Abstract
Data derived from transcranial magnetic stimulation (TMS) studies suggest that transcallosal inhibition mechanisms between the primary motor cortex of both hemispheres may contribute to the reduced motor performance of stroke patients. We here investigated the potential of modulating pathological interactions between cortical motor areas by means of repetitive TMS using functional magnetic resonance imaging (fMRI) and dynamic causal modeling (DCM). Eleven subacute stroke patients were scanned 1–3 months after symptom onset while performing whole hand fist closure movements. After a baseline scan, patients were stimulated with inhibitory 1-Hz rTMS applied over two different locations: (i) vertex (control stimulation) and (ii) primary motor cortex (M1) of the unaffected (contralesional) hemisphere. Changes in the endogenous and task-dependent effective connectivity were assessed by DCM of a bilateral network comprising M1, lateral premotor cortex, and the supplementary motor area (SMA). The results showed that rTMS applied over contralesional M1 significantly improved the motor performance of the paretic hand. The connectivity analysis revealed that the behavioral improvements were significantly correlated with a reduction of the negative influences originating from contralesional M1 during paretic hand movements. Concurrently, endogenous coupling between ipsilesional SMA and M1 was significantly enhanced only after rTMS applied over contralesional M1. Therefore, rTMS applied over contralesional M1 may be used to transiently remodel the disturbed functional network architecture of the motor system. The connectivity analyses suggest that both a reduction of pathological transcallosal influences (originating from contralesional M1) and a restitution of ipsilesional effective connectivity between SMA and M1 underlie improved motor performance.
Keywords: FMRI, DCM, Rehabilitation, Transcranial magnetic stimulation
Introduction
Focal cerebral ischemia within the territory of the middle cerebral artery (MCA) may cause serious disturbances within the motor system resulting in neurological deficits such as hemiparesis or loss of dexterity. Since the brain's capacity to compensate for such an ischemic lesion is limited, the majority of affected patients are left with a permanent motor deficit (Komitova et al., 2006; Kwakkel et al., 2002). However, not all of the observed motor deficits may result from direct damage to motor neurons and their descending axons. Rather, a growing body of evidence suggests that abnormal interactions among cortical regions remote from the ischemic lesion might also constitute an important pathophysiological factor for disability after stroke. For example, data obtained from transcranial magnetic stimulation (TMS) of the primary motor cortex (M1) suggest that motor output from the lesioned hemisphere may be additionally impaired by pathologically enhanced inhibition from the contralesional, i.e., intact motor cortex (Duque et al., 2005; Murase et al., 2004). This notion was recently corroborated using dynamic causal modeling (DCM) (Friston et al., 2003) to assess effective connectivity based upon functional magnetic resonance imaging (fMRI) data obtained in stroke patients (Grefkes et al., 2008b). Crucially, not only interhemispheric interactions among both M1, but also intra-hemispheric coupling within the lesioned hemisphere, e.g., among the supplementary motor area (SMA) and M1 (Grefkes et al., 2008b), was shown to be critically disturbed following subcortical stroke.
Repetitive transcranial magnetic stimulation (rTMS) protocols can be used to transiently modulate cortical excitability of motor areas (Maeda et al., 2000; Mansur et al., 2005; Nowak et al., 2008). This non-invasive technical approach to improve motor deficits after a stroke is based on the interhemispheric competition model for sensory and motor processing. Within this concept the equilibrium of transcallosal inhibition between the motor areas of the two hemispheres is disturbed due to the lesion with enhanced excitability of M1 in the unaffected hemisphere and abnormally increased interhemispheric inhibition exerted upon ipsilesional M1 when the paretic hand is moved (Duque et al., 2005; Murase et al., 2004). Indeed, proof-of-principle studies have been able to demonstrate that inhibitory 1-Hz rTMS applied over contralesional M1 may improve dexterity of the stroke-affected hand (Mansur et al., 2005; Nowak et al., 2008). Moreover, functional imaging data showed that such protocols may significantly reduce pathological over-activity in contralesional M1 (Nowak et al., 2008), and some studies also demonstrated distant rTMS effect, i.e., enhanced neural activity in ipsilesional M1 (Conchou et al., 2009). To date, however, the mechanisms underlying such rTMS induced effects and their influences upon the functional interactions within the motor networks remain poorly understood.
We hypothesized that the neuromodulatory effects induced by rTMS applied over contralesional M1 of stroke patients might be due to changes in both the region underneath the stimulation site and interconnected areas as demonstrated in a number of online rTMS-PET/fMRI studies (Lee et al., 2003; Chouinard et al., 2003; Bestmann et al., 2005; Conchou et al., 2009). We, hence, assumed that rTMS over contralesional M1 might modulate the connectivity from the contralesional primary motor cortex, in particular, suppress abnormal influences from this region on ipsilesional motor areas. This would then enable a more effective processing of motor-related information within the lesioned hemisphere.
Such changes in interregional interactions can be addressed by using fMRI and computational approaches like DCM estimating the intrinsic/endogenous and task-dependent influences that a particular area exerts over the activity of another area, i.e., effective connectivity (Friston et al., 2003; Grefkes et al., 2009; Penny et al., 2004; Stephan et al., 2007a,b). Accordingly, we acquired functional magnetic resonance imaging (fMRI) data in stroke patients who had suffered from a subcortical MCA stroke, and computed effective connectivity between key motor regions during visually paced rhythmic fist closures performed with the left, right or both hands. Studies in both humans and monkeys suggest that the ventral premotor cortex (PMC) is involved in the planning and execution of hand movements (Dum and Strick, 2002; for a review see Schubotz and von Cramon, 2003), while the SMA seems to be more engaged in the temporal organization of movements (Jenkins et al., 2000; Passingham et al., 1989). Both areas were strongly activated when healthy human subjects performed the same task as used in the present study, i.e., visually paced fist closures with the left or right hand (Grefkes et al., 2008a). Connectivity analyses in macaque monkeys identified significant reciprocal anatomical connections between M1 (corresponding to macaque area F1, respectively; Rizzolatti et al., 1998), superior ventral PMC (macaque area F4, respectively) and SMA (macaque area F3, respectively) within and across the hemispheres (Luppino et al., 1993; Rouiller et al., 1994; Boussaoud et al., 2005).
Based on this a priori knowledge, we constructed a bilaterally organized connectivity model comprising M1, superior ventral PMC and SMA to assess the effects of 1-Hz rTMS over contralesional M1 on the endogenous (intrinsic) connectivity and changes thereof induced by moving the paretic or non-paretic hand. We hypothesized that 1-Hz rTMS over contralesional M1 may modulate connectivity among motor regions in both the stimulated and the non-stimulated hemisphere, thereby correcting pathological changes in the functional network architecture contributing to the motor deficits of the paretic hand.
Materials and methods
Subjects
Eleven patients (mean age: 46 years, range 24–60 years) with mild to moderate unilateral hand weakness after a first-ever subcortical ischemic stroke in the left (n=8) or right (n= 3) MCA territory participated. The study was approved by the local ethics committee (RWTH Aachen file no. 07-170). Patients were selected according to the following criteria: i) stable (i.e., not fluctuating) unilateral motor deficit of the hand without spasticity 1–3 months after stroke (subacute phase), (ii) subcortical location of the ischemic lesion within the territory of the MCA, and (iii) no mirror movements.
