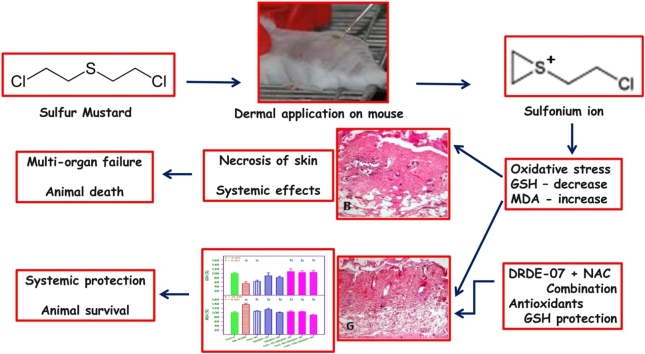
Graphical abstract
Keywords: Sulfur mustard, Antidote, N-acetylcysteine, DRDE-07, Glutathione, Malondialdehyde, Oxidative stress, Mice
Highlights
-
•
Sulfur mustard, an alkylating agent causes systemic toxicity by dermal application.
-
•
Decontamination upon contact is the recommended procedure.
-
•
Percutaneous administration in mice causes oxidative stress.
-
•
Decrease in glutathione and increase in malondialdehyde are the effects.
-
•
Combination of NAC and DRDE-07 showed good protection in mice.
Abstract
Introduction
Sulfur mustard (SM) is chemically, bis(2-chloroethyl) sulfide and a strong alkylating agent that causes cytotoxicity and blisters on skin. In laboratory animal models, SM is extremely lethal. Since no specific antidote has been proposed, decontamination upon contact is the recommended procedure. Several antidotes have been screened for SM, and in that sulfanyl compounds, N-acetyl-l-cysteine (NAC) and S-2(2-aminoethylamino) ethylphenyl sulfide (DRDE-07) showed good protection. Since they showed protection at high doses, the aim of this study was to evaluate the efficacy in combination at low dose, for percutaneously administered SM in mice.
Material and Methods
4 LD50 of SM (32.4 mg/kg) was administered, and NAC (50 mg/kg), DRDE-07 (25 and 50 mg/kg) and their combinations were evaluated as 30 min pre-treatment by single oral administration.
Result
After 72 h of SM exposure, significant decrease in body weight, decrease in hepatic reduced glutathione, and increase in hepatic malondialdehyde were observed (P < 0.001), showing oxidative stress. The combination of NAC (100 mg/kg) and DRDE-07 (50 mg/kg) showed significant protection (P < 0.01). The severe histopathological lesions induced by SM in liver, spleen and skin were also considerably reduced by the combination.
Conclusion
The combination of NAC and DRDE-07 having sulfanyl groups, will be promising antioxidants and an effective antidote for SM toxicity.
1. Introduction
Sulfur mustard (SM), a chemical warfare agent was used initially in World War I and thereafter in many conflicts [1]. SM is chemically bis(2-chloroethyl) sulfide and a strong alkylating agent, and causes cytotoxicity and blisters on human skin [2,3]. SM causes sterile blisters on human skin and becomes infectious once the blister ruptures [4]. In laboratory animal models, SM may not produce a typical blister, but it is extremely lethal. One microliter applied topically on a mouse or rat will cause progressive decrease in body weight and death in 10 days [5]. SM forms sulfonium ion with a strong electrophilic property in biological medium, and binds to many macromolecules including DNA, leading to DNA strand breaks [6]. The primary target organs for SM toxicity are skin, eyes and the respiratory system. In high concentrations, SM causes multiorgan failure and death [7,8].
Due to the strong alkylating property particularly with the DNA, SM can be used as an anticancer agent [9], but the limitation is that it is equally toxic to the normal cells, particularly the rapidly growing cells. In contrast some of the nitrogen mustards are used as anticancer agents [10]. A variety of synthetic and repurposed drugs were used in vitro and in vivo models to alleviate SM toxicity [11]. The therapeutic armamentarium of drugs includes sodium thiosulphate, antioxidants, cytoprotective agents, radical scavengers and sulfanyl compounds [12,13]. The tested molecules provided only minimum protection and a satisfactory molecule is yet to be identified. Since no specific antidote has been proposed, decontamination upon contact is the recommended procedure [11]. There are few drugs that have shown beneficial effect in SM human exposure. Among them sodium thiosulfate and N-acetyl-l-cysteine are mentionable [11]. In animal studies sulfanyl compounds like amifostine [S-2(3-aminopropylamino) ethyl phosphorothioate], DRDE-07 [S-2(2-aminoethylamino) ethylphenyl sulfide] and their analogues have been found to be very effective in protecting the lethality of several fold LD50 of dermally applied SM [14,15].
N-acetyl-l-cysteine (NAC) is a sulfanyl compound and an ester of the amino acid l-cysteine. It is an antioxidant, and prevents oxidative stress, inflammation and apoptosis. It is recommended for acetaminophen over dose. For several clinical conditions NAC has been found to be very effective [16,17]. NAC has several applications viz., as an antioxidant, free radical scavenger, anti-inflammatory agent, expectorant, mucolytic and vasodilator [18,19]. Several studies carried out on animals and humans have shown the promising role of NAC as an adjunct for a variety of clinical conditions, communicable and non-communicable diseases, and an antidote to many drug and chemical induced toxicities [20]. NAC has been shown to be beneficial in the management of toxicity due to heavy metals, pesticides and chemical warfare agents. It is also used as an effective adjunct for viral, bacterial and parasitic infections [21]. Animal studies and clinical trials have proved the effectiveness of NAC in many pulmonary and extrapulmonary complications. It is a very safe drug for humans and is available in the form of tablets, injectables and sprays.
DRDE-07 is a sulfanyl containing synthetic compound. Extensive preclinical studies have shown that it is safe as a prophylactic agent against SM and also against nitrogen mustards [22]. Among the various antidotes tested in animal models against percutaneous toxicity of SM, DRDE-07 and its analogues have given the highest protection [23]. DRDE-07 has also antioxidant, analgesic and anti-inflammatory properties that are additional benefits in SM and nitrogen mustard toxicities [24]. Studies carried out so far show that DRDE-07 protects SM and nitrogen mustard toxicity only at high doses [25]. Hence, the aim is to show the effectiveness of combined administration of NAC and DRDE-07 against percutaneous toxicity of SM in mice employing lower doses.
