Abstract
Probiotics are microorganisms that confer health benefits to host. Well-known examples include Bifidobacterium and Lactobacillus species. In recent years, interest in promoting our health with probiotics has grown as life expectancy and health awareness has increased. However, some concerns for safety and stability exist for these live organisms. Thus, “postbiotics” and “paraprobiotics,” non-viable heat-killed microbial cells or cell fractions that retain health benefits, are increasingly favored. Unfortunately, little information on clinical efficacy and mechanisms of action is available compared with many available probiotics. Lacticaseibacillus (previous name Lactobacillus) paracasei MCC1849 is a commonly used lactic acid bacterial strain in Japan that displays immuno-modulatory effects in humans in non-viable heat-killed form. This review discusses health benefits of heat-killed L. paracasei MCC1849 immune modulation and offers a theoretical basis for its mechanisms of action. We also discuss the feasibility of using heat-killed probiotics for application in food products.
Keywords: food application, heat-killed probiotics, immuno-modulation, Lacticaseibacillus paracasei MCC1849
Introduction
Gut microbiota influences essential human functions, including digestion, energy metabolism, and inflammation by modulating multiple endocrine, neural, and immune host pathways.1 Many studies show that dysbiosis of gut microbiota is closely related to diseases, such as inflammatory bowel disease, allergy, and obesity.2 Modulating gut microbiota composition through dietary intervention may thus become more popular as a way to maintain general health.3
Probiotics are defined as “live microorganisms, which, when administered in adequate amounts, confer a health benefit on the host.”4 Probiotic bacteria are found mainly in genera Lactobacillus and Bifidobacterium. Probiotics have been incorporated in various products such as milkbased drink, fermented milks, infant formula, and nutritional supplements. Probiotic supplementation may provide physiological benefits to hosts such as balancing and restoration of the gut microbiota,5 immuno-modulation,6 prevention of infection,7 antimicrobial effects,8 anti-obesity influences,9 improvement of hypercholesterolemics,10,11 and improved central nervous system function.12 Probiotic use still has issues despite demonstrating excellent physiological benefits. For example, probiotics have restrictions on commercialization and application because they are live strains.13 Furthermore, risks associated with the use of live strains, such as bacterial translocation, should be considered when administering probiotics to high-risk groups, such as elderly and acute-stage patients with reduced immunity.13 “Postbiotics” and “paraprobiotics” have attracted attention as possible solutions to such problems. Postbiotics are soluble factors (products or metabolic byproducts) secreted by live bacteria or released after bacterial lysis.14 Conversely, paraprobiotics are inactivated (non-viable) microbial intact cells, which when administered in sufficient amounts confer benefits to the consumer.15 In vitro and in vivo studies were reported that some postbiotics and paraprobiotics exhibit the physiological benefits.16 For example, oral intake of heat-killed Lactobacillus gasseri TMC0356 daily for 4 weeks by elderly subjects was reported to enhance natural defense mechanisms against pathogenic infections.17 Furthermore, inactivated Bifidobacterium longum BR-108 administration to obese diabetic mice was reported a significant decrease of body weight gain, adipose tissue mass, and blood glucose levels, as well as a significant reduction in blood glucose after a 5 weeks treatment.18
Various health benefits of heat-killed probiotic strains have been reported,17,19–23 but immuno-modulation has gained attention because it may prevent aging- or stress-related attenuation of immunity. This activity lessens the risk of infection in the elderly, leading to extended healthy life expectancy.24 Accumulating studies suggest that multiple bacterial components, such as peptide glycans, nucleic acids (DNA, RNA), lipoteichoic acids, and surface proteins, act in the digestive tract and systemic immune system via pattern recognition. Possible targets include toll-like receptors to regulate the balance of T helper 1 (Th1)/Th2 cells, as well as innate and acquired immunity.13 However, choosing suitable strains for specific efficacy is increasingly challenging because of differences in efficacy among strains, mechanisms of action, safety profiles, and origins.25 Many studies suggest that the effects of probiotics, either viable or heat-killed, are highly strain-specific and not equally safe and effective.26 Regarding heat-killed probiotic strains, efficacy and safety with supporting human data and mechanisms of action are often lacking. Therefore, a detailed review of beneficial properties of specific heat-killed probiotic strains is needed to inform appropriate heat-killed probiotic strain selection.
