To the editor,
Even in the era of these newer drugs, upfront autologous hematopoietic stem cell transplantation (AHCT) continues to confer benefits and remains a preferred strategy in transplant-eligible multiple myeloma (MM) patients1. Despite these effective interventions, MM invariably relapses after a period. The treatment landscape in relapsed MM continues to change with the introduction of several new agents. A second salvage AHCT (AHCT2) is another option for MM relapsing after a prior autotransplant. However, its use varies widely given the lack of modern randomized studies, and the availability of several different options in this therapeutic space2. We sought to assess the contemporary utility, safety, and clinical benefits of AHCT2 using the Center for International Blood and Marrow Transplant Research® (CIBMTR®) data.
Patients who underwent AHCT2 for MM between 2010 and 2015 in the US and Canada after relapse from first AHCT1 were included. Patients who received tandem transplants or an allogeneic transplant, who relapsed within 24 months after AHCT1, progressive disease at the time of AHCT2, and on dialysis were excluded (Supplemental Table 1).
Responses were defined according to the International Myeloma Working Group (IMWG) criteria3. Patient-, disease- and transplant-related factors were summarized using descriptive statistics. Probabilities of progression free survival (PFS) and overall survival (OS) were calculated using the Kaplan-Meier product limit estimate. The cumulative incidence of non-relapse mortality (NRM) and disease relapse/progression were estimated accounting for competing risks. Cox proportional hazard regression model was to understand the association between patient-, disease- and transplant-related factors with relapse/progression and OS using the following co-variates: hematopoietic cell transplantation co-morbidity index (HCT-CI) at the time of AHCT2, disease status prior to AHCT2, interval from AHCT1 to AHCT2, year of AHCT2 and planned consolidation/maintenance after AHCT2. All computations were made using the statistical package SAS version 9.
A total 975 patients met the inclusion criteria and were included in the analysis. Table 1 provides the patient-, disease- and transplant-related variables. The median age of the cohort was 62 years (range, 27–78), and 43% were females. Post AHCT2, 30% of the patient were reported to have planned consolidation/maintenance and the median follow up of survivors was 38 months (range, 1–83).
Table 1:
