Abstract
Background
Schistosomiasis (S. mansoni and S. haematobium) is an important neglected tropical disease in sub-Saharan Africa including Ethiopia. In Ethiopia, Schistosomiasis has been prioritized as neglected tropical disease and remained among major public health burden on school age children of the country. Few studies conducted on the association between prevalence of Schistosomiasis and gender of school age children have inconclusive finding about the association between these two variables. Therefore, this systematic review and meta-analysis was done to determine the pooled prevalence of Schistosomiasis and its association with gender of school age children in Ethiopia.
Methods
In this systematic review and meta-analysis, databases such as: Medline/PubMed, EMBASE, CINAHL, Cochrane Central library, Google Scholar, and HINARI were systematically searched. STATA version 14 was used to estimate pooled prevalence of Schistosomiasis using random effects model with 95% confidence interval. The results were presented by using forest plot and statistical heterogeneity was checked by using the Cochran Q test (chi-squared statistic), I2 test statistic and by visual examination of the forest plot.
Results
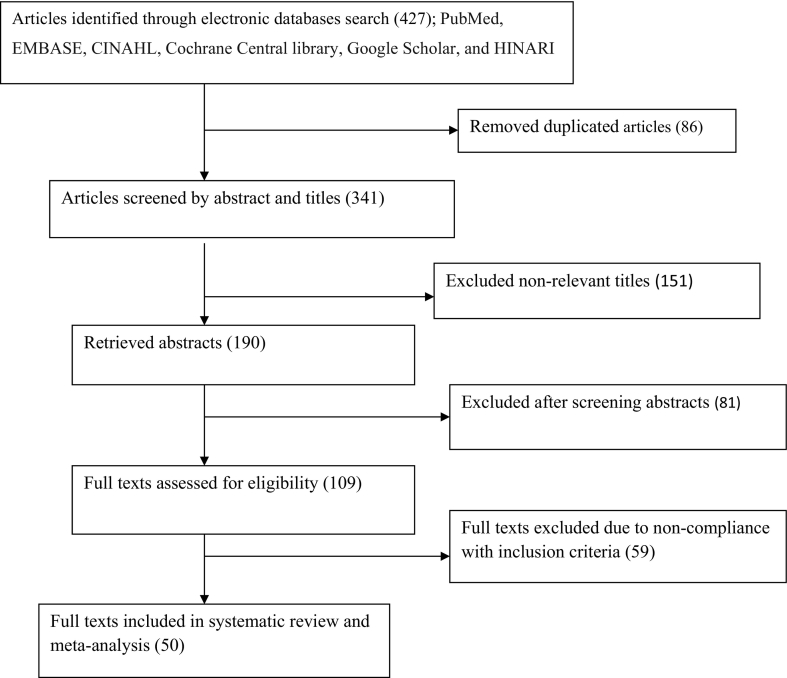
From the total of 427 studies identified for this review, 50 studies were included in the final analysis. The analysis noted that pooled prevalence of Schistosomiasis in Ethiopia was 28.78% (95% CI: 23.81, 33.74). The subgroup analysis indicated that extreme variability was observed in the prevalence of Schistosomiasis across the regions of the country. The highest (39.77%) prevalence of Schistosomiasis was reported from the southern region, whereas the lowest (14.95%) prevalence of Schistosomiasis was reported from Afar region. Male school age children were 58% more likely infected with Schistosomiasis than female school age children in Ethiopia (OR: 1.58, 95% CI: 1.33, 1.83).
Conclusions
The Prevalence of Schistosomiasis was higher than the 2018 report of the Ethiopian federal ministry of health. The prevalence of Schistosomiasis was predominant among male gender of the school age children. Therefore, sustainable control of Schistosomiasis requires the approaches that must go beyond current deworming program. Complementary prevention strategies including health education, safe water and adequate sanitary facilities provision should be simultaneously implemented. The underlining causes of variation in risk factors of Schistosomiasis among males and females should be further studied.
Keywords: Prevalence, Schistosomiasis, Meta-analysis, Systematic review, Ethiopia
1. Introduction
Neglected tropical diseases including Schistosomiasis are a group of chronic parasitic diseases and related conditions that are among the common illnesses of the world's poorest people (Amenu et al., 2017).
The burden of disease associated with Schistosomiasis and soil-transmitted helminthes (STH) infections is enormous, with at least 2 billion people affected globally. This is being progressively more recognized as a significant public health problem, particularly in developing countries where poverty, poor nutrition, inadequate sanitation, lack of clean drinking-water and minimal health care prevail. The highest rates of infection are often in children between the ages of 5 and 15 years (Organization WH and UNICEF, 2004).
Schistosomiasis is widely spread in South America, Middle East, Brazil and in many African countries including the Sudan, Kenya, Ethiopia and Madagascar. Facts show that the significant improvement in preventive chemotherapy (PC) coverage related to Schistosomiasis is continued being a challenge. Nowadays, it is believed that fewer than 10% of eligible populations, which might not contribute a significant figure for the reduction of Schistosomiasis, living in endemic regions of Africa are receiving PC for Schistosomiasis, intestinal helminthes infections, and/or trachoma (Hotez and Fenwick, 2009).
In Ethiopia, Schistosomiasis is most prevalent in agricultural communities along streams in the altitudes between 1300 and 2000 m above sea level and it is reported from all administrative regions (Kassa et al., 2005). Intestinal form of Schistosomiasis is broadly distributed while the uro-genital form is more restricted in distribution primarily to foci in the rift valley regions. There are an estimated 38.3 million people living in Schistosomiasis endemic areas, comprising 4.4 million preschool children, 12.3 million school-aged children, and 21.6 million adults (Ministry of Health, 2016).
Studies conducted in different time in Ethiopia showed different results: Schistosomiasis surveys carried out by the Institute of Pathobiology, Addis Ababa, in all 14 administrative regions between 1978 and 1982 found 15% of people infected with Schistosomiasis haematobium and the national S. mansoni survey of 1988–89 reported an overall prevalence of 25% (Deribe et al., 2012).
Preventive chemotherapy (PC) can reduce morbidity caused by schistosomiasis. According to the assessment of population requiring PC report in 2010, in addition to PC further efforts are required to control distribution of the disease among different social strata (Chala and Torben, 2018).
Prevalence of Schistosomiasis infection is higher in children and adolescents, because children have higher risk of contamination for different sanitation and hygiene problems like polluted water contact. Prevalence in males and in females is quite different, and not consistent in different studies. It was reported 42.4% and 26.5% for males and females respectively, and the prevalence of Schistosomiasis infection in another study using Kato-Katz method in Sanja primary school was high among male (79.5%) children but it was high among female (75%) children in Ewket Amba primary school (Deribe et al., 2012; Abera et al., 2013; Alebie et al., 2014).
Ethiopia has developed Schistosomiasis elimination strategic plan which may last 2016 to 2020. Yet, different facts of prevalence have been reported from different corners of the country (Ministry of Health, 2016).
The common assumption that men and boys are more heavily infected and affected than women and girls in endemic areas is not always correct. Despite social aspects of female and male genital schistosomiasis in school age children is understudied, particularly with regard to consequences, illness perceptions and social factors influencing access to treatment, controversial and inconsistent findings are reported about the gender as a risk factor for schistosomiasis. Otherwise, constructing pooled evidence on gender relation with the occurrence of the disease impacts the exposure and access to treatment and other control strategies (World Health Organization, 2008).