2. Material and methods
2.1. Animals
Swiss mice (between 25–30 g, female), randomly bred and maintained at Defence Research and Development Establishment (DRDE, India) were used. They were kept in polypropylene cages, three per cage on steam sterilised paddy husk and were given pellet feed (Ashirwad Ltd. India) and filtered water ad libitum. Appropriate Institutional Animal Ethics Committee approval was obtained for this study as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA, India). 24 h prior to SM exposure, hair on the dorsal side of the mice was closely clipped. To avoid influence of food on the absorption of the orally administered compounds, food material was removed 2–3 hr before.
2.2. Chemicals
S-2(2-aminoethylamino) ethylphenyl sulfide (DRDE-07) synthesized in the Synthetic Chemistry Division of DRDE was used [26]. The purity of DRDE-07 was found to be above 99 % (high performance liquid chromatographic analysis). SM was also synthesized (above 99 % by gas chromatographic analysis). N-acetylcysteine (NAC) was purchased from Sigma Chemicals (USA) and polyethylene glycol (PEG-300) was purchased from Merck (India). Other chemicals of analytical grade were procured from Qualigens (India).
2.3. Experimental design
The protective efficacy of NAC and DRDE-07 alone, and in combinations at different doses were studied against SM on selected haematological and biochemical variables. The following were the eight study groups (6 mice each):
-
A
Distilled water, p.o. + PEG-300 p.c. (control)
-
B
Distilled water, p.o. + SM, 4 LD50, p.c. (SM-4LD50)
-
C
NAC, 50 mg/kg, p.o. + SM, 4 LD50, p.c. (NAC-50)
-
D
DRDE-07, 25 mg/kg, p.o. + SM, 4 LD50, p.c. (DRDE07−25)
-
E
DRDE-0750 mg/kg, p.o.+ SM, 4 LD50, p.c. (DRDE07−50)
-
F
NAC,25 mg/kg, p.o.+ DRDE-0725 mg/kg, p.o.+ SM, 4 LD50, p.c. (NAC-25+DRDE07−25)
-
G
NAC,50 mg/kg, p.o.+ DRDE-0750 mg/kg, p.o.+ SM, 4 LD50, p.c. (NAC-50+DRDE07−50)
-
H
NAC,100 mg/kg, p.o.+ DRDE-0750 mg/kg, p.o.+ SM, 4 LD50, p.c. (NAC-100+DRDE07−50)
The antidotes were dissolved in water and administered orally once, 30 min prior to SM exposure using a 20-gauge stainless steel oral gavage cannula. Animals in control and SM-4LD50 groups received distilled water as pre-treatment. PEG-300 was applied on the dorsal side of mice in the control group. The SM dose applied was 4 LD50 (32.4 mg/kg, p.c.), and 1 LD50 of SM is 8.1 mg/kg, p.c. [25]. SM was diluted in PEG-300 and 30–50 μL was applied on the dorsal side of the mice on an area of 1.5 cm diameter, following all safety procedures [22]. Since NAC is a known compound and the effects have been proven against SM including humans, one dose of NAC, 50 mg/kg was used. This reduced the number of groups.
2.4. Sample collection
72 h after SM exposure, blood was taken from ocular plexus under anaesthesia. The mice were sacrificed and liver, spleen, kidney and skin were removed for evaluation. White blood cell (WBC) count, red blood cell (RBC) count and haemoglobin (Hb) were estimated in whole blood samples. Liver, spleen and kidney, and skin from the wound site were dissected out, cleaned and weighed. A portion of liver sample was used for the estimation of reduced glutathione (GSH) and malondialdehyde (MDA) levels. A portion of liver, spleen and skin were preserved in Bouin’s fluid for histological studies.
2.5. Organ to body weight ratio
It was calculated as percentage of organ weight divided by the animal weight for liver, spleen and kidney
2.6. Haematological and biochemical variables
RBC and WBC counts, and Hb of blood were estimated using Beckman Analyser (USA). Hisin and Hilf (1976) [27] method was used for the estimation of hepatic GSH. For this, 250 mg of liver sample was homogenized in 5 ml of phosphate ethylene diamine tetra acetic acid buffer (pH 8.0) and metaphosphoric acid (25 %). After centrifugation, ortho-phthaldialdehyde was added as a fluorescent dye to the supernatant. Lipid peroxidation was analysed by estimating the level of MDA, using the modified method of Easterbauer and Cheeseman (1990) [28]. Briefly, 200 mg of liver sample was homogenized in 0.15 M potassium chloride and 30 % trichloroacetic acid. 0.8 % thiobarbituric acid was added and boiled for 30 min. MDA was calculated by the molar extinction coefficient of 1.58 × 105/M per cm, by measuring the absorbance of the supernatant at 535 nm.
2.7. Histopathological observation
The samples of liver, spleen and skin were fixed in Bouin’s fluid. After fixation, small pieces of tissues were processed by dehydration in 30 %, 40 %, 50 %, 60 %, 70 %, 80 %, 90 % and absolute ethanol. After dehydration, the tissues were transferred to the clearing agent (toluene) and embedded in paraffin wax. Sections of 5−6 μm thickness from each block were taken and stained with haematoxylin and eosin for microscopic examination. The lesions were characterized using LEICA-QWIN-500 image analyzer and converted as percentages.
2.8. Statistical analysis
The haematological and biochemical data were analyzed using one-way ANOVA with Student Newman Keul’s multiple comparisons test. The hypotheses were tested comparing the control group with other groups (given as ‘a’), and comparing the SM exposed group with other groups (given as ‘b’). Using the mean value of the control group the other group values are converted to percentages and represented. For statistical significance P < 0.05 was taken. The statistical analyses and plotting of graph were done using SigmaPlot 13 (Systat Software, USA).
3. Results
The percent change in body weight, and liver, spleen and kidney weights are given in Table 1. One-way ANOVA showed significant difference in the body weight changes (P < 0.001). SM administration (4 LD50) showed 30 % decrease in the body weight in three days. There was no protection in the body weight change in DRDE07−25 and NAC-25+DRDE07−25 groups and they showed significant decrease in body weight when compared to the control group. DRDE07−50, NAC-50+DRDE07−50 and NAC-100+DRDE07−50 groups showed significant protection in body weight when compared to the SM group, showing that the combination is beneficial. Similar to body weight decrease, the percent liver weight and spleen weight were also decreased in SM group, but they were not statistically significant (P = 0.176 and 0.075 respectively). The percent kidney weight was significantly different among the groups (P = 0.007), and the SM group showed the lowest weight. NAC-50 and DRDE07−50 showed increased kidney weight compared to the control group. The decrease in spleen weight in SM group was more than the liver and kidney weights when compared to the control group (28 %).
