Abstract
Purpose
The purpose of this review is to give an overview on recently published articles investigating the associations of diet and dietary interventions with biomarkers of oxidative stress with special emphasis on different categories of redox biomarkers.
Findings
Intervention and observational studies both in healthy participants and patients that investigated associations of dietary habits, foodstuffs or isolated nutrients with biomarkers of oxidative stress were included in this review. Recently published observation studies confirm the inverse association between fruit and vegetable intake and oxidative stress markers. Studies investigating the effect of vitamin D and vitamin E, magnesium, zinc, chromium, selenium, probiotic supplementation and several phytochemicals reported consistent changes in redox biomarkers. Of 88 articles included in this review, only seven studies measured biomarkers from the three categories: oxidative damage, endogenous antioxidants, and exogenous antioxidants. Many studies rely on controversial assays for total antioxidant capacity, thus there is potential in many studies to improve biomarker repertoire to cover all three categories of biomarkers and to turn away from such assays.
Keywords: Oxidative stress, Biomarkers, Nutrition, Blood, Dietary intake, Intervention, Observational study
Graphical abstract
Highlights
-
•
Oxidative stress can be assessed by specific biomarker categories.
-
•
Three biomarker categories: oxidative damage, endogenous, exogenous antioxidants.
-
•
Only seven studies performed measurements of all three biomarker categories.
-
•
TAC, TRAP, FRAP, ORAC should not be used as stand-alone redox marker.
-
•
Several interventions reported improvements in markers of oxidative stress.
Abbreviations:
- AGE
Advanced glycation endproducts
- AFLD
Alcoholic induced fatty liver disease
- AOPP
Advanced oxidation protein products
- BAP
Biological antioxidant potential
- CAT
Catalase
- CFU
Colony forming units
- CHD
Coronary heart disease
- CML
Carboxymethyllysine
- DB
Double-blinded
- DHA
docosahexaenoic acid
- dROM
Derivatives of reactive oxygen metabolites
- EPA
eicosapentaenoic acid
- FFQ
Food frequency questionnaire
- FOX2
ferrous oxidation-xylenol orange 2
- FRAP
Ferric reducing ability of plasma
- F/V
Fruits and vegetables
- (8-)IsoP
(8-)isoprostanes
- GPx
Glutathione peroxidase
- GR
Glutathione reductase
- GSH
Glutathione
- GSSG
Glutathione dimer
- HD
Hemodialysis
- 4-HNE
4-hydroxynonenal
- IU
International units
- MDA
Malondialdehyde
- MetS
Metabolic syndrome
- NAFLD
Non-alcoholic fatty liver disease
- NB
Non-blinded
- NO
Nitric oxide
- 8OHdG
8-hydroxy-deoxyguanosine
- ORAC
Oxygen Radical Absorbance Capacity
- OS
Oxidative stress
- oxLDL
Oxidized low density lipoprotein
- PCs
Protein carbonyls
- PCOS
Polycystic ovary syndrome
- PON1
Paraoxonase and arylesterase 1
- PUFAs
Polyunsaturated fatty acids
- RFS
Recommended Food Score
- ROS
Reactive oxygen species
- SB
Single-blinded
- SOD
Superoxide dismutase
- TAC
Total antioxidant capacity
- TBARS
Thiobarbituric acid reactive substances
- TRAP
Total radical-trapping antioxidant parameter
1. Introduction
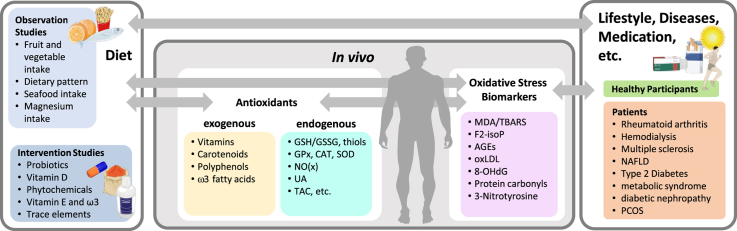
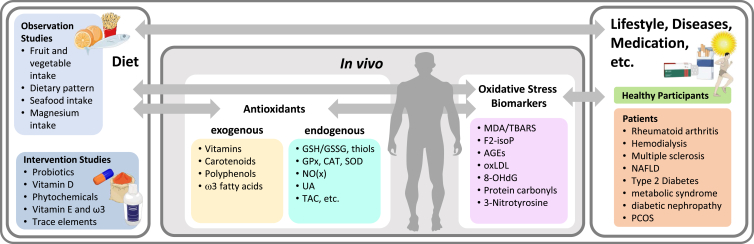
Oxidative stress (OS) is defined as an imbalance between pro- and antioxidants in favor of oxidative compounds such as free radicals and reactive oxygen species (ROS) [[1], [2], [3]]. The interaction of ROS with various biomolecules such as proteins, lipids, carbohydrates and nucleic bases leads to the formation of a variety of substances which are often referred to as biomarkers of OS. Those substances include in general products of enzymatic and non-enzymatic lipid peroxidation such as malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and isoprostanes (e.g. 8-isoprostane), DNA adducts like 8-hydroxy-deoxyguanosine (8OHdG), or specific advanced glycation endproducts (AGE) like carboxymethyllysine (CML). 3-Nitrotyrosine (3NT) and protein carbonyls (PCs) are further prominent biomarkers of OS, resulting from nitration and oxidation of proteins, respectively. Another way to measure redox status is via the activity of antioxidative enzymes like catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx). Many authors furthermore rely on the measurement of the so-called total antioxidant capacity (TAC). Hence, the possibilities to quantify OS are through the analyses of (1) exogenous antioxidants, (2) endogenous antioxidants, and (3) oxidative damage biomarkers in biological samples.
Even though it has been shown that elevated levels of OS occur in a number of various diseases such as Parkinson's disease, Alzheimer's disease [4], alcoholic and non-alcoholic induced fatty liver disease (AFLD and NAFLD), as well as in patients undergoing hemodialysis (HD), the often-propagated harmful effect of OS is still subject of controversial discussion. Severe OS has been reported to trigger necrosis, but moderate levels of ROS also have a regulatory function in apoptosis and in redox signaling [1]. Multiple studies investigated the influence of nutrition, especially the consumption of fruits and vegetables (F/V) on OS and found beneficial effects in participants with high F/V intake such as reduced levels of MDA and isoprostanes [5,6]. While elevated F/V consumption might modulate OS in a beneficial way, intervention studies focusing on isolated micronutrients often showed controversial results [7,8]. The aim of this review is to summarize the results of observational and intervention studies published over the last three years focusing on the association between diet/nutrition and OS. The substances on which this review will focus thereby range from typical nutritional antioxidants such as carotenoids and polyphenols as well as substances whose role in the modulation of OS is currently controversially discussed or has not yet been conclusively clarified including ω-3 fatty acids, vitamin D and probiotic supplementation.
We furthermore aimed to evaluate different categories of redox biomarkers.
2. Methods
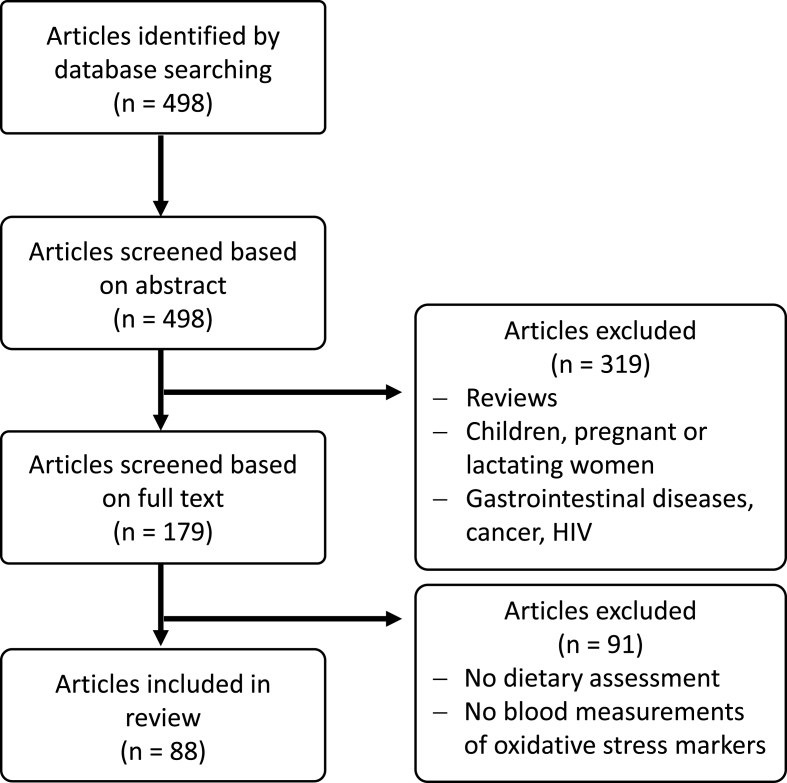
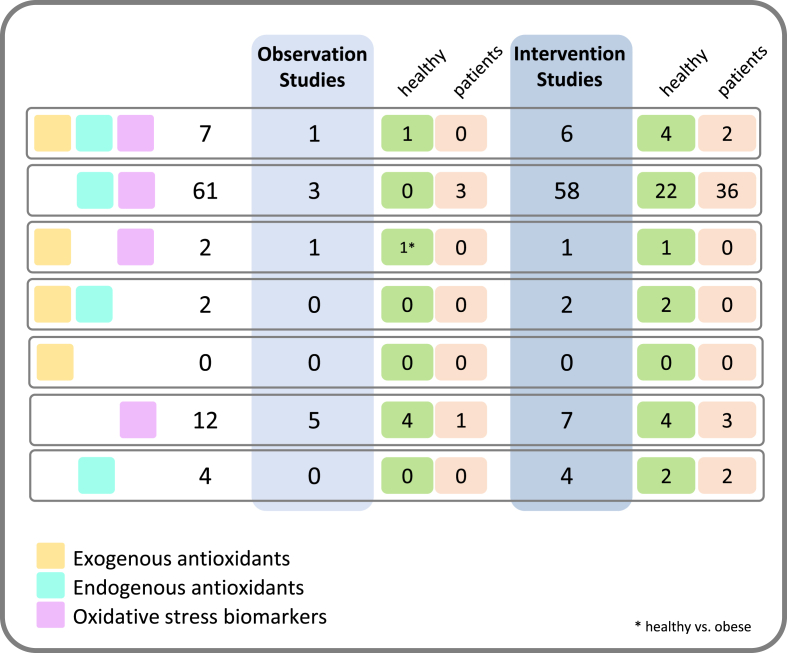
Literature search using PubMed was conducted on June 11th, 2020. Search terms included “nutrition” AND “oxidative stress” AND “biomarkers”. The search was restricted to articles published between January 1st, 2017 and the search date. A total of 498 articles were screened by two investigators based on abstracts. Inclusion criteria were: evaluation of dietary habits OR a dietary intervention AND measuring at least one OS biomarker of the following categories: (I) exogenous antioxidants, (II) endogenous antioxidants, and (III) oxidative damage biomarkers in biological samples. Articles that did not meet the inclusion criteria were removed. Studies assessing diet/interventions in children, pregnant or lactating women as well as those concerning patients suffering from cancer, HIV or gastrointestinal diseases were excluded. The selection process of articles used in this review is shown in Fig. 1. Studies were grouped into those reporting analyses of (I) exogenous antioxidants, (II) endogenous antioxidants, and (III) oxidative damage biomarkers in biological samples to assess overall quality of biomarker analyses (Fig. 2).
