Abstract
Introduction:
Sodium-glucose cotransporter-2 [SGLT2] inhibitors reduce cardiovascular events and mortality in patients with diabetes, particularly patients with established cardiovascular disease. Euglycemic diabetic ketoacidosis [euDKA], a complication of SGLT2 therapy, can be exacerbated by a low carbohydrate diet.
Case Report:
A 61-year-old man with a history of type 2 diabetes, taking an SGLT2 inhibitor empagliflozin 10 mg orally daily, presented to the emergency room with a 2-day history of nausea and chest pain. A week prior to presentation, he had started a ketogenic diet. He was initially admitted with a diagnosis of acute coronary syndrome. On initial assessment in the emergency room, his cardiac enzymes were normal and there were no ischemic changes in his ECG. As there was concern for unstable angina, he underwent cardiac catheterization, which showed a known total occlusion with collaterals and arteries with a non-obstructive disease without any evidence of acute plaque rupture. His baseline laboratory assessments revealed an elevated anion gap of 17, increased urinary and plasma ketones, and metabolic acidosis. His plasma glucose level was 84 mg/dL. The diagnosis of euDKA was made, and treatment with intravenous fluids and insulin was initiated. His chest pain and nausea subsequently resolved.
Conclusion:
We present a case of euDKA triggered by a ketogenic diet while on SGLT2 inhibitor therapy presenting as chest pain. The recognition of euDKA is important in the context of increased SGLT2 use for the management of cardiovascular risk for patients with diabetes.
Keywords: Acute coronary syndrome, chest pain, coronary artery disease, ketoacidosis, diabetes mellitus type 2, sodium-glucose transporter 2 inhibitors
1. INTRODUCTION
Several clinical trials have demonstrated clear cardiovascular benefits of sodium-glucose cotransporter 2 (SGLT2) inhibitors. Because of these benefits, SGLT2 inhibitors are now more frequently used in patients with type 2 diabetes and cardiovascular disease [1-3]. A known complication of SGLT2 inhibitor use is euglycemic diabetic ketoacidosis (euDKA) [4]. DKA is a serious and potentially life-threatening complication of diabetes with marked hyperglycemia (>250 mg/dl), increased plasma ketones, and aniongap acidosis. In comparison, euDKA can be more subtle, as it occurs in the presence of blood glucose values <250 mg/dL [4]. Mechanistically, SGLT2 inhibitors lower glucose by inhibiting glucose reabsorption in the proximal convoluted tubules in the kidney. By decreasing serum glucose levels, SGLT2 inhibitors lead to lower circulating insulin levels and increased glucagon secretion, which enhance lipolysis and free fatty acid production with greater subsequent ketogenesis [5]. Ketogenic or low-carbohydrate diets can be effective for weight loss [6]. However, a few case reports describe euDKA precipitated by carbohydrate-restricted diets in patients on SGLT2 inhibitors [4, 7]. To our knowledge, this is the first report of euglycemic DKA caused by an SGLT2 inhibitor during self-introduction of a ketogenic diet that has presented as concern for acute coronary syndrome (ACS).
2. PATIENT INFORMATION
A 61-year-old man with a history of type 2 diabetes with a hemoglobin A1c of 8.3%, hypertension, coronary artery disease (CAD), and hyperlipidemia presented to the emergency room with nausea and right-sided chest pain. The patient had a 30-year history of diabetes and was on metformin for years before starting insulin with an average daily dosage of 28 units. Three years ago, he started an SGLT2 inhibitor, improved his lifestyle and glycemic control, and stopped taking insulin. His home medications included empagliflozin 10 mg daily, metformin 500 mg twice a day, liraglutide 1.8 mg subcutaneously daily, rosuvastatin 5 mg daily, ezetimibe 10 mg daily, and omeprazole 40 mg daily.