Therefore, the literature was systematically reviewed on the prevalence of Schistosomiasis among school age children and its association with gender in Ethiopia with the objective of bringing different individual studies together and compute pooled estimate (Ministry of Health, 2016). Particular attention was given to the influence of gender as different studies showed inconsistent association on prevalence of Schistosomiasis between male and female. Further, the significance of this research was to show pooled estimate for controversial evidences from different studies so that gender specific interventions may be required to augment the national prevention and control strategies on schistosomiasis.
2. Method
2.1. Searching strategies
This systematic review and Meta-analysis was conducted to estimate the pooled prevalence of Schistosomiasis and its association with gender of school age children in Ethiopia. The protocol for this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO), University of York Centre for Reviews and Dissemination (Registration Number CRD42019129843) on the 7th of June 2019.The following databases were systematically searched to identify the literatures: Medline/PubMed, EMBASE, CINAHL, Cochrane Central Library, Google Scholar, and HINARI (Health Inter Network Access to Research Initiative) from November 1 to December 30, 2018. The reports were accessed using the following key terms: “Prevalence”, “Magnitude”, “determinants”, “associated factors”, “factors” “Schistosomiasis”, “S. mansoni” and “S. haematobium”, “sex”, “Gender” and “Ethiopia”. The key terms were used individually and in combination by using Boolean operators like “AND” and “OR” 1. (“Prevalence of schistosomiasis” OR “prevalence of schistosomiasis” OR “magnitude of schistosomiasis” AND “school age children” OR “5-14 years old children”) 2. (“Prevalence of schistosomiasis” OR “prevalence of schistosomiasis” OR “magnitude of schistosomiasis” AND “Ethiopia” 3. “Effect of gender” OR “Effect of gender” AND “Prevalence of schistosomiasis” OR “schistosomiasis” OR “prevalence of schistosomiasis” OR “magnitude of schistosomiasis” AND “school age children” OR “5–14 years old children” 4. “Effect of gender” OR “Effect of gender” OR “schistosomiasis” OR “mass drug administration” AND “school age children” OR “5–14 years old children” AND “Ethiopia”. The guideline followed was The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). In addition, after identification of studies and review articles, their lists of references were searched to identify more eligible studies. This systematic review and meta-analysis used the CoCoPop (Condition, Context, and Population) framework to determine the eligibility of the articles included. The condition was schistosomiasis (Co), the context was Ethiopia (Co), and the Population was school age children (Pop).
2.2. Study selection and eligibility criteria
The eligible studies for this systematic review and meta-analysis were selected after screening at four stages: screening for duplications, titles alone, abstracts, full-text articles, and based on inclusion criteria. All quantitative studies reported in English language, published and unpublished articles, and revealed the prevalence of Schistosomiasis as well the association between prevalence of Schistosomiasis and gender of school age children (Ministry of Health, 2016; Deribe et al., 2012; Abera et al., 2013; Alebie et al., 2014; Newcastle-Ottawa, n.d.; Modesti et al., 2016; Hardy and Thompson, 1998; Higgins et al., 2003; Knapp and Hartung, 2003; Tadesse et al., 2009) were included in this review. However, the studies which did not either report the prevalence of Schistosomiasis or did not report the association between Schistosomiasis and gender of school age children were excluded from this systematic review and meta-analysis. Moreover, articles which were not fully accessible, after at least two-email contact with the primary authors were excluded from this review. Exclusion of these articles was because of the inability to assess the quality of articles in absence of full text.
Potentially eligible articles were identified by two reviewers (DW and ZH), through independent reading of the titles and abstracts, which were searched and accessed broadly. Those two reviewers (DW, ZH) were independently reviewed the finally included articles against the inclusion criteria. In case of any discrepancies, a third reviewer (BS) was consulted.
2.3. Data extraction and quality assessment of the studies
Two authors (DW and BS) independently extracted all necessary data using the Microsoft excel sheet. Any disagreements at the time of data extraction were resolved by consulting the third author (YT). From each included study, features such as: (i) publication details (primary author's last name and year of publication), (ii) study setting, (iii) sample size and response rate, and (iv) prevalence of Schistosomiasis were extracted. The Newcastle-Ottawa Scale for cross-sectional studies quality assessment tool was adapted and used to assess the quality of each study (Newcastle-Ottawa, n.d.). The tool has three major sections: The first section graded from five stars focuses on the methodological quality of each study. The second section of the tool deals with the comparability of the study. The last section deals with the outcomes and statistical analysis of each original study. Two authors (DW and YT) independently assessed the quality of each original study using the tool. Disagreements between the two authors were resolved by taking the mean score of the two authors. Finally, article with a scale of greater than or equal to 6 out of 10 were included in this systematic review and Meta-analysis.
2.4. Statistical analysis
Data extraction were done using Microsoft Excel spread sheet, then analysis was done using STATA version 14 statistical software. For the analysis, standard error (SE) values were extracted from the studies, since they are more commonly reported with 95% confidence interval. When both SE and 95% CI were not provided, SE was calculated using the formula (SE = √ (P × (1 − P)/N), where P is the proportion of the cases reported and N is the denominator of the prevalence estimate (Modesti et al., 2016).
Statistical heterogeneity was assessed by using the Cochran Q test (chi-squared statistic), I2 test statistic and by visual examination of the forest plot (overlap of confidence intervals). Cochran's Q test was used to test the null hypothesis of no significant heterogeneity across the studies (Hardy and Thompson, 1998). Cochran's Q is calculated as the weighted sum of squared differences between individual study effects and the pooled effect across studies, with the weights being those used in the pooling method. Cochran's Q statistic follows a chi-squared distribution with k − 1 degree of freedom where k is the number of studies. Cochran's Q statistical heterogeneity test is considered as statistically significant.
The I2 statistic was also estimated because of the fact that the percentage of variation (inconsistency) in the measures of association across studies is due to heterogeneity rather than chance (Higgins et al., 2003). The I2 statistic is equal to the quantity of Cochran's Q minus its degree of freedom (df) divided by Cochran's Q times 100%, I2 = 100% × (Q − df)/Q. The value of I2 ranges between 0 and 100%, where 0% indicates no observed heterogeneity and large values indicate increasing heterogeneity (Higgins et al., 2003). An I2 value of 25%, 50%, and 75% is considered as low, moderate, and high heterogeneity respectively (Higgins et al., 2003). Egger's weighted regression and Begg's rank correlation tests were used to check for the publication bias (P < 0.05 is considered statistically significant).
In this review, test statistic showed there was a significant heterogeneity among the included studies (I2 = 99.5%, p < 0.001) as a result a random effects model was used to estimate the Der Simonian and Laird's pooled effect. To identify the possible source of heterogeneity, meta-regression was undertaken by taking the sample size and year of publication but none of them were found to be statistically significant(p = 0.246 and p = 0.587) (Table 2). The pooled effect was articulated in the form of odds ratio. (See Fig. 1.)
Table 3.
