Abstract
Non-small cell lung cancer (NSCLC) is a malignant tumor with high morbidity and mortality rates, which seriously endangers human health. Although treatment methods continue to evolve, the emergence of drug resistance is inevitable and seriously hinders the treatment of NSCLC. The tumor microenvironment (TME) protects tumor cells from the effects of chemotherapeutic drugs, which can lead to drug resistance. Cancer-associated fibroblasts (CAFs) are an important component of the TME, and various studies have demonstrated that CAFs play a crucial role in drug resistance in NSCLC. However, the drug resistance mechanism of CAFs and whether CAFs can be used as a target to reverse the resistance of tumor cells remain unclear. The present review discusses this issue and describes the heterogeneity of CAF markers, as well as their origins and resident organs, and the role and mechanism of this heterogeneity in NSCLC progression. Furthermore, the mechanism of CAF-mediated NSCLC resistance to chemotherapy, targeted therapy and immunotherapy is introduced, and strategies to reverse this resistance are described.
Keywords: cancer-associated fibroblasts, non-small cell lung cancer, resistance, mechanism, therapy strategy
1. Introduction
Lung cancer is a malignant disease with high morbidity and mortality rates, and non-small cell lung cancer (NSCLC) accounts for 80–85% of all lung cancer cases. Clinically, only a small percentage of patients with NSCLC are diagnosed at an early stage (I or II), at which tumors can be surgically removed. The majority of patients with NSCLC present with locally advanced or metastatic disease at the time of diagnosis, leaving chemotherapy, targeted therapy, and immunotherapy as the primary treatment strategies (1). However, primary resistance and acquired resistance after long-term drug usage are inevitable problems (2). Previous data suggest that the 5-year survival rate of patients with advanced NSCLC is <5% (3). Moreover, the occurrence of drug resistance is a major obstacle to successful treatment, which requires urgent medical attention (3,4).
Existing therapeutic approaches primarily counter drug resistance by targeting tumor cells and sparing those of the tumor microenvironment (TME). Since the concept of ‘seed and soil’ was proposed, the role of the TME in tumor drug resistance has received increasing attention. For example, a hypoxic microenvironment was found to induce cisplatin resistance in NSCLC (5,6). Collagen, a component of the extracellular matrix (ECM), induces NSCLC resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) by binding to the collagen receptor integrin α11β1 (7). In addition to these physical factors, stromal cells that surround the tumor, such as cancer stem cells (CSCs) (8) and stromal fibroblasts (9), can induce therapeutic resistance in NSCLC. Cancer-associated fibroblasts (CAFs) are an important component of the TME (10), which serve a primary role in drug resistance. Compared with tumor cells, CAFs are considered to be genetically stable with few mutations (11), and can influence tumor progression through the secretion of ECM proteins, proteases, cytokines, chemokines and growth factors (12,13). In addition, CAFs are involved in drug resistance in various malignancies, such as head and neck (14), breast (15), ovarian (13), gastrointestinal (16), pancreatic (17) and colorectal cancer (18), though the underlying mechanisms differ between tumor types. Thus, the mechanisms of CAF-mediated NSCLC resistance have gained considerable attention (19). The present review describes the functions and mechanisms of CAFs in NSCLC drug resistance, as well as potential strategies to reverse this effect.
2. Heterogeneity of CAFs
Numerous types of stromal cell, including fibroblasts, are present in the TME. Fibroblasts are activated in response to cancer cells, after which they are referred to as CAFs or myofibroblasts. CAFs are spindle-shaped cells of variable size and proliferative capacity (20,21). Moreover, CAFs exhibit high heterogeneity in terms of origin, surface markers and resident organs, which determines their functions in tumor progression. During the early stages of tumor development, CAFs play an antitumor role by promoting tissue repair. However, as the tumor progresses, CAFs promote tumor growth, metastasis and drug resistance.
Heterogeneity of CAF origins
Studies have demonstrated that cells, including resident tissue fibroblasts, bone marrow (BM)-derived mesenchymal stromal cells (MSCs), epithelial cells, endothelial cells, CSCs, hematopoietic stem cells (HSCs), vascular smooth muscle cells (VSMCs) and pericytes may act as the predecessors of CAFs (22–30). When healthy tissue is damaged and malignancy develops, immune cells are recruited to the site of injury, and via the release of specific mediators, activate the differentiation of resident fibroblasts into CAFs (22). In this manner, human breast fibroblasts gradually differentiate into CAFs, promoting tumor progression by establishing TGF-β and stromal-derived factor autocrine signals (23). TGF-β1 is the primary factor that activates the conversion of resident fibroblasts into CAFs. Moreover, hypoxia also promotes this process via the accumulation of reactive oxygen species (ROS) and the activation of the hypoxia-inducible factor (HIF)-1α-mediated signaling pathway (24). In addition, VSMCs and pericytes can differentiate into CAFs in breast cancer (25). BM-MSCs can also differentiate into CAFs. For example, CAFs with the phenotype and functional characteristics identical to those of BM-MSCs were isolated from primary human neuroblastoma tumors (26). In colon tumors, CAFs are generated via the activation of native mesenchymal cell populations and the recruitment of BM-MSCs (27). Furthermore, mouse-induced pluripotent stem cells were treated with conditioned media to generate CSC-like cells, which are a heterogeneous population surrounded by myofibroblast-like cells. At the same time, the expression of fibroblast activation protein (FAP), alpha-smooth muscle actin (α-SMA), and other key CAF markers was significantly increased, and for the first time, CSCs were confirmed to be the key origin of CAFs in the TME (28). Studies in two different pancreatic cancer mouse models also revealed that endothelial-mesenchymal transition transforms fibroblasts into CAFs after exposure to TGF-H1 (29). Moreover, fibroblast-specific protein-1+fibroblastscan be derived from epithelial-mesenchymal transformation (EMT) in the local environment (30). McDonald et al (31) found that CAFs derived from HSCs promoted the generation of tumor blood vessels. The origins of different CAFs populations are listed in Table I.
Table I.
Origins of CAFs.
| First author, year | Origin of CAFs | Cancer type | (Refs.) |
|---|---|---|---|
| Foster et al, 2018; | Resident tissue fibroblasts | Breast cancer | (22,23) |
| Kojima et al, 2010 | |||
| Borriello et al, 2017 | Marrow-derived mesenchymal stem cells | Neuroblastoma | (26) |
| Koliaraki et al, 2017 | Marrow-derived mesenchymal stem cells | Colon cancer | (27) |
| Zeisberg et al, 2007 | Endothelial cells | Pancreatic cancer | (29) |
| Nair et al, 2017 | Cancer stem cells | Breast cancer | (28) |
| McDonald et al, 2015 | Hematopoietic stem cells | Breast cancer | (31) |
| An et al, 2020 | Vascular smooth muscle cells | Breast cancer | (25) |
| An et al, 2020 | Pericytes | Breast cancer | (25) |
CAFs, cancer-associated fibroblasts.
Heterogeneity of CAF markers
The expression of CAF markers can be determined by immunofluorescence and immunohistochemical staining, and quantitatively detected by western blotting. As a heterogeneous cell population, different markers can be used to identify CAFs, the most common of which are podoplanin (PDPN), platelet-derived growth factor receptor (PDGF-R), vimentin, α-SMA and FAP. However, in isolation, none of these markers can specifically identify CAFs (32). PDPN+ CAFs are able to promote tumor formation (33). The expression of PDPN was detected in the CAFs of 177 patients with lung adenocarcinoma, and PDPN+ CAFs were found only in invasive rather than non-invasive adenocarcinoma (34). The expression of PDPN promotes platelet aggregation and contributes to cancer cell invasiveness (34). Therefore, PDPN+ CAFs are closely associated with the aggressiveness of various cancer types, including lung adenocarcinoma (33,34), breast cancer (35) and squamous cell carcinoma (36). PDGF-Rs can be categorized as PDGFR A and PDGFR B. PDGFR ligands include the PDGFs (PDGF-aa, PDGF-bb, PDGF-ab, PDGF-cc and PDGF-dd), and their expression is closely related to tumor occurrence and CAFs function (37). Vimentin is involved in the formation of cytoskeletal networks, especially in mesenchymal-derived cells. Due to their strong mesenchymal phenotype, vimentin is highly expressed in all types of fibroblasts, and has therefore been widely used for the identification of CAFs (38–40). Park et al (41) demonstrated that vimentin promotes lung cancer invasion and metastasis by promoting the recruitment of CAFs. CAFs are divided into two distinct clusters, namely C1-type and C2-type CAFs. Notably, the expression of α-SMA is lower in C1-type compared with the C2-type CAFs, though C1-type CAFs inhibit the self-renewal of oral cancer cells by releasing bone morphogenetic protein 4 (42). α-SMA is expressed by various cell types, including fibroblasts, making it impossible to use alone as a marker for CAF recognition. The upregulation of FAP is associated with poor prognosis in >90% of epithelial cancer types (43–45). Due to its high expression level in the tumor stroma, FAP has been used as a CAF identification marker in numerous studies (46). FAP+ CAFs control tumor progression by secreting chemokine (C-X-C motif) ligand 12 (CXCL12) and binding to its receptor chemokine (C-X-C motif) receptor 4 (CXCR4). FAP+CAFs increase T cell recruitment and promote the antitumor effect by mediating CXCL12/CXCR4 axis deletion in pancreatic ductal adenocarcinoma (47,48). In addition, FAP-activated prodrugs have been demonstrated as a feasible strategy for treating cancer, such as prostate cancer and breast cancer (49). Thapsigargin (TG) is a highly toxic natural plant product; a cytotoxic TG analog was coupled to FAP-selective peptide substrates to create inactive prodrugs, and when the prodrugs were activated, they led to apoptosis of prostate and breast cancer cells, but had no obvious toxicity to host cells (49). No single marker can mark all the CAFs, and not all CAFs express all potential marker proteins. Therefore, there is still a need to investigate CAFs-specific markers. In addition to the common CAFs markers, there are other less commonly used markers, such as microfibrillar-associated protein 5 (MFAP5), collagen type XI alpha 1 chain, tenascin-C, PDPN, integrin α11β1, neural/glial antigen, collagen 11-α1 and asporin (40). However, collagen 11-α1, MFAP5 and asporin are expressed only by CAFs, which can improve the specificity of their identification (50). Currently, a combination of markers, as well as cellular phenotype, is the most reliable method for the identification of CAFs.