Fig. 1.
Flowchart of the selection process.
Fig. 2.
Schematic overview of studies. Observation and intervention studies were evaluated in relation to measurements of different redox biomarkers. Depicted in light yellow are measured exogenous antioxidants in blood, turquoise shows endogenous antioxidants and oxidative stress markers are depicted by pink color. Both, healthy participants as well as patients were included. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Results
3.1. Observational studies
Ten observational studies focusing on general dietary patterns as well as isolated micronutrients were included in this review (Fig. 3). Although most studies were conducted with healthy participants two studies included HD patients and one study included patients suffering from coronary heart disease (CHD). The characteristics of each study are summarized in Table 1. Regarding F/V intake one observational study found significant negative correlations between individual plasma values of oxLDL and plasma levels of lutein as well as β-carotene and TAC [6]. These observed dietary habits were significantly sex-related with a higher F/V intake (p < 0.05) and higher plasma β-carotene (p < 0.001) in women compared to men. Additionally, levels of plasma lipid hydroperoxides were significantly lower in women compared to men. Likewise, MDA was higher in the cluster of obese participants and the obese participants had a lower F/V intake [9]. Examination of associations for diet quality and OS reported negative correlations between Recommended Food Score (RFS) and plasma MDA concentrations in participants suffering from “OS conditions” (obesity, hypertension, metabolic syndrome (MetS), diabetes and cancers), while no association between RFS and plasma MDA was found in the healthy control group [5]. One study found inverse associations between dietary intake of polyunsaturated fatty acids (PUFAs) and insoluble fiber with plasma isoprostanes concentrations [10]; a higher shellfish consumption was also reported to be associated with higher levels of plasma oxLDL in a Mediterranean population [11].
Fig. 3.
Number of articles that describe analyzing different biomarkers. Depicted in light yellow are studies that measured exogenous antioxidants in blood, turquoise shows which studies measured endogenous antioxidants and oxidative stress markers are depicted by pink color. Results are shown by observational studies (light blue) and intervention studies (darker blue), and separated by healthy participants (green) and patients (orange). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
Observational studies.
| Focus of Observation | Participants | N (age in years) | Biomarkers | Conclusion | Ref. |
|---|---|---|---|---|---|
| F/V intake (15-day dietary record and F/V questionnaire) | healthy | 83 (40 ± 10) | TAC, carotenoids, FOX2, oxLDL, PON1, dietary TAC | positive correlation between F/V and plasma carotenoids, negative correlation between F/V and oxLDL as well as between β-carotene and oxLDL | [6] |
| Dietary pattern (annual semi-quantitative FFQ with 160 items) |
healthy (obese) |
68: | MDA |
MDA higher in cluster with obese participants, obese had lower F/V intake |
[9] |
| 20 normal (56.4 ± 10.1) | |||||
| 35 overweight (51.7 ± 11.7) | |||||
| 13 obese (47.8 ± 10.2) | |||||
| Diet quality score (46 foods or food groups) |
healthy vs. persons with OS conditions |
1229: | carotenoids, MDA (plasma, urine and erythrocytes) |
only in the OS group: negative association between Recommended Food Score (RFS) and erythrocyte MDA concentrations but not with plasma MDA association between RFS and plasma carotenoids, especially total and β-carotene, in healthy and OS |
[5] |
| 823 healthy (45.3 ± 10.7) | |||||
| 406 OS (51.8 ± 11.2) | |||||
| Dietary pattern (assessed by diet history questionnaire, 124 food items) | healthy | 269 (59.8 ± 5.5) | 8-isoP | inverse associations between dietary intake of PUFAs, zinc and insoluble fiber with 8-isoP | [10] |
| Seafood intake (3-day dietary record) |
healthy |
81: men (39.9–49.3) | oxLDL, 8-isoP, TBARS |
shellfish consumption associated with elevated levels of plasma oxLDL |
[11] |
| women (36.6–47.9) | |||||
| Vitamin E intake (semi-quantitative FFQ, 146 items) | CHD | 1002 (20–75) | 3NT, GSH/GSSG, GPx | higher GPx activity associated with an inadequate intake of vitamin E | [12] |
| Magnesium intake (3-day food diary) |
healthy vs. obese |
83 women: | TBARS |
negative correlations between erythrocyte magnesium concentrations and plasma TBARS only in obese group |
[13] |
| 31 obese (34.7 ± 7.58) | |||||
| 52 control (35.4 ± 8.64) | |||||
| Dietary intake (dietary habits questionnaire) | healthy but cognitive frail | 815 (68.9 ± 6.1) | MDA | cognitive frail participants had lower intakes of niacin and riboflavin as well as higher plasma MDA | [14] |
| Nutritional status/intake (standardized 24h food record) | HD | 49 (18–65) | TAC, iron, Mn, Se, Zn, MDA, PCs, erythrocyte GPx | carbohydrate intake correlated positively with MDA and PCs, zinc intake correlated negatively with MDA and PCs | [15] |
| Dietary intake (semi-quantitative FFQ) | HD | 85 (62 ± 13.7) | TAC, SOD, MDA, GST, NO | low plasma NO associated with higher intake of nutrients with antioxidant properties (Cu, Mn, ω-3, ω-6, vitamin E) | [16] |
All biomarker measurements in blood (unless otherwise stated); men and women included (unless otherwise stated).
Focusing on single micronutrients, one study observed that higher plasma GPx activity levels were associated with an inadequate intake of vitamin E in patients with established cardiovascular disease [12]. Negative correlations between erythrocyte magnesium concentrations and plasma TBARS were found in obese and normal weight women although the correlation was only statistically significant in the obese group [13]. It was observed that cognitive frail participants had lower intakes of niacin and riboflavin as well as higher plasma MDA [14].
Concerning patients undergoing HD, Silva et al. [15] found no statistically significant associations between nutritional status and OS status in HD patients while Epifano et al. [16] found inverse associations between nutrient intake with antioxidant properties (Cu, Zn, Mn, vitamin C and ω-3, vitamin E) and plasma nitric oxide (NO) concentrations in HD patients.
3.2. Intervention studies with patients
3.2.1. Vitamin D supplementation
Multiple studies investigated the influence of high-dose vitamin D supplementation on OS status in patients with various diseases. Intervention doses ranged from 50000 IU vitamin D3 every two weeks to 1000 IU daily. Intervention duration ranged from eight weeks to six months. An overview of the study characteristics is shown in Table 2.
Table 2.
Vitamin D Supplementation in patients.
| Intervention | Disease | N (age in years) | Biomarkers | Effect of intervention (*) | Ref. |
|---|---|---|---|---|---|
| 1000 IU vitamin D3 + 1000 mg evening primrose oil daily, 12 wks, DB |
PCOS |
60 women: | TAC, GSH, MDA |
MDA decreased (#), GSH increased (#) |
[17] |
| 30 PG (25.4 ± 4.7) | |||||
| 30 IG (26.4 ± 8.1) | |||||
| 50000 IU vitamin D3 every 2 wks, 12 wks, DB | PCOS | 70 women (18–40) | TAC, GSH, MDA, NO | MDA lower (*) | [18] |
| 50000 IU vitamin D3 every 2 wks + 2x 1000 mg/day ω-3, 12 wks, DB | PCOS | 60 women (18–40) | TAC, GSH, MDA, NO | MDA decreased (#), TAC increased (#) | [19] |
| 50000 IU vitamin D3 every 2 wks, 12 wks, DB |
diabetic foot ulcer |
60: | TAC, GSH, MDA, NO |
MDA lower (*) |
[20] |
| 30 PG (58.6 ± 8.6) | |||||
| 30 IG (59.6 ± 8.2) | |||||
| 50000 IU vitamin D3/wk, 8 wks, DB |
diabetic nephropathy |
50: | TAC, SOD, CAT, GPX, MDA |
no effect |
[21] |
| 25 PG (43.7 ± 6.1) | |||||
| 25 IG (39.7 ± 7.3) | |||||
| 50000 IU vitamin D3 every 2 wks, 6 months, DB |
irritable bowel syndrome |
85: | TAC, MDA |
MDA decreased (#), TAC increased (#) |
[22] |
| 41 PG (38.3 ± 9.9) | |||||
| 44 IG (37.5 ± 8) | |||||
| 50000 IU vitamin D3 every 2 wks, 6 months, DB |
coronary artery disease |
60: | TAC, GSH, MDA, NO |
MDA lower (*), NO increased (#), GSH increased (#) |
[23] |
| 30 PG (63.0 ± 10.7) | |||||
| 30 IG (60.5 ± 8) | |||||
| 50000 IU vitamin D3 every 2 wks, 12 wks, DB | HD | 60 (18–80) | TAC, GSH, MDA, NO | TAC higher (*), MDA lower (*) | [24] |
| 50000 IU vitamin D3 every 2 wks + 2x 1000 mg/day ω-3, 12 wks, DB |
Multiple Sclerosis |
53: | TAC, GSH, MDA |
MDA lower (*) |
[25] |
| 27 PG (35.2 ± 9.2) | |||||
| 26 IG (33.3 ± 6.5) | |||||
| 50000 IU vitamin D3 every 2 wks, 12 wks, DB | methadone treatment patients | 68: | TAC, GSH, MDA, NO | TAC and GSH higher (*) | [26] |
| 34 PG (42.5 ± 8.9) | |||||
| 34 IG (40.1 ± 9.2) | |||||
All biomarkers measurements in blood (unless otherwise stated); men and women included (unless otherwise stated); PG (placebo group), IG (intervention group). DB (double-blind), SB (single blind), NB (not blinded); only significant results are mentioned. Significant changes are marked by: (*) vs. PG; (+) vs. baseline; (#) vs. baseline and PG.
