Abstract
Cardiovascular disease (CVD), including hypertensive disorders of pregnancy (HDP) and peripartum cardiomyopathy, is a leading cause of pregnancy-related death in the United States. Women who are African American or American Indian/Alaskan Native, have HDP, are medically underserved, are older, or are obese have a major risk for the onset and/or progression of CVD during and after pregnancy. Paradoxically, women with no preexisting chronic conditions or risk factors also experience significant pregnancy-related cardiovascular (CV) complications. The question remains whether substantial physiologic stress on the CV system during pregnancy reflected in hemodynamic, hematological, and metabolic changes uncovers subclinical prepregnancy CVD in these otherwise healthy women. Equally important and similarly understudied is the concept that women's long-term CV health could be detrimentally affected by adverse pregnancy outcomes, such as preeclampsia, gestational hypertension, and diabetes, and preterm birth. Thus, a critical life span perspective in the assessment of women's CV risk factors is needed to help women and health care providers recognize and appreciate not only optimal CV health but also risk factors present before, during, and after pregnancy. In this review article, we highlight new advancements in understanding adverse, pregnancy-related CV conditions and will discuss promising strategies or interventions for their prevention, diagnosis, and treatment.
Keywords: maternal mortality and morbidity, hypertensive disorders of pregnancy, peripartum cardiomyopathy, adverse pregnancy outcomes, long-term pregnancy-related cardiovascular disease
Introduction
The maternal mortality rate is rising in the United States even as rates decrease globally. Causes of maternal mortality are multifaceted, and in the United States, cardiovascular disease (CVD) is the primary cause of pregnancy-related mortality.1 The question remains whether substantial physiological stress on the cardiovascular (CV) system during pregnancy—reflected in hemodynamic, hematological, and metabolic changes—uncovers subclinical prepregnancy CVD in otherwise healthy women. Equally important and similarly understudied is the concept that women's long-term CV health could be detrimentally affected by adverse pregnancy outcomes (APOs), such as preeclampsia (PE), gestational hypertension, and diabetes, and preterm birth. Thus, a critical life span perspective in assessment of women's CV risk factors is needed.
We highlight advances in understanding the emergence of CV conditions during pregnancy and postpartum (specifically hypertensive disorders of pregnancy [HDP] and peripartum cardiomyopathy [PPCM]) and their association with CV risk over the life course. We explore how risk prediction using biomarkers could potentially facilitate stratification of antenatal care and testing of potential preventative and therapeutic interventions.
We also discuss opportunities for counseling, planning, and interventions to optimize treatments for underlying medical conditions associated with increased CV risk to improve pregnancy outcomes and long-term CV health. We also review follow-up care models and recommend strategies for implementing these models into clinical practice.
Hypertensive Disorders of Pregnancy
Hypertension is the most common complication observed during pregnancy, affecting 8%–10% of all pregnancies in the United States.2,3 HDP continue to be among the leading causes of pregnancy-related maternal mortality worldwide and contribute to 7%–12% of pregnancy-related maternal deaths in the United States annually.4–6 Importantly, the risk of developing CVD in women with a history of HDP is twice that seen in normotensive pregnancies.7 HDP can be classified into four general categories: (1) PE and eclampsia, (2) chronic hypertension (of any cause), (3) chronic hypertension with superimposed PE, and (4) gestational hypertension.8 Although we will reference other forms of HDP, we will predominantly focus on PE in this article.
New advancement in the pathogenesis of HDP
PE is classically defined as new-onset hypertension and proteinuria developing in the second half of pregnancy and resolving after delivery, yet it is multiorgan HDP that can present with systemic manifestations (e.g., elevated liver enzymes and low platelets) even before the development of proteinuria.2 According to the most recent definition endorsed by the International Society for the Study of Hypertension in Pregnancy, PE is defined as new-onset hypertension (systolic >140 mmHg and diastolic >90 mmHg) accompanied by one or more other features: proteinuria, other maternal organ dysfunction (including the liver, kidney, and nervous system), or hematological involvement, and/or uteroplacental dysfunction, such as fetal growth restriction and/or abnormal Doppler ultrasound findings of uteroplacental blood flow.9
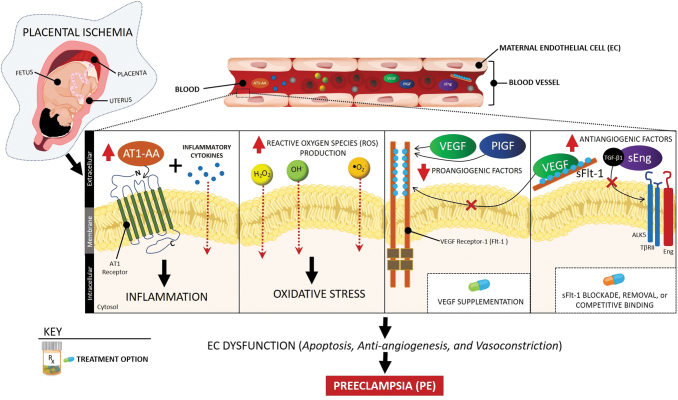
Although our understanding of the pathophysiology of PE has increased dramatically in the past 30 years, the precise mechanisms underlying this condition are still emerging. It is thought that one of the initiating events in PE is placental ischemia as a result of uteroplacental malperfusion and defective remodeling of the uterine spiral arteries due to insufficient trophoblast invasion.10 Studies in experimental models of PE have shown that placental ischemia is associated with oxidative stress and abnormal natural killer cells (NK cells) at the maternal–fetal interface.11,12 These changes, along with other genetic and environmental factors, lead to increased release of proinflammatory cytokines (e.g., TNF-α and IL-6), exosomes (including microRNAs), antiangiogenic factors (e.g., soluble fms-like tyrosine kinase-1 [sFlt-1] and soluble endoglin), autoantibodies to the angiotensin II type 1 receptor, and cell-free fetal DNA, as well as lower levels of proangiogenic factors (e.g., placental growth factor [PlGF]) in the maternal circulation.11,13,14 Imbalances in these pathways and differences in the maternal responses are thought to cause differential effects on maternal endothelial and metabolic dysfunction and, consequently, varying degrees in the severity of PE.12,15 In addition, although clinical observations and experimental studies point to the importance of nitric oxide during gestation, further studies are needed to examine whether its deficiency in mitigating oxidative stress and inflammation, as well as metabolic and endothelial dysfunction, could be a possible mechanism for PE.16,17 A few of the abovementioned pathways are illustrated in Figure 1.
