Abstract
Background
This meta-analysis on peripheral blood compounds in drug-naïve first-episode patients with either schizophrenia or major depressive disorder (MDD) examined which compounds change following psychopharmacological treatment.
Methods
The Embase, PubMed and PsycINFO databases were systematically searched for longitudinal studies reporting measurements of blood compounds in drug-naïve first-episode schizophrenia or MDD.
Results
For this random-effects meta-analysis, we retrieved a total of 31 studies comprising 1818 schizophrenia patients, and 14 studies comprising 469 MDD patients. Brain-derived neurotrophic factor (BDNF) increased following treatment in schizophrenia (Hedges' g (g): 0.55; 95% confidence interval (CI) 0.39–0.70; p < 0.001) and MDD (g: 0.51; CI 0.06–0.96; p = 0.027). Interleukin (IL)-6 levels decreased in schizophrenia (g: −0.48; CI −0.85 to −0.11; p = 0.011), and for MDD a trend of decreased IL-6 levels was observed (g: −0.39; CI −0.87 to 0.09; p = 0.115). Tumor necrosis factor alpha (TNFα) also decreased in schizophrenia (g: −0.34; CI −0.68 to −0.01; p = 0.047) and in MDD (g: −1.02; CI −1.79 to −0.25; p = 0.009). Fasting glucose levels increased only in schizophrenia (g: 0.26; CI 0.07–0.44; p = 0.007), but not in MDD. No changes were found for C-reactive protein, IL-1β, IL-2 and IL-4.
Conclusions
Psychopharmacological treatment has modulating effects on BDNF and TNFα in drug-naïve first-episode patients with either schizophrenia or MDD. These findings support efforts for further research into transdiagnostic preventive strategies and augmentation therapy for those with immune dysfunctions.
Key words: Cytokine, drug-naïve, first-episode, glucose metabolism, growth factors, immune system, major depressive disorder, neuroinflammation, psychopharmacological treatment, schizophrenia
Introduction
Historically, schizophrenia and major depressive disorder (MDD) have been regarded as separate clinical disorders, based on their distinct clinical presentation and course of the disorder. An obvious clinical argument for their distinction is the fact that both disorders are treated with pharmacologically distinct classes of therapeutics, i.e. dopamine antagonists for schizophrenia and enhancers of serotonin and/or noradrenalin activity for MDD. However, overlapping symptoms are observed such as psychotic or mood symptoms, apathy and cognitive impairment (Hill et al., 2009). Moreover, environmental and genetic risk factors are shared across both disorders (Buckholtz & Meyer-Lindenberg, 2012).
Genetic association studies have shown that patients with schizophrenia or MDD may have an immune system more prone to being activated, as expressed by major histocompatibility complex molecules (Debnath, Cannon, & Venkatasubramanian, 2013), its enhancers (Mokhtari & Lachman, 2016; Sekar et al., 2016) and complement factor 4 (Sekar et al., 2016) in schizophrenia, and associations between polymorphisms in immune genes and MDD (Barnes, Mondelli, & Pariante, 2017). Environmental stressors that activate the immune system in both disorders such as infection, trauma, stress or drug abuse, may put components of the immune system of the brain (i.e. microglia) in an altered state of activity (Barnes et al., 2017; Buckholtz & Meyer-Lindenberg, 2012; Fineberg & Ellman, 2013; Kahn et al., 2015; Lowe, Sasiadek, Coles, & George, 2019). As a result, microglia and other glia may reduce their neurotrophic function and produce less growth factors, such as brain-derived neurotrophic factor (BDNF) involved in neuroplasticity, leading to decreased neuron proliferation, resulting in reduced connectivity (Brites & Fernandes, 2015; Chew, Fusar-Poli, & Schmitz, 2013). Peripheral neurotoxic inflammatory factors may be increased by neuroinflammation (Drexhage et al., 2011; Monji, Kato, & Kanba, 2009). There is meta-analytic evidence that peripheral cytokines are altered in psychosis (Pillinger, D'Ambrosio, McCutcheon, & O, 2018a, 2018b) and in depression (Kohler et al., 2017). Meta-analytic evidence has also shown that patients with first-episode psychosis have an altered glucose homeostasis at disease onset (Greenhalgh et al., 2017; Kucukgoncu et al., 2019; Perry, McIntosh, Weich, Singh, & Rees, 2016; Pillinger et al., 2017), and that MDD increases the risks of hyperglycemia, insulin resistance and type 2 diabetes (Kan et al., 2013; Mezuk, Eaton, Albrecht, & Golden, 2008). Shared underlying factors may be responsible for the co-occurrence of immune and glucose metabolism disturbances often observed in schizophrenia and MDD (Bernardi, Marcuzzi, Piscianz, Tommasini, & Fabris, 2018; Milaneschi, Lamers, Berk, & Penninx, 2020; Nishiyama, Fujimoto, Takeuchi, & Azuma, 2018; Steiner et al., 2014).
Therefore, in recent years there has been an increasing interest in the transdiagnostic aspects of major psychiatric disorders (Van Os, Linscott, Myin-Germeys, Delespaul, & Krabbendam, 2009; Wigman et al., 2015). One of these aspects may be the effect of psychopharmacological treatment on peripheral blood compounds, as both antipsychotic and antidepressant treatment seem to have immunomodulatory properties (Capuzzi, Bartoli, Crocamo, Clerici, & Carrà, 2017; Köhler et al., 2018; Miller, Buckley, Seabolt, Mellor, & Kirkpatrick, 2011; Strawbridge et al., 2015; Wang et al., 2019). Psychopharmacological treatment also seems to have modulating effects on BDNF in schizophrenia and MDD (Fernandes et al., 2015; Sen, Duman, & Sanacora, 2008).
However, most studies report serum or plasma measurements of patients who already use psychotropic medication. Therefore, prior treatment, chronic illness or other factors associated with prolonged illness duration, such as social decline and poor lifestyle habits, could affect these measurements. Also, the similarity of altered peripheral blood compounds before and after treatment between schizophrenia and MDD has been sparsely explored. A better understanding of the underlying pathophysiology of both psychiatric disorders is mandatory in order to plan for potential broad preventive strategies and treatment regarding immune and metabolic dysfunctions prevalent in these disorders.
In the current meta-analysis, we aimed to answer the following question: which peripheral growth, immune or glucose metabolism compounds change following psychopharmacological treatment in drug-naïve first-episode patients with schizophrenia or MDD? Moreover, we aimed to answer the question whether these changes in compounds following treatment in schizophrenia or MDD are similar or dissimilar in direction and/or magnitude.
Methods
Literature search
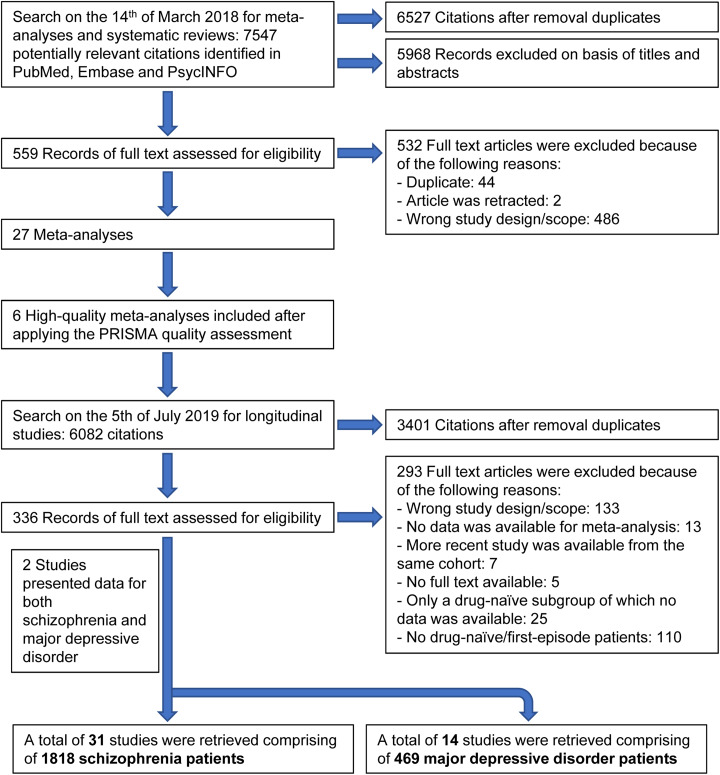
The literature was systematically reviewed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses and Meta-analysis of Observational Studies in Epidemiology guidelines (online Supplementary Tables S1 and S2) (Moher, Liberati, Tetzlaff, & Altman, 2009; Stroup et al., 2000). Two independent investigators (N. Ç. and N. v. B.) systematically searched the Embase, PubMed and PsycINFO databases from inception to the 5th of July 2019, without language restrictions. Details of the search strategy are provided in the online Supplementary Table S3 and Fig. 1. Finally, blood compounds were included if at least two studies were available investigating the same blood compound.
