Abstract
Along with the multiple neuroprotective effect, recent studies suggest that gintonin might increase the blood brain barrier permeability. We evaluated the effect of gintonin on the vascular permeability changes in different brain segments, using dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI). In this 8-week, randomized, open label pilot study, ten participants with subjective memory impairment but preserved cognitive function assigned to gintonin-enriched fraction (GEF) 300 mg/day or placebo groups. Korean versions of the Alzheimer's disease assessment scale (ADAS-K) and DCE-MRI parameters including Ktrans and Vp in different brain segments were evaluated at baseline and at 8 weeks after treatment. Nine participants completed the study protocol. No adverse events occurred during the observation period for 8 weeks in both groups. Following gintonin administration, increment trends of the brain permeability that did not reach a statistical significance were observed in the left hippocampus (Ktrans and Vp, both, p = 0.062), left thalamus and in left putamen (Ktrans, p = 0.062), and left insula and right amygdala (Vp, p = 0.062), but not in the control placebo group. The increment of the Ktrans value in the left thalamus from the baseline was highly correlated with the change of the ADAS scores (r = −0.900, p = 0.037). Gintonin might enhance the blood-brain barrier (BBB) permeability in the brain structures involved in cognitive functions. Further efficacy exploration for the synergistic effect of gintonin's BBB permeability enhancement to its other cognitive enhancing mechanisms are warranted.
Trial Registration
Clinical Research Information Service Identifier: KCT0003418
Keywords: Brain Permeability, Cognitive Dysfunction, Gintonin, Subjective Memory Impairment
INTRODUCTION
Dementia is a prevalent geriatric disorder with substantial clinical impact [1]. Recent studies demonstrate that pathologic, functional, and structural changes in the brain progress from early as twenty years before the clinical manifestation of dementia becomes evident [2]. Therefore, early recognition and intervention of the disease is increasingly recognized as the key to improve the clinical course of dementia [3]. It is important to continually protect the cognitive function from no symptom or very mild stages of cognitive deterioration, but the drugs for the dementia treatment such as donepezil, rivastigmine, galantamine, and memantine do not have a long-term cognitive protective effect that exceeds drug side effects [4,5,6,7]. Although the role of functional foods that can be used relatively safe, there is not enough research on dietary supplements that can help improve cognitive function [8].
Ginseng extracts have been reported to improve cognitive function in humans [9,10]. Recently, ginseng contains a high concentration of lysophosphatidic acid (LPA), a G protein-coupled receptor ligand, and it is known that ginseng-containing LPA improves cognitive function by a mechanism different from conventional saponin.[11] Gintonin is a high concentration extract of ginseng-containing LPA [12,13]. In gintonin, LPA binds to ginseng major latex-like protein 151 (GLP151), a ginseng-specific glycoprotein, which shows high stability and binding affinity with LPA receptor [11,12,14]. Gintonin inhibits β-amyloid (Aβ) production by activating LPA receptors in neurons and promotes the release of soluble amyloid precursor protein α (sAPPα) with neuroprotective action [14]. Gintonin has also been shown to increase acetylcholine synthesis instead of inhibiting acetylcholinease [13,14]. Recently, the effect of gintonin in improving cognitive function was established in subjects with mild dementia [15].
High levels of LPA receptors are expressed in brain endothelial cells, and animal studies have shown that gintonin acts on LPA receptors to induce blood-brain barrier (BBB) opening [16,17]. Considering that the efficient delivery of drugs beyond the BBB into the brain parenchyma is a fundamental issue of drug treatment for central nervous system diseases, the permeability enhancing effect of gintonin is an important feature that can significantly increase the clinical efficacy [18].
Recently, measurement of BBB has been tried in human subjects, to demonstrate the pathophysiology of central nervous system disease in relation with BBB dysfunction. In this regard, dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) is a new technique for evaluating vascular permeability. DCE MRI specifically measures the BBB permeability by quantitative measure of the signals across the BBB. By measuring DCE parameters across the brain and overlaying them to the structural MRI image, we can assess the permeability of BBB in each anatomical structures of the brain [19].
In this study, we used DCE MRI to evaluate the changes in the BBB permeability in cognition-associated brain regions after gintonin-enriched fraction (GEF) treatment in subjective memory impairment, and its relationship with the changes in the cognitive function.
METHODS
Study participants
In this pilot study, subjects who were aged between 50 and 85 years, with complaining memory impairment but without dementia (baseline Mini-Mental State Examination-Korean version [K-MMSE] scores of 23 or higher) were recruited from the Neurology department of the Seoul National University Hospital. We excluded subjects who (1) those who are taking ginseng or health functional foods containing ginseng components; (2) have allergies or hypersensitivity to ginseng; (3) have clinically significant liver or kidney disease; (4) A person who has undergone surgery or the like that cannot interfere with food absorption or cannot be orally administered; (5) The person is being treated for major mental disorders such as manic depression and schizophrenia; (6) Those who are receiving drugs and dietary supplements that may affect memory and cognitive function within 4 weeks of the test, or other test drugs related to cognitive improvement; (7) alcohol or drug addicts.
This study was registered at Clinical Research Information Service; Korea Center for Disease Control and Prevention, Ministry of Health and Welfare, Republic of Korea (KCT0003418, date of registration:10/01/2019). This study protocol and supporting documentation were approved by Institutional Review Board (IRB) of Seoul National University Hospital (SNUH; H-1711-092-901) and the study was performed in compliance with the SNUH IRB regulations and the International Conference on Harmonisation guideline for Good Clinical Practice. Written and informed consent to participate was obtained from all enrolled participants. As all of the participants were without dementia, obtaining informed consent from their guardian or legally authorized representative was not required.