Table 1.
Percent change in body weight, liver weight, spleen weight and kidney weight.
| Group | Body weight (%) | Liver weight (%) | Spleen weight (%) | Kidney weight (%) |
|---|---|---|---|---|
| Control | 100 + 2.8 | 100 + 2.0 | 100 + 8.5 | 100 + 3.7 |
| SM-4LD50 | 70 + 2.0a | 82 + 2.4 | 72 + 6.2 | 84 + 6.0 |
| NAC-50 | 88 + 5.2 | 90 + 7.2 | 84 + 14.5 | 115 + 10.9b |
| DRDE07−25 | 80 + 2.9a | 85 + 5.7 | 68 + 7.2 | 94 + 6.5 |
| DRDE07−50 | 92 + 3.0b | 88 + 2.0 | 79 + 10.7 | 110 + 4.1b |
| NAC-25+DRDE07−25 | 71 + 3.9a | 86 + 4.4 | 91 + 12.1 | 87 + 5.5 |
| NAC-50+DRDE07−50 | 95 + 6.1b | 91 + 3.0 | 95 + 9.2 | 109 + 7.1 |
| NAC-100+DRDE07−50 | 90 + 8.8b | 87 + 4.8 | 107 + 4.4 | 86 + 6.7 |
| F = | 5.215 | 1.559 | 2.026 | 3.349 |
| P = | < 0.001 | 0.176 | 0.075 | 0.007 |
Values are mean + SEM (n = 6 each).
Significantly different from control group.
Significantly different from SM group.
The percent change in WBC, RBC and Hb are given in Table 2. The WBC count and Hb did not show any statistically significant difference among the groups (P = 0.155 and 0.260 respectively), but statistically significant difference was seen in RBC count (P = 0.006). A statistically significant increase in RBC count was observed in NAC-50 and DRDE07−50 groups when compared to the control. Though, not statistically significant, the SM group showed increased RBC count and Hb with a decrease in WBC count.
Table 2.
Percent change in white blood cell count, red blood cell count and blood haemoglobin.
| Group | WBC (%) | RBC (%) | Hb (%) |
|---|---|---|---|
| Control | 100 + 11.0 | 100 + 2.9 | 100 + 3.5 |
| SM-4LD50 | 75 + 7.3 | 118 + 8.8 | 116 + 4.1 |
| NAC-50 | 73 + 9.0 | 147 + 12.8a | 115 + 6.6 |
| DRDE07−25 | 71 + 9.0 | 120 + 6.8 | 122 + 6.03 |
| DRDE07−50 | 64 + 9.2 | 143 + 12.5a | 113 + 7.3 |
| NAC-25+DRDE07−25 | 112 + 23.8 | 98 + 5.7 | 96 + 3.9 |
| NAC-50+DRDE07−50 | 100 + 23.4 | 102 + 5.9 | 95 + 6.3 |
| NAC-100+DRDE07−50 | 119 + 24.9 | 114 + 17.3 | 109 + 19.8 |
| F = P = |
1.629 0.155 |
3.441 0.006 |
1.335 0.260 |
Control values -.
White blood cell count (WBC) = 12.5 + 0.3 × 103 cells/μL.
Red blood cell count (RBC) = 8.6 + 0.1 × 106 cells/μL.
Blood haemoglobin (Hb) = 12.4 + 0.2 g/dL.
Values are mean + SEM (n = 6 each).
bSignificantly different from SM group.
Significantly different from control group.
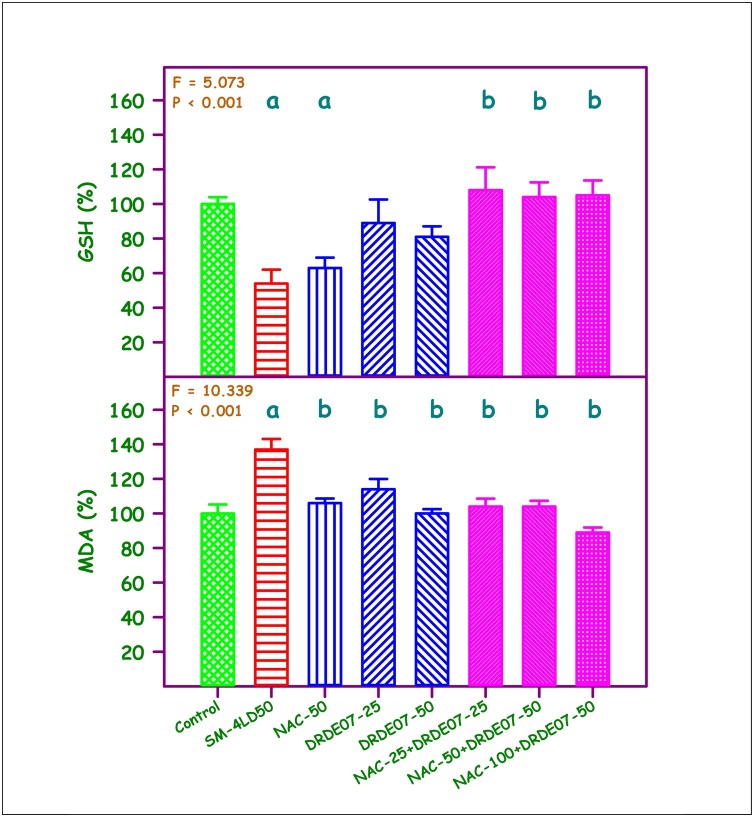
The percent change in liver GSH and MDA are given in Fig. 1. A statistically significant difference was observed in liver GSH among the experimental groups (P < 0.001). Compared to the control group, the SM group and the NAC-50 group showed 46 % and 37 % decrease respectively. The combination groups viz., NAC-25+DRDE07−25, NAC-50+DRDE07−50 and NAC-100+DRDE07−50 showed statistically significant protection in liver GSH when compared to the SM group (P < 0.05). A significant protection was also observed when compared to NAC-50 alone group (P < 0.05), but not with DRDE07−50 alone group. A 37 % increase in liver MDA level was observed in SM group (P < 0.001). All the treatment groups showed significant decrease in the liver MDA level compared to the SM group (P < 0.05). The combination of NAC-100+DRDE07−50 group showed the highest protection.
Fig. 1.