Fig. 1.
Study selection.
Inclusion criteria
Inclusion criteria were (1) longitudinal studies presenting peripheral blood compound measurements before and after antipsychotic treatment in schizophrenia or antidepressant treatment in MDD; (2) a diagnosis of schizophrenia, schizophreniform disorder or MDD according to the Diagnostic and Statistical Manual of Mental Disorders or International Statistical Classification of Diseases; (3) growth and immune compounds; (4) glucose homeostasis compounds under fasting conditions; (5) antipsychotic-naïve patients with schizophrenia and antidepressant-naïve patients with MDD; (6) first-episode of schizophrenia or MDD; (7) studies reported information to calculate common effect size statistics of change scores, i.e. means and standard deviations before and after treatment; exact p, t or z values; or corresponding authors could supply these data upon request.
Data extraction and processing
Two authors (N. Ç. and N. v. B.) independently extracted data from the retrieved longitudinal studies. We used data of the last observation carried forward analysis and data of completer analyses when provided. If multiple publications from the same cohort were available, we extracted data from the largest and/or most recent dataset.
In a sensitivity analysis we re-ran our analyses including only high-quality studies as assessed with the Newcastle-Ottawa quality assessment scale (NOS) (Wells et al., 2009) for which all studies should meet at least two-thirds of the NOS criteria, implying a cut-off score of 6.
Statistical analysis
We included a blood compound for analysis if at least two studies reported measurements on the respective compound. Standardized mean differences were calculated, represented as Hedges' g (g), using a random effects model. We assessed heterogeneity across studies using the Cochran Q statistic (Bowden, Tierney, Copas, & Burdett, 2011). Inconsistency across studies was assessed with the I2 statistic, assigning adjectives of low, moderate and high heterogeneity to I2 values of 0 to <25%, 25–75% and >75%, respectively. (Higgins, Thompson, Deeks, & Altman, 2003). Potential publication bias was assessed using the Egger test of the intercept if 10 or more studies were analyzed for the same compound and represented diagrammatically with funnel plots (online Supplementary Fig. S1) (Egger, Davey Smith, Schneider, & Minder, 1997), and the trim and fill method (Duval & Tweedie, 2000). Meta-regression of continuous moderators was performed if at least six studies were available (Fu et al., 2011). Following this rule, we assessed the effects of age, body-mass index (BMI), illness duration, treatment duration, total baseline severity score, positive symptoms, negative symptoms [as measured with the Positive and Negative Syndrome Scale (PANSS)], Hamilton Rating Scale for Depression and sex (% males). Additionally, we compared the effect sizes statistically of each compound between schizophrenia and MDD using a Wald-type test (Pillinger et al., 2018a). Next, we statistically compared the subgroup summary effect sizes of growth, immune and glucose, by running a combined analysis of all studies assigned to the respective subgroup. Finally, we restricted our analyses to high-quality studies only to reassess the direction of change and magnitude of the compounds in schizophrenia and MDD. Results of meta-analysis and meta-regression with a p value <0.05 were considered significant. All analyses were performed using R software 3.4.0 and Comprehensive Meta-Analysis version 3.0 (Borenstein, Hedges, Higgins, & Rothstein, 2013; R Development Core Team, 2008).
Results
Retrieved studies
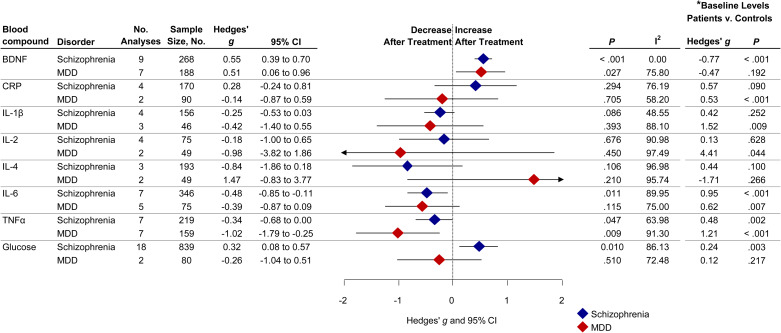
A total of 31 studies were retrieved comprising 1818 drug-naïve first-episode schizophrenia patients, and a total of 14 studies were retrieved comprising 469 drug-naïve first-episode MDD patients. From the following eight peripheral blood compounds at least two studies were available for meta-analysis for both disorders: BDNF, C-reactive protein (CRP), interleukin (IL)-1β, IL-2, IL-4, IL-6, tumor necrosis factor alpha (TNFα) and fasting glucose concentration. The study selection process is presented in Fig. 1. The effect sizes of change for peripheral blood compounds following treatment in schizophrenia and MDD are presented in Fig. 2. Figure 2 also shows the results of (baseline) blood compounds in drug-naïve first-episode patients with schizophrenia or MDD compared with healthy controls, as published in a previous meta-analysis (Çakici et al., 2020).
Fig. 2.
Forest plot showing effect sizes of the change of blood compounds following treatment in drug-naïve first-episode schizophrenia and MDD. Diamonds illustrate the summary effect sizes of change, the middle of each diamond represents the summary effect size, and the width of the lines depicts the width of the overall 95% CI. BDNF, brain-derived neurotrophic factor; CRP, C-reactive protein; IL, interleukin; MDD, major depressive disorder; P, p value; TNFα, tumor necrosis factor alpha. *Baseline levels in drug-naïve first-episode patients measured before treatment compared with healthy controls (Çakici et al., 2020).
Additional study information such as catchment area, patient characteristics, psychopharmacological treatment and treatment duration are provided in online Supplementary Tables S4–S6.
Peripheral growth compounds: BDNF
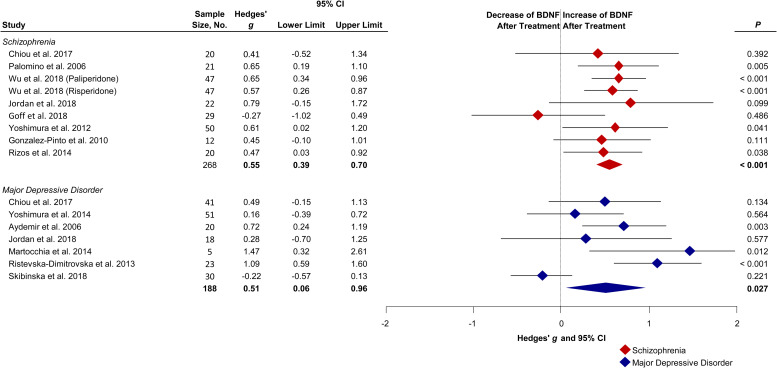
BDNF increased in schizophrenia patients following treatment with a medium effect size (g: 0.55; 95% CI (CI), 0.39 to 0.70; p < 0.001; I2 = 0%, Fig. 3). MDD patients also showed increased BDNF following treatment with a similar effect size as compared with schizophrenia (g: 0.51; CI 0.06–0.96; p = 0.027; I2 = 76%).
Fig. 3.
Forest plot showing effect sizes of the change of BDNF following treatment in drug-naïve first-episode schizophrenia and MDD. Baseline levels in drug-naïve first-episode schizophrenia (Hedges' g = −0.77; p < 0.001) and MDD (Hedges' g = −0.47; p = 0.192) patients measured before treatment compared with healthy controls (Çakici et al., 2020). Diamonds illustrate the summary effect sizes of change, the middle of each diamond represents the summary effect size, and the width of the lines depicts the width of the overall 95% CI. BDNF, brain-derived neurotrophic factor; P, p value.
