Abstract
Atherosclerosis is a systemic chronic inflammatory disease. Many antioxidants including alpha-lipoic acid (LA), a product of lipoic acid synthase (Lias), have proven to be effective for treatment of this disease. However, the question remains whether LA regulates the immune response as a protective mechanism against atherosclerosis. We initially investigated whether enhanced endogenous antioxidant can retard the development of atherosclerosis via immunomodulation.
To explore the impact of enhanced endogenous antioxidant on the retardation of atherosclerosis via immune regulation, our laboratory has recently created a double mutant mouse model, using apolipoprotein E-deficient (Apoe−/−) mice crossbred with mice overexpressing lipoic acid synthase gene (LiasH/H), designated as LiasH/HApoe−/− mice. Their littermates, Lias+/+Apoe−/− mice, served as a control. Distinct redox environments between the two strains of mice have been established and they can be used to facilitate identification of antioxidant targets in the immune response.
At 6 months of age, LiasH/HApoe−/− mice had profoundly decreased atherosclerotic lesion size in the aortic sinus compared to their Lias+/+Apoe−/− littermates, accompanied by significantly enhanced numbers of regulatory T cells (Tregs) and anti-oxidized LDL autoantibody in the vascular system, and reduced T cell infiltrates in aortic walls.
Our results represent a novel exploration into an environment with increased endogenous antioxidant and its ability to alleviate atherosclerosis, likely through regulation of the immune response. These outcomes shed light on a new therapeutic strategy using antioxidants to lessen atherosclerosis.
Keywords: Regulatory T cells, Oxidized LDL autoantibody, Antioxidant mouse models, Atherosclerosis, And immune regulation
1. Introduction
α-Lipoic acid (LA) is a potent antioxidant synthesized by lipoic acid synthase (Lias) in mitochondria [1]. It is a co-factor for several enzymes including pyruvate dehydrogenase complex and alpha-ketoglutarate dehydrogenase complex, both of which participate in ATP production. LA as an antioxidant is well-established [3, 4], but its function in immune regulation remains to be elucidated. In an effort to study the role of LIAS in immune regulation during the development of atherosclerosis, we generated genetic mouse models by modifying the 3′ untranslated regions (3′-UTR) of the Lias gene that influence Lias mRNA copy number, thus affecting Lias protein expression to obtain LiasH/H mice. LiasH/H mice display ~160% of Lias protein expression confirmed by Western blot, with corresponding low levels of antioxidant and oxidative stress [9]. LiasH/H mice were crossed with an atherosclerosis-prone mouse model, Apoe−/− mice, to test whether enhanced antioxidant capacity can attenuate the onset and development of atherosclerosis in the double mutant LiasH/HApoe−/− mice. Our novel LiasH/HApoe−/− mice mimic gain-of-function of antioxidant by creating a strengthened endogenous antioxidant microenvironment. They may amplify subtle changes that may not be apparent under basal conditions and will be helpful to identify LA targeted diagnostic biomarkers in mitochondria, and decipher the mechanisms of immune regulation during development of the disease.
Broad evidence supports that atherosclerosis is a systemic chronic inflammatory disease [10], characterized by the formation of an immune cell infiltration dominated by lipid-laden macrophage foam cells and T cells, and elicits lipid retention and oxidative modification of low-density lipoprotein (LDL) in the arterial wall [11]. These modifications trigger both innate and adaptive immune responses [12]. Adaptive immunity has been shown to participate in all stages of the disease. T lymphocytes are among the earliest cells recruited into the atherosclerotic plaque [13], predominated by a large number of CD4+ T cells in the atherosclerotic plaques in humans and mice [14]. Adoptive transfer of CD4+ T lymphocytes increases the progression of atherosclerosis in immunodeficient mice [15]. In response to the local milieu of cytokines, naïve CD4+ T lymphocytes differentiate into T helper (Th) cell 1 (Th1), Th2, Th17, and Treg lineages. The Th1 response, including the production of interferon-γ (IFN-γ), is prevalent during atherosclerosis [16] and has been shown to promote atherosclerosis [17]. Tregs can suppress the activity of effector T cells and are known to play a critical role in the T-cell homeostasis. Treg cells also mediate suppressor function through the production of IL-10 and TGF-β, respectively [18,19]. As a result, Tregs are considered to be atheroprotective. Evidence shows that numbers of circulating Tregs decline in patients with coronary artery disease or atherosclerosis [20,21]. Since autoimmune antibodies are found in the circulation of patients with atherosclerosis, autoimmune mechanisms may contribute to the pathogenesis of disease [22]. These antibodies react against a wide variety of oxidized lipid-protein adducts, mainly derived from oxidized low-density lipoproteins (oxLDLs). OxLDLs are no longer recognized as self-molecules by the body’s immune system after either enzymatic or non-enzymatic modification [23]. 1,4-dihydropyridine-type MDA-acet-aldehyde-Lys adduct (MAA), which is a form of oxLDL, has been proposed to be one of the most potent atherogenic protein adducts caused by lipid peroxidation and appear to play a critical role in atherogenesis [24].
To date, little is known about whether antioxidants can regulate the immune system during the development of atherosclerosis. In particular, how antioxidants regulate T helper cell (Th) lineages during atherosclerosis progression remains to be elucidated. It is well-known that under the influence of their microenvironment, Th cell subsets with their signature cytokine profiles can switch from one lineage to another [25]. In addition, T helper activation is strongly dependent on the redox potential of the microenvironment [26]. We hypothesized that generation of type and amount of T cell subsets, and plasticity of the signature cytokines were influenced and regulated by the antioxidant microenvironment and higher expression of LIAS will promote Treg cell production. In our current study, we found that Tregs and MAA autoantibodies in circulation were significantly increased while lymphocyte infiltration was significantly decreased in aortic walls of LiasH/HApoe−/− mice compared with Lias+/+Apoe−/− mice. These results raised a possibility that LA alleviates atherosclerosis by manipulating the immune response.