Heterogeneity of CAF resident organs
Though CAFs lack specific markers, literature reports that CAF markers may possess organs heterogeneity, and that CAFs expressing the same marker may possess different functions in different organs. For example, in ovarian cancer, PDGF-R+ CAFs promote tumor progression by remodeling the ECM (51). However, CAFs expressing PDGF increase the levels of the Puma in myofibroblasts, which subsequently activates Bak, a pro-apoptotic protein that induce cholangiocarcinoma cell apoptosis (52). Similarly, CAFs may express different markers in different organs. PDGF-R is expressed by a variety of different CAFs; however, those originating from BM-MSCs do not express PDGF-Rα in breast tumors and lung metastases (53). At present, CD200 is only known to be highly expressed in CAFs derived from NSCLC and can promote the sensitivity of NSCLC to EGFR-TKIs (54). Su et al (8) demonstrated that CD10+/GPR77+CAFs continually promote p65 phosphorylation and acetylation by binding GPR77 receptor C5a, thereby promoting the self-renewal of tumor stem cells and enhancing drug resistance in patients with lung and breast cancer.
Role of CAF heterogeneity in NSCLC
Heterogeneity between origins, markers and resident organs determines the different functions of CAFs. Genetically engineered mouse models and clinical studies have demonstrated that at least two types of CAFs with different functions exist, namely pro-cancer CAFs (pCAF) and anticancer CAFs (rCAF) (55). CAFs also exhibit different functions in tumor progression. In NSCLC, the functional heterogeneity of CAFs primarily results from differences in expression markers, and there are few studies on the functional differences caused by the heterogeneity of origins. CAFs that exert pro-tumor effects include α-SMA+PDPN+, FAP+, CD34+ and CD10+/GPR77+CAFs. CAFs with antitumor properties include CD200+ and CD99+CAFs. The pro/anti-tumor effect of CAFs in NSCLC progression are summarized in Table II.
Table II.
Pro- and antitumor effects of CAFs in non-small cell lung cancer progression.
| First author, year | CAF markers | Samples, n | Pro/antitumor effect | (Refs.) |
|---|---|---|---|---|
| Alcaraz et al, 2019 | α-SMA | 220 | Pro | (56) |
| Yoshida et al, 2015; | Podoplanin | 177 | Pro | (61,62) |
| Neri et al, 2015 | ||||
| Kilvaer et al, 2015; | FAP | 536 | Pro | (59,60) |
| Cohen et al, 2008 | ||||
| Schulze et al, 2020 | CD34 | 304 | Pro | (57) |
| Su et al, 2018 | CD10+/GPR77+ | Pro | (8) | |
| Ishibashi et al, 2017 | CD200 | Anti | (54) | |
| Edlund et al, 2012 | CD99 | Anti | (63) |
CAF, cancer-associated fibroblast; α-SMA: α-smooth muscle actin; FAP, fibroblast activation protein.
Pro-tumor effect of CAFs
CAFs promote tumor growth and metastasis, as well as tumor cell drug resistance. Tissue analysis revealed a high mortality rate among 220 patients with NSCLC and high α-SMA expression, indicating that α-SMA is associated with poor survival time (56). Immunohistochemical analysis of 304 patients with pTNM stage I–III NSCLC revealed that CAF-associated CD34 expression was an independent prognostic factor for stage I–III NSCLC, and that SMA+CAFs were associated with higher tumor stages and promoted tumor progression (57). The 28 patients with NSCLC were divided into two CAF subgroups, the high desmoplastic CAFs (HD-CAFs) and low desmoplastic CAFs (LD-CAFs), according to the obtained scores and classification based on desmoplasia. Compared with LD-CAFs, HD-CAFs exhibited a higher collagen matrix remodeling rate and promoted tumor invasion and growth (58). The immunohistochemical analysis of tumor samples from 536 patients with NSCLC indicated that CAFs expressing FAP-1 were associated with poor patient prognosis, which has also been demonstrated in patients with pancreatic cancer. In addition, FAP+CAFs have been associated with reduced survival time (59,60). Yoshida et al (61) demonstrated that compared with the control group, lung adenocarcinoma cells co-cultured with PDPN+ CAFs possessed greater drug resistance properties. In patients with postoperative recurrence, compared with the PDPN-CAF group, the PDPN+ group showed a lower treatment response to EGFR-TKIs. These results suggest that PDPN+ CAFs are involved in primary drug resistance to these compounds. Using the collagen invasion assay model of co-culture between cancer cells and CAFs, CAFs were found to invade local tissues through ECM remodeling, followed by subsequent cancer cell invasion. Furthermore, the down regulation of CAF-associated PDPN reduced the invasiveness of CAFs and cancer cells (62). In addition, CD10+/GPR77+CAFs maintain the stemness of CSCs and promote drug resistance in patients with lung cancer (8). Therefore, it is undeniable that heterogeneity of origin is an important influencing factor of CAF function, which is a valuable future research direction.
Anti-tumor effect of CAFs
In addition to their tumor-promoting functions, CAFs also possess antitumor properties. For example, CD200+ CAFs enhanced the sensitivity of lung cancer to gefitinib. Moreover, individuals with CD200+ CAFs exhibit longer progression-free survival after gefitinib treatment following post-surgical relapse. The binding of CD200 to its receptor CD200R1, which is expressed by immune cells, triggers an immunosuppressive response, leading to an antitumor effect (54). In addition, CD99 is a newly discovered CAF marker, the overexpression of which may inhibit tumor progression (63). However, the tumor-suppressive mechanism of CAFs remains unclear, and requires further investigation.
3. Roles and mechanisms of CAFs in NSCLC drug resistance
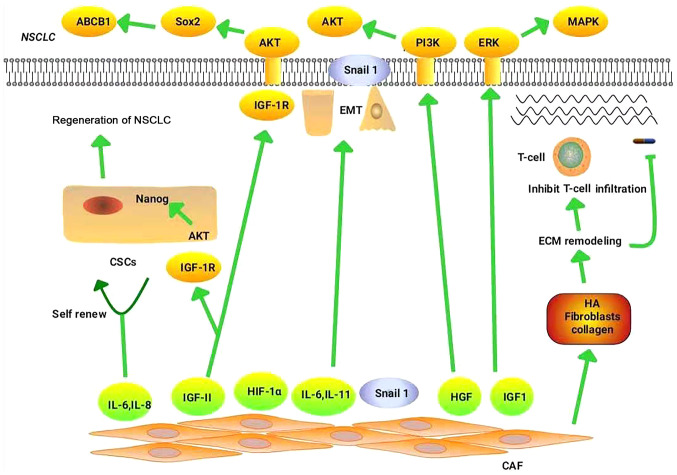
CAFs influence tumor formation by promoting drug resistance, though the associated underlying mechanisms remain unclear. Clarifying these mechanisms may help to prevent the occurrence of drug resistance in NSCLC. Next, the mechanisms by which CAFs mediate the resistance of NSCLC to chemotherapy, targeted therapy and immunotherapy, are explored. Fig. 1 illustrates the mechanisms by which CAFs regulate drug resistance in NSCLC.
Figure 1.