Heat-killed Lacticaseibacillus paracasei MCC 1849 (hereafter designated as heat-killed MCC1849) is a well-established heat-killed probiotic strain with immuno-modulatory properties. The strain has a long history of use in human foods and is reported as safe in single-dose and long-term repeated dose safety trials.27 In this review, we summarize beneficial effects of heat-killed MCC1849 for humans mainly focusing on immuno-modulation, safety, and use in various commercial products.
Lacticaseibacillus paracasei MCC1849
Origin and characteristics
L. paracasei MCC1849 is a probiotic strain isolated from the intestine of a healthy adult. It has been commercially available in Japan since 2014 and is currently used in various kinds of products including yogurt, juice, bread, and soup. MCC1849 is a non-motile, non-spore-forming, rod-shaped anaerobic Gram-positive bacterium. It was identified as L. paracasei based on morphological, physiological, and genetic characteristics. MCC1849 is reported to induce high levels of interleukin-12 (IL-12) in a non-viable heat-killed form.28 IL-12 is a cytokine released from antigen-presenting cells that acts on naïve T cells to promote differentiation into Th1 cells29 and follicular helper T (Tfh) precursor cells.30 Th1 cells activate various immune cells, including natural killer (NK) cells and macrophages, that are responsible for innate immunity and removing pathogenic bacteria, viruses, and infected cells.29 Tfh cells play an important role in acquired immunity by promoting B cell maturation, thereby facilitating antigen-specific antibody responses to viral, bacterial, parasite, and fungal infections.31 In one experiment, MCC1849 or other bacteria such as Lactobacillus and Lacticaseibacillus strains were cultured for 16 h at 37°C in Lactobacilli-MRS broth, collected via centrifugation, washed twice with phosphate-buffered saline (PBS), and then washed twice with sterile distilled water. The organisms were suspended in distilled water and were killed by heating them at 100°C for 30 min. A portion of each heated suspension was lyophilized to measure the dry weight of the bacterial cells in the suspension. The pasteurized cells were counted directly under the microscope (Figure 1). These heat-killed microorganisms (10 μg/ml) obtained thereby were added to spleen cells derived from BALB/c mice. A significant increase in IL-12 concentration was observed in culture supernatant 48 h after addition of MCC1849, as compared with other strains,28 indicating immuno-modulatory effects.
Figure 1.
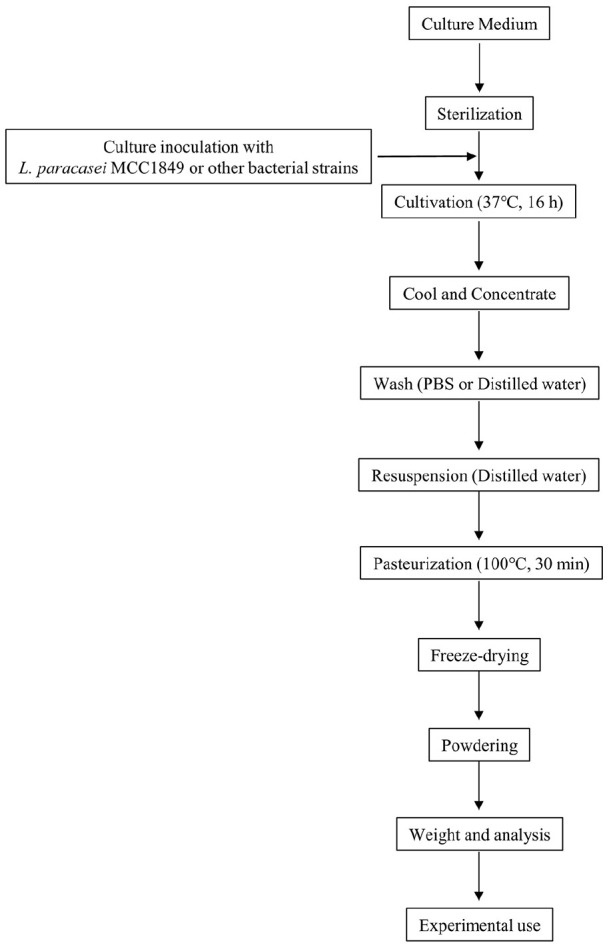
Flowchart of the preparation process for the heat-killed probiotic bacteria.28
Safety
L. paracasei species are granted qualified presumption of safety status by the European Food Safety Authority, with the generic qualification that “the strains should not harbor any acquired antimicrobial resistance genes to clinically relevant antimicrobials,” indicating a lack of safety concerns.32 L. paracasei is consumed globally as a component of yogurt, other fermented foods, and various dietary supplements.32