2. Materials and methods
2.1. Animals and experimental design
Apoe−/− mice on C57BL/6 J background were purchased from Jackson Laboratories (B6.129P2-Apoetm1Unc/J, Stock No:002052, Bar Harbor, ME). LiasH/H mice on C57BL/6 background were generated via C57BL/6 embryonic stem cells as we described previously [9]. When the mice were engineered by genetic modification of the 3′-untranslated region (3′-UTR), the endogenous Lias gene expression was increased by ~ 160% in LiasH/H mice [9]. Correspondingly Lias protein expression levels and antioxidant content in LiasH/H mice increased (9) as confirmed by RT-PCR and Western blot. LiasH/H mice were then crossed with Apoe−/− mice on the same genetic background to achieve a double mutant LiasH/HApoe−/− mouse model. Lias+/+Apoe−/− mice, as controls, were littermates of LiasH/HApoe−/− mice obtained during the mating process and all mice were sacrificed at 6 months of age. Male animals were used in the study. All animals were bred and housed in a pathogen-free animal facility and fed normal chow in a temperature-controlled environment (22–25 °C) with a 12-h light/dark cycle. The animals received regular chow diet and water ad libitum. Animal care and experimental protocols (19–111; PI: Xianwen Yi) were approved by the Institutional Animal Care and Use Committee of the University of North Carolina, Chapel Hill.
2.2. Plasma lipid profile
Blood samples were collected using a submandibular bleeding method and centrifuged (1500 × g, 20 min) to obtain plasma samples. Plasma total cholesterol (Chol) and triglycerides (TG) were quantified using colorimetric kits from Wako (Richmond, VA) based on enzymatic reactions according to the manufacturer’s protocols. Plasma high-density lipoprotein (HDL) and low-density lipoprotein (LDL)/Very-LDL (VLDL) cholesterol were separated and measured using HDL and LDL/VLDL cholesterol assay kit (Abeam, Cambridge, MA).
2.3. Oxidative stress status
Plasma lipid peroxide content was determined as 4-hydroxynonenal (4-HNE), a commonly used lipid peroxidation marker, assayed by using an ELISA Kit following the manufacturer’s protocol (Cell Biolabs. Inc., San Diego, CA). Blood total antioxidant capacity was measured by Antioxidant Assay Kit (Cayman Chemical, Ann Arbor, MI). The assay is based on the ability of antioxidants in the sample to inhibit the oxidation of ABTS® (2,2′-azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS® + by metmyoglobin.
2.4. Quantitation of atherosclerotic lesions
Mice were perfused with PBS containing heparin, followed by 10% buffered formalin (pH 7.4) under physiological pressure for histological and immunohistochemical staining. Mouse hearts were collected after perfusion, embedded in optimal cutting temperature (OCT) compound and were snap frozen. Sequential cryosections of OCT-embedded aortic roots were cut such that all three leaflets of the aortic valve could be visualized. The frozen heart blocks were cut at 10 μm thickness and cryosections were first stained with Oil Red O and then counterstained with hematoxylin. Images of aortic root were captured with an Olympus BX61 microscope. The average lesion area from three continuous cross-sections of the aortic root at an anatomical position defined by the presence of all three mitral valves, was measured in each animal. Quantitative morphometric analysis of lesion area using NIH Imaging Software was carried out by a histologist blinded to the sample genotypes. Atherosclerosis at the remainder of the aortic tree is not sufficiently large in the mice on normal chow and was not used for quantitation.
2.5. Measurement of circulating Tregs using flow cytometry
Mouse peripheral blood was collected by submandibular bleeding into EDTA micro tubes with 500 μl PBS and mixed well. Lymphocytes were obtained by centrifugation over Lympholyte®-Mammal Separation Media (Cedarlane Laboratories Ltd., Burlington, NC) at 800 × g for 20 min at room temperature. The isolated lymphocytes were washed 3 times with FACS wash buffer.
Lymphocytes isolated from blood were stained with CD3-APC-eFluor780, CD4-FITC, CD25-PE-Cyanine 7 for 30 min in the dark. Nuclear staining of forkhead box protein 3 (FoxP3), cells were fixed in Fixation/Permeabilization Buffer (eBioscience) for one hour, followed by staining with FoxP3-PE-eFluor 610 for one hour in the dark. All antibodies were from eBioscience (San Diego, CA). Cells were then examined with Cyan ADP (Dako) and analyzed with Summit software.
2.6. IFN-γ and IL-10 in serum
Serum IL-10 and IFN-γ were measured by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Each sample was assayed in duplicate. Absorbance was measured at 450 nm by means of a spectrophotometer (SpectraMax M5, Molecular Devices).
2.7. Determination of MAA-specific antibody titers
MAA-adducts were synthesized as described previously [27]. Briefly, ε-aminocaproic acid (6-ACA), acetaldehyde, and malondialdehyde were incubated at 37 °C for 3 days. MAA-6ACA was purified by HPLC. MAA-6ACA was conjugated with bovine serum albumin (BSA) as reported previously [27]. MAA-6ACA-BSA was seeded in 96-well plates and incubated at 4 °C overnight. After blocking with 3% BSA, serum samples diluted at 1:320 ratio by 1% BSA in PBS were incubated at 4 °C overnight. After washing, peroxidase-labeled secondary antibody (Envision + Single) was used with anti-mouse (IgG)-HRP (Code K4001, Dako North America, Inc., Carpentaria, CA) and incubated for 1 h. After washing the wells, 3,3′,5,5′-tetra-methylbenzidine (TMB)/H2O2 substrate was added to the wells and kept at room temperature for 30 min. The plates were then read with a plate reader (Vmax Kinetic Microplate Reader, Molecular Devices, Sunnyvale, CA) at a wavelength of 650 nm.
2.8. Aortic lymphocyte infiltration
2.8.1. Flow Cytometry analysis of immune cells within mouse aortic walls
Immune cells within murine aortas were analyzed using flow cytometry following a published protocol [28]. Briefly, entire aortas were perfused and attached adipose tissue was carefully removed under a dissection microscope. After blood was completely flushed out of the aortas, the aortas were dissected and digested with the enzyme cocktail containing 450 U/ml collagenase type I, 125 U/ml collagenase type XI, 60 U/ml hyaluronidase type I-s and 60 U/ml DNase 1 in PBS containing 20 mM Hepes at 37 °C for 1 h. A cell suspension was obtained by mashing the aorta through a 70-μm strainer. The lymphocytes were fractioned by density gradient centrifugation using Lympholyte®-Mammal Separation Media (Cedarlane Laboratories Ltd., Burlington, NC). Aortic cells were stained with CD3-APC and CD4-PE-Cy7 for T cells, and CD45-FITC as a lymphocyte common antigen to identify all lymphocytes in the aortas. Cells were analyzed by flow cytometry. All subsequent analyses were performed on cells gated to CD45.