Percent change in reduced glutathione (GSH) and malondialdehyde (MDA) levels in liver.
Control values - GSH = 2.79 + 0.12 μmol/g tissue, MDA = 1.17 + 0.17 nmol/g tissue.
Values are mean + SEM (n = 6 each).
aSignificantly different from control group.
bSignificantly different from SM group.
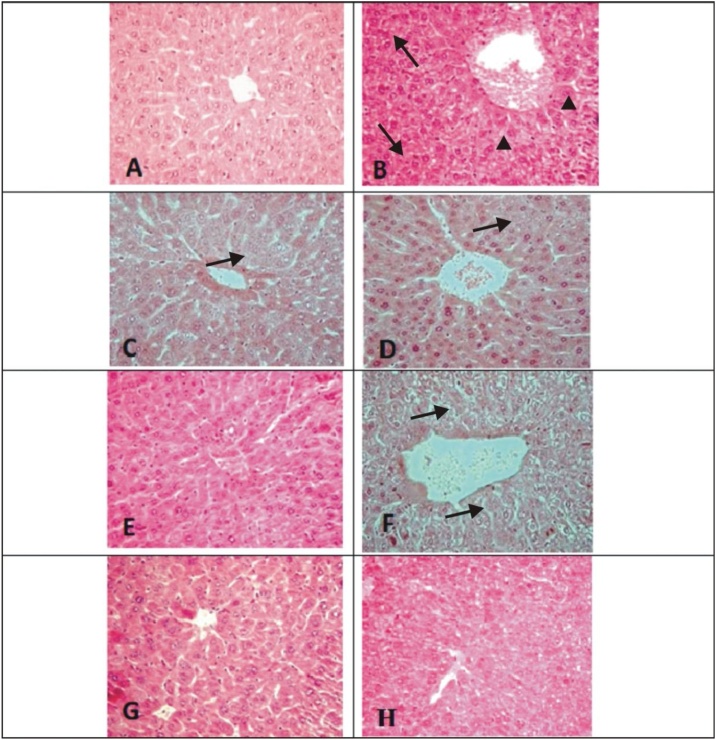
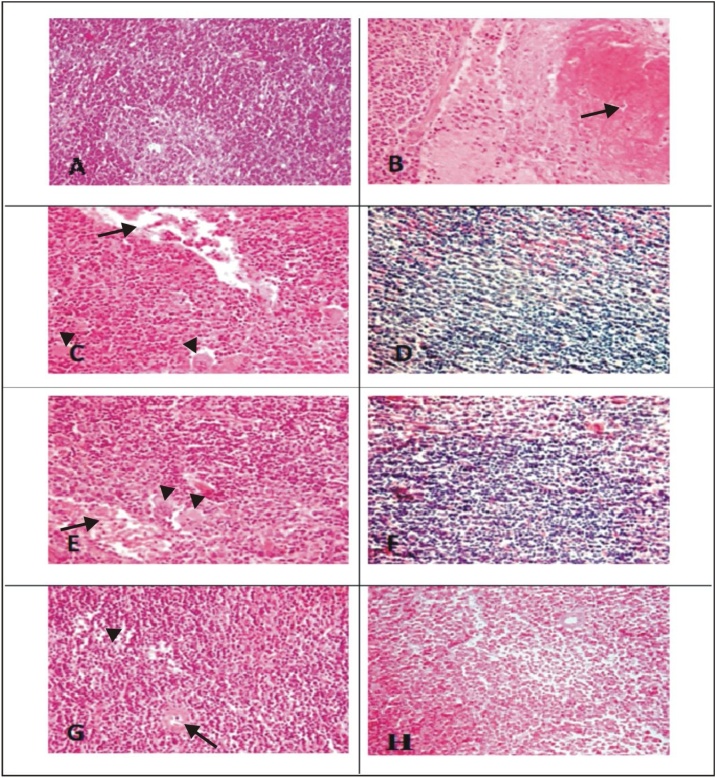
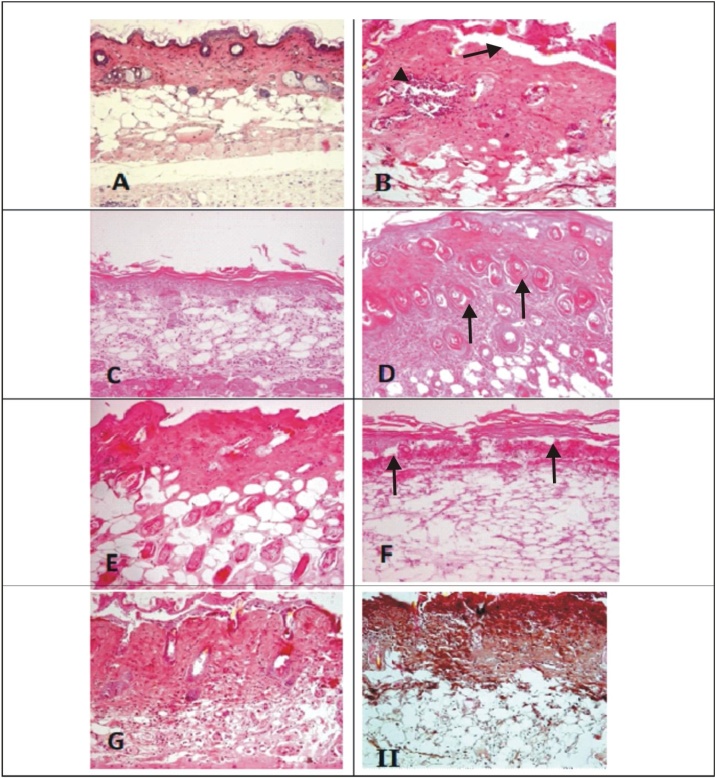
The histological observations of liver, spleen and skin of control, SM and the treatment groups are given in Figs. 2, 3 and 4 . The liver of control mice showed the lobules, radially arranged hepatocytes from the central canal and the portal triad. Liver sections of mice with SM (4 LD50) showed massive disorganized hepatocytes, hypertrophied Kupffer cells, infiltration of inflammatory cells, vacuolation and necrosis. Severity of the lesions was less in the treatment groups. The combination groups of NAC with DRDE-07 showed considerable reduction in hepatic lesions. Control mice spleen showed the germinal centre, red pulp and white pulp. Depletion and loss of lymphoid follicle with accumulation of fibrinoid material was observed in SM group (4 LD50). The treatment groups showed lesser lesions and the combination groups of NAC and DRDE-07 showed substantial decrease in the lesions with marked protection. The control mice skin showed epidermis comprising of stratified epithelium, adnexal tissue (hair follicle and sebaceous glands) and the connective tissue. Following SM (4 LD50) exposure, coagulative necrosis of epidermal cells extending to the dermis, loss of connection from basement membrane, infiltration of inflammatory cells in the dermis and edema were observed in the area surrounding the wound. Leucocytes and RBCs in a network of fibrin covered the degenerated dermo-epidermal region. Severe adnexal atrophy was observed in all the SM exposed mice. The treatment groups also showed more or less similar severity of lesions on the skin with the combination treatment groups showed reduced severity of necrosis.