FIG. 1.
Signaling pathways, potential biomarkers, and therapeutic targets in PE. Placental ischemia-derived imbalance in proangiogenic (VEGF and PlGF) and antiangiogenic (sFlt-1 and sEng) factors, oxidative stress, and inflammation led to systemic vascular dysfunction reflected in hypertension and proteinuria in PE. Potential targeted interventions include blockade, removal, or competitive binding of sFlt-1 or supplementation of recombinant isoforms of proangiogenic factors. AT1-AA, AT1 receptor autoantibodies; PE, preeclampsia; PlGF, placental growth factor; sEng, soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase-1; VEGF, vascular endothelial growth factor.
Potential biomarkers for prediction, early detection, and prognosis of HDP
Numerous studies have evaluated the role of biochemical and biophysical markers individually, in combination, and with the addition of clinical history in an attempt to improve risk prediction.2,18,19 Overall, clinical, biochemical, and epidemiological findings suggest that PE is not a single disorder and that different pathways may converge on a common syndromic end point.20 Identifying these disease subtypes may allow development of novel biomarkers and therapies.
Some of the most commonly studied biophysical markers include the mean arterial pressure and uterine artery pulsatility index for early detection of alterations in uteroplacental blood flow.2 However, uterine artery Doppler studies alone have a low predictive value for the development of early-onset PE and an even lower value for late-onset PE.2 Commonly studied biochemical markers include PlGF, sFlt-1, and soluble endoglin2,18,19 and their ratios21 as early indicators of abnormal cellular signaling pathways (Fig. 1).
Regardless of the parameters used, screening for PE in low-risk women is associated with very low positive predictive values ranging from 8% to 33%.18 In general, biomarkers perform better in the prediction of early-onset PE (using first- and second-trimester parameters) than late-onset PE. Combinations of biomarkers fare better, with prediction rates of early-onset PE ranging from 30% to 100% in small studies.22 Verification of these findings in large prospective studies has not yet occurred.
At present, further studies to identify and validate novel biomarkers with high predictive value are warranted. In addition, evidence that accurate prediction of early-onset PE can be followed by interventions that improve maternal or fetal outcomes is limited, and this area remains an important research opportunity.
Novel treatment strategies for HDP
Current recommendations for treating PE are focused on managing hypertension and preventing seizures and, if necessary, delivering the fetus. Few interventions specifically act on factors in the pathogenesis of PE, rather than simply manage the symptoms and adverse maternal and fetal outcomes. Novel treatment strategies for PE target imbalances in several potential pathways.
Clinical studies suggest that an imbalance in prostacyclin (platelet inhibitor and vasodilator) and thromboxane A2 (platelet activator and vasoconstrictor) is involved in the pathogenesis of PE.23,24 Thus, recent clinical guidelines recommend low-dose aspirin to reduce the incidence of PE because low-dose aspirin can inhibit thromboxane A2 without altering secretion of endothelial prostacyclin.25
In addition, imbalances of angiogenic and antiangiogenic factors are novel targets for PE prevention. In PE, elevated levels of sFlt-1 during pregnancy mediate hypertension, proteinuria, and glomerular endotheliosis by inhibiting downstream signaling of vascular endothelial growth factor (VEGF) and PlGF (Fig. 1).26,27 In preclinical animal models of PE, administration of recombinant isoforms of these angiogenic factors or the interruption of sFlt-1 production with small interfering RNA (siRNA) molecules and small-molecule inhibitors—such as phosphodiesterase 5, proton pump, or sodium–potassium pump inhibitors—has shown promise in reducing blood pressure, abnormal urinary protein, and kidney damage.26,27 Statins, such as pravastatin, may inhibit sFlt-1 production in animals models of PE and pregnant women with severe PE.27,28 Other treatment strategies with the potential to lower hypertension include removing excess circulating sFlt-1 with antibodies and extracorporeal techniques, such as dextran sulfate apheresis or adsorption columns.27
Although ongoing work on novel treatment strategies for HDP appears to be promising, further research is needed to establish effective, evidence-based treatment recommendations during pregnancy in women with HDP, which do not harm the expecting mother or unborn child.