2.8.2. Immune cell quantification in aortic atherosclerotic lesions
Contiguous 10 μm frozen sections of the ascending aortic root were stained with H&E using standard protocols. Quantification of immune cells was performed on contiguous 10 μm frozen sections of the aortic root by identification of mononuclear cell nuclei apart from surrounding structures using positive pixel intensity defined algorithm. Briefly, H&E stained slides were digitized using a Nikon Eclipse E800 microscope with a Nikon DS-Ri2 camera. The anonymized tiff files were then analyzed by NIS Elements Software BR 4.30.02 (Nikon Instruments Inc.) using the positive pixel intensity algorithm to define positive mononuclear cell nuclei areas in the manually defined region of interest and represented as mean of the percentage of aortic area for positive inflammatory cells by pixel intensity per mouse aortic vessel in a blinded manner. Immune cell quantification in aortic vessels with atherosclerotic lesions was performed in 3 biological replicates per mouse from each group, at the original magnification (4 ×).
2.9. Immunohistochemistry
Immunohistochemical analyses were performed on 10 μm frozen sections of aortic root. Macrophages were detected using rat monoclonal anti-mouse CD68 (Novus Biologicals, Littleton, CO) and rabbit antibody against pMAA-6ACA-BSA antigens. The rabbit anti-MAA polyclonal antibody was kindly provided by Dr. Todd A. Wyatt (University of Nebraska).
All secondary antibodies were conjugated with horseradish peroxidase (HRP). Endogenous peroxidase activity or endogenous biotin sites were quenched with 3% H2O2/PBS or with the avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). Images were taken under an Olympus BX61 microscope.
2.10. Statistical analysis
Statistical analysis was performed using Students’ t-test. All data are represented as the mean ± standard error of mean (SEM). P < 0.05 values were considered to be statistically significant.
3. Results
3.1. Reduced atherosclerotic lesion size in LiasH/HApoe−/− mice
Atherosclerotic plaques at the aortic sinus of each mouse fed standard chow were histologically analyzed at 6 months of age. Fig. 1A and 1B are representative photomicrographs of the aortic sinus plaque in LiasH/HApoe−/− mice (A) and Lias+/+Apoe−/− mice (B). Lesions are detected in red after Oil Red O staining. The mean size of lesions at the aortic sinus was 3.4 ± 0.7 μm2 × 105 and 4.9 ± 0.9 μm2 × 105 (P < 0.05) in 6-month-old LiasH/HApoe−/− mice and Lias+/+Apoe−/− mice, respectively. LiasH/HApoe−/− mice had a 31% decrease in atherosclerotic plaque area compared to their Lias+/+Apoe−/− counterparts (P < 0.05) (Fig. 1C), suggesting that increased steady-state levels of Lias transcript increased endogenous antioxidant capacity and contributed to a reduction in atherosclerotic lesion size.
Fig. 1.
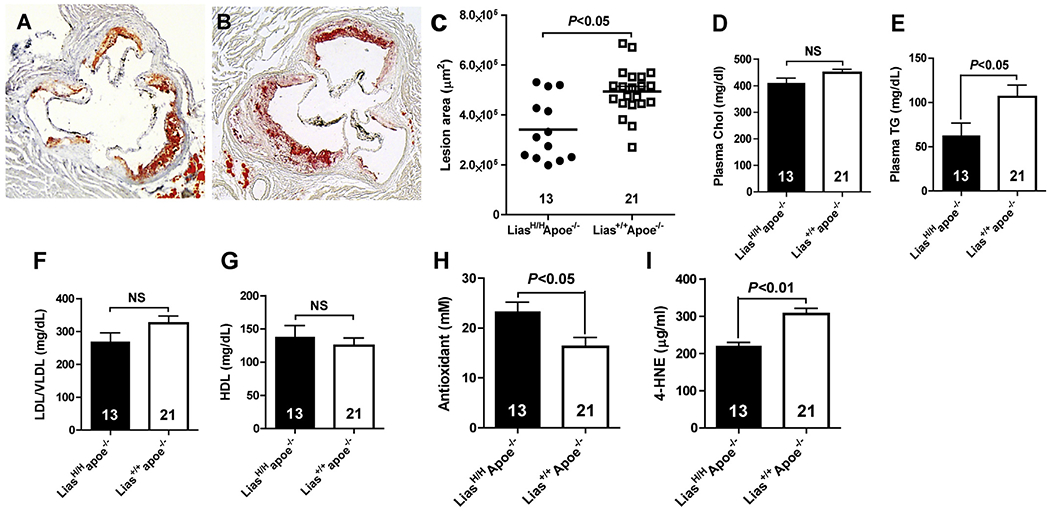
Representative light photomicrographs of aortic sinus plaque, plasma lipid profile and redox status. Aortic sinus plaque in LiasH/HApoe−/− mice (A) Lias+/+Apoe−/− mice (B). Original magnification 40×. Sections are stained with Oil Red O and counterstained with hematoxylin. C) Average atherosclerosis plaques at the aortic roots of 6-month-old male LiasH/HApoe−/− (n = 13) and Lias+/+Apoe−/− (n = 21) mice. Each point represents the lesion size of the individual mouse and the horizontal bars stand for mean lesion size. (D) Plasma total cholesterol (Chol). (E) Plasma triglyceride (TG). (F) VLDL/LDL cholesterol. (G) HDL cholesterol. (H) Total antioxidant content and (I) Lipid peroxidation marker, 4-HNE, concentration in 6-month-old male LiasH/HApoe−/− (closed bars) and Lias+/+Apoe−/− mice (open bars). Numbers inside bars indicate the number of animals. Results are expressed as mean ± SE. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Plasma triglyceride and cholesterol LiasH/HApoe−/− mice
Since lipoic acid has anti-hyperlipidemia function [29] and Treg cells can influence cholesterol metabolism [30], we examined the effect of increased Lias gene expression on plasma cholesterol (Chol) and triglyceride (TG) levels. Plasma TG level was significantly lower (Fig. 1E), but total Chol level was not (Fig. 1D) in LiasH/HApoe−/− compared to Lias+/+Apoe−/− mice at the end of the observed period. Plasma cholesterol associated with VLDL/LDL was higher in LiasH/HApoe−/− mice compared with Lias+/+Apoe−/− mice (Fig. 1F) but HDL value was the opposite in the two strains, and both values did not reach significance (Fig. 1G). Since most TG is carried by the VLDL particle, which is the major proatherogenic lipoprotein particle in Apoe−/− mice, higher levels of TG may contribute to the larger atherosclerotic lesions in Lias+/+Apoe−/− mice.