Fig. 2.
Photomicrograph of liver tissue (H & E; 100x).
Representative image of each group
(A) Control mice showing normal hepatic chord, hepatocytes, central canal and kupffer cells; (B) SM-4LD50 arrow showing severe necrosis, arrowheads showing ballooning of hepatocytes and distortion of lobular pattern; (C) NAC-50 arrow showing reduction of severity of lesions as compared to B; (D) DRDE07−25 arrow showed slight decrease in severity of degenerative changes than B; (E) DRDE07−50; (F) NAC-25+DRDE07−25 arrow showing minimal degenerative changes as compared to B; (G) NAC-50+DRDE07−50; (H) NAC-100+DRDE07−50
Fig. 3.
Photomicrograph of spleen tissue (H & E;100x).
Representative image of each group
(A) Control mice spleen showing normal spleenic histology with germinal center, red pulp and marginal zone of white pulp; (B) SM-4LD50 arrow showing severe necrosis/apoptosis; (C) NAC-50 arrow showing fibrinous exudates and arrowheads showing necrosis/apoptosis; (D) DRDE07−25; (E) DRDE07−50 arrow showing fibrinous exudates and arrowheads showing necrosis/apoptosis; (F) NAC-25+DRDE07−25 showing lymphoid depletion; (G) NAC-50+DRDE07−50 arrow showing fibrinous and arrowheads showing lymphoid depletion ; (H) NAC-100+DRDE07−50
Fig. 4.
Photomicrograph of skin (H & E; 100x).
Representative image of each group
(A) Control mice skin section showing normal arrangement of epidermis, dermis, hair follicles and sebaceous glands; (B) SM-4LD50 arrow showing inflammation, atrophy dermoepidermal sepration; (C) NAC-50 showing hyalinization; (D) DRDE07−25; (E) DRDE07−50 (F) NAC-25+DRDE07−25 arrow showing coagulative necrosis of epidermis penetrating deep into dermis along with necrotic inflammatory cells; (G) NAC-50+DRDE07−50 showing mild to moderate decrease in severity of skin lesions compared to B; (H) NAC-100+DRDE07−50.
The severity of lesions in control, SM and the treatment groups on liver, spleen and skin are summarised in Table 3. In general, SM administration showed more than 45 % lesion severity in liver, spleen and skin. The individual treatments of NAC-50, DRDE07−25 and DRDE07−50 groups showed less than 45 % severity of lesions. The combination groups viz., NAC-25+DRDE07−25, NAC-50+DRDE07−50 and NAC-100+DRDE07−50 groups showed less than 22 % severity of lesions, showing the protective effect of the combination of NAC and DRDE-07. The severity of skin lesions was more or less similar in all the experimental groups.
Table 3.
Lesions in liver, spleen and kidney tissues.
| Lesions | ABCDEFGH |
|---|---|
| Liver | |
| Necrosis/apoptosis | -+++++++++++++++ |
| Inflammatory foci | -++++++++++++ |
| Hepatocyte vacuolation | -+++++++++++++ |
| Kupffer cell hyperplasia | -+++++++++++++++ |
| Hepatocellular degeneration | -+++++++++++++++ |
| Granulovacuolar degeneration | -++++++++++++++ |
| Spleen | |
| Lymphoid depletion | -++++++++++++++ |
| Necrosis/ apoptosis | -++++++++++++++ |
| Exudation of fibrinous exudates | -+++++++++++++- |
| Depletion of germinal centre | -++++++++++++ |
| Skin | |
| Inflammation | -++++++++++++++++++ |
| Epidermal necrosis | -++++++++++++++++++ |
| Vacuolization of keratinocytes | -++++++++++++++ |
| Edema | -++++++++++++ |
| Hyperplasia of basal cells | -++++++++++++ |
| Dermoepidermal separation | -++++++++++++++++++++ |
| Adnexal atrophy | -++++++++++++++++++++ |
A = Control; B = SM-4LD50; C = NAC-50; D = DRDE07−25; E = DRDE07−50.
F = NAC-25+DRDE07−25; G = NAC-50+DRDE07−50; H = NAC-100+DRDE07−50.
Average of 6 observations in each group.
- nil; + minimal (< 12 %); ++mild (< 22 %); +++moderate (< 45 %); ++++ severe (> 45 %).
4. Discussion
Percutaneously administered SM is extremely toxic. Earlier publications show that major quantity of SM evaporates and only a small quantity is absorbed [29]. However, being a highly lipophilic compound, SM quickly binds to the skin and subsequently absorbed. Hence physical decontamination should be done very quickly [11]. In biological system SM forms sulfonium ion with very high electrophilic property and binds to majority of cellular macromolecules [30]. Other than decontamination a better therapeutic approach is to restrict the secondary biochemical events of alkylation and prevent cell death. The toxicity of SM in humans is generally confined to skin, eyes and respiratory system when exposed to vapours or droplets [31]. Contamination with large quantity leads to systemic poisoning with multiorgan failure and death [32]. The most interesting phenomenon is that in animal models (mouse, rat and rabbit), SM exposure by percutaneous route is more toxic than the subcutaneous, oral or intraperitoneal routes irrespective of the diluent used [5]. Some of the nitrogen mustards also show similar peculiar phenomenon [33,34]. In animal models though skin blisters are not generally visible but systemic toxicity is very profound. This shows that the breakdown products of SM alkylation from the skin induce more damage than the subcutaneously injected SM, bypassing the skin.
