Abstract
Significance: Staphylococcus aureus is among the leading causes of bacterial infections worldwide. The high burden of S. aureus among human and animal hosts, which includes asymptomatic carriage and infection, is coupled with a notorious ability of the microbe to become resistant to antibiotics. Notably, S. aureus has the ability to produce molecules that promote evasion of host defense, including the ability to avoid killing by neutrophils.
Recent Advances: Significant progress has been made to better understand S. aureus–host interactions. These discoveries include elucidation of the role played by numerous S. aureus virulence molecules during infection. Based on putative functions, a number of these virulence molecules, including S. aureus alpha-hemolysin and protein A, have been identified as therapeutic targets. Although it has not been possible to develop a vaccine that can prevent S. aureus infections, monoclonal antibodies specific for S. aureus virulence molecules have the potential to moderate the severity of disease.
Critical Issues: Therapeutic options for treatment of methicillin-resistant S. aureus (MRSA) are limited, and the microbe typically develops resistance to new antibiotics. New prophylactics and/or therapeutics are needed.
Future Directions: Research that promotes an enhanced understanding of S. aureus–host interaction is an important step toward developing new therapeutic approaches directed to moderate disease severity and facilitate treatment of infection. This research effort includes studies that enhance our view of the interaction of S. aureus with human neutrophils. Antioxid. Redox Signal. 34, 452–470.
Keywords: Staphylococcus aureus, MRSA, CA-MRSA, antibiotic resistance, neutrophil, PMN
Introduction
Antibiotic resistance in bacteria is a serious problem globally. According to the 2019 AR Threats Report published by the Centers for Disease Control and Prevention (CDC, Atlanta, GA), 2.8 million people in the United States acquire an antibiotic-resistant infection annually, and there are an estimated 35,000 associated deaths. The problem is similar in other parts of the world. For example, in Europe, there are an estimated 33,000 annual deaths attributed to antimicrobial resistant infections (21). In addition to the reported morbidity and mortality, antibiotic resistant bacteria cause a significant economic burden. For instance, the annual cost burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) in the United States was estimated to be as great as $13.8 billion (97).
S. aureus resistance to penicillin was reported in the 1940s, and infections with antibiotic resistant S. aureus remain a problem today (81, 166). Indeed, MRSA is currently classified as a “serious threat” by the CDC, and threat level is based on seven factors including clinical impact, incidence, transmissibility, and available treatments. Consistent with this assessment, S. aureus is one of the six “ESCAPE bugs”—those that cause the majority of nosocomial infections in the United States and are resistant to antibiotics (16). Inasmuch as S. aureus has remained a threat to human health throughout recorded history, and because the microbe can readily develop resistance to antibiotics, S. aureus will be considered as a model organism for the purpose of this review.
S. aureus is a Commensal Microbe and an Opportunistic Pathogen
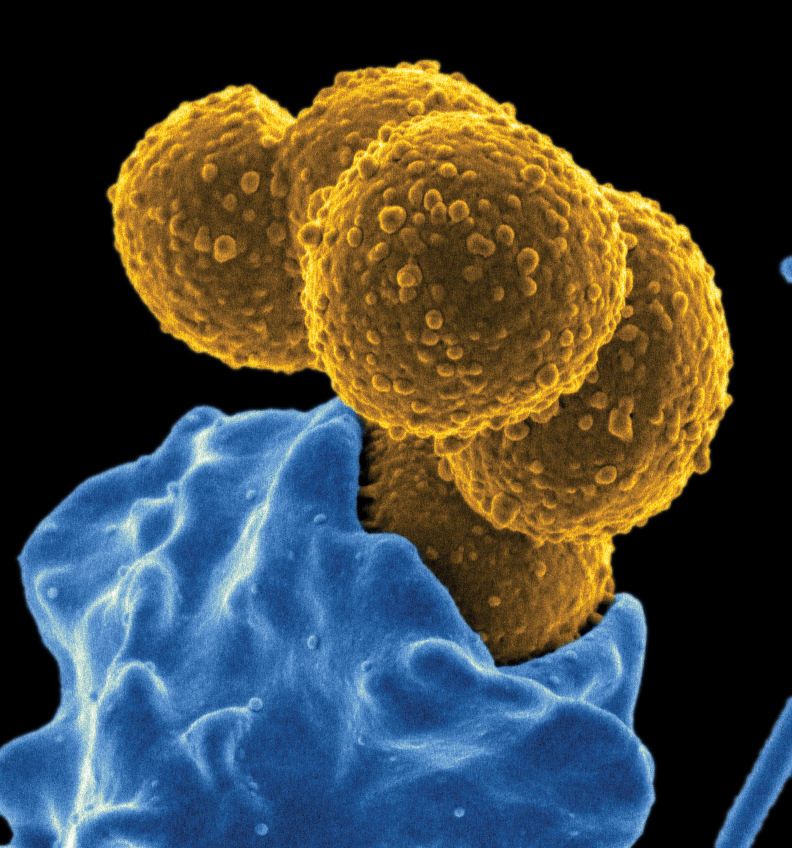
S. aureus is a gram-positive, catalase-positive, facultative anaerobic bacterium that colonizes healthy humans and animals. The microbe is also an important opportunistic pathogen. S. aureus was first identified in pus from a leg abscess in the late 19th century by Alexander Ogston (122). The bacterium is named for the grape-like appearance of multiple cocci under the microscope and for the golden color of colonies on solid media (staphyle is Greek for grape and aureus is Latin for golden) (Fig. 1). Approximately 30% of people are asymptomatically colonized by S. aureus in the nares and another third are at least transiently colonized (57). In recent years, studies have investigated the interaction of S. aureus with the nasal microbiota and found that certain nasal commensal microbes enhance colonization with S. aureus, whereas others inhibit colonization (91). This knowledge can potentially be exploited for the development of novel decolonization strategies that are employed to decrease the burden of S. aureus carriage in targeted populations (120).
FIG. 1.
Staphylococcus aureus. Scanning electron micrograph of S. aureus (yellow) bound to a human neutrophil (blue).
Recent studies have demonstrated that S. aureus colonization of humans is more extensive than previously known. In addition to the nose, S. aureus can be isolated from many other body sites, including the oropharynx, axillae, groin area, perineum, rectum, and intestine (2, 115). Asymptomatic colonization with S. aureus is a risk factor for subsequent infection with the colonizing strain (71, 183). However, infections in colonized individuals are typically less severe compared with those in individuals who are not colonized with S. aureus, perhaps because such individuals have developed some protective immunity against severe infection. Other risk factors for S. aureus infection include damaged skin or mucous membranes, immunosuppression (e.g., due to chemotherapy, age, congenital neutrophil disorders, or acquired immunodeficiency syndrome), the presence of catheters or prosthetics, and underlying diseases such as cancer or diabetes (106, 178). S. aureus is transmitted by direct skin-to-skin contact or via contaminated fomites. Thus, S. aureus carriers play an important epidemiological role in transmission and perpetuation of disease.