3.3. Decreased oxidative stress in LiasH/HApoe−/− mice
The LiasH/HApoe−/− mice showed an increased plasma antioxidant capacity (Fig. 1H) and reduced oxidative stress (Fig. 1I) compared with Lias+/+Apoe−/− mice, as measured by Antioxidant Assay Kit (Cayman Chemical, Ann Arbor, MI) and 4-Hydroxynonenal (4-HNE) Assay Kit (Cell Biolabs, San Diego, CA), respectively.
3.4. Flow cytometry analysis detected T lymphocytes and total mononuclear cell count within aortic walls
Mononuclear cells include macrophages and lymphocytes. Mononuclear cell infiltration is characteristic of inflammatory lesions. We observed more mononuclear cell infiltration in the aortic root of Lias+/+Apoe−/− (Fig. 2A) than in LiasH/HApoe−/− mice (Fig. 2B) by counting the mononuclear cell nuclei apart from surrounding structures using a positive pixel intensity defined algorithm. The pixel intensity of the (replicate) mean percentage of positive inflammatory cells in the aortic roots in Lias+/+Apoe−/− mouse was significantly higher than in LiasH/HApoe−/− mice (Fig. 2C), consistent with a reduction in inflammatory cells in atherosclerotic lesions of LiasH/HApoe−/− mice.
Fig. 2.
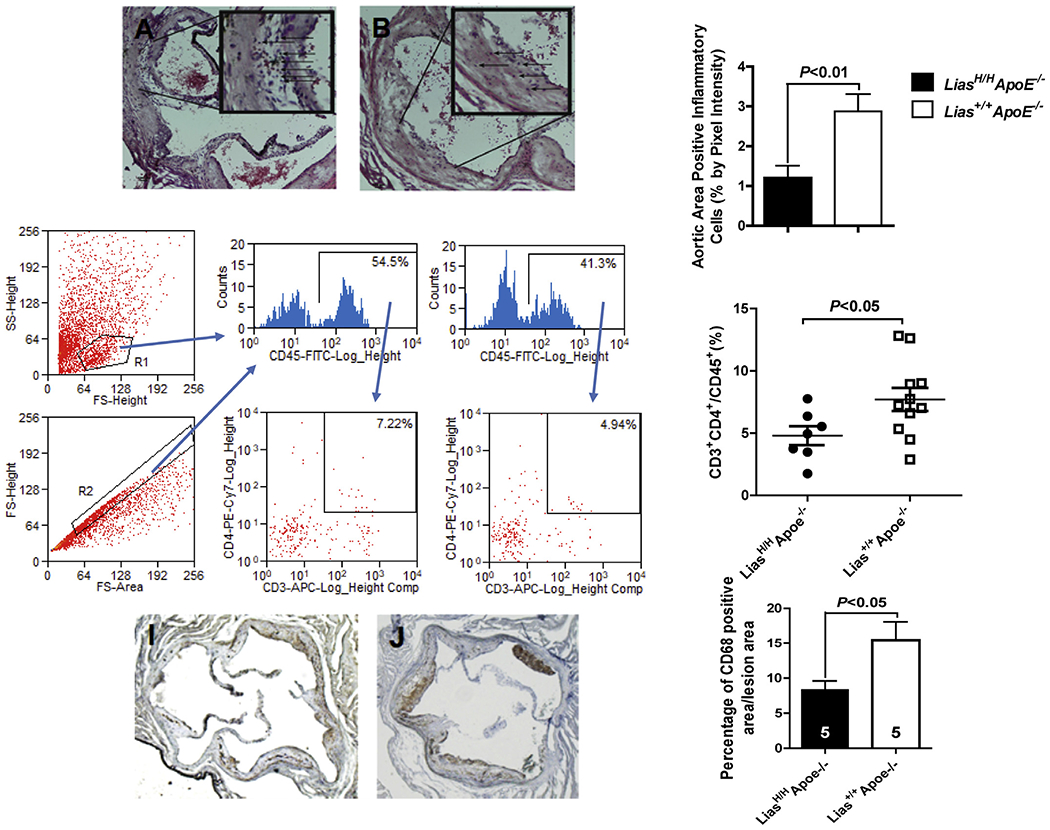
Histological analysis of the ascending aortic root. (A). Representative Lias+/+Apoe−/− aortic root (arrows in insert delineate to identified mononuclear cell nuclei). (B). Representative LiasH/HApoe−/− aortic root (arrows in insert delineate to identified the nuclei). (C). Student’s t-test was used to determine differences in aortic root cellular nuclei in LiasH/HApoe−/− compared to controls. Original magnification 40×.
Evidence showed the presence of large numbers of T cells in all stages of lesion development, which may contribute to atherosclerosis [31]. Relative T-lymphocyte density in the aortic wall was analyzed by flow cytometry after enzyme digestion to determine the percentage of CD4+ T lymphocytes of LiasH/HApoe−/− and Lias+/+Apoe−/− in aortic connective tissues. Staining for the CD45+ common lymphocyte antigen allowed the identification of aortic leukocytes; CD4+ T cells were detected by CD3+ and CD4+ double staining. The percentage of CD3+ / CD4+ T lymphocytes of the total CD45+ cells in the LiasH/HApoe−/− aortic wall was significantly reduced (Fig. 2G and H) compared with Lias+/+Apoe−/− mice (Fig. 2F and 2H). The data suggest that enhanced endogenous antioxidant level decreased inflammatory cell infiltration, which may contribute to the suppression of atherogenesis. We also found the positive area stained by antibody against CD68+ monocytes/macrophages are reduced in LiasH/HApoe−/− mice (Fig. 2I) compared to Lias+/+Apoe−/− mice (Fig. 2J). Quantitative analysis of the area stained with CD68 relative to the total plaque area was significantly less in LiasH/HApoe−/− mice (8.4 ± 1.2%) compared to Lias+/+Apoe−/− mice (15.4 ± 2.6%, P < 0.05) (Fig. 2K). Together these studies demonstrate that increasing Lias expression (LiasH/HApoe−/−) results in decreased CD4+ T cells and CD68+ monocytes/macrophages atherosclerotic plaques compared to Apoe−/− strain-matched controls.