Studies on acute and subchronic toxicity of viable MCC1849 revealed that both single and repeated oral administration of MCC1849 did not cause death and any symptoms of toxicity in a rat model.27 In an acute toxicity study, groups of five male and female 6 week-old Crj:CD (SD) rats were orally administered a single dose of 6000 mg/kg (2.21 × 1012 CFU/kg) of MCC1849 and examined for clinical signs of acute toxicity for 14 days. No abnormalities or histopathological findings attributable were found in any organ throughout the test period.27 Furthermore, in a 90 day study, oral administration of MCC1849 with repeated doses (1000 mg/kg; 3.27 × 1011 CFU/kg) to 10 male and female 6 week-old Crj:CD (SD) rats did not cause any toxicologically relevant adverse impacts.27
As mentioned in the introduction, administration of live probiotics to people with weak immunity may be hazardous.33 In such cases, non-viable heat-killed probiotics could be an alternative since no safety concerns of heat-killed probiotics are reported to date. Heat-killed bacteria might thus display safety advantages over live probiotics.33 Taken together, non-viable heat-killed L. paracasei MCC1849 is likely to be safe for human consumption.
Efficacy of heat-killed Lacticaseibacillus paracasei MCC1849
Immuno-modulation
Several interventional clinical studies suggested that heat-killed L. paracasei MCC1849 could potentiate immunity or prevent infection. In a randomized, double-blind, placebo-controlled trial, intake of heat-killed MCC1849-containing jelly improved responsiveness of vaccinations in the elderly as compared with the placebo group.34 The study included 45 elderly subjects aged 65 years or older. Participants were randomly divided into two groups: MCC1849 group, provided with jelly containing 1 × 1010 heat-killed MCC1849 cells, and placebo group, provided with the same jelly without MCC1849. They were instructed to consume jelly once a day for 6 weeks from autumn to winter. All participants received influenza vaccination (A/H1N1, A/H2N3, and B) 3 weeks after the start of the experiment. Efficacy of vaccination decreases in elderly populations because of reduced immune function associated with aging.35 Yet, subgroup analysis of subjects over 85 (MCC1849 group: 16, placebo group: 11) showed numbers of blood vaccine antigens significantly higher in the MCC1849 group subjects. Likewise, impaired antibody responses to the A/H1N1 and B antigens were improved only in the MCC1849 group.34 MCC1849 intake may affect responsiveness to vaccinations, particularly in populations with reduced immunity.
Another randomized controlled study also indicated an ability of heat-killed MCC1849 (1 × 1010 cells per day) to potentiate immunity in the elderly.36 Sixty-two subjects aged 65 years or older were randomly allocated to an MCC1849 group supplemented with a oral nutritional supplement containing 1 × 1010 heat-killed MCC1849 cells per day and control group supplemented with fruit juice group without MCC1849. MCC1849 group participants exhibited significantly higher combined immunological scores from eight-point immunity subunit scores, including blood T cell number and NK cell number35 after 4 weeks of treatment. Further investigation is needed, however, the nutritional supplement containing MCC1849, may show positive effects on the immune system in the elderly.