Baseline characteristics:
| Baseline characteristics | N (%) |
|---|---|
| No. of patients | 975 |
| Median age, years (range) | 62 (27–78) |
| Female sex | 423 (43) |
| Race | |
| Caucasian | 723 (74) |
| Black | 130 (13) |
| Hispanic | 62 (6) |
| Others/Not reported | 25 (2.5)/35 (4) |
| KPS | |
| <90 | 426 (44) |
| ≥90 | 530 (54) |
| NR | 19 (2) |
| HCT-CI | |
| 0 | 252 (26) |
| 1 | 108 (11) |
| 2 | 126 (13) |
| ≥3 | 398 (41) |
| NR | 91 (9) |
| Immunochemical subtype | |
| IgG | 532 (55) |
| Non-IgG | 354 (36) |
| Non-secretory | 88 (9) |
| Stage III at diagnosis (ISS/DSS) | 503 (52) |
| Time from diagnosis to 2nd HCT ≥60 months | 591 (61) |
| Time from 1st HCT to 2nd HCT, months | |
| 24–36 | 120 (12) |
| 36–48 | 245 (25) |
| 48–60 | 197 (20) |
| ≥60 | 413 (42) |
| Conditioning regimen | |
| Melphalan | 880 (90) |
| Melphalan +Other | 95 (10) |
| Disease status prior to HCT | |
| CR/sCR | 111 (11) |
| VGPR | 226 (23) |
| PR | 467 (48) |
| SD | 155 (16) |
| NR | 16 (2) |
| Year of transplant | |
| 2010 | 100 (10) |
| 2011 | 125 (13) |
| 2012 | 153 (16) |
| 2013 | 163 (17) |
| 2014 | 202 (21) |
| 2015 | 232 (24) |
| Consolidation/Maintenance post AHCT2 | 297 (30) |
| Median follow up of survivors, months (range) | 35 (1–83) |
KPS: Karnofsky performance status; HCT-CI: Hematopoietic cell transplantation co-morbidity index; ISS: International Staging System; DSS: Durie Salmon Staging System; CR/sCR: Complete response/ stringent complete response; VGPR: Very good partial response; PR: partial response; SD: stable disease and NR: not reported
The rates of NRM at day 100, 1 year and 3 years were 1% (95% CI, 0%−1%), 1% (95% CI, 1%−2%) and 2% (95% CI, 1% −4%) respectively (Figure 1A). The cumulative incidence of relapse/progression (Figure 1B) at 1- and 3-years were 49% (95% CI, 46%−52%) and 84% (95% CI, 82%−87%) respectively. Patients relapsing ≥36 months from first AHCT had significantly lower incidence of relapse/progression after AHCT2 compared to those relapsing 24–35 months (3-year incidence of relapse 82% vs. 88%; p=0.02)
Figure 1:
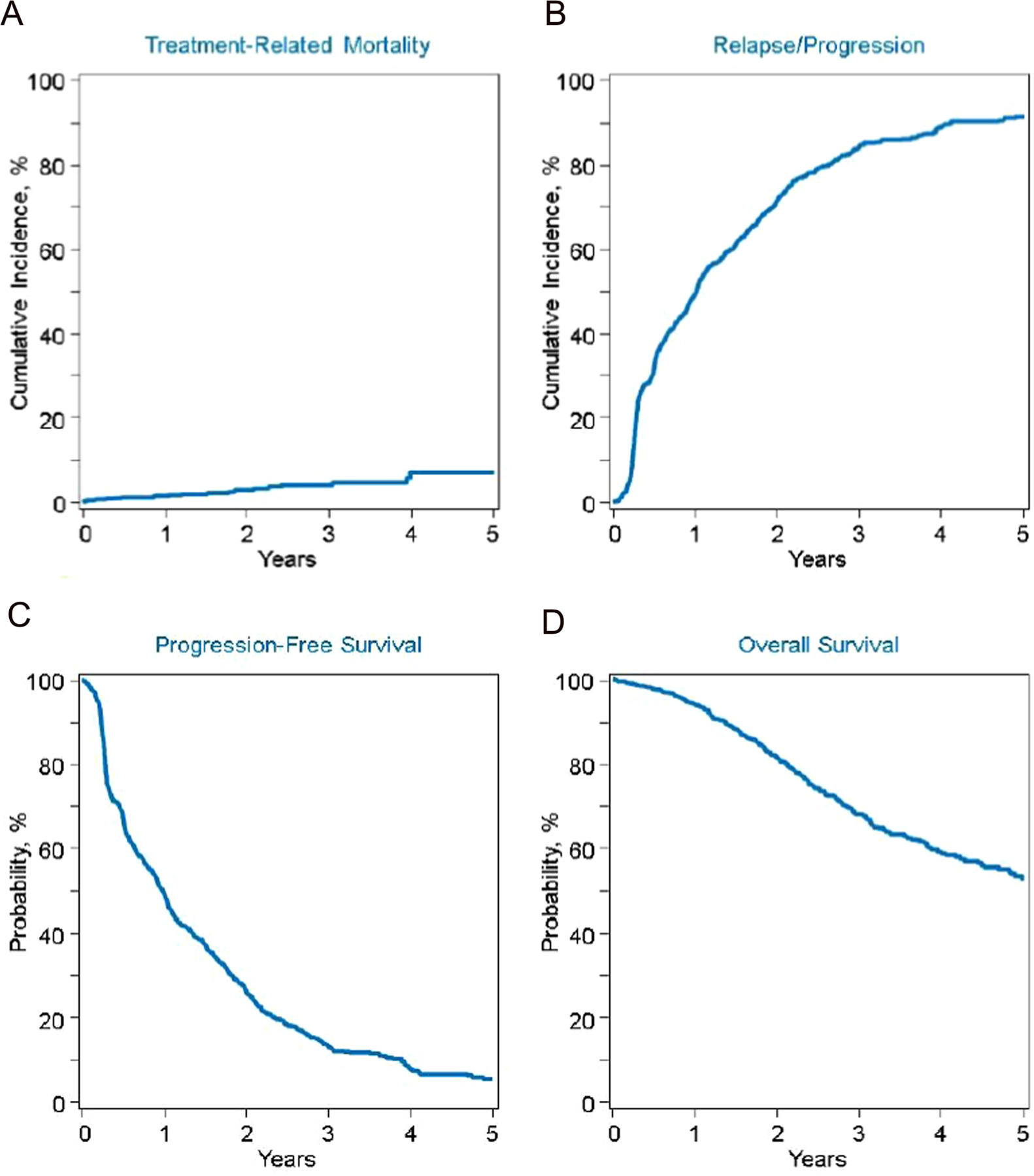
A Cumulative incidence of NRM; B relapse/progression; C: Progression free survival and D: Overall survival
The 1-year and 3-year PFS and OS outcomes were 50% (95% CI, 46%−53%); 13% (95% CI, 11%−16%) and 94% (95% CI, 92%−95%); 68% (95% CI, 64%−71%) respectively (Figure 1C and 1D). Patients relapsed ≥36 months after first AHCT had significantly better PFS and OS than those relapsing earlier (3-year PFS, 16% vs. 9%; p=0.01); (3-year OS, 72% vs. 61%; p= 0.004) respectively (Supplemental Figure 1).