The increased presence of CD3+ CD4+ cells in the aortic walls of LiasH/HApoe−/− mice. Following enzymatic digestion, cells released from aortic walls were stained with antibodies conjugated to fluorescent dyes for flow cytometry analysis. Cells in the aortic walls were gated by scattering light (D) and CD45 staining (E). Percentage of CD3+ and CD4+ double-positive T cells in lymphocytes of aortic walls in (F) LiasH/HApoe−/− and (G) Lias+/+Apoe−/− mice. (H) A population of CD3+ and CD4+ double-positive T cells in relation to all T cells (or divided by) all T cells in aortic walls of mice (n = 6 for LiasH/HApoe−/− and n = 11 for Lias+/+Apoe−/− mice). The horizontal bars indicate mean ± SE.
Representative immunohistochemical images. CD68 antibody staining in aortic root sections of LiasH/HApoe−/− (I) and Lias+/+Apoe−/− (J) was detected by staining the aortic root brown with DAPI and counterstaining with hematoxylin. Original magnification 40 ×. Two aortic sections were examined per mouse, five mice per group. (K) Quantification of CD68-positive area relative to plaque area. Results are expressed as mean ± SE.
3.5. Increased Tregs in peripheral blood in LiasH/HApoe−/− mice
It is very demanding to obtain enough aortic lymphocytes to functionally address their characteristics using flow cytometry because there are a very limited number of resident and infiltrating lymphocytes in the aortic walls to acquire reliable analysis of Tregs. Thus, we used peripheral blood lymphocytes to uncover the mechanism behind the observed atherosclerotic phenotypes. The total Treg population was identified as CD3+ CD4+ FOXP3+ cells. The results showed that the proportion of total peripheral blood Tregs was 11.3% in LiasH/HApoe−/− mice (Fig. 3B) compared to 6.2% in the control Lias+/+Apoe−/− mice (Fig. 3C), reflecting a significant increased Treg population in LiasH/HApoe−/− mice (Fig. 3D).
Fig. 3.
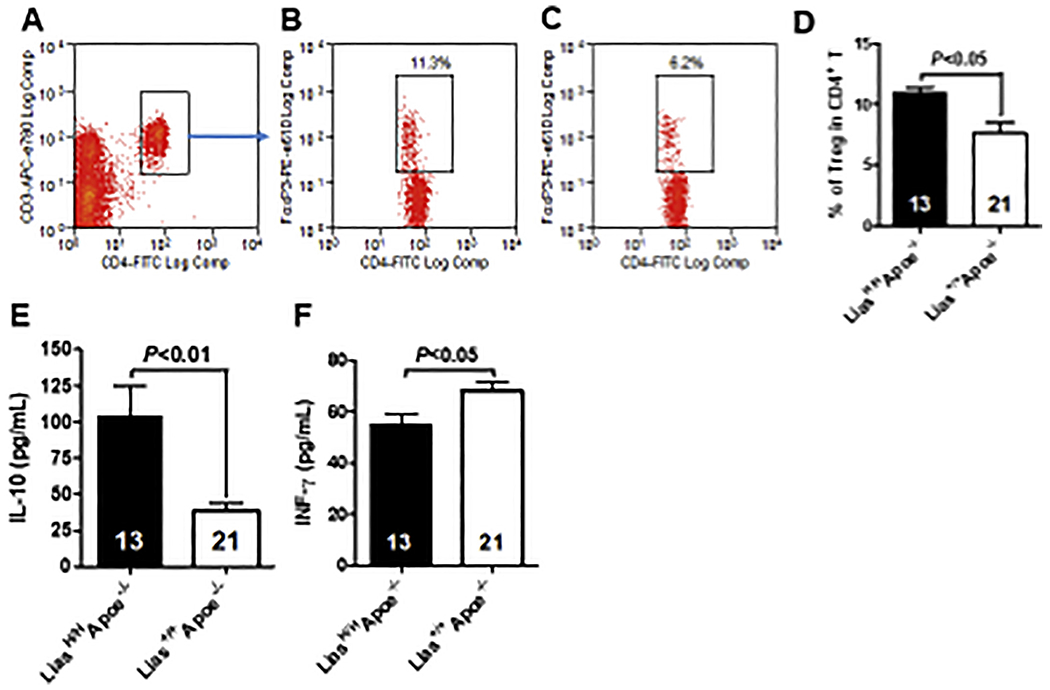
Tregs in peripheral blood of LiasH/HApoe−/− and Lias+/+Apoe−/− mice. Blood was collected from each experimental group and lymphocytes isolated from blood were stained with CD3-APC-eFluor780, CD4-FITC. Cells were fixed, permeabilized and stained intracellularly with FoxP3-PE-eFluor 610 and flow cytometry analysis. Cells were gated on CD4 and CD3 (A) and FoxP3 with LiasH/HApoe−/− (B) and Lias+/+Apoe−/− (C). Quantitative analysis of Tregs in the two groups of mice (D). Results are expressed as mean ± SE. Serum levels of interleukin-10 (E) and interferon γ (F) in 6-month-old male LiasH/HApoe−/− (closed bar) and Lias+/+Apoe−/− mice (open bar). Results are expressed as mean ± SE.
3.6. Circulating IFN-γ and IL-10
Th1 CD4+ T cell subset secretes pro-inflammatory cytokines such as IFN-γ, whereas Tregs produce the anti-inflammatory cytokine IL-10. Tregs and serum IL-10 are decreased in vulnerable patients that have had recurrent cardiac events in comparison with stable patients [32]. Thus, we measured the level of these two types of cytokines in peripheral blood to determine the influence of two environments of different redox status generated by different levels of Lias gene expression. Furthermore, LiasH/HApoe−/− mice serum had significantly increased IL-10 and IFN-γ levels compared to Lias+/+Apoe−/− mice (Fig. 3E and 3F). Therefore, increased antioxidant level in LiasH/HApoe−/− mice enhances both immunosuppressive Treg and immunosuppressive cytokine IL-10.