Study design and procedures
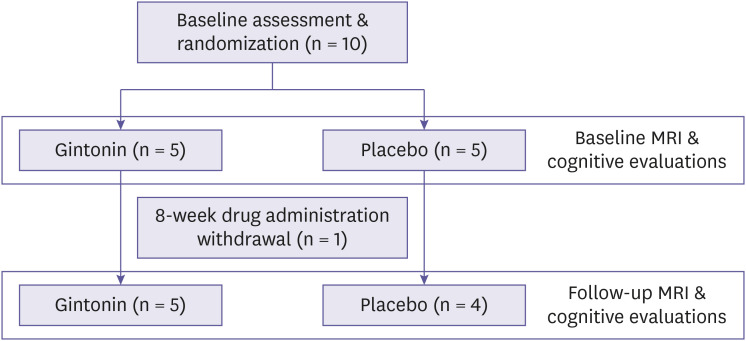
This study was designed as a randomized blind-labeled fashion. Ten participants who met the inclusion criteria were then randomly assigned to GEF or the control placebo groups. At baseline, participants' medical histories, laboratory examinations, scores of cognitive evaluations, and the laterality of the dominant hand were obtained. One subject was dropped-out due to the withdrawal of the consent and remaining nine participant completed the study. Before the intake of GEF or placebo, all the participants had performed DCE MRI study as a baseline. The test group received two 400 mg tablets containing GEF 150 mg and excipient compound 250 mg once daily, whereas the placebo group received two 400 mg tablets containing crystalline cellulose 374 mg and excipient compound 26 mg once daily for 8 weeks. GEF and placebo tablets used for the study were supplied by Gintonin KU Biotech Co., LTD. (Republic of Korea). The designated intake dose is the result of substituting a human equivalent dose of 25–100 mg/kg/day in a 60 kg adult for a significant improvement in cognitive function through mouse in vivo experiments [13]. The GEF and the placebo tablets were identical in appearance. At 4 weeks after treatment, participants were followed up with physical and laboratory examinations and checked for any adverse event. At 8 weeks after treatment, participants were followed up again with physical and laboratory examinations, checked for any adverse event, and performed follow-up DCE MRI evaluation. Basal and follow-up cognitive evaluations were also performed (Fig. 1). The follow-up interval of 8-weeks were designated according to our previous study observed that 4-week oral administration of the same dose of gintonin to this study significantly improved the cognitive function measured by K-MMSE scores and this effect was still significant at 8-week [20]. Although the pharmacodynamic profiles of gintonin was not demonstrated, our previous data indicates that the 8-week continuous administration of gintonin might be sufficient to evaluate the effect gintonin on BBB permeability. The design of this study was approved by the Institutional Review Board of the SNUH.
Figure 1. A flow chart illustrating the study process.
MRI, magnetic resonance imaging.
MRI protocol
All study participants underwent DCE MRI studies with a 3-T imaging unit with a 32-channel head coil (Discovery 750; GE Healthcare, Milwaukee, WI, USA). The MRI protocol included pre- and post-contrast 3-dimensional fast spoiled gradient echo sequences with multi-planar reconstructions of the sagittal, coronal, and transverse T1-weighted images and fluid-attenuated inversion recovery images. The specific imaging parameters were demonstrated in the previous study [21]. DCE MRI was performed after intravenous administration of gadobutrol (Gadovist®; Bayer Schering Pharma, Berlin, Germany) according to the protocol reported in the previous study [21]. To minimize the potential confounding effect of diurnal alterations of the cerebral blood flow and blood pressure, the baseline MRI was obtained between 9 A.M. and 13 A.M. and the time of the follow-up MRI evaluation was matched to the time of the baseline MRI evaluation [22].
MRI analysis
All image analyses were performed by expert neuro-radiologists who were blinded to clinical information. The detailed procedures of the DCE MRI analysis was described in the previous report [21]. In brief, automatic cortical reconstruction and volumetric segmentation were performed on the T1-weighted image by using the open-source software for the structural neuroimaging analysis, FreeSurfer (version 6.0; Laboratory for Computational Neuroimaging, Boston, MA, USA) according to the procedures as follows: motion correction and averaging; removal of nonbrain tissue; segmentation of the brain structures; intensity normalization; tessellation of the gray matter-white matter boundaries; automated topology correction; surface deformation; and registration of the brain images to a Destrieux atlas. DCE MRI data were processed by using a commercial software package (NordicICE, version 2.3.12; NordicNeuroLab, Bergen, Norway). The arterial input function (AIF) was obtained with the DCE module and all of the pharmacokinetic parameter maps were obtained using the AIF. The DCE parameters included Ktrans (the volume transfer constant for gadolinium between blood plasma and the tissue extravascular extracellular space) and Vp (the fractional plasma volume). They were obtained based on a 2 compartments pharmacokinetic model and was calculated by using the fixed T1 of 1,000 msec.
Co-linking between brain region masks and the Ktrans and Vp maps was performed using the software NordicICE. On every axial image, Ktrans and Vp were calculated for the mask on a pixel-by-pixel basis and summated to obtain total parameter values from all of the brain segments. Ktrans and Vp maps were derived using the software NordicICE (Fig. 2). Histograms of Ktrans and Vp were plotted and the mean values of Ktrans and Vp were derived from the histograms. The reproducibility of was established to be high [21].