On multivariate analysis, disease status prior to AHCT2 was the only variable prognostic for relapse/progression, PFS and OS (Supplemental Table 2). Compared to patients with ≥VGPR prior to AHCT2, the risk of relapse/progression was significantly higher in patients with partial response (PR) [hazard ratio, HR 1.49 (1.27–1.75); p <.0001 and in stable disease (SD) [1.64 (1.33–2.04); p<.0001]. Similarly, patients in PR [HR 1.46 (1.24– 1.71), p<.0001 or in SD [HR 1.61 (1.31–1.99); p<.0001] had significantly worse PFS compared to those in ≥VGPR. Further, those patients in PR [HR 1.75 (1.31–2.31); p<.0001] and in SD [HR 1.77 (1.22–2.55); p=0.002] had significantly higher hazards of mortality compared to those in ≥VGPR.
A total of 69 (7%) developed second primary malignancies (SPMs) (Supplemental Table 3). Majority of the patients only one SPM (N=57) with myelodysplastic syndrome (MDS)/myeloproliferative neoplasms being the most common (N=14), followed by genitourinary (GU) (N=16). At the time of the last follow up, 232 patients had died and 83% of them from myeloma progression (Supplemental Table 4) while SPMs accounted for only 2% of the deaths.
To our knowledge, these data represent the largest series evaluating the role of AHCT2 in the novel agent era demonstrating its safety and clinical benefit in MM patients’ relapse after a first AHCT. With a 1-year PFS of 50% and OS of 94%, this modality compares favorably with several other approved regimens using newer agents. Since the depth of response prior to AHCT2 predicted for superior PFS and OS, using novel combinations for re-induction might result in even better outcomes. The rates of SPMs after AHCT2 were at 7% but accounted for only 2% of total deaths. These results provide a benchmark for future prospective studies evaluating the role of AHCT2 in relapsed MM.
Choosing therapy in relapsed MM is becoming increasingly complex in the crowded space of emerging and existing therapies2. In this context, the specific patient population that would benefit from AHCT2 is not clearly defined as none of these studies assessed the role of AHCT2. However, in terms of timing the duration of remission of AHCT1 (vary from 12–36 months) has consistently been predictive of PFS after AHCT24. In this analysis, the only variable that was predictive of outcomes on multivariate analysis was the depth of response prior to AHCT2 (when including patients >24 months remission since AHCT1).