Mechanism of CAFs in NSCLC drug resistance. CD10+/GPR77+CAFs can maintain the stemness of CSCs by secreting IL-6 and IL-8, thereby promoting drug resistance in patients with NSCLC. CAFs promote NSCLC resistance mainly through the following pathways: HGF/PI3K/AKT, IGF-II/IGF1R/Nanog, IGF-II/IGF-1R/AKT/Sox2/ABCB1 and IGF1/IGF1R/ERK/MAPK. CAFs increase TGF-β1-induced EMT in NSCLC by secreting IL-6. CAFs promote NSCLC drug resistance by regulating the hypoxic microenvironment through high expression of HIF-1α. CAFs deliver Snail to lung cancer cells through exosomes, which induce EMT in these cells and promote drug resistance. CAFs increase the stiffness of the matrix by enhancing ECM components (such as HA, fibroblasts and collagen), thereby preventing the binding of immune checkpoint inhibitors to their receptors, and prevent the infiltration and migration of immune cells, thereby promoting immune escape. In addition, ECM stiffness functions as a barrier to tumor cell drug absorption. CAF, cancer-associated fibroblast; NSCLC, non-small cell lung cancer; CSCs, cancer stem cells; IL, interleukin; HGF, hepatocyte growth factor; PI3K, phosphatidylinositol 3 kinase; AKT, protein kinase B; IGF-II, insulin-like growth factor-II; IGF1R, insulin-like growth factor 1 receptor; ABCB1, ATP-binding cassette sub-family B member 1; ERK, extracellular signal-regulated kinases; MAPK, mitogen-activated protein kinase; TGF-β1, transforming growth factor-β1; HIF-1α, hypoxia-inducible factor-1α; EMT, epithelial-mesenchymal transition; ECM, extracellular matrix; HA, hyaluronic acid.
Roles and mechanisms of CAF-associated chemotherapeutic resistance
CAFs promote the resistance of NSCLC to chemotherapy primarily by mediating EMT (19), remodeling the ECM (7,11), maintaining the stemness of CSCs (8) and promoting metabolic reprogramming (64–66).
EMT
EMT is an important developmental process that is closely associated with drug resistance (67,68). During EMT, epithelial cell markers, such as N-and E-cadherin, are downregulated, while mesenchymal cell markers, such as vimentin and fibronectin, are upregulated. Previous studies have reported that EMT is associated with drug resistance in pancreatic (69), bladder (70), breast (71) and colorectal cancer (72). Compared with the control group, the expression of E-cadherin in the indirect CAF co-culture group was reduced, the expression of vimentin was enhanced, and migration and invasion ability were correspondingly augmented (20). Therefore, CAFs are among the factors mediating EMT. Studies have shown that CAFs regulate EMT and promote drug resistance by secreting IL-6 and hepatocyte growth factor (HGF) (73). For example, CAFs significantly increased TGF-β1-induced EMT in cancer cells by secreting IL-6, thereby contributing to cisplatin resistance in NSCLC (74). This process involves the expression of TGF-β1, and silencing TGF-β1 reverses EMT and thus increases the sensitivity of NSCLC to cisplatin (74). HGF, also known as scatter factor, is a member of the fibrinogen family that which functions to activate EMT (75). Ying et al (19) investigated the functions of HGF in the paclitaxel resistance of NSCLC by constructing a three-dimensional microfluidic chip. The high levels of HGF secreted by CAFs enhanced the phosphatidylinositol 3 kinase/protein kinase B (PI3K/AKT) activation, as well as the expression of GRP78, and promoted the resistance of NSCLC to paclitaxel. CAFs were also found to induce EMT in NSCLC cells, inducing resistance to chemotherapy. Therefore, targeting CAFs may enhance the therapeutic effect of drugs towards NSCLC.
CSCs
Studies have reported the presence of white blood cells with stem cell-like properties in patients with acute myeloid leukemia, which are designated as CSCs (76,77). CSCs are a subgroup of tumor cells that exhibit strong resistance to chemotherapy (77,78), of which there are two primary underlying mechanisms. The first outlines that in a hypoxic microenvironment, CSCs remain quiescent in a non-permanent dormant state, and that chemotherapeutic drugs primarily target rapidly dividing cancer cells, allowing quiescent stem cells to survive and regenerate to form tumors at a later point in time (79). Another mechanism is the use of ATP-binding box (ABC) transporters to expel chemotherapeutic drugs, resulting in drug resistance (80). CAFs primarily promote NSCLC drug resistance by maintaining the stemness of CSCs, and stimulating their self-renewal. When CAFs are co-cultured with CSCs, CAF-associated insulin-like growth factor-II (IGF-II) activates the insulin-like growth factor 1 receptor (IGF1R) on CSCs, thereby activating the IGF-II/IGF1R/Nanog signaling pathway to maintain CSCs stemness, both in vivo and in vitro. In turn, CSCs promote CAF-associated IGF-II secretion via cytokines such as basic fibroblast growth factor. The IGF-II/IGF1R axis promotes the expression of Nanog in cancer cells, and blocking the IGF-II/IGF1R/Nanog pathway reduces the stemness of CSCs (12). CAFs exhibit high CD44 expression in tumor hypoxic and avascular areas, and CAF CD44 expression is significantly increased following treatment with an angiogenesis inhibitor. Through co-cultures and tumor sphere formation assays, CAFs were found to maintain the stemness of CSCs and enhance the resistance of tumor cells to anticancer drugs, properties that were not exhibited by CD44-deficient CAFs (81). In addition, CD10+/GPR77+CAFs can maintain the stemness of CSCs by secreting IL-6 and IL-8, thereby promoting drug resistance in patients with lung cancer (8). According to these studies, CAFs can promote the chemotherapeutic drug resistance of NSCLC by regulating CSCs.
ECM remodeling
Under normal physiological conditions, the ECM supports the proliferation and migration of surrounding cells. Cancer tissue is generally stiffer than normal tissue. The stiffness of the ECM is primarily attributed to the accumulation of hyaluronic acid (HA) at its core, which can withstand the compressive stress of the tumor, while the accumulation of collagen and fibronectin in the periphery promotes resistance to tensile stress. ECM stiffness functions as a barrier to tumor cell drug absorption (82). CAFs promote tumor resistance by increasing matrix stiffness through the enhancement of ECM components such as HA and collagen. Collagen is resistant to tensile stress, as it becomes harder on stretching (11). HA is also resistant to stress. Integrin α11β1 is a specific collagen receptor associated with increased collagen stiffness. CAFs can affect the stiffness of interstitial collagen by expressing high levels of integrin α11, which promotes the progression of NSCLC tumors (7). NSCLC cells cultured on a semi-solid growth substrate (to simulate the stiffness of the matrix in the TME) can promote the resistance of NSCLC to chemotherapeutic drugs (83). In lung adenocarcinoma, PDPN+ CAFs physically remodel the ECM (62). Therefore, CAFs can promote NSCLC resistance to chemotherapy via ECM remodeling.
Metabolic reprogramming
Due to mitochondrial defects, cancer cell metabolism is altered, the ability to oxidize glucose to CO2 is inhibited, and the propensity to convert glucose into lactic acid increases. These phenomena are collectively known as the Warburg effect (64,84), which is mediated by pyruvate kinase M2 (PKM2). PKM2 is upregulated in NSCLC cell lines and can promote NSCLC resistance to cisplatin (65). Under hypoxic conditions, cisplatin-resistant cells secrete exosomes containing high concentrations of PKM2, which are absorbed by cisplatin-sensitive cells. Exosomal PKM2 also regulates glycolysis in treatment-sensitive cells, promoting cell survival and inhibiting apoptosis. Secondly, in the tumor microenvironment, exosomes secreted by the cisplatin-resistant cells deliver PKM2 to CAFs, and the metabolically reprogrammed CAFs release pyruvate and lactate, promoting chemotherapeutic resistance (66). In addition, CAF autophagy releases lactic acid, ketone bodies and glutamine to create a nutrient-rich microenvironment that supports tumor growth (64).
Roles and mechanisms of CAFs in the resistance to targeted therapy
CAFs mainly promote tumor EMT (85–92) and create a hypoxic microenvironment (93–96) to render NSCLC cells resistant to targeted therapy.
EMT
EMT is a reversible process regulated by several EMT-related transcription factors (EMT-TFs), such as Snail, Slug, Twist and zinc finger E-box-binding homeobox 1 (ZEB1). EMT enhances the migration and invasiveness, as well as the resistance of cancer cells to targeted therapy (85). For example, the A549 lung cancer cell line developed drug resistance after long-term treatment with gefitinib. These gefitinib-resistant cells showed reduced expression of E-cadherin and vimentin, indicating the occurrence of EMT (86). Moreover, the expression of the EMT regulator ZEB1 was increased in the HCC4006ER erlotinib-resistant cell line. HCC4006ER cells acquired an EMT phenotype and were able to activate the TGF-β1/SMAD pathway (87). Snail, a key transcription factor for EMT, is closely associated with chemotherapy resistance. CAFs deliver Snail to lung cancer cells through exosomes, which induce EMT in these cells and promote drug resistance (88). However, whether CAFs can also promote NSCLC drug resistance by regulating other EMT-TFs remains to be elucidated. EMT is associated with NSCLC-targeted drug resistance, which is primarily achieved through extracellular signal-regulated kinases/mitogen-activated protein kinase (ERK/MAPK), Hedgehog (Hh) and other related signaling pathways. CAFs can activate corresponding receptors in NSCLC through the upregulation of growth factors such as HGF and IGF-1, and also regulate EMT and gefitinib resistance in a paracrine manner (89). IGF1R induces EMT in NSCLC cells and increases their resistance to EGFR-TKIs by enhancing the ERK/MAPK signaling pathway, small interfering (si)RNAs targeting IGF1R reversed the EMT phenotype and resistance to EGFR-TKIs (90). Choe et al (91) reported that co-culturing CAFs with NSCLC stimulated CAFs to induce EMT by activating the Hh signaling pathway, making PC9 cells resistant to erlotinib. A combination of the cell surface molecules Patched and Smoothened with the ligands sonic hedgehog, Indian hedgehog and desert hedgehog activates the transcription factor GLI1, thereby activating the Hh pathway and mediating tumor cell resistance to EGFR-TKIs by inducing EMT (92). In summary, CAFs can promote NSCLC resistance to targeted therapy by regulating EMT-TFs, and by activating multiple pathways, which also indicates that CAFs play an important role in the resistance of NSCLC to targeted therapy.