3.7. MAA-specific antibody titers in the serum of LiasH/HApoe−/− and Lias+/+Apoe−/− mice
To determine the effect of increased Lias gene expression on oxLDL autoantibody in peripheral blood, we examined the serum MAA antibody titer using ELISA in LiasH/HApoe−/− and Lias+/+Apoe−/− mice. The concentration of MAA-specific IgG in the serum of LiasH/HApoe−/− mice is significantly higher than Lias+/+Apoe−/− mice at 6 months of age (Fig. 4). However, IgM did not show a significant difference between the two groups (data not shown). This result suggests that an enhanced level of antioxidant increased oxLDL autoantibody production, which may contribute to attenuation of atherosclerosis.
Fig. 4.
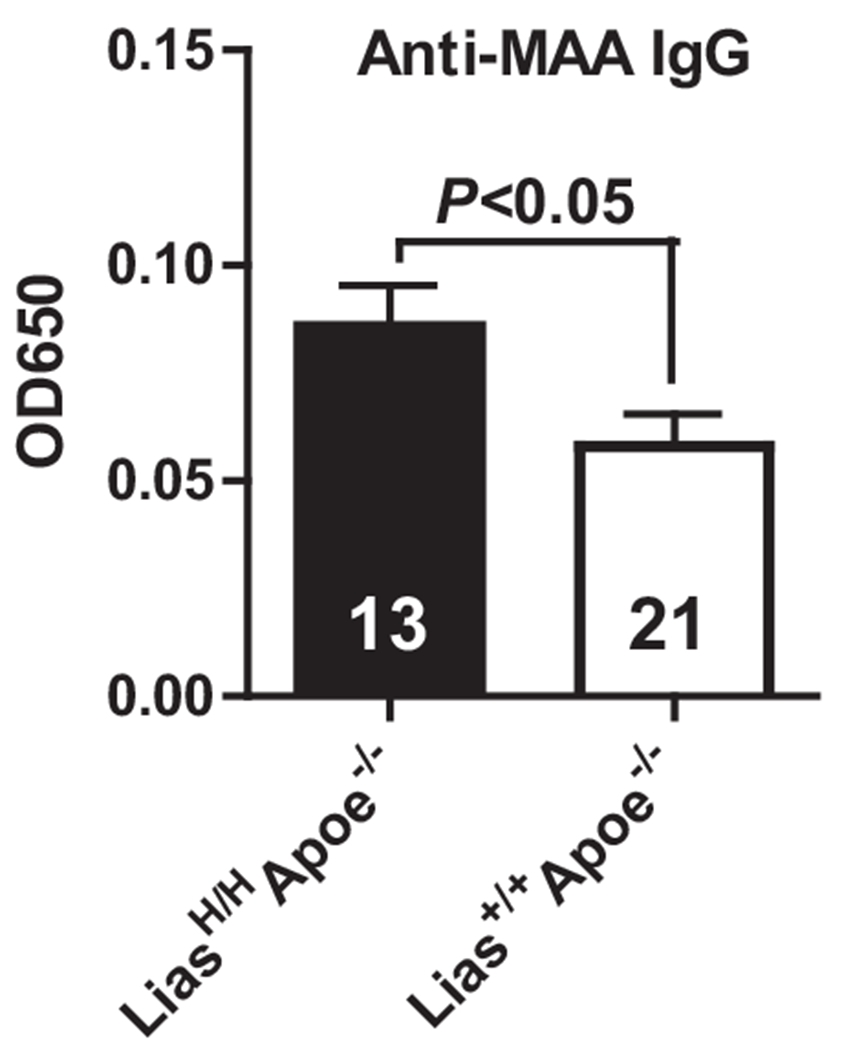
An ELISA method for characterizing the concentration of anti-MAA antibodies. Serum IgG antibody levels to MAA were determined by ELISA at 6 months of age in male LiasH/HApoe−/− (closed bar) and Lias+/+Apoe−/− mice (open bar). Results are expressed as mean ± SE.
4. Discussion
We previously reported that reduced Lias gene expression resulted in larger atherosclerotic lesions in Lias−/−Apoe−/− mice (8). To further define the role of endogenous antioxidant, this study was designed to test our hypothesis that strengthened endogenous antioxidant capacity in LiasH/HApoe−/− mice was able to retard the development of atherosclerosis via immune regulation. Our data showed that LiasH/HApoe−/− mice with overexpression of the Lias gene displayed a correspondingly lower lipid peroxidation level and higher antioxidant capacity in circulation than Lias+/+Apoe−/− mice as a control. Additionally, overexpression of the Lias gene significantly alleviated atherosclerosis in the aortic sinus, co-committed with reduced plasma triglycerides, increased number of Tregs, decreased infiltration of CD4+ T cells and elevated MAA antibody in LiasH/HApoe−/− mice, compared to their counterparts. Thus, our results provide more evidence for potential antioxidant therapy for human atherosclerosis.
Our results indicate that both altered innate and adaptive immune response manipulated by overexpressing Lias gene is involved in the attenuation of atherosclerosis. Leukocytes play a pivotal role in modulating atherosclerotic pathogenesis and inflammation. Under healthy conditions, a small number of leukocytes including macrophages, dendritic cells, neutrophils, and lymphocytes reside within the walls of the aorta [33]. Dynamic changes in the leukocyte population occur within the aorta during atherosclerosis [33]. The process of atherosclerotic plaque formation is characterized by the influx of monocytes/macrophages and T cells into lesion sites [34]. To understand the effect of antioxidant on T cell infiltration in arterial walls, critical for the development of atherosclerosis, we quantified the total lymphocyte numbers in atherosclerotic artery walls in the two different endogenous antioxidant environments based on Galkina’s method [35]. We found a significant decrease in the percentage of CD4+ T cells within the atherosclerotic wall in LiasH/HApoe−/− mice, compared with Lias+/+Apoe−/− mice. The result suggests that increased endogenous antioxidant can suppress pro-atherogenesis via decreasing infiltrating CD4+ T cells. This method has its limitation because it releases both resident and infiltrating T cells in the artery walls. Nevertheless, it has been an accepted method [35].