Immune compounds: CRP, cytokines and TNFα
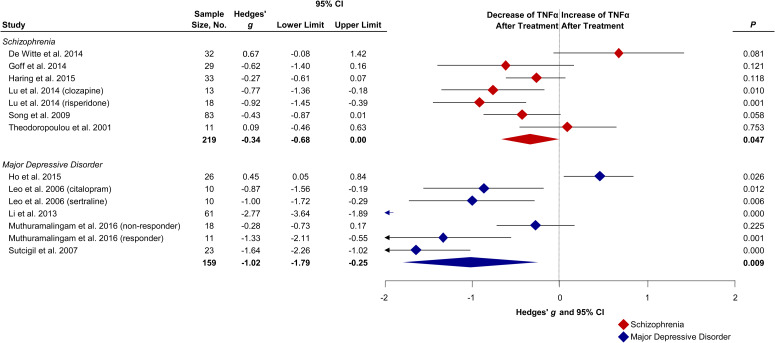
CRP remained unchanged following treatment in schizophrenia (g: 0.28; CI −0.24 to 0.81; p = 0.294; I2 = 76%) and in MDD (g: −0.14; CI −0.87 to 0.59; p = 0.705; I2 = 58%). IL-1β did not significantly change following treatment in schizophrenia (g: −0.25; CI −0.53 to 0.04; p = 0.086; I2 = 49%) and in MDD (g: −0.42; CI −1.40 to 0.55; p = 0.393; I2 = 88%). IL-2 was not significantly altered after treatment in schizophrenia patients (g: −0.18; CI −1.00 to 0.65; p = 0.676; I2 = 91%) nor after treatment in MDD patients (g: −0.98; CI −3.82 to 1.86; p = 0.50; I2 = 97%). IL-4 decreased non-significantly following treatment in schizophrenia (g: −0.84; CI −1.86 to 0.18; p = 0.11; I2 = 97%) whereas in MDD IL-4 increased non-significantly (g: 1.47; CI −0.83 to 3.78; p = 0.210; I2 = 96%). The Wald type test did not find a significant difference between groups (p = 0.070). IL-6 decreased in schizophrenia patients following treatment with a medium effect size (g: −0.48; CI −0.85 to −0.11; p = 0.011; I2 = 90%). In MDD we observed a trend of decreased IL-6 levels with a small to medium effect size (g: −0.39; CI −0.87 to 0.09; p = 0.115; I2 = 75%). TNFα decreased following treatment in schizophrenia with a small to medium effect size (g: −0.34; CI −0.68 to −0.01; p = 0.047; I2 = 64%, Fig. 4). MDD patients also showed decreased TNFα after treatment with a large effect size (g: −1.02; CI −1.79 to −0.25; p = 0.009; I2 = 91%) compared with schizophrenia patients. See online Supplementary Figs S2–S6 for effect size estimates for individual studies.
Fig. 4.
Forest plot showing effect sizes of the change of TNFα following treatment in drug-naïve first-episode schizophrenia and MDD. Baseline levels in drug-naïve first-episode schizophrenia (Hedges' g = 0.48; p = 0.002) and MDD (Hedges' g = 1.21; p < 0.001) patients measured before treatment compared with healthy controls (Çakici et al., 2020). Diamonds illustrate the effect sizes of change, the middle of each diamond represents the effect size, the width of the lines depicts the width of the 95% CI, and the width of the summary effect size diamond depicts the overall 95% CI. TNFα, tumor necrosis factor alpha; P, p value.
Glucose metabolism compounds: glucose
Glucose increased in schizophrenia patients following antipsychotic treatment with a small to medium effect size (g: 0.32; CI 0.08–0.57; p = 0.010; I2 = 86%). Indication of publication bias was found (Egger test: p < 0.001). After correcting for publication bias glucose remained increased following treatment in schizophrenia (g: 0.32; CI 0.08–0.57). Glucose remained unchanged in MDD patients following treatment (g: −0.26; CI −1.04 to 0.52; p = 0.51; I2 = 72%). See online Supplementary Fig. S7 for effect size estimates for individual studies.
Effects of moderators
Meta-regression analysis showed that schizophrenia patients with a high symptom severity score before treatment had a greater change of IL-6 levels following treatment (g: −0.03; CI −0.04 to −0.01; p = 0.002) (Table 1, online Supplementary Fig. S8). This effect was greatly influenced by the negative symptom score before treatment, i.e. patients with more negative symptoms before treatment showed a greater change in IL-6 levels after treatment (g: −0.13; −0.21 to −0.06; p < 0.001; online Supplementary Fig. S9). Younger patients with schizophrenia showed more change in TNFα levels following treatment compared with older patients with schizophrenia (g: 0.14; CI 0.02–0.26; p = 0.019; online Supplementary Fig. S10). A longer treatment duration was associated with a greater change in TNFα levels after treatment in MDD patients (g: −0.64; CI −0.91 to −0.37; p < 0.001; online Supplementary Fig. S11). Schizophrenia patients with lower BMI before treatment showed a greater change in glucose levels following treatment (g: −0.53; CI −0.87 to −0.18; p = 0.003; online Supplementary Fig. S12). Male schizophrenia patients tended to have a smaller change in TNFα after treatment compared with female schizophrenia patients (g: 0.08; CI 0.03–0.12; p < 0.001; online Supplementary Fig. S12). Besides these effects, meta-regression analysis showed no significant effects for illness duration, change of BMI and change of symptom severity on the changes in blood compounds levels following treatment.
Table 1.
Effects of moderators
| Schizophrenia | Major Depressive Disorder | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood Compound | No. Analyses | Coefficient | 95% Lower Limit | 95% Upper Limit | P | Blood compound | No. Analyses | Coefficient | 95% Lower Limit | 95% Upper Limit | P |
| BDNF | BDNF | ||||||||||
| Age | 8 | 0.04 | -0.04 | 0.11 | 0.341 | Age | 7 | 0.03 | -0.01 | 0.07 | 0.135 |
| Sex (% males) | 6 | 0.00 | -0.01 | 0.02 | 0.660 | Sex (% males) | 7 | 0.01 | −0.01 | 0.02 | 0.537 |
| Treatment duration | 9 | 0.00 | −0.01 | 0.01 | 0.824 | Treatment duration | 7 | 0.08 | −0.22 | 0.38 | 0.606 |
| CRP | n/a | CRP | n/a | ||||||||
| Glucose | Glucose | n/a | |||||||||
| Age | 17 | 0.01 | −0.09 | 0.10 | 0.912 | ||||||
| Treatment duration | 17 | −0.04 | −0.12 | 0.05 | 0.401 | IL-1β | n/a | ||||
| BMI, before treatment | 9 | −0.53 | −0.87 | −0.18 | 0.003 | ||||||
| BMI, change score | 6 | 0.04 | −0.53 | 0.61 | 0.886 | IL-4 | n/a | ||||
| Illness duration | 6 | −0.22 | −1.44 | 0.99 | 0.720 | ||||||
| PANSS total, pre-treatment | 8 | −0.01 | −0.03 | 0.02 | 0.693 | IL-6 | n/a | ||||
| PANSS total, change score | 6 | 0.01 | −0.02 | 0.03 | 0.703 | ||||||
| PANSS positive, pre-treatment | 7 | 0.02 | −0.07 | 0.10 | 0.740 | TNFα | |||||
| PANSS positive, change score | 6 | 0.02 | −0.05 | 0.09 | 0.623 | Treatment duration | 7 | −0.64 | −0.91 | −0.37 | < 0.001 |
| PANSS negative, pre-treatment | 7 | −0.03 | −0.14 | 0.09 | 0.651 | ||||||
| PANSS negative, change score | 6 | 0.02 | −0.09 | 0.13 | 0.729 | ||||||
| Sex (% males) | 13 | −0.01 | −0.03 | 0.01 | 0.253 | ||||||
| IL-1β | n/a | ||||||||||
| IL-4 | n/a | ||||||||||
| IL-6 | |||||||||||
| Age | 7 | −0.06 | −0.15 | 0.04 | 0.243 | ||||||
| Treatment duration | 7 | 0.02 | −0.02 | 0.05 | 0.360 | ||||||
| PANSS total, pre-treatment | 7 | −0.03 | −0.04 | −0.01 | 0.002 | ||||||
| PANSS total, change score | 6 | −0.01 | −0.04 | 0.02 | 0.449 | ||||||
| PANSS positive, pre-treatment | 7 | −0.10 | −0.20 | 0.01 | 0.061 | ||||||
| PANSS negative, pre-treatment | 7 | −0.13 | −0.21 | −0.06 | 0.001 | ||||||
| PANSS negative, change score | 6 | −0.05 | −0.14 | 0.04 | 0.291 | ||||||
| Sex (% males) | 7 | 0.04 | −0.03 | 0.10 | 0.302 | ||||||
| TNFα | |||||||||||
| Age | 7 | 0.14 | 0.02 | 0.26 | 0.019 | ||||||
| Sex (% males) | 6 | 0.08 | 0.03 | 0.12 | < 0.001 | ||||||
| Treatment duration | 7 | 0.00 | −0.05 | 0.04 | 0.930 | ||||||
BMI, body-mass index; BDNF, brain-derived neurotrophic factor; CRP, C-reactive protein; IL, interleukin; n/a, not enough studies available for analysis; PANSS, Positive and Negative Syndrome Scale; TNFα, tumor necrosis factor alpha.
Meta-regression of continuous moderators was performed if at least six studies were available.
Statistical comparison of effect sizes
For IL-4 we observed a non-significant difference of the overall effect size in schizophrenia v. MDD (Wald score: −1.80; p = 0.070; online Supplementary Table S7). For all other compounds there was no significant difference between the overall effect size of alterations in schizophrenia v. MDD. There was no significant difference between the overall effect size of alterations of immune compounds in schizophrenia v. MDD.
Effect of study quality
In a sensitivity analysis including only high-quality studies (online Supplementary Tables S8 and S9), our results of increased BDNF after treatment in schizophrenia remained the same with a similar effect size. Similar to the main analysis, we observed decreased TNFα in MDD after treatment, but with a larger effect size (g: −2.25; p = 0.045). In contrast to the initial findings, we did not observe any changes for IL-6, TNFα and glucose in schizophrenia, nor for BDNF and IL-6 in MDD. However, due to the relatively small number of available studies for this sensitivity analysis we probably had insufficient power (as evidenced by the generally smaller effect sizes) to make conclusive statements.