The subgroup prevalence of Schistosomiasis in Ethiopia, 1997–2019.
| Variables | Characteristics | No. studies included | Sample size | Prevalence (95% CI) |
|---|---|---|---|---|
| By region | Amhara | 20 | 25,565 | 29.07 (20.52–37.61) |
| SNNPR | 8 | 3440 | 39.77 (20.47–59.00) | |
| Oromia | 8 | 3548 | 32.57 (18.07–47.08) | |
| Tigray | 8 | 3186 | 20.85 (12.49–29.22) | |
| Afar | 4 | 2722 | 14.95 (6.80–23.11) | |
| Others* | 2 | 817 | 25.80 (6.33–45.27) | |
| By sample size | ≥600 | 12 | 24,417 | 15.37 (11.031–19.71) |
| <600 | 38 | 14,861 | 33.05 (23.81–33.74) | |
| Year | Before 2015 | 38 | 18,958 | 30.96 (24.01–37.91) |
| After 2015 | 12 | 20,320 | 23.82 (17.07–30.57) |
Others*: Gambela, Somali, SNNPR = South nations, nationalities and people of Ethiopia.
Fig. 1.
Flow chart of study selection for systematic review and meta-analysis of the prevalence of Schistosomiasis among school age children in Ethiopia, 1997–2019.
Egger's test of the intercept in random effects model was used to check publication bias (Knapp and Hartung, 2003). As the results of the test suggested a possible existence of a significant publication bias (p = 0.000 in Egger's test), the final effect size was determined by applying Duval and Tweedie's Trim and Fill analysis in the random-effects model.
2.5. Classification of regions
The classification of the regions in Ethiopia as agrarian and emerging is based on their need for special support for health and other development services as a result of huge inequity of health and other development status in this regions due to the community in these regions are pastoralists/semi pastoralists. As we indicated in the table four of the manuscript, emerging regions = Afar, Gambela, Somalia, these regions are known as emerging regions in Ethiopia where more affirmative actions are warranted to address vertical equity.
3. Result
3.1. Characteristics of the original studies
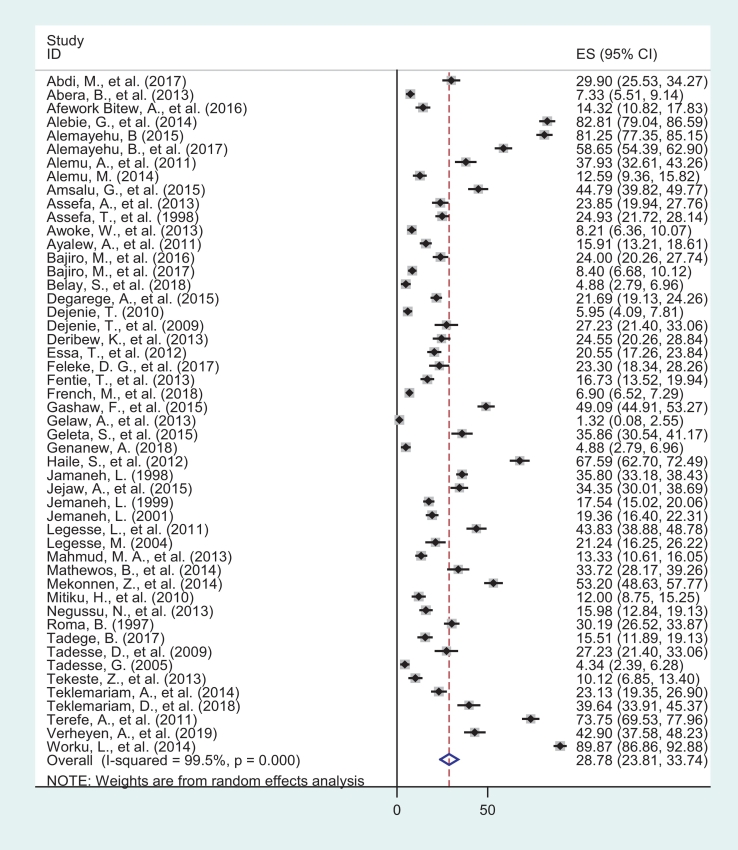
This systematic review and Meta-analysis has been reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA). The publication year of the included studies range from 1997 to 2019. The minimum sample size (school age children) of individual studies included in this systematic review was 224 whereas the maximum sample size for the included study was 15,455 (Tadesse et al., 2009; French et al., 2018). From the included studies, majority of them were from Amhara regional state (Abera et al., 2013; Alebie et al., 2014; French et al., 2018; Abdi et al., 2017; Afework Bitew et al., 2016; Alemu et al., 2011; Amsalu et al., 2015; Assefa et al., 1998; Ayalew et al., 2011; Essa et al., 2012; Feleke et al., 2017; Fentie et al., 2013; Gashaw et al., 2015; Gelaw et al., 2013; Jamaneh, 1998; Jemaneh, 1999; Jemaneh, 2001; Mathewos et al., 2014; Tekeste et al., 2013; Worku et al., 2014) and nearly half of the studies were from the three regions: South nation's nationalities and people region (SNNPR) (Alemayehu and Tomass, 2015; Alemayehu et al., 2017; Alemu, 2014; Jejaw et al., 2015; Mitiku et al., 2010; Roma and Worku, 1997; Tadege and Shimelis, 2017; Terefe et al., 2011), Oromia (Bajiro et al., 2016; Bajiro et al., 2017; Haile et al., 2012; Legesse and Erko, 2004; Mekonnen et al., 2014; Tadesse, 2005; Teklemariam et al., 2018; Verheyen et al., 2019), and Tigray region (Tadesse et al., 2009; Assefa et al., 2013; Belay et al., 2018; Dejenie et al., 2009; Genanew and Teshale, 2018; Legesse et al., 2011; Mahmud et al., 2013; Teklemariam et al., 2014). In this systematic review and meta-analysis, 39,278 school age children were included to estimate the pooled prevalence of Schistosomiasis in Ethiopia. Both the lowest and highest prevalence of Schistosomiasis was observed in study conducted in Amhara region, 1.32% in Gondar (Gelaw et al., 2013) and 89.87% in Sanja area (Worku et al., 2014) (Table 1).
Table 1.
Descriptive summary of the included studies in the systematic review and meta-analysis of the prevalence of Schistosomiasis (S. mansoni and S. haematobium) in Ethiopia 1997–2019.