Studies investigating the influence of high-dose vitamin D supplementation on OS in women suffering from polycystic ovary syndrome (PCOS) found consistent results regarding significantly reduced plasma MDA levels compared to the placebo group [[17], [18], [19]]. No effect was found on NO concentrations between placebo and intervention [18,19], while few studies reported positive effects on TAC and GSH levels [17,19]. In diabetic patient's high-dose vitamin D supplementation showed different effects on OS markers. Even though plasma MDA was significantly reduced in patients with diabetic foot ulcer compared to the placebo group [20], no effect on OS markers were observed in patients with diabetic nephropathy [21]. Consensus results on plasma MDA reduction in the intervention group were found in multiple studies including patients suffering from coronary artery disease, irritable bowel syndrome, multiple sclerosis as well as patients undergoing HD after long-term (three - six months) high-dose vitamin D supplementation [[22], [23], [24], [25]]. Vitamin D intervention in methadone treated patients lead to a significant increase in plasma GSH and TAC levels while plasma MDA levels were not affected compared to the placebo group [26].
3.2.2. Probiotic supplementation
Probiotic supplementation was performed in multiple studies by daily administration of one capsule probiotic supplement containing 2x109 colony forming units (CFU) of Lactobacillus species L.casei, L.acidophilus or L.fermentum as well as Bifidobacterium bifidum for twelve weeks (Table 3). Another way of probiotic supplementation was performed by administering probiotic drinks or yoghurt [[27], [28], [29]] for seven–eight weeks.
Table 3.
Probiotic supplementation in patients.
| Intervention | Disease | N (age in years) | Biomarkers | Effect of intervention | Ref. |
|---|---|---|---|---|---|
| 100 ml Sorghum drink with probiotics, 3–4x/wk, 2 pks on day of HD and the day after, 7 wks, SB |
CKD/HD |
58: | TAC, MDA, SOD, polyphenols |
TAC higher (*), MDA lower (*), SOD higher (*) |
[27] |
| 29 PG (63 ± 10.6) | |||||
| 29 IG (63.2 ± 11.2) | |||||
| Probiotic supplements daily (one capsule/day), 12 wks, DB |
HD |
60: | TAC, MDA, NO, GSH |
MDA lower (*), TAC lower |
[30] |
| 30 PG (59.4 ± 16.0) | |||||
| 30 IG (54.0 ± 16.0) | |||||
| 100 mg Lactobacillus coagulants tablet, 2 per day, 8 wks, DB |
HD |
46 (63 ± 17) | MDA |
MDA decreased (#) |
[31] |
| 23 PG | |||||
| 23 IG | |||||
| Probiotic capsule(s) supplements daily, 12 wks, DB |
diabetic nephropathy |
60: | TAC, MDA, GSH, NO, AGEs |
NO increased, GSH increased, MDA reduced, AGEs reduced (*) |
[32] |
| 30 PG (60.9 ± 4.4) | |||||
| 30 IG (58.9 ± 8.8) | |||||
| 200 ml probiotic soy milk/day, 8 wks (vs. soy milk), SB |
diabetic kidney disease |
40: | TAC, MDA, GPx, GSH/GSSG, GR, 8-isoP |
increase in GSH, GPx and GR, decrease in GSSG (+) |
[28] |
| 20 PG (53.6 ± 1.6) | |||||
| 20 IG (56.9 ± 1.8) | |||||
| 220 g yoghurt or milk per day, 24 wks, NB |
NAFLD and MetS |
92 women (36–66) | SOD, GPx |
SOD, GPx increased in yoghurt vs. milk group after 24 wks (+) |
[29] |
| 44 PG | |||||
| 48 IG | |||||
| Syn-/Probiotic supplements daily, 12 wks, DB |
overweight, diabetes and CHD |
60: | TAC, MDA, NO, GSH |
NO increased, MDA decreased (#) |
[33] |
| 30 PG (64.0 ± 11.7) | |||||
| 30 IG (64.2 ± 12.0) | |||||
| Probiotic supplement capsules daily, 12 wks, DB |
Multiple Sclerosis |
60: | TAC, MDA, NO, GSH |
differences in adjusted changes between MDA and NO (*) |
[34] |
| 30 PG (33.8 ± 8.9) | |||||
| 30 IG (34.4 ± 9.2) | |||||
| Probiotic supplements capsule daily, 12 wks, DB |
diabetic foot ulcer |
60: | TAC, MDA, NO, GSH |
higher TAC, NO, lower MDA (*) |
[35] |
| 29 PG (58.5 ± 11.0) | |||||
| 30 IG (62.6 ± 9.7) | |||||
| Probiotic supplements daily, 12 wks, DB | PCOS | 60: | TAC, MDA, NO, GSH | higher TAC, GSH lower MDA (*) | [36] |
| 30 PG (27.7 ± 4.7) | |||||
| 30 IG (27.2 ± 4.6) |
All biomarkers measurements in blood (unless otherwise stated); men and women included (unless otherwise stated); PG (placebo group), IG (intervention group). DB (double-blind), SB (single blind), NB (not blinded); only significant results are mentioned. Significant changes are marked by: (*) vs. PG; (+) vs. baseline; (#) vs. baseline and PG.
Three studies investigated the effect of probiotic supplementation in patients undergoing HD and reported consistent results regarding an improved OS status in patients receiving probiotic treatment. Each study reported significantly reduced plasma MDA levels in the intervention group compared to the placebo group [27,30] or compared to baseline [31]. An increase in plasma TAC in the intervention group was found in two studies [27,30], while an additional increase in SOD activity was reported by Lopes et al. [27]. Probiotic supplementation in patients suffering from diabetic kidney disease led to mixed results. Although daily administration of probiotic supplements for twelve weeks showed a significant increase in plasma GSH with simultaneous decrease in plasma MDA [32], no effect on plasma MDA was observed after daily consumption of probiotic soy milk for eight weeks, while plasma GSH as well as GR and GPx activity showed a significant increase [28]. Increased activity of plasma GPx and SOD as well as reduced 8-isoP levels were observed in patients with MetS and NAFLD after intervention with 220 g yoghurt per day for 24 weeks [29]. Reduced plasma MDA levels as well as elevated NO levels were reported in overweight patients with diagnosed CHD and diabetes mellitus [33] and in patients with multiple sclerosis [34]. Improvements in OS status after twelve weeks of probiotic intervention have also been shown in the clinical pictures of diabetic foot ulcer and PCOS resulting in reduced plasma MDA levels [35,36] as well as an increase in TAC [35].
3.2.3. Vitamin E and ω-3 supplementation
ω-3 supplementation was mainly performed by administration of 1000 mg flaxseed oil, rich in α-linolenic acid, each day for twelve weeks [[37], [38], [39], [40]] whereby two supplements contained additional 400 IU vitamin E [37,39]. Co-supplementation of long-chain PUFA and vitamin E delivering 180 mg eicosapentaenoic acid (EPA), 120 mg docosahexaenoic acid (DHA) and 9 IU vitamin E per day for eight weeks was performed in one study [41] while isolated vitamin E supplementation in high doses of 400 and 800 IU per day for twelve weeks was conducted in two studies [42,43]. Study details are enlisted in Table 4.
Table 4.
Interventions with vitamin E and/or ω-3 supplementation in patients.
| Intervention | Disease | N (age in years) | Biomarkers | Effect of intervention | Ref. |
|---|---|---|---|---|---|
| 1000 mg ω-3 + 400 IU vitamin E, 12 wks, DB |
Fibrocystic breast disease |
56: | TAC, MDA, NO, GSH |
NO higher (*) |
[37] |
| 28 PG (47.6 ± 5.8) | |||||
| 28 IG (45.3 ± 7.2) | |||||
| 1000 mg flaxseed oil/day, 12 wks, DB |
diabetic nephropathy |
60: | TAC, MDA, NO, GSH |
no effect |
[38] |
| 30 PG (62.4 ± 9.6) | |||||
| 30 IG (62.9 ± 10.5) | |||||
| 1000 mg flaxseed oil + 400 IU vitamin E/day, 12 wks, DB |
PCOS |
68: | TAC, MDA, GSH |
TAC increased (#), MDA decreased (#) |
[39] |
| 34 PG (26.6 ± 5.6) | |||||
| 34 IG (24.9 ± 5.5) | |||||
| 2x1000 mg/day flaxseed oil ω-3 supplements, 12 wks, DB |
PCOS |
60: | NO |
no effect |
[40] |
| 30 PG (27.0 ± 3.2) | |||||
| 30 IG (28.4 ± 6.4) | |||||
| 180 mg EPA, 120 mg DHA, 2 mg vitamin E, 3x/day, 8 wks, DB |
Type 2 diabetes |
30: | TBARS, 8-isoP, TRAP, SOD, uric acid |
no effect |
[41] |
| 15 PG (50.5 ± 6.1) | |||||
| 15 IG (50.7 ± 6.7) | |||||
| 400 IU vitamin E, 12 wks, DB |
implantation failure |
40: | MDA |
MDA lower (*) |
[42] |
| 20 IG (32.2 ± 2.3) | |||||
| 20 PG (31.5 ± 2.3) | |||||
| 800 IU vitamin E, 12 wks, DB |
diabetic nephropathy |
54: | TAC, MDA, NO, GSH, vitamin E |
GSH higher (*) |
[43] |
| 27 PG (64.5 ± 9.2) | |||||
| 27 IG (62.2 ± 9.8) | |||||
| (A) 30 g isolated soy protein + flaxseed oil | wound healing of burn patients | 73: | MDA, SOD | no effect | [44] |
| (B) isolated soy protein + corn oil | A 25 (36.8 ± 11.7) | ||||
| (C) wheat flour + corn oil | B 24 (32.5 ± 9.9) | ||||
| 12 wks, DB | C 24 (35.4 ± 10.3) |
All biomarkers measurements in blood (unless otherwise stated); men and women included (unless otherwise stated); PG (placebo group), IG (intervention group). DB (double-blind), SB (single blind), NB (not blinded); only significant results are mentioned. Significant changes are marked by: (*) vs. PG; (+) vs. baseline; (#) vs. baseline and PG.
Findings on the effect of isolated ω-3-administration on OS status have been mostly consistent. Administration of 1000 mg flaxseed oil/day for twelve weeks neither showed an effect on OS status in women with PCOS nor in patients with diabetic nephropathy [38,40]. The same was observed after even higher dosage of 30 g flaxseed oil/day for three weeks in wound healing patients [44]. The co-supplementation of 1000 mg flaxseed oil and 400 IU vitamin E in patients diagnosed with fibrocystic breast disease on the other hand led to a significant increase in plasma NO in the intervention group, but had no effect on plasma MDA [37]. Contrary, flaxseed oil and vitamin E co-supplementation significantly lowered plasma MDA levels in PCOS patients, but no effect on plasma GSH was observed [39]. Intervention with long-chain PUFA and low-dose vitamin E in type 2 diabetic patients again showed no significant effect on plasma TBARS [41]. High-dose vitamin E supplementation for twelve weeks in implantation failure patients significantly lowered plasma MDA levels [42], while no effect on OS markers were observed in diabetic nephropathy patients with exception of significantly elevated plasma GSH levels [43].
