOI] [PubMed] [Google Scholar]
- 86.Soeiro MNC, Werbovetz K, Boykin DW, Wilson WD, Wang MZ, Hemphill A. Novel amidines and analogues as promising agents against intracellular parasites: a systematic review. Parasitology. 2013;140(8):929–951. doi: 10.1017/S0031182013000292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Da Batista GJ, Batista MM, Oliveira GM, et al. Combined treatment of heterocyclic analogues and benznidazole upon Trypanosoma cruzi in vivo. Moreno SN, ed. PLoS ONE. 2011;6(7):e22155. doi: 10.1371/journal.pone.0022155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Da batista DGJ, Batista MM, De oliveira GM, et al. Arylimidamide DB766, a potential chemotherapeutic candidate for Chagas’ disease treatment. Antimicrob Agents Chemother. 2010;54(7):2940–2952. doi: 10.1128/AAC.01617-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Z, Wenzler T, Brun R, Zhu X, Boykin DW. Synthesis and antiparasitic activity of new bis-arylimidamides: DB766 analogs modified in the terminal groups. Eur J Med Chem. 2014;83:167–173. doi: 10.1016/j.ejmech.2014.06.022 [DOI] [PubMed] [Google Scholar]
- 90.da Silva CF, Da Batista DGJ, Oliveira GM, et al. In vitro and in vivo investigation of the efficacy of arylimidamide DB1831 and its mesylated salt form–DB1965–against Trypanosoma cruzi infection. PLoS One. 2012;7(1). doi: 10.1371/journal.pone.0030356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.da Silva CF, de Batista DGJ, de Araújo JS, et al. Phenotypic evaluation and in silico ADMET properties of novel arylimidamides in acute mouse models of Trypanosoma cruzi infection. Drug Des Devel Ther. 2017;11:1095–1105. doi: 10.2147/DDDT.S120618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Timm BL, da Silva PB, Batista MM, et al. In vitro and in vivo biological effects of novel arylimidamide derivatives against Trypanosoma cruzi. Antimicrob Agents Chemother. 2014;58(7):3720–3726. doi: 10.1128/AAC.02353-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Álvarez G, Varela J, Cruces E, et al. Identification of a new amide-containing thiazole as a drug candidate for treatment of Chagas’ disease. Antimicrob Agents Chemother. 2015;59(3):1398–1404. doi: 10.1128/AAC.03814-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Santos CC, Lionel JR, Peres RB, et al. In vitro, in silico, and in vivo analyses of novel aromatic amidines against Trypanosoma cruzi. Antimicrob Agents Chemother. 2018;62:2. doi: 10.1128/AAC.02205-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saccoliti F, Di Santo R, Costi R. Recent advancement in the search of innovative antiprotozoal agents targeting trypanothione metabolism. ChemMedChem. 2020;cmdc.202000325. doi: 10.1002/cmdc.202000325 [DOI] [PubMed] [Google Scholar]
- 96.de Moreira TLB, Barbosa AFS, Veiga-Santos P, et al. Effect of thiazolidine LPSF SF29 on the growth and morphology of Trypanosoma cruzi. Int J Antimicrob Agents. 2013;41(2):183–187. doi: 10.1016/j.ijantimicag.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 97.Olmo F, Cussó O, Marín C, et al. In vitro and in vivo identification of tetradentated polyamine complexes as highly efficient metallodrugs against Trypanosoma cruzi. Exp Parasitol. 2016;164:20–30. doi: 10.1016/j.exppara.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 98.Martín-Escolano R, Cebrián R, Maqueda M, et al. Assessing the effectiveness of AS-48 in experimental mice models of Chagas’ disease. J Antimicrob Chemother. 2020;75(6):1537–1545. doi: 10.1093/jac/dkaa030 [DOI] [PubMed] [Google Scholar]
- 99.Martín-Escolano R, Cebrián R, Martín-Escolano J, et al. Insights into Chagas treatment based on the potential of bacteriocin AS-48. Int J Parasitol Drugs Drug Resist. 2019;10:1–8. doi: 10.1016/j.ijpddr.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paixão DA, Lopes CD, Carneiro ZA, et al. In vitro anti-Trypanosoma cruzi activity of ternary copper(II) complexes and in vivo evaluation of the most promising complex. Biomed Pharmacother. 2019;109:157–166. doi: 10.1016/j.biopha.2018.10.057 [DOI] [PubMed] [Google Scholar]
- 101.Guedes PMM, Oliveira FS, Gutierrez FRS, et al. Nitric oxide donor trans-[RuCl([15]aneN)NO] as a possible therapeutic approach for Chagas’ disease. Br J Pharmacol. 2010;160(2):270–282. doi: 10.1111/j.1476-5381.2009.00576.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silva JJN, Guedes PMM, Zottis A, et al. Novel ruthenium complexes as potential drugs for Chagas’s disease: enzyme inhibition and in vitro/in vivo trypanocidal activity. Br J Pharmacol. 2010;160(2):260–269. doi: 10.1111/j.1476-5381.2009.00524.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Silva JJN, Pavanelli WR, Pereira JCM, Silva JS, Franco DW. Experimental chemotherapy against Trypanosoma cruzi infection using ruthenium nitric oxide donors. AAC. 2009;53(10):4414–4421. doi: 10.1128/AAC.00104-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Silva JJN, Osakabe AL, Pavanelli WR, Silva JS, Franco DW. In vitro and in vivo antiproliferative and trypanocidal activities of ruthenium NO donors. Br J Pharmacol. 2007;152(1):112–121. doi: 10.1038/sj.bjp.0707363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neitz RJ, Chen S, Supek F, et al. Lead identification to clinical candidate selection: drugs for Chagas disease. J Biomol Screen. 2015;20(1):101–111. doi: 10.1177/1087057114553103 [DOI] [PubMed] [Google Scholar]