| S. No | Primary Author/s | Publication year | Study area | Study design | Response rate | Sample size | Prevalence with 95%CI |
|---|---|---|---|---|---|---|---|
| 1 | Abdi et al. (2017) | 2017 | Zegie | Cross sectional | 96.68 | 408 | 29.9 (25.53–34.27) |
| 2 | Abera et al. (2013) | 2013 | Bahir Dar | Cross sectional | 98.36 | 778 | 7.326 (5.511–9.142) |
| 3 | Afework Bitew et al. (2016) | 2016 | Around Lake Tana | Cross sectional | 100 | 384 | 14.32 (10.82–17.83) |
| 4 | Alebie et al. (2014) | 2014 | Sanja | Cross sectional | 100 | 384 | 76.3 (72.05–80.56) |
| 5 | Alemayehu and Tomass (2015) | 2015 | Woliata | Cross sectional | 100 | 384 | 81.25 (77.35–85.15) |
| 6 | Alemayehu et al. (2017) | 2017 | Woliata | Cross sectional | 97.67 | 503 | 58.65 (54.39–62.9) |
| 7 | Alemu et al. (2011) | 2011 | Zerma | Cross sectional | 100 | 319 | 37.93 (32.61–43.26) |
| 8 | Alemu (2014) | 2014 | Umolante | Cross sectional | 100 | 405 | 12.59 (9.361–15.82) |
| 9 | Amsalu et al. (2015) | 2015 | Hayk | Cross sectional | 100 | 384 | 44.79 (39.82–49.77) |
| 10 | Assefa et al. (2013) | 2013 | Mekelle | Cross sectional | 100 | 457 | 23.85 (19.94–27.76) |
| 11 | Assefa et al. (1998) | 1998 | Wollo | Cross sectional | 100 | 698 | 24.93 (21.72–28.14) |
| 12 | Awoke et al. (2013) | 2013 | Amibera | Cross sectional | 98.57 | 828 | 8.213 (6.356–10.07) |
| 13 | Ayalew et al. (2011) | 2011 | Delgi | Cross sectional | 100 | 704 | 15.91 (13.21–18.61) |
| 14 | Bajiro et al. (2016) | 2016 | Manna District | Cross sectional | 100 | 500 | 24 (20.26–27.74) |
| 15 | Bajiro et al. (2017) | 2017 | Jimma | Cross sectional | 100 | 1000 | 8.4 (6.681–10.12) |
| 16 | Belay et al. (2018)) | 2018 | Medebay Zana | Cross sectional | 100 | 410 | 4.88 (2.793–6.963) |
| 17 | Degarege et al. (2015) | 2015 | Afar | Cross sectional | 89.03 | 885 | 20.79 (18.27–23.31) |
| 18 | Dejenie and Asmelash (2010) | 2010 | Afar | Cross sectional | 100 | 622 | 5.949 (4.09–7.807) |
| 19 | Dejenie et al. (2009) | 2009 | Waja | Cross sectional | 100 | 224 | 29.91 (23.91–35.91) |
| 20 | Deribew et al. (2013) | 2013 | Amibara | Cross sectional | 100 | 387 | 24.55 (20.26–28.84) |
| 21 | Essa et al. (2012) | 2012 | Gorgora | Cross sectional | 100 | 579 | 20.55 (17.26–23.84) |
| 22 | Feleke et al. (2017) | 2017 | Haike | Cross sectional | 100 | 279 | 23.3 (18.34–28.26) |
| 23 | Fentie et al. (2013) | 2013 | Lake Tana Basen | Cross sectional | 100 | 520 | 16.73 (13.52–19.94) |
| 24 | French et al. (2018) | 2018 | Amhara | Cross sectional | 91.15 | 15,455 | 6.904 (6.522–7.286) |
| 25 | Gashaw et al. (2015)) | 2015 | Maksegnit | Cross sectional | 100 | 550 | 49.09 (44.91–53.27) |
| 26 | Gelaw et al. (2013) | 2013 | Gonder | Cross sectional | 93.25 | 304 | 1.316 (0.079–2.553) |
| 27 | Geleta et al., 2015 | 2015 | Abobo | Cross sectional | 97.12 | 304 | 35.86 (30.54–41.17) |
| 28 | Genanew and Teshale (2018) | 2018 | Medebay Zana | Cross sectional | 100 | 410 | 4.878 (2.793–6.963) |
| 29 | Haile et al. (2012) | 2012 | Finchaa | Cross sectional | 92.31 | 324 | 67.59 (62.7–72.49) |
| 30 | Jamaneh (1998) | 1998 | Dembia | Cross sectional | 100 | 1282 | 35.8 (33.18–38.43) |
| 31 | Jejaw et al. (2015) | 2015 | Mizan-Aman | Cross sectional | 100 | 460 | 34.35 (30.01–38.69) |
| 32 | Jemaneh (1999)) | 1999 | Gonder | Cross sectional | 100 | 878 | 17.54 (15.02–20.06) |
| 33 | Jemaneh (2001) | 2001 | Chilga | Cross sectional | 100 | 687 | 19.36 (16.4–22.31) |
| 34 | Legesse et al. (2011) | 2011 | Adwa | Cross sectional | 98.7 | 381 | 57.74 (52.81–62.67) |
| 35 | Legesse and Erko (2004)) | 2004 | Lake Langano | Cross sectional | 100 | 259 | 21.24 (16.25–26.22) |
| 36 | Mahmud et al. (2013) | 2013 | Northern | Cross sectional | 100 | 600 | 13.33 (10.61–16.05) |
| 37 | Mathewos et al. (2014) | 2014 | Gonder | Cross sectional | 93.55 | 261 | 33.72 (28.17–39.26) |
| 38 | Mekonnen et al. (2014) | 2014 | Fincha | Cross sectional | 98.91 | 453 | 53.2 (48.63–57.77) |
| 39 | Mitiku et al. (2010) | 2010 | Tikur Woha | Cross sectional | 97.66 | 375 | 12 (8.75–15.25) |
| 40 | Negussu et al. (2013) | 2013 | Afder & Goda | Cross sectional | 98.09 | 513 | 15.98 (12.84–19.13) |
| 41 | Roma and Worku (1997)) | 1997 | Wondo-Genet | Cross sectional | 86.67 | 520 | 30.19 (26.52–33.87) |
| 42 | Tadege and Shimelis (2017) | 2017 | Hawassa | Cross sectional | 97.4 | 374 | 31.02 (26.39–35.64) |
| 43 | Tadesse et al. (2009)) | 2009 | Waja | Cross sectional | 100 | 224 | 27.23 (21.4–33.06) |
| 44 | Tadesse (2005)) | 2005 | Babile | Cross sectional | 98.34 | 415 | 4.337 (2.394–6.281) |
| 45 | Tekeste et al., 2013 | 2013 | Gorgora | Cross sectional | 100 | 326 | 10.12 (6.848–13.4) |
| 46 | Teklemariam et al. (2014) | 2014 | Enderta | Cross sectional | 100 | 480 | 23.13 (19.35–26.9) |
| 47 | Teklemariam et al. (2018)) | 2018 | Ziway | Cross sectional | 100 | 280 | 39.64 (33.91–45.37) |
| 48 | Terefe et al. (2011) | 2011 | Bushulo | Cross sectional | 100 | 419 | 73.75 (69.53–77.96) |
| 49 | Verheyen et al. (2019) | 2019 | Yachi | Cross sectional | 95.48 | 317 | 42.9 (37.58–48.23) |
| 50 | Worku et al. (2014) | 2014 | Sanja | Cross sectional | 100 | 385 | 89.87 (86.86–92.88) |
3.2. Meta-analysis
This meta-analysis found that the pooled prevalence of Schistosomiasis among school age children in Ethiopia was found to be 28.77% (95% CI: 23.81, 33.74). The degree of heterogeneity between studies was high (I2 = 99.5% with Cochrane Q-statistics p-value <0.000). This was not unexpected as each study differs in terms of study area, sample size and year of the study. Because of this, final overall prevalence was computed based on a random effects meta-analysis model. Moreover, advanced statistical meta-analysis model was conducted to identify the possible sources of random variations across primary studies such as a univariate meta-regression model by considering publication year and sample size as covariates. These variables (publication year and sample size) were not statistically significant source of heterogeneity, P = 0.587 and P = 0.246 respectively (Table 2). Additionally, Begg's correlation and Egger's regression tests was used to identify the possible publication bias. The test results, showed that there was statistically significant publication bias across the included studies (p-values = 0.000, for both tests). Finally, the pooled prevalence of Schistosomiasis among school age children was estimated by using trim and fill analysis in random effect model (Fig. 2).