3.2.4. Supplementation with polyphenol-rich plant extracts or single phytochemicals
Extracts from various fruits containing high amounts of polyphenols such as pomegranate, mulberry and blueberry have been evaluated for their influence on the OS status in multiple diseases as well as isolated phytochemicals such as genistein, gallic acid, curcumin (from the curcuma root), crocin (crocus and gardenia flowers) or silybin (from the milk thistle seeds) leading to various improvements in OS markers (see Table 5).
Table 5.
Supplementation with phytochemicals in patients.
| Intervention | Disease | N (age in years) | Biomarkers | Effect of intervention | Ref. |
|---|---|---|---|---|---|
| 2 capsules 250 mg pomegranate extract/day, 8 wks, DB |
rheumatoid arthritis |
55: | GPx, MDA |
GPx higher (*) |
[45] |
| 30 IG (48.4 ± 11.4) | |||||
| 25 PG (49.1 ± 12.2) | |||||
| 100 ml pomegranate juice, 3x/wk, crossover (8 wks IG/PG, 4 wks wash-out, 8 wks IG/PG), NB |
HD |
41 (18–65) | TAC, MDA, IL-6, blood pressure |
TAC increased, MDA decreased (#) |
[46] |
| 22 IG | |||||
| 19 PG | |||||
| 300 mg mulberry extract/day, 12 wks, DB |
diabetic nephropathy |
60: | TAC, NO, GSH, MDA |
IG: NO increased (+), GSH increased (#), MDA decreased (#) PG: MDA increased (#) |
[47] |
| 30 IG (63.7 ± 10.8) | |||||
| 30 PG (63.1 ± 9.6) | |||||
| 200 ml agraz (blueberry) nectar daily, crossover (4 wks intervention, 4 wks washout, 4 wks intervention), DB | MetS | 40 women (47 ± 9) | TAC (AC by DPPH), TBARS, urinary 8OHdG, 8-isoP | higher TAC, lower 8OHdG (*) | [48] |
| 3 g (=3 capsules) Melissa officinalis extract/day, 8 wks, DB |
chronic stable angina pectoris |
73: | MDA, PON1 |
MDA lower (*) PON1 higher (*) |
[49] |
| 35 IG (58.8 ± 8.3) | |||||
| 38 PG (56.5 ± 8.9) | |||||
| Genistein supplementation 2 capsules/day (54 mg), 12 wks, DB |
Type 2 diabetes |
54 women (47–69) | TAC, MDA |
MDA decreased (+), TAC higher (*) |
[50] |
| 28 IG | |||||
| 36 PG | |||||
| 2x 500 mg curcumin capsules daily, 12 wks vs. placebo, DB |
β-Thalassemia |
61: | TAC, MDA, CAT, vitamin E |
MDA decreased (#), TAC increased (+) |
[51] |
| 31 IG (25.97 ± 6.92) | |||||
| 30 PG (27.61 ± 6.23) | |||||
| 30 mg crocin/day, 4 wks, DB |
Multiple sclerosis |
40: | TAC, MDA, total thiols, DNA damage |
MDA decreased in PG (+) and IG (#) DNA damage lower in PG and IG (*) | [52] |
| 20 IG (29 ± 5) | Total thiols lower only in IG (*) | ||||
| 20 PG (31.5 ± 5.3) |
TAC increased in IG (*) |
||||
| 15 mg/day gallic acid, 1 wk, crossover, DB | type 2 diabetes | 19 (66.6 ± 7.5) | MDA, FRAP, oxLDL | oxLDL reduction in IG (+) | [53] |
| 2/day, 6 months (303 mg silybin, 10 μg vitamin D, 15 mg vitamin E), 6 months additional follow-up with no intervention vs. healthy control and vs. untreated patients, NB | NAFLD | 90 (18–85) | TBARS | TBARS “improved” by 80% in treated group after 6 months (+); significance is not clear | [54] |
| 60 received treatment, 30 did not, vs. 60 healthy controls | |||||
All biomarkers measurements in blood (unless otherwise stated); men and women included (unless otherwise stated); PG (placebo group), IG (intervention group). DB (double-blind), SB (single blind), NB (not blinded); only significant results are mentioned. Significant changes are marked by: (*) vs. PG; (+) vs. baseline; (#) vs. baseline and PG.
Intervention with 250 mg pomegranate extract twice a day for eight weeks in patients with rheumatoid arthritis showed no effect on plasma MDA in the intervention group while plasma GPx activity was significantly elevated [45]. In contrast, 100 ml pomegranate juice three times a day for three weeks led to significant reduction in plasma MDA compared to the placebo group in patients undergoing HD [46]. Likewise, administration of 300 mg mulberry extract per day for twelve weeks reduced plasma MDA while increasing plasma GSH in diabetic kidney disease patients [47]. Daily administration of 200 ml blueberry nectar for four weeks significantly increased TAC while plasma TBARS and isoprostanes were not affected [48]. 3 g of Melissa officinalis extract per day for eight weeks was reported to significantly reduce plasma MDA levels in patients with chronic stable angina pectoris while increasing PON1 activity [49]. Studies concerning supplementation with isolated phytochemicals reported almost consistent beneficial results. Thus, a reduction of plasma MDA with simultaneous increase in TAC was observed for genistein supplementation in type 2 diabetic patients, after curcumin supplementation in β-thalassemia patients and after crocin supplementation in multiple sclerosis patients [[50], [51], [52]]. In a crossover study, the daily administration of 15 mg gallic acid significantly reduced plasma oxLDL levels while plasma MDA and FRAP levels were unchanged [53]. Intervention with 303 mg silybin-phospholipid twice a day for four weeks significantly reduced plasma TBARS in NAFLD patients [54].
3.2.5. Trace and bulk element supplementation
The influence of the bulk element magnesium on OS was the research objective in three intervention studies (Table 6). Magnesium was always co-supplemented with calcium, vitamin E or zinc. Individually performed intervention study on the antioxidative effect of trace elements have been performed for selenium and chromium. Duration of the interventions ranged from four to twelve weeks.
Table 6.
Trace and bulk element supplementation in patients.
| Intervention | Disease | N (age in years) | Biomarkers | Effect of intervention | Ref. |
|---|---|---|---|---|---|
| 250 mg magnesium oxide +220 mg ZnSO4 2x/day, 12 wks, DB |
PCOS |
60 women (18–40) | TAC, GSH, MDA, NO, PCs |
GSH higher (*), PCs lower (*) |
[55] |
| 30 IG | |||||
| 30 PG | |||||
| 250 mg magnesium oxide + 400 IU vitamin E/day, 12 wks, DB |
PCOS |
60 women: | TAC, GSH, MDA, NO |
TAC and NO increased (#) |
[56] |
| 30 IG (26 ± 3.7) | |||||
| 30 PG (27.2 ± 7.1) | |||||
| 100 mg magnesium, 4 mg zinc, 400 mg calcium + 200 IU vitamin D 2x/day, 12 wks, DB |
PCOS |
60 women: | TAC, GSH, MDA, NO |
MDA lower (*), TAC higher (*) |
[57] |
| 30 PG (24.8 ± 4.8) | |||||
| 30 IG (23.8 ± 5.7) | |||||
| 200 μg/day chromium, 8 wks, DB | PCOS | 40 women (18–40) | TAC, GSH, MDA | TAC higher (*), MDA lower (*) | [58] |
| 200 μg selenium yeast/day, 4 wks, DB | cardiac bypass | 33 women: | TAC, GSH, MDA, NO | GSH higher (*), MDA lower (*) | [59] |
| 17 IG (61.2 ± 4.6) | |||||
| 16 PG (62.6 ± 11.6) | |||||
All biomarkers measurements in blood (unless otherwise stated); men and women included (unless otherwise stated); PG (placebo group), IG (intervention group). DB (double-blind), SB (single blind), NB (not blinded); only significant results are mentioned. Significant changes are marked by: (*) vs. PG; (+) vs. baseline; (#) vs. baseline and PG.
Studies investigating the influence of magnesium co-supplementation on OS markers in women diagnosed with PCOS reported inconsistent changes in different biomarkers [[55], [56], [57]]. While co-supplementation of 250 magnesium oxide with 220 mg zinc sulfate twice a day for twelve weeks resulted in a significant reduction in PCs and elevated levels of plasma GSH, no effect on plasma TAC, NO and MDA was observed [55]. Whereas administration with 100 mg magnesium, 4 mg zinc, 400 mg calcium and 200 IU vitamin D3 twice a day for twelve weeks led to significant reductions in plasma MDA and increased plasma TAC, while GSH levels were unchanged [57]. Intervention with 250 mg magnesium oxide and 400 IU vitamin E improved plasma TAC and NO values in the intervention group while plasma MDA and GSH were unaffected [56]. Regarding trace elements, daily supplementation with 200 μg chromium for eight weeks significantly lowered plasma MDA levels in women with PCOS while simultaneously increasing plasma GSH levels [58]. Similar effects were observed after intervention with 200 μg/day selenium as selenium yeast for four weeks in patients undergoing coronary artery bypass grafting which significantly reduced plasma MDA levels while significantly increasing plasma GSH [59].
3.3. Intervention studies in healthy participants
3.3.1. Interventions with foodstuffs
Table 7 shows interventions in healthy participants with foodstuffs. Multiple studies investigating long- and short-term effects on green tea or mate extract consumption in healthy participants found consistent improvements in different markers of OS. Daily consumption of 450 mg green tea extract (Camellia sinensis) or sour tea extract (Hibiscus sabdariffa) for six months significantly reduced plasma MDA and increased TAC in the respective intervention groups compared to the placebo group [60]. Improvements of OS status were also reported in single-dose ingestion of 10 g matisha powder (Camellia sinensis) that resulted in significantly reduced plasma oxLDL until 6 h after intake [61]. After single consumption of ice cream containing green tea extract, increase in plasma FRAP was observed after 2 h of intake while plasma derivatives of reactive oxygen metabolites (dROM) and H2O2 were reduced [62]. Administration of 200 ml mate tea (Ilex paraguariensis) three times a day for one week significantly improved the ratio between reduced and oxidized glutathione (GSH:GSSG ratio) [63], the ingestion of 250 mg mate tea extract nine times a day for 60 days showed beneficial effects on various markers of OS including SOD, CAT, GPx and PON1 activity as well as elevated plasma GSH concentrations [64]. Daily intake of 3 g cardamom powder for twelve weeks was reported to significantly reduce plasma MDA [65].
Table 7.