- 106.Hulpia F, Van Hecke K, da França Silva C, et al. Discovery of Novel 7-Aryl 7-Deazapurine 3′-deoxy-ribofuranosyl nucleosides with potent activity against Trypanosoma cruzi. J Med Chem. 2018;61(20):9287–9300. doi: 10.1021/acs.jmedchem.8b00999 [DOI] [PubMed] [Google Scholar]
- 107.Lin C, Hulpia F, da Silva CF, et al. Discovery of Pyrrolo[2,3- b]pyridine (1,7-Dideazapurine) nucleoside analogues as anti- trypanosoma cruzi agents. J Med Chem. 2019;62(19):8847–8865. doi: 10.1021/acs.jmedchem.9b01275 [DOI] [PubMed] [Google Scholar]
- 108.Aguirre-Alvarado C, Zaragoza-Martínez F, Rodríguez-Páez L, et al. Trypanocidal activity of the ethyl esters of N-propyl and N-isopropyl oxamates on intracellular amastigotes of Trypanosoma cruzi acute infected mice. J Enzyme Inhib Med Chem. 2010;25(1):111–115. doi: 10.3109/14756360903027741 [DOI] [PubMed] [Google Scholar]
- 109.Chena MA, Elizondo S, Rodríguez-Páez L, Nogueda B, Baeza I, Wong C. Trypanocidal activity of N-isopropyl oxamate on cultured epimastigotes and murine trypanosomiasis using different Trypanosoma cruzi strains. J Enzyme Inhib Med Chem. 2005;20(2):189–197. doi: 10.1080/14756360500047019 [DOI] [PubMed] [Google Scholar]
- 110.Wong-Baeza C, Nogueda-Torres B, Serna M, Meza-Toledo S, Baeza I, Wong C. Trypanocidal effect of the benzyl ester of N-propyl oxamate: a bi-potential prodrug for the treatment of experimental Chagas disease. BMC Pharmacol Toxicol. 2015;16:10. doi: 10.1186/s40360-015-0010-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nefertiti ASG, Batista MM, Da Silva PB, et al. In vitro and in vivo studies of the trypanocidal effect of novel quinolines. Antimicrob Agents Chemother. 2017;62(2):e01936–17. doi: 10.1128/AAC.01936-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vasconcelos JF, Meira CS, Silva DN, et al. Therapeutic effects of sphingosine kinase inhibitor N,N-dimethylsphingosine (DMS) in experimental chronic Chagas disease cardiomyopathy. Sci Rep. 2017;7(1):6171. doi: 10.1038/s41598-017-06275-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olmo F, Rotger C, Ramírez-Macías I, et al. Synthesis and biological evaluation of N,N’-squaramides with high in vivo efficacy and low toxicity: toward a low-cost drug against Chagas disease. J Med Chem. 2014;57(3):987–999. doi: 10.1021/jm4017015 [DOI] [PubMed] [Google Scholar]
- 114.Nagendar P, Gillespie JR, Herbst ZM, et al. Triazolopyrimidines and imidazopyridines: structure–activity relationships and in vivo efficacy for trypanosomiasis. ACS Med Chem Lett. 2019;10(1):105–110. doi: 10.1021/acsmedchemlett.8b00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Khare S, Nagle AS, Biggart A, et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature. 2016;537(7619):229–233. doi: 10.1038/nature19339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de LF Diniz, IS Caldas, PM M da Guedeset al. Effects of Ravuconazole treatment on parasite load and immune response in dogs experimentally infected with Trypanosoma cruzi. AAC. 2010;54(7):2979–2986. doi: 10.1128/AAC.01742-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Urbina JA, Payares G, Sanoja C, Lira R, Romanha AJ. In vitro and in vivo activities of ravuconazole on Trypanosoma cruzi, the causative agent of Chagas disease. Int J Antimicrob Agents. 2003;21(1):27–38. doi: 10.1016/S0924-8579(02)00273-X [DOI] [PubMed] [Google Scholar]
- 118.da Diniz LF, Mazzeti AL, Caldas IS, Ribeiro I, Bahia MT. Outcome of E1224-benznidazole combination treatment for infection with a multidrug-resistant trypanosoma cruzi strain in mice. Antimicrob Agents Chemother. 2018;62(6):e00401–18. doi: 10.1128/AAC.00401-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guedes-da-silva FH, Da batista GJ, Da Silva CF, et al. Successful aspects of the coadministration of Sterol 14α-Demethylase Inhibitor VFV and benznidazole in experimental mouse models of chagas disease caused by the drug-resistant strain of Trypanosoma cruzi. ACS Infect Dis. 2019;5(3):365–371. doi: 10.1021/acsinfecdis.8b00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bastos JK, Albuquerque S, Silva MLA. Evaluation of the Trypanocidal activity of lignans isolated from the leaves of zanthoxylum naranjillo. Planta Med. 1999;65(6):541–544. doi: 10.1055/s-1999-14012 [DOI] [PubMed] [Google Scholar]
- 121.Ferreira ME, Nakayama H, de Arias AR, et al. Effects of canthin-6-one alkaloids from Zanthoxylum chiloperone on Trypanosoma cruzi-infected mice. J Ethnopharmacol. 2007;109(2):258–263. doi: 10.1016/j.jep.2006.07.028 [DOI] [PubMed] [Google Scholar]