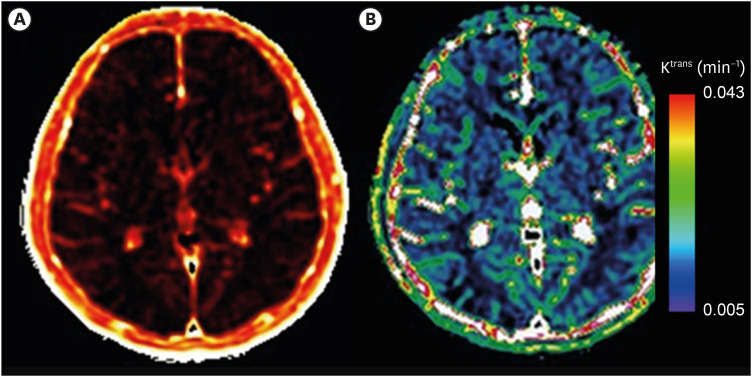
Figure 2. Examples of DCE MRI mapping for Ktrans.
Mapping images showing Ktrans measurement values in the brain. The brightness in (A) and the colors in (B) represents the values of Ktrans overlaid on the structural MRI images. In (B), red color indicates the higher value of Ktrans that can be interpreted that flow of the contrast materials is greater across the BBB whereas the lower values shows less movement of materials showing purple color.
DCE, dynamic contrast-enhanced; MRI, magnetic resonance imaging; BBB, blood-brain barrier.
Clinical measurements
At baseline, at 4 weeks, and at 8 weeks after treatment, K-MMSE, clinical dementia rating (CDR), and the Korean version of the Alzheimer's disease assessment scale (ADAS-K). In particular, ADAS test is a frequently used tool to evaluate the cognitive enhancing effect in clinical trials. This test mainly focused on language and memory functions and has merits in more detailed evaluation than the K-MMSE or CDR. The cognitive items of ADAS test include short-term memory, language (speech, comprehension, naming, and word-finding abilities), praxis, visuospatial functions, orientation, and word recognition. ADAS test also contains non-cognitive domains such as behavioral problem or emotional abnormalities. Scoring is ranged from 0 to 70, with higher score is greater cognitive dysfunction. We also analyzed the association of ADAS score changes with the changes in the Ktrans and Vp values in each regions of interest (ROI) of the brain MRI.
Laboratory measurement included complete blood count and serum panels including electrolyte, glucose, uric acid, total protein, albumin creatinine level, liver enzymes, and cholesterol profiles, routine urinalysis, systolic/diastolic blood-pressure and pulse rate, and routine 12-lead electrocardiography. The treatment-emergent adverse events were recorded using the preferred term of the medical dictionary for regulatory activities (MedDRA).
ROIs of the brain
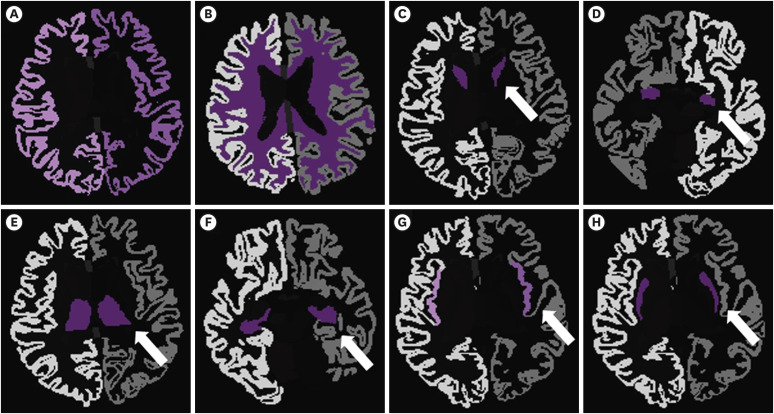
The ROIs were categorized into right and left hemisphere, respectively. The specific brain regions were as follows: cerebral cortex, white matter, caudate, amygdala, thalamus, hippocampus, insular, and putamen, which cover cortical and subcortical structures in the supratentorial areas which mainly mediate the cognitive functions (Fig. 3).
Figure 3. Regions of interest.
Eight regions in the brain were evaluated separately for the right and the left hemispheres. (A) Cerebral cortex, (B) white matter, (C) caudate nucleus, (D) amygdala, (E) thalamus, (F) hippocampus, (G) insula, and (H) putamen.
Statistical analysis
Statistical Package for the Social Sciences version 25.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Baseline characteristics were compared using the Fisher's exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. The change of the ADAS-K score and the DCE MRI parameters from baseline to 8 weeks after treatment were evaluated using the Wilcoxon rank sum test and inter-group comparison using the repeated measure analysis of variance. The correlation between the change of the each ADAS-K scores and the DCE MRI parameters was evaluated using the Spearman correlation coefficient. As a pilot study, power analyses for the minimal sample size to reach a statistical significance were calculated using allocation ratio of 1:1, power of 0.8, and α error probability of 0.05. All statistical evaluations were two-tailed and p-values of < 0.05 was considered as statistically significant.
Availability of data
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
RESULTS
Participants and demographic findings
Between October 2018 and May 2019, 9 participants completed this study. Five participants were randomized into the gintonin group and the remaining 4 into the placebo group. Demographic and baseline clinical characteristics were comparable between the study groups (Table 1). Every participant was right-handed and no one was ambidextrous. No significant clinical or laboratory adverse event was observed during the 8-week follow-up period. Nine out of 10 participants completed and endured the study protocol.