Interventions with foodstuffs in healthy participants.
| Intervention | N (age in years) | Biomarkers | Effect of intervention | Ref |
|---|---|---|---|---|
| 450 mg/day green tea extract (GTE) or 450 mg/day sour tea extract (STE) vs. placebo, 6 wks, DB |
18 GTE (20.94 ± 1.43) | TAC, MDA |
MDA decreased (#) and TAC increased (#) in both GTE and STE group |
[60] |
| 18 STE (20.71 ± 1.26) | ||||
| 18 PG (21.19 ± 2.16) | ||||
| 10 g matisha powder, single-dose, NB | 17 men (20–40) | oxLDL | oxLDL reduced until 6 h after intake (+) | [61] |
| Antioxidant ice cream with green tea extract, single-dose, SB | 14 (38 ± 3) | FRAP, NOx, H2O2, dROM, polyphenols | FRAP increased, H2O2, dROM decrease 2 h after antioxidant ice cream consumption (+) | [62] |
| 200 ml mate tea 3x/day, 7 days vs. control, NB | 9 men (25 ± 3) | GSH/GSSG | Lower GSH (*) | [63] |
| 250 mg yerba mate extract 9x/day, 60 d, NB | 14 (age not mentioned) | GSH, GPx, SOD, CAT, PON1, LOOH, TBARS | Improvement in all markers (+) | [64] |
| 3 g cardamom powder intake, 12 wks, DB |
40: | TAC, MDA, SOD, PCs, GR |
MDA lower (*) |
[65] |
| 20 IG (48.3 ± 10.4) | ||||
| 20 PG 47.5 ± 10.3) | ||||
| Beetroot juice; 70 ml 2x/day, DB | 20 (60–75) | 3NT, NOx | no effect | [66] |
| 480 ml tart cherry juice/day, 12 wks, NB | 37 (65–80) | 8OHdG, MDA, 8-oxoguanine glycosylase, oxLDL, 4-HNE | no effect | [67] |
| Cranberry extract beverage 450 ml/day, 8 wks, DB |
78: (43.1 ± 1.1) | GSH/GSSG, GPx, SOD |
no effect |
[68] |
| 38: PG | ||||
| 40: IG | ||||
| 500 mg Aronia extract/day, 12 wks, DB |
49: | oxLDL, CAT, GPx, SOD |
no effect |
[69] |
| 25 IG (32.6 ± 2.6) | ||||
| 24 PG (37.4 ± 3.0) | ||||
| 600 ml berry beverage 3x/day, 5 wks, NB | 40 (63 ± 1) | oxLDL, MDA | no effect | [70] |
| Prune essence concentrates 100 ml or 50 ml or 50 ml placebo per day, 4 wks, NB |
60 (18–53): | TAC, TBARS |
no effect |
[71] |
| 20 in each group | ||||
| 750 ml anthocyanin-rich fruit juice, 8 wks, NB | 62 men (20–50) | SOD, CAT, oxLDL, urine anthocyanins | no effect | [72] |
| 500 mL of pomegranate juice once before exercise training, NB | 9 (21 ± 1) | MDA, CAT, GPx | Lower MDA, higher CAT GPX (*) | [73] |
| 200 g of açai pulp/day, 4 wks, NB | 40 women (24 ± 3) | TAC, MDA, oxLDL, PON1, ROS | oxLDL, MDA decreased, TAC, PON1 increased (+) | [74] |
| 500 ml of a fermented orange juice beverage a day, 2 wks, NB | 30 (33.9 ± 6.9) | TAC, ORAC, SOD, GPx, GR (glutathione reductase), GSH, TBARS, oxLDL | ORAC higher (*) | [75] |
| Korean black raspberry powder, 30g/day, 2 wks, NB | 102 (30–60) | MDA, GSH/GSSG, erythrocyte: CAT, SOD, GPx | Higher GSH/GSSG ratio in erythrocytes, lower plasma MDA (*) | [76] |
| 22 g freeze-dried highbush blueberry powder/day, 8 wks, DB | 40 (45–65) | TBARS, oxLDL, SOD, GPx, GR, 8-isoP, 8OHdG | short-term effect on 8OHdG, reduction after 4 wks intervention but not 8 wks (*) | [77] |
| 16.9 g in slowly digestible starch/day, 3 wks, NB | 20 (20–65) | MDA, GSH, urinary isoP | MDA increased, GSH decreased (+) | [78] |
| 70 g oat porridge/day, 4 wks, NB | 24 (30–60) | ORAC, FRAP, MDA | increased ORAC and FRAP (+) | [79] |
| Wine grape beef burger daily, 1 month, NB | 34 men (25–65) | TRAP, MDA, oxLDL, AOPPs, tocopherols, vitamin C | AOPP, oxLDL lower (*) | [80] |
| 90 g raisins/day, 4 wks, NB |
36: | MDA, AOPPs, NO |
no effect |
[81] |
| 14 PG (29.8 ± 1.4) | ||||
| 22 IG (30.8 ± 1.6) | ||||
| 100 mg seaweed/day, 8 wks, DB, crossover | 80 (30–65) | TAC, polyphenols | no effect | [82] |
All biomarkers measurements in blood (unless otherwise stated); men and women included (unless otherwise stated); PG (placebo group), IG (intervention group). DB (double-blind), SB (single blind), NB (not blinded); only significant results are mentioned. Significant changes are marked by: (*) vs. PG; (+) vs. baseline; (#) vs. baseline and PG.
Studies on the beneficial effects of polyphenol-rich juices and fruit extracts on the OS status in healthy participants came to different results. Interventions with 70 ml beetroot juice for two days [66], 480 ml tart cherry juice a day for twelve weeks [67], 450 ml cranberry extract a day for eight weeks [68], 500 mg Aronia extract a day for twelve weeks [69], 600 ml of a mixed berry beverage three times a day for five weeks [70], 100 ml prune extract per day for four weeks [71] as well as daily intake of 750 ml of an mixed anthocyanin-rich fruit juice for eight weeks [72] showed no effect on the OS status of healthy participants. In contrast, some studies reported improvements. Single-dose administration of 500 ml pomegranate juice after exercise significantly attenuated increase of plasma MDA as well as CAT and GPx activity [73]. Also, administration of 200 g acai pulp per day for four weeks resulted in a significant decrease in plasma MDA and oxLDL levels with increases in TAC and PON1 activity [74]. Intake of 250 ml fermented orange juice twice a day for two weeks increased plasma ORAC significantly while other biomarkers were unchanged [75]. Intervention with 30 g of freeze-dried black raspberry powder per day for two weeks significantly reduced plasma MDA levels and improved GSH:GSSG ratio in erythrocytes [76], but in a similar study, the ingestion of 22 g of freeze-dried highbush blueberry powder daily for eight weeks only showed short-term effects on plasma 8OHdG levels, and other markers like plasma TBARS, isoprostanes, oxLDL, SOD and GPx activity were unaffected [77].
Intake of 16.9 g of slowly digestible starch per day for three weeks showed slightly adverse effects on OS status as plasma MDA levels increased and plasma GSH levels decreased after intervention [78]. Administration of 70 g oat porridge per day for four weeks on the other hand was reported to increase plasma ORAC and FRAP, while plasma MDA levels were unchanged [79]. Intake of beef burgers prepared with wine grape pomace flour on a daily basis for one month reduced plasma oxLDL as well as advanced oxidation protein products (AOPP) after intervention [80]. No significant effect on participants redox status was observed in two studies investigation the effect of a daily intake of 90 g raisins for four weeks [81] or 100 mg sea weed for eight weeks [82].
3.3.2. Interventions with single supplements or multi-nutrient supplements
Table 8 shows interventions in healthy participants with supplements. Several studies investigated the influence of single supplements on the OS status of healthy participants while mainly focusing on supplements rich in antioxidants such as anthocyanins, flavonoids or carotenoids.
Table 8.
Interventions with single supplements or multi-nutrient supplements in healthy participants.
| Intervention | N (age in years) | Biomarkers | Effect of intervention | Ref |
|---|---|---|---|---|
| 60 mg/day anthocyanins or 6 mg lutein | 76 (50–70) |
TAC, total polyphenols, vitamin C |
TAC increased (+) |
[83] |
| +2 mg Zeaxanthin/day or both combined, NB | ||||
| 11 mg lycopene, phytoene, and phytofluene/day, 4 wks, DB |
20 | FRAP, oxLDL, 8OHdG, carotenoids |
no effect |
[84] |
| 10 M (36.8 ± 7.0) | ||||
| 10 F (37.9 ± 5.1) | ||||
| Ferulic acid 1000 mg/day, 6 wks, DB |
48: | MDA, oxLDL, dROM, BAP |
BAP increased, dROM, MDA, oxLDL decreased (#) |
[85] |
| 24 PG (45,9 ± 7,8), | ||||
| 24 IG (48.7 ± 7.6) | ||||
| 15 mg hydroxytyrosol from olive extract/day, 3 wks, DB, crossover | 28 (32.0 ± 12.2) | TAC, MDA, nitrite/nitrate, oxLDL, SOD | MDA decreased, nitrite/nitrate decreased, TAC, SOD increased (#) | [86] |
| 3.6 g α-linolenic acid + 190 mg Quercetin/day, 8 wks, DB, crossover | 67 (19–35) | retinol, α-tocopherol, β-carotene, oxLDL, flavonols | no effect | [87] |
| Eriomin (containing eriocitrin, hesperidin, and naringin) 200/400/800 mg/placebo, once every 2 wks, 12 wks, DB | 103 (49 ± 10) | TAC, MDA | MDA decreased, TAC increased (#) | [88] |
| 80 mg curcumin nanomicelle/day, 10 wks, DB |
56: | TAC, MDA |
MDA lower, TAC higher (*) |
[89] |
| 28 IG (30.54 ± 4.03) | ||||
| 85 PG (30 ± 3.96) | ||||
| 1720 mg DHA and 600 mg EPA/day, 18 months, DB |
391: | MDA |
no effect |
[90] |
| 195 IG (73.1 ± 5.4) | ||||
| 196 PG (73.1 ± 5.7) | ||||
|
l-Carnitine 1500 mg per day, 24 wks, NB |
20 (65–70) | PCs, uric acid, myeloperoxidase, trimethylamine N-oxide |
no effect |
[91] |
| 11 IG | ||||
| 9 PG | ||||
| Micronutrient pack daily, 3 months, DB | 98 (65–100) | MDA, SOD, GSH | MDA lower TAC, GSH higher (*) | [92] |
| Multivitamin/micronutrient supplement Mind Master®, 80 ml/day, 8 wks, DB |
58: | TBARS, GPx, SOD, oxLDL, PCs, urinary isoP, 8OHdG |
no effect |
[93] |
| 28 PG (32.9 ± 5.6) | ||||
| 30 IG (34.9 ± 5.8) | ||||
| Antioxidant supplement before exercise (300 mg of α-lipoic acid, 500 mg vitamin C and 200 IU vitamin E) once, DB, crossover | 19 (25.7 ± 1.2) | TBARS | no effect | [94] |
Daily supplementation with 60 mg anthocyanins or 6 mg lutein plus 2 mg zeaxanthin or a combination was reported to significantly increase plasma TAC after four and eight months of intervention in all three groups [83], while no significant effect on OS markers was found after combined intervention with 11 mg lycopene, phytoene and phytofluene per day for four weeks [84]. Intervention with ferulic acid 1000 mg/day for six weeks significantly lowered plasma MDA and oxLDL and plasma dROM while elevating biological antioxidant potential (BAP) at the same time [85]. In a similar way daily intake of 7.5 mg hydroxytyrosol was reported to lower plasma MDA while improving plasma TAC in a three-week intervention study [86]. No effect on redox status was observed after combined supplementation of 190 mg quercetin with 3.6 g α-linolenic acid a day for eight weeks [87]. A decrease in MDA and an increase in TAC were reported both for different intakes of flavonoid-rich supplement Eriomin® over twelve weeks [88] and 80 mg curcumin a day for ten weeks [89]. No effect on redox status of healthy participants was observed for supplementation with 1720 mg DHA and 600 mg EPA daily for 18 months [90] as well as daily intake of 1500 mg l-carnitine for 24 weeks [91].