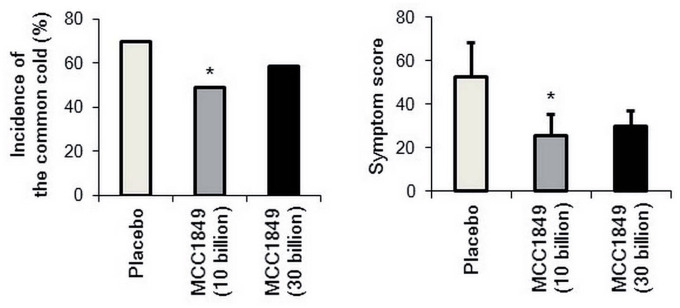
Another randomized, placebo-controlled, double-blind comparison study, including healthy young adults, reported that heat-killed MCC1849 was effective in preventing common colds.37 The study involved 241 healthy women over 18 years of age, who were randomly divided into three groups (30 billion group: 3 × 1010 heat-killed MCC1849 cells, 10 billion group: 1 × 1010 heat-killed MCC1849 cells, and placebo group: dextrin) based on the occurrence of common colds in the previous year.37 In the subgroup of individuals susceptible to colds in the previous year (n = 150), ingestion of 1 × 1010 MCC1849 cells per day resulted in a significant improvement in the incidence of the common cold (placebo group: 69.8%, 10 billion group: 49.0%, and 30 billion group: 58.7%) and symptom score (placebo group: 52.8 ± 15.2, 10 billion group: 25.5 ± 9.7, and 30 billion group: 29.7 ± 7.1) after 12 weeks of intervention (Figure 2).37 No adverse events associated with the consumption of heat-killed MCC1849 were reported in either study summarized above. Findings serve as a basis for the use of MCC1849 as a safe immune modulator for both the elderly and adults.
Figure 2.
Changes in the common cold incidence and symptom score.37 *P < 0.05 versus placebo group by Pearson’s chi square test.
Deduced mechanisms of action for immuno-modulation
Heat-killed MCC1849 induced significant IL-12, previously reported to induce differentiation from naïve T cells into Tfh precursor cells.30 In Peyer’s patches, key players of gut mucosal immune host response toward antigens and bacteria,38 Tfh cells induce differentiation into IgA-positive B cells, and antigen-specific IgA production.31,39 IgA plays a major role in preventing initial infection by eliminating and neutralizing foreign pathogens at mucosal immune tissue.40
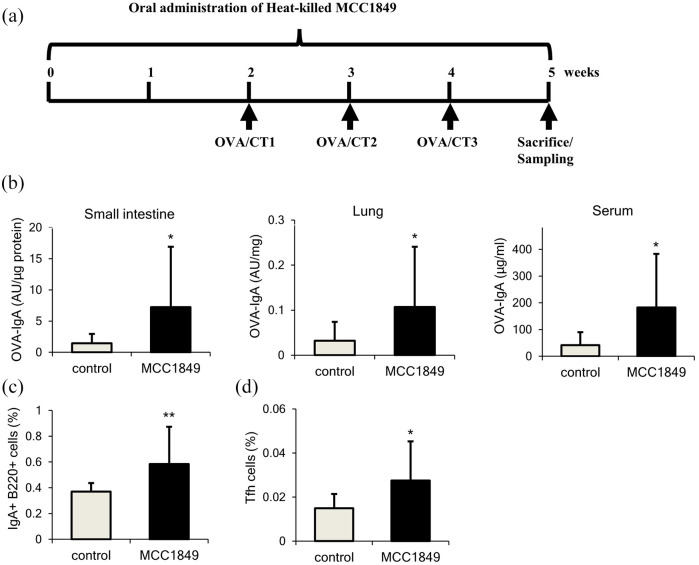
In vivo model, oral supplementation with heat-killed MCC1849 for 5 weeks exhibited potential for enhancing differentiation of B cells into IgA-positive B cells via activation by Tfh cells in Peyer’s patches. Control animals were administered with the same diet without heat-killed MCC1849. Antigen stimulation was induced by oral administration of ovalbumin and cholera toxin (Figure 3a).28 Antigen-specific IgA production and intestinal immune cells were then evaluated by enzyme-linked immunosorbent assay and flow cytometry analysis. Administration of heat-killed MCC1849 significantly increased antigen-specific IgA in the small intestine, lung, and serum (Figure 3b).28 Heat-killed MCC1849 ingestion also induced a significant increase of IgA-positive B cells in Peyer’s patch (Figure 3c).28 Also, the percentage of Tfh cells, which promote differentiation into IgA-positive B cells, was also found to increase significantly in animals receiving heat-killed MCC1849 (Figure 3d).28 Moreover, significant increases in cytokine gene expression, including IL-12 and IL-21 expression, in Peyer’s patch cells were observed in MCC1849 group as determined using quantitative polymerase chain reaction (PCR).28 IL-21 is a cytokine secreted from Tfh cells that facilitates differentiation into IgA-positive B cells.41 Expression of Bcl-6, a transcription factor highly expressed in Tfh cells, was also found to increase after MCC1849 ingestion.28 These findings suggest that heat-killed MCC1849 intake induces differentiation of IgA-positive B cells via Tfh cells and possibly promotes antigen-specific IgA production in the small intestine. Also, MCC1849 increased IgA levels in the serum and lungs, indicating that MCC1849 modulates not only gut mucosal immunity but also respiratory and systemic immunity.