Manifestations of S. aureus infection
S. aureus infections can be mild to life-threatening, localized or disseminated, and affect any organ of the body (178). Thus, symptoms and pathologies caused by S. aureus are manifold. The most common presentations of S. aureus infection are skin and soft tissue infections (SSTIs), bacteremia, endocarditis, pneumonia, device- or prosthetic-related infections, and osteoarticular infections (33, 106, 178). Here, we highlight a selected number of infections or syndromes for which S. aureus is notorious.
Moran et al. reported that MRSA—primarily USA300 strains—accounted for 59% of all purulent SSTIs reporting to emergency departments in the United States in 2004 (116). These data provided strong support to the idea that S. aureus is the leading cause of community bacterial infections. Talan et al. made similar findings for 2008, which indicated the high incidence of CA-MRSA had remained unchanged (174). Although S. aureus remains the leading cause of SSTIs in the United States, recent data indicate the incidence of SSTIs in emergency departments decreased from 2009 to 2014 (117). These findings are consistent with the reported trends for decreased MRSA SSTIs in this setting (94). Collectively, these and other reports suggest the epidemic of CA-MRSA infections peaked before 2010.
S. aureus is also a leading cause of bloodstream infections (BSIs). For instance, recent multicenter studies reported that S. aureus is the most abundant cause of health care-associated BSIs (4, 87). In 75% of cases, S. aureus BSI results from a primary infection such as SSTI, pneumonia, or an infected indwelling catheter, whereas in 25% of cases, the primary source of infection remains unknown (178). Other known risk factors for S. aureus BSI include human immunodeficiency virus infection, hemodialysis, cancer, recent organ transplantation, and injection drug use (95, 175). Bacteremia caused by S. aureus is typically associated with a relatively high mortality rate, which is varied and depends on multiple factors including patient age and comorbidities (178).
S. aureus is the most abundant cause of infective endocarditis in the industrialized world. Such infections represent more than ∼25% of all infective endocarditis cases (178), and they are most often health care-associated (49, 178). For example, during the time period of 1998–2009, 28.7% of infective endocarditis cases in the United States were caused by S. aureus (during the same time frame, 24.7% were caused by streptococci, which ranked second) (15). Notably, there was a significant increase in the percentage of S. aureus infective endocarditis cases during this time period (15). Bor et al. suggested that the noted increase in cases is linked to an increase in cardiac devices and patient implants. Consistent with this idea, patients with prosthetic heart valves and/or S. aureus bacteremia are known to be at risk for infective endocarditis (43). S. aureus infective endocarditis has an overall mortality rate of 22%–66%, which is greater than that for other pathogens (178).
Pneumonia is an important manifestation of S. aureus infection, and S. aureus pneumonia occurs in both community and health care settings (178). For example, in a large retrospective cohort study, Kollef et al. reported that S. aureus is the leading cause (among bacterial pathogens) of health care-associated, hospital acquired, and ventilator-associated pneumonias in the United States (86). MRSA accounted for ∼34%–57% of these S. aureus pneumonia cases (86). Magill et al. reported similar findings for a study that surveyed 199 hospitals in 2015 (108). Mortality rates for health care-associated and hospital-acquired pneumonias have been reported as ∼18%–19%, thereby underscoring the importance of S. aureus as a cause of severe respiratory infections.
The incidence of S. aureus community-acquired pneumonia is less than that of health care-associated or hospital-acquired pneumonias (87), although it can also be severe and/or fatal (50, 62). A characteristic difference between health care-associated or hospital-acquired pneumonias and community-associated/acquired pneumonias is the lack of patient comorbidities in the latter group. That said, CA-MRSA pneumonia-related fatalities have often been associated with antecedent influenza virus infection (53, 62). There is also compelling evidence that S. aureus contributed to (or was the underlying cause of) severe or fatal pneumonia during influenza A virus pandemics that occurred in the 20th century (27, 143, 156).
Inasmuch as S. aureus is a leading cause of BSIs, it is not surprising that it causes bone and joint infections. Indeed, the organism is the most frequently isolated agent from individuals with infectious osteomyelitis and prosthetic joint infection in multiple regions of the world (89, 138, 145, 178). S. aureus can disseminate to bones and joints via the bloodstream or direct placement of contaminated prosthetic devices (178). Osteomyelitis is often difficult to treat and can become chronic (138). S. aureus can form a biofilm within the bone, (123), rendering the organism less susceptible to host defenses and antibiotics. In addition, S. aureus can form small colony variants (SCVs), which are phenotypically characterized by reduced metabolic activity and slow growth (and thus form small colonies on agar plates) and are more resistant to killing than non-SCV S. aureus (180). SCVs have been implicated in the pathogenesis of chronic osteomyelitis, thereby contributing to the difficulty with treatment (77).
History and Mechanisms of Antibiotic Resistance in S. aureus
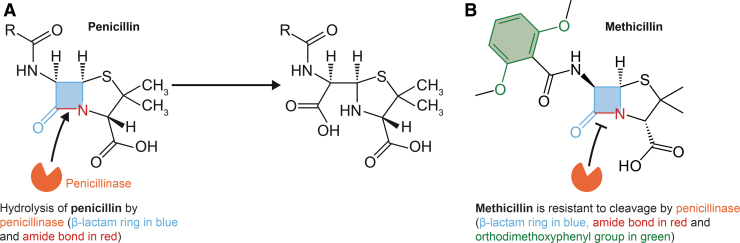
S. aureus was susceptible to most clinically useful antibiotics before the modern antibiotic era. Indeed, penicillin was used widely and successfully to treat S. aureus (and other) infections in the 1940s and 1950s. Such widespread use of this important antibiotic led ultimately to the development of resistance among many bacterial pathogens including S. aureus (81). Kirby discovered that S. aureus resistance to penicillin was due to the activity of a penicillinase that he isolated from resistant clinical isolates (81). This penicillinase, now more commonly known as a type of β-lactamase, is an enzyme encoded by the blaZ gene, which in S. aureus is often located on a plasmid within a transposon (118). Penicillinase hydrolyzes the amide bond of the β-lactam ring of penicillin and ampicillin (126) (Fig. 2A). Penicillinase production is varied among S. aureus strains, but those with high production are also more likely to be resistant to other antibiotics, such as tetracycline and streptomycin (142).
FIG. 2.