We have already reported rTMS effects on movement kinematics and changes in BOLD activity for this cohort of patients in a previous paper (Nowak et al., 2008). Furthermore, connectivity data from the baseline sessions of 9 subjects were compared with healthy controls in another paper (Grefkes et al., 2008b). We report here the rTMS effects of rTMS on interregional connectivity compared to both a control stimulation site (vertex stimulation) and prior to any rTMS intervention. All analyses were re-computed according to the model selection procedure (see below), and hence all data presented in the paper are new.
Experimental paradigm
Patients were scanned with fMRI under 3 conditions: (1) immediately prior to rTMS (baseline condition), (2) following rTMS applied over the vertex (control stimulation), and (3) following rTMS applied over the contralesional M1. The rTMS stimulation conditions were separated by at least 120 min and the sequence of application was counterbalanced across subjects (Nowak et al., 2008). TMS was performed using a 70-mm figure-of-eight coil and a Magstim Rapid stimulator (Magstim Company, Dyfed, Wales) (see Nowak et al., 2008 for technical details).
rTMS was applied over the contralesional M1 (“motor hotspot” of the hand area) at a rate of 1-Hz at 100% resting motor threshold for 10 min (= 600 pulses). The resting motor threshold was defined for each participant as the lowest stimulator output that elicited motor evoked potentials with peak-to-peak amplitude of at least 50 μV in the contralateral first dorsal interosseus muscle in at least 5 of ten trials. Control stimulation was applied by medially shifting the coil to the vertex within the same coronal plane as the hotspot. Post-hoc analyses in each subject showed that the M1 activation cluster (which roughly coincides with the TMS motor hotspot; Sparing et al., 2008) and hence the vertex stimulation site was located on average 1.5–2 cm posterior to the SMA cluster in the interhemispheric fissure. Furthermore, no muscle twitches of the leg were observed during control stimulation using identical stimulation parameters as for the hand area. All rTMS sessions were performed in the anteroom of the MR scanner. The mean time lag from the end of the rTMS session and start of the fMRI measurements was less than 3 min owing to optimized preparation procedures for time consuming settings such as mirror adjustments and orientation of acquisition planes.
Functional magnetic resonance imaging
Subjects were informed by written instructions on a monitor visible through a mirror whether to move the left, right or both hands in the upcoming task-block (Grefkes et al., 2008a,b). The main reason for investigating both hands (affected and unaffected) in the fMRI task was to identify hand-specific differences in interregional coupling. We, hence, considered movements of the unaffected hand as “within-subject” control condition for movements of the affected hand. Likewise, we used the bimanual condition to explore whether unilateral rTMS over contralesional M1 has a specific impact on neural coupling during synchronous movements of the hands.
Subjects then performed stereotypical whole hand fist closures and openings for 15 s at a frequency of 1.55 Hz (= 23 movements) indicated by a blinking red circle. Blocks of movements were separated by resting baselines of 15 s in which subjects were shown a white screen until the next block of movements was announced. In order to reduce task-related movements of the head, we used both foam wedges at the jaws and rigid fixation bars between the head coil and the subjects' temples. Task performance was measured by recording the number of fist closures per block over all fMRI sessions. The experimenter analyzing task performance in the scanner was blinded to the kind of stimulation condition (vertex or M1) the patient had received prior to scanning. The whole fMRI session lasted approximately 12.5 min. Note that the relatively short duration of the fMRI sessions also contributed to minimizing discomfort in the scanner, and hence helped to limit head motion.
Functional MR images were acquired on a Siemens Trio 3.0 T whole-body scanner using a gradient echo planar imaging (EPI) sequence (TR=1600 ms, TE= 30 ms, 26 axial slices à 3.0 mm, inplane resolution=3.1×3.1 mm, flip angle=90°; 457 volumes+4 dummy scans). Additional high-resolution T1 (MPRAGE, magnetization prepared rapid acquisition gradient echo) and FLAIR (fluid attenuated inversion recovery) volumes were acquired after the baseline fMRI scan (parameters as in Grefkes et al., 2008b).
For MR image preprocessing and statistical analysis, we used the SPM software package (SPM5; Wellcome Department of Imaging Neuroscience, London/UK). After removing the first four volumes of a session (dummy images), all EPI volumes were realigned to the now first EPI of the time series to correct for head movements. All subjects did not move more than 2 mm in x, y, z direction, and also head rotation was within acceptable limits (< 1°). After coregistration with the anatomical 3D image, all volumes were spatially normalized to the standard template of the Montreal Neurological Institute (MNI, Canada) using the unified segmentation approach (Ashburner and Friston, 2005). An isotropic smoothing kernel of 8 mm full width half maximum (FWHM) was applied to the EPI images to suppress noise and effects due to residual differences in functional and gyral anatomy. Box-car vectors for each condition were convolved with a canonical hemodynamic response function to create the regressors of interest for the subsequent general linear model (Kiebel and Holmes, 2004). Head motion parameters were added as covariates to exclude movement related variance from the time series. Voxels were identified as significant on the single subject level if their t values passed a height threshold of t=3.43 (P<0.001, uncorrected).
Connectivity analysis
Dynamic causal modeling (DCM; Friston et al., 2003) was used to assess effective connectivity between the cortical motor areas activated by the aforementioned task (i) at baseline, (ii) after vertex stimulation and (iii) after contralesional M1 stimulation. DCM allows the estimation of task-independent intrinsic couplings (from now on referred to as “endogenous coupling”), and the changes in coupling induced by a specific task (e.g., moving the right hand). For the biophysical background of DCM and its advantages and disadvantages compared to other approaches of inferring effective connectivity from fMRI, the reader is referred to, e.g., Friston et al. (2003), Penny et al. (2004) and Stephan et al. (2007a,b).
We focused our analysis on core regions of the cortical motor system as identified in both hemispheres of each subject (Eickhoff et al., 2008; Grefkes et al., 2008a,b): primary motor cortex (M1), supplementary motor area (SMA), and lateral premotor cortex (PMC). Strongest activation within the visual cortex was found in extrastriate cortex with a local maximum in area V5, which is in good agreement to the known responsiveness of this region to moving or flickering/blinking stimuli (Jantzen et al., 2005; Zeki et al., 1991) and allowed for a reliable identification of this area at the single subject level (in contrast to the variable activation peaks in V1 along the calcarine sulcus). It is essential to note that coupling parameters obtained from DCM refer to functional interactions, but do not necessarily reflect direct axonal connection. The effects of relay regions can (usually) be neglected in models of effective connectivity (Eickhoff et al., 2009). Hence, the relay of visual information towards the premotor regions, e.g., via parietal regions which were not explicitly modeled in the DCM should be implicitly reflected in the derived rate constants of our model for effective connectivity within the cortical motor system.