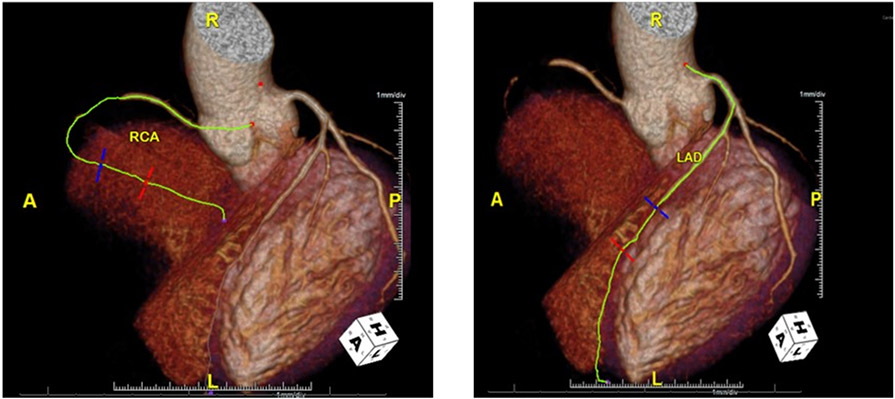
Two weeks prior to presentation, he visited his primary care physician with complaints of vague right-sided, non-radiating chest pain. Although his symptoms were deemed most consistent with gastroesophageal reflux disease, he underwent further evaluation of his chest pain. An electrocardiogram (ECG) revealed left axis deviation and inferior infarct, unchanged from prior ECGs. He was subsequently referred for a cardiology consultation. Coronary computed tomography angiography (CCTA) showed a noncalcified plaque, causing total occlusion of the mid-right coronary artery, less than 30% stenosis in the proximal left anterior descending (LAD), and 50% stenosis in the middle LAD (Fig. 1). His total coronary artery calcium score was 0, despite significant CAD on CCTA. He underwent an exercise stress test that demonstrated ST elevations in leads II, III, and AVF, as well as stress-induced wall motion abnormalities. He was advised to start aspirin and was subsequently scheduled for an elective left heart catheterization in one week.
Fig. (1).
CT Coronary angiogram displays a total occlusion of middle right coronary artery and 50% occlusion of middle left anterior descending artery. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In the interim, he self-adopted a ketogenic diet, limiting his daily carbohydrate intake to under 40 grams. Five days later, he developed persistent, non-radiating, and vague right-sided chest pain along with nausea, which felt different from his original symptoms. There were no aggravating or alleviating factors. He denied shortness of breath, sweating, heartburn, fevers, chills, recent travel, alcohol use, vomiting, or diarrhea. His wife reported an odd breath odor.
3. DIAGNOSTIC ASSESSMENT
His vital signs showed: temperature 36.7°C (98°F), heart rate 89 beats/minute, respiration rate 18 breaths/minute, blood pressure 142/80, oxygen saturation 97% on room air, and body mass index 24.41 kg/m2. His troponin was negative and ECG showed inferior Q waves similar to prior ECGs. Labs showed: glucose 84 (70-100 mg/dl), anion gap 17 (6-14 mmol/L), bicarbonate 17 (22-29 mmol/L). He had a high quantity of urinary ketones, as well as elevated plasma beta-hydroxybutyric acid 4.1 (normal <0.3). He was diagnosed with euDKA. Given the concern for (ACS), he was administered 1 liter of normal saline and underwent cardiac catheterization, which showed the known complete total occlusion of the right coronary artery with collaterals and no change in other diseased arteries. No revascularization was performed.
4. THERAPEUTIC INTERVENTION
Empagliflozin was stopped, 3 liters of intravenous normal saline was given, and an insulin drip with dextrose was started to maintain euglycemia while he was on insulin.
5. FOLLOW-UP AND OUTCOMES
The next morning, his chest pain and ketoacidosis resolved. Upon discharge, his SGLT2 inhibitor was stopped and he was maintained on a low dose of insulin and informed about the risks of a ketogenic diet while on SGLT2 inhibitor therapy.