The effect of SM starts 24 h after dermal application with weight loss. The weight loss is progressive and in 12–14 days, about 50 % of weight loss was observed. The animals would appear extremely weak and emaciated. In sublethal LD50 doses, the animals recover and gain weight [15]. The body weight loss is one good indicator of the toxic effects of SM. In the present study, within 3 days more than 30 % decrease in body weight was observed. Though, NAC and DRDE-07 improved the body weight, the combinations of NAC and DRDE-07 showed significant improvement, showing antagonistic effect on SM toxicity. The loss of body weight in SM is due to reduced consumption of food and water, which is also reflected on the vital organs. About 20–30 % decrease in the organ weights was observed in the present study. NAC with DRDE-07 combinations showed reduced effects. SM administration increases membrane permeability, reduction of extra cellular volume, free radical generation and oxidative stress [35]. This may be the reason for reduced body weight as well as the organ weights in the unprotected animals. Many of the earlier reports showed that the antidotes studied were effective only prophylactically and not therapeutically against SM toxicity in vivo showing that once the cascade of events are initiated it is rather difficult to intervene [36].
GSH exerts cytoprotective activity by inhibiting reactive oxygen species, restoration of damaged molecules by hydrogen donation, reduction of peroxidases, and maintenance of protein thiols in the reduced state [37]. SM forms sulfonium ions in the body and binds with the sulfhydryl groups [38]. This results in oxidative stress with a decrease in the level of GSH and an increase in lipid peroxidation [22,39]. Several in vitro and in vivo studies have reported that GSH is increased following DRDE-07 treatment, thereby preventing oxidative stress [14,34,39].
NAC acts as an antioxidant directly and is also a precursor for the synthesis GSH. The amino acid cysteine is one of the components of GSH which is a tripeptide. The availability of cysteine is generally less and NAC supplementation compensates for the synthesis of glutathione. NAC provides protection from toxic liver damage by elevating intracellular GSH levels [40]. As a source of SH groups, NAC can enhance glutathione-S- transferase activity, promote detoxification, and act directly on reactive oxygen free radicals [41]. SM toxicity increases lipid peroxidation which increases membrane permeability. This leads to an increase in RBC count and Hb due to haemoconcentration [42]. The combined administration of NAC and DRDE-07 protected all the effects. These results were supported by histopathological examination which revealed recovery from necrosis to normal architecture, particularly in the liver and spleen. The effect of SM on the skin is more of a local effect causing severe necrosis and break down of cellular components [43]. This leads to the cascade of systemic effects with oxidative stress and lipid peroxidation which is protected by the sulfanyl compounds. Hence, the protection of skin lesions by the antidotes are not complete, though systemic toxicity is reduced. The combination therapy showed better improvement particularly in the GSH level than the mono therapy and hence is more suitable.
NAC has additional benefits of directly binding to toxic metabolites like N-acetyl-p-benzoquinoneimine in acetaminophen over dose, mucolytic property by breaking the disulphide bonds and reducing the viscosity of mucus and vasodilatation due to production of nitric oxide [21]. As a mucolytic and antioxidant, NAC can be supplemented with steroids and antibiotics for effective treatment of SM toxicity in humans. Several studies have shown that NAC administration by oral, intraperitoneal, intravenous and by inhalation is very effective as an antioxidant, anti-inflammatory and cytoprotectant for SM toxicity [44]. NAC is an effective drug for phosgene and aluminium phosphide poisoning also [45,46].
Lipophilicity is an important property for the effectiveness of the compound. Compared to amifostine, DRDE-07 is more lipophilic, and hence can cross the cell membrane and protect from SM toxicity [47]. This explains the better prophylactic capability of orally administered DRDE-07. Amifostine, which is an analogues to DRDE-07 is effective as a cytoprotectant against cisplatin when it was given intravenously and not orally [48].
Presently, in several disease conditions the calcium permeable transient receptor potential channels are implicated. The transient receptor potential ankyrin 1 (TRPA1) is involved in pain and inflammation. SM activated human TRPA1 (hTRPA1) channels and NAC administration directly inhibited SM induced stimulation of hTRPA1 [49]. TRPA1 and TRPV1 (transient receptor potential vanilloid 1) channels are involved in itch and common skin diseases and NAC inhibited the TRP channels [50].
NAC is also an effective drug for influenza and other viral diseases and hence is in clinical trial for COVID-19 infection [51]. NAC is an approved drug and available in the form of tablets, injectables and spray. Though, DRDE-07 is in preclinical stage, amifostine which is analogues to DRDE-07 is used as a cytoprotectant for cisplatin, cyclophosphamide, and radiation therapy [52].
5. Conclusion
From the present study, it is concluded that the combination of NAC and DRDE-07 having sulfanyl groups will be a promising and effective antidote for SM toxicity. They can also be considered for other conditions in which oxidative stress is a cause for the toxicity.
Source of funding
Nil.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Edited by: DR. A.M Tsatsaka
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2021.03.011.
Contributor Information
Alka Gupta, Email: alka0207@gmail.com.
Rajagopalan Vijayaraghavan, Email: jai_vijay@hotmail.com.