We defined the individual ROIs according to the next local maximum in relation to the group coordinates. We used the following anatomical constraints (Grefkes et al., 2008a): M1 located at the “hand knob” of the precentral gyrus; PMC in lateral precentral cortex/sulcus at the level of the inferior frontal sulcus (corresponding to the superior ventral PMC; Tomassini et al., 2007; Schubotz and von Cramon, 2003); SMA in the dorsal medial wall within the interhemispheric fissure; V5 at the occipitotemporal junction. The latter region, which – in contrast to early retinotopic cortex – showed a well defined local maximum in neural activity across all subjects, was defined as input region since subjects used the visual pacing cue as signal for moving the respective hand. For each subject, the time series of all ROIs were extracted in a sphere region (radius=4 mm) from the “effects of interest” F-contrast (P<0.001, uncorrected), and adjusted for effects of no interest (e.g., realignment parameters for head movements).
Model definition
Based on published data on anatomical connectivity in macaque monkeys, we assumed intrinsic/endogenous connections between SMA and ipsilateral and contralateral M1 (Rouiller et al., 1994), between SMA and ipsilateral (Luppino et al., 1993) as well as contralateral PMC (Boussaoud et al., 2005), between PMC and both ipsilateral and contralateral M1 (Rouiller et al., 1994), as well as homotopic transcallosal connections between M1–M1 (Rouiller et al., 1994), SMA–SMA (McGuire et al., 1991; Rouiller et al., 1994) and PMC–PMC (Boussaoud et al., 2005). Evidently, the condition-specific modulations of interregional coupling may not necessarily affect all intrinsic anatomical connections. We, therefore, constructed 4 alternative models (Supplementary Fig. 1), and used Bayesian model selection (Penny et al., 2004) to determine the model which provides the best fit between accuracy and generalizability (based on the baseline fMRI data). Simpler models are often more effective in DCM analyses but given the wealth of experiments using fMRI, TMS and EEG which demonstrate a bilateral involvement of the cortical motor system in the control of unimanual movements, we always included interhemispheric and ipsilateral modulations in our models in order to acknowledge the observation that even simple hand movements involve areas in both hemispheres. The models, nevertheless, vary in terms of the complexity of interhemispheric interactions considering only a few interhemispheric connections (model 1) to a fully connected model (model 4). Furthermore, model 1 and model 2 assume a lateralized modulation of connectivity while model 3 and 4 assume that the same network is modulated by right, left and bilateral hand movements. We, therefore, suggest that our alternative models represent biologically plausible models representing a balanced trade-off between considering known anatomical connections (at least in macaques), functional properties of the motor cortex as derived from neuroimaging studies and a relative simplicity.
For consistency and comparability with earlier data (Eickhoff et al., 2008; Grefkes et al., 2008a,b) the model evidence was approximated using both the Bayesian Information Criterion (BIC) and the Akaike Information Criterion (AIC), and a decision was only made if BIC and AIC concurred (Penny et al., 2004; Stephan et al., 2007a,b). The “winning” model should then represent the best balance between the relative fit and complexity of the model (Stephan et al., 2007a,b). We additionally calculated the positive evidence ratio (PER) for each model comparison: The PER represents the number of subjects in whom the Bayes factor gave a positive evidence for model A in relation to the number of subjects showing positive evidence for the alternative model B (Stephan et al., 2007a,b). Note that the model selection rests on a fixed effects procedure whereas subsequent inference on parameters employs a random effects procedure. This means that the inference on model structure is specific for the particular sample of patients studied. Recent advances for group analyses in DCM overcome this limitation by using a random effects approach based on the conditional density of the models per se (Stephan et al., 2009). However, in the present study the model with the highest ABF was also always superior to all other models according to PER. Therefore, outliers are very unlikely to have influenced the results of the BMS procedure.
All three task conditions (movement of the affected hand, the unaffected hand, and bimanually) were modeled as experimental perturbations of the cortical network. Data from patients with right hemisphere lesions were flipped rendering the comparison affected vs. unaffected hemisphere rather than left vs. right hemisphere. The model comparison procedure showed that one model (Fig. 1) yielded the highest evidence according to both average Bayes factors and positive evidence ratio (Supplementary Table 1). DCMs of the winning model were then estimated separately for each of the three sessions in each subject, thereby allowing an identification of changes in interregional coupling induced by rTMS. Coupling parameter estimates were compared across sessions by means of one-sample 2-sided t-tests (software SPSS 17.0.1 for Windows, SPSS Inc.). Parameter estimates were considered statistically significant when passing a threshold of P< 0.05 (Bonferroni corrected for multiple comparisons).
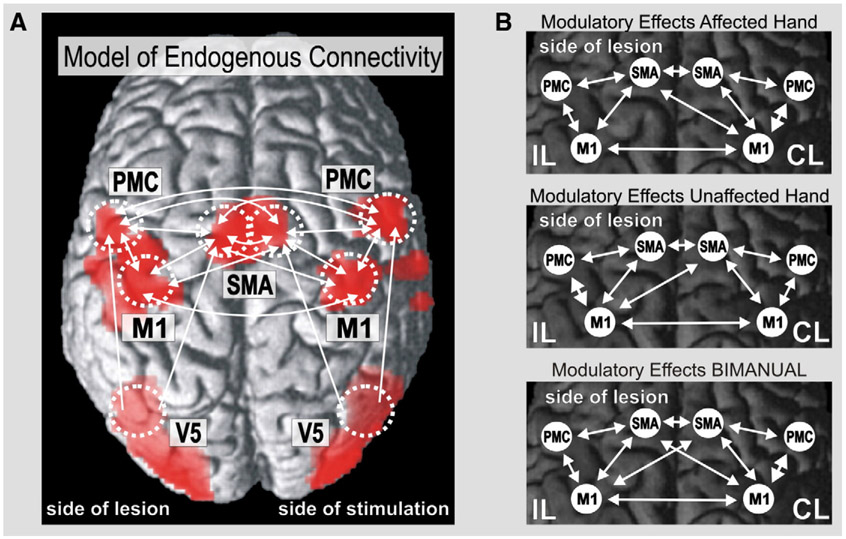
Fig. 1.
Regions of interest (ROI) and connectivity model used for estimating interregional coupling. Scans from patients with right-sided lesions were flipped at the midsagittal plane. Moving the left or right hand yielded significant activations in primary sensorimotor cortex (with local maxima in M1), lateral PMC, SMA and visual cortex. (A) Connectivity model for endogenous neural coupling (connections between V5 and contralateral premotor areas not shown). (B) Task-dependent modulations of connectivity for movements of the affected, unaffected and bilateral hands.
In order to identify those connections which were specifically modulated by rTMS applied over contralesional M1 compared to both vertex stimulation and baseline, we performed a conjunction analysis across significant differences in interregional coupling between (i) M1 stimulation versus vertex stimulation, and (ii) M1 stimulation versus baseline (prior to intervention). This means only connections significantly modulated in the M1 stimulation condition compared to both control stimulation and baseline (pair-wise t-tests, P< 0.05 for each comparison) were considered to reflect rTMS-specific effects on effective connectivity. This conservative approach ensured that (i) learning or habituation effects between baseline and M1 stimulation and (ii) effects specifically induced by stimulating the vertex (e.g., spread to adjacent cortex on the paracentral lobule) did not influence the statistical results. Correlation analyses (Pearson) between coupling parameters and task performance were computed using SPSS 17.0.1 for Windows (SPSS Inc.).
Results
The clinical data of the stroke patients are summarized in Table 1.