Table 1. Demographic and clinical characteristics.
| Variables | Gintonin (n = 5) | Placebo (n = 4) | p value |
|---|---|---|---|
| Male sex | 1 (20.0) | 0 (0.0) | 1.000 |
| Age (yr) | 72.0 ± 4.6 | 77.3 ± 3.6 | 0.106 |
| Hypertension | 1 (20.0) | 1 (25.0) | 1.000 |
| Diabetes | 1 (20.0) | 0 (0.0) | 1.000 |
| Hyperlipidemia | 1 (20.0) | 1 (25.0) | 1.000 |
| Heart disease | 0 (0.0) | 0 (0.0) | - |
| Alcohol drink | 0 (0.0) | 0 (0.0) | - |
| Smoking | 0 (0.0) | 0 (0.0) | - |
| Baseline K-MMSE score | 27 [26–28.5] | 28 [24–29] | 0.894 |
| Baseline ADAS-K score | 16.75 [8.75–25.5] | 20.75 [16.75–25.5] | 0.503 |
| Follow-up ADAS-K score | 9.75 [8.2–18.75] | 18.75 [16.25–26.25] | 0.108 |
| ADAS-K score change | −3 [−10.25–0.75] | 1.5 [−6.25–2.25] | 0.354 |
Data are reported as number (%) or mean ± standard deviation or as median [interquartile range]. K-MMSE: Korean version of Mini-Mental State Examination and ADAS-K: Korean versions of the Alzheimer's disease assessment scale.
Cognitive changes following gintonin administration.
At baseline, the total ADAS-K scores were similar between the gintonin and the placebo groups (16.75 [interquartile range; IQR, 8.75–25.5] in the gintonin group and 20.75 [16.75–25.5] in the control group). At 8 weeks following gintonin administration, ADAS-K total score was lowered by to 9.75 (8.2–18.75) (% mean change reduction 41.8% of from baseline), whereas in the control placebo group, the mean score decreased to 18.75 (16.25–26.25), 9.6% from the baseline (p = 0.108).
Ktrans and Vp changes in DCE MRI analysis
Ktrans and Vp values, which indicate rate of efflux (Ktrans) or volume fraction (Vp) cross the BBB in each ROI ara, were compared before and following 8-week gintonin or placebo administration (Table 2). Although most ROIs did not reach to the statistical significance of p-value < 0.05, increment trends that did not reach a statistical significance of the Ktrans and Vp were observed in the left hippocampus (both, p = 0.062), of Ktrans in the left thalamus and the left putamen (both, p = 0.062), and of Vp in the left insula and the right amygdala (both, p = 0.062). These finding were not observed in the placebo group. In the power analysis, the total number of study subjects required to observe a statistically significant increments of Ktrans and Vp in the left hippocampus, Ktrans in the left thalamus and the left putamen, and Vp in the left insula and the right amygdala by using gintonin was 28.
Table 2. Baseline and follow-up Ktrans and Vp values in different brain segments.
| Variables | Ktrans (baseline) | Ktrans (follow-up) | p value* | Vp (baseline) | Vp (follow-up) | p value* | |
|---|---|---|---|---|---|---|---|
| Left amygdala | Gintonin | 0.0006 (0.0003–0.0007) | 0.0008 (0.0006–0.0009) | 0.313 | 1.1502 (0.6298–2.3247) | 2.0611 (0.7836–2.1226) | 0.188 |
| Placebo | 0.0009 (0.0003–0.002) | 0.0008 (0.0002–0.0011) | 1.000 | 1.9432 (0.933–2.9708) | 1.266 (1.0504–2.682) | 0.875 | |
| p value† | 0.556 | 1.000 | 0.657‡ | 0.286 | 1.000 | 0.695‡ | |
| Right amygdala | Gintonin | 0.0005 (0.0003–0.0013) | 0.0006 (0.0005–0.0010) | 1.000 | 1.3441 (0.7076–1.6929) | 1.5965 (0.9359–2.1468) | 0.063 |
| Placebo | 0.0008 (0.0001–0.0016) | 0.0005 (0.0004–0.0006) | 0.375 | 2.1113 (1.1162–2.6397) | 1.4716 (1.0439–2.7518) | 0.875 | |
| p value† | 0.730 | 0.286 | 0.372‡ | 0.191 | 1.000 | 0.850‡ | |
| Left caudate | Gintonin | 0.0003 (0.0002–0.0008) | 0.0007 (0.0005–0.0015) | 0.188 | 1.2969 (0.7379–3.5888) | 1.8859 (1.2903–2.0889) | 0.625 |
| Placebo | 0.0009 (0.0002–0.0024) | 0.0006 (0.0003–0.0011) | 0.875 | 2.0913 (1.2898–2.5865) | 1.414 (1.1902–1.6014) | 0.250 | |
| p value† | 0.286 | 0.730 | 0.943‡ | 0.413 | 0.111 | 0.070‡ | |
| Right caudate | Gintonin | 0.0005 (0.0002–0.0011) | 0.0009 (0.0005–0.0015) | 0.438 | 1.7182 (0.9853–2.1940) | 2.0762 (1.5270–2.4733) | 0.188 |
| Placebo | 0.0006 (0.0001–0.0013) | 0.0004 (0.0001–0.0009) | 0.625 | 2.0831 (1.343–2.5126) | 1.7191 (0.8854–2.3618) | 0.875 | |
| p value† | 1.000 | 0.286 | 0.165‡ | 0.413 | 0.556 | 0.526‡ | |
| Left cerebral cortex | Gintonin | 0.0012 (0.0006–0.0014) | 0.0010 (0.0010–0.0015) | 0.438 | 1.7483 (1.4185–2.6308) | 2.3271 (1.7160–2.4052) | 0.188 |