6. DISCUSSION
This case highlights that euDKA can be triggered by ketogenic diets in patients on SGLT2 inhibitors and can present with chest pain. This finding may have important implications because this class of medication is increasingly prescribed for patients with underlying cardiovascular disease [1, 2, 8], since they are associated with a reduction in major cardiovascular events, cardiovascular mortality, as well as reduction in hospitalization for heart failure, strokes, and all-cause mortality [9, 10]. Clinicians must be aware that euDKA can present with symptoms mimicking ACS.
The coronary artery calcium (CAC) score is an independent predictor of the risk of major cardiovascular events. A CAC score of 0 is associated with a very low risk of future coronary events. Our patient had a known coronary artery occlusion, but a CAC score of 0. In the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry, which consists of 10,037 subjects without known CAD, coronary stenosis of ≥ 50% occurred in 3.5% of subjects and coronary stenosis of ≥ 70% occurred in 1.4% of subjects who had a CAC score of 0 [11]. Although uncommon, patients such as ours may have a CAC score 0 and still have CAD.
Clinicians may not initially consider euDKA if blood glucose levels are normal. Because there was a concern for ACS in our patient with euDKA, he underwent cardiac catheterization. As patients with euDKA are usually dehydrated, a risk factor for contrast-induced nephropathy, it was imperative our patient received intravenous fluids before his cardiac catheterization. SGLT2 inhibitors promote a negative fluid and sodium balance, which can contribute to a hypovolemic state. Hypovolemia can drive elevations in glucagon, cortisol, and epinephrine, which further increase insulin resistance, lipolysis, and ketogenesis [4]. For these reasons, it was important to recognize euDKA before administering intravenous contrast for cardiac catheterization.
DKA presenting as chest pain has been reported, but rarely. One case report describes a 44-year-old man with type 2 diabetes who presented to the emergency room with chest pain and was initially treated for ACS. He did not have ST-T changes on his ECG nor elevated troponins on his laboratory, but he did have elevated serum glucose levels [307 mg/dL], elevated anion gap metabolic acidosis, and elevated urine ketones. Prior to admission, he did not take his insulin during the daytime while fasting to prevent hypoglycemia. Unlike our case, this patient had DKA with marked hyperglycemia and was not on an SGLT2 inhibitor. Similar to our case, this patient had a change in diet prior to admission, and his chest pain resolved after intravenous fluids and insulin infusion for treatment [12].
There are also reports of acute myocardial infarction complicated by DKA [13, 14]. One proposed mechanism suggests that ketoacidosis contributes to hyperviscosity, increased coagulation, and thrombosis. Furthermore, ketoacidosis can contribute to coronary spasm through endothelial dysfunction. These mechanisms may have contributed to chest pain in our patient even though he likely did not have ACS [13-15]. Alternatively, ACS may promote DKA since cardiac ischemia can lead to an increase in stress hormones [16] and free fatty acid release from an adipose tissue [17, 18].
Clinicians should use caution when prescribing SGLT2 inhibitors to patients at greater risk for euDKA, such as those with an infection. Sick-day strategies during acute illness for those taking SGLT2 inhibitors should be discussed with patients, such as temporarily discontinuing SGLT2 inhibitors, staying well hydrated, and contacting their provider [19, 20]. Patients should also avoid excess alcohol consumption and very low carbohydrate/ketogenic diets [21]. This is particularly important, as carbohydrate-restrictive diets, a popular approach to weight loss, with SGLT2 inhibitors can lead to euDKA. [7, 22-24]. A randomized, open-label study investigating the safety and efficacy of a SGLT2 inhibitor in individuals with differing carbohydrate intakes found that ketone bodies were significantly higher in the low carbohydrate group compared to the high carbohydrate group and concluded that a strict low carbohydrate diet while on SGLT2 inhibitor therapy should be avoided to prevent DKA [25].