The role of AHCT2 in MM is mainly derived from retrospective studies and one prospective randomized study comparing ACHT2 versus standard chemotherapy5. The prospective randomized clinical trial (BSBMT/UK Myeloma X) showed a PFS (19 months vs. 11 months; p<0.001) and OS (67 months vs. 52 months; p=0.02) benefit for AHCT2 when compared to cyclophosphamide 400 mg/m2 weekly for 12 weeks after initial induction with bortezomib, doxorubicin and dexamethasone6. In the current treatment landscape, the choice of induction and cyclophosphamide consolidation strategies used in the non-AHCT arm remains questionable; however, the time to second objective progression was significantly better, and this underscores the potential continued benefit of AHCT2 in eligible patients. The outcomes reported in our study are comparable to some of the new approved FDA regimens in that space. In the relapsed setting, there are several key trials with the backbone of lenalidomide and dexamethasone (Rd), specifically carfilzomib-Rd (KRd) (ASPIRE)7; daratumumab- Rd (DRd) (POLLUX)8, daratumumab with bortezomib and dexamethasone (CASTOR)9, and several newer immunotherapeutic approaches CAR-Ts, ADCs and TCEs2. The reported 2-year survival in ASPIRE with KRd was 73%, and 1-year PFS for POLLUX and CASTOR trials were 83% and 60% with triplet combinations, respectively. The 1-years PFS of 50% and 3-year OS of 68% achieved with ACHT2 in our study indicates clinical benefit. Additionally, AHCT2 is likely a cost-effective option compared to other expensive novel combinations available in relapsed setting10. However, most centers collect enough stem cells for >1 transplant, and a recent study from a single center reported a high cost of storage of those cells, but with low utilization rate suggesting potential underutilization of AHCT2 11.
The depth of response prior to AHCT2 was the only significant factor determining the outcomes in our study and is consistent with several other studies12–14. The exact agents used for induction regimen were not available in the majority, but our observations underscore the use of effective re-induction strategies to achieve the best possible response in eligible patients for AHCT2. Several clinical trials are underway evaluating the role of AHCT2 in the context of novel drug induction, and consolidation/maintenance.
The NRM rates vary from 0–8% in AHCT2, including promising rates of 0–3% in the largest studies4,14. The NRM rates of 1-year and 3-years of 1% and 3% in our large patient population affirms the safety. Disease relapse (83%) was the common cause of death in our study, followed by infections of 6% and the remaining causes in the range of 1–2%. Another concern i.e. the risk of SPMs was seen in 7% of the patients, and which is much higher than 3% reported in Myeloma X trial6. The role of maintenance/consolidation in SPMs is not clear, as recent studies that routinely used consolidation/maintenance after AHCT2 did not report the SPM rates12,13. The cumulative risk of death (2%) from SPMS was outweighed by myeloma, and the fact that these new SPMs were not confirmed by pathology reports might have potential led to an overestimation. There is a wide heterogeneity in clinical practice15 in the post ACHT2 consolidation/maintenance (only 30% planned in our study) and is being addressed in future trials.
The study has several limitations. First of all, as a retrospective study, it is subject to inherent data limitations. Second, the study does not provide evidence on the timing of AHCT2, and whether incorporation of AHCT2 in second line vs. later lines is differentially beneficial cannot be answered. Third, detailed data on re-induction regimens, cytogenetic risk and maintenance/consolidation are lacking in the majority of patients. Despite these limitations, this study is one of the largest studies reporting the role of AHCT2 in relapsed MM in a contemporary era and establishes the safety and efficacy of AHCT2.
Supplementary Material
Footnotes
CONFLICTS
Dr. Dhakal has served on the advisory board of Takeda, Amgen, and Jansen. He has received honorarium from Celgene.
D’Souza has received research support from Sanofi, Mundipharma EDO, TeneoBio and Takeda. She has received consultancy fees from Pfizer, Imbrium, Akcea and Janssen.