Figure 3.
(a) Fowchart of the animal experiment. (b) Antigen-specific IgA amount in small intestine, lungs, and serum after MCC1849 ingestion. (c) Proportion of IgA + B cells. (d) Tfh cells in Peyer’s patch after MCC1849 ingestion.28 *P < 0.05, **P < 0.01 versus control group by Student’s t-test.
In another in vivo model study, administration of heat-killed MCC1849 starting before infection with the influenza virus significantly suppresses pulmonary viral presence 6 days after infection. Thus, MCC1849 exerts an anti-viral effect against influenza in respiratory tract mucosa.28
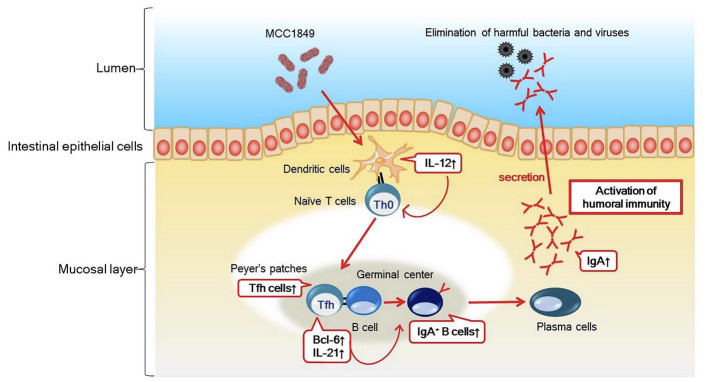
One potential mechanism underlying heat-killed MCC1849 immuno-modulatory effects is shown in Figure 4. Orally administered heat-killed MCC1849 is recognized by antigen-presenting cells, such as dendritic cells, in Peyer’s patches to produce IL-12. In addition to an increased expression of IL-12, expression of Bcl-6 and IL-21 induces the formation of Tfh cells from naïve T cells. Promotion of antigen-specific IgA production in intestinal tissue then occurs by facilitating differentiation into IgA-positive B cells in Peyer’s patches. IgA production is likely a mechanism for immuno-modulation exhibited following heat-killed MCC1849 ingestion (Figure 4).28
Figure 4.
Estimated mechanism of action of immuno-modulatory effect by heat-killed MCC1849.
Impact on quality of life and mood status
Besides immuno-modulatory effects of heat-killed MCC1849, some clinical studies mentioned above showed psychological effects on humans. For example, one randomized controlled study involving elderly subjects reported significant improvement in subjective symptoms associated with quality of life (QOL) after 4 weeks of ingestion. Responses such as “laughing less than before” and “tired for no obvious reason” were significantly reduced in subjects receiving heat-killed MCC1849.36 Moreover, in another randomized, placebo-controlled, double-blind comparison study including healthy young adults under stress due to an upcoming examination, POMS 2 mood profile assessment showed significantly higher scores in “Vigor-Activity” and “Friendliness” at weeks 6 and 12 in subjects receiving MCC1849 (1 × 1010 cells per day) compared with placebo.37 Heat-killed MCC1849 may thus help maintain QOL and positive mood both in the elderly and adults.
Heat-killed Lactobacillus gasseri CP2305, another probiotic strain, ameliorated stress-related symptoms in adults possibly by modulating autonomic nerves via the vagus nerve that connects the intestine and brain.42 Modulation of the vagus nerves, as well as regulation of the hypothalamo-pituitary-adrenal axis involved in stress modulation, could be affected by probiotics or their components43; however, no studies that provide mechanistic insights to support psychological effects of heat-killed MCC1849 are currently available. Additional well-designed randomized controlled trials and mechanistic studies are needed to develop conclusive evidence on psychological effects of heat-killed MCC1849.