Fig. 2.
Forest plot of pooled epidemiology of Schistosomiasis among school age children in Ethiopia, 1997–2019.
3.3. Sub group analysis
We performed sub group analysis using the region of the study, the sample size and year of mass drug administration started. Accordingly, the highest prevalence was observed in SNNPR (39.77% (20.47–59.00) whereas as the lowest prevalence was observed in Afar region (14.95% (6.80–23.11)). Regarding to sample size of the study, the prevalence of Schistosomiasis among school age children was higher in studies having sample size <600 study participants, 33.05% (95% CI: 23.81, 33.74) compared to those studies having sample size ≥600 study participants, 15.37% (95% CI: 11.031, 19.71). Moreover, this systematic review and meta-analysis identified that the Schistosomiasis prevalence was higher before starting mass drug administration in Ethiopia (before 2015), 30.96 (24.01–37.91) compared to after mass drug administration started in Ethiopia (after 2015) 23.82 (17.07–30.57) (Table 3).
Table 2.
Related factors with heterogeneity of prevalence of Schistosomiasis in Ethiopia the current meta-analysis (based on univariate meta-regression), 1997–2019.
| Variable | Coefficient | p-value |
|---|---|---|
| Publication year | 0.3107278 | 0.587 |
| Sample size | −0.0017061 | 0.246 |
3.4. Association between gender of school age children and Schistosomiasis prevalence in Ethiopia
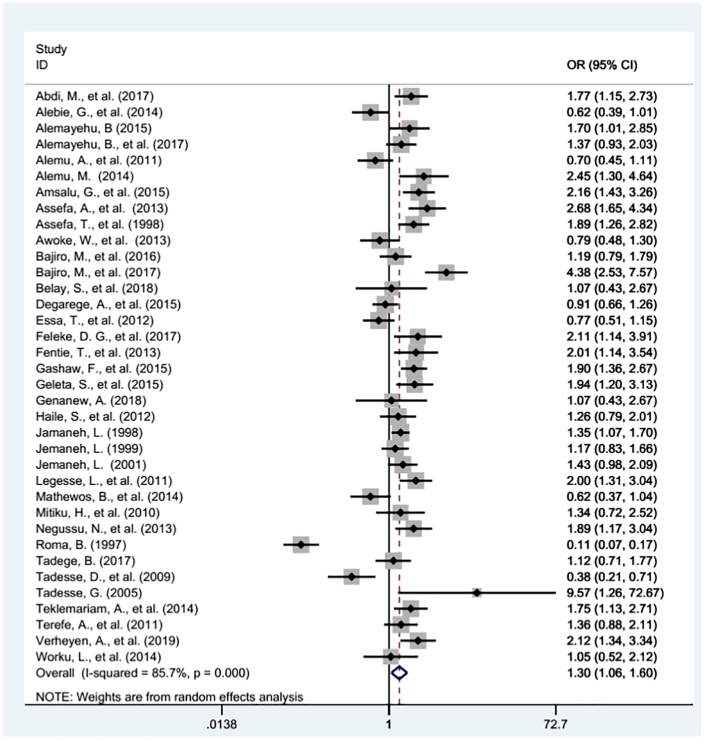
Thirty six studies were examined for the association between gender of school age children and Schistosomiasis prevalence in Ethiopia (Alebie et al., 2014; Tadesse et al., 2009; Abdi et al., 2017; Alemu et al., 2011; Amsalu et al., 2015; Assefa et al., 1998; Essa et al., 2012; Feleke et al., 2017; Fentie et al., 2013; Gashaw et al., 2015; Jamaneh, 1998; Jemaneh, 1999; Jemaneh, 2001; Mathewos et al., 2014; Worku et al., 2014; Alemayehu and Tomass, 2015; Alemayehu et al., 2017; Alemu, 2014; Mitiku et al., 2010; Roma and Worku, 1997; Tadege and Shimelis, 2017; Terefe et al., 2011; Bajiro et al., 2016; Bajiro et al., 2017; Haile et al., 2012; Tadesse, 2005; Verheyen et al., 2019; Assefa et al., 2013; Belay et al., 2018; Genanew and Teshale, 2018; Legesse et al., 2011; Teklemariam et al., 2014; Awoke et al., 2013; Degarege et al., 2015; Geleta et al., 2015; Negussu et al., 2013). In this meta-analysis highest heterogeneity was observed (I2 = 85.7% and p- value <0.000) across the studies. Hence, a random effect meta-analysis model was used to estimate the pooled effect of Schistosomiasis infection across the gender of school age children. Publication bias was checked by using Begg's test and Egger's regression analysis. The analysis revealed that there was significant publication bias with p - value of 0.000. Finally, trim and fill analysis were used to adjust the publication bias. As a result, the pooled odds ratio has been changed from (OR: 1.30, 95% CI: 1.06, 1.60) to (OR: 1.58, 95% CI: 1.33, 1.83) (Fig. 3). Therefore, male school age children were 58% more likely infected with Schistosomiasis than female school age children in Ethiopia (OR: 1.58, 95% CI: 1.33, 1.83). Analysis was done to see whether there is difference between the regions and the years before and after mass drug administration in Ethiopia, 2015. The two categories of regions in Ethiopia (agrarian and emerging regions) and, time before and since mass drug administration were considered to see the association between gender and schistosomiasis. Accordingly, studies conducted in agrarian regions, and after 2015 showed a significant association between gender and Schistosomiasis infection, male school age children were 31% more likely infected with Schistosomiasis (OR: 1.31, 95% CI: 1.04, 1.64) and 63% more likely infected with Schistosomiasis (OR: 1.63, 95% CI: 1.32, 2.03) than female school age children in agrarian regions and since 2015 respectively in Ethiopia. (Table 4).
Fig. 3.
The pool odd ratio of the association between prevalence of Schistosomiasis and gender of school age children in Ethiopia, 1997–2019.
Table 4.
The association between gender and Schistosomiasis infection before and after 2015 and between agrarian and emerging regions in Ethiopia, 1997–2019.
| Characteristics | No. studies included (36) | Sample size | OR (95% CI) |
|---|---|---|---|
| Before 2015 | 22 | 11,331 | 1.13 (0.84, 1.52) |
| After 2015 | 14 | 6708 | 1.63 (1.32, 2.03) |
| Agrarian regions | 32 | 15,512 | 1.31 (1.04, 1.64) |
| Emerging regions | 4 | 2530 | 1.26 (0.80, 1.98) |
Agrarian regions = Oromia, Amhara, SNNPR, Tigray; Emerging regions = Afar, Gambela, Somalia.