CONCLUSION
SGLT2 inhibitors are increasingly used to improve cardiovascular outcomes for patients with diabetes. However, there is a small but real risk for euDKA with their use. Clinicians should include euDKA in their differential diagnosis when patients on SGLT2 inhibitors present with acute symptoms, as symptoms of euDKA may be protean and difficult to detect. In our case, euDKA presented with an atypical symptom such as chest pain. It is also important to detect euDKA and ensure these patients are well hydrated as precardiac catheterization may limit the risk of contrast-induced nephropathy. While patients with type 2 diabetes and atherosclerotic cardiovascular disease should always be counselled on the importance of lifestyle modifications, this case emphasizes the need for clinicians to specifically address and caution against very low-carbohydrate or ketogenic diets for patients on SGLT2 inhibitors, as this may be an underrecognized risk factor for euDKA.
Acknowledgments
FUNDING
Dr. Dorcely is supported by postdoctoral training grant T32 HL098129 from the NHLBI.
LIST OF ABBREVIATIONS AND ACRONYMS
- ACS
Acute Coronary Syndrome
- CAC
Coronary Artery Calcium
- CAD
Coronary Artery Disease
- euDKA
Euglycemic Diabetes Ketoacidosis
- SGLT2
Sodium-glucose Cotransporter-2
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
REFERENCES
- [1].Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015; 373(22): 2117–28. 10.1056/NEJMoa1504720 PMID: 26378978 [DOI] [PubMed] [Google Scholar]
- [2].Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017; 377(7): 644–57. 10.1056/NEJMoa1611925 PMID: 28605608 [DOI] [PubMed] [Google Scholar]
- [3].10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019; 42(Suppl. 1): S103–23. 10.2337/dc19-S010 PMID: 30559236 [DOI] [PubMed] [Google Scholar]
- [4].Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care 2015; 38(9): 1687–93. 10.2337/dc15-0843 PMID: 26078479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: Basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev 2017; 33(5): 33. [5]. 10.1002/dmrr.2886 PMID: 28099783 [DOI] [PubMed] [Google Scholar]
- [6].Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr 2013; 67(8): 789–96. 10.1038/ejcn.2013.116 PMID: 23801097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hayami T, Kato Y, Kamiya H, et al. Case of ketoacidosis by a sodium-glucose cotransporter 2 inhibitor in a diabetic patient with a low-carbohydrate diet. J Diabetes Investig 2015; 6(5): 587–90. 10.1111/jdi.12330 PMID: 26417418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019; 42(Suppl. 1): S90–S102. 10.2337/dc19-S009 PMID: 30559235 [DOI] [PubMed] [Google Scholar]
- [9].Scheen AJ. Cardiovascular Effects of New Oral Glucose-Lowering Agents: DPP-4 and SGLT-2 Inhibitors. Circ Res 2018; 122(10): 1439–59. 10.1161/CIRCRESAHA.117.311588 PMID: 29748368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Newman JD, Vani AK, Aleman JO, Weintraub HS, Berger JS, Schwartzbard AZ. The Changing Landscape of Diabetes Therapy for Cardiovascular Risk Reduction: JACC State-of-the-Art Review. J Am Coll Cardiol 2018; 72(15): 1856–69. 10.1016/j.jacc.2018.07.071 PMID: 30286929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Villines TC, Hulten EA, Shaw LJ, et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J Am Coll Cardiol 2011; 58(24): 2533–40. 10.1016/j.jacc.2011.10.851 PMID: 22079127 [DOI] [PubMed] [Google Scholar]