- 122.Jimenez-Coello M, Acosta-Viana KY, Guzman-Marin E, Perez Gonzalez C, Salud Perez GM. Anti-trypanosomal activity of (8-hydroxymethylen)-trieicosanyl acetate against infective forms of Trypanosoma cruzi. Pharm Biol. 2010;48(6):666–671. doi: 10.3109/13880200903241853 [DOI] [PubMed] [Google Scholar]
- 123.Ferreira ME, Cebrián-Torrejón G, Corrales AS, et al. Zanthoxylum chiloperone leaves extract: first sustainable Chagas disease treatment. J Ethnopharmacol. 2011;133(3):986–993. doi: 10.1016/j.jep.2010.11.032 [DOI] [PubMed] [Google Scholar]
- 124.Marín C, Ramírez-Macías I, López-Céspedes A, et al. In vitro and in vivo trypanocidal activity of flavonoids from Delphinium staphisagria against Chagas disease. J Nat Prod. 2011;74(4):744–750. doi: 10.1021/np1008043 [DOI] [PubMed] [Google Scholar]
- 125.Jiménez-Coello M, Acosta-Viana K, Pérez M, Del Guzmán-Marín S. In vivo activity of (8-Hydroxymethylen)-Trieicosanyl Acetate against Trypanosoma cruzi during acute phase of the infection. Afr J Trad Compl Alt Med. 2011;8(5S). doi: 10.4314/ajtcam.v8i5S.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meira CS, Guimarães ET, Bastos TM, et al. Physalins B and F, seco-steroids isolated from Physalis angulata L., strongly inhibit proliferation, ultrastructure and infectivity of Trypanosoma cruzi. Parasitology. 2013;140(14):1811–1821. doi: 10.1017/S0031182013001297 [DOI] [PubMed] [Google Scholar]
- 127.Jimenez-Coello M, Acosta-Viana KY, Ortega-Pacheco A, Perez-Gutierrez S, In Vivo G-ME. Antiprotozoal activity of the chloroform extract from carica papaya seeds against amastigote stage of trypanosoma cruzi during indeterminate and chronic phase of infection. Evid Based Complement Alternat Med. 2014;2014:458263. doi: 10.1155/2014/458263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Varela J, Serna E, Torres S, et al. In vivo anti-Trypanosoma cruzi activity of hydro-ethanolic extract and isolated active principles from Aristeguietia glutinosa and mechanism of action studies. Molecules. 2014;19(6):8488–8502. doi: 10.3390/molecules19068488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.da Rocha CQ, Queiroz EF, Meira CS, et al. Dimeric flavonoids from Arrabidaea brachypoda and assessment of their anti-Trypanosoma cruzi activity. J Nat Prod. 2014;77(6):1345–1350. doi: 10.1021/np401060j [DOI] [PubMed] [Google Scholar]
- 130.Meira CS, Guimarães ET, Jaf DS, et al. In vitro and in vivo antiparasitic activity of Physalis angulata L. concentrated ethanolic extract against Trypanosoma cruzi. Phytomedicine. 2015;22(11):969–974. doi: 10.1016/j.phymed.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 131.Lozano E, Strauss M, Spina R, et al. The in vivo trypanocidal effect of the diterpene 5-epi-icetexone obtained from Salvia gilliesii. Parasitol Int. 2016;65(1):23–26. doi: 10.1016/j.parint.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 132.García-Huertas P, Olmo F, Sánchez-Moreno M, Dominguez J, Chahboun R, Triana-Chávez O. Activity in vitro and in vivo against Trypanosoma cruzi of a furofuran lignan isolated from Piper jericoense. Exp Parasitol. 2018;189:34–42. doi: 10.1016/j.exppara.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 133.Tempone AG, Sartorelli P, Mady C, Fernandes F. Natural products to anti-trypanosomal drugs: an overview of new drug prototypes for American Trypanosomiasis. Cardiovasc Hematol Agents Med Chem. 2007;5(3):222–235. doi: 10.2174/187152507781058726 [DOI] [PubMed] [Google Scholar]
- 134.Varela J, Cerecetto H, Slowed GM. Development of natural products for chagas disease, how to move forward?. In: Nissapatorn V, Oz HS editors. . Chagas Disease - Basic Investigations and Challenges. InTech;2018. doi: 10.5772/intechopen.77234 [DOI] [Google Scholar]
- 135.Llurba Montesino N, Kaiser M, Brun R, Schmidt TJ. Search for antiprotozoal activity in herbal medicinal preparations; new natural leads against neglected tropical diseases. Molecules. 2015;20(8):14118–14138. doi: 10.3390/molecules200814118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mishina YV, Krishna S, Haynes RK, Meade JC. Artemisinins inhibit Trypanosoma cruzi and Trypanosoma brucei rhodesiense in vitro growth. AAC. 2007;51(5):1852–1854. doi: 10.1128/AAC.01544-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chadwick M, Trewin H, Gawthrop F, Sesquiterpenoids Lactones: WC. Benefits to plants and people. Int J Mol Sci. 2013;14(6):12780–12805. doi: 10.3390/ijms140612780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Branquinho RT, Mosqueira VCF, de Oliveira-silva JCV, Simões-Silva MR, Saúde-Guimarães DA, de Lana M. Sesquiterpene lactone in nanostructured parenteral dosage form is efficacious in experimental Chagas disease. Antimicrob Agents Chemother. 2014;58(4):2067–2075. doi: 10.1128/AAC.00617-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cgc DM, Branquinho RT, Oliveira MT, et al. Efficacy of lychnopholide polymeric nanocapsules after oral and intravenous administration in murine experimental chagas disease. Antimicrob Agents Chemother. 