| Placebo | 0.0012 (0.0006–0.0023) | 0.0011 (0.0009–0.0013) | 1.000 | 2.6482 (1.6822–3.2229) | 2.065 (1.5909–2.4081) | 0.375 | |
| p value† | 0.905 | 1.000 | 0.962‡ | 0.286 | 0.556 | 0.281‡ | |
| Right cerebral cortex | Gintonin | 0.0011 (0.0005–0.0013) | 0.0011 (0.0010–0.0015) | 0.438 | 1.9754 (1.4508–3.0824) | 2.4540 (1.6824–2.7634) | 0.438 |
| Placebo | 0.0015 (0.0004–0.002) | 0.0011 (0.001–0.0012) | 0.625 | 3.3234 (1.8568–3.6139) | 2.2525 (1.6462–2.558) | 0.250 | |
| p value† | 0.413 | 0.905 | 0.542‡ | 0.191 | 0.556 | 0.349‡ | |
| Left cerebral white matter | Gintonin | 0.0004 (0.0002–0.0004) | 0.0004 (0.0003–0.0005) | 1.000 | 0.6722 (0.6132–0.9448) | 0.8385 (0.6631–0.8834) | 0.813 |
| Placebo | 0.0005 (0.0003–0.0008) | 0.0004 (0.0003–0.0005) | 0.625 | 1.0049 (0.7566–1.7476) | 0.8724 (0.6709–1.1154) | 0.625 | |
| p value† | 0.730 | 0.907 | 1.000‡ | 0.191 | 0.556 | 0.849‡ | |
| Right cerebral white matter | Gintonin | 0.0004 (0.0002–0.0006) | 0.0004 (0.0002–0.0005) | 1.000 | 0.7100 (0.6863–0.9417) | 0.8129 (0.6263–0.9446) | 0.625 |
| Placebo | 0.0004 (0.0001–0.0007) | 0.0004 (0.0003–0.0004) | 0.875 | 1.3006 (0.7654–1.4763) | 0.9304 (0.6928–1.0289) | 0.250 | |
| p value† | 0.905 | 0.413 | 0.668‡ | 0.064 | 0.413 | 0.742‡ | |
| Left hippocampus | Gintonin | 0.0005 (0.0004–0.0010) | 0.0009 (0.0007–0.0013) | 0.063 | 1.6071 (0.8137–3.2338) | 2.9265 (1.3222–3.7655) | 0.063 |
| Placebo | 0.001 (0.0002–0.0021) | 0.0007 (0.0005–0.001) | 0.625 | 3.7953 (1.4476–5.8162) | 1.6762 (1.6539–4.4797) | 0.625 | |
| p value† | 0.556 | 0.191 | 0.259‡ | 0.191 | 0.730 | 0.693‡ | |
| Right hippocampus | Gintonin | 0.0006 (0.0004–0.0025) | 0.0010 (0.0007–0.0012) | 0.813 | 1.9241 (1.7171–2.7636) | 3.4669 (1.5562–4.7605) | 0.125 |
| Placebo | 0.0011 (0.0001–0.0019) | 0.0007 (0.0005–0.0008) | 0.625 | 3.4643 (2.2179–4.3159) | 2.4579 (2.1958–5.1764) | 0.875 | |
| p value† | 0.905 | 0.111 | 0.052‡ | 0.111 | 0.730 | 0.292‡ | |
| Left insula | Gintonin | 0.0008 (0.0004–0.0010) | 0.0009 (0.0007–0.0018) | 0.438 | 3.0056 (2.1105–3.2942) | 3.4208 (2.5679–3.7976) | 0.063 |
| Placebo | 0.0012 (0.0001–0.002) | 0.0009 (0.0005–0.0013) | 0.875 | 2.9661 (2.6221–3.4981) | 3.002 (2.6796–3.3429) | 1.000 | |
| p value† | 0.905 | 0.730 | 0.833‡ | 0.905 | 0.413 | 0.132‡ | |
| Right insula | Gintonin | 0.0006 (0.0003–0.0012) | 0.0008 (0.0006–0.0013) | 0.625 | 2.3256 (1.1931–4.2462) | 2.4483 (1.5931–3.4674) | 0.625 |
| Placebo | 0.0009 (0.0000–0.0014) | 0.0008 (0.0007–0.0009) | 0.625 | 2.9719 (2.0078–5.4536) | 2.1806 (1.3857–3.1026) | 0.313 | |
| p value† | 0.548 | 0.841 | 0.642‡ | 0.222 | 0.841 | 0.779‡ | |
| Left putamen | Gintonin | 0.0004 (0.0003–0.0007) | 0.0007 (0.0004–0.0010) | 0.063 | 1.5185 (0.9837–2.3280) | 1.8308 (1.5427–2.3805) | 0.313 |
| Placebo | 0.0008 (0.0001–0.0011) | 0.0005 (0.0004–0.0007) | 0.625 | 2.2691 (1.2971–2.9007) | 1.9166 (1.6388–2.0534) | 0.875 | |
| p value† | 0.413 | 0.556 | 0.454‡ | 0.286 | 0.730 | 0.728‡ | |
| Right putamen | Gintonin | 0.0004 (0.0003–0.0008) | 0.0006 (0.0005–0.0010) | 0.813 | 1.4997 (0.9487–2.1690) | 2.0293 (1.4487–2.5527) | 0.125 |
| Placebo | 0.0008 (0.0000–0.0011) | 0.0006 (0.0004–0.0007) | 0.625 | 2.839 (1.6464–3.4225) | 1.917 (1.6372–2.5571) | 0.250 | |
| p value† | 0.556 | 0.730 | 1.000‡ | 0.064 | 0.905 | 0.509‡ | |
| Left thalamus | Gintonin | 0.0004 (0.0003–0.0007) | 0.0007 (0.0004–0.0011) | 0.063 | 1.4282 (0.9386–1.9946) | 2.0601 (1.2905–2.2366) | 0.188 |
| Placebo | 0.0006 (0.0003–0.0011) | 0.0005 (0.0004–0.0009) | 0.875 | 2.5125 (1.2939–3.3996) | 1.6827 (1.3105–2.2861) | 0.625 | |
| p value† | 1.000 | 0.556 | 0.293‡ | 0.191 | 1.000 | 0.850‡ | |
| Right thalamus | Gintonin | 0.0005 (0.0004–0.0011) | 0.0007 (0.0005–0.0013) | 1.000 | 1.7745 (1.3341–1.9812) | 2.3085 (1.3219–2.6296) | 0.125 |
| Placebo | 0.0005 (0.0001–0.0011) | 0.0006 (0.0005–0.0007) | 0.750 | 2.9895 (1.5972–3.1871) | 1.9596 (1.3244–1.9789) | 0.500 | |
| p value† | 1.000 | 0.393 | 0.246‡ | 0.250 | 0.571 | 0.654‡ | |
Data are reported as median (range). *p value for the change from baseline by Wilcoxon rank sum test, †p value for the gintonin group and the placebo group by Wilcoxon rank sum test, and ‡p value for the test group and the placebo group by repeated-measure ANOVA.