- [12].Mohamad NAR, Cheah PK. Chest pain in emergency department: A diagnosis of diabetic ketoacidosis must be ruled out. Int J Case Rep Imag 2010; 1(3): 6–9. 10.5348/ijcri-2010-11-5-CR-2 [DOI] [Google Scholar]
- [13].Dai Z, Nishihata Y, Kawamatsu N, et al. Cardiac arrest from acute myocardial infarction complicated with sodium-glucose cotransporter 2 inhibitor-associated ketoacidosis. J Cardiol Cases 2016; 15(2): 56–60. 10.1016/j.jccase.2016.10.006 PMID: 30546697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Batra AS, Acherman RJ, Wong P, Silka MJ. Acute myocardial infarction in a 12-year-old as a complication of hyperosmolar diabetic ketoacidosis. Pediatr Crit Care Med 2002; 3(2): 194–6. 10.1097/00130478-200204000-00021 PMID: 12780995 [DOI] [PubMed] [Google Scholar]
- [15].Carl GF, Hoffman WH, Passmore GG, et al. Diabetic ketoacidosis promotes a prothrombotic state. Endocr Res 2003; 29(1): 73–82. 10.1081/ERC-120018678 PMID: 12665320 [DOI] [PubMed] [Google Scholar]
- [16].Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000; 355(9206): 773–8. 10.1016/S0140-6736(99)08415-9 PMID: 10711923 [DOI] [PubMed] [Google Scholar]
- [17].Kurien VA, Oliver MF. Serum-free-fatty-acids after acute myocardial infarction and cerebral vascular occlusion. Lancet 1966; 2(7455): 122–7. 10.1016/S0140-6736(66)92420-2 PMID: 4161376 [DOI] [PubMed] [Google Scholar]
- [18].Schrieks IC, Nozza A, Stähli BE, et al. Adiponectin, Free Fatty Acids, and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Acute Coronary Syndrome. Diabetes Care 2018; 41(8): 1792–800. 10.2337/dc18-0158 PMID: 29903845 [DOI] [PubMed] [Google Scholar]
- [19].Wilding J, Fernando K, Milne N, et al. SGLT2 Inhibitors in Type 2 Diabetes Management: Key Evidence and Implications for Clinical Practice. Diabetes Ther 2018; 9(5): 1757–73. 10.1007/s13300-018-0471-8 PMID: 30039249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fitchett D A safety update on sodium glucose co-transporter 2 inhibitors. Diabetes Obes Metab 2019; 21(S2)(Suppl. 2): 34–42. 10.1111/dom.13611 PMID: 31081590 [DOI] [PubMed] [Google Scholar]
- [21].Handelsman Y, Henry RR, Bloomgarden ZT, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Position Statement on The Association of SGLT-2 Inhibitors and Diabetic Ketoacidosis. Endocr Pract 2016; 22(6): 753–62. 10.4158/EP161292.PS PMID: 27082665 [DOI] [PubMed] [Google Scholar]
- [22].Sood M, Simon BF Ryan K, Zebrower M Euglycemic Diabetic Ketoacidosis with Sglt2 Inhibitor Use in a Patient on the Atkins Diet: A Unique Presentation of a Known Side Effect. AACE Clin Case Rep 2018; 4: e104–7. 10.4158/EP171860.CR [DOI] [Google Scholar]
- [23].Taylor SI, Blau JE, Rother KI. SGLT2 Inhibitors May Predispose to Ketoacidosis. J Clin Endocrinol Metab 2015; 100(8): 2849–52. 10.1210/jc.2015-1884 PMID: 26086329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Saponaro C, Pattou F, Bonner C. SGLT2 inhibition and glucagon secretion in humans. Diabetes Metab 2018; 44(5): 383–5. 10.1016/j.diabet.2018.06.005 PMID: 30017776 [DOI] [PubMed] [Google Scholar]
- [25].Yabe D, Iwasaki M, Kuwata H, et al. Sodium-glucose co-transporter-2 inhibitor use and dietary carbohydrate intake in Japanese individuals with type 2 diabetes: A randomized, open-label, 3-arm parallel comparative, exploratory study. Diabetes Obes Metab 2017; 19(5): 739–43. 10.1111/dom.12848 PMID: 27990776 [DOI] [PMC free article] [PubMed] [Google Scholar]