Anshoo Gautam, Email: anshoo.gautam@gmail.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Romano J.A., Lukey B.J., Salem H. CRC Press; Boca Raton FL. USA: 2008. Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology, and Therapeutics. [Google Scholar]
- 2.Kehe K., Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Saladi R.N., Smith E., Persaud A.N. Mustard: a potential agent of chemical warfare and terrorism. Clin. Exp. Dermatol. 2006;31:1–5. doi: 10.1111/j.1365-2230.2005.01945.x. [DOI] [PubMed] [Google Scholar]
- 4.Graham J.S., Chilcott R.P., Rice P., Milner S.M., Hurst C.G., Maliner B.I. Wound healing of cutaneous sulfur mustard injuries: strategies for the development of improved therapies. J. Burns Wounds. 2005;4 e1. Published 2005 Jan 5. [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayaraghavan R., Kulkarni A., Pant S.C., Kumar P., Rao P.V., Gupta N. Differential toxicity of sulfur mustard administered through percutaneous, subcutaneous, and oral routes. Toxicol. Appl. Pharmacol. 2005;202:180–188. doi: 10.1016/j.taap.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Jan Y.H., Heck D.E., Laskin D.L., Laskin J.D. DNA damage signaling in the cellular responses to mustard vesicants. Toxicol. Lett. 2020;326:78–82. doi: 10.1016/j.toxlet.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghasemi H., Owlia P., Jalali-Nadoushan M.R., Pourfarzam S., Azimi G., Yarmohammadi M.E. A clinicopathological approach to sulfur mustard-induced organ complications: a major review. Cutan. Ocul. Toxicol. 2013;32:304–324. doi: 10.3109/15569527.2013.781615. [DOI] [PubMed] [Google Scholar]
- 8.Panahi Y., Shahbazi A., Naderi M., Jadidi K., Sahebkar A. Sulfur mustard-related ocular complications: a review of proteomic alterations and pathways involved. Curr. Pharm. Des. 2018;24:2849–2854. doi: 10.2174/1381612824666180903112218. [DOI] [PubMed] [Google Scholar]
- 9.Smith S.L. War! What is it good for? Mustard gas medicine. CMAJ. 2017;189:E321–E322. doi: 10.1503/cmaj.161032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh R.K., Kumar S., Prasad D.N., Bhardwaj T.R. Therapeutic journery of nitrogen mustard as alkylating anticancer agents: historic to future perspectives. Eur. J. Med. Chem. 2018;151:401–433. doi: 10.1016/j.ejmech.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Etemad L., Moshiri M., Balali-Mood M. Advances in treatment of acute sulfur mustard poisoning - a critical review. Crit. Rev. Toxicol. 2019;49:191–214. doi: 10.1080/10408444.2019.1579779. [DOI] [PubMed] [Google Scholar]
- 12.Pohanka M. Antioxidants countermeasures against sulfur mustard. Mini Rev. Med. Chem. 2012;12:742–748. doi: 10.2174/138955712801264783. [DOI] [PubMed] [Google Scholar]
- 13.Beigi Harchegani A., Khor A., Tahmasbpour E., Ghatrehsamani M., Bakhtiari Kaboutaraki H., Shahriary A. Role of oxidative stress and antioxidant therapy in acute and chronic phases of sulfur mustard injuries: a review. Cutan. Ocul. Toxicol. 2019;38:9–17. doi: 10.1080/15569527.2018.1495230. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharya R., Rao P.V., Pant S.C., Kumar P., Tulsawani R.K., Pathak U. Protective effects of amifostine and its analogues on sulfur mustard toxicity in vitro and in vivo. Toxicol. Appl. Pharmacol. 2001;176:24–33. doi: 10.1006/taap.2001.9252. [DOI] [PubMed] [Google Scholar]
- 15.Vijayaraghavan R., Kumar P., Joshi U., Raza S.K., Lakshmana Rao P.V., Malhotra R.C. Prophylactic efficacy of amifostine and its analogues against sulphur mustard toxicity. Toxicology. 2001;163:83–91. doi: 10.1016/s0300-483x(01)00369-9. [DOI] [PubMed] [Google Scholar]
- 16.Vijayaraghavan R., Sivanesan S. N-Acetyl-L-Cysteine (NAC) is a promising adjunct for pulmonary and extrapulmonary complications of chemical toxicity and microbial infections. World J. Pharm. Pharm. Sci. 2020;9(9):1410–1433. [Google Scholar]
- 17.Šalamon Š, Kramar B., Marolt T.P., Poljšak B., Milisav I. Medical and dietary uses of N-Acetylcysteine. Antioxidants (Basel) 2019;8:111. doi: 10.3390/antiox8050111. Published 2019 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adil M., Amin S.S., Mohtashim M. N-acetylcysteine in dermatology. Indian J. Dermatol. Venereol. Leprol. 2018;84:652–659. doi: 10.4103/ijdvl.IJDVL_33_18. [DOI] [PubMed] [Google Scholar]
- 19.Dludla P.V., Orlando P., Silvestri S., Mazibuko-Mbeje S.E., Johnson R., Marcheggiani F., Cirilli I., Muller C.J.F., Louw J., Obonye N., Nyawo T., Nkambule B.B., Tiano L. N-Acetyl cysteine ameliorates hyperglycemia-induced cardiomyocyte toxicity by improving mitochondrial energetics and enhancing endogenous Coenzyme Q9/10 levels. Toxicol. Rep. 2019;5(November 6):1240–1245. doi: 10.1016/j.toxrep.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dash M., Maity M., Dey A., Perveen H., Khatun S., Jana L., Chattopadhyay S. The consequence of NAC on sodium arsenite-induced uterine oxidative stress. Toxicol. Rep. 2018;13(February 5):278–287. doi: 10.1016/j.toxrep.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millea P.J. N-acetylcysteine: multiple clinical applications. Am. Fam. Physician. 2009;80:265–269. [PubMed] [Google Scholar]
- 22.Sharma M., Vijayaraghavan R., Agrawal O.P. Comparative toxic effect of nitrogen mustards (HN-1, HN-2, and HN-3) and sulfur mustard on hematological and biochemical variables and their protection by DRDE-07 and its analogues. Int. J. Toxicol. 2010;29:391–401. doi: 10.1177/1091581810365730. [DOI] [PubMed] [Google Scholar]
- 23.Gautam A., Vijayaraghavan R. Drde-07: a possible antidote for sulphur mustard toxicity. Cell. Mol. Biol. (Noisy-le-grand) 2010;56 Suppl: OL1334-OL1340. Published 2010 Sep 11. [PubMed] [Google Scholar]
- 24.Bhutia Y.D., Vijayaraghavan R., Pathak U. Analgesic and anti-inflammatory activity of amifostine, DRDE-07, and their analogs, in mice. Indian J. Pharmacol. 2010;42:17–20. doi: 10.4103/0253-7613.62401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar P., Vijayaraghavan R., Kulkarni A.S., Pathak U., Raza S.K., Jaiswal D.K. In vivo protection by amifostine and DRDE-07 against sulphur mustard toxicity. Hum. Exp. Toxicol. 2002;21:371–376. doi: 10.1191/0960327102ht250oa. [DOI] [PubMed] [Google Scholar]