Correlation of parameters of DCE MRI and ADAS score
For the brain segments with increasing trends that did not reach a statistical significance of the brain permeability parameters following gintonin treatment, further correlations analysis between the changes of the DCE MRI parameters and the changes of the ADAS-K scores were performed. As the result, changes in the Ktrans value in the left thalamus from the baseline was highly correlated with the change of the ADAS scores (r = −0.900, p = 0.037), while the changes in other brain segments were not significantly associate with the ADAS-K score changes (Table 3).
Table 3. Correlation analysis between the change of the ADAS-K score and the dynamic contrast-enhanced magnetic resonance imaging parameters.
| ADAS-K score change | Left hippocampus Ktrans | Left putamen Ktrans | Left thalamus Ktrans | Right amygdala Vp | Left hippocampus Vp | Left insula Vp |
|---|---|---|---|---|---|---|
| r | −0.300 | −0.700 | −0.900 | −0.300 | −0.500 | 0.500 |
| p value | 0.624 | 0.188 | 0.037 | 0.624 | 0.391 | 0.391 |
ADAS-K, Korean versions of the Alzheimer's disease assessment scale.
DISCUSSION
This is a pilot study that investigated whether gintonin (GEF) alters the human BBB. Using DCE MRI, we evaluated the GEF effects on the BBB permeability in human beings. In addition, given that GEF improves human cognitive function, we also assessed the changes in the cognitive scores after using GEF for 8 weeks. When compared to the placebo, we observed an increment trends that did not reach a statistical significance of the brain permeability in the left hippocampus (both by Ktrans and by Vp), left thalamus and putamen (by Ktrans), and the left insula and right amygdala (by Vp). In particular, the degree of parameter changes of DCE MRI (Ktrans) in left thalamus was highly correlated with cognitive improvement of the ADAS score, indicating that the enhancement of the permeability in this area might be one of the potential mechanism of gintonin's beneficial effect in the cognitive function [23].
Previous studies have demonstrated the effect of gintonin on the BBB permeability based on the in vitro or the in vivo models [16,18,24,25]. In a study using human brain microvascular endothelial cells, gintonin induced widening of the junctional spaces, modulated the expression levels of the endothelial junctional proteins such as cadherin, occludin, zonula occludens 1, and claudin-5, and also enabled the entrance of large molecules to enter the cells [24]. In a rodent model, the fluorescent-labeled gintonin well distributed throughout the endothelium, neurons and glial cells after intravenous injection [18]. It is also found that the permeability enhancing effect of gintonin is mediated by its binding with the highly expressed LPA receptors in the cerebrovascular endothelium, the LPA 1/3, and secondary increment of VEGF [16,25]. Significance of our studies have also proved the effect in the human BBB, supporting that it modulates the BBB trafficking.
Gintonin has been reported to increase cognitive function. The previous proposed mechanisms that underly the beneficial effects included the followings: inhibition of toxic Aβ production, stimulation of the neuroprotective sAPPα production, improvement of the synaptic function, and modulation of the acetylcholine metabolism [13,14,26,27]. In our study, the regions which showed incremental trends in the BBB permeability without clinical significance were closely involved in the cognitive function [23,28,29,30]. Those brain regions also enriched with acetylcholine and its receptors [20]. Regional difference in the density of the LPA receptor expression or compensatory mechanism to overcome the subclinical cholinergic dysfunction might be the possible explanations for the inter-structural difference of the gintonin's effect on BBB permeability. Especially, increment of the Ktrans value in the left thalamus was highly correlated with the improvement of the ADAS scores. Thalamus is a central integrator of global cognitive function such as learning, memory, and flexible adaption [23]. Thalamus is highly abundant with cholinergic innervation, which constitutes a bottom-up cholinergic system mediating global attention and perceptional functions [31]. Therefore, enhancement of the BBB permeability in thalamus might substantially augment the effects of gintonin.