Hypoxic microenvironment
Hypoxia is a hallmark feature of the TME, and is considered to be one of the key factors for drug resistance in tumors (97). Cancer cells are often in a state of hypoxia that promotes tumor growth (98). In rapidly growing tumors, the distance between cells and blood vessels increases, which in turn impedes drug absorption into the tumor, especially in a hypoxic environment (99). For example, EGFR-mutated NSCLC cell lines exposed to high concentrations of gefitinib under low oxygen conditions acquired drug-resistant cells, known as gefitinib-resistant persistent cells (GRPs). Moreover, stem cell-associated genes are highly expressed in GRPs. This process is primarily mediated by an upregulation in IGF1 expression by HIF1, which in turn activates IGF1R on GRPs, thereby promoting NSCLC resistance to gefitinib and increasing CSCs numbers (93). The expression level of HIF-1α is upregulated in CAFs (94), indicating that these cells may promote NSCLC drug resistance by regulating the hypoxic microenvironment. In addition, HIF-1 can promote NSCLC drug resistance by inducing the expression of ABC transporters (99). EBC-1R is an NSCLC cell line resistant to the EMT inhibitors PHA-665752 and crizotinib, which possesses the characteristics of CSCs, and forms spheres (95) in which the expression of ATP-binding cassette sub-family B member 1 (ABCB1) is upregulated. Drug resistance is reversed following treatment with the ABCB1 inhibitor elacridar (95). IGF-II is an insulin-like hormone that plays an important role in regulating cellular proliferation, differentiation, senescence and drug resistance. CAFs regulate NSCLC cell drug resistance by secreting IGF-II and binding to the membrane receptor IGF-1R, in addition to activating the IGF-II/IGF-1R/AKT/Sox2/ABCB1 pathway in cancer cells, which in turn upregulates the expression of P-glycoprotein (96).
Roles and mechanisms of CAFs in immunotherapeutic resistance
Over the past few years, immune checkpoint inhibitors have played an important role in clinical trials, and have been approved as the standard therapy for advanced NSCLC (100). For instance, nivolumab and pembrolizumab targeting programmed cell death protein 1 (PD-1), atezolizumab targeting programmed cell death ligand 1 (PD-L1), and tremelimumab targeting cytotoxic T-lymphocyte antigen 4 have been approved by the United States Food and Drug Administration for NSCLC treatment (101). However, only 15–25% of patients with NSCLC respond to immune checkpoint inhibitors, the majority of which experience primary drug resistance (102). At present, only a limited number of studies have reported CAF-mediated NSCLC resistance to immune checkpoint inhibitors, although this has also been reported for other tumor species. In NSCLC, CAFs primarily prevent the infiltration and migration of immune cells by remodeling the ECM and preventing the binding of immune checkpoint inhibitors to their receptors, thus prompting immune escape. The density and direction of the ECM influence the behavior and migration of T cells in human lung cancer. T cells generally accumulate in areas with loose stromal fibers (103), and a dense ECM serves as a contact barrier between T cells and the tumor cells (104). It also prevents T cells from binding PD-1 inhibitors, and thus promotes the resistance of tumor cells to immune checkpoint inhibitors (103). The ECM includes collagen, laminin and fibronectin. Lysyl oxidase crosslinks collagen molecules into fibers to form a dense ECM, which inhibits the migration of T cells and reduces the effect of PD-1 inhibitors (104). In a xenograft model of NSCLC, CAFs overexpressing lysyl oxidase like-1 were found to remodel the collagen matrix in vivo (105), suggesting that CAFs promote NSCLC resistance to immunotherapeutic drugs through the ECM. CAFs are the primary producers of TGF-β (106) and can influence T cell infiltration via TGF-β. TGF-β signaling was demonstrated to inhibit T cell infiltration in breast mouse tumor models (104). CAFs specifically inhibit CD8+ T cell infiltration, thereby promoting tumor resistance to different immunosuppressive agents (107). In addition, CAFs can function as antigen-presenting cells and induce T-cell death in an antigen-dependent manner via PD-L2 and FASL (108). Compared with patients with PD-L1-CAFs, those with PD-L1+ CAFs exhibited significantly prolonged relapse-free survival, and the expression of PD-L1 in CAFS was affected by IFN-γ (109). At present, literature reporting the correlation between CAFs and immune checkpoint inhibitors is limited. The correlation between CAF-associated surface markers and immune markers was studied in 536 patients with NSCLC, and the results indicated that CAFs had little effect on immune cell infiltration in NSCLC (110). Therefore, whether CAFs also promote the drug resistance of tumor cells by inhibiting T cell infiltration requires investigated further.
4. Therapeutic strategies
The strategy for reversing NSCLC drug resistance is displayed in Table III.
Table III.
Strategies to reverse non-small cell lung cancer drug resistance.
| First author, year | Factor | Mechanisms | Resistant to | Inhibitor of | (Refs.) |
|---|---|---|---|---|---|
| Shien et al, 2017 | IL-6 | OSMRs/JAK1/STAT3 | Chemotherapy | JAK1 | (119) |
| Rotow et al, 2017 | HGF | HGF/ERK | Targeted therapy | HGF | (2) |
| Tao et al, 2016 | IL-11 | IL-11R/STAT3 | Chemotherapy | STAT3 | (118) |
| Zhang et al, 2018 | IGF | IGF1R/AKT/Sox2/P-GP | Chemotherapy | IGF2 | (96) |
| Wang et al, 2019 | ANXA3 | ANXA3/JNK | Chemotherapy | JNK | (116) |
| Wei et al, 2020 | GGT5 | Chemotherapy | GGT5 | (117) | |
| Najafi et al, 2019; Rebelo et al, 2018 | MMPs | Degradation of the ECM | Chemotherapy/immunotherapy | (82,121) |
OSMRs, oncostatin-M; JAK1, Janus kinase1; STAT3, signal transducer and activator of transcription 3; HGF, hepatocyte growth factor; IGF, insulin-like growth factor; IGF1R, insulin-like growth factor receptor-1; P-GP, P-glycoprotein; ANXA3, Annexin A3; GGT5, γ-glutamyl transferase 5; MMP, matrix metalloproteinase; ECM, extracellular matrix.
Targeting CAFs
There are currently two available strategies for reversing drug resistance by targeting CAFs, one of which is to inhibit the production of CAFs, while the other is to block the pathways downstream of them.
Fibroblast to CAFs transformation relies on the expression of TGF-β. Treatment with TGF-β rapidly activates the TGF-β signaling pathway, resulting in the transformation of fibroblasts into myofibroblast phenotype. Myofibroblast transdifferentiation requires the production of ROS, and the expression of NAD(P)H Oxidase-4 (NOX4) is associated with the CAF marker α-SMA. A NOX4 inhibitor (GKT137831) or targeted NOX4-knockout (short hairpin RNA and siRNA) reduced the accumulation of CAFs. Therefore, CAF generation can be inhibited by decreasing NOX4 expression, which may reduce the occurrence of NSCLC drug resistance (111). Pirfenidone is a pyridine compound that inhibits fibroblast proliferation and CAF differentiation and activation (112). FAP is expressed by the majority of CAFs, and T cells can be genetically modified to express FAP-specific chimeric antigen receptors. These FAP-specific T cells recognize and destroy FAP+CAFs with subsequent antitumor effects (113). Some CAFs possess myofibroblast characteristics and express α-SMA, which can significantly promote NSCLC resistance to chemotherapy via the expression of high levels of inflammatory cytokines and chemokines (114). Plasminogen activator inhibitor-1 (PAI-1) can promote the MF characteristics of CAFs, and the expression of PAI-1 in CAFs is correlated with the expression of α-SMA (114). PAI-1 inhibitors also decrease the expression levels of α-SMA and inhibit the MF characteristics of CAFs, improving chemotherapeutic efficacy in NSCLC (114). Thus inhibiting the MF properties of CAFs may be a novel therapeutic strategy for the treatment of chemotherapy-resistant NSCLC (114).