Interaction of S. aureus penicillinase with penicillin and methicillin. Penicillinase hydrolyzes the amide bond (highlighted in red) of the β-lactam ring of penicillin and ampicillin (A). Methicillin is resistant to cleavage by S. aureus penicillinase (a β-lactamase) due to the presence of an ortho-dimethoxyphenyl group (highlighted in green) that sterically hinders the enzyme from hydrolyzing its target amide bond (B).
S. aureus strains can be naturally resistant to penicillin, or they can acquire resistance during penicillin treatment, as was demonstrated by Spink and Ferris in 1947 (165). Penicillin-resistant S. aureus was first isolated in hospitals but did not remain constrained to the health care setting and ultimately caused infections in the community. These infections were largely caused by strains belonging to the phage type 80/81, which was later characterized as clonal complex 30 (CC30) by molecular typing methods (144, 151). The phage-type 80/81 strain was particularly virulent and transmissible, and ultimately became pandemic (144).
Inasmuch as the majority of phage-type 80/81 clinical isolates were penicillin resistant, a new therapeutic was needed to treat S. aureus infections. To that end, methicillin (marketed as Celbenin [BRL 1241] by Beecham Research Laboratories, Inc.—now GlaxoSmithKline), a semisynthetic β-lactam antibiotic, was introduced in 1959/1960 as a treatment for infections caused by penicillin-resistant staphylococci (82). Methicillin is resistant to cleavage by S. aureus β-lactamase due to the presence of an ortho-dimethoxyphenyl group that sterically hinders the enzyme from hydrolyzing its target amide bond (168) (Fig. 2B). Within a year after the introduction of methicillin, MRSA were isolated from hospitalized patients (8, 73).
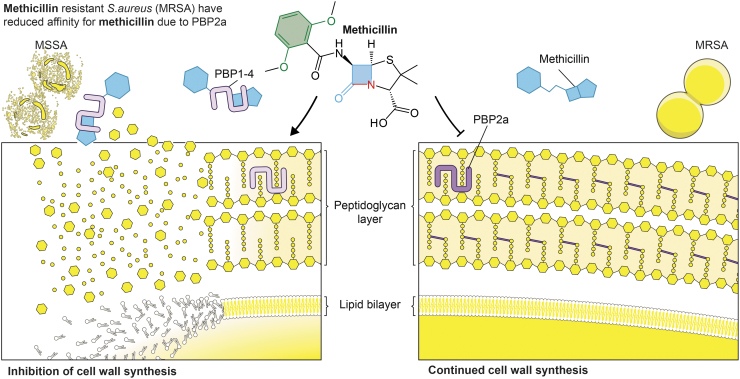
Methicillin resistance is conferred by the mecA gene, which is located on a mobile genetic element known as staphylococcal cassette chromosome mec (96). As such, the mecA gene is acquired via horizontal gene transfer. The mecA gene encodes a transpeptidase known as PBP2a, an altered penicillin binding protein that has low affinity for β-lactam antibiotics (23, 46). The reduced binding of PBP2a with β-lactam antibiotics circumvents the ability of these antibiotics to inhibit cell wall synthesis (Fig. 3). Importantly, PBP2a confers resistance to all β-lactam antibiotics—not just methicillin (23). A recent study by Harkins et al. suggested that MRSA was present before the use of methicillin as a therapeutic agent (64). This finding changed the long-standing notion that clinical use of methicillin was the underlying factor in the rapid emergence of MRSA. Rather, Harkin et al. proposed that it was the widespread use of penicillin that selected for mecA-positive S. aureus in the 1940s (64).
FIG. 3.
S. aureus methicillin resistance is conferred by PBP2a, which has reduced affinity for methicillin. MSSA (left side) contains PBP1–4 that are readily bound by methicillin, a process that inhibits peptidoglycan and cell wall synthesis. By comparison, PBP2a has reduced affinity for methicillin, and thus PBP2a can participate in cell wall synthesis. MSSA, methicillin-susceptible S. aureus; PBP2a, penicillin-binding protein 2a; PBP1–4, penicillin binding proteins 1–4.
MRSA epidemiology and subtypes
MRSA has remained a significant problem globally since the 1960s. The pathogen is endemic in hospitals worldwide and is often the leading cause of infections in this setting. CA-MRSA was epidemic in the United States and in other regions of the world in the first decade of the 21st century (24). More recently, the CDC estimated that there were 323,700 cases of MRSA in hospitalized patients in the United States in 2017, and these cases resulted in an estimated 10,600 deaths. The overall prevalence of MRSA among S. aureus isolates in 11 countries representing Central America and South America was reported as ∼48% for 2004–2007 (149). These findings are consistent with a subsequent study by Reyes et al., who reported that MRSA comprised 41% of all S. aureus isolates from 32 tertiary hospitals in Columbia, Ecuador, Peru, and Venezuela (139). In Europe, the prevalence of MRSA-invasive infection differs by country and ranges widely (e.g., the occurrence is 0.9% in the Netherlands and 56% in Romania). As of 2014, Scandinavian countries had the lowest rates of invasive MRSA infections, whereas southern and southeastern European countries have the highest rates of such infections (greater than 25% occurrence).
The sustained high prevalence of MRSA, coupled with the emergence of CA-MRSA, led to the implementation of more robust infection control and prevention measures, and an increased awareness of the scope of the problem (196). Consistent with the implementation of these measures, the number of estimated hospital MRSA infections in the United States has decreased steadily since 2005 (88). Kourtis et al. used data that were collected from 400 acute care hospitals participating in the CDCs Emerging Infections Program population surveillance study to estimate MRSA BSI rates in the United States over an 11-year period (88). Notably, there were dramatic reductions in hospital-onset and community-onset MRSA BSIs in the United States during the period of 2005–2012, but no change in infection rates during 2012–2017 (88). The reason for the lack of continued reduction in MRSA BSIs is not clear. There is also evidence that infection rates for MRSA are on the decline in countries outside the United States. For example, a similar decline in MRSA BSIs was reported by Wyllie et al. for hospitals in Oxford, United Kingdom, from 1998 to 2010 (196).
Compared with health care-associated MRSA infections, there has been little change in the rate of CA-MRSA BSIs in the United States since 2005. Many factors likely contribute to the unchanging rate of CA-MRSA infections, including the lack of a controlled environment for infection control measures and the strain of S. aureus. As suggested by Kourtis et al., this differential is perhaps explained by a decrease in infections caused by pulsed-field type USA100 strains, which are primarily in the health care setting.