2016;60(9):5215–5222. doi: 10.1128/AAC.00178-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Branquinho RT, Roy J, Farah C, et al. Biodegradable polymeric nanocapsules prevent cardiotoxicity of anti-Trypanosomal Lychnopholide. Sci Rep. 2017;7:44998. doi: 10.1038/srep44998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Laurella LC, Cerny N, Bivona AE, et al. Assessment of sesquiterpene lactones isolated from Mikania plants species for their potential efficacy against Trypanosoma cruzi and Leishmania sp. Schallig HDFH, ed. PLoS Negl Trop Dis. 2017;11(9):e0005929. doi: 10.1371/journal.pntd.0005929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Alberti AS, Cerny N, Bivona A, Cazorla SI. Antitrypanosomal and antileishmanial activities. In: Sülsen VP, Martino VS editors. Sesquiterpene Lactones. Springer International Publishing; 2018:175–196. doi: 10.1007/978-3-319-78274-4_8. [DOI] [Google Scholar]
- 143.Gonçalves-Santos E, Vilas-Boas DF, Diniz LF, et al. Sesquiterpene lactone potentiates the immunomodulatory, antiparasitic and cardioprotective effects on anti-Trypanosoma cruzi specific chemotherapy. Int Immunopharmacol. 2019;77:105961. doi: 10.1016/j.intimp.2019.105961 [DOI] [PubMed] [Google Scholar]
- 144.Branquinho RT, de Mello CGC, Oliveira MT, et al. Lychnopholide in Poly(D,L-Lactide)- Block -polyethylene glycol nanocapsules cures infection with a drug-resistant Trypanosoma cruzi strain at acute and chronic phases. Antimicrob Agents Chemother. 2020;64(4):e01937. doi: 10.1128/AAC.01937-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Milagre MM, Branquinho RT, Gonçalves MF, et al. Activity of the sesquiterpene lactone goyazensolide against Trypanosoma cruzi in vitro and in vivo. Parasitology. 2020;147(1):108–119. doi: 10.1017/S0031182019001276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McCabe RE, Remington JS, Araujo FG. In vitro and in vivo effects of itraconazole against Trypanosoma cruzi. Am J Trop Med Hyg. 1986;35(2):280–284. doi: 10.4269/ajtmh.1986.35.280 [DOI] [PubMed] [Google Scholar]
- 147.McCabe RE, Remington JS, Araujo FG. Ketoconazole promotes parasitological cure of mice infected with Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1987;81(4):613–615. doi: 10.1016/0035-9203(87)90430-5 [DOI] [PubMed] [Google Scholar]
- 148.Goad LJ, Berens RL, Marr JJ, Beach DH, Holz GG. The activity of ketoconazole and other azoles against Trypanosoma cruzi: biochemistry and chemotherapeutic action in vitro. Mol Biochem Parasitol. 1989;32(2–3):179–189. doi: 10.1016/0166-6851(89)90069-8 [DOI] [PubMed] [Google Scholar]
- 149.Lazardi K, Urbina JA, de Souza W. Ultrastructural alterations induced by two ergosterol biosynthesis inhibitors, ketoconazole and terbinafine, on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob Agents Chemother. 1990;34(11):2097–2105. doi: 10.1128/aac.34.11.2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Araujo MSS, Martins-Filho OA, Pereira MES, Brener Z. A combination of benznidazole and ketoconazole enhances efficacy of chemotherapy of experimental Chagas’ disease. J Antimicrob Chemother. 2000;45(6):819–824. doi: 10.1093/jac/45.6.819 [DOI] [PubMed] [Google Scholar]
- 151.Molina J. In vivo activity of the bis-triazole D0870 against drug-susceptible and drug-resistant strains of the protozoan parasite Trypanosoma cruzi. J Antimicrob Chemother. 2000;46(1):137–140. doi: 10.1093/jac/46.1.137 [DOI] [PubMed] [Google Scholar]
- 152.de Toledo O, MT Bahia, CM Carneiroet al. Chemotherapy with benznidazole and itraconazole for mice infected with different Trypanosoma cruzi clonal genotypes. Antimicrob Agents Chemother. 2003;47(1):223–230. doi: 10.1128/aac.47.1.223-230.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.PMM da Guedes, JA Urbina, de Lana Met al. Activity of the New Triazole Derivative Albaconazole against Trypanosoma (Schizotrypanum) cruzi in Dog Hosts. AAC. 2004;48(11):4286–4292. doi: 10.1128/AAC.48.11.4286-4292.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Benaim G, Sanders JM, Garcia-Marchán Y, et al. Amiodarone has intrinsic anti- Trypanosoma cruzi activity and acts synergistically with posaconazole †. J Med Chem. 2006;49(3):892–899. doi: 10.1021/jm050691f [DOI] [PubMed] [Google Scholar]