Table 1.
BG= basal ganglia; IC= internal capsule; CR= corona radiata; MMS= Mini Mental status according to Folstein et al., 1975; NIHSS= National Institutes of Health Stroke Scale according to Brott et al., 1989; Modified Rankin Score according to Bonita and Beaglehole, 1988; ARAT= Action Research Arm Test according to Lyle, 1981; MRC= Medical Research Council Score of wrist extension according to Medical Research Council, 1976; Hamilton Rating Scale for Depression.
| Patient | Age (years) |
Gender | Stroke localization |
Disease duration (months) |
Affected hand |
Hand dominance |
MMS score |
NIHSS score |
Modified Rankin scale |
ARAT score | MRC score |
Hamilton Rating Scale |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | m | BG, CR | 2 | Left | Right | 27 | 4 | 1 | 42 | 4 | 2 |
| 2 | 37 | m | BG, IC,CR | 1 | Right | Right | 30 | 3 | 2 | 45 | 5 | 0 |
| 3 | 48 | m | IC | 2 | Right | Right | 29 | 4 | 2 | 41 | 4 | 1 |
| 4 | 42 | m | CR | 1 | Right | Right | 27 | 2 | 1 | 57 | 5 | 0 |
| 5 | 53 | m | CR | 3 | Left | Right | 28 | 1 | 2 | 44 | 4 | 6 |
| 6 | 45 | m | BG | 2 | Left | Right | 29 | 6 | 2 | 47 | 4-5 | 0 |
| 7 | 47 | m | BG, IC | 2 | Right | Right | 30 | 2 | 2 | 53 | 4-5 | 1 |
| 8 | 48 | f | IC | 2 | Right | Right | 30 | 5 | 3 | 40 | 4 | 3 |
| 9 | 60 | m | IC | 2 | Right | Right | 29 | 1 | 2 | 44 | 4 | 1 |
| 10 | 51 | m | CR | 3 | Right | Right | 28 | 1 | 1 | 47 | 4-5 | 1 |
| 11 | 24 | f | BG | 1 | Right | Right | 30 | 1 | 1 | 40 | 4 | 0 |
Behavioral data
All patients showed motor deficits at the stroke-affected hand (Table 1). These impairments also influenced the fist closure frequency of the paretic hand, i.e., except for one patient none of the patients could achieve the requested maximum frequency of 1.55 Hz (= 23 closures in a 15 s block). Repeated measures ANOVA on the frequencies of fist closures with the factors “session” (baseline, vertex stimulation, M1 stimulation) and “hand” (affected, unaffected, bimanual) revealed a significant main effect of both factors (“session” F2,20= 6.00; P< 0.01; “hand” F2,20= 13.40; P< 0.01) and a significant session-by-hand interaction (F4,40= 6.67; P<0.001). Post-hoc t-tests (Bonferroni corrected) revealed that compared to baseline (mean fist closures per block±standard deviation: 19.94±2.43; range: 16–23) stimulation over the vertex did not significantly change movement performance of the affected hand (20.01±2.41; t= −0.97; P= 0.38). By contrast, stimulation of contralesional M1 induced a small but significant increase of paretic hand performance (mean 20.66±2.44) compared to both baseline (t= − 4.94; P< 0.01) and vertex stimulation (t= − 3.51; P< 0.01) in almost every patient (10/11). Importantly, we observed no statistically significant difference in clenching performance between the first and the last block in the scanner (P>0.05) indicating that there was no relevant drop of motor performance at the end of the fMRI sessions. Movements of the unaffected hand (baseline: 22.94±0.63; vertex: 22.91±0.31, M1: 22.89±0.27) or bilateral movements (baseline: 20.76±2.44; vertex: 20.81±2.39, M1: 20.96±2.37) were not statistically different between any of the three sessions (P> 0.05 for all comparisons). Mirror movements were not detected (by visual inspection) in any of the three sessions.
Connectivity prior to rTMS intervention
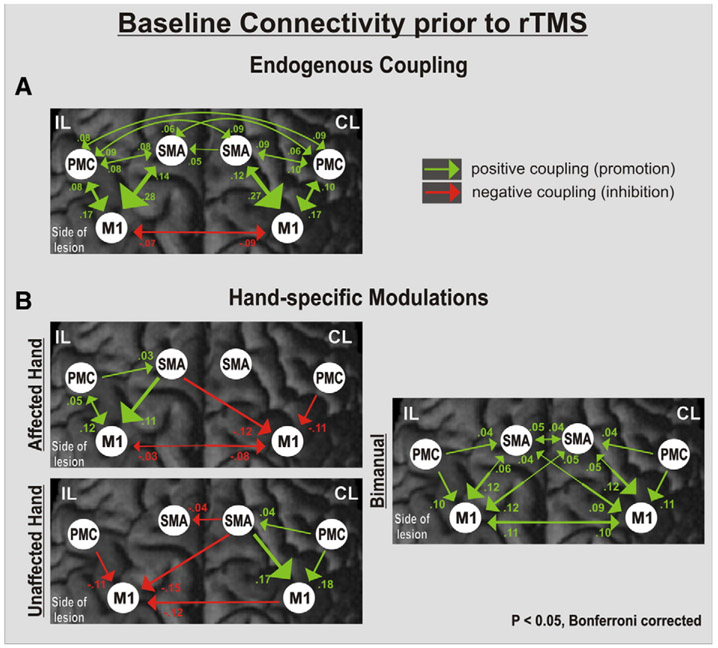
Fig. 2A demonstrates the pattern of endogenous connectivity (the A matrix) among the motor areas in the baseline session, i.e., prior to rTMS. The endogenous connectivity between the motor areas of interest was symmetrically organized across the hemispheres. The only negative, i.e., inhibitory coupling with endogenous ipsilesional M1 activity was exerted by its contralateral counterpart. Correlating the degree of motor impairment with neural coupling rates at baseline yielded a significant positive relationship between the individual fist closure frequency of the paretic hand and the strength of the SMA–M1 connection (r= 0.68, P< 0.05).
Fig. 2.
Interregional connectivity at baseline. Coupling parameters (rate constants in 1/s) indicate connection strength (changes in activation per second), which is also coded in the size and color of the arrows representing effective connectivity. Positive (green) values represent facilitatory, negative (red) values inhibitory influences on neuronal activity. The greater the absolute value (reflecting the rate constant of the observed influence in 1/s), the more predominant the effect one area has over another. (A) Endogenous coupling of motor areas irrespective of hand movements. (B) Hand-specific modulation of connections. Note the additional inhibitory coupling between contralesional (CL) M1 and ipsilesional (IL) M1 for movements of the affected hand.