Peripartum Cardiomyopathy
PPCM is a leading cause of maternal mortality.29 PPCM is defined as systolic heart failure (left ventricular ejection fraction <45% and/or fractional shortening <30% on echocardiogram) presented in the last month of pregnancy or the first 5 months after delivery in otherwise healthy women.30 Overall, the incidence of PPCM has been increasing in the United States, although estimates range from 1 in 1000 to 1 in 4000 live births,31 with significant variation by geographic location.32 The predisposing factors include advanced maternal age, PE, chronic hypertension, and multiple-gestation pregnancies.33 Furthermore, the incidence of PPCM is higher in African American women, who also have worse outcomes and higher mortality.34,35
Pathophysiology of PPCM
The cause of PPCM is largely unknown. Several genetic variations have been associated with PPCM.36–38 Notably, familial clustering of PPCM has been shown,39–41 and some women with PPCM have a truncated mutation in TTN, the gene encoding titin, a sarcomeric protein involved in the structural, mechanical, and regulatory functions of cardiac muscle.38 However, not all women with these genetic variations develop PPCM, so other contributing and/or precipitating factors might be involved. Some postulated mechanisms involve hemodynamic stress of pregnancy, malnutrition, or myocarditis, but evidence is insufficient to support their major role.
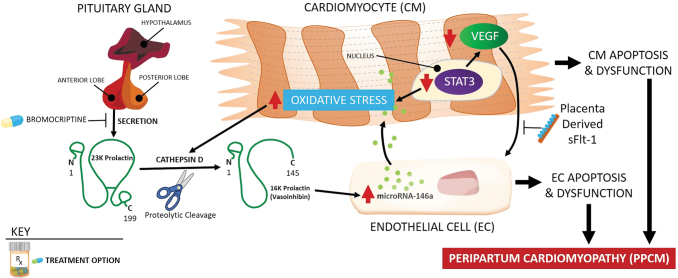
One major PPCM research effort has been directed toward exploring the hormonal/vascular hypothesis (Fig. 2). It has been suggested that pregnant mice lacking antioxidative signal transducer and activator of transcription 3 (STAT3) developed cardiomyopathy due to the presence of a cathepsin D-cleaved 16-kDa prolactin product implicated in endothelial cell and cardiomyocyte apoptosis and dysfunction. Low STAT3 levels in the myocardium and the presence of circulating 16-kDa prolactin fragments also were detected in PPCM patients undergoing heart transplants.42 In addition, the role of microRNA146a in the cross talk between endothelial and cardiomyocyte cells was implied in prolactin-induced PPCM.43
FIG. 2.
Pathophysiology of peripartum cardiomyopathy. In pathogenesis of peripartum cardiomyopathy, the role of cathepsin D-cleaved 16-kDa prolactin fragment, microRNA146a, and decreased VEGF signaling has been suggested. Suppression of prolactin production by bromocriptine had positive therapeutic effects in some preclinical and clinical studies. STAT3, signal transducer and activator of transcription 3.
On the other hand, reduced levels of VEGF were identified in PPCM patients, supporting the hypothesis that PPCM is a systemic vascular disease stemming from impaired VEGF signaling in endothelial cells.44 Moreover, the late gestation increase in placenta-derived circulating antiangiogenic sFlt-1, which binds to and inhibits VEGF, may work in concert with an already present, toxic shorter form of prolactin. Both effects may converge into a potent CV insult that leads to PPCM.45 Importantly, sFlt-1 is increased in PE46 and multiple-gestation pregnancy,47 and these women are also more prone to develop PPCM. However, sFlt-1 is elevated only in a subset of women with PPCM,45 and the origin of sFlt-1 in women after delivery is unknown. It is important to note that an sFlt-1 increase can also be observed in nonpregnant patients with heart failure,48 suggesting a common pathophysiological mechanism for PPCM and other forms of heart failure. Additional research is needed to verify the implication of sFlt-1 in advancing PPCM diagnosis and treatment.
Challenges in establishing diagnostic and prognostic biomarkers for PPCM
Unfortunately, PPCM is still a diagnosis of exclusion, and because it is a rare disease, establishing prognostic markers is a challenge. Further studies are needed to validate the routine use of microRNA146a, along with 16-kDa prolactin, and cathepsin D in diagnostic strategies (Fig. 2) and to establish whether genetic testing can identify women at risk.
Although recovery is expected in most patients,49 the presence of a TTN gene truncation38 or higher levels of sFlt-150 correlated with worse cardiac function and adverse clinical events. An ejection fraction lower than 20% and larger heart size at presentation are prognostic for poor recovery.49,51 On the other hand, a higher level of relaxin-2, with effects that are mediated in part by VEGF, was associated with improved 2-month recovery.50 African American women with PPCM have worse outcomes, sometimes despite adequate therapy.34,49,52,53 Socioeconomic factors, access to medical care, and genetic factors may contribute to these worse outcomes in women of African descent.37,54
Treatment concepts for PPCM
Because PPCM has no specific treatment, its management is similar to other common forms of systolic heart failure (fluid management with diuretics, vasopressin, and inotropes, with the addition of beta-blockers, vasodilators, and aldosterone antagonists, and, in extreme cases, use of mechanical supportive devices), with attention to drugs contraindicated for pregnant and lactating women (such as ACE inhibitors/angiotensin II receptor blockers).