Currently, the primary methods of reversing NSCLC drug resistance are via the inhibition CAF downstream pathways. According to literature, resistance can be reversed by targeted inhibition of the cytokines secreted by CAFs, such as IL-6 (115), IGFII (96), HGF (2), Annexin A3 (ANXA3) (116) and γ-glutamyl transferase 5 (GGT5) (117). CAFs express IL-6 to upregulate Bcl-2 and Mcl-1, reduce the sensitivity of NSCLC to cisplatin, and protect NSCLC cells from apoptosis (115). A combination of IL-6-targeted inhibitors and cisplatin can either reduce or inhibit the cisplatin resistance in NSCLC (115). CAFs regulate NSCLC cell drug resistance through the secretion of IGF2 and by binding IGF-1R, activating the AKT/Sox2/P-GP pathway in cancer cells. Traditional chemotherapeutic regimes, combined with IGF2-targeted inhibitors, may serve as an innovative therapeutic strategy for NSCLC (96). CAFs activate ERK by secreting HGF, which contributes to the resistance of NSCLC cells to EGFR-TKIs, and combination therapy with HGF-targeted drugs restores the sensitivity of cancer cells to EGFR-TKIs (2). In addition, the expression of ANXA3 is higher in CAFs than in normal fibroblasts (NFs). Furthermore, the overexpression of ANXA3 increased the cisplatin resistance of lung cancer cells. The underlying mechanism was that CAFs enhanced chemotherapeutic resistance by activating the ANXA3/JNK signaling pathway to inhibit cisplatin-induced apoptosis. The resistance of cancer cells to cisplatin can also be decelerated using JNK-targeting inhibitors (116). GGT5 is a member of the γ-glutamyl transpeptidase family that is abundantly expressed by CAFs, promoting NSCLC resistance to paclitaxel and cisplatin. NSCLC regains its sensitivity to chemotherapy drugs when GGT5 is blocked (117). Furthermore, CAFs secrete IL-11, which activates the IL-11R/STAT3 anti-apoptotic signaling pathway by binding to IL-11R, thereby promoting the chemotherapeutic resistance of NSCLC. STAT3 inhibitors can obstruct this process and reverse drug resistance (118). With further understanding of the roles of CAFs in NSCLC drug resistance, targeted inhibition of CAFs and their secreted cytokines can serve as suitable candidates for the treatment of drug resistance.
Targeting EMT
CAFs secrete several types of cytokines, such as Snail and IL-6, to remodel the EMT (73,88). The secretion of IL-6 by CAFs induces EMT and promotes cisplatin resistance in NSCLC cells (73). Co-culturing of NSCLC with CAFs results in the secretion of IL-6 and oncostatin-M (OSM) from CAFs, which in turn activates STAT3. The activated OSM receptors (OSMR)/JAK1/STAT3 pathway contributes to NSCLC cell resistance to chemotherapy drugs. However, this process can be effectively blocked by the JAK1 inhibitor filgotinib (119). In addition, CAFs deliver Snai1 to cancer cells through exosomes to induce cancer cell EMT. However, CAFs can also inhibit EMT when treated with the exosome release inhibitor GW4869, restoring NSCLC drug sensitivity (88). CAFs also promote EMT by secreting TGF-β, indicating that the ability of the TEM to support tumor cells can be reduced by inhibiting TGF-β (120).
Targeting the ECM
According to the aforementioned findings, T cell migration, and the efficacy of anti-PD-1 blockers, can be improved by reducing ECM content and matrix stiffness, which can improve the sensitivity of NSCLC cells to chemotherapy and immunotherapy. Matrix metalloproteinases (MMPs), ERK1/2, JNK, and HIF-1 have been proven to promote ECM degradation (82). However, CAFs primarily degrade the ECM by secreting MMPs, and CAFs co-cultured with NSCLC cells promote the expression of MMP1 and MMP9 (121), effectively reversing drug resistance.
Targeting CSCs
CAFs can facilitate CSC-induced drug resistance in NSCLC in various ways. Therefore, targeting CSCs can improve the therapeutic effect on tumors. For example, CD10+/GPR77+CAFs can promote the self-renewal of CSCs and enhance drug resistance in patients with lung cancer. According to these findings, GPR77 monoclonal antibody therapy may destroy the ecological niche of CSCs, thus retarding the formation of tumors and reversing chemotherapeutic resistance (8). In addition, as aforementioned, CAFs maintain the stemness of CSCs and promote NSCLC drug resistance through the IGF-II/IGF1R/Akt/Nanog signaling pathway. However, the inhibition of this pathway reverses drug resistance to NSCLC (12).
5. Conclusions
CAFs can promote NSCLC drug resistance by inducing EMT, increasing CSC stiffness, remodeling the ECM, and creating a hypoxic microenvironment. These functions are crucial for the role of CAFs in NSCLC drug resistance. The heterogeneity of CAFs is an important factor in the failure of cancer treatment. The lack of reliable markers to identify CAF cell populations has further hindered our understanding of the relationship between CAFs and therapeutic resistance. Therefore, identifying CAF-specific surface markers is key for the future direction of this research field. Due to the heterogeneity of CAFs, targeted inhibitors have yet to be discovered. However drug resistance can be reversed by reducing the accumulation of CAFs, as well as targeted inhibition of their downstream pathways. In addition, the drug sensitivity of NSCLC can be restored by inhibiting EMT, degrading the ECM and destroying the ecological niche of CSCs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors contributions
CC was involved in conceptualization, collection and review of the literature, interpretation, drafting the manuscript, writing and critical revision. JH and SY were involved in critically revising the manuscript for important intellectual content. WL, XW, HS, TQ and FXC were involved in drafting the manuscript or revising it critically for important intellectual content. HG and ZL were involved in conceptualization, figure preparation, writing and critical revision, resource provision and supervision. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer. 2017;17:637–658. doi: 10.1038/nrc.2017.84. [DOI] [PubMed] [Google Scholar]
- 3.Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: A review. JAMA. 2019;322:764–774. doi: 10.1001/jama.2019.11058. [DOI] [PubMed] [Google Scholar]
- 4.Holohan C, Schaeybroeck SV, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 5.Salem A, Asselin MC, Reymen B, Jackson A, Lambin P, West CM, OConnor JP, Faivre-Finn C. Targeting hypoxia to improve non-small cell lung cancer outcome. J Natl Cancer Inst. 2018;110 doi: 10.1093/jnci/djx160. [DOI] [PubMed] [Google Scholar]
- 6.Fischer C, Leithner K, Wohlkoenig C, Quehenberger F, Bertsch A, Olschewski A, Olschewski H, Hrzenjak A. Panobinostat reduces hypoxia-induced cisplatin resistance of non-small cell lung carcinoma cells via HIF-1α destabilization. Mol Cancer. 2015;14:4. doi: 10.1186/1476-4598-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navab R, Strumpf D, To C, Pasko E, Kim KS, Park CJ, Hai J, Liu J, Jonkman J, Barczyk M, et al. Integrin α11β1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer. Oncogene. 2016;35:1899–1908. doi: 10.1038/onc.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su S, Chen J, Yao H, Liu J, Yu S, Lao L, Wang M, Luo M, Xing Y, Chen F, et al. CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172:841–856.e16. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Li Q, Yamada T, Matsumoto K, Matsumoto I, Oda M, Watanabe G, Kayano Y, Nishioka Y, Sone S, Yano S. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2009;15:6630–6638. doi: 10.1158/1078-0432.CCR-09-1001. [DOI] [PubMed] [Google Scholar]
- 10.Radisky DC. Fibroblasts act as co-conspirators for chemotherapy resistance. Cancer Biol Ther. 2008;7:1348–1349. doi: 10.4161/cbt.7.9.6850. [DOI] [PubMed] [Google Scholar]
- 11.Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;99:186–196. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Chen WJ, Ho CC, Chang YL, Chen HY, Lin CA, Ling TY, Yu SL, Yuan SS, Chen YJ, Lin CY, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun. 2014;5:3472. doi: 10.1038/ncomms4472. [DOI] [PubMed] [Google Scholar]
- 13.Leung CS, Yeung TL, Yip KP, Wong KK, Ho SY, Mangala LS, Sood AK, Lopez-Berestein G, Sheng J, Wong ST, et al. Cancer-associated fibroblasts regulate endothelial adhesion protein LPP to promote ovarian cancer chemoresistance. J Clin Invest. 2017;128:589–606. doi: 10.1172/JCI95200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.New J, Arnold L, Ananth M, Alvi S, Thornton M, Werner L, Tawfik O, Dai H, Shnayder Y, Kakarala K, et al. Secretory autophagy in cancer-associated fibroblasts promotes head and neck cancer progression and offers a novel therapeutic target. Cancer Res. 2017;77:6679–6691. doi: 10.1158/0008-5472.CAN-17-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allaoui R, Bergenfelz C, Mohlin S, Hagerling C, Salari K, Werb Z, Anderson RL, Ethier SP, Jirström K, Påhlman S, et al. Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat Commun. 2016;7:13050. doi: 10.1038/ncomms13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Wang R, Qiao M, Xu Y, Guan W, Wang L. Cancer associated fibroblasts tailored tumor microenvironment of therapy resistance in gastrointestinal cancers. J Cell Physiol. 2018;233:6359–6369. doi: 10.1002/jcp.26433. [DOI] [PubMed] [Google Scholar]