Fig. 2B demonstrates the task-specific modulatory effects on interregional coupling depending on which hand was moved by the patient at baseline, i.e., prior to the rTMS sessions (B matrix). Movements of the paretic hand specifically enhanced neural coupling within the ipsilesional hemisphere with an additional inhibitory influence of contralesional M1 upon ipsilesional M1, which was not present for the corresponding connection during movements of the unaffected hand, and which significantly correlated with the individual movement frequency of the paretic hand (Pearson's r= 0.66, P< 0.05).
rTMS effects on endogenous connectivity
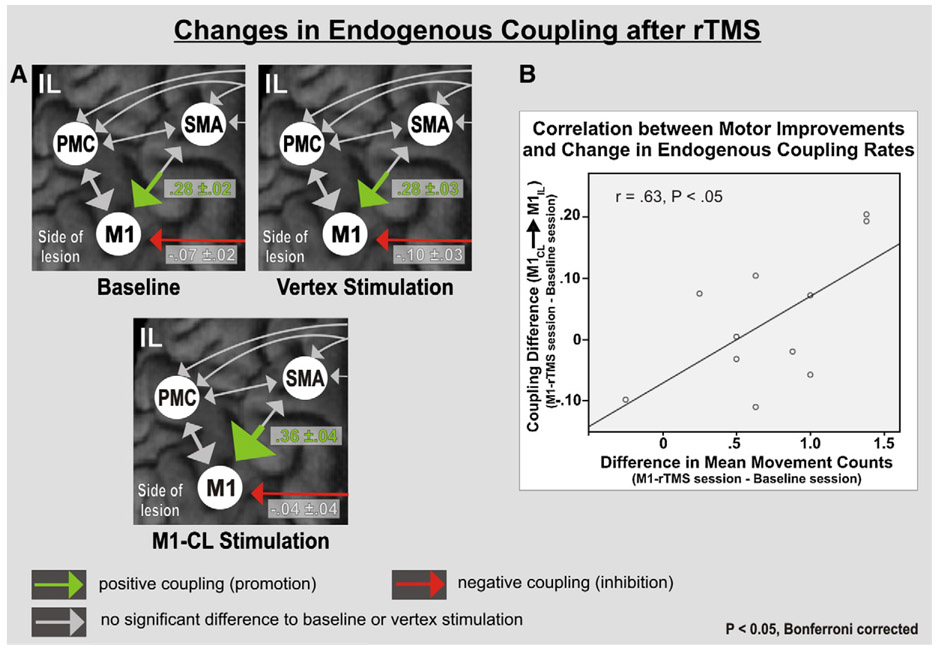
One hertz rTMS applied over the vertex did not induce a significant change in endogenous coupling (A matrix) for any of the motor areas compared to baseline (P> 0.05; Bonferroni corrected). By contrast, 1 rTMS applied over contralesional M1 was associated with a significant increase in the endogenous coupling between ipsilesional SMA and M1 compared to both baseline and vertex stimulation (P< 0.05 for each comparison, Fig. 3A). Furthermore, while the reduction in inhibitory influence observed for the connection between contralesional and ipsilesional M1 did not pass the a priori statistical thresholds [i.e., only a trend towards statistical significance compared to vertex stimulation, P= 0.09], there was a statistically significant correlation between the decrease in M1–M1 inhibition (i.e., reduced inhibition of ipsilesional M1) and the behavioral improvement of paretic hand function after rTMS over contralesional M1 compared to baseline (r= 0.63, P< 0.05; Fig. 3B). The coupling parameters of the remaining connections were not significantly different between M1 stimulation and vertex stimulation or baseline.
Fig. 3.
Significant changes in endogenous coupling after stimulation of contralesional M1. (A) Significant increase in ipsilesional SMA–M1 coupling compared to both baseline and vertex stimulation. Significant trend (P= .09) of reduced endogenous inhibition exerted from M1-CL over M1-IL between vertex and M1 stimulation. (B) The reduction in inhibitory influences from M1-C1 upon M1-IL (red arrow in A) after rTMS over M1-CL significantly correlated with improvements in task performance (Pearson correlation).
rTMS effects on hand-specific modulations in connectivity
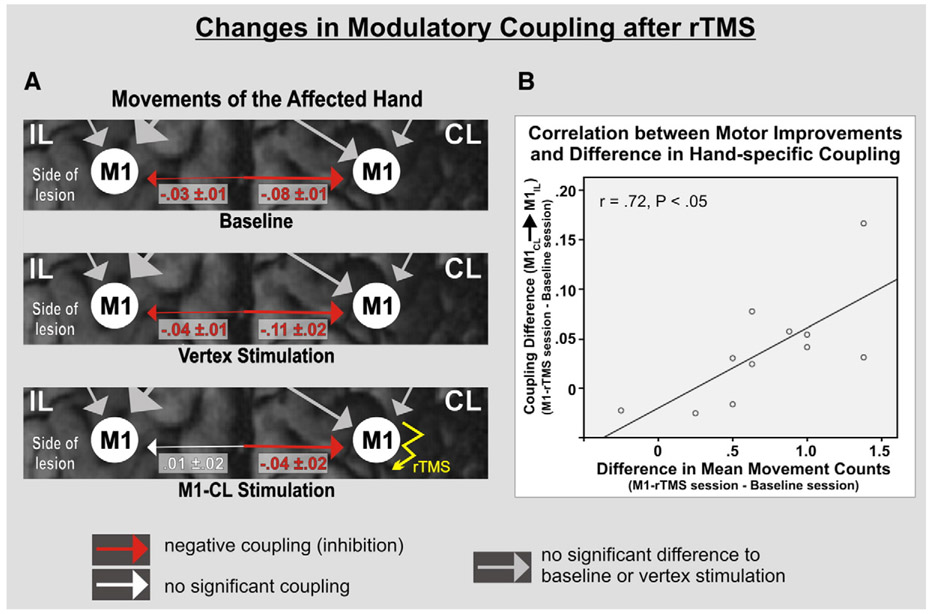
Stimulation over the vertex did not induce a significant difference in the task-dependent modulations of interregional coupling (B matrix) compared to baseline, and especially the negative coupling originating from contralesional M1 during movements of the paretic hand was not affected (relative to baseline; P> 0.05 for each connection). By contrast, rTMS over contralesional M1 significantly reduced this pathological inhibitory coupling compared to both baseline (P= 0.016) and vertex stimulation (P= 0.019) (Fig. 4A). The magnitude of the reduction (i.e., change) of pathological inhibition was correlated with the change in motor performance of the paretic hand (r= 0.72; P< 0.05; Fig. 4B). There was also a trend for a significant reduction of the inhibitory influences of ipsilesional M1 upon contralesional M1 activity compared to baseline connectivity (P= 0.053) which reached statistical significance when comparing M1 stimulation to vertex stimulation (P= 0.033) (Fig. 4A), indicating a general suppression of inhibitory M1–M1 interactions following rTMS over contralesional M1. Coupling rates for the unaffected hand or during bilateral movements were not statistically different compared to baseline and vertex stimulation. Hence, rTMS applied over contralesional M1 had a task-specific effect only for movements of the paretic hand.
Fig. 4.
Significant differences in modulatory influences during movements of the affected hand. (A) Pathological inhibition exerted from M1-CL over M1-IL at baseline that persisted after vertex stimulation. This influence was not significant after rTMS over M1-CL compared to both baseline (P= .02) and vertex stimulation (P= .02). (B) The degree of reduction in pathological coupling (disinhibition) significantly correlated with improvements in task performance of the paretic hand (Pearson correlation).