Several studies tested bromocriptine intervention in suppressing prolactin production in women with PPCM (Fig. 2). Nonrandomized studies in South Africa and Germany demonstrated improvement, but not a full recovery, of left ventricular function among women treated with bromocriptine.55,56 In a subsequent, larger, prospective, nonplacebo-controlled, randomized clinical trial of patients with PPCM, the addition of bromocriptine to standard therapy for heart failure was associated with a high recovery rate and a very low rate of adverse outcomes up to 6 months postpartum.57 Spontaneous recovery occurs in a large proportion of patients, thereby complicating interpretations of nonplacebo-controlled studies and trials, as well as future trials evaluating such treatments as bromocriptine. Predictors for recovery are not established.
Nevertheless, as promising as these studies are, caution also should be exercised because 5-year follow-up data of a German PPCM cohort showed a frequent persisting or de novo hypertension and arrhythmia.44 These studies further underline an urgent need for additional validation of the current therapeutic approaches as well as exploration of novel pharmacological targets and drug repurposing that are safe for both mothers and babies.
Adverse Pregnancy Outcomes and Long-Term Risk for CVD
Several large cohort studies and meta-analyses have found links between APOs and long-term risk for CVD in women.58–65 These adverse outcomes include PE and other HDP,58,60,64 gestational diabetes mellitus (GDM),59,63 preterm birth,60,62,66,67 pregnancy loss,61,63 and small for gestational-age infants.67
In studies assessing long-term (10–20 years after pregnancy) risk, women diagnosed with PE have an estimated two- to fourfold increased risk of future CV or cerebrovascular events compared with unaffected women. The risk for future CV events is similar for women diagnosed with gestational hypertension and increased even more when HDP are associated with other adverse outcomes such as iatrogenic preterm birth and stillbirth.68,69 Several recent studies demonstrate short-term CV risk as well,60,70 revealing an approximate 50% increase in the diagnosis of CVD within 5 years70 and a two- to threefold increase in the diagnosis of hypertension within 2–7 years after pregnancy for affected women.60
Researchers are now attempting to determine whether underlying pathology for CVD exists preconceptionally versus whether the APO acts directly on maternal cardiometabolic function to cause future CVD. HDP and subsequent CVD share common predisposing factors. Several studies suggest that pregnancy likely unmasks existing pathology and that APOs are not causative, although they may hasten and/or mediate the process toward CVD.59,63,71 Regardless, controlling CV risk and aggressive treatment of APOs are important strategies to improve pregnancy and immediate and long-term CV outcomes in women.
Prepregnancy characteristics underlying associations
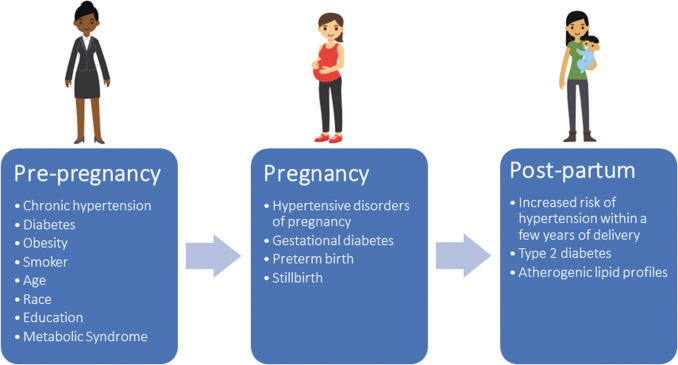
Women with chronic conditions (e.g., hypertension, diabetes, and renal, lung, and autoimmune diseases72–75), obese women,3,59,60 and smokers76 are at higher risk for pregnancy complications; these same factors also are associated with CVD.58 Some nonmodifiable factors—such as race, education, geography, and age—are also associated with elevated risk for APOs and future CVD (Fig. 3).60,77–80
FIG. 3.
Accumulation of cardiovascular risk factors from preconception through pregnancy and postpartum periods. Demographic, clinical, behavioral, social, and environmental risk factors for cardiovascular disease may contribute to adverse pregnancy outcomes. These may persist throughout pregnancy and postpartum periods. Additional cardiovascular risk may be acquired or uncovered during pregnancy, including maternal conditions (hypertensive disorders of pregnancy and gestational diabetes) and fetal conditions (small for gestational age). Preconception and pregnancy risks may persist during the postpartum period and/or emergence of additional cardiovascular risk factors may occur, including maternal conditions (hypertension, diabetes, and dyslipidemia).