Vancomycin-intermediate S. aureus and vancomycin-resistant S. aureus
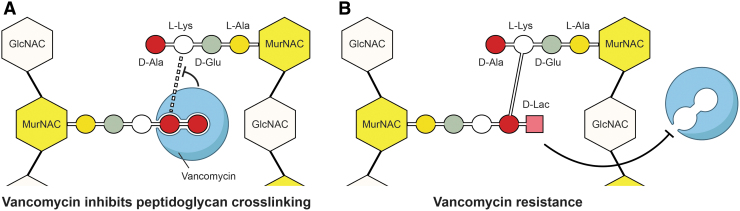
The glycopeptide antibiotic vancomycin continues to be important for treatment of severe MRSA infections (103). Vancomycin inhibits cell wall synthesis by binding to D-Ala-D-Ala residues of peptidoglycan precursor molecules, thereby blocking peptidoglycan synthesis (111) (Fig. 4A). As with virtually all clinically useful antibiotics, S. aureus can become resistant to vancomycin. Vancomycin resistance in S. aureus can be separated into two categories: (i) complete vancomycin resistance conferred by the vanA gene operon—hereafter referred to as vancomycin-resistant S. aureus (VRSA) and (ii) vancomycin-intermediate S. aureus (VISA).
FIG. 4.
Antibacterial action of vancomycin and mechanism of resistance. Vancomycin inhibits cell wall synthesis by binding to D-Ala-D-Ala residues of peptidoglycan precursor molecules (A). In vancomycin-resistant S. aureus, D-Ala-D-Ala residues of the peptidoglycan precursor molecule are replaced by D-Ala-D-Lac residues, which do not bind vancomycin. Peptidoglycan cross-linking occurs normally (B).
In VRSA, the D-Ala-D-Ala residues of the peptidoglycan precursor molecule are replaced by D-Ala-D-Lac residues, which prevent binding of vancomycin (111) (Fig. 4B). The minimum inhibitory concentration of vancomycin for VRSA is ≥16 μg/mL, whereas that for VISA strains is 4–8 μg/mL. VRSA was first reported in the United States in 2002 (22) and in Europe in 2013 (52). To date, only 15 VRSA cases have been reported in the United States (188). VISA were first isolated in Japan in 1996 and are now widespread (67, 199). In contrast to VRSA, our understanding of the mechanisms that contribute to the development of VISA is incomplete. Previous studies have demonstrated that the VISA phenotype typically develops as a series of stepwise mutations in genes involved (directly or indirectly) in cell wall biosynthesis (78, 119) and/or occurs by transient changes in the S. aureus transcriptome (61, 69). These attributes, along with an often heterogeneous phenotype during the early stages of the development of VISA, make detection difficult. Although VRSA infections are difficult to treat, there appears to be a fitness cost associated with vanA-mediated resistance, and to date, no major outbreaks of VRSA have occurred (32, 48). By comparison, the prevalence of VISA is much greater. According to a systematic review by Zhang et al., who considered 91 studies on VISA from different countries during 1997 and 2014, the prevalence of VISA has been estimated as comprising ∼7.9% of MRSA isolates recovered for 2010–2014 in Asia, Oceania, India, Europe, and North America (199).
Livestock-associated MRSA
Livestock-associated MRSA (LA-MRSA) are transmitted from livestock (pigs, poultry, and cattle) to humans. This zoonotic transmission pattern was first observed in the mid-2000s and led to the introduction of the term LA-MRSA (76, 184). As is the case with S. aureus in general, colonization with LA-MRSA is a risk factor for subsequent infection (54). Farmers, butchers, abattoir workers, and veterinarians are especially at risk for transmission and subsequent colonization and infection (14, 72, 195). Indeed, carriage rates among pig farmers have been shown to be high in Canada, the United States, and certain European countries (54, 80, 161). In Europe and the United States, predominant LA-MRSA clones that cause human infection are classified by multilocus sequence typing (MLST or ST) as ST398 or CC398 (54, 55, 161). Genomic studies have provided strong evidence that LA-MRSA CC398 evolved from a human-tropic MSSA CC398 lineage, which lost human-specific immune modulators and acquired resistance to antibiotics (137, 181).
The need for new treatments and prophylactics
Most S. aureus infections can be treated successfully with appropriate antibiotics. However, MRSA often harbor resistance to multiple antibiotics, and this attribute combined with patient comorbidities makes treatment difficult and sometimes unsuccessful. Hence, there is a need to develop new therapeutics and prophylactics (i.e., vaccines) that can reduce MRSA morbidity and mortality. The need for novel treatment options is underscored by the fact that S. aureus can develop resistance to the newest antimicrobials such as ceftaroline, a fifth-generation cephalosporin that is active against MRSA (190). In 2017, the WHO published a list of antibiotic-resistant priority pathogens for which new antimicrobials are needed. This list includes MRSA, VISA, and VRSA. Therapeutic and/or prophylactic approaches that target S. aureus virulence molecules and/or those that promote bacterial survival in the host are potentially a viable alternative to (or could be used in combination with) antibiotic therapies. Inasmuch as many S. aureus virulence molecules have evolved to promote evasion of human innate host defenses such as polymorphonuclear leukocytes (PMNs or neutrophils), we provide a concise overview of the role of neutrophils in host defense against S. aureus as relevant background.
Neutrophils in Host Defense Against Bacterial Infection
Neutrophils are short-lived, professional phagocytes, and the most abundant cells of the innate immune system. They are recruited rapidly to sites of infection or injury and are highly effective at ingesting and destroying bacteria and fungi. The importance of neutrophils in host defense is underscored by the fact that individuals with neutropenia or inherited neutrophil disorders often suffer from recurring bacterial infections and notably those caused by S. aureus (68).
Neutrophils comprise 55%–70% of circulating white cells in human peripheral blood. They are terminally differentiated leukocytes and have a relatively short life span during steady-state conditions. Neutrophils develop as postmitotic granulocyte precursors in bone marrow for several days (7.5 days as mitotic precursors and 6.5 days as postmitotic cells), and mature PMNs are then released into the bloodstream, where they circulate for half a day (7). These cells then enter tissues where they remain for 1–2 days, ultimately undergo apoptosis and are removed by mononuclear phagocytes.
Granulocyte turnover is extraordinary in healthy individuals, ∼1.8 × 109 cells/kg/day, and the majority of hematopoiesis is dedicated to their production (6). Maturation from pluripotent hematopoietic stem cells in the bone marrow is tightly regulated by transcription factors and cytokines such as PU.1, C/EBPα, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor. As granulocyte precursors mature in bone marrow, they develop antimicrobial capacity, which includes formation of cytoplasmic granules (7). During infection or insult, neutrophil maturation can be shortened significantly and the egress of neutrophils into the bloodstream expedited. This process is known as emergency granulopoiesis.