Discussion
We used dynamic causal modeling (DCM) to assess the influence of low-frequency repetitive transcranial stimulation (rTMS) on effective connectivity within the cortical motor system in patients with motor impairments due to a subcortical stroke. The data suggest that rTMS over contralesional M1 does not only reduce inhibitory influences of this region on ipsilesional M1 activity, but also enables a more effective motor processing in areas of the lesioned hemisphere as implied by the enhanced coupling of SMA and M1. These remote rTMS effects on cortical motor processing might constitute a relevant mechanism for improved motor performance.
Effects of stroke on motor networks
Task-dependent over-activation within several motor areas is a consistent finding in stroke patients (Chollet et al., 1991; Weiller et al., 1992). Typically, a “bilateralization” of neural activity during movements of the paretic hand is observed which is not evident in healthy subjects or when the patients move their unaffected hand (Bütefisch et al., 2005; Feydy et al., 2002; Nowak et al., 2008; Ameli et al., 2009). Patients with better functional outcome, however, exhibit a progressive lateralization of neural activation towards the ipsilesional hemisphere (Carey et al., 2002; Marshall et al., 2000; Nelles, 2004; Ward et al., 2003). The observation of enhanced neural activity for movements of the stroke-affected hand has stimulated a discussion regarding the role of contralesional and non-primary motor areas for recovery of motor function (Johansen-Berg et al., 2002; O'Shea et al., 2007). TMS experiments in chronic stroke patients indicate that contralesional M1 may exert an abnormally high inhibition on the ipsilesional motor cortex, thereby possibly contributing to the impaired motor function of the paretic hand (Murase et al., 2004). Therefore, it has been suggested that the downregulation of contralesional cortical excitability may help to promote functional recovery of the affected hand following stroke (Hummel and Cohen, 2006; Ziemann, 2005). In fact, an increasing number of studies have consistently demonstrated improvements in dexterous movements of the stroke-affected hand when neural processing in contralesional M1 was transiently inhibited by rTMS (Mansur et al., 2005; Takeuchi et al., 2005). Nearly all subjects in the present study showed a small increase in clenching performance during scanning subsequent to a single rTMS session over contralesional M1. Bimanual performance (i.e., number of fist closures) and bimanual connectivity were not significantly modulated, indicating that rTMS over contralesional M1 might especially promote neural mechanisms involved in unimanual tasks. Such a view is supported by the DCM analysis showing that bimanual movement induced a positive enhancement of interregional coupling for all connections analyzed, i.e., there was no pathological inhibitory coupling among the M1–M1 regions which could have been modified by rTMS. Furthermore, stimulation over the vertex did not change motor performance compared to baseline indicating that a putative spread of the magnetic field from the vertex towards the SMA was not strong enough to elicit a behavioral effect in the control condition.
Modulation of cortical networks with rTMS
The current data imply that rTMS-mediated improvements might be based on a more efficient interaction of key motor areas in both the ipsilesional and contralesional hemisphere. The hypothesis that rTMS over motor regions may induce changes in both neural activity and connectivity in local and remote motor regions has already been suggested by several neuroimaging studies (Lee et al., 2003; Pleger et al., 2006; Rowe et al., 2006). In line with these concepts, our connectivity analyses indicate that neural responses after rTMS applied over contralesional M1 are reduced not only in the cortex underlying the stimulated area, but also in more distant sites, implying a “network effect” of focal rTMS (Nowak et al., 2008).
There is ample evidence that TMS can effectively modulate interhemispheric interactions, e.g., on the level of M1 (Lee et al., 2003; Rowe et al., 2006). TMS studies employing paired-pulse protocols in stroke patients suggest a relevant role of contralesional M1 for interhemispheric inhibition and paretic hand function (Duque et al., 2005; Gilio et al., 2003; Murase et al., 2004; Pal et al., 2005). The present connectivity analysis adds a mechanistic model for these effects by demonstrating that rTMS over contralesional M1 may reduce the pathological inhibition exerted from contralesional M1 upon ipsilesional M1 selectively for movements of the affected hand. Note that this abnormal inhibitory influence of the motor cortex ipsilateral to the paretic hand is not observed when the healthy hand is moved or when healthy subjects perform the identical task (Grefkes et al., 2008a). Therefore, despite the lack of a healthy control group in the current study, these rTMS effects on interhemispheric interactions seem to be specific for stroke patients. As the reduction of endogenous inhibition exerted from contralesional M1 on ipsilesional M1 was significantly correlated with improved motor performance of the affected hand, a general disinhibition of ipsilesional M1 activity might constitute the critical feature for improved motor performance. In fact, rTMS protocols as used in the present study do not only affect interhemispheric inhibition, but may also enhance excitability of the motor cortex contralateral to stimulation (Lee et al., 2003). Such findings are in good accordance to the connectivity data of the present study showing a significantly stronger coupling between ipsilesional SMA and ipsilesional M1 after rTMS applied over contralesional M1 (Fig. 3A), which could – at least in principle – also account for enhanced excitability of ipsilesional M1. Therefore, motor improvements of the affected hand observed after 1-Hz rTMS over contralesional M1 might result not only from a decrease in transcallosal inhibition, but also from a more efficient coupling of areas in the lesioned hemisphere, thereby enabling a better motor performance of the paretic hand. Although we found a correlation of the patients' motor performance and ipsilesional SMA–M1 coupling in the baseline session, increases in coupling after rTMS over contralesional M1 were not correlated with improvements in motor performance (in contrast to M1–M1 interaction as discussed above). Hence, the current study cannot finally prove whether the increase in SMA–M1 coupling after rTMS over contralesional M1 is an effect specific to TMS-evoked modulations of pathological connectivity in stroke patients or rather represents a more general effect of rTMS on the hemisphere contralateral to stimulation which might also have occurred in healthy subjects.