Identification and prevention strategies for women at risk for APO-associated, future maternal CVD
Identifying a woman's risk before or early in pregnancy provides additional time to implement strategies to prevent APOs and perhaps slow progression to CVD. There is growing recognition that the occurrence of pregnancy complications reliably identifies women with underlying, often unrecognized CV risk factors, who may benefit from screening and preventive actions, such as preconception counseling to address risk and prepregnancy control of blood pressure, blood glucose, lipids, and weight. Women with a previous APO are at a higher risk in a subsequent pregnancy, a variable that can be used to identify risk preconceptionally. Biomarkers may be useful, particularly for nulliparous women who have no apparent risk factors. Established (e.g., hsCRP,60,62,81 triglycerides,82 lipids,59,82 and glucose59,60,81) and novel CV risk biomarkers (e.g., sFlt-1, PlGF, VEGF, and endoglin)83 have been found to be altered in early pregnancy in women who later have an APO. However, more work is needed to identify which biomarkers or combinations of biomarkers (and their timing) are most predictive of risk.
Early intervention has the potential to reduce both APOs for women who become pregnant again and their lifetime risk of CVD.84–86 Medications and nonpharmacological strategies may improve pregnancy outcomes and risk factors for future CVD.84,86 Low-dose aspirin is recommended for at-risk pregnancies with PE, diabetes, multifetal gestations, and renal disease, as well as, more recently, to reduce the risk of preterm birth.23,24 Statins have shown promise to address potentially abnormal vascular function seen in PE and antiphospholipid syndrome.87,88
CV assessment and follow-up at 3 months postpartum for women with HDP, GDM, fetal growth restriction, and preterm birth are recommended.89 This screening should include a medical/pregnancy history, physical examination, biochemical testing, and nutrition assessment. Women should be counseled with regard to their individual identified risks and opportunities for prevention.
Importantly, women with healthier lifestyle profiles across all reproductive stages (i.e., preconception, pregnancy, and postpartum) have lower risks of APOs as well as lower risks for future CVD.59,63 The American Heart Association's Life's Simple 7 defines ideal CV health based on behavioral (smoking status, physical activity, and diet) and clinical (weight, blood glucose, cholesterol, and blood pressure) risk factors that can be improved through lifestyle changes.90
Women should be encouraged to adhere to a healthy diet,91,92 get regular physical activity to decrease risks for APOs, address sleep-disordered breathing,93 and breastfeed, which lowers risks for CVD (including hypertension and hyperlipidemia), type 2 diabetes, and cancer.59,92,94–96 Maintaining a normal weight before, during, and after pregnancy is important. Obese women and those with excessive weight gain during pregnancy and postpartum weight retention are at higher risk for poorer outcomes.3,59,60 In addition, pregnant women with increased leisure-time physical activity early in pregnancy had low rates of GDM, similar to women with higher patterns of activity, suggesting that increased activity early in pregnancy may improve pregnancy outcomes.97 Importantly, physical activity moderately attenuated the association of GDM with myocardial infarction and stroke.63
Conclusions
Further research is needed to establish effective, evidence-based treatment recommendations during pregnancy, which do not harm the mother or unborn child. Work on novel treatment strategies for peripartum CV-related conditions appears promising. However, pregnant and lactating women are usually excluded from participating in drug trials, a conundrum that the Task Force on Research Specific to Pregnant and Lactating Women is tackling now.98 Carefully planned prospective studies to address these research opportunities in pregnant and lactating women are needed. After pregnancy, comprehensive CV evaluations beyond the immediate postpartum period may provide the opportunity for counseling and interventions to mitigate the underlying risk factors to improve future pregnancy outcomes and lifelong CV health.
Acknowledgment
The authors thank Cheryl Kassed for her input and critical review of this article.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No specific funding was received for this work.
References
- 1. Mehta LS, Warnes CA, Bradley E, et al. Cardiovascular considerations in caring for pregnant patients: A scientific statement from the American Heart Association. Circulation 2020;141:e884–e903 [DOI] [PubMed] [Google Scholar]
- 2. ACOG Practice Bulletin No. 222: Gestational hypertension and preeclampsia. Obstet Gynecol 2020;135:e237–e260 [DOI] [PubMed] [Google Scholar]
- 3. Hauspurg A, Countouris ME, Catov JM. Hypertensive disorders of pregnancy and future maternal health: How can the evidence guide postpartum management? Curr Hypertens Rep 2019;21:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berg CJ, Callaghan WM, Henderson Z, Syverson C. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol 2011;117:1230. [DOI] [PubMed] [Google Scholar]
- 5. Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol 2017;130:366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah S, Gupta A. Hypertensive disorders of pregnancy. Cardiol Clin 2019;37:345–354 [DOI] [PubMed] [Google Scholar]
- 7. McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am Heart J 2008;156:918–930 [DOI] [PubMed] [Google Scholar]