Discussion
In this meta-analysis, we found that drug-naïve first-episode patients with either schizophrenia or MDD both show changes of peripheral blood compounds in a similar direction following treatment: growth factor BDNF increased with a similar medium effect size and immune factor TNFα decreased in schizophrenia with a small to medium effect size and in MDD with a large effect size. Glucose metabolism factor fasting glucose increased in schizophrenia patients after treatment with a small to medium effect size, but not in MDD patients. We found no significant differences among the overall effect sizes of all compounds between both disorders.
Below we will discuss findings concerning growth, immune and glucose metabolism factors separately in more detail.
Impaired neuroplasticity
Impairment of neuroplasticity has been implicated in the pathophysiology of both schizophrenia and MDD (Rao et al., 2017). BDNF, among other neurotrophic factors, contribute to maintaining the plasticity of neurons and is therefore a key component for cognition (Benraiss, Chmielnicki, Lerner, Roh, & Goldman, 2001; Pencea, Bingaman, Wiegand, & Luskin, 2001). There is meta-analytic evidence that BDNF is decreased in both schizophrenia and MDD present from disease-onset (Çakici et al., 2020; Fernandes et al., 2015; Molendijk et al., 2014).
In the current study, BDNF significantly increased following treatment in both disorders. These findings suggest that treating a current first-episode of schizophrenia or MDD using regular psychopharmacological treatment tends to restore BDNF levels. It is unclear what the exact nature of this restoration is. We could not discern whether increased BDNF levels are caused by psychopharmacological treatment or by other factors associated with reduction of symptom severity. Rat studies have shown that second-generation antipsychotics (SGAs) have neuroprotective effects through modulation of BDNF levels (Angelucci, Mathe, & Aloe, 2000; Bai, Chlan-Fourney, Bowen, Keegan, & Li, 2003; Wakade, Mahadik, Waller, & Chiu, 2002), and that antidepressants increase total BDNF messenger RNA expression in astrocytes and microglia (Hisaoka-Nakashima et al., 2016).
Mounting evidence shows that hippocampal atrophy is associated with MDD (Duman & Monteggia, 2006; Schmidt & Duman, 2007), already from disease-onset (Cole, Costafreda, McGuffin, & Fu, 2011) and that antidepressants can block or even reverse hippocampal atrophy (Schmidt & Duman, 2007). It is thought that the neurotrophic actions of antidepressants upregulate BDNF which, in turn, increases neurogenesis within the hippocampus (Schmidt & Duman, 2007). Additionally, electroconvulsive therapy can increase brain volume in the limbic structures, including the hippocampus and amygdala (Takamiya et al., 2018), and increase BDNF among patients with MDD (Birkenhager, Geldermans, Van den Broek, van Beveren, & Fekkes, 2012; Rocha et al., 2016).
According to a meta-analysis of longitudinal studies, BDNF might be regarded as a biomarker for successful treatment of MDD (Polyakova et al., 2015). Polyakova and colleagues showed that BDNF was initially decreased in acute MDD and subsequently increased following antidepressant treatment in patients that in particular responded to treatment (Polyakova et al., 2015). A meta-analysis of drug-free psychosis patients showed that BDNF increased after antipsychotic treatment in psychosis patients, however treatment response was not related to the increase of BDNF over the course of treatment (Fernandes et al., 2015).
Immune system dysfunctions, partly modifiable?
A growing body of evidence shows that signs of low-grade peripheral inflammation are seen in both schizophrenia and MDD from disease-onset (Çakici et al., 2020; Goldsmith, Rapaport, & Miller, 2016; Kohler et al., 2017; Upthegrove, Manzanares-Teson, & Barnes, 2014). In a previous meta-analysis we showed that increased immune factors IL-6 and TNFα are present in drug-naïve first-episode patients with schizophrenia or MDD compared with healthy controls (Çakici et al., 2020). In the current study, we found that TNFα substantially decreased following treatment in both drug-naïve first-episode patient groups. IL-6 decreased following treatment in schizophrenia patients and for MDD patients we observed a trend of decreased IL-6 following treatment. Two studies which were not included in the main analysis investigated the influence of non-pharmacological treatment on IL-6 in drug-naïve first-episode patients with MDD (Gazal et al., 2013; Keri, Szabo, & Kelemen, 2014), using cognitive-behavioral therapy (CBT), a widely used evidence-based treatment for MDD (American Psychiatric Association, 2000). Interestingly, when adding these CBT studies in a post-hoc analysis to the main IL-6 meta-analysis (Gazal et al., 2013; Keri et al., 2014), we observed a significant decrease of IL-6 following treatment in MDD patients (online Supplementary Table S6B). These CBT findings may suggest that the modulating effects on IL-6 and possibly other immune markers are not limited to solely treatment with medication (Keri et al., 2014). Indeed, other factors associated with reduction of symptom severity, such as improvement in sleep and diet, or diminished stress, may also have beneficial anti-inflammatory effects.
Both in vitro and in vivo research has shown that particularly atypical antipsychotics possess anti-inflammatory properties. Clozapine displays anti-inflammatory and neuroprotective effects (Hu et al., 2012), but also olanzapine, aripiprazole and risperidone have shown to possess anti-inflammatory effects (Juncal-Ruiz et al., 2018; Obuchowicz, Bielecka-Wajdman, Paul-Samojedny, & Nowacka, 2017; Stapel et al., 2018). In MDD, antidepressants have proven to counteract inflammation and hypothalamic–pituitary–adrenal axis activation (Eskeland, Halvorsen, & Tanum, 2017; Roumestan et al., 2007). However, it was not possible in our meta-analysis to differentiate between the anti-inflammatory actions across the different types of psychopharmacological treatments, since SGA and selective serotonin reuptake inhibitor (SSRI) studies are overrepresented in the literature and other agents are underrepresented (online Supplementary Table S10).
We like to point out that the effect sizes of change point consistently into the direction of normalization. In other words, when the direction of effect size of a compound was increased or decreased at baseline, the direction of effect size of this compound after treatment seemed to decrease or increase respectively. This was most notable with BDNF, TNFα and IL-6. Although not significant, IL-4 seemed to be altered in opposite directions before treatment in both disorders, and subsequently changed in opposite directions after treatment in both disorders. These observations should be cautiously interpreted due to the paucity of available data and subsequent lack of power in this meta-analysis, but merit further investigation.
In a similar meta-analysis, IL-6 and IL-1β decreased after 4 weeks of antipsychotic treatment (Capuzzi et al., 2017). Furthermore, they presented decreased IL-2 and no change of TNFα after treatment. These differences may be explained because we included more studies for each compound and extracted data from the largest or most recent dataset. Similar to our findings, another meta-analysis reported increased IL-6, decreased IL-1β and no change of IL-2 following treatment in acute psychosis, and decreased IL-6 and unaltered IL-2 following treatment in acute depression (Goldsmith et al., 2016). In contrast to our study, no change in TNFα was reported in both disorders and an increase of IL-1β was observed in acute depression. These differences could have been influenced by effects of medication or prolonged disease duration, as the majority of the included studies was not performed in drug-naïve subjects, and not all patients were in their first-episode of disease (Goldsmith et al., 2016). A more recent meta-analysis investigated the effects of antidepressant treatment on cytokines and chemokines in MDD patients – of whom the majority were not drug-naïve or in their first-episode (Köhler et al., 2018). Similar to our study, they presented decreased IL-6 and TNFα, and no changes for IL-1β, IL-2 and IL-4 following treatment in MDD (Köhler et al., 2018).
Taken together, both immune factors IL-6 and TNFα are increased in schizophrenia and MDD at disease onset. Treatment seems to normalize IL-6 and TNFα in both disorders.
Inflammatory subtypes
Signs of low-grade inflammation could indicate that immune alterations are disease-inherent abnormalities, however immune alterations could also be present only in some patients. A meta-analysis of immune parameters observed lower variability of especially IL-6 in psychosis patients, possibly indicating that immune alterations in schizophrenia patients are truly a sign of intrinsic immune dysfunction (Pillinger et al., 2018a, 2018b). Conversely, another study found genetic evidence for an immune-related subgroup of schizophrenia (Trossbach et al., 2019).
MDD is a highly heterogeneous disorder and the presence of an inflammatory MDD subtype could be relevant. Lamers and colleagues presented evidence that patients with specific atypical features show associations with immuno-metabolic outcomes including IL-6, CRP and metabolic syndrome components, both cross-sectionally and longitudinally (Lamers et al., 2020). Hence, they proposed an immunometabolic depression model – including chronic low-grade inflammation, oxidative stress, disruption of neuroendocrine regulators (leptin and insulin resistance) and biomolecules (dyslipidemia) involved in energy metabolism – predominantly present in patients with atypical behavioral symptoms which could explain the considerable comorbidity between depression and cardiometabolic conditions (e.g. obesity, metabolic syndrome and diabetes) (Milaneschi et al., 2020). However, a meta-analysis looking at variability of immune parameters in untreated depression, found that depression is associated with a pro-inflammatory state, rather than with an immune subgroup, as some of the inflammatory markers elevated in depression, including CRP and IL-12, showed low variability (Osimo et al., 2020).