4. Discussion
We conducted this systematic review and meta-analysis to estimate the pooled prevalence of Schistosomiasis (S. mansoni and S. haematobium) and its association with gender of school age children in Ethiopia. The result of 50 included studies noted that the pooled prevalence of Schistosomiasis in Ethiopia was 28.77% (95% CI: 23.81, 33.74%). This find was lower than a national survey of Mozambique (47%) (Augusto et al., 2009) and Sierra Leone (69.0% & 42.2%) (Hodges et al., 2012; Bah et al., 2019). whereas, the prevalence of Schistosomiasis in Ethiopia was higher than the study conducted in Kenya (2.1%) (Mwandawiro et al., 2013), Chad (1%) (Beasley et al., 2002) and Mali (12.7%) (Landouré et al., 2012). Findings from different countries indicate that prevalence of Schistosomiasis considerably varies among countries. Differences in the prevalence of Schistosomiasis among these different countries might be associated with environmental sanitation, water supply, and the socioeconomic status of countries. Moreover, the above variations could be due to methodological differences (i.e., data analysis and sampling of study participants) and health service utilization culture of the countries.
In this study, we also performed sub-group analysis based on the study areas (i.e. regions of the country) where the studies were conducted. The findings of the subgroup analysis indicated that extreme variability was observed in the prevalence of Schistosomiasis across the regions of the country. The highest (39.77%) prevalence of Schistosomiasis was reported from the southern nation, nationality and people region, whereas the lowest (14.95%) prevalence of Schistosomiasis was reported from Afar region. The possible explanation for this variation could be due to the cultural variation across the regions of the country and geographical differences across these different regions of the country.
The current meta-analysis was also examined the association between prevalence of Schistosomiasis and gender of school age children in the context of Ethiopia. Accordingly, gender of school age children was significantly associated with prevalence of Schistosomiasis. Male school age children were 58% more likely to be infected with Schistosomiasis than female school age children in Ethiopia. This finding was consistent with a study conducted in Zimbabwe (Taylor and Makura, 1985). The obvious explanation for this could be male school age children had higher frequency of contact with contaminated water bodies than female while helping their family in outdoor activities such as herding cattle, fishing and farming. Moreover, male children usually play outdoors, and engage in outdoor activities compared to their female counterparts, which may predispose them to higher risks of Schistosomiasis infection. In this review, studies conducted in agrarian regions showed that male school age children were 31% more likely infected with Schistosomiasis than female school age children. This finding is consistent with recommendation from study conducted in 16 countries where there authors recommended context specific studies for gender inequality in neglected tropical diseases service utilization (Cohn et al., 2019). In this review, studies conducted since 2015 in which Ethiopia started national mass drug administration following WHO recommendation (https://espen.afro.who.int/system/files/content/resources/ETHIOPIA_NTD_Master_Plan_2016_220.pdf, n.d.) showed that male school age children were 63% more likely infected with Schistosomiasis than female school age children. This finding is consistent with study conducted in 16 countries where male gender had lower preventive chemotherapy coverage than Female for all diseases (Cohn et al., 2019). This might be due to strong women empowerment activities at school level and at community for health services utilization.
5. Conclusion
The prevalence of Schistosomiasis (S. mansoni and S. haematobium) in Ethiopia was higher than the 2018 report of the Ethiopian federal ministry of health. The prevalence of Schistosomiasis was predominant in male gender school age children, since 2015 and in agrarian regions of Ethiopia. Beyond current mass drug administration program, interventions against Schistosomiasis should be culture and context specific, to address the gender related risk to Schistosomiasis. Further researches should be encouraged to identify context specific factors related to gender differences in occurrence of schistosomiasis.
Acknowledgments
Acknowledgements
We would like to acknowledge all Liberians for their cooperation.
Limitations
This systematic review and meta-analysis has the following limitations: only English language articles or reports and published articles were considered to conduct this review, the majority of the studies included in this review were cross-sectional in nature as a result; the outcome variable might be affected by other confounding variables. Furthermore, this meta-analysis represented only studies reported from seven regions of the country. Therefore, the regions might be under-represented due to the limited number of studies included.
References
- Abdi M., Nibret E., Munshea A. Prevalence of intestinal helminthic infections and malnutrition among schoolchildren of the Zegie Peninsula, northwestern Ethiopia. J. Infect. Public Health. 2017;10(1):84–92. doi: 10.1016/j.jiph.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Abera B., Alem G., Yimer M., Herrador Z. Epidemiology of soil-transmitted helminths, Schistosoma mansoni, and haematocrit values among schoolchildren in Ethiopia. J. Infect. Dev. Countr. 2013;7(3):253–260. doi: 10.3855/jidc.2539. [DOI] [PubMed] [Google Scholar]
- Afework Bitew A., Abera B., Seyoum W., Endale B., Kiber T., Goshu G. Soil-transmitted Helminths and Schistosoma mansoni infections in Ethiopian orthodox church students around Lake Tana, Northwest Ethiopia. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alebie G., Erko B., Aemero M., Petros B. Epidemiological study on Schistosoma mansoni infection in Sanja area, Amhara region, Ethiopia. Parasit. Vect. 2014;7:15. doi: 10.1186/1756-3305-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemayehu B., Tomass Z. Schistosoma mansoni infection prevalence and associated risk factors among schoolchildren in Demba Girara, Damot Woide District of Wolaita zone, southern Ethiopia. Asian Pac J Trop Med. 2015;8(6):457–463. doi: 10.1016/j.apjtm.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Alemayehu B., Tomass Z., Wadilo F., Leja D., Liang S., Erko B. Epidemiology of intestinal helminthiasis among school children with emphasis on Schistosoma mansoni infection in Wolaita zone, southern Ethiopia. BMC Public Health. 2017;17(1):587. doi: 10.1186/s12889-017-4499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemu A., Atnafu A., Addis Z., Shiferaw Y., Teklu T., Mathewos B. Soil transmitted helminths and schistosoma mansoni infections among school children in Zarima town, Northwest Ethiopia. BMC Infect. Dis. 2011;11:189. doi: 10.1186/1471-2334-11-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemu M. Prevalence of intestinal Schistosomiasis and soil transmitted Helminthiasis among primary school children in Umolante District, South Ethiopia. Clin. Med. Res. 2014;3(6):174. [Google Scholar]
- Amenu K., Getachew T., Bekele A., Defar A., Taddesse M., Teklie H. Neglected Tropical Diseases (NTD) service availability at health facilities in Ethiopia: evidence from 2014 Ethiopian service provision assessment. Ethiop. J. Health Dev. 2017;31(1):378–383. [Google Scholar]
- Amsalu G., Mekonnen Z., Erko B. A new focus of schistosomiasis mansoni in Hayk town, northeastern Ethiopia. BMC Res. Notes. 2015;8:22. doi: 10.1186/s13104-014-0965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa A., Dejenie T., Tomass Z. Infection prevalence of Schistosoma mansoni and associated risk factors among schoolchildren in suburbs of Mekelle city, Tigray, Northern Ethiopia. Momona Ethiop. J. Sci. 2013;5(1):174–188. [Google Scholar]
- Assefa T., Woldemichael T., Dejene A. Intestinal parasitism among students in three localities in south Wello, Ethiopia. Ethiopian Journal of Health Development. 1998;12(3):231. [Google Scholar]
- Augusto G., Nalá R., Casmo V., Sabonete A., Mapaco L., Monteiro J. Geographic distribution and prevalence of schistosomiasis and soil-transmitted helminths among schoolchildren in Mozambique. Am. J. Trop. Med. Hyg. 2009;81(5):799–803. doi: 10.4269/ajtmh.2009.08-0344. [DOI] [PubMed] [Google Scholar]
- Awoke W., Bedimo M., Tarekegn M. Prevalence of schistosomiasis and associated factors among students attending at elementary schools in Amibera District, Ethiopia. Open J. Prevent. Med. 2013;3(2):199–204. [Google Scholar]