- 8. American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–1131 [DOI] [PubMed] [Google Scholar]
- 9. Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 2018;72:24–43 [DOI] [PubMed] [Google Scholar]
- 10. Cornelius DC, Cottrell J, Amaral LM, LaMarca B. Inflammatory mediators: A causal link to hypertension during preeclampsia. Br J Pharmacol 2019;176:1914–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ Res 2019;124:1094–1112 [DOI] [PubMed] [Google Scholar]
- 12. Redman CW, Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol 2015;213(4 Suppl):10. [DOI] [PubMed] [Google Scholar]
- 13. Amaral LM, Wallace K, Owens M, LaMarca B. Pathophysiology and current clinical management of preeclampsia. Curr Hypertens Rep 2017;19:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019;366:12381. [DOI] [PubMed] [Google Scholar]
- 15. Costa RA, Hoshida MS, Alves EA, Zugaib M, Francisco RP. Preeclampsia and superimposed preeclampsia: The same disease? The role of angiogenic biomarkers. Hypertens Pregnancy 2016;35:139–149 [DOI] [PubMed] [Google Scholar]
- 16. Dorniak-Wall T, Grivell RM, Dekker GA, Hague W, Dodd JM. The role of l-arginine in the prevention and treatment of pre-eclampsia: A systematic review of randomised trials. J Hum Hypertens 2014;28:230–235 [DOI] [PubMed] [Google Scholar]
- 17. Possomato-Vieira JS, Khalil RA. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. Adv Pharmacol 2016;77:361–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Espinoza J. Recent biomarkers for the identification of patients at risk for preeclampsia: The role of uteroplacental ischemia. Expert Opin Med Diagn 2012;6:121–130 [DOI] [PubMed] [Google Scholar]
- 19. Wright D, Wright A, Nicolaides KH. The competing risk approach for prediction of preeclampsia. Am J Obstet Gynecol 2020;223:10. [DOI] [PubMed] [Google Scholar]
- 20. Myatt L, Redman CW, Staff AC, et al. Strategy for standardization of preeclampsia research study design. Hypertension 2014;63:1293–1301 [DOI] [PubMed] [Google Scholar]
- 21. Perales A, Delgado JL, de la Calle M, et al. sFlt-1/PlGF for prediction of early-onset pre-eclampsia: STEPS (Study of Early Pre-eclampsia in Spain). Ultrasound Obstet Gynecol 2017;50:373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuc S, Wortelboer EJ, van Rijn BB, Franx A, Visser GH, Schielen PC. Evaluation of 7 serum biomarkers and uterine artery Doppler ultrasound for first-trimester prediction of preeclampsia: A systematic review. Obstet Gynecol Surv 2011;66:225–239 [DOI] [PubMed] [Google Scholar]
- 23. ACOG Committee Opinion No. 743: Low-dose aspirin use during pregnancy. Obstet Gynecol 2018;132:e44–e52 [DOI] [PubMed] [Google Scholar]
- 24. Hoffman MK, Goudar SS, Kodkany BS, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): A randomised, double-blind, placebo-controlled trial. Lancet 2020;395:285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Atallah A, Lecarpentier E, Goffinet F, Doret-Dion M, Gaucherand P, Tsatsaris V. Aspirin for prevention of preeclampsia. Drugs 2017;77:1819–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dhariwal NK, Lynde GC. Update in the management of patients with preeclampsia. Anesthesiol Clin 2017;35:95–106 [DOI] [PubMed] [Google Scholar]
- 27. Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol 2019;15:275–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karumanchi SA, Granger JP. Preeclampsia and pregnancy-related hypertensive disorders. Hypertension 2016;67:238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petersen EE, Davis NL, Goodman D, et al. Vital signs: Pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep 2019;68:423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pearson GD, Veille JC, Rahimtoola S, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA 2000;283:1183–1188 [DOI] [PubMed] [Google Scholar]
- 31. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation 2016;133:1397–1409 [DOI] [PubMed] [Google Scholar]
- 32. Krishnamoorthy P, Garg J, Palaniswamy C, et al. Epidemiology and outcomes of peripartum cardiomyopathy in the United States: Findings from the Nationwide Inpatient Sample. J Cardiovasc Med (Hagerstown) 2016;17:756–761 [DOI] [PubMed] [Google Scholar]
- 33. Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: Diagnosis, prognosis, and management. J Am Coll Cardiol 2011;58:659–670 [DOI] [PubMed] [Google Scholar]
- 34. Goland S, Modi K, Hatamizadeh P, Elkayam U. Differences in clinical profile of African-American women with peripartum cardiomyopathy in the United States. J Card Fail 2013;19:214–218 [DOI] [PubMed] [Google Scholar]
- 35. Petersen EE, Davis NL, Goodman D, et al. Racial/ethnic disparities in pregnancy-related deaths: United States, 2007–2016. MMWR Morb Mortal Wkly Rep 2019;68:762–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horne BD, Rasmusson KD, Alharethi R, et al. Genome-wide significance and replication of the chromosome 12p11.22 locus near the PTHLH gene for peripartum cardiomyopathy. Circ Cardiovasc Genet 2011;4:359–366 [DOI] [PubMed] [Google Scholar]
- 37. Sheppard R, Hsich E, Damp J, et al. GNB3 C825T polymorphism and myocardial recovery in peripartum cardiomyopathy: Results of the Multicenter Investigations of Pregnancy-Associated Cardiomyopathy Study. Circ Heart Fail 2016;9:e002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ware JS, Li J, Mazaika E, et al. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med 2016;374:233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fett JD, Sundstrom BJ, Etta King M, Ansari AA. Mother-daughter peripartum cardiomyopathy. Int J Cardiol 2002;86:331–332 [DOI] [PubMed] [Google Scholar]