The Ktrans or Vp changes in other brain regions which showed incremental trends in the BBB permeability after gintonin and were also associated with the cognitive function, such as hippocampus, caudate, and amygdala did not correlate with the ADAS-K score change improvement. Although not elucidated in this study, the major cause of this negative association might be the small number of participants. Additionally, as thalamus is excusive supplied by the small perpetrating arteriole ramifying directly from the large basal cerebral arteries, the function and integrity of BBB in thalamus might be more susceptible than other structures to the gintonin's effects exerted on the vascular endothelium [32].
As one of therapeutic strategy in the cognitive impaired subjects, our study enables us to consider potential application of gintonin, associated with BBB permeability. The FDA approved medications established as effective in treating Alzheimer's disease (AD) as inhibiting acetylcholinesterase inhibitors, such as donepezil, rivastigmine, or galantamine. The main problem of these medications that reduce compliance is adverse events, mostly gastrointestinal complains due to increase level of acetylcholine, since increase level of acetylcholine dose not only affect brain in central, but also in peripheral digestive system. Therefore, some studies returned negative results for their efficacy in treating MCI or subjective memory impairment [4,5,6,7], of these disease entity is important as the initial stage of AD. This is because when the initial cognitive function is normal or the degree of deterioration is slight, it is difficult to evaluate the effect of the drug through the degree of change in the score. Also in these cases it is difficult to ascertain the effectiveness of drugs that outweigh the risk of side effects that AD therapeutic drugs may have at normal use doses. In this regard, the permeability enhancing effect of gintonin suggests that gintonin might provide a synergistic effect with those established drugs for AD by enhancing the delivery of the drugs into the structures highly involved in cognitive function and also enable achieving the same pharmacological effect in the brain using lower doses. Therefore, gintonin might serve as a feasible option as an add-on therapy to overcome the safety issues of the agents for AD treatment. As described earlier in this study, AD treatment agents on the wide range of cognitive functions mediated by the cholinergic neurons in the thalamus.
This study has several limitations to be addressed. First, we did not reach to the statistically significant p-values to demonstrate the BBB permeability enhancing effect of gintonin. The main cause might be the pilot study design including small number of participants, and the observations in this study should be interpretated with cautions. Second, increment trends without a statistical significance were also observed in other comparisons without any clear relevance to the study hypothesis. For example, Vp at baseline was marginally lower in gintonin group in right cerebral white matter and in right putamen. Those non-relevant finding might lower the clinical certainty of the major findings of the current study. However, as the power analysis returned that minimal number of 28 participants is required to reach a statistical significance, future studies including larger number of participants is warranted to establish the BBB permeability enhancing effect of gintonin over those non-relevant findings. Third, this study used only a single dosage of GEF. As gintonin was also tolerable in the higher doses, future studies might include multiple dosage regimens with higher doses of GEF. As gintonin was safe without provoking adverse events, gintonin might have a potential clinical utility in the long-term management of the cognitive dysfunction, as a combination treatment added to the conventional treatment agents, for example, to determine the efficacy of the brain entrance of the co-administered donepezil [18].
In conclusion, we have demonstrated gintonin alters human BBB permeability in the major structures involving in the global cognitive function. It might potentially promote their clinical utility, as it can facilitate the delivery of other AD treatment agents into the brain, when used in combination [18].
Footnotes
Funding: This research was supported by the grant from Gintonin-KU. This paper was supported by Konkuk University Researcher Fund in 2019.
Reviewer: This article was reviewed by peer experts who are not TCP editors.
Conflict of Interest: - Authors: Nothing to declare
- Reviewers: Nothing to declare
- Editors: Nothing to declare
- Conceptualization: Kim M, Nah SY.
- Data curation: Shin YW, Kim M, Nah SY.
- Formal analysis: Lee WJ, Shin YW.
- Investigation: Lee WJ, Shin YW, Chang H, Shin HR.
- Methodology: Chang H, Choi SH.
- Project administration: Shin HR.
- Resources: Choi SH, Kim M, Nah SY.
- Software: Choi SH.
- Supervision: Kim M, Nah SY.
- Validation: Nah SY.
- Writing - original draft: Lee WJ.
- Writing - review & editing: Shin YW, Kim M, Nah SY.