- 155.Cencig S, Coltel N, Truyens C, Carlier Y. Evaluation of benznidazole treatment combined with nifurtimox, posaconazole or AmBisome® in mice infected with Trypanosoma cruzi strains. Int J Antimicrob Agents. 2012;40(6):527–532. doi: 10.1016/j.ijantimicag.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 156. .L F de Diniz, JA Urbina, IM de Andradeet al. Benznidazole and posaconazole in experimental chagas disease: positive interaction in concomitant and sequential treatments. Rodrigues MM, ed. PLoS Negl Trop Dis. 2013;7(8):e2367. doi: 10.1371/journal.pntd.0002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Assíria Fontes Martins T, de Figueiredo Diniz L, Mazzeti AL, et al. Benznidazole/Itraconazole combination treatment enhances anti-trypanosoma cruzi activity in experimental chagas disease. Costa FTM, ed. PLoS ONE. 2015;10(6):e0128707. doi: 10.1371/journal.pone.0128707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Buckner FS, Bahia MT, Suryadevara PK, et al. Pharmacological characterization, structural studies, and in vivo activities of anti-chagas disease lead compounds derived from Tipifarnib. Antimicrob Agents Chemother. 2012;56(9):4914–4921. doi: 10.1128/AAC.06244-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hucke O, Gelb MH, Verlinde CLMJ, Buckner FS. The protein farnesyltransferase inhibitor tipifarnib as a new lead for the development of drugs against Chagas Disease. J Med Chem. 2005;48(17):5415–5418. doi: 10.1021/jm050441z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kraus JM, Tatipaka HB, McGuffin SA, et al. Second generation analogues of the cancer drug clinical candidate tipifarnib for anti-chagas disease drug discovery. J Med Chem. 2010;53(10):3887–3898. doi: 10.1021/jm9013136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bellera CL, Balcazar DE, Vanrell MC, et al. Computer-guided drug repurposing: identification of trypanocidal activity of clofazimine, benidipine and saquinavir. Eur J Med Chem. 2015;93:338–348. doi: 10.1016/j.ejmech.2015.01.065 [DOI] [PubMed] [Google Scholar]
- 162.Sbaraglini ML, Bellera CL, Fraccaroli L, et al. Novel cruzipain inhibitors for the chemotherapy of chronic Chagas disease. Int J Antimicrob Agents. 2016;48(1):91–95. doi: 10.1016/j.ijantimicag.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 163.Benaim G, Paniz-Mondolfi AE, Sordillo EM, Martinez-Sotillo N. Disruption of intracellular calcium homeostasis as a therapeutic target against Trypanosoma cruzi. Front Cell Infect Microbiol. 2020;10:46. doi: 10.3389/fcimb.2020.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sbaraglini ML, Bellera CL, Quarroz Braghini J, et al. Combined therapy with Benznidazole and repurposed drugs Clofazimine and Benidipine for chronic Chagas disease. Eur J Med Chem. 2019;184:111778. doi: 10.1016/j.ejmech.2019.111778 [DOI] [PubMed] [Google Scholar]
- 165.Simões-Silva MR, De Araújo JS, Peres RB, et al. Repurposing strategies for Chagas disease therapy: the effect of imatinib and derivatives against Trypanosoma cruzi. Parasitology. 2019;146(8):1006–1012. doi: 10.1017/S0031182019000234 [DOI] [PubMed] [Google Scholar]
- 166.Ferreira DD, Mesquita JT, da Costa Silva TA, et al. Efficacy of sertraline against Trypanosoma cruzi: an in vitro and in silico study. J Venom Anim Toxins Incl Trop Dis. 2018;24:30. doi: 10.1186/s40409-018-0165-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Planer JD, Hulverson MA, Arif JA, Ranade RM, Don R, Buckner FS. Synergy testing of FDA-approved drugs identifies potent drug combinations against Trypanosoma cruzi. PLoS Negl Trop Dis. 2014;8(7):e2977. doi: 10.1371/journal.pntd.0002977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marr JJ, Berens RL, Nelson DJ. Antitrypanosomal effect of allopurinol: conversion in vivo to aminopyrazolopyrimidine nucleotides by Trypanosoma curzi. Science. 1978;201(4360):1018–1020. doi: 10.1126/science.356267 [DOI] [PubMed] [Google Scholar]
- 169.Avila J, Avila A. Trypanosoma cruzi: allopurinol in the treatment of mice with experimental acute Chagas disease. Exp Parasitol. 1981;51(2):204–208. doi: 10.1016/0014-4894(81)90109-0 [DOI] [PubMed] [Google Scholar]
- 170.Gobbi P, Lo Presti MS, Fernández AR, et al. Allopurinol is effective to modify the evolution of Trypanosoma cruzi infection in mice. Parasitol Res. 2007;101(5):1459–1462. doi: 10.1007/s00436-007-0644-2 [DOI] [PubMed] [Google Scholar]
- 171.Raviolo MA, Solana ME, Novoa MM, Gualdesi MS, Alba-Soto CD, Briñón MC. Synthesis, physicochemical properties of allopurinol derivatives and their biological activity against Trypanosoma cruzi. Eur J Med Chem. 2013;69:455–464. doi: 10.1016/j.ejmech.2013.08.045 [DOI] [PubMed] [Google Scholar]
- 172.Mazzeti AL, de Diniz LF, Gonçalves KR, et al. Synergic effect of allopurinol in combination with nitroheterocyclic compounds against Trypanosoma cruzi. Antimicrob Agents Chemother. 2019;63(6):e02264–18. doi: 10.1128/AAC.02264-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.da Silva MTA, Silva-Jardim I, Portapilla GB, et al. In vivo and in vitro auranofin activity against Trypanosoma cruzi: possible new uses for an old drug. Exp Parasitol. 2016;166:189–193. doi: 10.1016/j.exppara.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 174.Rivarola HW, Bustamante JM, Lo Presti S, et al. Trypanosoma cruzi: chemotherapeutic effects of clomipramine in mice infected with an isolate obtained from an endemic area. Exp Parasitol. 2005;111(2):80–86. doi: 10.1016/j.exppara.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 175.Fauro R, Lo Presti S, Bazan C, et al. Use of clomipramine as chemotherapy of the chronic phase of Chagas disease. Parasitology. 2013;140(7):917–927. doi: 10.1017/S0031182013000103 [DOI] [PubMed] [Google Scholar]