- Ayalew A., Debebe T., Worku A. Prevalence and risk factors of intestinal parasites among Delgi school children, North Gondar, Ethiopia. J. Parasitol. Vect. Biol. 2011;3(5):75–81. [Google Scholar]
- Bah Y.M., Paye J., Bah M.S., Conteh A., Saffa S., Tia A. Schistosomiasis in school age children in Sierra Leone after 6 years of mass drug administration with Praziquantel. Front. Public Health. 2019;7 doi: 10.3389/fpubh.2019.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajiro M., Dana D., Ayana M., Emana D., Mekonnen Z., Zawdie B. Prevalence of Schistosoma mansoni infection and the therapeutic efficacy of praziquantel among school children in Manna District, Jimma zone, Southwest Ethiopia. Parasit. Vectors. 2016;9(1):560. doi: 10.1186/s13071-016-1833-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajiro M., Dana D., Levecke B. Prevalence and intensity of Schistosoma mansoni infections among schoolchildren attending primary schools in an urban setting in southwest, Ethiopia. BMC Res. Notes. 2017;10(1):677. doi: 10.1186/s13104-017-3023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley M., Brooker S., Ndinaromtan M., Madjiouroum E.M., Baboguel M., Djenguinabe E. First nationwide survey of the health of schoolchildren in Chad. Tropical Med. Int. Health. 2002;7(7):625–630. doi: 10.1046/j.1365-3156.2002.00900.x. [DOI] [PubMed] [Google Scholar]
- Belay S., Tadesse D., Awala A., Teklay G., Hailu T., Alemu M. Multivariate analysis of factors associated with Schistosoma mansoni and hookworm infection among primary school children in rural Bahir Dar, Northwest Ethiopia. BMC Res. Notes. 2018;4:4. doi: 10.1186/s40794-018-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chala B., Torben W. An epidemiological trend of urogenital schistosomiasis in Ethiopia. Front. Public Health. 2018 Mar 5;6:60. doi: 10.3389/fpubh.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D.A., Kelly M.P., Bhandari K., Zoerhoff K.L., Batcho W.E., Drabo F., Negussu N., Marfo B., Goepogui A., Lemoine J.F., Ganefa S. Gender equity in mass drug administration for neglected tropical diseases: data from 16 countries. Int. Health. 2019 Sep 2;11(5):370–378. doi: 10.1093/inthealth/ihz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degarege A., Mekonnen Z., Levecke B., Legesse M., Negash Y., Vercruysse J. Prevalence of Schistosoma haematobium infection among school-age children in Afar area, Northeastern Ethiopia. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0133142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejenie T., Asmelash T. Schistosomiasis mansoni among school children of different water source users in Tigray, Northern Ethiopia. Momona Ethiop. J. Sci. 2010;2(1) [Google Scholar]
- Dejenie T., Asmelash T., Teferi M. Intestinal helminthes infections and re-infections with special emphasis on schistosomiasis mansoni in Waja, North Ethiopia. Momona Ethiop. J. Sci. 2009;1(2) [Google Scholar]
- Deribe K., Meribo K., Gebre T., Hailu A., Ali A., Aseffa A. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasit. Vectors. 2012;5(1):240. doi: 10.1186/1756-3305-5-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deribew K., Tekeste Z., Petros B., Huat L.B. Urinary schistosomiasis and malaria associated anemia in Ethiopia. Asian Pac. J. Trop. Biomed. 2013;3(4):307–310. doi: 10.1016/S2221-1691(13)60068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essa T., Birhane Y., Endris M., Moges A., Moges F. Current status of Schistosoma mansoni infections and associated risk factors among students in Gorgora town, Northwest Ethiopia. ISRN Infect Dis. 2012;2013 [Google Scholar]
- Feleke D.G., Arega S., Tekleweini M., Kindie K., Gedefie A. Schistosoma mansoni and other helminthes infections at Haike primary school children, North-East, Ethiopia: a cross-sectional study. BMC Res. Notes. 2017;10(1):609. doi: 10.1186/s13104-017-2942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentie T., Erqou S., Gedefaw M., Desta A. Epidemiology of human fascioliasis and intestinal parasitosis among schoolchildren in Lake Tana Basin, Northwest Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 2013;107(8):480–486. doi: 10.1093/trstmh/trt056. [DOI] [PubMed] [Google Scholar]
- French M., Dorny P., Vercruysse J., Levecke B., Nute A.W., Endeshaw T. Prevalence of soil-transmitted helminths and Schistosoma mansoni among a population-based sample of school-age children in Amhara region, Ethiopia. PLoS Negl. Trop. Dis. 2018;11(1):431. doi: 10.1186/s13071-018-3008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashaw F., Aemero M., Legesse M., Petros B., Teklehaimanot T., Medhin G. Prevalence of intestinal helminth infection among school children in Maksegnit and Enfranz towns, northwestern Ethiopia, with emphasis on Schistosoma mansoni infection. Parasit. Vectors. 2015;8:567. doi: 10.1186/s13071-015-1178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelaw A., Anagaw B., Nigussie B., Silesh B., Yirga A., Alem M. Prevalence of intestinal parasitic infections and risk factors among schoolchildren at the University of Gondar Community School, Northwest Ethiopia: a cross-sectional study. BMC Public Health. 2013;13(1):304. doi: 10.1186/1471-2458-13-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleta S., Alemu A., Getie S., Mekonnen Z., Erko B. Prevalence of urinary schistosomiasis and associated risk factors among Abobo primary school children in Gambella Regional State, southwestern Ethiopia: a cross sectional study. Parasit. Vectors. 2015;8:215. doi: 10.1186/s13071-015-0822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genanew A., Teshale T. Prevalence of intestinal helminths and associated factors among school children of Medebay Zana wereda; North Western Tigray, Ethiopia 2017. Trop. Dr. 2018;11(1):444. doi: 10.1186/s13104-018-3556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile S., Golassa L., Mekonnen Z. Prevalence of Schistosoma mansoni and effectiveness of Praziquantel in school children in Finchaa valley, Ethiopia. J. Parasitol. Vect. Biol. 2012;4(3):25–30. [Google Scholar]
- Hardy R.J., Thompson S.G. Detecting and describing heterogeneity in meta-analysis. Stat. Med. 1998;17(8):841–856. doi: 10.1002/(sici)1097-0258(19980430)17:8<841::aid-sim781>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges M.H., Dada N., Warmsley A., Paye J., Bangura M.M., Nyorkor E. Mass drug administration significantly reduces infection of Schistosoma mansoni and hookworm in school children in the national control program in Sierra Leone. BMC Infect. Dis. 2012;12(1):16. doi: 10.1186/1471-2334-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Fenwick A. Public Library of Science; 2009. Schistosomiasis in Africa: An Emerging Tragedy in our New Global Health Decade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://espen.afro.who.int/system/files/content/resources/ETHIOPIA_NTD_Master_Plan_2016_220.pdf
- Jamaneh L. Schistosomiasis manosni and geo-helminthiasis in school children in the Dembia plains. Northwest Ethiopia. Ethiop. J. Health Dev. 1998;12(3):237–244. [Google Scholar]
- Jejaw A., Zemene E., Alemu Y., Mengistie Z. High prevalence of Schistosoma mansoni and other intestinal parasites among elementary school children in Southwest Ethiopia: a cross-sectional study. BMC Public Health. 2015;15:600. doi: 10.1186/s12889-015-1952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemaneh L. Intestinal helminth infections in schoolchildren in Gonder town and surrounding areas, Northwest Ethiopia. SINET: Ethiop. J. Sci. 1999;22(2):209–220. [Google Scholar]
- Jemaneh L. Soil-transmitted helminth infections and Schistosomiasis mansoni in school children from Chilga District, northwest Ethiopia. Ethiop. J. Health Sci. 2001;11(2) [Google Scholar]
- Kassa Laikemariam, Omer Anteneh, Tafesse Wutet, Taye Tadele, Fekadu Kebebew A.B. Guideline. 2005. Schistosomiasis. [Google Scholar]
- Knapp G., Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003;22(17):2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- Landouré A., Dembélé R., Goita S., Kané M., Tuinsma M., Sacko M. Significantly reduced intensity of infection but persistent prevalence of schistosomiasis in a highly endemic region in Mali after repeated treatment. PLoS Negl. Trop. Dis. 2012;6(7) doi: 10.1371/journal.pntd.0001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legesse L., Erko B., Hailu A. Current status of intestinal Schistosomiasis and soiltransmitted helminthiasis among primary school children in Adwa Town, Northern Ethiopia. Ethiopian J. Health Dev. 2011;24(3) [Google Scholar]
- Legesse M, Erko B. Prevalence of intestinal parasites among schoolchildren in a rural area close to the southeast of Lake Langano, Ethiopia. Ethiop. J. Health Dev. 18: 116. 2004;120.