High inflammation before the start of treatment in both depression and schizophrenia seems to be associated with worse treatment response. A multinational, multi-center, randomized, double-blind study of first-episode psychosis patients showed that those with the most severe symptoms before treatment presented with higher pro-inflammatory compounds and were the most at risk of non-remission compared to psychosis patients with less severe symptoms (Martinuzzi et al., 2019). Additionally, in a longitudinal study of first-episode psychosis patients an association was found between increased high-sensitivity CRP, triglycerides and BMI at baseline, and higher PANSS scores and reduced treatment response at 1-year follow-up (Nettis et al., 2019).
In depression, regular antidepressant treatment fails for over 30% of the patients, whereby those with high inflammation and/or high BMI are in particular at risk for resistance to treatment (Bekhbat et al., 2018; Haroon et al., 2018). A longitudinal study showed that high IL-6 at baseline could predict a chronic course of depression (Lamers et al., 2019). Moreover, a meta-analysis showed that increased inflammation is contributory to treatment resistance in depression (Strawbridge et al., 2015).
In our meta-regression analyses, the changes in blood compounds were merely related to symptom severity. The presence of inflammatory subtypes could be an explanatory factor, however, to evaluate predictors of response we would need individual patient data, which is beyond the scope of the current study.
Glucose homeostasis
In this meta-analysis, we observed an increase of fasting glucose levels following treatment in drug-naïve first-episode schizophrenia patients, but not in those with MDD. Meta-analytic evidence has confirmed an altered glucose homeostasis is already present in schizophrenia from disease onset (Çakici et al., 2020; Greenhalgh et al., 2017; Kucukgoncu et al., 2019; Perry et al., 2016; Pillinger et al., 2017), even in drug-naïve patients (Çakici et al., 2020). Indeed, long before the advent of antipsychotics an increased incidence of diabetes among schizophrenia patients has been described (Kohen, 2004). SGAs, especially olanzapine and clozapine, have well-known side-effects such as obesity and metabolic syndrome, in particular abnormal glucose and lipid metabolism (Pramyothin & Khaodhiar, 2010). Given the evidence that an altered glucose homeostasis is already present at disease onset in schizophrenia (Çakici et al., 2020; Pillinger et al., 2017), antipsychotic treatment could be a contributory factor to further compromising the glucose homeostasis, resulting in diabetes or other metabolic diseases, often observed in chronic schizophrenia.
In contrast, an altered glucose homeostasis does not seem to be present in first-onset MDD, and, neither does treatment in first-episode MDD contribute to glucose homeostasis alteration. It should be noted that for the current meta-analysis only two studies were available that investigated the effects of antidepressant treatment on glucose changes in MDD patients. Additionally, metabolic dysregulation or disorders are often seen in patients who have been affected by MDD for a longer time (Lasserre et al., 2017) Additionally, metabolic diseases are often seen in patients who have been affected by MDD for a longer time (Lasserre et al., 2017). A bidirectional relationship might exist between obesity-related traits and MDD with atypical features, as a large international consortium study showed that patients with atypical depression carried a higher number of genetic risk variants for disturbances in BMI, CRP and leptin (Milaneschi et al., 2017). Also, a cross-disorder proteomics analysis, using the same multi-analyte platform, showed that increased levels of insulin and leptin are present in both schizophrenia and MDD (Çakici et al., 2019a).
Taken together, evidence was found for a dysfunctional glucose metabolism in drug-naïve first-episode schizophrenia patients before and after psychotropic treatment, but not in those with depression. However, impaired glucose homeostasis and other metabolic alterations are often observed in patients with chronic MDD. Diabetes and cardiovascular diseases are more common in schizophrenia and MDD compared to the healthy population, and contribute to a decreased lifespan in patients with schizophrenia or MDD. It is unclear whether this association is simply reflective of side effects of psychotropic medication or an unhealthy lifestyle, or whether glucose and metabolic alterations are also the results of specific disease-inherent abnormalities.
Clinical implications: paving the way for personal diagnostics and targeted treatment
Given the possibility of common underlying pathways for immune dysfunction and impaired plasticity between schizophrenia and MDD, it is worthwhile to consider the clinical relevance of our findings for treatment. The presence of inflammation before the start of treatment in both depression and schizophrenia seems to be associated with worse treatment response, as outlined in the section ‘Inflammatory subtypes’. Signs of inflammation in schizophrenia and MDD readily suggest augmentation therapy for those patients in which the underlying pathophysiology is related to inflammatory dysfunctions. Although antipsychotics already have some anti-inflammatory actions (Hu et al., 2012), a meta-analysis on the efficacy of augmentation with anti-inflammatory agents for schizophrenia patients showed that some anti-inflammatory agents improved symptomatology, i.e. aspirin, estrogens, minocycline and N-acetylcysteine (Çakici, van Beveren, Judge-Hundal, Koola, & Sommer, 2019b). TNF antagonism seems to be particularly effective in patients with treatment-resistant depression, signs of inflammation (CRP > 5 mg/L) and elevated plasma lipids and cholesterols (Bekhbat et al., 2018). Furthermore, there is meta-analytic evidence that anti-inflammatory add-on treatment to antidepressants may have beneficial effects on improving symptom severity in MDD (Köhler-Forsberg et al., 2019).
Taken together, our findings support the hypothesis that factors associated with neuroplasticity and inflammation are dysregulated in the early-phase of both schizophrenia and MDD. Although regular treatment appears to normalize some growth and immune factors, anti-inflammatory augmentation therapy seems beneficial in schizophrenia and MDD, especially given that an inflammatory subtype is associated with worse treatment response. Further research is warranted to identify which biological underlying disease mechanisms are responsible. The latter readily suggests research into advanced diagnostics and augmentation therapy for inflammatory or metabolic dysfunctions present in schizophrenia and MDD. Further research may elucidate a serum-based clinical decision support system in schizophrenia and MDD to identify inflammatory and/or metabolic aberrant subgroups, in order to augment their regular therapy with anti-inflammatory or metabolic medications.
Limitations
To our best knowledge, this is the first meta-analysis evaluating changes in peripheral growth, immune and glucose metabolism compounds following psychopharmacological treatment in drug-naïve first-episode patients with schizophrenia or MDD. Based on our approach to include only drug-naïve first-episode patients, we could make a better estimation of the alterations in compounds following treatment measured in peripheral blood, without the influence of prior treatment, chronic illness or other factors associated with prolonged illness duration, such as social decline, poorer dietary habits and insufficient physical activity. Ideally, for a meta-analysis, a sufficient number of studies and sample sizes is needed (e.g. at least five studies). For BDNF and TNFα there was a sufficient number of studies available for analysis. The other peripheral blood compounds, that were analyzed in less than five studies (such as IL-6), merit further investigation. Unfortunately, evidence regarding the change of blood compounds following treatment in drug-naïve first-episode patients with schizophrenia or MDD is relatively scarce. Therefore, we could not include other peripheral blood compounds that are often associated with schizophrenia (e.g. nerve growth factor (NGF), IL-8 and insulin) (Çakici et al., 2020; Upthegrove et al., 2014) or MDD (e.g. soluble IL-2 receptor, NGF and vascular endothelial growth factor) (Çakici et al., 2020; Kohler et al., 2017). Furthermore, since SGA and SSRI studies are overrepresented in the literature, we could not differentiate between the anti-inflammatory actions across the different types of psychopharmacological treatments (online Supplementary Table S10).
Another important limitation is that we did not include other major psychiatric disorders such as bipolar disorder. Generally, schizophrenia and bipolar disorder are seen as more related to one another than schizophrenia and MDD. As a consequence, there is a substantial body of research investigating similarities between schizophrenia and bipolar disorder, both from a clinical perspective, as well as the biological underpinnings of both disorders. Our study focused on schizophrenia and MDD, but future projects are warranted to compare bipolar disorder with these disorders. Current findings point at transdiagnostic biological disturbances, underscoring the importance of studying neurobiologic disturbance more broadly in psychiatric disorders.
Conclusions
In this meta-analysis, we found evidence that growth factor BDNF increase and immune factor TNFα decrease following treatment in drug-naïve first-episode patients with either schizophrenia or MDD. BDNF and TNFα are possibly shared markers of an active first-episode of schizophrenia and MDD, since these blood compounds tend to normalize following treatment. An altered glucose homeostasis might be uniquely present at the onset of schizophrenia, with further dysregulation by antipsychotic treatment. Our findings support efforts for further research into transdiagnostic preventive strategies and augmentation therapy for those with high inflammation or metabolic disturbances. Currently, it may help patients with schizophrenia or MDD to accept and continue regular (psychopharmacological) treatment when they are told that dysregulated growth and immune factors tend to normalize during treatment.