- 176.Cunha ELA, da Torchelsen FKVS, Cunha LM, et al. Benznidazole, itraconazole and their combination in the treatment of acute experimental chagas disease in dogs. Exp Parasitol. 2019;204:107711. doi: 10.1016/j.exppara.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 177.Moreira da Silva R, LT Oliveira, Silva Barcellos NM, de Souza J, de Lana M. Preclinical monitoring of drug association in experimental chemotherapy of chagas’ disease by a new HPLC-UV method. Antimicrob Agents Chemother. 2012;56(6):3344–3348. doi: 10.1128/AAC.05785-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cunha ELA, da Torchelsen FKVS, Cunha LM, et al. Benznidazole, itraconazole and their combination in the treatment of acute experimental Chagas disease in dogs. MethodsX. 2019;6:2544–2552. doi: 10.1016/j.mex.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Echeverría LE, González CI, Hernandez JCM, et al. Efficacy of the Benznidazole+Posaconazole combination therapy in parasitemia reduction: an experimental murine model of acute Chagas. Rev Soc Bras Med Trop. 2020;53:e20190477. doi: 10.1590/0037-8682-0477-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gulin JEN, Eagleson MA, López-Muñoz RA, Solana ME, Altcheh J, García-Bournissen F. In vitro and in vivo activity of voriconazole and benznidazole combination on trypanosoma cruzi infection models. Acta Trop. 2020;211:105606. doi: 10.1016/j.actatropica.2020.105606 [DOI] [PubMed] [Google Scholar]
- 181.Rial MS, Scalise ML, López Alarcón M, et al. Experimental combination therapy using low doses of benznidazole and allopurinol in mouse models of Trypanosoma cruzi chronic infection. Parasitology. 2019;146(3):305–313. doi: 10.1017/S0031182018001567 [DOI] [PubMed] [Google Scholar]
- 182.Grosso NL, Alarcon ML, Bua J, Laucella SA, Riarte A, Fichera LE. Combined treatment with benznidazole and allopurinol in mice infected with a virulent Trypanosoma cruzi isolate from Nicaragua. Parasitology. 2013;140(10):1225–1233. doi: 10.1017/S0031182013000176 [DOI] [PubMed] [Google Scholar]
- 183.Veiga-Santos P, Barrias ES, Santos JFC, et al. Effects of amiodarone and posaconazole on the growth and ultrastructure of Trypanosoma cruzi. Int J Antimicrob Agents. 2012;40(1):61–71. doi: 10.1016/j.ijantimicag.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 184.Strauss M, Lo Presti MS, Bazán PC, et al. Clomipramine and benznidazole association for the treatment of acute experimental Trypanosoma cruzi infection. Parasitol Int. 2013;62(3):293–299. doi: 10.1016/j.parint.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 185.Cevey ÁC, Mirkin GA, Donato M, et al. Treatment with Fenofibrate plus a low dose of Benznidazole attenuates cardiac dysfunction in experimental Chagas disease. Int J Parasitol Drugs Drug Resist. 2017;7(3):378–387. doi: 10.1016/j.ijpddr.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.González-Herrera F, Cramer A, Pimentel P, et al. Simvastatin attenuates endothelial activation through 15-Epi-Lipoxin A4 production in murine chronic chagas cardiomyopathy. Antimicrob Agents Chemother. 2017;61(3):e02137-16, e02137-16. doi: 10.1128/AAC.02137-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Santeliz S, Caicedo P, Giraldo E, et al. Dipyridamole potentiated the trypanocidal effect of nifurtimox and improved the cardiac function in NMRI mice with acute chagasic myocarditis. Mem Inst Oswaldo Cruz. 2017;112(9):596–608. doi: 10.1590/0074-02760160499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Providello MV, Carneiro ZA, Portapilla GB, et al. Benefits of ascorbic acid in association with low-dose benznidazole in treatment of chagas disease. Antimicrob Agents Chemother. 2018;62(9):e00514–18. doi: 10.1128/AAC.00514-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Strauss M, Rodrigues JHS, Lo Presti MS, et al. In vitro and in vivo drug combination for the treatment of Trypanosoma cruzi infection: a multivariate approach. Exp Parasitol. 2018;189:19–27. doi: 10.1016/j.exppara.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 190.Madigan R, Majoy S, Ritter K, et al. Investigation of a combination of amiodarone and itraconazole for treatment of American trypanosomiasis (Chagas disease) in dogs. J Am Vet Med Assoc. 2019;255(3):317–329. doi: 10.2460/javma.255.3.317 [DOI] [PubMed] [Google Scholar]
- 191.Sass G, Madigan RT, Joubert L-M, et al. A combination of itraconazole and amiodarone is highly effective against trypanosoma cruzi infection of human stem cell-derived cardiomyocytes. Am J Trop Med Hyg. 2019;101(2):383–391. doi: 10.4269/ajtmh.19-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rocha Simões-Silva M, Brandão peres R, Britto C, et al. Impact of levamisole in co-administration with benznidazole on experimental Chagas disease. Parasitology. 2019;146(8):1055–1062. doi: 10.1017/S0031182019000374 [DOI] [PubMed] [Google Scholar]