Stimuli that recruit neutrophils to inflammatory sites can be derived from damaged tissue, resident immune cells, and/or invading microorganisms. To reach inflamed tissue, neutrophils need to exit the vasculature. This multistep process encompasses rolling, adhesion, crawling, and transmigration and is tightly regulated by the expression of selectins and integrins present on the surface of PMNs and endothelial cells (38). After extravasation, neutrophils migrate along a chemoattractant gradient that involves polarized expression of receptors, actin polymerization, and intracellular calcium mobilization (133). Chemoattractants include bacteria-derived N-formylated peptides such as N-formyl-methionyl-leucyl-phenylalanine (fMLF), host complement components including C5a, membrane-derived lipid mediators, and chemokines (133). There is a hierarchy of neutrophil chemoattractants—for example, bacteria-derived signals such as N-formylated peptides and C5a are dominant over host-derived chemokines (133). In addition, neutrophil LYN kinase, an SRC family kinase, can function as redox sensor for H2O2 released by damaged host cells, thereby promoting chemotaxis (36).
Neutrophils are professional phagocytes that efficiently recognize, phagocytose, and kill invading bacteria and fungi. Pattern recognition receptors (PRRs) on the neutrophil surface recognize and bind conserved bacterial structures, such as flagellin, LPS, peptidoglycan, lipoproteins, and β-glucans. Toll-like receptors (TLRs) are among these important neutrophil PRRs. Indeed, survival of S. aureus-infected mice lacking TLR2 is reduced significantly compared with wild-type infected mice (173).
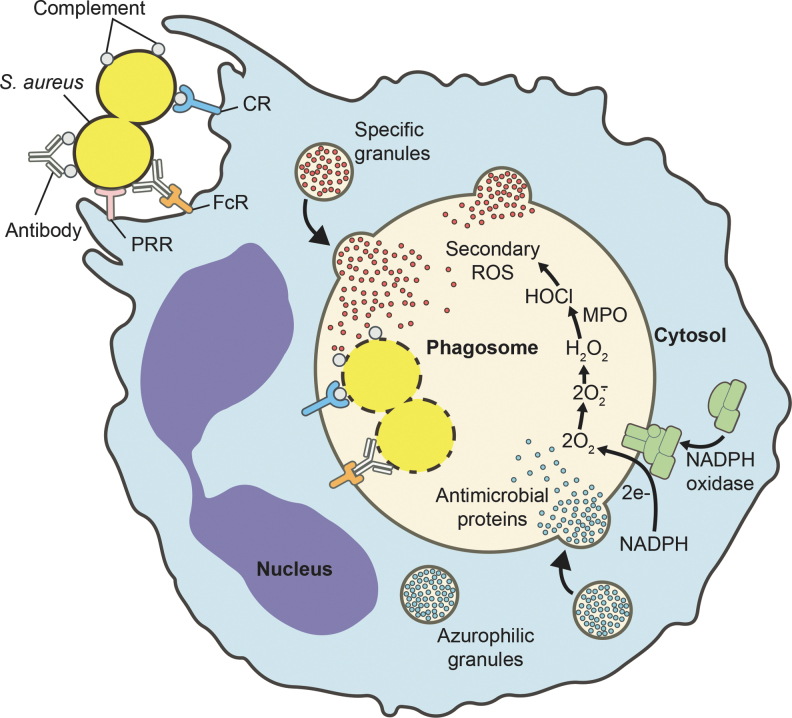
Although PRRs are important for recognition of microbes and priming of neutrophils for enhanced function, phagocytosis (uptake) is enhanced significantly by coating of microbes with host opsonins such as antibody and serum complement components. For example, studies performed in vitro have shown that phagocytosis of S. aureus by human neutrophils in suspension is increased significantly by opsonization in human serum (107). Such opsonins are recognized by receptors for antibody and serum complement, which when activated drive uptake or phagocytosis. Neutrophils possess several antibody Fc receptors, including three different IgG receptors and an IgA receptor, and several complement receptors (e.g., CD11b/CD18 or complement receptor 3 [CR3]). During phagocytosis, microbes are sequestered within a membrane-bound organelle known as a phagosome. Phagosome maturation involves fusion with cytoplasmic granules, thereby enriching the phagosome lumen with microbicidal peptides and proteins (38). Transmembrane components of the NADPH-dependent oxidase are also enriched in the phagosome membrane by fusion with specific and gelatinase-containing granules (38). These processes are important for neutrophil microbicidal activity.
Killing of microbes within the phagosome occurs by the combination of oxygen-dependent and oxygen-independent processes (Fig. 5). Oxygen-dependent killing is dependent on an NADPH oxidase, which transfers electrons from cytoplasmic NADPH to molecular oxygen, thereby producing superoxide. The increased oxygen consumption that accompanies production of superoxide is known as the respiratory burst. Superoxide is converted rapidly to hydrogen peroxide and other microbicidal reactive oxygen species (ROS), including singlet oxygen, chloramines, hydroxyl radicals, and hypochlorous acid (38). The importance of the NADPH oxidase in host defense is underscored by the observation that patients with chronic granulomatous disease (CGD), a genetic disorder characterized by the inability of phagocytes to produce superoxide, are more susceptible to bacterial and fungal infections (68).
FIG. 5.
Neutrophil phagocytosis and activation. Neutrophil phagocytosis of S. aureus and subsequent intracellular microbicidal processes. Specific and azurophilic granules fuse with the phagosome, thereby enriching the lumen of the vacuole with antimicrobial peptides and proteins. In addition, the NADPH oxidase assembles at the phagosome membrane and produces superoxide, which is converted to other ROS. CR, complement receptor; FcR, antibody Fc receptor; MPO, myeloperoxidase; PRR, pattern recognition receptor; ROS, reactive oxygen species.
Oxygen-independent killing of microbes by PMNs relies on fusion of cytoplasmic granules (azurophilic and specific granules) with the phagosome, and the resultant release of antimicrobial peptides (AMPs) and proteins into the phagosome. Azurophilic granules enrich the phagosome with numerous microbicidal molecules, including α-defensin, cathepsin, proteinase 3, elastase, and bactericidal/permeability-increasing protein, whereas specific granules contribute lysozyme, lactoferrin, calprotectin, hCAP-18 (unprocessed form of LL-37), and others (38). Additionally, some of the granule proteins restrict intraphagosomal bacterial growth. The combination of ROS and antimicrobial proteins of granules is highly effective at killing ingested bacteria and fungi.
Neutrophils also have the ability to ensnare extracellular microbes via the formation of extracellular traps (17). Neutrophil extracellular traps (NETs) are web-like structures consisting of chromatin, histones, and neutrophil granule proteins that are released from the cell under certain conditions or with specific stimuli (38). Decondensed chromatin acts as a “net” that immobilizes and kills microbes. It is worth noting that the ability of NETs to kill ensnared microbes directly has been debated (113). Although NETs can contribute to host defense, formation of NETs is accompanied by release of cytotoxic molecules that are nonspecific and can damage host tissues and/or promote development of autoimmune-mediated diseases (98).