References
- 1.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 4.Tricco AC, Soobiah C, Berliner S, Ho JM, Ng CH, Ashoor HM, et al. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis. CMAJ. 2013;185:1393–1401. doi: 10.1503/cmaj.130451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doody RS, Ferris SH, Salloway S, Sun Y, Goldman R, Watkins WE, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72:1555–1561. doi: 10.1212/01.wnl.0000344650.95823.03. [DOI] [PubMed] [Google Scholar]
- 6.Winblad B, Gauthier S, Scinto L, Feldman H, Wilcock GK, Truyen L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70:2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- 7.Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63:651–657. doi: 10.1212/01.wnl.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- 8.Barnard ND, Bush AI, Ceccarelli A, Cooper J, de Jager CA, Erickson KI, et al. Dietary and lifestyle guidelines for the prevention of Alzheimer's disease. Neurobiol Aging. 2014;35(Suppl 2):S74–S78. doi: 10.1016/j.neurobiolaging.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy DO, Scholey AB. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav. 2003;75:687–700. doi: 10.1016/s0091-3057(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 10.Park KC, Jin H, Zheng R, Kim S, Lee SE, Kim BH, et al. Cognition enhancing effect of panax ginseng in Korean volunteers with mild cognitive impairment: a randomized, double-blind, placebo-controlled clinical trial. Transl Clin Pharmacol. 2019;27:92–97. doi: 10.12793/tcp.2019.27.3.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang SH, Shin TJ, Choi SH, Cho HJ, Lee BH, Pyo MK, et al. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi SH, Jung SW, Lee BH, Kim HJ, Hwang SH, Kim HK, et al. Ginseng pharmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front Pharmacol. 2015;6:245. doi: 10.3389/fphar.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HJ, Shin EJ, Lee BH, Choi SH, Jung SW, Cho IH, et al. Oral administration of gintonin attenuates cholinergic impairments by scopolamine, amyloid-β protein, and mouse model of Alzheimer's disease. Mol Cells. 2015;38:796–805. doi: 10.14348/molcells.2015.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang SH, Shin EJ, Shin TJ, Lee BH, Choi SH, Kang J, et al. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimers Dis. 2012;31:207–223. doi: 10.3233/JAD-2012-120439. [DOI] [PubMed] [Google Scholar]
- 15.Moon J, Choi SH, Shim JY, Park HJ, Oh MJ, Kim M, et al. Gintonin administration is safe and potentially beneficial in cognitively impaired elderly. Alzheimer Dis Assoc Disord. 2018;32:85–87. doi: 10.1097/WAD.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 16.Kim DG, Jang M, Choi SH, Kim HJ, Jhun H, Kim HC, et al. Gintonin, a ginseng-derived exogenous lysophosphatidic acid receptor ligand, enhances blood-brain barrier permeability and brain delivery. Int J Biol Macromol. 2018;114:1325–1337. doi: 10.1016/j.ijbiomac.2018.03.158. [DOI] [PubMed] [Google Scholar]
- 17.On NH, Savant S, Toews M, Miller DW. Rapid and reversible enhancement of blood-brain barrier permeability using lysophosphatidic acid. J Cereb Blood Flow Metab. 2013;33:1944–1954. doi: 10.1038/jcbfm.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi SH, Lee NE, Cho HJ, Lee RM, Rhim H, Kim HC, et al. Gintonin facilitates brain delivery of donepezil, a therapeutic drug for Alzheimer disease, through lysophosphatidic acid 1/3 and vascular endothelial growth factor receptors. J Ginseng Res. 2019 doi: 10.1016/j.jgr.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsson HB, Courivaud F, Rostrup E, Hansen AE. Measurement of brain perfusion, blood volume, and blood-brain barrier permeability, using dynamic contrast-enhanced T 1-weighted MRI at 3 tesla. Magn Reson Med. 2009;62:1270–1281. doi: 10.1002/mrm.22136. [DOI] [PubMed] [Google Scholar]
- 20.Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen EG, Lockridge O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience. 2002;110:627–639. doi: 10.1016/s0306-4522(01)00613-3. [DOI] [PubMed] [Google Scholar]
- 21.Kim YS, Kim M, Choi SH, You SH, Yoo RE, Kang KM, et al. Altered vascular permeability in migraine-associated brain regions: evaluation with dynamic contrast-enhanced MRI. Radiology. 2019;292:713–720. doi: 10.1148/radiol.2019182566. [DOI] [PubMed] [Google Scholar]
- 22.Hodkinson DJ, O’Daly O, Zunszain PA, Pariante CM, Lazurenko V, Zelaya FO, et al. Circadian and homeostatic modulation of functional connectivity and regional cerebral blood flow in humans under normal entrained conditions. J Cereb Blood Flow Metab. 2014;34:1493–1499. doi: 10.1038/jcbfm.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolff M, Vann SD. The cognitive thalamus as a gateway to mental representations. J Neurosci. 2019;39:3–14. doi: 10.1523/JNEUROSCI.0479-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang SH, Lee BH, Choi SH, Kim HJ, Won KJ, Lee HM, et al. Effects of gintonin on the proliferation, migration, and tube formation of human umbilical-vein endothelial cells: involvement of lysophosphatidic-acid receptors and vascular-endothelial-growth-factor signaling. J Ginseng Res. 2016;40:325–333. doi: 10.1016/j.jgr.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi SH, Kim HJ, Cho HJ, Park SD, Lee NE, Hwang SH, et al. Gintonin-mediated release of astrocytic vascular endothelial growth factor protects cortical astrocytes from hypoxia-induced cell damages. J Ginseng Res. 2019;43:305–311. doi: 10.1016/j.jgr.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park H, Kim S, Rhee J, Kim HJ, Han JS, Nah SY, et al. Synaptic enhancement induced by gintonin via lysophosphatidic acid receptor activation in central synapses. J Neurophysiol. 2015;113:1493–1500. doi: 10.1152/jn.00667.2014. [DOI] [PubMed] [Google Scholar]
- 27.Shin TJ, Kim HJ, Kwon BJ, Choi SH, Kim HB, Hwang SH, et al. Gintonin, a ginseng-derived novel ingredient, evokes long-term potentiation through N-methyl-D-aspartic acid receptor activation: involvement of LPA receptors. Mol Cells. 2012;34:563–572. doi: 10.1007/s10059-012-0254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Jong LW, van der Hiele K, Veer IM, Houwing JJ, Westendorp RG, Bollen EL, et al. Strongly reduced volumes of putamen and thalamus in Alzheimer's disease: an MRI study. Brain. 2008;131:3277–3285. doi: 10.1093/brain/awn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- 31.Kim K, Müller ML, Bohnen NI, Sarter M, Lustig C. Thalamic cholinergic innervation makes a specific bottom-up contribution to signal detection: Evidence from Parkinson's disease patients with defined cholinergic losses. Neuroimage. 2017;149:295–304. doi: 10.1016/j.neuroimage.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Percheron G. The anatomy of the arterial supply of the human thalamus and its use for the interpretation of the thalamic vascular pathology. Z Neurol. 1973;205:1–13. doi: 10.1007/BF00315956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.