Acknowledgements
We thank J. G. Daams for his help in data identification. We thank the following authors for providing additional data for this meta-analysis: A. González-Pinto, L. Haring, Y. Lin, A. Muthuramalingam, C. Noto, M. Skibinska and X. Zhou.
Financial support
This study was supported by the Amsterdam Neuroscience (Amsterdam Neuroscience Alliance Project 2017) to L. de Haan, B. W. J. H. Penninx and N. J. M. van Beveren. N. Çakici was funded by an unrestricted personal grant to N. J. M. van Beveren by Antes, Center for Mental Health Care. These funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the manuscript and in the decision to submit the paper for publication.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291721000155.
click here to view supplementary material
Conflict of interest
None.
References
- American Psychiatric Association (2000). Practice guideline for the treatment of patients with major depressive disorder (revision). American Journal of Psychiatry, 157(4 Suppl), 1–45. [PubMed] [Google Scholar]
- Angelucci, F., Mathe, A. A., & Aloe, L. (2000). Brain-derived neurotrophic factor and tyrosine kinase receptor TrkB in rat brain are significantly altered after haloperidol and risperidone administration. Journal of Neuroscience Research, 60(6), 783–794. doi: [DOI] [PubMed] [Google Scholar]
- Bai, O., Chlan-Fourney, J., Bowen, R., Keegan, D., & Li, X. M. (2003). Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. Journal of Neuroscience Research, 71(1), 127–131. doi: 10.1002/jnr.10440 [DOI] [PubMed] [Google Scholar]
- Barnes, J., Mondelli, V., & Pariante, C. M. (2017). Genetic contributions of inflammation to depression. Neuropsychopharmacology, 42(1), 81–98. doi: 10.1038/npp.2016.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat, M., Chu, K., Le, N. A., Woolwine, B. J., Haroon, E., Miller, A. H., & Felger, J. C. (2018). Glucose and lipid-related biomarkers and the antidepressant response to infliximab in patients with treatment-resistant depression. Psychoneuroendocrinology, 98, 222–229. doi: 10.1016/j.psyneuen.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss, A., Chmielnicki, E., Lerner, K., Roh, D., & Goldman, S. A. (2001). Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. Journal of Neuroscience, 21(17), 6718–6731. doi: 10.1523/jneurosci.21-17-06718.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi, S., Marcuzzi, A., Piscianz, E., Tommasini, A., & Fabris, B. (2018). The complex interplay between lipids, immune system and interleukins in cardio-metabolic diseases. International Journal of Molecular Sciences, 19(12), 4058. doi: 10.3390/ijms19124058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenhager, T. K., Geldermans, S., Van den Broek, W. W., van Beveren, N., & Fekkes, D. (2012). Serum brain-derived neurotrophic factor level in relation to illness severity and episode duration in patients with major depression. Journal of Psychiatric Research, 46(3), 285–289. doi: 10.1016/j.jpsychires.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Borenstein, M., Hedges, L., Higgins, J., & Rothstein, H. (2013). Comprehensive meta-analysis version 3. Englewood, NJ: Biostat. [Google Scholar]
- Bowden, J., Tierney, J. F., Copas, A. J., & Burdett, S. (2011). Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Medical Research Methodology, 11, 41. doi: 10.1186/1471-2288-11-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brites, D., & Fernandes, A. (2015). Neuroinflammation and depression: Microglia activation, extracellular microvesicles and microRNA dysregulation. Frontiers in Cellular Neuroscience, 9, 476. doi: 10.3389/fncel.2015.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz, J. W., & Meyer-Lindenberg, A. (2012). Psychopathology and the human connectome: Toward a transdiagnostic model of risk for mental illness. Neuron, 74(6), 990–1004. doi: 10.1016/j.neuron.2012.06.002 [DOI] [PubMed] [Google Scholar]
- Çakici, N., Bot, M., Lamers, F., Janssen, T., van der Spek, P. J., de Haan, L., … van Beveren, N. J. M. (2019a). Increased serum levels of leptin and insulin in both schizophrenia and major depressive disorder: A cross-disorder proteomics analysis. European Neuropsychopharmacology, 29(7), 835–846. doi: 10.1016/j.euroneuro.2019.05.010 [DOI] [PubMed] [Google Scholar]
- Çakici, N., Sutterland, A. L., Penninx, B., Dalm, V. A., de Haan, L., & van Beveren, N. J. M. (2020). Altered peripheral blood compounds in drug-naïve first-episode patients with either schizophrenia or major depressive disorder: A meta-analysis. Brain, Behavior, and Immunity, 88, 547–558. doi: 10.1016/j.bbi.2020.04.039. [DOI] [PubMed] [Google Scholar]
- Çakici, N., van Beveren, N. J. M., Judge-Hundal, G., Koola, M. M., & Sommer, I. E. C. (2019b). An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: A meta-analysis. Psychological Medicine, 49(14), 2307–2319. doi: 10.1017/s0033291719001995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuzzi, E., Bartoli, F., Crocamo, C., Clerici, M., & Carrà, G. (2017). Acute variations of cytokine levels after antipsychotic treatment in drug-naïve subjects with a first-episode psychosis: A meta-analysis. Neuroscience and Biobehavioral Reviews, 77, 122–128. doi: 10.1016/j.neubiorev.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Chew, L. J., Fusar-Poli, P., & Schmitz, T. (2013). Oligodendroglial alterations and the role of microglia in white matter injury: Relevance to schizophrenia. Developmental Neuroscience, 35(2–3), 102–129. doi: 10.1159/000346157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, J., Costafreda, S. G., McGuffin, P., & Fu, C. H. (2011). Hippocampal atrophy in first episode depression: A meta-analysis of magnetic resonance imaging studies. Journal of Affective Disorders, 134(1–3), 483–487. doi: 10.1016/j.jad.2011.05.057 [DOI] [PubMed] [Google Scholar]
- Debnath, M., Cannon, D. M., & Venkatasubramanian, G. (2013). Variation in the major histocompatibility complex [MHC] gene family in schizophrenia: Associations and functional implications. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 42, 49–62. doi: 10.1016/j.pnpbp.2012.07.009 [DOI] [PubMed] [Google Scholar]
- Drexhage, R. C., Weigelt, K., van Beveren, N., Cohen, D., Versnel, M. A., Nolen, W. A., & Drexhage, H. A. (2011). Immune and neuroimmune alterations in mood disorders and schizophrenia. International Review of Neurobiology, 101, 169–201. doi: 10.1016/b978-0-12-387718-5.00007-9 [DOI] [PubMed] [Google Scholar]
- Duman, R. S., & Monteggia, L. M. (2006). A neurotrophic model for stress-related mood disorders. Biological Psychiatry, 59(12), 1116–1127. doi: 10.1016/j.biopsych.2006.02.013 [DOI] [PubMed] [Google Scholar]
- Duval, S., & Tweedie, R. (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463. doi: 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Egger, M., Davey Smith, G., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskeland, S., Halvorsen, J. A., & Tanum, L. (2017). Antidepressants have anti-inflammatory effects that may be relevant to dermatology: A systematic review. Acta Dermato-Venereologica, 97(8), 897–905. doi: 10.2340/00015555-2702 [DOI] [PubMed] [Google Scholar]
- Fernandes, B. S., Steiner, J., Berk, M., Molendijk, M. L., Gonzalez-Pinto, A., Turck, C. W., … Goncalves, C. A. (2015). Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: Meta-analysis and implications. Molecular Psychiatry, 20(9), 1108–1119. doi: 10.1038/mp.2014.117 [DOI] [PubMed] [Google Scholar]
- Fineberg, A. M., & Ellman, L. M. (2013). Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biological Psychiatry, 73(10), 951–966. doi: 10.1016/j.biopsych.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, R., Gartlehner, G., Grant, M., Shamliyan, T., Sedrakyan, A., Wilt, T. J., … Trikalinos, T. A. (2011). Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. Journal of Clinical Epidemiology, 64(11), 1187–1197. doi: 10.1016/j.jclinepi.2010.08.010 [DOI] [PubMed] [Google Scholar]