Apoptosis and resolution of the inflammatory response
As described in brief above, neutrophils possess and/or produce numerous molecules that are cytotoxic to host cells. Therefore, multiple mechanisms exist to prevent unintended release of these molecules into host tissues. Indeed, during normal steady-state processes (noninflammatory states), neutrophils undergo constitutive (spontaneous) apoptosis at the end of their lifespan and are removed by mononuclear phagocytes (153, 154). As neutrophils undergo apoptosis, functional capacity is decreased and proinflammatory capacity is downregulated (193). Thus, apoptosis and removal of apoptotic neutrophils by macrophages is a mechanism to facilitate nonphlogistic turnover of ∼1011 neutrophils each day in a healthy adult.
Neutrophil apoptosis can be accelerated by phagocytosis. Watson et al. first reported that phagocytosis of bacteria (Escherichia coli) induces rapid apoptosis in human PMNs (191), and this phenomenon has since been extended to include numerous other bacteria and fungi. The acceleration of neutrophil apoptosis after phagocytosis has been termed phagocytosis-induced cell death (PICD) (198). Studies by Zhang et al. demonstrated that the production of neutrophil ROS is associated with PICD, and subsequent work by Kobayashi et al. reported that neutrophils from patients with X-linked CGD fail to undergo PICD (85, 198). These findings, coupled with earlier work by Watson et al. (191) and Simons et al. (159), in which PICD (or lack of) was dictated in part by bacteria-to-neutrophil ratios, indicate ROS are important for triggering PICD. Inasmuch as effete neutrophils need to be cleared from infected tissues, it is likely that PICD is a process that promotes safe clearance of spent neutrophils from such sites, thus contributing to the resolution of infection.
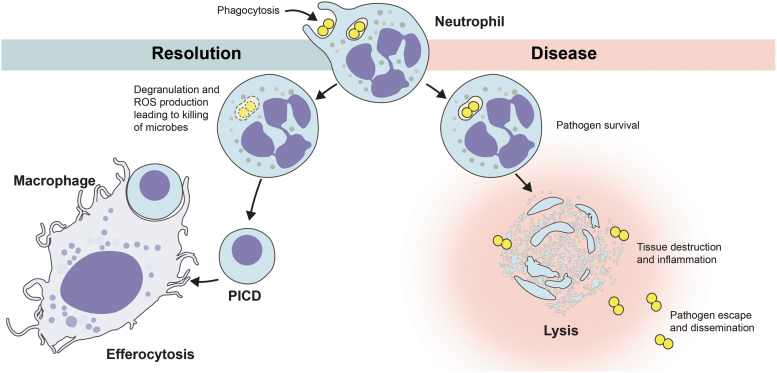
Neutrophils are the predominant cellular defense against bacteria and fungi, and therefore, it is perhaps not surprising that some bacterial pathogens have evolved means to alter normal neutrophil apoptosis and turnover. For example, pathogens such as Anaplasma phagocytophilum (197) and Francisella tularensis (155) delay neutrophil apoptosis. However, a few bacterial pathogens, including S. aureus, cause rapid neutrophil lysis after phagocytosis. In either case, modulation of PICD by pathogens can lead to pathogen dissemination and disease. As an example, possible outcomes of the neutrophil phagocytosis of S. aureus are depicted in Figure 6.
FIG. 6.
Possible outcomes of neutrophil phagocytosis of S. aureus. Efferocytosis is the phagocytosis of cells undergoing apoptosis. PICD, phagocytosis-induced cell death.
S. aureus Molecules That Target Neutrophils and Neutrophil Functions
S. aureus is known for its myriad virulence factors and immune evasion molecules, and the topic is too extensive for the purpose of this review. Therefore, we focus on selected virulence factors that target neutrophils (Table 1).
Table 1.
Staphylococcus aureus Molecules That Target Neutrophils and/or Neutrophil Functions
| Staphylococcus aureus molecule | Target molecule | Targeted function | References |
|---|---|---|---|
| Staphylococcal enterotoxin-like toxin X (SEIX) | P-selectin glycoprotein ligand-1 (PSGL-1) | Recruitment | (45) |
| Staphylococcal superantigen-like 5 (SSL5) | P-selectin glycoprotein ligand-1 (PSGL-1) | Recruitment | (12) |
| Chemotaxis inhibitory protein of S. aureus (CHIPS) | Formyl peptide receptor (FPR), complement component fragment 5a receptor (C5aR) | Chemotaxis | (34) |
| Formyl peptide receptor-like 1 inhibitor (FLIPr, FLIPr-like) | Formyl peptide receptor-like 1 (FPRL1), formyl peptide receptor (FPR) | Chemotaxis | (135, 136) |
| Staphopain A (ScpA) | C-X-C motif chemokine receptor 2 (CXCR2) | Chemotaxis | (93) |
| Capsule polysaccharide (Cps) | — | Phagocytosis | (121, 176) |
| Protein A (SpA) | IgG | Phagocytosis | (47, 84) |
| Second immunoglobulin-binding protein (Sbi) | IgG | Phagocytosis | (160) |
| Clumping factor A (ClfA) | — | Phagocytosis | (65) |
| α-Type phenol soluble modulin (PSMα) | Formyl peptide receptor 2 (FPR2), plasma membrane | PMN lysis | (130) |
| Panton–Valentine leukocidin (PVL) | Complement component fragment 5a receptor (C5aR1, C5aR2) | PMN lysis | (162) |
| Leukocidin ED (LukED) | C-X-C motif chemokine receptor (CXCR1, CXCR2), CCR5 | PMN lysis | (3, 140) |
| Leukocidin GH/AB (LukGH/LukAB) | CD11b | PMN lysis | (41, 182) |
| γ-Hemolysin AB, BC (HlgAB, HlgBC) | C-X-C motif chemokine receptor (CXCR1, CXCR2, CXCR4), C-C motif chemokine receptor 2 (CCR2) | PMN lysis | (164) |
| Staphyloxanthin | Superoxide | ROS-mediated killing | (28, 105) |
| Superoxide dismutase (sodA, sodM) | Superoxide | ROS-mediated killing | (63) |
| Catalase (KatA) | Hydrogen peroxide | ROS-mediated killing | (110) |
| Alkyl hydroperoxide reductase (AhpCF) | Hydrogen peroxide | ROS-mediated killing | (30) |
| Staphylococcal peroxidase inhibitor (SPIN) | Myeloperoxidase (MPO) | ROS-mediated killing | (35) |
| Flavohaemoglobin (Hmp) | Nitrogen radicals | Nitrosative stress | (56, 141) |
| D-alanylation operon (Dlt) | — | Killing via AMPs | (131) |
| Peptidoglycan O-acetyltransferase (OatA) | — | Killing via AMPs | (10) |
| Multiple peptide resistance factor (MprF) | — | Killing via AMPs | (92, 129) |
| Staphylokinase | α-defensins | Killing via AMPs | (74) |
| Aureolysin | Cathelicidin LL37 | Killing via AMPs | (158) |
| Extracellular adherence protein (Eap), extracellular adherence protein homologue (EapH, EapH2) | Elastase, proteinase 3, and cathepsin G | Killing via AMPs | (167) |
| ABC transporter (VraFG) | — | Killing via AMPs | (99) |
| Nuclease | NETs | Trapping by NETs | (11) |
| Adenosine synthase A (AdsA) | NETs | Trapping by NETs | (177) |
AMPs, antimicrobial peptides; NETs, neutrophil extracellular traps; PMN, polymorphonuclear leukocyte; ROS, reactive oxygen species.