- Mahmud M.A., Spigt M., Mulugeta Bezabih A., Lopez Pavon I., Dinant G.J., Blanco Velasco R. Risk factors for intestinal parasitosis, anaemia, and malnutrition among school children in Ethiopia. Pathog. Glob. Health. 2013;107(2):58–65. doi: 10.1179/2047773213Y.0000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewos B., Alemu A., Woldeyohannes D., Alemu A., Addis Z., Tiruneh M. Current status of soil transmitted helminths and Schistosoma mansoni infection among children in two primary schools in North Gondar, Northwest Ethiopia: a cross sectional study. BMC Res. Notes. 2014;7:88. doi: 10.1186/1756-0500-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekonnen Z., Meka S., Zeynudin A., Suleman S. Schistosoma mansoni infection and undernutrition among school age children in Fincha’a sugar estate, rural part of West Ethiopia. BMC Res. Notes. 2014;7:763. doi: 10.1186/1756-0500-7-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health . 2nd edition. 2016. National Neglected Tropical Diseases Master Plan; pp. 1–78. [Google Scholar]
- Mitiku H., Legesse M., Teklemariam Z., Erko B. Transmission of Schistosoma mansoni in Tikur Wuha area, southern Ethiopia. Ethiop. J. Health Dev. 2010;24(3) [Google Scholar]
- Modesti P.A., Reboldi G., Cappuccio F.P., Agyemang C., Remuzzi G., Rapi S. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwandawiro C.S., Nikolay B., Kihara J.H., Ozier O., Mukoko D.A., Mwanje M.T. Monitoring and evaluating the impact of national school-based deworming in Kenya: study design and baseline results. Parasit. Vectors. 2013;6(1):198. doi: 10.1186/1756-3305-6-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negussu N., Wali M., Ejigu M., Debebe F., Aden S., Abdi R. Prevalence and distribution of schistosomiasis in afder and Gode zone of Somali region, Ethiopia. J. Global Infect. Dis. 2013;5(4):149–152. doi: 10.4103/0974-777X.122007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcastle-Ottawa Scale customized for cross-sectional studies In. Available from https://static-content.springer.com/esm/.../12889_2012_5111_MOESM3_ESM.doc.
- Organization WH, UNICEF . World Health Organization; Geneva: 2004. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis: World Health Organization/Unicef Joint Statement. [PubMed] [Google Scholar]
- Roma B., Worku S. Magnitude of Schistosoma mansoni and intestinal helminthic infections among school children in Wondo-Genet Zuria, southern Ethiopa. Ethiop. J. Health Dev. 1997;11:125–130. [Google Scholar]
- Tadege B., Shimelis T. Infections with Schistosoma mansoni and geohelminths among school children dwelling along the shore of the Lake Hawassa, southern Ethiopia. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0181547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse D., Tsehaye A., Mekonnen T. Intestinal helminthes infections and re-infections with special emphasis on Schistosomiasis mansoni in Waja, North Ethiopia. Momona Ethiop. J. Sci. 2009;1(2):4–16. [Google Scholar]
- Tadesse G. The prevalence of intestinal helminthic infections and associated risk factors among school children in Babile town, eastern Ethiopia. Ethiop. J. Health Dev. 2005;19(2):140–147. [Google Scholar]
- Taylor P., Makura O. Prevalence and distribution of schistosomiasis in Zimbabwe. Ann. Trop. Med. Parasitol. 1985;79(3):287–299. doi: 10.1080/00034983.1985.11811921. [DOI] [PubMed] [Google Scholar]
- Tekeste Z., Belyhun Y., Gebrehiwot A., Moges B., Workineh M., Ayalew G. Epidemiology of intestinal schistosomiasis and soil transmitted helminthiasis among primary school children in Gorgora, Northwest Ethiopia. Asian Pac. J. Trop. Dis. 2013;3(1):61–64. [Google Scholar]
- Teklemariam A., Dejenie T., Tomass Z. Infection prevalence of intestinal helminths and associated risk factors among schoolchildren in selected kebeles of Enderta district, Tigray, Northern Ethiopia. J. Parasitol. Vect. Biol. 2014;6(11):166–173. [Google Scholar]
- Teklemariam D., Legesse M., Degarege A., Liang S., Erko B. Schistosoma mansoni and other intestinal parasitic infections in schoolchildren and vervet monkeys in Lake Ziway area, Ethiopia. BMC Res.Notes. 2018;11(1):146. doi: 10.1186/s13104-018-3248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terefe A., Shimelis T., Mengistu M., Hailu A., Erko B. Schistosomiasis mansoni and soil-transmitted helminthiasis in Bushulo village, southern Ethiopia. Ethiop. J. Health Dev. 2011;25(1):46–50. [Google Scholar]
- Verheyen A., Dana D., Van Dorst B., Mekonnen Z., Levecke B., Stuyver L.J. Transmission of Schistosoma mansoni in Yachi areas, southwestern Ethiopia: new foci. Parasit. Vectors. 2019;8(1):1. [Google Scholar]
- Worku L., Damte D., Endris M. Schistosoma mansoni infection and associated determinant factors among school children in Sanja town. Northwest Ethiopia. 2014;2014:792536. doi: 10.1155/2014/792536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2008. The Social Context of Schistosomiasis and its Control: An Introduction and Annotated Bibliography. [Google Scholar]