The question arises whether the changes in coupling parameters are directly related to the impact of rTMS on cortical excitability or rather reflect indirect effects evoked by the better motor performance of the paretic hand (e.g., stronger efference copy signals or proprioceptive feedback). Although we cannot exclude that the small but significant increases in motor output might have introduced a “bottom-up effect” on neural activity and connectivity, two aspects speak against a dominant influence: First, the enhanced motor output of the affected hemisphere after rTMS over contralesional M1 was not associated with a stronger inhibitory influence exerted from ipsilesional on contralesional areas. Thus, the reduction of the pathological influences originating from contralesional M1 is likely to result from the 1 Hz rTMS effect on cortical excitability. Furthermore, the increase in endogenous coupling observed between ipsilesional SMA and M1 was independent from which hand was moved (which defines “endogenous” or “intrinsic” connectivity), and hence cannot be explained by the enhanced motor output of the paretic hand after rTMS over contralesional M1. Such changes might rather reflect a general improvement of interregional processing evoked by rTMS. This notion is supported by fMRI studies in rodents indicating that the restitution of neural networks in the ipsilesional hemisphere constitutes a key factor for successful recovery of function (Weber et al., 2008). That (r)TMS may have remote effects on motor areas has been demonstrated for several cortical and subcortical regions (Bestmann et al., 2005; Lee et al., 2003; Siebner et al., 2003), and indicates that behavioral effects evolving after stimulation might be based on a fast re-modeling of the whole network rather than being caused by a single motor region only (Chen et al., 2003; Rowe et al., 2006)
Limitations and open questions
The behavioral rTMS effects observed for the hand clenching task during the fMRI sessions were relatively small (up to 7% performance increase) and cannot be explained by a drop of motor performance at the end of the fMRI sessions due to diminishing rTMS effects (as there was no difference compared to the first fMRI blocks). Although almost all patients showed reduced task performance at the paretic hand at baseline, motor deficits were generally mild (cf. Table 1) which might have limited possible rTMS effects (ceiling effects). However, the statistical significance of the difference compared to baseline or control stimulation indicates that stimulating contralesional M1 has indeed induced changes in the cortical network architecture which we aimed to explore with our DCM analyses and which are difficult to be assessed with “conventional” TMS-paradigms (e.g., ipsilesional SMA–M1 interaction). As discussed above, we cannot claim that all changes in effective connectivity observed after rTMS over contralesional M1 are specific to stroke patients or would appear similarly in a healthy control group following the same stimulation protocol. Furthermore, a longer stimulation period or tasks targeting more isolated finger movements such as index tapping or pinching movements might have yielded better behavioral effects for a single rTMS session (Talelli and Rothwell, 2006; Nowak et al., 2008). As our pilot fMRI scans showed that a finger tapping task evoked much weaker BOLD activity, we decided to use the fist closure task to activate the motor system, as a high anatomical–functional precision in the identification of the motor ROIs is essential for DCM analyses and was better warranted by the latter task. One could argue that the rTMS-mediated changes in the frequency of ballistic fist closure movements (facilitated by lowered thresholds of excitability in the lesioned hemisphere after contralesional rTMS) do not automatically imply congruent effects for more skillful movements such as finger sequences or reach-grasp tasks. However, we have already reported the behavioral effects of the identical rTMS protocol as used in the present study in an earlier publication (Nowak et al., 2008). We showed that not only simple motor tasks like finger tapping but also motor performance in an object-grasping-task, i.e., a more relevant task with regard to activities of daily living, was significantly improved after rTMS over contralesional M1. Improvement rates were even higher than for the fist closure task performed in the scanner. Therefore, more complex motor tasks like reaching-and-grasping tasks might be even better suited to demonstrate a behaviorally relevant effect of rTMS but such kinds of paradigms are difficult to perform in an MRI environment due to spatial restrictions of the scanner and the induction of hand-movement related head motion artifacts (which are difficult if not impossible to remove from an image time series).
The question arises to which extent changes in effective connectivity are mirrored by changes in neurophysiological data, i.e., how changes in endogenous or task-dependent coupling relate to changes in cortical excitability which was not tested in the current study. Therefore, combined DCM–TMS studies evaluating the relationship between DCM coupling parameters and electrophysiological measures (Bestmann et al., 2005; Lee et al., 2003; Siebner et al., 2003; Ziemann, 2005) are needed. Although the results of the current study support the concept of disturbed interhemispheric inhibition, the issue of the significance of contralesional M1 for functional recovery is still under debate as not all studies published on this issue point to a negative role of contralesional M1. For example, disruption of contralesional M1 by fMRI activation guided triple-pulse TMS during execution of a complex sequential finger movement task reduced the performance of the affected hand in stroke patients (Lotze et al., 2006). A generally negative role of contralesional M1 for ipsilesional motor output is also questioned by a recent study with rats showing that transient inactivation of the contralesional motor cortex by means of lidocaine injection may deteriorate recovered motor performance of the stroke-affected limb in strongly impaired animals (Biernaskie et al., 2005). These data indicate that at least for some patients the contralesional M1 may have a supportive role for the motor output of the ipsilesional hemisphere. Furthermore, it remains unclear why a subcortical lesion in the depth of the white matter may trigger pathological transcallosal interactions among the hemispheres (Duque et al., 2005; Murase et al., 2004). Hence, longitudinal studies employing different modalities and covering the whole period from early post-ischemic changes to the chronic stage are needed in order to explore and evaluate the development of pathological interactions after stroke (Ward et al., 2003). In this context the current study aimed at extending previous interhemispheric competition models by implementing also other areas than M1 into the model, e.g., SMA and PMC, which might yield a better description of the networks underlying motor behavior. Therefore, the combination of computational approaches such as DCM with brain mapping tools, such as MRI, EEG or (r)TMS, seems to offer a great potential to yield a better understanding of the processes promoting recovery of function after stroke.
Supplementary Material
Acknowledgments
S.B.E. was funded by the Human Brain Project (R01-MH074457-01A1) and the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model).
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2009.12.029.
References
- Ameli M, Grefkes C, Kemper F, Riegg F, Rehme AK, Karbe H, Fink GR, Nowak DA, 2009. Differential effects of high-frequency rTMS over ipsilesional primary motor cortex in cortical and subcortical MCA stroke. Ann. Neurol 66 (3), 298–309. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K, 2005. Unified segmentation. Neuroimage 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Biernaskie J, Szymanska A, Windle V, Corbett D, 2005. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. J. Neurosci 21, 989–999. [DOI] [PubMed] [Google Scholar]
- Bonita R, Beaglehole R, 1988. Modification of Rankin Scale: recovery of motor function after stroke. Stroke 19, 1497–1500. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J, 2005. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage 28, 22–29. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Tanne-Gariepy J, Wannier T, Rouiller EM, 2005. Callosal connections of dorsal versus ventral premotor areas in the macaque monkey: a multiple retrograde tracing study. BMC Neurosci. 6, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brott T, Adams HP Jr., Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, 1989. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20, 864–870. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, Kleiser R, Müller K, Wittsack HJ, Hömberg V, Seitz RJ, 2005. Recruitment of contralesional motor cortex in stroke patients with recovery of hand function. Neurology 64, 1067–1069. [DOI] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K, 2002. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain 125, 773–788. [DOI] [PubMed] [Google Scholar]
- Chen WH, Mima T, Siebner HR, Oga T, Hidemi H, Satow T, Begum T, Nagamine T, Shibasaki H, 2003. Low-frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clin. Neurophysiol 114, 1628–1637. [DOI] [PubMed] [Google Scholar]
- Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS, 1991. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann. Neurol 29, 63–71. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Van Der Werf YD, Leonard G, Paus T, 2003. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J. Neurophysiol 90, 1071–1083. [DOI] [PubMed] [Google Scholar]