Chemotaxis inhibitors
S. aureus produces several molecules that have the potential to inhibit neutrophil chemotaxis and recruitment. For example, chemotaxis inhibitory protein of S. aureus blocks neutrophil chemotaxis by binding competitively to the formyl peptide receptor (FPR) and the C5a receptor (C5aR) on the neutrophil surface (134). In addition, FPRs, which bind bacteria-derived fMLF, a strong chemoattractant, are targeted by FLIPr and FLIPr-like proteins, which effectively block fMLF-mediated neutrophil chemotaxis in vitro (135, 136). Neutrophil recruitment is also negatively influenced by staphylococcal enterotoxin-like toxin X (SEIX) and staphylococcal superantigen-like 5, both of which bind P-selectin glycoprotein ligand-1 (PSGL-1, CD162) expressed on phagocytes (12, 45). The interaction of neutrophil PSGL-1 with P-selectin on the surface of capillary endothelial cells is an important step in the initial stages of neutrophil recruitment from the bloodstream. The cysteine protease staphopain A can cleave the chemokine receptor CXCR2 (IL-8 receptor) on human neutrophils, thus preventing the binding of chemokines and subsequent chemotaxis (93).
Interaction with host opsonins
S. aureus produces numerous surface molecules that bind host serum proteins such as fibrinogen, plasminogen, and antibody. Such surface-bound host proteins can mask bacterial surface molecules, thereby reducing recognition by neutrophils. For example, S. aureus protein A (SpA) binds the Fc-region of IgG, an interaction that prevents antibody binding with neutrophil Fc receptors. It is because of this characteristic that SpA has been shown to decrease opsonophagocytosis (47). Indeed, S. aureus strains with high production of SpA were phagocytosed more slowly by neutrophils than strains that produce low SpA concentrations by comparison (132). Consistent with these observations, S. aureus-mutant strains lacking SpA have decreased virulence in animal infection models (40, 124, 127).
More recently, Falugi et al. used a mouse infection model to demonstrate that S. aureus SpA dampens the humoral immune response to S. aureus (44). Subsequent work by Pauli et al. (128) revealed that SpA alters the antibody response to S. aureus in humans, findings compatible with those of Falugi et al.. Inasmuch as neutralization of SpAs ability to sequester IgG and dampen antibody production would likely be beneficial to the outcome of infection and success of a potential S. aureus vaccine, SpA has been investigated as a potential therapeutic target. For example, Chen et al. showed that a recombinant monoclonal antibody (mAb) specific for SpA promotes opsonophagocytic killing of MRSA in mouse and human blood and leads to decolonization of S. aureus from the murine pharynx and gastrointestinal tract (25). S. aureus has a second immunoglobulin-binding protein, Sbi, which binds IgG and has the ability to decrease opsonophagocytosis in vitro (160). Compared with SpA, less is known about the role of Sbi during human infections.
S. aureus produces clumping factor A (ClfA), a fibrinogen binding protein and adhesin that promotes colonization of the host and can inhibit phagocytosis in vitro (65). Notably, ClfA has been targeted with numerous vaccine approaches over the past two decades (5, 18, 75, 102). Results of such studies and clinical trials have met with mixed success. Josefsson et al. were among the first to report that experimental vaccination with S. aureus ClfA or antibodies specific for ClfA moderate the severity of S. aureus infection in a mouse model (75). More recently, Li et al. found that active or passive vaccination against ClfA provided little or no protection in other mouse infection models (102). Li et al. hypothesized that use of Freund's adjuvant in mouse protection studies may have contributed to previous positive results with ClfA vaccine approaches. Freund's adjuvant is not used in human vaccine formulations, and results in human clinical trials have failed to provide protection and/or meet endpoint criteria. For example, DeJonge et al. tested the ability of intravenous immune globulin (IVIG) containing high titers of ClfA antibodies to protect infants against late-onset sepsis (37). The proportion of infants who developed late-onset sepsis was similar in placebo and IVIG treatment groups (5% and 6%, respectively) (37). Consistent with those findings, a phase II clinical trial with a humanized mAb specific for ClfA failed to reveal significant protection against S. aureus bacteremia (192).
Most recently, a 4-antigen S. aureus vaccine (SA4Ag), which includes recombinant mutant ClfA, showed promising results in preclinical studies and induced functional antibodies that persisted for up to 3 years after vaccination (9, 31). Despite these encouraging results, the vaccine was discontinued in a phase 2b clinical trial because it did not achieve predetermined endpoint objectives (PF-06290510). Inasmuch as the success of the preclinical studies for SA4Ag was in part determined by opsonophagocytic activity, the lack of efficacy in human clinical trials is perhaps not surprising. Although S. aureus produces many molecules that have the ability to inhibit phagocytosis in vitro, it has long been known that S. aureus is ingested readily by phagocytes—most notably neutrophils—from healthy adults (18–65 years, the population used in the clinical trial) (148, 185). Thus, a vaccine directed largely to promote opsonophagocytic activity is not optimal. More work is needed to fully understand the contribution of ClfA in human infections.
Cytolytic toxins
Virtually all S. aureus strains have the ability to produce multiple cytolytic toxins (157). Alpha-hemolysin (or alpha-toxin, Hla) is one of the best characterized and highly conserved S. aureus toxins (13). The role of Hla in virulence has been tested in multiple animal infection models over the years, including models of S. aureus SSTI, bacteremia, peritonitis, and pneumonia (19, 79, 172). The toxin contributes significantly to virulence in each of these models and is therefore a reasonable target for therapeutic approaches. Indeed, passive and active vaccination approaches that target Hla have shown good success in animal infection models especially when combined with other antigens (1, 39, 66, 70, 79, 114). Early studies by Adlam et al. demonstrated that immunization of rabbits with purified Hla protects rabbits against lethal experimental mastitis (1). Subsequent work by Menzies and Kernodle demonstrated that rabbit antiserum elicited by immunization with an Hla toxoid protects mice against lethal challenge with S. aureus (114). Consistent with those findings, Bubeck Wardenburg and Schneewind showed that immunization of mice with Hla toxoid protects animals against S. aureus pneumonia (20). Kennedy et al. then reported that active and passive vaccine approaches directed against Hla protect mice from severe and soft tissue infections caused by the USA300 epidemic strain (79). Collectively, these studies set the stage for development of therapeutic approaches directed against Hla.