- 40. Morales A, Painter T, Li R, et al. Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation 2010;121:2176–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, et al. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation 2010;121:2169–2175 [DOI] [PubMed] [Google Scholar]
- 42. Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007;128:589–600 [DOI] [PubMed] [Google Scholar]
- 43. Halkein J, Tabruyn SP, Ricke-Hoch M, et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest 2013;123:2143–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moulig V, Pfeffer TJ, Ricke-Hoch M, et al. Long-term follow-up in peripartum cardiomyopathy patients with contemporary treatment: Low mortality, high cardiac recovery, but significant cardiovascular co-morbidities. Eur J Heart Fail 2019;21:1534–1542 [DOI] [PubMed] [Google Scholar]
- 45. Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012;485:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011;123:2856–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bdolah Y, Lam C, Rajakumar A, et al. Twin pregnancy and the risk of preeclampsia: Bigger placenta or relative ischemia? Am J Obstet Gynecol 2008;198:428..e1–428.e6. [DOI] [PubMed] [Google Scholar]
- 48. Hammadah M, Georgiopoulou VV, Kalogeropoulos AP, et al. Elevated soluble Fms-like tyrosine kinase-1 and placental-like growth factor levels are associated with development and mortality risk in heart failure. Circ Heart Fail 2016;9:e002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McNamara DM, Elkayam U, Alharethi R, et al. Clinical outcomes for peripartum cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol 2015;66:905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Damp J, Givertz MM, Semigran M, et al. Relaxin-2 and soluble Flt1 levels in peripartum cardiomyopathy: Results of the Multicenter IPAC Study. JACC Heart Fail 2016;4:380–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goland S, Modi K, Bitar F, et al. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail 2009;15:645–650 [DOI] [PubMed] [Google Scholar]
- 52. Harper MA, Meyer RE, Berg CJ. Peripartum cardiomyopathy: Population-based birth prevalence and 7-year mortality. Obstet Gynecol 2012;120:1013–1019 [DOI] [PubMed] [Google Scholar]
- 53. Irizarry OC, Levine LD, Lewey J, et al. Comparison of clinical characteristics and outcomes of peripartum cardiomyopathy between African American and non-African American women. JAMA Cardiol 2017;2:1256–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Azibani F, Pfeffer TJ, Ricke-Hoch M, et al. Outcome in German and South African peripartum cardiomyopathy cohorts associates with medical therapy and fibrosis markers. ESC Heart Fail 2020;7:512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sliwa K, Blauwet L, Tibazarwa K, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: A proof-of-concept pilot study. Circulation 2010;121:1465–1473 [DOI] [PubMed] [Google Scholar]
- 56. Haghikia A, Podewski E, Libhaber E, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol 2013;108:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hilfiker-Kleiner D, Haghikia A, Berliner D, et al. Bromocriptine for the treatment of peripartum cardiomyopathy: A multicentre randomized study. Eur Heart J 2017;38:2671–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gastrich MD, Zinonos S, Bachmann G, et al. Preeclamptic women are at significantly higher risk of future cardiovascular outcomes over a 15-year period. J Womens Health (Larchmt) 2020;29:74–83 [DOI] [PubMed] [Google Scholar]
- 59. Gunderson EP, Chiang V, Pletcher MJ, et al. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: The Coronary Artery Risk Development in Young Adults study. J Am Heart Assoc 2014;3:e000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Haas DM, Parker CB, Marsh DJ, et al. Association of adverse pregnancy outcomes with hypertension 2 to 7 years postpartum. J Am Heart Assoc 2019;8:e013092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Horn J, Tanz LJ, Stuart JJ, et al. Early or late pregnancy loss and development of clinical cardiovascular disease risk factors: A prospective cohort study. BJOG 2019;126:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tanz LJ, Stuart JJ, Williams PL, et al. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. Circulation 2017;135:578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tobias DK, Stuart JJ, Li S, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med 2017;177:1735–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2017;10:e003497. [DOI] [PubMed] [Google Scholar]
- 65. Catov JM, Dodge R, Barinas-Mitchell E, et al. Prior preterm birth and maternal subclinical cardiovascular disease 4 to 12 years after pregnancy. J Womens Health (Larchmt) 2013;22:835–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Catov JM, Wu CS, Olsen J, Sutton-Tyrrell K, Li J, Nohr EA. Early or recurrent preterm birth and maternal cardiovascular disease risk. Ann Epidemiol 2010;20:604–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Silverberg O, Park AL, Cohen E, Fell DB, Ray JG. Premature cardiac disease and death in women whose infant was preterm and small for gestational age: A retrospective cohort study. JAMA Cardiol 2018;3:247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): Population-based retrospective cohort study. Lancet 2005;366:1797–1803 [DOI] [PubMed] [Google Scholar]