- 193.Pereira RS, Malvezi AD, Lovo-Martins MI, et al. Combination therapy using benznidazole and aspirin during the acute phase of experimental chagas disease prevents cardiovascular dysfunction and decreases typical cardiac lesions in the chronic Phase. Antimicrob Agents Chemother. 2020;64(7):e00069–20. doi: 10.1128/AAC.00069-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Machado YA, Bahia MT, Caldas IS, et al. Amlodipine increases the therapeutic potential of ravuconazole upon Trypanosoma cruzi Infection. Antimicrob Agents Chemother. 2020;64(8):e02497–19. doi: 10.1128/AAC.02497-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.DNDi. The BENDITA study | DNDi. Drugs for Neglected Diseases initiative (DNDi). 2019. Accessed December16, 2020. https://dndi.org/publications/2019/bendita-study/
- 196.Filardi LS, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans R Soc Trop Med Hyg. 1987;81(5):755–759. doi: 10.1016/0035-9203(87)90020-4 [DOI] [PubMed] [Google Scholar]
- 197.Rial MS, Arrúa EC, Natale MA, et al. Efficacy of continuous versus intermittent administration of nanoformulated benznidazole during the chronic phase of Trypanosoma cruzi Nicaragua infection in mice. J Antimicrob Chemother. 2020;75(7):1906–1916. doi: 10.1093/jac/dkaa101 [DOI] [PubMed] [Google Scholar]
- 198.Perin L, da Fonseca KS, De Carvalho TV, et al. Low-dose of benznidazole promotes therapeutic cure in experimental chronic Chagas’ disease with absence of parasitism in blood, heart and colon. Exp Parasitol. 2020;210:107834. doi: 10.1016/j.exppara.2020.107834 [DOI] [PubMed] [Google Scholar]
- 199.Bustamante JM, Sanchez-Valdez F, Padilla AM, White B, Wang W, Tarleton RL. A modified drug regimen clears active and dormant trypanosomes in mouse models of Chagas disease. Sci Transl Med. 2020;12:567. doi: 10.1126/scitranslmed.abb7656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Molina I, Perin L, Aviles AS, et al. The effect of benznidazole dose among the efficacy outcome in the murine animal model. A quantitative integration of the literature. Acta Trop. 2020;201:105218. doi: 10.1016/j.actatropica.2019.105218 [DOI] [PubMed] [Google Scholar]
- 201.Mazzeti AL, de Diniz LF, Gonçalves KR, et al. Time and dose-dependence evaluation of nitroheterocyclic drugs for improving efficacy following Trypanosoma cruzi infection: a pre-clinical study. Biochem Pharmacol. 2018;148:213–221. doi: 10.1016/j.bcp.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 202.Mosqueira VCF, Mazzeti AL, Bahia MT. Nanomedicines against Chagas disease. In: Applications of Nanobiotechnology for Neglected Tropical Diseases. Elsevier; 2021:169–189. doi: 10.1016/B978-0-12-821100-7.00008-X [DOI] [Google Scholar]
- 203.Davies C, Simonazzi A, Micheloud JF, et al. Benznidazole/Poloxamer 407 solid dispersion as a new strategy to improve the treatment of experimental trypanosoma cruzi infection. J Parasitol. 2020;106(3):323–333. doi: 10.1645/19-80 [DOI] [PubMed] [Google Scholar]
- 204.Manarin R, Lamas MC, Bottasso E, Serra E, Revelli S, Salomón CJ. Efficacy of novel benznidazole solutions during the experimental infection with Trypanosoma cruzi. Parasitol Int. 2013;62(1):79–81. doi: 10.1016/j.parint.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 205.Yardley V, Croft SL. In vitro and in vivo activity of amphotericin B-lipid formulations against experimental Trypanosoma cruzi infections. Am J Trop Med Hyg. 1999;61(2):193–197. doi: 10.4269/ajtmh.1999.61.193 [DOI] [PubMed] [Google Scholar]
- 206.Cencig S, Coltel N, Truyens C, Carlier Y. Parasitic loads in tissues of mice infected with Trypanosoma cruzi and treated with AmBisome. PLoS Negl Trop Dis. 2011;5(6):e1216. doi: 10.1371/journal.pntd.0001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Clemons KV, Sobel RA, Martinez M, Correa-Oliveira R, Stevens DA. Lack of efficacy of liposomal Amphotericin B against acute and chronic Trypanosoma cruzi infection in mice. Am J Trop Med Hyg. 2017;97(4):1141–1146. doi: 10.4269/ajtmh.16-0975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Morilla MJ, Montanari JA, Prieto MJ, Lopez MO, Petray PB, Romero EL. Intravenous liposomal benznidazole as trypanocidal agent: increasing drug delivery to liver is not enough. Int J Pharm. 2004;278(2):311–318. doi: 10.1016/j.ijpharm.2004.03.025 [DOI] [PubMed] [Google Scholar]
- 209.Morilla MJ, Montanari J, Frank F, et al. Etanidazole in pH-sensitive liposomes: design, characterization and in vitro/in vivo anti-Trypanosoma cruzi activity. J Controlled Release. 2005;103(3):599–607. doi: 10.1016/j.jconrel.2004.12.012 [DOI] [PubMed] [Google Scholar]
- 210.Oliveira LT, Mosqueira VCF, Castanheira R WO2015039199 Micro- and nanostructured pharmaceutical and veterinary compositions, containing benznidazole and derivatives thereof, which form micro- and nanostructures in the gastrointestinal tract, and biological uses thereof. Patent Scop e. 2014. Available from: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015039199 Accessed November26, 2020.