Evidence shows that impaired function or reduced number of Tregs is likely one of the mechanisms for the increased local inflammatory response in atherosclerosis [33]. Our data demonstrate that the frequency of circulating Treg cells in LiasH/HApoe−/− mice were significantly increased (vs. Lias+/+Apoe−/− mice) suggesting that the LIAS-altered endogenous antioxidant environment influences Treg differentiation and proliferation. Tregs suppress Th1 that produces IFN-γ and promotes atherosclerosis [17]. Treg cells also mediate suppressor function through the production of IL-10 [18,19]. Thus, increased IL-10 production in LiasH/HApoe−/− mice provided additional evidence that the activity of Tregs in circulation was elevated. In addition, we previously found that all major organs and cells in LiasH/HApoe−/− mice including Tregs had upregulated Lias gene expression (160 ± 10% of that in wild type mice). Therefore, it is also possible that because of the upregulation of Lias gene in Tregs, the metabolic changes of Tregs in LiasH/HApoe−/− mice can better meet the requirement of bioenergetic demand upon activation. Furthermore, our results agree with other studies using a different antioxidant (40). For example, one study revealed that the administration of resveratrol was associated with small but significant increases in the proportion of circulating Treg cells. This result was corroborated by in vitro experiments, which demonstrated the more efficient proliferation of Treg cells when T cell receptor-activated peripheral blood mononuclear cells were cultured in the presence of resveratrol [36]. However, it is still not clear why antioxidants can increase numbers of Tregs. It is reported that T cell commitment to Th1 or Th2 appears to crucially depend on the activation of redox-sensitive signaling cascades, where oxidative stress supports Th1 development while antioxidative milieu leads to a shift to allergic Th2 responses [37,38]. Our animal models provided such an antioxidative milieu for Treg inducement. Moreover, lymphocytes require a reducing milieu for optimal activation. For instance, immunization is associated with a striking increase in free thiols in lymphoid tissue (41; 42). Furthermore, LA is a sulfur-containing compound so it may provide extra protection. Additional studies will be necessary to prove the hypotheses.
Our data demonstrate that MAA autoantibody was significantly increased in the blood of LiasH/HApoe−/− mice. MAA is a form of oxLDL proposed to be one of the most potent atherogenic protein adducts caused by lipid peroxidation and appears to play a critical role in atherogenesis [24]. Thus, elevated anti-MAA antibody production may represent an attempt by the body to block this antigen and increase clearance of circulating oxidized LDL particles [39].
Our current work disclosed that potential effect of LA attenuated atherosclerosis via immune regulation using our new antioxidant/atherosclerosis mouse model. However, this study is still in its initial stage. We are not certain whether Lias gene expression is also increased in regulatory T cells per se in LiasH/H mice although changes of Lias gene expression occurred at a similar level in all major organs in the genetic mutant Lias mice. In addition, in addition to being an antioxidant, LA is also a cofactor in pyruvate dehydrogenase complex and alpha-ketoglutarate dehydrogenase complex, both of which are mitochondrial enzymes and participate in ATP production. Thus, it is possible that the impact of LA on atherosclerosis is through the regulation of energy homeostasis of Tregs. Furthermore, our results also revealed that plasma TG level was significantly lower but Chol level was not significantly different in the Lias overexpressing mice. Potential mechanisms for LA mediated Chol and TG remains unclear but TG is not thought as a causal risk factor for atherosclerosis [40].
In conclusion, using the novel antioxidant model, we found that overexpression of Lias gene and resulting elevation of intrinsic antioxidant levels ameliorates atherogenic properties of Apoe−/− mice. One of the plausible mechanisms is the suppression of inflammatory response via potential regulation of the immune system, including the increased population of Treg cells and circulating autoantibody levels and decreased infiltration of T cells. The outcome of this work would provide a potentially novel preventive and therapeutic strategy for clinical applications by lipoic acid treatment.
Acknowledgments
Funding sources
This study was supported by a Grant-in-Aid Award 13GRNT17120056 from the American Heart Association to X.Y. and HL042630 from the National Institute of Health to N.M. JSPS KAKENHI (to J.N. Grant Number 17H07027) and the Health and Labor Science Research Grants from Ministry of Health, Labor and Welfare of the Japanese Government (to J.N.).
Abbreviations:
- Chol
cholesterol
- FoxP3
forkhead box protein 3
- 4-HNE
4-hydroxynonenal
- IFN-γ
interferon-γ
- LA
α-lipoic acid
- Lias
lipoic acid synthase
- MAA
1,4-dihydropyridine-type MDA-acetaldehyde-Lys adduct
- oxLDL
oxidized low-density lipoprotein
- Tregs
regulatory T cells
Footnotes
Declaration of Competing Interest
The authors declare no conflicts of interests.
References
- [1].Reed LJ, From lipoic acid to multi-enzyme complexes, Protein Sci. 7 (1998) 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang WJ, Bird KE, McMillen TS, LeBoeuf RC, Hagen TM, Frei B, Dietary alpha-lipoic acid supplementation inhibits atherosclerotic lesion development in apolipoprotein E-deficient and apolipoprotein E/low-density lipoprotein receptor-deficient mice, Circulation 117 (2008) 421–428. [DOI] [PubMed] [Google Scholar]
- [4].Namazi N, Larijani B, Azadbakht L, Alpha-lipoic acid supplement in obesity treatment: a systematic review and meta-analysis of clinical trials, Clin. Nutr (2018) 419–428. [DOI] [PubMed] [Google Scholar]
- [9].Xu L, Hiller S, Simington S, Nickeleit V, Maeda N, James LR, Yi X, Influence of different levels of Lipoic acid synthase gene expression on diabetic nephropathy, PLoS One 11 (2016) e0163208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Paoletti R, Gotto AM Jr., Hajjar DP, Inflammation in atherosclerosis and implications for therapy, Circulation 109 (2004) III20–III26. [DOI] [PubMed] [Google Scholar]
- [11].Tabas I, Williams KJ, Boren J, Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications, Circulation 116 (2007) 1832–1844. [DOI] [PubMed] [Google Scholar]