- Conchou F, Loubinoux I, Castel-Lacanal E, Le TA, Gerdelat-Mas A, Faure-Marie N, Gros H, Thalamas C, Calvas F, Berry I, Chollet F, Simonetta MM, 2009. Neural substrates of low-frequency repetitive transcranial magnetic stimulation during movement in healthy subjects and acute stroke patients. A PET study. Hum. Brain Mapp 30, 2542–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL, 2002. Motor areas in the frontal lobe of the primate. Physiol. Behav 77, 677–682. [DOI] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG, 2005. Transcallosal inhibition in chronic subcortical stroke. Neuroimage 28, 940–946. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Dafotakis M, Grefkes C, Shah NJ, Zilles K, Piza-Katzer H, 2008. Central adaptation following heterotopic hand replantation probed by fMRI and effective connectivity analysis. Exp. Neurol 212, 132–144. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K, 2009. A systems perspective on the effective connectivity of overt speech production. Philos. Transact. A Math Phys. Eng. Sci 367, 2399–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feydy A, Carlier R, Roby-Brami A, Bussel B, Cazalis F, Pierot L, Burnod Y, Maier MA, 2002. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke 33 (6), 1610–1617. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. Mini-Mental State. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W, 2003. Dynamic causal modelling. Neuroimage 19, 1273–1302. [DOI] [PubMed] [Google Scholar]
- Gilio F, Rizzo V, Siebner HR, Rothwell JC, 2003. Effects on the right motor hand-area excitability produced by low-frequency rTMS over human contralateral homologous cortex. J. Physiol 551, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR, 2008a. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage 41, 1382–1394. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, Karbe H, Fink GR, 2008b. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann. Neurol 63, 236–246. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Wang LE, Eickhoff SB, Fink GR, 2009. Noradrenergic modulation of cortical networks engaged in visuomotor processing. Cereb Cortex. in press doi: 10.1093/cercor/bhp144. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG, 2006. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 5, 708–712. [DOI] [PubMed] [Google Scholar]
- Jantzen KJ, Steinberg FL, Kelso JAS, 2005. Functional MRI reveals the existence of modality and coordination-dependent timing networks. Neuroimage 25, 1031–1042. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ, 2000. Self-initiated versus externally triggered movements II. The effect of movement predictability on regional cerebral blood flow. Brain 123 (Pt. 6), 1216–1228. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM, 2002. The role of ipsilateral premotor cortex in hand movement after stroke. Proc. Natl. Acad. Sci. U. S. A 99, 14518–14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebel S, Holmes A, 2004. The General Linear Model, In: Frackowiak RS, Friston K, Frith CD, Dolan RJ, Price CJ, Zeki S, Ashburner J, Penny W (Eds.), Human Brain Function, 2nd edition. Elsevier Academic Press, San Diego, pp. 725–761. [Google Scholar]
- Komitova M, Johansson BB, Eriksson PS, 2006. On neural plasticity, new neurons and the postischemic milieu: an integrated view on experimental rehabilitation. Exp. Neurol 199, 42–55. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, Wagenaar RC, 2002. Long term effects of intensity of upper and lower limb training after stroke: a randomised trial. J. Neurol. Neurosurg. Psychiatry 72, 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Siebner HR, Rowe JB, Rizzo V, Rothwell JC, Frackowiak RS, Friston KJ, 2003. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J. Neurosci 23, 5308–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C, 2006. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J. Neurosci 26, 6096–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G, 1993. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J. Comp. Neurol 1993, 114–140. [DOI] [PubMed] [Google Scholar]
- Lyle RC, 1981. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int. J. Rehabil. Res 4, 483–492. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A, 2000. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin. Neurophysiol 111, 800–805. [DOI] [PubMed] [Google Scholar]
- Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci-Neto J, Santos CM, Wagner T, Rigonatti SP, Marcolin MA, Pascual-Leone A, 2005. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology 64, 1802–1804. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL, 2000. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke 31, 656–661. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Bates JF, Goldman-Rakic PS, 1991. Interhemispheric integration: I. Symmetry and convergence of the corticocortical connections of the left and the right principal sulcus (PS) and the left and the right supplementary motor area (SMA) in the rhesus monkey. Cereb. Cortex 1, 390–407. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG, 2004. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol 55, 400–409. [DOI] [PubMed] [Google Scholar]
- Nelles G, 2004. Cortical reorganization—effects of intensive therapy. Restor. Neurol. Neurosci 22, 239–244. [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, Küst J, Karbe H, Fink GR, 2008. Improving dexterity following stroke: effects of low-frequency rTMS over contralesional M1 on movement kinematics and neural activity in subcortical stroke. Arch. Neurol 65, 741–747. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Johansen-Berg H, Trief D, Gobel S, Rushworth MF, 2007. Functionally specific reorganization in human premotor cortex. Neuron 54, 479–490. [DOI] [PubMed] [Google Scholar]
- Pal PK, Hanajima R, Gunraj CA, Li JY, Wagle-Shukla A, Morgante F, Chen R, 2005. Effect of low-frequency repetitive transcranial magnetic stimulation on interhemispheric inhibition. J. Neurophysiol 94, 1668–1675. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Chen YC, Thaler D, 1989. Supplementary motor cortex and self-initiated movement. In: Ito M (Ed.), Neural Programming. Karger, Tokyo, pp. 13–24. [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ, 2004. Modelling functional integration: a comparison of structural equation and dynamic causal models. Neuroimage 23 (Suppl. 1), S264–S274. [DOI] [PubMed] [Google Scholar]
- Pleger B, Blankenburg F, Bestmann S, Ruff CC, Wiech K, Stephan KE, Friston KJ, Dolan RJ, 2006. Repetitive transcranial magnetic stimulation-induced changes in sensorimotor coupling parallel improvements of somatosensation in humans. J. Neurosci 26, 1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M, 1998. The organization of the cortical motor system: new concepts. Neuron 31, 889–901. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M, 1994. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp. Brain Res 102, 227–243. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Siebner H, Filipovic SR, Cordivari C, Gerschlager W, Rothwell J, Frackowiak R, 2006. Aging is associated with contrasting changes in local and distant cortical connectivity in the human motor system. Neuroimage 32, 747–760. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY, 2003. Functional–Anatomical concepts of human premotor cortex: evidence from fMRI and PET studies. Neuroimage 20 (Suppl. 1), S120–S131. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Peller M, Lee L, 2003. Applications of combined TMS-PET studies in clinical and basic research. Suppl. Clin. Neurophysiol 56, 63–72. [DOI] [PubMed] [Google Scholar]
- Sparing R, Buelte D, Meister IG, Paus T, Fink GR, 2008. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum. Brain Mapp 29 (1), 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Fink GR, Marshall JC, 2007a. Mechanisms of hemispheric specialization: insights from analyses of connectivity. Neuropsychologia 45, 209–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Weiskopf N, Drysdale PM, Robinson PA, Friston KJ, 2007b. Comparing hemodynamic models with DCM. Neuroimage 38, 387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ, 2009. Bayesian model selection for group studies. Neuroimage 46 (4), 1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K, 2005. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke 36, 2681–2686. [DOI] [PubMed] [Google Scholar]
- Talelli P, Rothwell J, 2006. Does brain stimulation after stroke have a future? Curr. Opin. Neurol 19, 543–550. [DOI] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Klein JC, Behrens TE, Pozzilli C, Matthews PM, Rushworth MF, Johansen-Berg H, 2007. Diffusion-weighted imaging tractography-based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomicaland functional specializations. J. Neurosci 27 (38), 10259–10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS, 2003. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 126, 2476–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R, Ramos-Cabrer P, Justicia C, Wiedermann D, Strecker C, Sprenger C, Hoehn M, 2008. Early prediction of functional recovery after experimental stroke: functional magnetic resonance imaging, electrophysiology, and behavioral testing in rats. J. Neurosci 28, 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RSJ, 1992. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann. Neurol 31, 463–472. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS, 1991. A direct demonstration of functional specialization in human visual cortex. J. Neurosci 11, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, 2005. Improving disability in stroke with rTMS. Lancet Neurol 4, 454–455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.