- 17.von Ahrens D, Bhagat TD, Nagrath D, Maitra A, Verma A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J Hematol Oncol. 2017;10:76. doi: 10.1186/s13045-017-0448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, Wang Y, Wang T, Hou Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8:3932–3948. doi: 10.7150/thno.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ying L, Zhu Z, Xu Z, He T, Li E, Guo Z, Liu F, Jiang C, Wang Q. Cancer associated fibroblast-derived hepatocyte growth factor inhibits the paclitaxel-induced apoptosis of lung cancer A549 cells by up-regulating the PI3K/Akt and GRP78 signaling on a microfluidic platform. PLoS One. 2015;10:e0129593. doi: 10.1371/journal.pone.0129593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shan T, Chen S, Chen X, Lin WR, Li W, Ma J, Wu T, Ji H, Li Y, Cui X, Kang Y. Prometastatic mechanisms of CAF-mediated EMT regulation in pancreatic cancer cells. Int J Oncol. 2017;50:121–128. doi: 10.3892/ijo.2016.3779. [DOI] [PubMed] [Google Scholar]
- 21.Lai D, Ma L, Wang F. Fibroblast activation protein regulates tumor-associated fibroblasts and epithelial ovarian cancer cells. Int J Oncol. 2012;41:541–550. doi: 10.3892/ijo.2012.1475. [DOI] [PubMed] [Google Scholar]
- 22.Foster DS, Jones RE, Ransom RC, Longaker MT, Norton JA. The evolving relationship of wound healing and tumor stroma. JCI Insight. 2018;3:e99911. doi: 10.1172/jci.insight.99911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louault K, Li R, DeClerck YA. Cancer-associated fibroblasts: Understanding their heterogeneity. Cancers (Basel) 2020;12:3108. doi: 10.3390/cancers12113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An Y, Liu F, Chen Y, Yang Q. Crosstalk between cancer-associated fibroblasts and immune cells in cancer. J Cell Mol Med. 2020;24:13–24. doi: 10.1111/jcmm.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borriello L, Nakata R, Sheard MA, Fernandez GE, Sposto R, Malvar J, Blavier L, Shimada H, Asgharzadeh S, Seeger RC, DeClerck YA. Cancer-associated fibroblasts share characteristics and protumorigenic activity with mesenchymal stromal cells. Cancer Res. 2017;77:5142–5157. doi: 10.1158/0008-5472.CAN-16-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koliaraki V, Pallangyo CK, Greten FR, Kollias G. Mesenchymal cells in colon cancer. Gastroenterology. 2017;152:964–979. doi: 10.1053/j.gastro.2016.11.049. [DOI] [PubMed] [Google Scholar]
- 28.Nair N, Calle AS, Zahra MH, Prieto-Vila M, Oo AKK, Hurley L, Vaidyanath A, Seno A, Masuda J, Iwasaki Y, et al. A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Sci Rep. 2017;7:6838. doi: 10.1038/s41598-017-07144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 30.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI0215518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald LT, Russell DL, Kelly RR, Xiong Y, Motamarry A, Patel RK, Jones JA, Watson PM, Turner DP, Watson DK, et al. Hematopoietic stem cell-derived cancer-associated fibroblasts are novel contributors to the pro-tumorigenic microenvironment. Neoplasia. 2015;17:434–448. doi: 10.1016/j.neo.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016;30:1002–1019. doi: 10.1101/gad.279737.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoshino A, Ishii G, Ito T, Aoyagi K, Ohtaki Y, Nagai K, Sasaki H, Ochiai A. Podoplanin-positive fibroblasts enhance lung adenocarcinoma tumor formation: Podoplanin in fibroblast functions for tumor progression. Cancer Res. 2011;71:4769–4779. doi: 10.1158/0008-5472.CAN-10-3228. [DOI] [PubMed] [Google Scholar]
- 34.Kawase A, Ishii G, Nagai K, Ito T, Nagano T, Murata Y, Hishida T, Nishimura M, Yoshida J, Suzuki K, Ochiai A. Podoplanin expression by cancer associated fibroblasts predicts poor prognosis of lung adenocarcinoma. Int J Cancer. 2008;123:1053–1059. doi: 10.1002/ijc.23611. [DOI] [PubMed] [Google Scholar]
- 35.Schoppmann SF, Berghoff A, Dinhof C, Jakesz R, Gnant M, Dubsky P, Jesch B, Heinzl H, Birner P. Podoplanin-expressing cancer-associated fibroblasts are associated with poor prognosis in invasive breast cancer. Breast Cancer Res Treat. 2012;134:237–244. doi: 10.1007/s10549-012-1984-x. [DOI] [PubMed] [Google Scholar]
- 36.Ono S, Ishii G, Nagai K, Takuwa T, Yoshida J, Nishimura M, Hishida T, Aokage K, Fujii S, Ikeda N, Ochiai A, et al. Podoplanin-positive cancer-associated fibroblasts could have prognostic value independent of cancer cell phenotype in stage I lung squamous cell carcinoma: Usefulness of combining analysis of both cancer cell phenotype and cancer-associated fibroblast phenotype. Chest. 2013;143:963–970. doi: 10.1378/chest.12-0913. [DOI] [PubMed] [Google Scholar]
- 37.Heldin CH. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal. 2013;11:97. doi: 10.1186/1478-811X-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsia LT, Ashley N, Ouaret D, Wang LM, Wilding J, Bodmer WF. Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers. Proc Natl Acad Sci USA. 2016;113:E2162–E2171. doi: 10.1073/pnas.1603534113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herrera M, Islam AB, Herrera A, Martín P, García V, Silva J, Garcia JM, Salas C, Casal I, de Herreros AG, et al. Functional heterogeneity of cancer-associated fibroblasts from human colon tumors shows specific prognostic gene expression signature. Clin Cancer Res. 2013;19:5914–5926. doi: 10.1158/1078-0432.CCR-13-0694. [DOI] [PubMed] [Google Scholar]
- 40.Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: Cancer-associated fibroblasts and their markers. Int J Cancer. 2020;146:895–905. doi: 10.1002/ijc.32193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park SY, Kim HM, Koo JS. Differential expression of cancer-associated fibroblast-related proteins according to molecular subtype and stromal histology in breast cancer. Breast Cancer Res Treat. 2015;149:727–741. doi: 10.1007/s10549-015-3291-9. [DOI] [PubMed] [Google Scholar]
- 42.Patel AK, Vipparthi K, Thatikonda V, Arun I, Bhattacharjee S, Sharan R, Arun P, Singh S. A subtype of cancer-associated fibroblasts with lower expression of alpha-smooth muscle actin suppresses stemness through BMP4 in oral carcinoma. Oncogenesis. 2018;7:78. doi: 10.1038/s41389-018-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Cell Biol. 2014;211:1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012;11:257–266. doi: 10.1158/1535-7163.MCT-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huber MA, Kraut N, Park JE, Schubert RD, Rettig WJ, Peter RU, Garin-Chesa P. Fibroblast activation protein: Differential expression and serine protease activity in reactive stromal fibroblasts of melanocytic skin tumors. J Investig Dermatol. 2003;120:182–188. doi: 10.1046/j.1523-1747.2003.12035.x. [DOI] [PubMed] [Google Scholar]
- 46.Berdiel-Acer M, Sanz-Pamplona R, Calon A, Cuadras D, Berenguer A, Sanjuan X, Paules MJ, Salazar R, Moreno V, Batlle E, et al. Differences between CAFs and their paired NCF from adjacent colonic mucosa reveal functional heterogeneity of CAFs, providing prognostic information. Mol Oncol. 2014;8:1290–1305. doi: 10.1016/j.molonc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res. 2014;2:187–193. doi: 10.1158/2326-6066.CIR-14-0002. [DOI] [PubMed] [Google Scholar]
- 48.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brennen WN, Rosen DM, Wang H, Isaacs JT, Denmeade SR. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J Natl Cancer Inst. 2012;104:1320–1334. doi: 10.1093/jnci/djs336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida GJ. Regulation of heterogeneous cancer-associated fibroblasts: The molecular pathology of activated signaling pathways. J Exp Clin Cancer Res. 2020;39:112. doi: 10.1186/s13046-020-01611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raghavan S, Snyder CS, Wang A, McLean K, Zamarin D, Buckanovich RJ, Mehta G. Carcinoma-associated mesenchymal stem cells promote chemoresistance in ovarian cancer stem cells via PDGF signaling. Cancers (Basel) 2020;12:2063. doi: 10.3390/cancers12082063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rizvi S, Mertens JC, Bronk SF, Hirsova P, Dai H, Roberts LR, Kaufmann SH, Gores GJ. Platelet-derived growth factor primes cancer-associated fibroblasts for apoptosis. J Biol Chem. 2014;289:22835–22849. doi: 10.1074/jbc.M114.563064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raz Y, Cohen N, Shani O, Bell RE, Novitskiy SV, Abramovitz L, Levy C, Milyavsky M, Leider-Trejo L, Moses HL, et al. Bone marrow-derived fibroblasts are a functionally distinct stromal cell population in breast cancer. J Exp Med. 2018;215:3075–3093. doi: 10.1084/jem.20180818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishibashi M, Neri S, Hashimoto H, Miyashita T, Yoshida T, Nakamura Y, Udagawa H, Kirita K, Matsumoto S, Umemura S, et al. CD200-positive cancer associated fibroblasts augment the sensitivity of epidermal growth factor receptor mutation-positive lung adenocarcinomas to EGFR tyrosine kinase inhibitors. Sci Rep. 2017;7:46662. doi: 10.1038/srep46662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, Esaki N, Ushida K, Mii S, Shiraki Y, et al. Meflin-positive cancer-associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res. 2019;79:5367–5381. doi: 10.1158/0008-5472.CAN-19-0454. [DOI] [PubMed] [Google Scholar]