Supplementations with multi-nutrient packs showed inconsistent results. While a three-month intervention with daily intake of one multi-micronutrient pack significantly decreased plasma MDA and increasing plasma TAC and GSH levels [92], eight-week intervention with daily consumption of 80 ml Mind Master® containing a mixture of vitamins and trace elements showed no effect on plasma biomarker of OS [93]. A similar observation was made after single administration of an antioxidant supplement containing vitamin C, E and α-linolenic acid where no changes in plasma MDA were observed [94].
4. Discussion
The interplay between dietary factors and lifestyle factors including exercise, sun exposure, diseases and medication and resulting biomarkers is complex. An overview of the study characteristics – diet and supplements, diseases, biomarkers – included in this review is given in Fig. 2. Recent observational studies were able to show inverse association between F/V intake and plasma markers of OS such as oxLDL and MDA. The observed effects were assigned to the antioxidative capacity of F/V's phytochemicals such as carotenoids as well as their content in insoluble fiber. Interestingly intervention studies supplementing isolated carotenoids were not able to find strong improvements in the redox status of healthy participants showing a possible advantage of consumption of whole F/V compared to isolated phytochemicals.
Intervention studies with patients showed almost uniformly positive effects on the participants redox status by supplementation with vitamin D, independently of the disease. Vitamin D was administered in a dosage of 50.000 IU every two weeks over a timespan of usually 8–12 weeks. Likewise, beneficial effects on patients redox status were reported for intervention with fruit extracts (pomegranate, mulberry), probiotic supplementation with cultures of L.casei, L.acidophilus, L.fermentum and Bifidobacterium bifidum as well as bulk element magnesium and trace elements selenium and chromium.
Consistently no effects on redox biomarkers in patients were observed for intervention studies focusing only on ω-3 fatty acids. It should be noted that the majority of the intervention studies with patients included in this review were conducted by single or collaborating research groups from Iran.
One study described their study population as under “OS conditions”. This included MetS, obesity, and diseases such as type 2 diabetes, hypertension, dyslipidemia, cardiovascular/neurovascular diseases, or diet-related cancers (liver, colon, stomach, breast, prostate, and lung) [5]. However, these diseases are heterogenous and therefore difficult to combine into one group.
Concerning study sample sizes, the majority included 100 participants or less. The largest observational study had 1229 participants [5], while the largest intervention study with healthy subjects had 391 participants [90]. Thus, this is something to keep in mind, especially when interpreting studies.
Intervention studies conducted with healthy participants were able to show positive effects on redox status by intake of tea extracts derived from Camelia sinensis and Ilex paraguariensis regardless of the duration. Observations on redox status after intake of polyphenol-rich juices and fruit extracts have been mostly inconsistent because the number of studies showing improvements and those with no effect are balanced. The same applies to multi-nutrient intervention studies with healthy participants included in this review which in general express the overriding problem that observed improvements in participant's redox status cannot be tracked down to one component and are therefore difficult to interpret.
It is encouraging that the vast majority of the studies included in this review rely on the measurement of multiple biomarkers to determine the redox status of their participants rather than focusing on one isolated biomarker as OS should not be regarded as a closed system and until now no known biomarker of OS can be viewed as a stand-alone “gold standard” [[95], [96], [97]].
For the determination of the endogenous antioxidative defense multiple studies relied on rather unspecific assays based on simple redox reactions like TRAP, FRAP, TEAC or ORAC which can be regarded as insufficient for capturing the in vivo redox state as they are heavily influenced by plasma uric acid [98] and do not account for the activity of enzymes with antioxidative properties such as SOD, CAT and GPx. While the use of assays like TRAP, FRAP, TEAC and ORAC is appropriate for in vitro applications like determination of the antioxidative potential of beverages or plant extracts they should not be used as a stand-alone marker for in vivo antioxidative capacity and at least be combined with the measurement of CAT, SOD or GPx activities [95].
Only seven studies were identified that analyzed blood biomarkers of every category: exogenous antioxidants, endogenous antioxidants and OS (see Fig. 3). Four of these were intervention studies with healthy participants [62,72,80,84], two were interventions in patients [[27], [51]]. One observation study with healthy participants also used all three categories [6] but again, measurement of endogenous antioxidants was based on TAC, FOX2 (ferrous oxidation-xylenol orange 2) and dietary TAC, thus limiting results. Of the 7 studies four studies did not only rely on TAC/FRAP/ORAC assays as endogenous antioxidant measure [[21], [51], [62], [72]].
One should keep in mind, that some biomarkers should only be analyzed in specific specimen, for instance, the GSH:GSSG ratio should be measured in whole blood or red blood cells as GSH is a major intracellular non-protein thiol whose concentration is known to be strongly affected by hemolysis of plasma/serum samples as well as storage time [99] while MDA, due to the autoxidation during agglutination is best measured in plasma. Thus, the sample type must be considered when carrying out and interpreting human studies.
The most commonly used biomarkers in the selected studies were TAC, MDA and GSH. These are relatively easy methods but are limited in informative value and come with limitations. Again, many of the intervention studies came from the same research group from Iran, thus applying similar study protocols and similar sample sizes.
5. Conclusion
Current observational studies strengthen the already known beneficial effects of a sufficient intake of F/V by showing inverse association between F/V intake and markers of OS. Nevertheless, the significance of these findings could be improved by conducting observational studies with larger study populations and using biomarkers of all three categories. Generally, it should be possible when taking a blood sample, to split the sample and use it for different assays. Nutrients which have been reported to modulate the redox status in a positive way include vitamin D and E, magnesium, zinc, chromium and selenium as well as probiotic supplementation and several phytochemicals. Regarding foodstuffs, the intake of extracts derived from Camelia sinensis and Ilex paraguariensis were shown to improve markers of OS. Larger study populations and using biomarkers of all three categories could improve meaningfulness of studies and allow better comparison of studies.
Overall, we recommend that researchers look for alternatives to TAC to analyze endogenous antioxidant status, such as GHS:GSSH ratio. Our recommendations are summarized in an Expert Opinion Box. As we are at the edge of a true demographic explosion, it would be very useful, if scientists were be able to include high-quality studies in future reviews based on these criteria.
Expert Opinion Box
Human observational studies on redox biomarkers lose interpretability if they:
-
•
do not involve a broad spectrum of both biomarker categories: antioxidants and damage biomarkers
-
•
only use total antioxidant capacity (TAC) measures or general, highly sensitive, unstable or poorly specific biomarkers
-
•
do not include a rigorous assessment of the participants, especially age and sex
-
•
do not include any parameters associated with biomarkers and health condition (lifestyle habits and season for healthy individuals and disease-specific markers for patients)
-
•
do not analyze results in the context of overall lifestyle habits and comorbidities (particularly relevant as the world's population is rapidly expanding and at disease risk, i.e. persons aged 80 years and older, or multimorbid)
Human intervention studies on redox biomarkers may lose interpretability if they:
-
•
use supplements with single compounds unless there is a specific research question
-
•
use a mixture of substances independent from lifestyle in general and natural nutrition in particular, unless there is a specific biochemical reason
Furthermore, biomarkers from all three categories should be used. Ideally, these biomarkers would be measured with standard protocols, in the appropriate sample (serum/plasma/whole blood) and units would be published in comparable manner (i.e. units vs. change % etc.).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 2.Sies H. Biochemistry of oxidative stress. Angew Chem. Int. Ed. Engl. 1986;25(12):1058–1071. [Google Scholar]
- 3.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21(7):363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A. Advanced glycation end products and protein carbonyl levels in plasma reveal sex-specific differences in Parkinson's and Alzheimer's disease. Redox Biol. 2020;34:101546. doi: 10.1016/j.redox.2020.101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y. Combination of diet quality Score, plasma carotenoids, and lipid peroxidation to monitor oxidative stress. Oxid. Med. Cell Longev. 2018;2018:8601028. doi: 10.1155/2018/8601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacchetti T. Relationship of fruit and vegetable intake to dietary antioxidant capacity and markers of oxidative stress: a sex-related study. Nutrition. 2019;61:164–172. doi: 10.1016/j.nut.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Zhang P., Omaye S.T. Antioxidant and prooxidant roles for beta-carotene, alpha-tocopherol and ascorbic acid in human lung cells. Toxicol. Vitro. 2001;15(1):13–24. doi: 10.1016/s0887-2333(00)00054-0. [DOI] [PubMed] [Google Scholar]
- 8.Block G. The effect of vitamins C and E on biomarkers of oxidative stress depends on baseline level. Free Radic. Biol. Med. 2008;45(4):377–384. doi: 10.1016/j.freeradbiomed.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Navarro T. Different intestinal microbial profile in over-weight and obese subjects consuming a diet with low content of fiber and antioxidants. Nutrients. 2017;9(6) doi: 10.3390/nu9060551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arikawa A.Y. Plasma F2-isoprostanes are positively associated with glycemic load, but inversely associated with dietary polyunsaturated fatty acids and insoluble fiber in postmenopausal women. J. Nutr. 2017;147(9):1693–1699. doi: 10.3945/jn.117.254631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aranda N. Consumption of seafood and its estimated heavy metals are associated with lipid profile and oxidative lipid damage on healthy adults from a Spanish Mediterranean area: a cross-sectional study. Environ. Res. 2017;156:644–651. doi: 10.1016/j.envres.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 12.Corina A. Low intake of vitamin E accelerates cellular aging in patients with established cardiovascular disease: the CORDIOPREV study. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74(6):770–777. doi: 10.1093/gerona/gly195. [DOI] [PubMed] [Google Scholar]