- Gazal, M., Souza, L. D., Fucolo, B. A., Wiener, C. D., Silva, R. A., Pinheiro, R. T., … Kaster, M. P. (2013). The impact of cognitive behavioral therapy on IL-6 levels in unmedicated women experiencing the first episode of depression: A pilot study. Psychiatry Research, 209(3), 742–745. doi: 10.1016/j.psychres.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Goldsmith, D. R., Rapaport, M. H., & Miller, B. J. (2016). A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Molecular Psychiatry, 21(12), 1696–1709. doi: 10.1038/mp.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh, A. M., Gonzalez-Blanco, L., Garcia-Rizo, C., Fernandez-Egea, E., Miller, B., Arroyo, M. B., & Kirkpatrick, B. (2017). Meta-analysis of glucose tolerance, insulin, and insulin resistance in antipsychotic-naïve patients with nonaffective psychosis. Schizophrenia Research, 179, 57–63. doi: 10.1016/j.schres.2016.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon, E., Daguanno, A. W., Woolwine, B. J., Goldsmith, D. R., Baer, W. M., Wommack, E. C., … Miller, A. H. (2018). Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology, 95, 43–49. doi: 10.1016/j.psyneuen.2018.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, S. K., Reilly, J. L., Harris, M. S., Rosen, C., Marvin, R. W., Deleon, O., & Sweeney, J. A. (2009). A comparison of neuropsychological dysfunction in first-episode psychosis patients with unipolar depression, bipolar disorder, and schizophrenia. Schizophrenia Research, 113(2–3), 167–175. doi: 10.1016/j.schres.2009.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisaoka-Nakashima, K., Kajitani, N., Kaneko, M., Shigetou, T., Kasai, M., Matsumoto, C., … Nakata, Y. (2016). Amitriptyline induces brain-derived neurotrophic factor (BDNF) mRNA expression through ERK-dependent modulation of multiple BDNF mRNA variants in primary cultured rat cortical astrocytes and microglia. Brain Research, 1634, 57–67. doi: 10.1016/j.brainres.2015.12.057 [DOI] [PubMed] [Google Scholar]
- Hu, X., Zhou, H., Zhang, D., Yang, S., Qian, L., Wu, H. M., … Hong, J. S. (2012). Clozapine protects dopaminergic neurons from inflammation-induced damage by inhibiting microglial overactivation. Journal of Neuroimmune Pharmacology, 7(1), 187–201. doi: 10.1007/s11481-011-9309-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncal-Ruiz, M., Riesco-Davila, L., Ortiz-Garcia de la Foz, V., Martinez-Garcia, O., Ramirez-Bonilla, M., Ocejo-Vinals, J. G., … Crespo-Facorro, B. (2018). Comparison of the anti-inflammatory effect of aripiprazole and risperidone in 75 drug-naive first episode psychosis individuals: A 3 months randomized study. Schizophrenia Research, 202, 226–233. doi: 10.1016/j.schres.2018.06.039 [DOI] [PubMed] [Google Scholar]
- Kahn, R. S., Sommer, I. E., Murray, R. M., Meyer-Lindenberg, A., Weinberger, D. R., Cannon, T. D., … Insel, T. R. (2015). Schizophrenia. Nature Reviews Disease Primers, 1, 15067. doi: 10.1038/nrdp.2015.67 [DOI] [PubMed] [Google Scholar]
- Kan, C., Silva, N., Golden, S. H., Rajala, U., Timonen, M., Stahl, D., & Ismail, K. (2013). A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care, 36(2), 480–489. doi: 10.2337/dc12-1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri, S., Szabo, C., & Kelemen, O. (2014). Expression of Toll-like receptors in peripheral blood mononuclear cells and response to cognitive-behavioral therapy in major depressive disorder. Brain, Behavior, and Immunity, 40, 235–243. doi: 10.1016/j.bbi.2014.03.020 [DOI] [PubMed] [Google Scholar]
- Kohen, D. (2004). Diabetes mellitus and schizophrenia: Historical perspective. The British Journal of Psychiatry. Supplement, 47, S64–S66. [DOI] [PubMed] [Google Scholar]
- Köhler-Forsberg, O., N, C., Hjorthøj, L., Nordentoft, C., Mors, M., & Benros, O., & E, M. (2019). Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: Meta-analysis of clinical trials. Acta Psychiatrica Scandinavica, 139(5), 404–419. doi: 10.1111/acps.13016 [DOI] [PubMed] [Google Scholar]
- Kohler, C. A., Freitas, T. H., Maes, M., de Andrade, N. Q., Liu, C. S., Fernandes, B. S., … Carvalho, A. F. (2017). Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatrica Scandinavica, 135(5), 373–387. doi: 10.1111/acps.12698 [DOI] [PubMed] [Google Scholar]
- Köhler, C. A., Freitas, T. H., Stubbs, B., Maes, M., Solmi, M., Veronese, N., … Carvalho, A. F. (2018). Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: Systematic review and meta-analysis. Molecular Neurobiology, 55(5), 4195–4206. doi: 10.1007/s12035-017-0632-1 [DOI] [PubMed] [Google Scholar]
- Kucukgoncu, S., Kosir, U., Zhou, E., Sullivan, E., Srihari, V. H., & Tek, C. (2019). Glucose metabolism dysregulation at the onset of mental illness is not limited to first episode psychosis: A systematic review and meta-analysis. Early Intervention in Psychiatry, 13(5), 1021–1031. doi: 10.1111/eip.12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers, F., Milaneschi, Y., Smit, J. H., Schoevers, R. A., Wittenberg, G., & Penninx, B. (2019). Longitudinal association between depression and inflammatory markers: Results from the Netherlands study of depression and anxiety. Biological Psychiatry, 85(10), 829–837. doi: 10.1016/j.biopsych.2018.12.020 [DOI] [PubMed] [Google Scholar]
- Lamers, F., Milaneschi, Y., Vinkers, C. H., Schoevers, R. A., Giltay, E. J., & Penninx, B. (2020). Depression profilers and immuno-metabolic dysregulation: Longitudinal results from the NESDA study. Brain, Behavior, and Immunity, 88, 174–183. doi: 10.1016/j.bbi.2020.04.002 [DOI] [PubMed] [Google Scholar]
- Lasserre, A. M., Strippoli, M. F., Glaus, J., Gholam-Rezaee, M., Vandeleur, C. L., Castelao, E., … Preisig, M. (2017). Prospective associations of depression subtypes with cardio-metabolic risk factors in the general population. Molecular Psychiatry, 22(7), 1026–1034. doi: 10.1038/mp.2016.178 [DOI] [PubMed] [Google Scholar]
- Lowe, D. J. E., Sasiadek, J. D., Coles, A. S., & George, T. P. (2019). Cannabis and mental illness: A review. European Archives of Psychiatry and Clinical Neuroscience, 269(1), 107–120. doi: 10.1007/s00406-018-0970-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinuzzi, E., Barbosa, S., Daoudlarian, D., Bel Haj Ali, W., Gilet, C., Fillatre, L., … Glaichenhaus, N. (2019). Stratification and prediction of remission in first-episode psychosis patients: The OPTiMiSE cohort study. Translational Psychiatry, 9(1), 20. doi: 10.1038/s41398-018-0366-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezuk, B., Eaton, W. W., Albrecht, S., & Golden, S. H. (2008). Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care, 31(12), 2383–2390. doi: 10.2337/dc08-0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi, Y., Lamers, F., Berk, M., & Penninx, B. (2020). Depression heterogeneity and its biological underpinnings: Toward immunometabolic depression. Biological Psychiatry, 88(5), 369–380. doi: 10.1016/j.biopsych.2020.01.014 [DOI] [PubMed] [Google Scholar]
- Milaneschi, Y., Lamers, F., Peyrot, W. J., Baune, B. T., Breen, G., Dehghan, A., … Penninx, B. (2017). Genetic association of major depression with atypical features and obesity-related immunometabolic dysregulations. JAMA Psychiatry, 74(12), 1214–1225. doi: 10.1001/jamapsychiatry.2017.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, B. J., Buckley, P., Seabolt, W., Mellor, A., & Kirkpatrick, B. (2011). Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biological Psychiatry, 70(7), 663–671. doi: 10.1016/j.biopsych.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Journal of Clinical Epidemiology, 62(10), 1006–1012. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- Mokhtari, R., & Lachman, H. M. (2016). The major histocompatibility complex (MHC) in schizophrenia: A review. Journal of Clinical & Cellular Immunology, 7(6), 479. doi: 10.4172/2155-9899.1000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk, M. L., Spinhoven, P., Polak, M., Bus, B. A., Penninx, B. W., & Elzinga, B. M. (2014). Serum BDNF concentrations as peripheral manifestations of depression: Evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Molecular Psychiatry, 19(7), 791–800. doi: 10.1038/mp.2013.105 [DOI] [PubMed] [Google Scholar]
- Monji, A., Kato, T., & Kanba, S. (2009). Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry and Clinical Neurosciences, 63(3), 257–265. doi: 10.1111/j.1440-1819.2009.01945.x [DOI] [PubMed] [Google Scholar]
- Nettis, M. A., Pergola, G., Kolliakou, A., O'Connor, J., Bonaccorso, S., David, A., … Mondelli, V. (2019). Metabolic-inflammatory status as predictor of clinical outcome at 1-year follow-up in patients with first episode psychosis. Psychoneuroendocrinology, 99, 145–153. doi: 10.1016/j.psyneuen.2018.09.005 [DOI] [PubMed] [Google Scholar]