- 56.Alcaraz J, Carrasco JL, Millares L, Luis IC, Fernández-Porras FJ, Martínez-Romero A, Diaz-Valdivia N, De Cos JS, Rami-Porta R, Seijo L, et al. Stromal markers of activated tumor associated fibroblasts predict poor survival and are associated with necrosis in non-small cell lung cancer. Lung Cancer. 2019;135:151–160. doi: 10.1016/j.lungcan.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 57.Schulze AB, Schmidt LH, Heitkötter B, Huss S, Mohr M, Marra A, Hillejan L, Görlich D, Barth PJ, Rehkämper J, Evers G. Prognostic impact of CD34 and SMA in cancer-associated fibroblasts in stage I–III NSCLC. Thorac Cancer. 2020;11:120–129. doi: 10.1111/1759-7714.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hao J, Zeltz C, Pintilie M, Li Q, Sakashita S, Wang T, Cabanero M, Martins-Filho SN, Wang DY, Pasko E, et al. Characterization of distinct populations of carcinoma-associated fibroblasts from non-small cell lung carcinoma reveals a role for ST8SIA2 in cancer cell invasion. Neoplasia. 2019;21:482–493. doi: 10.1016/j.neo.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kilvaer TK, Khanehkenari MR, Hellevik T, Al-Saad S, Paulsen EE, Bremnes RM, Busund LT, Donnem T, Martinez IZ. Cancer associated fibroblasts in stage I–IIIA NSCLC: Prognostic impact and their correlations with tumor molecular markers. PLoS One. 2015;10:e0134965. doi: 10.1371/journal.pone.0134965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, Hoffman JP, Weiner LM, Cheng JD. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37:154–158. doi: 10.1097/MPA.0b013e31816618ce. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida T, Ishii G, Goto K, Neri S, Hashimoto H, Yoh K, Niho S, Umemura S, Matsumoto S, Ohmatsu H, et al. Podoplanin-positive cancer-associated fibroblasts in the tumor microenvironment induce primary resistance to EGFR-TKIs in lung adenocarcinoma with EGFR mutation. Clin Cancer Res. 2015;21:642–651. doi: 10.1158/1078-0432.CCR-14-0846. [DOI] [PubMed] [Google Scholar]
- 62.Neri S, Ishii G, Hashimoto H, Kuwata T, Nagai K, Date H, Ochiai A. Podoplanin-expressing cancer-associated fibroblasts lead and enhance the local invasion of cancer cells in lung adenocarcinoma. Int J Cancer. 2015;137:784–796. doi: 10.1002/ijc.29464. [DOI] [PubMed] [Google Scholar]
- 63.Edlund K, Lindskog C, Saito A, Berglund A, Pontén F Göransson-Kultima H, Isaksson A, Jirström K, Planck M, Johansson L, et al. CD99 is a novel prognostic stromal marker in non-small cell lung cancer. Int J Cancer. 2012;131:2264–2273. doi: 10.1002/ijc.27518. [DOI] [PubMed] [Google Scholar]
- 64.Mitchell MI, Engelbrecht AM. Metabolic hijacking: A survival strategy cancer cells exploit? Crit Rev Oncol Hematol. 2017;109:1–8. doi: 10.1016/j.critrevonc.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki A, Puri S, Leland P, Puri A, Moudgil T, Fox BA, Puri RK, Joshi BH. Subcellular compartmentalization of PKM2 identifies anti-PKM2 therapy response in vitro and in vivo mouse model of human non-small-cell lung cancer. PLoS One. 2019;14:e0217131. doi: 10.1371/journal.pone.0217131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang D, Zhao C, Xu F, Zhang A, Jin M, Zhang K, Liu L, Hua Q, Zhao J, Liu J, et al. Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics. 2021;11:2860–2875. doi: 10.7150/thno.51797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–344. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mallini P, Lennard T, Kirby J, Meeson A. Epithelial-to-mesenchymal transition: What is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev. 2014;40:341–348. doi: 10.1016/j.ctrv.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 72.Ye Q, Su L, Chen D, Zheng W, Liu Y. Astragaloside IV Induced miR-134 expression reduces EMT and increases chemotherapeutic sensitivity by suppressing CREB1 signaling in colorectal cancer cell line SW-480. Cell Physiol Biochem. 2017;43:1617–1626. doi: 10.1159/000482025. [DOI] [PubMed] [Google Scholar]
- 73.Ding X, Ji J, Jiang J, Cai Q, Wang C, Shi M, Yu Y, Zhu Z, Zhang J. HGF-mediated crosstalk between cancer-associated fibroblasts and MET-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death Dis. 2018;9:867. doi: 10.1038/s41419-018-0922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shintani Y, Fujiwara A, Kimura T, Kawamura T, Funaki S, Minami M, Okumura M. IL-6 secreted from cancer-associated fibroblasts mediates chemoresistance in NSCLC by increasing epithelial-mesenchymal transition signaling. J Thorac Oncol. 2016;11:1482–1492. doi: 10.1016/j.jtho.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 75.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: Rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 76.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 77.Leon G, MacDonagh L, Finn SP, Cuffe S, Barr MP. Cancer stem cells in drug resistant lung cancer: Targeting cell surface markers and signaling pathways. Pharmacol Ther. 2016;158:71–90. doi: 10.1016/j.pharmthera.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 78.Shafee N, Smith CR, Wei S, Kim Y, Mills GB, Hortobagyi GN, Stanbridge EJ, Lee EY. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–3250. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schöning JP, Monteiro M, Gu W. Drug resistance and cancer stem cells: The shared but distinct roles of hypoxia-inducible factors HIF1α and HIF2α. Clin Exp Pharmacol Physiol. 2017;44:153–161. doi: 10.1111/1440-1681.12693. [DOI] [PubMed] [Google Scholar]
- 80.Chen Z, Shi T, Zhang L, Zhu P, Deng M, Huang C, Hu T, Jiang L, Li J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016;370:153–164. doi: 10.1016/j.canlet.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 81.Kinugasa Y, Matsui T, Takakura N. CD44 expressed on cancer-associated fibroblasts is a functional molecule supporting the stemness and drug resistance of malignant cancer cells in the tumor microenvironment. Stem Cells. 2014;32:145–156. doi: 10.1002/stem.1556. [DOI] [PubMed] [Google Scholar]
- 82.Najafi M, Farhood B, Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. 2019;120:2782–2790. doi: 10.1002/jcb.27681. [DOI] [PubMed] [Google Scholar]
- 83.Keeratichamroen S, Lirdprapamongkol K, Svasti J. Mechanism of ECM-induced dormancy and chemoresistance in A549 human lung carcinoma cells. Oncol Rep. 2018;39:1765–1774. doi: 10.3892/or.2018.6258. [DOI] [PubMed] [Google Scholar]
- 84.De Rosa V, Iommelli F, Monti M, Fonti R, Votta G, Stoppelli MP, Del Vecchio S. Reversal of warburg effect and reactivation of oxidative phosphorylation by differential inhibition of EGFR signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21:5110–5120. doi: 10.1158/1078-0432.CCR-15-0375. [DOI] [PubMed] [Google Scholar]
- 85.Iderzorig T, Kellen J, Osude C, Singh S, Woodman JA, Garcia C, Puri N. Comparison of EMT mediated tyrosine kinase inhibitor resistance in NSCLC. Biochem Biophys Res Commun. 2018;496:770–777. doi: 10.1016/j.bbrc.2018.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rho JK, Choi YJ, Lee JK, Ryoo BY, Na II, Yang SH, Kim CH, Lee JC. Epithelial to mesenchymal transition derived from repeated exposure to gefitinib determines the sensitivity to EGFR inhibitors in A549, a non-small cell lung cancer cell line. Lung Cancer. 2009;63:219–226. doi: 10.1016/j.lungcan.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 87.Yoshida T, Song L, Bai Y, Kinose F, Li J, Ohaegbulam KC, Muñoz-Antonia T, Qu X, Eschrich S, Uramoto H, et al. ZEB1 mediates acquired resistance to the epidermal growth factor receptor-tyrosine kinase inhibitors in non-small cell lung cancer. PLoS One. 2016;11:e0147344. doi: 10.1371/journal.pone.0147344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.You J, Li M, Cao LM, Gu QH, Deng PB, Tan Y, Hu CP. Snail1-dependent cancer-associated fibroblasts induce epithelial-mesenchymal transition in lung cancer cells via exosomes. QJM. 2019;112:581–590. doi: 10.1093/qjmed/hcz093. [DOI] [PubMed] [Google Scholar]