- 211.Spósito PÁF, Mazzeti Silva AL, De oliveira Faria C, et al. Ravuconazole self-emulsifying delivery system: in vitro activity against Trypanosoma cruzi amastigotes and in vivo toxicity. IJN. 2017;12:3785–3799. doi: 10.2147/IJN.S133708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mazzeti AL, Oliveira LT, Gonçalves KR, Schaun GC, Mosqueira VCF, Bahia MT. Benznidazole self-emulsifying delivery system: a novel alternative dosage form for Chagas disease treatment. Eur J Pharm Sci. 2020;145:105234. doi: 10.1016/j.ejps.2020.105234 [DOI] [PubMed] [Google Scholar]
- 213.Esteva MI, Rial MS, Scalise ML, Fichera LE, Arrúa EC, Salomon CJ. Promising efficacy of benznidazole nanoparticles in acute Trypanosoma cruzi murine model: in-vitro and in-vivo studies. Am J Trop Med Hyg. 2016;95(2):388–393. doi: 10.4269/ajtmh.15-0889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Abriata JP, Eloy JO, Riul TB, Campos PM, Baruffi MD, Marchetti JM. Poly-epsilon-caprolactone nanoparticles enhance ursolic acid in vivo efficacy against Trypanosoma cruzi infection. Mater Sci Eng C Mater Biol Appl. 2017;77:1196–1203. doi: 10.1016/j.msec.2017.03.266 [DOI] [PubMed] [Google Scholar]
- 215.Saraiva J, Lira AAM, Esperandim VR, et al. (-)-Hinokinin-loaded poly(D,-lactide-co-glycolide) microparticles for Chagas disease. Parasitol Res. 2010;106(3):703–708. doi: 10.1007/s00436-010-1725-1 [DOI] [PubMed] [Google Scholar]
- 216.Molina J. Cure of experimental Chagas’ disease by the bis-triazole DO870 incorporated into “stealth” polyethyleneglycol-polylactide nanospheres. J Antimicrob Chemother. 2001;47(1):101–104. doi: 10.1093/jac/47.1.101 [DOI] [PubMed] [Google Scholar]
- 217.Rolón M, Serrano DR, Lalatsa A, et al. Engineering oral and parenteral amorphous Amphotericin B Formulations against experimental Trypanosoma cruzi infections. Mol Pharm. 2017;14(4):1095–1106. doi: 10.1021/acs.molpharmaceut.6b01034 [DOI] [PubMed] [Google Scholar]
- 218.Parra FL, Frank FM, Alliani BF, Romero EL, Petray PB. Imiquimod-loaded nanoarchaeosomes as a promising immunotherapy against Trypanosoma cruzi infection. Colloids Surf B Biointerfaces. 2020;189:110850. doi: 10.1016/j.colsurfb.2020.110850 [DOI] [PubMed] [Google Scholar]
- 219.Campos MCO, Leon LL, Taylor MC, Kelly JM. Benznidazole-resistance in Trypanosoma cruzi: evidence that distinct mechanisms can act in concert. Mol Biochem Parasitol. 2014;193(1):17–19. doi: 10.1016/j.molbiopara.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Petravicius PO, Costa-Martins AG, Silva MN, et al. Mapping benznidazole resistance in trypanosomatids and exploring evolutionary histories of nitroreductases and ABCG transporter protein sequences. Acta Trop. 2019;200:105161. doi: 10.1016/j.actatropica.2019.105161 [DOI] [PubMed] [Google Scholar]
- 221.Zingales B. Trypanosoma cruzi genetic diversity: something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018;184:38–52. doi: 10.1016/j.actatropica.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 222.Franco J, Ferreira RC, Ienne S, Zingales B. ABCG-like transporter of Trypanosoma cruzi involved in benznidazole resistance: gene polymorphisms disclose inter-strain intragenic recombination in hybrid isolates. Infect Genet Evol. 2015;31:198–208. doi: 10.1016/j.meegid.2015.01.030 [DOI] [PubMed] [Google Scholar]
- 223. .SMF Murta, MA Krieger, LR Montenegroet al. Deletion of copies of the gene encoding old yellow enzyme (TcOYE), a NAD(P)H flavin oxidoreductase, associates with in vitro-induced benznidazole resistance in Trypanosoma cruzi. Mol Biochem Parasitol. 2006;146(2):151–162. doi: 10.1016/j.molbiopara.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 224.Nogueira FB, Krieger MA, Nirdé P, Goldenberg S, Romanha AJ, Murta SMF. Increased expression of iron-containing superoxide dismutase-A (TcFeSOD-A) enzyme in Trypanosoma cruzi population with in vitro-induced resistance to benznidazole. Acta Trop. 2006;100(1–2):119–132. doi: 10.1016/j.actatropica.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 225.Dumoulin PC, Vollrath J, Tomko SS, Wang JX, Burleigh B. Glutamine metabolism modulates azole susceptibility in Trypanosoma cruzi amastigotes. eLife. 2020;9:e60226. doi: 10.7554/eLife.60226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sánchez-Valdéz FJ, Padilla A, Wang W, Orr D, Tarleton RL. Spontaneous dormancy protects Trypanosoma cruzi during extended drug exposure. Elife. 2018;7. doi: 10.7554/eLife.34039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Romanha AJ, de Castro SL, De Soeiro M, et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz. 2010;105(2):233–238. doi: 10.1590/S0074-02762010000200022 [DOI] [PubMed] [Google Scholar]
- 228.Vannier-Santos MA, Brunoro VG, de Nazaré C, et al.. Parasite, compartments, and molecules: trick versus treatment on chagas disease. In: editor, De Souza W. Biology of Trypanosoma Cruzi. IntechOpen; 2019. doi: 10.5772/intechopen.84472 [DOI] [Google Scholar]