Application of heat-killed probiotics to food products
Live bacteria are highly susceptible to heat and low pH and require advanced technologies to remain stable for long periods in food products.44 This issue places a significant constraint on product composition and manufacturing processing. Addition of live bacteria to products and processes can change the flavor and physical properties of the final products and may shorten the product lifespan.45 Conversely, heat-killed probiotics are sterile and can be used in any product regardless of food formulation or product lines. Contamination risk by live bacteria at the time of manufacturing is low. Furthermore, the likelihood of flavor being affected is extremely low. Therefore paraprobiotic products are more simple and convenient in regard to the industrial handling when compared to the probiotic products.46 Heat-killed L. paracasei MCC1849 maintains its physiological efficacy in aluminum storage bags as well as in neutral liquid foods, such as dairy drinks and nutritional supplements, across a wide range of temperatures and after prolonged storage.47 Heat-killed MCC1849 is used in various popular food products, such as miso soup, soups, confectioneries, tofu, and seasonings, into which live bacteria are traditionally difficult to incorporate.47
Despite many advantages, some issues for using heat-killed bacteria remain. One is that no defined procedure for bacterial count of heat-killed probiotics. Measurement methods such as hemocytometer,48 quantitative PCR,49 and flow cytometry48 are being developed. We need to choose a method that is appropriate for each type of product. Another issue is that guidelines and product standards for heat-killed probiotics have not been established. If these problems are solved, more heat-killed probiotics can be expected to be introduced into a variety of foods.
Conclusion
This paper describes heat-killed L. paracasei MCC1849 as an immuno-modulator that can be easily and safely consumed daily. Products containing MCC1849 may be an effective tool for maintaining health in an aging society. Heat-killed L. paracasei may prevent aging- or stress-related attenuation of immunity and risk of infection in the elderly and lead to extended healthy life expectancy.24 Psychological effects of heat-killed MCC1849 are also reported, including improvement of mood state and QOL.36,37
Despite promising results, clinical studies summarized here have limitations in insufficient sample size and target populations. Furthermore, the mechanism of action for immuno-modulatory properties of heat-killed MCC1849 may involve Tfh cell induction and the promotion of IgA production in Peyer’s patches27; however, mechanistic insights are not robust. Therefore, additional well-designed randomized controlled trials with larger sample size and further mechanistic studies on active ingredients in heat-killed MCC1849 and the molecular basis of induction of Tfh differentiation are needed to understand the full potential of heat-killed MCC1849 as an immuno-modulator.
In conclusion, although further investigations are required to clarify how heat-killed MCC1849 confers health benefits to humans; still, heat-killed MCC1849 has the potential to modulate immunity and maintain desirable psychological state.
Acknowledgments
The authors are grateful to all the researchers whom we cited in this review for their significant and valuable research. We thank Enago (www.enago.jp) for reviewing the English language of the whole manuscripts.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors H.M., S.A., N.I., and F.A. are employees of Morinaga Milk Industry Co., Ltd.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Satoshi Arai  https://orcid.org/0000-0002-0700-6830
https://orcid.org/0000-0002-0700-6830
References
- 1. Wang B, Yao M, Lv L, et al. (2017) The human microbiota in health and disease. Engineering 3: 71–82. [Google Scholar]
- 2. Carding S, Verbeke K, Vipond DT, et al. (2015) Dysbiosis of the gut microbiota in disease. Microbial Ecology in Health and Disease 26: 26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shen TC. (2017) Diet and gut microbiota in health and disease. Intestinal Microbiome: Functional Aspects in Health and Disease 88: 117–126. [DOI] [PubMed] [Google Scholar]
- 4. Joint FAO/WHO Working Group Report (2002) Guidelines for the Evaluation of Probiotics in Food. London, Ontario: FAO/WHO, pp.1–11. [Google Scholar]