- Nishiyama, K., Fujimoto, Y., Takeuchi, T., & Azuma, Y. T. (2018). Aggressive crosstalk between fatty acids and inflammation in macrophages and their influence on metabolic homeostasis. Neurochemical Research, 43(1), 19–26. doi: 10.1007/s11064-017-2269-x [DOI] [PubMed] [Google Scholar]
- Obuchowicz, E., Bielecka-Wajdman, A. M., Paul-Samojedny, M., & Nowacka, M. (2017). Different influence of antipsychotics on the balance between pro- and anti-inflammatory cytokines depends on glia activation: An in vitro study. Cytokine, 94, 37–44. doi: 10.1016/j.cyto.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Osimo, E. F., Pillinger, T., Rodriguez, I. M., Khandaker, G. M., Pariante, C. M., & Howes, O. D. (2020). Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5166 patients and 5083 controls. Brain, Behavior, and Immunity, 87, 901–909. doi: 10.1016/j.bbi.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencea, V., Bingaman, K. D., Wiegand, S. J., & Luskin, M. B. (2001). Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. Journal of Neuroscience, 21(17), 6706–6717. doi: 10.1523/jneurosci.21-17-06706.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, B. I., McIntosh, G., Weich, S., Singh, S., & Rees, K. (2016). The association between first-episode psychosis and abnormal glycaemic control: Systematic review and meta-analysis. The Lancet. Psychiatry, 3(11), 1049–1058. doi: 10.1016/s2215-0366(16)30262-0 [DOI] [PubMed] [Google Scholar]
- Pillinger, T., Beck, K., Gobjila, C., Donocik, J. G., Jauhar, S., & Howes, O. D. (2017). Impaired glucose homeostasis in first-episode schizophrenia: A systematic review and meta-analysis. JAMA Psychiatry, 74(3), 261–269. doi: 10.1001/jamapsychiatry.2016.3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillinger, T., D'Ambrosio, E., McCutcheon, R., & O, D. H. (2018a). Is psychosis a multisystem disorder? A meta-review of central nervous system, immune, cardiometabolic, and endocrine alterations in first-episode psychosis and perspective on potential models. Molecular Psychiatry, 24(6), 776–794. doi: 10.1038/s41380-018-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillinger, T., Osimo, E. F., Brugger, S., Mondelli, V., McCutcheon, R. A., & Howes, O. D. (2018b). A meta-analysis of immune parameters, variability, and assessment of modal distribution in psychosis and test of the immune subgroup hypothesis. Schizophrenia Bulletin, 45(5), 1120–1133. doi: 10.1093/schbul/sby160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyakova, M., Stuke, K., Schuemberg, K., Mueller, K., Schoenknecht, P., & Schroeter, M. L. (2015). BDNF as a biomarker for successful treatment of mood disorders: A systematic & quantitative meta-analysis. Journal of Affective Disorders, 174, 432–440. doi: 10.1016/j.jad.2014.11.044 [DOI] [PubMed] [Google Scholar]
- Pramyothin, P., & Khaodhiar, L. (2010). Metabolic syndrome with the atypical antipsychotics. Current Opinion in Endocrinology, Diabetes and Obesity, 17(5), 460–466. doi: 10.1097/MED.0b013e32833de61c [DOI] [PubMed] [Google Scholar]
- Rao, S., Martinez-Cengotitabengoa, M., Yao, Y., Guo, Z., Xu, Q., Li, S., … Zhang, F. (2017). Peripheral blood nerve growth factor levels in major psychiatric disorders. Journal of Psychiatric Research, 86, 39–45. doi: 10.1016/j.jpsychires.2016.11.012 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2008). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. ISBN 3-900051-07-0, Retrieved from http://www.R-project.org [Google Scholar]
- Rocha, R. B., Dondossola, E. R., Grande, A. J., Colonetti, T., Ceretta, L. B., Passos, I. C., … da Rosa, M. I. (2016). Increased BDNF levels after electroconvulsive therapy in patients with major depressive disorder: A meta-analysis study. Journal of Psychiatric Research, 83, 47–53. doi: 10.1016/j.jpsychires.2016.08.004 [DOI] [PubMed] [Google Scholar]
- Roumestan, C., Michel, A., Bichon, F., Portet, K., Detoc, M., Henriquet, C., … Mathieu, M. (2007). Anti-inflammatory properties of desipramine and fluoxetine. Respiratory Research, 8(1), 35. doi: 10.1186/1465-9921-8-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, H. D., & Duman, R. S. (2007). The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behavioural Pharmacology, 18(5–6), 391–418. doi: 10.1097/FBP.0b013e3282ee2aa8 [DOI] [PubMed] [Google Scholar]
- Sekar, A., Bialas, A. R., de Rivera, H., Davis, A., Hammond, T. R., Kamitaki, N., … McCarroll, S. A. (2016). Schizophrenia risk from complex variation of complement component 4. Nature, 530(7589), 177–183. doi: 10.1038/nature16549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, S., Duman, R., & Sanacora, G. (2008). Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Meta-analyses and implications. Biological Psychiatry, 64(6), 527–532. doi: 10.1016/j.biopsych.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapel, B., Sieve, I., Falk, C. S., Bleich, S., Hilfiker-Kleiner, D., & Kahl, K. G. (2018). Second generation atypical antipsychotics olanzapine and aripiprazole reduce expression and secretion of inflammatory cytokines in human immune cells. Journal of Psychiatric Research, 105, 95–102. doi: 10.1016/j.jpsychires.2018.08.017 [DOI] [PubMed] [Google Scholar]
- Steiner, J., Bernstein, H. G., Schiltz, K., Muller, U. J., Westphal, S., Drexhage, H. A., & Bogerts, B. (2014). Immune system and glucose metabolism interaction in schizophrenia: A chicken-egg dilemma. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 48, 287–294. doi: 10.1016/j.pnpbp.2012.09.016 [DOI] [PubMed] [Google Scholar]
- Strawbridge, R., Arnone, D., Danese, A., Papadopoulos, A., Herane Vives, A., & Cleare, A. J. (2015). Inflammation and clinical response to treatment in depression: A meta-analysis. European Neuropsychopharmacology, 25(10), 1532–1543. doi: 10.1016/j.euroneuro.2015.06.007 [DOI] [PubMed] [Google Scholar]
- Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., … Thacker, S. B. (2000). Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA, 283(15), 2008–2012. [DOI] [PubMed] [Google Scholar]
- Takamiya, A., Chung, J. K., Liang, K. C., Graff-Guerrero, A., Mimura, M., & Kishimoto, T. (2018). Effect of electroconvulsive therapy on hippocampal and amygdala volumes: Systematic review and meta-analysis. British Journal of Psychiatry, 212(1), 19–26. doi: 10.1192/bjp.2017.11 [DOI] [PubMed] [Google Scholar]
- Trossbach, S. V., Hecher, L., Schafflick, D., Deenen, R., Popa, O., Lautwein, T., … Korth, C. (2019). Dysregulation of a specific immune-related network of genes biologically defines a subset of schizophrenia. Translational Psychiatry, 9(1), 156. doi: 10.1038/s41398-019-0486-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upthegrove, R., Manzanares-Teson, N., & Barnes, N. M. (2014). Cytokine function in medication-naive first episode psychosis: A systematic review and meta-analysis. Schizophrenia Research, 155(1-3), 101–108. doi: 10.1016/j.schres.2014.03.005 [DOI] [PubMed] [Google Scholar]
- Van Os, J., Linscott, R. J., Myin-Germeys, I., Delespaul, P., & Krabbendam, L. (2009). A systematic review and meta-analysis of the psychosis continuum: Evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychological Medicine, 39(2), 179–195. doi: 10.1017/s0033291708003814 [DOI] [PubMed] [Google Scholar]
- Wakade, C. G., Mahadik, S. P., Waller, J. L., & Chiu, F. C. (2002). Atypical neuroleptics stimulate neurogenesis in adult rat brain. Journal of Neuroscience Research, 69(1), 72–79. doi: 10.1002/jnr.10281 [DOI] [PubMed] [Google Scholar]
- Wang, L., Wang, R., Liu, L., Qiao, D., Baldwin, D. S., & Hou, R. (2019). Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: A systematic review and meta-analysis. Brain, Behavior, and Immunity, 79, 24–38. doi: 10.1016/j.bbi.2019.02.021 [DOI] [PubMed] [Google Scholar]
- Wells, G. A., Shea, B., O'Connell, D., Peterson, J., Welch, V., Losos, M., & Tugwell, P. (2009). The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Retrieved from http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- Wigman, J. T., van Os, J., Borsboom, D., Wardenaar, K. J., Epskamp, S., Klippel, A., … Wichers, M. (2015). Exploring the underlying structure of mental disorders: Cross-diagnostic differences and similarities from a network perspective using both a top-down and a bottom-up approach. Psychological Medicine, 45(11), 2375–2387. doi: 10.1017/s0033291715000331 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291721000155.
click here to view supplementary material