- 26.Pathak U., Raza S.K., Kulkarni A.S., Vijayaraghvan R., Kumar P., Jaiswal D.K. Novel S-substituted aminoalkylamino ethanethiols as potential antidotes against sulfur mustard toxicity. J. Med. Chem. 2004;47:3817–3822. doi: 10.1021/jm030099v. [DOI] [PubMed] [Google Scholar]
- 27.Hissin P.J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 28.Esterbauer H., Cheeseman K.H. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 29.Papirmeister B., Feister A.J., Robinson S.I., Ford R.D. CRC Press; Boca Raton, FL: 1991. Medical Defence Against Mustard Gas: Toxic Mechanism and Pharmacological Implications. [Google Scholar]
- 30.Lakshmana Rao P.V., Vijayaraghavan R., Bhaskar A.S. Sulphur mustard induced DNA damage in mice after dermal and inhalation exposure. Toxicology. 1999;139:39–51. doi: 10.1016/s0300-483x(99)00097-9. [DOI] [PubMed] [Google Scholar]
- 31.Jenner J., Graham S.J. Treatment of sulphur mustard skin injury. Chem. Biol. Interact. 2013;206:491–495. doi: 10.1016/j.cbi.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Balali-Mood M., Hefazi M. Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic Clin. Pharmacol. Toxicol. 2006;99:273–282. doi: 10.1111/j.1742-7843.2006.pto_429.x. [DOI] [PubMed] [Google Scholar]
- 33.Sharma M., Vijayaraghavan R., Gautam A. DRDE-07 and its analogues as promising cytoprotectants to nitrogen mustard (HN-2)--an alkylating anticancer and chemical warfare agent. Toxicol. Lett. 2009;188:243–250. doi: 10.1016/j.toxlet.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Kumar P., Gautam A., Prakash C.J., Kumar A., Ganesan K., Pathak U. Ameliorative effect of DRDE 07 and its analogues on the systemic toxicity of sulphur mustard and nitrogen mustard in rabbit. Hum. Exp. Toxicol. 2010;29:747–755. doi: 10.1177/0960327109359641. [DOI] [PubMed] [Google Scholar]
- 35.Long L., Li W., Chen W., Li F.F., Li H., Wang L.L. Dynamic cytotoxic profiles of sulfur mustard in human dermal cells determined by multiparametric high-content analysis. Toxicol. Res. (Camb) 2016;5:583–593. doi: 10.1039/c5tx00305a. Published 2016 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gautam A., Vijayaraghavan R. Prophylactic effect of gossypin against percutaneously administered sulfur mustard. Biomed. Environ. Sci. 2007;20:250–259. [PubMed] [Google Scholar]
- 37.Navarro J., Obrador E., Pellicer J.A., Aseni M., Viña J., Estrela J.M. Blood glutathione as an index of radiation-induced oxidative stress in mice and humans. Free Radic. Biol. Med. 1997;22:1203–1209. doi: 10.1016/s0891-5849(96)00554-0. [DOI] [PubMed] [Google Scholar]
- 38.Vijayaraghavan R., Gautam A., Sharma M., Satish H.T., Pant S.C., Ganesan K. Comparative evaluation of some flavonoids and tocopherol acetate against the systemic toxicity induced by sulphur mustard. Indian J. Pharmacol. 2008;40:114–120. doi: 10.4103/0253-7613.42304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gautam A., Gupta A., Lomash V., Pant S.C., Vijayaraghavan R. Prophylactic efficacy of combination of DRDE-07 and its analogues with amifostine against sulphur mustard induced systemic toxicity. Indian J. Exp. Biol. 2010;48:752–761. [PubMed] [Google Scholar]
- 40.Das S.K., Mukherjee S., Smith M.G., Chatterjee D. Prophylactic protection by N-acetylcysteine against the pulmonary injury induced by 2-chloroethyl ethyl sulfide, a mustard analogue. J. Biochem. Mol. Toxicol. 2003;17:177–184. doi: 10.1002/jbt.10076. [DOI] [PubMed] [Google Scholar]
- 41.De Vries N., De Flora S. N-acetyl-l-cysteine. J. Cell. Biochem. 1993;17F:270–277. doi: 10.1002/jcb.240531040. Suppl. [DOI] [PubMed] [Google Scholar]
- 42.Dacre J.C., Goldman M. Toxicology and pharmacology of the chemical warfare agent sulfur mustard. Pharmacol. Rev. 1996;48:289–326. [PubMed] [Google Scholar]
- 43.Kehe K., Balszuweit F., Steinritz D., Thiermann H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–19. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 44.Shohrati M., Karimzadeh I., Saburi A., Khalili H., Ghanei M. The role of N-acetylcysteine in the management of acute and chronic pulmonary complications of sulfur mustard: a literature review. Inhal. Toxicol. 2014;26:507–523. doi: 10.3109/08958378.2014.920439. [DOI] [PubMed] [Google Scholar]
- 45.Sciuto A.M., Hurt H.H. Therapeutic treatments of phosgene-induced lung injury. Inhal. Toxicol. 2004;16:565–580. doi: 10.1080/08958370490442584. [DOI] [PubMed] [Google Scholar]
- 46.Gheshlaghi F., Lavasanijou M.R., Moghaddam N.A., Khazaei M., Behjati M., Farajzadegan Z. N-acetylcysteine, ascorbic acid, and methylene blue for the treatment of aluminium phosphide poisoning: still beneficial? Toxicol. Int. 2015;22:40–44. doi: 10.4103/0971-6580.172255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulkarni A.S., Vijayaraghavan R., Anshoo G., Satish H.T., Pathak U., Raza S.K. Evaluation of analogues of DRDE-07 as prophylactic agents against the lethality and toxicity of sulfur mustard administered through percutaneous route. J. Appl. Toxicol. 2006;26:115–125. doi: 10.1002/jat.1114. [DOI] [PubMed] [Google Scholar]
- 48.Bonner H.S., Shaw L.M. New dosing regimens for amifostine: a pilot study to compare the relative bioavailability of oral and subcutaneous administration with intravenous infusion. J. Clin. Pharmacol. 2002;42:166–174. doi: 10.1177/00912700222011201. [DOI] [PubMed] [Google Scholar]
- 49.Stenger B., Popp T., John H., Siegert M., Tsoutsoulopoulos A., Schmidt A. N-Acetyl-L-cysteine inhibits sulfur mustard-induced and TRPA1-dependent calcium influx. Arch. Toxicol. 2017;91:2179–2189. doi: 10.1007/s00204-016-1873-x. [DOI] [PubMed] [Google Scholar]
- 50.Fernandes E.S., Vong C.T., Quek S., Cheong J., Awal S., Gentry C. Superoxide generation and leukocyte accumulation: key elements in the mediation of leukotriene B₄-induced itch by transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1. FASEB J. 2013;27:1664–1673. doi: 10.1096/fj.12-221218. [DOI] [PubMed] [Google Scholar]
- 51.Clinical Trials.gov . Clinical Trials.gov Identifier: NCT04374461 Memorial Sloan Kettering Cancer Center; 2020. A Study of N-acetylcysteine in Patients With COVID-19 Infection. May. [Google Scholar]
- 52.Capizzi R.L., Oster W. Chemoprotective and radioprotective effects of amifostine: an update of clinical trials. Int. J. Hematol. 2000;72:425–435. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.