- 13.Morais J.B.S. Magnesium status and its association with oxidative stress in obese women. Biol. Trace Elem. Res. 2017;175(2):306–311. doi: 10.1007/s12011-016-0797-x. [DOI] [PubMed] [Google Scholar]
- 14.Malek Rivan N.F. Cognitive frailty among Malaysian older adults: baseline findings from the LRGS TUA cohort study. Clin. Interv. Aging. 2019;14:1343–1352. doi: 10.2147/CIA.S211027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva R.E. Potential role of nutrient intake and malnutrition as predictors of uremic oxidative toxicity in patients with end-stage renal disease. Oxid. Med. Cell Longev. 2019;2019:7463412. doi: 10.1155/2019/7463412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos Epifanio A.P. Metabolic, inflammatory and oxidative stress markers in the nitric oxide variation of hemodialysis subjects. Nutr. Hosp. 2018;35(1):176–184. doi: 10.20960/nh.1319. [DOI] [PubMed] [Google Scholar]
- 17.Nasri K. The effects of vitamin D and evening primrose oil co-supplementation on lipid profiles and biomarkers of oxidative stress in vitamin D-deficient women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Endocr. Res. 2018;43(1):1–10. doi: 10.1080/07435800.2017.1346661. [DOI] [PubMed] [Google Scholar]
- 18.Maktabi M., Chamani M., Asemi Z. The effects of vitamin D supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm. Metab. Res. 2017;49(7):493–498. doi: 10.1055/s-0043-107242. [DOI] [PubMed] [Google Scholar]
- 19.Jamilian M. The influences of vitamin D and omega-3 co-supplementation on clinical, metabolic and genetic parameters in women with polycystic ovary syndrome. J. Affect. Disord. 2018;238:32–38. doi: 10.1016/j.jad.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Razzaghi R. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J. Diabet. Complicat. 2017;31(4):766–772. doi: 10.1016/j.jdiacomp.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Barzegari M. The effects of vitamin D supplementation on lipid profiles and oxidative indices among diabetic nephropathy patients with marginal vitamin D status. Diabetes Metab. Syndr. 2019;13(1):542–547. doi: 10.1016/j.dsx.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Amani R. Vitamin D3 induced decrease in IL-17 and malondialdehyde, and increase in IL-10 and total antioxidant capacity levels in patients with irritable bowel syndrome. Iran J. Immunol. 2018;15(3):186–196. doi: 10.22034/IJI.2018.39388. [DOI] [PubMed] [Google Scholar]
- 23.Farrokhian A. Long-term vitamin D supplementation affects metabolic status in vitamin D-deficient type 2 diabetic patients with coronary artery disease. J. Nutr. 2017;147(3):384–389. doi: 10.3945/jn.116.242008. [DOI] [PubMed] [Google Scholar]
- 24.Tamadon M.R. Clinical trial on the effects of vitamin D supplementation on metabolic profiles in diabetic hemodialysis. Horm. Metab. Res. 2018;50(1):50–55. doi: 10.1055/s-0043-119221. [DOI] [PubMed] [Google Scholar]
- 25.Kouchaki E. High-dose omega-3 fatty acid plus vitamin D3 supplementation affects clinical symptoms and metabolic status of patients with multiple sclerosis: a randomized controlled clinical trial. J. Nutr. 2018;148(8):1380–1386. doi: 10.1093/jn/nxy116. [DOI] [PubMed] [Google Scholar]
- 26.Ghaderi A. Clinical trial of the effects of vitamin D supplementation on psychological symptoms and metabolic profiles in maintenance methadone treatment patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;79(Pt B):84–89. doi: 10.1016/j.pnpbp.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Lopes R. Evaluation of the health benefits of consumption of extruded tannin sorghum with unfermented probiotic milk in individuals with chronic kidney disease. Food Res. Int. 2018;107:629–638. doi: 10.1016/j.foodres.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Miraghajani M. The impact of probiotic soy milk consumption on oxidative stress among type 2 diabetic kidney disease patients: a randomized controlled clinical trial. J. Ren. Nutr. 2017;27(5):317–324. doi: 10.1053/j.jrn.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y. Yogurt improves insulin resistance and liver fat in obese women with nonalcoholic fatty liver disease and metabolic syndrome: a randomized controlled trial. Am. J. Clin. Nutr. 2019;109(6):1611–1619. doi: 10.1093/ajcn/nqy358. [DOI] [PubMed] [Google Scholar]
- 30.Soleimani A. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017;91(2):435–442. doi: 10.1016/j.kint.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 31.Kooshki A., Tofighiyan T., Miri M. A synbiotic supplement for inflammation and oxidative stress and lipid abnormalities in hemodialysis patients. Hemodial. Int. 2019;23(2):254–260. doi: 10.1111/hdi.12748. [DOI] [PubMed] [Google Scholar]
- 32.Mafi A. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food Funct. 2018;9(9):4763–4770. doi: 10.1039/c8fo00888d. [DOI] [PubMed] [Google Scholar]
- 33.Farrokhian A. The effects of synbiotic supplementation on carotid intima-media thickness, biomarkers of inflammation, and oxidative stress in people with overweight, diabetes, and coronary heart disease: a randomized, double-blind, placebo-controlled trial. Probiotics Antimicrob. Proteins. 2019;11(1):133–142. doi: 10.1007/s12602-017-9343-1. [DOI] [PubMed] [Google Scholar]
- 34.Kouchaki E. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2017;36(5):1245–1249. doi: 10.1016/j.clnu.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Mohseni S. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Diabetes Metab. Res. Rev. 2018;34(3) doi: 10.1002/dmrr.2970. [DOI] [PubMed] [Google Scholar]
- 36.Karamali M. Effects of probiotic supplementation on hormonal profiles, biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Arch. Iran. Med. 2018;21(1):1–7. [PubMed] [Google Scholar]
- 37.Mirhashemi S.M. Metabolic response to omega-3 fatty acids and vitamin E Co-supplementation in patients with fibrocystic breast disease: a randomized, double-blind, placebo-controlled trial. Arch. Iran. Med. 2017;20(8):466–473. [PubMed] [Google Scholar]
- 38.Soleimani A. Metabolic response to omega-3 fatty acid supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2017;36(1):79–84. doi: 10.1016/j.clnu.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Rahmani E. The effects of omega-3 fatty acids and vitamin E co-supplementation on gene expression of lipoprotein(a) and oxidized low-density lipoprotein, lipid profiles and biomarkers of oxidative stress in patients with polycystic ovary syndrome. Mol. Cell. Endocrinol. 2017;439:247–255. doi: 10.1016/j.mce.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Mirmasoumi G. The effects of flaxseed oil omega-3 fatty acids supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp. Clin. Endocrinol. Diabetes. 2018;126(4):222–228. doi: 10.1055/s-0043-119751. [DOI] [PubMed] [Google Scholar]
- 41.Fayh A.P.T. Effects of n-3 fatty acids and exercise on oxidative stress parameters in type 2 diabetic: a randomized clinical trial. J. Int. Soc. Sports Nutr. 2018;15:18. doi: 10.1186/s12970-018-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashemi Z. The effects of vitamin E supplementation on endometrial thickness, and gene expression of vascular endothelial growth factor and inflammatory cytokines among women with implantation failure. J. Matern. Fetal Neonatal Med. 2019;32(1):95–102. doi: 10.1080/14767058.2017.1372413. [DOI] [PubMed] [Google Scholar]
- 43.Aghadavod E. Effects of high-dose vitamin E supplementation on markers of cardiometabolic risk and oxidative stress in patients with diabetic nephropathy: a randomized double-blinded controlled trial. Iran J. Kidney Dis. 2018;12(3):156–162. [PubMed] [Google Scholar]
- 44.Babajafari S. The effect of isolated soy protein adjunctive with flaxseed oil on markers of inflammation, oxidative stress, acute phase proteins, and wound healing of burn patients; a randomized clinical trial. Burns. 2018;44(1):140–149. doi: 10.1016/j.burns.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Ghavipour M. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients. Eur. J. Clin. Nutr. 2017;71(1):92–96. doi: 10.1038/ejcn.2016.151. [DOI] [PubMed] [Google Scholar]
- 46.Barati Boldaji R. Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: a randomized crossover trial. J. Sci. Food Agric. 2020;100(2):846–854. doi: 10.1002/jsfa.10096. [DOI] [PubMed] [Google Scholar]
- 47.Taghizadeh M. Metabolic response to mulberry extract supplementation in patients with diabetic nephropathy: a randomized controlled trial. Iran J. Kidney Dis. 2017;11(6):438–446. [PubMed] [Google Scholar]
- 48.Espinosa-Moncada J. Evaluation of agraz consumption on adipocytokines, inflammation, and oxidative stress markers in women with metabolic syndrome. Nutrients. 2018;10(11) doi: 10.3390/nu10111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Javid A.Z. The effects of Melissa officinalis (lemon balm) in chronic stable angina on serum biomarkers of oxidative stress, inflammation and lipid profile. Asia Pac. J. Clin. Nutr. 2018;27(4):785–791. doi: 10.6133/apjcn.022018.01. [DOI] [PubMed] [Google Scholar]
- 50.Braxas H. Effectiveness of genistein supplementation on metabolic factors and antioxidant status in postmenopausal women with type 2 diabetes mellitus. Can. J. Diabetes. 2019;43(7):490–497. doi: 10.1016/j.jcjd.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Nasseri E. Benefits of curcumin supplementation on antioxidant status in beta-thalassemia major patients: a double-blind randomized controlled clinical trial. Ann. Nutr. Metab. 2017;71(3–4):136–144. doi: 10.1159/000479634. [DOI] [PubMed] [Google Scholar]
- 52.Ghiasian M. Effects of crocin in reducing DNA damage, inflammation, and oxidative stress in multiple sclerosis patients: a double-blind, randomized, and placebo-controlled trial. J. Biochem. Mol. Toxicol. 2019;33(12) doi: 10.1002/jbt.22410. [DOI] [PubMed] [Google Scholar]
- 53.Ferk F. Gallic acid improves health-associated biochemical parameters and prevents oxidative damage of DNA in type 2 diabetes patients: results of a placebo-controlled pilot study. Mol. Nutr. Food Res. 2018;62(4) doi: 10.1002/mnfr.201700482. [DOI] [PubMed] [Google Scholar]
- 54.Federico A. Evaluation of the effect derived from silybin with vitamin D and vitamin E administration on clinical, metabolic, endothelial dysfunction, oxidative stress parameters, and serological worsening markers in nonalcoholic fatty liver disease patients. Oxid. Med. Cell Longev. 2019;2019:8742075. doi: 10.1155/2019/8742075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Afshar Ebrahimi F. The effects of magnesium and zinc Co-supplementation on biomarkers of inflammation and oxidative stress, and gene expression related to inflammation in polycystic ovary syndrome: a randomized controlled clinical trial. Biol. Trace Elem. Res. 2018;184(2):300–307. doi: 10.1007/s12011-017-1198-5. [DOI] [PubMed] [Google Scholar]