- 5. Ogata T, Nakamura T, Anjitsu K, et al. (1997) Effect of Bifidobacterium longum BB536 administration on the intestinal environment, defecation frequency and fecal characteristics of human volunteers. Bioscience and Microflora 16(2): 53–58. [Google Scholar]
- 6. Iwabuchi N, Shimizu K(Xiao JZ) (2010) Immuno-modulating effects of Bifidobacterium longum BB536 and the mechanisms. Milk Science 59: 275–281. [Google Scholar]
- 7. Kafshdooz T, Akbarzadeh A, Seghinsara AM, et al. (2017) Role of probiotics in managing of Helicobacter pylori infection: A review. Drug Research 67: 88–93. [DOI] [PubMed] [Google Scholar]
- 8. Shafi A, Raja HN, Farooq U, et al. (2019) Antimicrobial and antidiabetic potential of synbiotic fermented milk: A functional dairy product. International Journal of Dairy Technology 72: 15–22. [Google Scholar]
- 9. Kondo SK, Xiao JX, Satoh TS, et al. (2010) Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Bioscience, Biotechnology, and Biochemistry 74: 1656–1661. [DOI] [PubMed] [Google Scholar]
- 10. Xiao JZ, Kondo S, Takahashi N, et al. (2003) Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. Journal of Dairy Science 86: 2452–2461. [DOI] [PubMed] [Google Scholar]
- 11. Sarfraz F, Farooq U, Shafi A, et al. (2019) Hypolipidaemic effects of synbiotic yoghurt in rabbits. International Journal of Dairy Technology 72: 545–550. [Google Scholar]
- 12. Kobayashi Y, Sugahara H, Shimada K, et al. (2017) Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Scientific Reports 7(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berlanga M, Miñana-galbis D. (2019) Health benefits of heat-killed (tyndallized) probiotics: An overview. International Journal of Molecular Sciences 20(10): 2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, et al. (2018) Postbiotics: An evolving term within the functional foods field. Trends in Food Science & Technology 75: 105–114. [Google Scholar]
- 15. Taverniti V, Guglielmetti S. (2011) The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes & Nutrition 6: 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cuevas-González PF, Liceaga AM, Aguilar-Toalá JE. (2020) Postbiotics and paraprobiotics: From concepts to applications. Food Research International 136: 109502. [DOI] [PubMed] [Google Scholar]
- 17. Miyazawa K, Kawase M, Kubota A, et al. (2015) Heat-killed Lactobacillus gasseri can enhance immunity in the elderly in a double-blind, placebo-controlled clinical study. Beneficial Microbes 6: 441–449. [DOI] [PubMed] [Google Scholar]
- 18. Othman MB, Sakamoto K. (2020) Effect of inactivated Bifidobacterium longum intake on obese diabetes model mice (TSOD). Food Research International 129: 108792. [DOI] [PubMed] [Google Scholar]
- 19. Hirose Y, Yamamoto Y, Yoshikai Y, et al. (2013) Oral intake of heat-killed Lactobacillus plantarum L-137 decreases the incidence of upper respiratory tract infection in healthy subjects with high levels of psychological stress. Journal of Nutritional Science 2: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sugimura T, Takahashi H, Jounai K, et al. (2015) Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. British Journal of Nutrition 114(5): 727–733. [DOI] [PubMed] [Google Scholar]
- 21. Maekawa T, Ishijima SA, Ida M, et al. (2016) Prophylactic effect of Lactobacillus pentosus strain S-PT84 on Candida Infection and Gastric Inflammation in a Murine Gastrointestinal Candidiasis Model [Errata]. Medical Mycology Journal 57(4): E81–E92. [DOI] [PubMed] [Google Scholar]
- 22. Pedret A, Valls RM, Calderón-pérez L, et al. (2019) Effects of daily consumption of the probiotic Bi fi dobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: A randomized controlled trial. International Journal of Obesity 43(9): 1863–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakamura F, Ishida Y, Aihara K, et al. (2016) Effect of fragmented Lactobacillus amylovorus CP1563 on lipid metabolism in overweight and mildly obese individuals: A randomized controlled trial. Microbial Ecology in Health and Disease 27(1): 30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Landete JM, Gaya P, Rodríguez E, et al. (2017) Probiotic bacteria for healthier aging: Immunomodulation and metabolism of phytoestrogens. BioMed Research International. Epub ahead of print 2017. DOI: 10.1155/2017/5939818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wong CB, Odamaki T, Xiao JZ. (2019) Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action. Journal of Functional Foods 54: 506–519. [Google Scholar]