- [12].Weber C, Zernecke A, Libby P, The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models, Nat. Rev. Immunol 8 (2008) 802–815. [DOI] [PubMed] [Google Scholar]
- [13].Shimokama T, Haraoka S, Watanabe T, Immunohistochemical and ultra-structural demonstration of the lymphocyte-macrophage interaction in human aortic intima, Mod. Pathol 4 (1991) 101–107. [PubMed] [Google Scholar]
- [14].Roselaar SE, Kakkanathu PX, Daugherty A, Lymphocyte populations in atherosclerotic lesions of apoE −/− and LDL receptor −/− mice. Decreasing density with disease progression, Arterioscler. Thromb. Vasc. Biol 16 (1996) 1013–1018. [DOI] [PubMed] [Google Scholar]
- [15].Zhou X, Nicoletti A, Elhage R, Hansson GK, Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice, Circulation 102 (2000) 2919–2922. [DOI] [PubMed] [Google Scholar]
- [16].de Boer OJ, Becker AE, van der Wal AC, T lymphocytes in atherogenesis-functional aspects and antigenic repertoire, Cardiovasc. Res 60 (2003) 78–86. [DOI] [PubMed] [Google Scholar]
- [17].Mallat Z, Taleb S, Ait-Oufella H, Tedgui A, The role of adaptive T cell immunity in atherosclerosis, J. Lipid Res 50 (Suppl) (2009) S364–S369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mays LE, Chen YH, Maintaining immunological tolerance with Foxp3, Cell Res. 17 (2007) 904–918. [DOI] [PubMed] [Google Scholar]
- [19].Wen K, Li G, Yang X, Bui T, Bai M, Liu F, Kocher J, Yuan L, CD4+ CD25-FoxP3+ regulatory cells are the predominant responding regulatory T cells after human rotavirus infection or vaccination in gnotobiotic pigs, Immunology 137 (2012) 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ammirati E, Moroni F, Pedrotti P, Scotti I, Magnoni M, Bozzolo EP, Rimoldi OE, Camici PG, Non-invasive imaging of vascular inflammation, Front. Immunol 5 (2014) 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Potekhina AV, Pylaeva E, Provatorov S, Ruleva N, Masenko V, Noeva E, Krasnikova T, Arefieva T, Treg/Th17 balance in stable CAD patients with different stages of coronary atherosclerosis, Atherosclerosis 238 (2015) 17–21. [DOI] [PubMed] [Google Scholar]
- [22].Gistera A, Hermansson A, Strodthoff D, Element ML, Hedin U, Fredrikson GN, Nilsson J, Hansson GK, Ketelhuth DF, Vaccination against T-cell epitopes of native ApoB100 reduces vascular inflammation and disease in a humanized mouse model of atherosclerosis, J. Intern. Med 281 (2017) 383–397. [DOI] [PubMed] [Google Scholar]
- [23].Lim H, Kim YU, Sun H, Lee JH, Reynolds JM, Hanabuchi S, Wu H, Teng BB, Chung Y, Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo, Immunity 40 (2014) 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gonen A, Hansen LF, Turner WW, Montano EN, Que X, Rafia A, Chou MY, Wiesner P, Tsiantoulas D, Corr M, VanNieuwenhze MS, Tsimikas S, Binder CJ, Witztum JL, Hartvigsen K, Atheroprotective immunization with malondialdehyde-modified LDL is hapten specific and dependent on advanced MDA adducts: implications for development of an atheroprotective vaccine, J. Lipid Res 55 (2014) 2137–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang C, Collins M, Kuchroo VK, Effector T cell differentiation: are master regulators of effector T cells still the masters? Curr. Opin. Immunol 37 (2015) 6–10. [DOI] [PubMed] [Google Scholar]
- [26].Yan Z, Garg SK, Kipnis J, Ranerjee R, Extracellular redox modulation by regulatory T cells, Nat. Chem. Biol 5 (2009) 721–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shimomoto T, Collins LR, Yi X, Holley DW, Zhang Z, Tian X, Uchida K, Wang C, Horkko S, Willis MS, Gold A, Bultman SJ, Nakamura J, A purified MAA-based ELISA is a useful tool for determining anti-MAA antibody titer with high sensitivity, PLoS One 12 (2017) e0172172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Butcher MJ, Herre M, Ley K, Galkina E, Flow cytometry analysis of immune cells within murine aortas, J. Vis. Exp 53 (2011) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yi X, Maeda N, Alpha-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet, Diabetes 55 (2006) 2238–2244. [DOI] [PubMed] [Google Scholar]
- [30].Klingenberg R, Gerdes N, Radeau RM, Gistera A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Luscher TF, Jauhiainen M, Sparwasser T, Hansson GK, Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis, J. Clin. Invest 123 (2013) 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hansson GK, Holm J, Jonasson L, Detection of activated T lymphocytes in the human atherosclerotic plaque, Am. J. Pathol 135 (1989) 169–1675. [PMC free article] [PubMed] [Google Scholar]
- [32].George J, Schwartzenberg S, Medvedovsky D, Jonas M, Charach G, Afek A, Shamiss A, Regulatory T cells and IL-10 levels are reduced in patients with vulnerable coronary plaques, Atherosclerosis 222 (2012) 519–523. [DOI] [PubMed] [Google Scholar]
- [33].Galkina E, Ley K, Immune and inflammatory mechanisms of atherosclerosis, Annu. Rev. Immunol 27 (2009) 165–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ross R, Atherosclerosis – an inflammatory disease, N. Engl. J. Med 340 (1999) 115–126. [DOI] [PubMed] [Google Scholar]
- [35].Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K, Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent, J. Exp. Med 203 (2006) 1273–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Espinoza JL, Trung LQ, Inaoka PT, Yamada K, An DT, Mizuno S, Nakao S, Takami A, The repeated administration of resveratrol has measurable effects on circulating T-cell subsets in humans, Oxidative Med. Cell. Longev 2017 (2017) 6781872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C, Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns, Proc. Natl. Acad. Sci. U. S. A 95 (1998) 3071–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hasan AA, Ghaemmaghami AM, Fairclough L, Robins A, Sewell HF, Shakib F, Allergen-driven suppression of thiol production by human dendritic cells and the effect of thiols on T cell function, Immunobiology 214 (2009) 2–16. [DOI] [PubMed] [Google Scholar]
- [39].Schiopu A, Frendeus B, Jansson R, Soderberg I, Ljungcrantz I, Araya Z, Shah PK, Carlsson R, Nilsson J, Fredrikson GN, Recombinant antibodies to an oxidized low-density lipoprotein epitope induce rapid regression of atherosclerosis in apobec-1(−/−;)/low-density lipoprotein receptor(−/−) mice, J. Am. Coll. Cardiol 50 (2007) 2313–2318. [DOI] [PubMed] [Google Scholar]
- [40].Libby P, Ridker PM, Hansson GK, Progress and challenges in translating the biology of atherosclerosis, Nature 473 (2011) 317–325. [DOI] [PubMed] [Google Scholar]