- 56.Shokrpour M., Asemi Z. The effects of magnesium and vitamin E Co-supplementation on hormonal status and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. Biol. Trace Elem. Res. 2019;191(1):54–60. doi: 10.1007/s12011-018-1602-9. [DOI] [PubMed] [Google Scholar]
- 57.Maktabi M., Jamilian M., Asemi Z. Magnesium-zinc-calcium-vitamin D Co-supplementation improves hormonal profiles, biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res. 2018;182(1):21–28. doi: 10.1007/s12011-017-1085-0. [DOI] [PubMed] [Google Scholar]
- 58.Jamilian M. The influences of chromium supplementation on glycemic control, markers of cardio-metabolic risk, and oxidative stress in infertile polycystic ovary syndrome women candidate for in vitro fertilization: a randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res. 2018;185(1):48–55. doi: 10.1007/s12011-017-1236-3. [DOI] [PubMed] [Google Scholar]
- 59.Kamali A., Amirani E., Asemi Z. Effects of selenium supplementation on metabolic status in patients undergoing for coronary artery bypass grafting (CABG) surgery: a randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res. 2019;191(2):331–337. doi: 10.1007/s12011-019-1636-7. [DOI] [PubMed] [Google Scholar]
- 60.Hadi A. The effect of green tea and sour tea (Hibiscus sabdariffa L.) supplementation on oxidative stress and muscle damage in athletes. J. Diet. Suppl. 2017;14(3):346–357. doi: 10.1080/19390211.2016.1237400. [DOI] [PubMed] [Google Scholar]
- 61.Papada E. Bioavailability of terpenes and postprandial effect on human antioxidant potential. An open-label study in healthy subjects. Mol. Nutr. Food Res. 2018;62(3) doi: 10.1002/mnfr.201700751. [DOI] [PubMed] [Google Scholar]
- 62.Sanguigni V. Natural antioxidant ice cream acutely reduces oxidative stress and improves vascular function and physical performance in healthy individuals. Nutrition. 2017;33:225–233. doi: 10.1016/j.nut.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Panza V.P. Effect of mate tea (Ilex paraguariensis) on the expression of the leukocyte NADPH oxidase subunit p47(phox) and on circulating inflammatory cytokines in healthy men: a pilot study. Int. J. Food Sci. Nutr. 2019;70(2):212–221. doi: 10.1080/09637486.2018.1486393. [DOI] [PubMed] [Google Scholar]
- 64.Becker A.M. Spray-dried yerba mate extract capsules: clinical evaluation and antioxidant potential in healthy individuals. Plant Foods Hum. Nutr. 2019;74(4):495–500. doi: 10.1007/s11130-019-00764-4. [DOI] [PubMed] [Google Scholar]
- 65.Kazemi S. Cardamom supplementation improves inflammatory and oxidative stress biomarkers in hyperlipidemic, overweight, and obese pre-diabetic women: a randomized double-blind clinical trial. J. Sci. Food Agric. 2017;97(15):5296–5301. doi: 10.1002/jsfa.8414. [DOI] [PubMed] [Google Scholar]
- 66.Oggioni C. Dietary nitrate does not modify blood pressure and cardiac output at rest and during exercise in older adults: a randomised cross-over study. Int. J. Food Sci. Nutr. 2018;69(1):74–83. doi: 10.1080/09637486.2017.1328666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chai S.C. Effects of tart cherry juice on biomarkers of inflammation and oxidative stress in older adults. Nutrients. 2019;11(2) doi: 10.3390/nu11020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chew B. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: a randomized controlled trial. Eur. J. Nutr. 2019;58(3):1223–1235. doi: 10.1007/s00394-018-1643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie L. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: a randomized controlled trial. Nutr. Res. 2017;37:67–77. doi: 10.1016/j.nutres.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Nilsson A. Effects of a mixed berry beverage on cognitive functions and cardiometabolic risk markers; A randomized cross-over study in healthy older adults. PloS One. 2017;12(11) doi: 10.1371/journal.pone.0188173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiu H.F. Regulatory/modulatory effect of prune essence concentrate on intestinal function and blood lipids. Pharm. Biol. 2017;55(1):974–979. doi: 10.1080/13880209.2017.1285323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bakuradze T. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radic. Res. 2019;53(sup1):1045–1055. doi: 10.1080/10715762.2019.1618851. [DOI] [PubMed] [Google Scholar]
- 73.Ammar A. Effects of pomegranate juice supplementation on oxidative stress biomarkers following weightlifting exercise. Nutrients. 2017;9(8) doi: 10.3390/nu9080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pala D. Acai (Euterpe oleracea Mart.) dietary intake affects plasma lipids, apolipoproteins, cholesteryl ester transfer to high-density lipoprotein and redox metabolism: a prospective study in women. Clin. Nutr. 2018;37(2):618–623. doi: 10.1016/j.clnu.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 75.Escudero-Lopez B. Consumption of orange fermented beverage improves antioxidant status and reduces peroxidation lipid and inflammatory markers in healthy humans. J. Sci. Food Agric. 2018;98(7):2777–2786. doi: 10.1002/jsfa.8774. [DOI] [PubMed] [Google Scholar]
- 76.Kim Y.J. Integration of traditional and metabolomics biomarkers identifies prognostic metabolites for predicting responsiveness to nutritional intervention against oxidative stress and inflammation. Nutrients. 2017;9(3) doi: 10.3390/nu9030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson S.A. Effects of daily blueberry consumption on circulating biomarkers of oxidative stress, inflammation, and antioxidant defense in postmenopausal women with pre- and stage 1-hypertension: a randomized controlled trial. Food Funct. 2017;8(1):372–380. doi: 10.1039/c6fo01216g. [DOI] [PubMed] [Google Scholar]
- 78.Lambert-Porcheron S. Modulation of starch digestibility in breakfast cereals consumed by subjects with metabolic risk: impact on markers of oxidative stress and inflammation during fasting and the postprandial period. Mol. Nutr. Food Res. 2017;61(12) doi: 10.1002/mnfr.201700212. [DOI] [PubMed] [Google Scholar]
- 79.Pavadhgul P. Oat porridge consumption alleviates markers of inflammation and oxidative stress in hypercholesterolemic adults. Asia Pac. J. Clin. Nutr. 2019;28(2):260–265. doi: 10.6133/apjcn.201906_28(2).0008. [DOI] [PubMed] [Google Scholar]
- 80.Urquiaga I. The consumption of beef burgers prepared with wine grape pomace flour improves fasting glucose, plasma antioxidant levels, and oxidative damage markers in humans: a controlled trial. Nutrients. 2018;10(10) doi: 10.3390/nu10101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanellos P.T. The effect of raisins on biomarkers of endothelial function and oxidant damage; an open-label and randomized controlled intervention. Food Res. Int. 2017;102:674–680. doi: 10.1016/j.foodres.2017.09.061. [DOI] [PubMed] [Google Scholar]
- 82.Baldrick F.R. Impact of a (poly)phenol-rich extract from the brown algae Ascophyllum nodosum on DNA damage and antioxidant activity in an overweight or obese population: a randomized controlled trial. Am. J. Clin. Nutr. 2018;108(4):688–700. doi: 10.1093/ajcn/nqy147. [DOI] [PubMed] [Google Scholar]
- 83.Estevez-Santiago R. Lack of a synergistic effect on cardiometabolic and redox markers in a dietary supplementation with anthocyanins and xanthophylls in postmenopausal women. Nutrients. 2019;11(7) doi: 10.3390/nu11071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nieman D.C. Effect of 4-week ingestion of tomato-based carotenoids on exercise-induced inflammation, muscle damage, and oxidative stress in endurance runners. Int J Sport Nutr Exerc Metab. 2018;28(3):266–273. doi: 10.1123/ijsnem.2017-0272. [DOI] [PubMed] [Google Scholar]
- 85.Bumrungpert A. Ferulic acid supplementation improves lipid profiles, oxidative stress, and inflammatory status in hyperlipidemic subjects: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2018;10(6) doi: 10.3390/nu10060713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Colica C. Antioxidant effects of a hydroxytyrosol-based pharmaceutical formulation on body composition, metabolic state, and gene expression: a randomized double-blinded, placebo-controlled crossover trial. Oxid. Med. Cell Longev. 2017;2017:2473495. doi: 10.1155/2017/2473495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burak C. Effect of alpha-linolenic acid in combination with the flavonol quercetin on markers of cardiovascular disease risk in healthy, non-obese adults: a randomized, double-blinded placebo-controlled crossover trial. Nutrition. 2019;58:47–56. doi: 10.1016/j.nut.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 88.Ribeiro C.B. Effectiveness of Eriomin(R) in managing hyperglycemia and reversal of prediabetes condition: a double-blind, randomized, controlled study. Phytother Res. 2019;33(7):1921–1933. doi: 10.1002/ptr.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alizadeh F. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: a randomized clinical trial. Phytother Res. 2018;32(3):514–521. doi: 10.1002/ptr.5998. [DOI] [PubMed] [Google Scholar]
- 90.Danthiir V. An 18-mo randomized, double-blind, placebo-controlled trial of DHA-rich fish oil to prevent age-related cognitive decline in cognitively normal older adults. Am. J. Clin. Nutr. 2018;107(5):754–762. doi: 10.1093/ajcn/nqx077. [DOI] [PubMed] [Google Scholar]
- 91.Olek R.A. Increased trimethylamine N-oxide is not associated with oxidative stress markers in healthy aged women. Oxid. Med. Cell Longev. 2019;2019:6247169. doi: 10.1155/2019/6247169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ren Q. Effect of micronutrient pack on micronutrient status and antioxidant capacities among institutional older adults in Shanghai, China. Asia Pac. J. Clin. Nutr. 2019;28(3):457–466. doi: 10.6133/apjcn.201909_28(3).0005. [DOI] [PubMed] [Google Scholar]
- 93.Fragopoulou E. Suppression of DNA/RNA and protein oxidation by dietary supplement which contains plant extracts and vitamins: a randomized, double-blind, placebo-controlled trial. Lipids Health Dis. 2018;17(1):187. doi: 10.1186/s12944-018-0836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kappus R.M. No evidence of racial differences in endothelial function and exercise blood flow in young, healthy males following acute antioxidant supplementation. Int. J. Sports Med. 2017;38(3):193–200. doi: 10.1055/s-0042-119203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sies H. Total antioxidant capacity: appraisal of a concept. J. Nutr. 2007;137(6):1493–1495. doi: 10.1093/jn/137.6.1493. [DOI] [PubMed] [Google Scholar]
- 96.Frijhoff J. Clinical relevance of biomarkers of oxidative stress. Antioxidants Redox Signal. 2015;23(14):1144–1170. doi: 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kochlik B., Grune T., Weber D. New findings of oxidative stress biomarkers in nutritional research. Curr. Opin. Clin. Nutr. Metab. Care. 2017;20(5):349–359. doi: 10.1097/MCO.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 98.Lotito S.B., Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006;41(12):1727–1746. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 99.Giustarini D. Interference of plasmatic reduced glutathione and hemolysis on glutathione disulfide levels in human blood. Free Radic. Res. 2004;38(10):1101–1106. doi: 10.1080/10715760400008854. [DOI] [PubMed] [Google Scholar]