REFERENCES:
- 1.Dhakal B, Szabo A, Chhabra S, Hamadani M, D’Souza A, Usmani S et al. Autologous Transplantation for Newly Diagnosed Multiple Myeloma in the Era of Novel Agent Induction: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4(3):343–50. doi: 10.1001/jamaoncol.2017.4600 [published Online First: 2018/01/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chim CS, Kumar SK, Orlowski RZ,Cook G, Richardson P, Gertz MA et al. Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond. Leukemia 2018;32(2):252–62. doi: 10.1038/leu.2017.329 [published Online First: 2017/12/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016;17(8):e328–e46. doi: 10.1016/S1470-2045(16)30206-6 [DOI] [PubMed] [Google Scholar]
- 4.Michaelis LC, Saad A, Zhong X,Le-Rademacher J,Freytes C, Marks D et al. Salvage second hematopoietic cell transplantation in myeloma. Biol Blood Marrow Transplant 2013;19(5):760–6. doi: 10.1016/j.bbmt.2013.01.004 [published Online First: 2013/01/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giralt S, Garderet L, Durie B,Cook G, Gahrton G, Bruno B et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on Salvage Hematopoietic Cell Transplantation in Patients with Relapsed Multiple Myeloma. Biol Blood Marrow Transplant 2015;21(12):2039–51. doi: 10.1016/j.bbmt.2015.09.016 [published Online First: 2015/10/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook G, Ashcroft AJ, Cairns DA,Williams C, Brown J, Cavenagh J et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol 2016;3(7):e340–51. doi: 10.1016/S2352-3026(16)30049-7 [published Online First: 2016/07/05] [DOI] [PubMed] [Google Scholar]
- 7.Stewart AK, Rajkumar SV, Dimopoulos MA,Masszi T, Spicka I, Oriol A et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 2015;372(2):142–52. doi: 10.1056/NEJMoa1411321 [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos MA, Oriol A, Nahi H,San-Miguel J, Bahlsi N, Usmani S et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med 2016;375(14):1319–31. doi: 10.1056/NEJMoa1607751 [DOI] [PubMed] [Google Scholar]
- 9.Palumbo A, Chanan-Khan A, Weisel K,Nooka A, Masszi T, Beask M et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med 2016;375(8):754–66. doi: 10.1056/NEJMoa1606038 [DOI] [PubMed] [Google Scholar]
- 10.Niphadkar S, Varadarajan I, Pompa T, Hou K, Degen K, Vazquez-Martinez et al. Autologous Stem Cell Transplant: A Cost Effective and Efficacious Treatment for Newly Diagnosed Multiple Myeloma. Blood 2016;128(22):1.27389536 [Google Scholar]
- 11.Ahmed N, Li L, Malek E, Caimi P, Covut F, Reese-Koc J et al. Value and Cost Effectiveness of Storing Peripheral Blood Progenitor Cells for Salvage Autologous Stem Cell Transplant in Multiple Myeloma. Biology of Blood and Marrow Transplantation 2020;26(3) [Google Scholar]
- 12.Auner HW, Szydlo R, Rone A, Chaidos A, Giles C, Kafner Ed et al. Salvage autologous stem cell transplantation for multiple myeloma relapsing or progressing after up-front autologous transplantation. Leuk Lymphoma 2013;54(10):2200–4. doi: 10.3109/10428194.2013.773998 [published Online First: 2013/02/08] [DOI] [PubMed] [Google Scholar]
- 13.Gossi U, Jeker B, Mansouri Taleghani B, Bacher U,Novak U, Betticher D et al. Prolonged survival after second autologous transplantation and lenalidomide maintenance for salvage treatment of myeloma patients at first relapse after prior autograft. Hematol Oncol 2018;36(2):436–44. doi: 10.1002/hon.2490 [published Online First: 2018/01/25] [DOI] [PubMed] [Google Scholar]
- 14.Sellner L, Heiss C, Benner A,Raab M, Hillengass J, Hose D et al. Autologous retransplantation for patients with recurrent multiple myeloma: a single-center experience with 200 patients. Cancer 2013;119(13):2438–46. doi: 10.1002/cncr.28104 [published Online First: 2013/04/12] [DOI] [PubMed] [Google Scholar]
- 15.Gonsalves WI, Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK et al. Second auto-SCT for treatment of relapsed multiple myeloma. Bone Marrow Transplant 2013;48(4):568–73. doi: 10.1038/bmt.2012.183 [published Online First: 2012/09/25] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