- 26. Mcfarland LV, Evans CT, Goldstein EJ. (2018) Strain-specificity and disease-specificity of probiotic efficacy: A systematic review and meta-analysis. Frontiers in Medicine 5: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arai S, Minami J, Sato Y, et al. (2018) Safety evaluation of Lactobacillus paracasei MCC1849 by oral toxicity tests using rats. 薬理と治療 46: 27–36. [Google Scholar]
- 28. Arai S, Iwabuchi N, Takahashi S, et al. (2018) Orally administered heat-killed Lactobacillus paracasei MCC1849 enhances antigen-specific IgA secretion and induces follicular helper T cells in mice. PLoS One 13(6): e0199018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trinchieri G. (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Reviews Immunology 3: 133–146. [DOI] [PubMed] [Google Scholar]
- 30. Nakayamada S, Kanno Y, Takahashi H, et al. (2012) Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 35: 919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crotty S. (2019) T follicular helper cell biology a decade of discovery and diseases. Immunity 50: 1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barlow S, Chesson A, Collins JD, et al. (2007) Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA—Opinion of the Scientific Committee. The EFSA Journal 587: 1–16. [Google Scholar]
- 33. Akter S, Park J, Jung HK. (2020) Potential health-promoting benefits of paraprobiotics, inactivated probiotic cells. Journal of Microbiology Biotechnology 30: 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maruyama M, Abe R, Shimono T, et al. (2016) The effects of non-viable Lactobacillus on immune function in the elderly: A randomised, double-blind, placebo-controlled study. International Journal of Food Sciences and Nutrition 67: 67–73. [DOI] [PubMed] [Google Scholar]
- 35. Hirokawa K, Utsuyama M, Kikuchi Y, et al. (2009) Assessment of age-related decline of immunological function and possible methods for immunological restoration in elderly. In: T. Fulop (ed.) Handbook on Immunosenescence. Dordrecht: Springer, pp.1547–1570. [Google Scholar]
- 36. Shimono T, Hoshino T, Takara T. (2019) Effects of an oral nutritional supplement drink containing Lactobacillus paracasei MCC1849 on improving the immune system of elderly people—A randomized open-label trial. Japanese Pharmacology and Therapeutics 47: 97–113. [Google Scholar]
- 37. Murata M, Kondo J, Iwabuchi N, et al. (2018) Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Beneficial Microbes 9: 855–864. [DOI] [PubMed] [Google Scholar]
- 38. Jung C, Hugot J-P, Barreau F. (2010) Peyer’s patches: The immune sensors of the intestine. International Journal of Inflammation 82371: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lycke NY, Bemark M. (2012) The role of Peyer’s patches in synchronizing gut IgA responses. Frontiers in Immunology 3: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fagarasan S, Honjo T. (2003) Intestinal IgA synthesis regulation of front-line body defences. Nature Reviews Immunology 3: 63–72. [DOI] [PubMed] [Google Scholar]
- 41. Fazilleau N, Mark L, McHeyzer-Williams LJ, et al. (2009) Follicular helper T cells: Lineage and location. Immunity 30: 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. 樋口敏将, 藤原茂 (2017) Lactobacillus gasseri CP2305株の機能性とその応用. 生物工会誌 95: 586–589. [Google Scholar]
- 43. Berm LG, Salinas E, Ortiz GG, et al. (2019) From probiotics to psychobiotics: Live beneficial bacteria which act on the brain-gut axis. Nutrients 11(4): 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Terpou A, Papadaki A, Lappa IK, et al. (2019) Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 11(7): 1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xiao JZ. (2017) Distribution and strategies for prevention of bacteriophage contamination in yogurt factories. Milk Science 66: 39–44. [Google Scholar]
- 46. Barros CP, Guimarães JT, Esmerino EA, et al. (2020) Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Current Opinion in Food Science 32: 1–8. [Google Scholar]
- 47. Maehata H, Murata M. (2019) Immuno-modulatory effect of Lactobacillus paracasei MCC1849 and its application to food products. Milk Science 68: 179–186. [Google Scholar]
- 48. Vembadi A, Menachery A, Qasaimeh MA. (2019) Cell cytometry: Review and perspective on biotechnological advances. Frontiers in Bioengineering and Biotechnology 7: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Soejima T, Xiao J, Abe F. (2016) A novel mechanism for direct real-time polymerase chain reaction that does not require DNA isolation from prokaryotic cells. Scientific Reports 6(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]