- 89.Yi Y, Zeng S, Wang Z, Wu M, Ma Y, Ye X, Zhang B, Liu H. Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim Biophys Acta Mol Basis Dis. 2018;1864:793–803. doi: 10.1016/j.bbadis.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 90.Zhou J, Wang J, Zeng Y, Zhang X, Hu Q, Zheng J, Chen B, Xie B, Zhang WM. Implication of epithelial-mesenchymal transition in IGF1R-induced resistance to EGFR-TKIs in advanced non-small cell lung cancer. Oncotarget. 2015;6:44332–44345. doi: 10.18632/oncotarget.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choe C, Shin YS, Kim C, Choi SJ, Lee J, Kim SY, Cho YB, Kim J. Crosstalk with cancer-associated fibroblasts induces resistance of non-small cell lung cancer cells to epidermal growth factor receptor tyrosine kinase inhibition. Oncol Targets Ther. 2015;8:3665–3678. doi: 10.2147/OTT.S89659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Della Corte CM, Bellevicine C, Vicidomini G, Vitagliano D, Malapelle U, Accardo M, Fabozzi A, Fiorelli A, Fasano M, Papaccio F, et al. SMO gene amplification and activation of the hedgehog pathway as novel mechanisms of resistance to anti-epidermal growth factor receptor drugs in human lung cancer. Clin Cancer Res. 2015;21:4686–4697. doi: 10.1158/1078-0432.CCR-14-3319. [DOI] [PubMed] [Google Scholar]
- 93.Murakami A, Takahashi F, Nurwidya F, Kobayashi I, Minakata K, Hashimoto M, Nara T, Kato M, Tajima K, Shimada N, et al. Hypoxia increases gefitinib-resistant lung cancer stem cells through the activation of insulin-like growth factor 1 receptor. PLoS One. 2014;9:e86459. doi: 10.1371/journal.pone.0086459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petrova V, Annicchiarico-petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. doi: 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sugano T, Seike M, Noro R, Soeno C, Chiba M, Zou F, Nakamichi S, Nishijima N, Matsumoto M, Miyanaga A, et al. Inhibition of ABCB1 overcomes cancer stem cell-like properties and acquired resistance to MET inhibitors in non-small cell lung cancer. Mol Cancer Ther. 2015;14:2433–2440. doi: 10.1158/1535-7163.MCT-15-0050. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Q, Yang J, Bai J, Ren J. Reverse of non-small cell lung cancer drug resistance induced by cancer-associated fibroblasts via a paracrine pathway. Cancer Sci. 2018;109:944–955. doi: 10.1111/cas.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li F, Mei H, Gao Y, Xie X, Nie H, Li T, Zhang H, Jia L. Co-delivery of oxygen and erlotinib by aptamer-modified liposomal complexes to reverse hypoxia-induced drug resistance in lung cancer. Biomaterials. 2017;145:56–71. doi: 10.1016/j.biomaterials.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 98.Bridgford JL, Xie SC, Cobbold SA, Pasaje CFA, Herrmann S, Yang T, Gillett DL, Dick LR, Ralph SA, Dogovski C, et al. Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nat Commun. 2018;9:3801. doi: 10.1038/s41467-018-06221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chou CW, Wang CC, Wu CP, Lin YJ, Lee YC, Cheng YW, Hsieh CH. Tumor cycling hypoxia induces chemoresistance in glioblastoma multiforme by upregulating the expression and function of ABCB1. Neuro Oncol. 2012;14:1227–1238. doi: 10.1093/neuonc/nos195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raju S, Joseph R, Sehgal S. Review of checkpoint immunotherapy for the management of non-small cell lung cancer. Immunotargets Ther. 2018;7:63–75. doi: 10.2147/ITT.S125070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kloten V, Lampignano R, Krahn T, Schlange T. Circulating tumor Cell PD-L1 expression as biomarker for therapeutic efficacy of immune checkpoint inhibition in NSCLC. Cells. 2019;8:809. doi: 10.3390/cells8080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pu X, Wu L, Su D, Mao W, Fang B. Immunotherapy for non-small cell lung cancers: Biomarkers for predicting responses and strategies to overcome resistance. BMC Cancer. 2018;18:1082. doi: 10.1186/s12885-018-4990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F, Donnadieu E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nicolas-Boluda A, Vaquero J, Barrin S, Kantari-Mimoun C, Ponzo M, Renault G, Deptuła P, Pogoda K, Bucki R, Cascone I, et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. Cold Spring Harbor. 2020 doi: 10.7554/eLife.58688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeltz C, Pasko E, Cox TR, Navab R, Tsao MS. LOXL1 is regulated by integrin α11 and promotes non-small cell lung cancer tumorigenicity. Cancers (Basel) 2019;11:705. doi: 10.3390/cancers11050705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saunier EF, Akhurst RJ. TGF beta inhibition for cancer therapy. Curr Cancer Drug Targets. 2006;6:565–578. doi: 10.2174/156800906778742460. [DOI] [PubMed] [Google Scholar]
- 107.Ford K, Hanley CJ, Mellone M, Szyndralewiez C, Heitz F, Wiesel P, Wood O, Machado M, Lopez MA, Ganesan AP, et al. NOX4 inhibition potentiates immunotherapy by overcoming cancer-associated fibroblast-mediated CD8 T-cell exclusion from tumors. Cancer Res. 2020;80:1846–1860. doi: 10.1158/0008-5472.CAN-19-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 + T Cells to protect tumour cells. Nat Commun. 2018;9:948. doi: 10.1038/s41467-018-03347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Teramoto K, Igarashi T, Kataoka Y, Ishida M, Hanaoka J, Sumimoto H, Daigo Y. Clinical significance of PD-L1-positive cancer-associated fibroblasts in pN0M0 non-small cell lung cancer. Lung Cancer. 2019;137:56–63. doi: 10.1016/j.lungcan.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 110.Kilvaer TK, Rakaee M, Hellevik T, Østman A, Strell C, Bremnes RM, Busund LT, Dønnem T, Martinez-Zubiaurre I. Tissue analyses reveal a potential immune-adjuvant function of FAP-1 positive fibroblasts in non-small cell lung cancer. PLoS One. 2018;13:e0192157. doi: 10.1371/journal.pone.0192157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hanley CJ, Mellone M, Ford K, Thirdborough SM, Mellows T, Frampton SJ, Smith DM, Harden E, Szyndralewiez C, Bullock M, et al. Targeting the myofibroblastic cancer-associated fibroblast phenotype through inhibition of NOX4. J Natl Cancer Inst. 2018;110:109–120. doi: 10.1093/jnci/djx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fujiwara A, Funaki S, Fukui E, Kimura K, Kanou T, Ose N, Minami M, Shintani Y. Effects of pirfenidone targeting the tumor microenvironment and tumor-stroma interaction as a novel treatment for non-small cell lung cancer. Sci Rep. 2020;10:10900. doi: 10.1038/s41598-020-67904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kakarla S, Chow K, Mata M, Shaffer DR, Song XT, Wu MF, Liu H, Wang LL, Rowley DR, Pfizenmaier K, Gottschalk S. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther. 2013;21:1611–1620. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masuda T, Nakashima T, Namba M, Yamaguchi K, Sakamoto S, Horimasu Y, Miyamoto S, Iwamoto H, Fujitaka K, Miyata Y, et al. Inhibition of PAI-1 limits chemotherapy resistance in lung cancer through suppressing myofibroblast characteristics of cancer-associated fibroblasts. J Cell Mol Med. 2019;23:2984. doi: 10.1111/jcmm.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duan S, Tsai Y, Keng P, Chen Y, Lee SO, Chen Y. IL-6 signaling contributes to cisplatin resistance in non-small cell lung cancer via the up-regulation of anti-apoptotic and DNA repair associated molecules. Oncotarget. 2015;6:27651–27660. doi: 10.18632/oncotarget.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang L, Li X, Ren Y, Geng H, Zhang Q, Cao L, Meng Z, Wu X, Xu M, Xu K. Cancer-associated fibroblasts contribute to cisplatin resistance by modulating ANXA3 in lung cancer cells. Cancer Sci. 2019;110:1609–1620. doi: 10.1111/cas.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wei JR, Dong J, Li L. Cancer-associated fibroblasts-derived gamma-glutamyltransferase 5 promotes tumor growth and drug resistance in lung adenocarcinoma. Aging (Albany NY) 2020;12:13220–13233. doi: 10.18632/aging.103429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tao L, Huang G, Wang R, Pan Y, He Z, Chu X, Song H, Chen L. Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Sci Rep. 2016;6:38408. doi: 10.1038/srep38408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shien K, Papadimitrakopoulou VA, Ruder D, Behrens C, Shen L, Kalhor N, Song J, Lee JJ, Wang J, Tang X, et al. JAK1/STAT3 activation through a proinflammatory cytokine pathway leads to resistance to molecularly targeted therapy in non-small cell lung cancer. Mol Cancer Ther. 2017;16:2234–2245. doi: 10.1158/1535-7163.MCT-17-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Foster JG, Wong SC, Sharp TV. The hypoxic tumor microenvironment: Driving the tumorigenesis of non-small-cell lung cancer. Future Oncol. 2014;10:2659–2674. doi: 10.2217/fon.14.201. [DOI] [PubMed] [Google Scholar]
- 121.Rebelo SP, Pinto C, Martins TR, Harrer N, Estrada MF, Loza-Alvarez P, Cabeçadas J, Alves PM, Gualda EJ, Sommergruber W, Brito C. 3D-3-culture: A tool to unveil macrophage plasticity in the tumour microenvironment. Biomaterials. 2018;163:185–197. doi: 10.1016/j.biomaterials.2018.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.