- 69. Riise HKR, Sulo G, Tell GS, et al. Association between gestational hypertension and risk of cardiovascular disease among 617,589 Norwegian women. J Am Heart Assoc.2018;7:e008337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, Louis JM. Pregnancy as a window to future health: Maternal placental syndromes and short-term cardiovascular outcomes. Am J Obstet Gynecol 2016;215:484..e1–484.e14. [DOI] [PubMed] [Google Scholar]
- 71. Lane-Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ. Long-term cardiovascular risks associated with adverse pregnancy outcomes: JACC review topic of the week. J Am Coll Cardiol 2019;73:2106–2116 [DOI] [PubMed] [Google Scholar]
- 72. Battarbee AN, Sinkey RG, Harper LM, Oparil S, Tita ATN. Chronic hypertension in pregnancy. Am J Obstet Gynecol 2020;222:532–541 [DOI] [PubMed] [Google Scholar]
- 73. Grieger JA, Bianco-Miotto T, Grzeskowiak LE, et al. Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: A prospective cohort of nulliparous women. PLoS Med 2018;15:e1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hui D, Hladunewich MA. Chronic kidney disease and pregnancy. Obstet Gynecol 2019;133:1182–1194 [DOI] [PubMed] [Google Scholar]
- 75. Williams A, Grantz K, Seeni I, et al. Obstetric and neonatal complications among women with autoimmune disease. J Autoimmun 2019;103:102287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gould GS, Havard A, Lim LL; The Psanz Smoking in Pregnancy Expert Group; Kumar R. Exposure to tobacco, environmental tobacco smoke and nicotine in pregnancy: A pragmatic overview of reviews of maternal and child outcomes, effectiveness of interventions and barriers and facilitators to quitting. Int J Environ Res Public Health 2020;17:2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ghosh G, Grewal J, Mannisto T, et al. Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethn Dis 2014;24:283–289 [PMC free article] [PubMed] [Google Scholar]
- 78. Kent ST, McClure LA, Zaitchik BF, Gohlke JM. Area-level risk factors for adverse birth outcomes: Trends in urban and rural settings. BMC Pregnancy Childbirth 2013;13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Metcalfe A, Lail P, Ghali WA, Sauve RS. The association between neighbourhoods and adverse birth outcomes: A systematic review and meta-analysis of multi-level studies. Paediatr Perinat Epidemiol 2011;25:236–245 [DOI] [PubMed] [Google Scholar]
- 80. Schaaf JM, Liem SM, Mol BW, Abu-Hanna A, Ravelli AC. Ethnic and racial disparities in the risk of preterm birth: A systematic review and meta-analysis. Am J Perinatol 2013;30:433–450 [DOI] [PubMed] [Google Scholar]
- 81. Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: The Avon Longitudinal Study of Parents and Children. Circulation 2012;125:1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Parikh NI, Laria B, Nah G, et al. Cardiovascular disease-related pregnancy complications are associated with increased maternal levels and trajectories of cardiovascular disease biomarkers during and after pregnancy. J Womens Health (Larchmt) 2020;29:1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation 2010;122:478–487 [DOI] [PubMed] [Google Scholar]
- 84. Berks D, Hoedjes M, Raat H, et al. Feasibility and effectiveness of a lifestyle intervention after complicated pregnancies to improve risk factors for future cardiometabolic disease. Pregnancy Hypertens 2019;15:98–107 [DOI] [PubMed] [Google Scholar]
- 85. Hauspurg A, Ying W, Hubel CA, Michos ED, Ouyang P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clin Cardiol 2018;41:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e13–e115 [DOI] [PubMed] [Google Scholar]
- 87. Costantine MM, Cleary K, Hebert MF, et al. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: A pilot randomized controlled trial. Am J Obstet Gynecol 2016;214:720..e1–720.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lefkou E, Mamopoulos A, Dagklis T, Vosnakis C, Rousso D, Girardi G. Pravastatin improves pregnancy outcomes in obstetric antiphospholipid syndrome refractory to antithrombotic therapy. J Clin Invest 2016;126:2933–2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. ACOG Practice Bulletin No. 212: Pregnancy and heart disease. Obstet Gynecol 2019;133:e320–e356 [DOI] [PubMed] [Google Scholar]
- 90. Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613 [DOI] [PubMed] [Google Scholar]
- 91. Grieger JA, Grzeskowiak LE, Clifton VL. Preconception dietary patterns in human pregnancies are associated with preterm delivery. J Nutr 2014;144:1075–1080 [DOI] [PubMed] [Google Scholar]
- 92. Stang J, Huffman LG. Position of the Academy of Nutrition and Dietetics: Obesity, reproduction, and pregnancy outcomes. J Acad Nutr Diet 2016;116:677–691 [DOI] [PubMed] [Google Scholar]
- 93. Facco FL, Parker CB, Reddy UM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol 2017;129:31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nguyen B, Jin K, Ding D. Breastfeeding and maternal cardiovascular risk factors and outcomes: A systematic review. PLoS One 2017;12:e0187923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol 2009;113:974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA 2005;294:2601–2610 [DOI] [PubMed] [Google Scholar]
- 97. Catov JM, Parker CB, Gibbs BB, et al. Patterns of leisure-time physical activity across pregnancy and adverse pregnancy outcomes. Int J Behav Nutr Phys Act 2018;15:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Eunice Kennedy Shriver National Institute of Child Health and Human Development. Task Force on Research Specific to Pregnant Women and Lactating Women (PRGLAC). 2020. Available at: https://www.nichd.nih.gov/about/advisory/PRGLAC Accessed June7, 2020