Indeed, the human anti-Hla mAb MEDI4893 has been tested in various animal infection models both as a prophylactic and a therapeutic treatment option and been found to reduce disease severity significantly, especially when combined with antibiotics (66). A phase 1 study in humans revealed that intravenous administration of MEDI4893 resulted in long-lasting anti-Hla neutralizing antibody levels (152), and a phase 2 study demonstrated reduction of S. aureus pneumonia in high-risk intensive care unit patients treated with MEDI4893 (51). Thus, Hla is a promising target for passive and active immunization approaches.
The bicomponent leukocidins are group of well-studied S. aureus cytotoxins that cause lysis of white blood cells. They consist of two subunits, known as S and F, which are secreted separately but assemble in the target cell membrane to form β-barrel pores that can disrupt cell homeostasis and cause cell lysis (157). S. aureus bicomponent leukocidins include Panton–Valentine leukocidin (PVL), leukocidin ED (LukED), LukGH/LukAB, gamma-hemolysin AB (HlgAB), and HlgCB (163). Each has a slightly different host cell target spectrum, and some leukotoxins are host specific and leukocyte lysis is species specific (163). A number of leukocyte transmembrane receptors for these leukotoxins have been identified recently, including C5a and IL-8 receptors, and CD11b (163). The contribution of leukocidins to S. aureus virulence has been investigated extensively in animal infection models. Based on such studies, one can argue that each of these leukotoxins has a role in S. aureus virulence under certain conditions. That said, the role played by each of these molecules in human infections remains unknown or incompletely determined and more work is needed.
Despite this lack of knowledge, S. aureus cytotoxins are being considered as targets for possible therapeutic approaches. Studies by Rouha et al. showed that a combination of two human mAbs (named ASN100), which are together specific for six S. aureus cytotoxins (Hla, LukSF-PV, LukED, HlgAB, HlgCB, and LukGH/AB), inhibits lysis of host epithelial cells and leukocytes—including PMNs—in vitro (150). These mAbs prevented lethality in a rabbit model of severe S. aureus pneumonia (169), and more recently, showed promising results for safety, tissue penetration, and functional activity in healthy human volunteers (109). The approach to target S. aureus cytotoxins was corroborated by Vu et al., who demonstrated the ability of MEDI4893 (the aforementioned anti-Hla antibody) and a mAb specific for PVL, LukED, and HlgAB/CB to moderate the severity of disease in a rabbit model of S. aureus necrotizing pneumonia (187). Tran et al. showed further that a combination of cytotoxin antigens (Hla and PVL subunit toxoids) led to greater protection against lethal USA300 pneumonia in rabbits than any of the antigens alone, findings compatible with earlier work by Rouha et al. and Stulik et al. (150, 169, 179). Taken together, these studies provide compelling support for a prophylactic and/or therapeutic that targets multiple S. aureus cytotoxins by active or passive vaccination.
α-Type phenol-soluble modulin (PSMα) peptides are secreted, short amphipathic α-helical peptides that can lyse many types of host cells, including neutrophils (189). In contrast to the bicomponent leukotoxins, PSMα-mediated cytolysis is likely receptor independent and attributed to its surfactant-like properties. However, at sublytic concentrations, PSMα peptides can bind FPR2 on neutrophils and elicit proinflammatory processes (26, 90). Although psm loci are present in the core genome of virtually all S. aureus lineages, high PSM expression levels are associated with the most prominent CA-MRSA strains, suggesting a link between PSM production and the success of CA-MRSA (100).
The expression of PSMα peptides as well as other important S. aureus virulence factors is regulated by the accessory gene regulator (Agr) quorum-sensing system, which has also been investigated as a potential therapeutic target (194). For example, Sully et al. identified a small-molecule inhibitor, savarin, that targets AgrA, the transcription regulator of the agr operon, and thus prevents transcription of hla, psm-α, and lukS/F-PV (170). In addition to blocking lysis of human PMNs in vitro, administration of savarin to mice increased bacterial clearance and reduced tissue injury in S. aureus skin infection models (170). Inasmuch as Agr regulates the expression of a number of S. aureus virulence factors, targeting the Agr quorum sensing system is potentially a promising approach for the development of novel prophylactics and therapeutics directed to moderate severity of S. aureus infections.
Moderation of ROS and resistance to AMPs
S. aureus possesses multiple factors to protect itself from ROS and reactive nitrogen radicals, including genes encoding superoxide dismutases (SodA and SodM), catalase (KatA), alkyl hydroperoxide reductase (AhpCF), staphylococcal peroxidase inhibitor (SPIN), flavohaemoglobin (Hmp), and staphyloxanthin. Some of these molecules are upregulated following phagocytosis of S. aureus by human neutrophils (185), and the importance of some of these proteins has been tested in animal infection models. For example, SPIN is highly conserved among S. aureus lineages, and expression is upregulated within 20 min after phagocytosis (35). SPIN binds MPO and actively prevents formation of hypochlorous acid, the product of the MPO-halide system originally described by Seymour Klebanoff (35). A SPIN deletion mutant is more susceptible to killing by neutrophils compared with the S. aureus wild-type strain (35).
The structural similarity between staphylococcal dehydrosqualene synthase (CrtM), which is important for production of staphyloxanthin, and human squalene synthase (SQS), an enzyme involved in cholesterol synthesis, has been exploited as a potential therapeutic by Liu et al. (104). Certain phosphosulfonates, which act as cholesterol synthesis inhibitors in humans, inhibit staphyloxanthin synthesis in S. aureus and thereby render the bacterium more susceptible to killing by ROS (104). Mice treated with phosphosulfonate had reduced bacterial burden in a mouse kidney infection model. Considering that cholesterol-lowering drugs are widely used in humans, exploiting antistaphylococcal capacity is an intriguing therapeutic approach that merits further research.
In addition to the ability to moderate ROS, S. aureus has evolved multiple mechanisms to circumvent killing by AMPs and proteins. Here, we provide selected examples of these S. aureus AMP resistance mechanisms. For instance, S. aureus can modify cell wall composition, cleave AMPs, and bind and/or export them from the cytoplasm via efflux pumps. S. aureus encodes a three-component AMP sensing system that controls expression of anti-AMP molecules (99, 101). Cell wall modifications include D-alanylation of teichoic acid and incorporation of cationic lysyl-phosphatidyl glycerol into the bacterial membrane as a means to neutralize the negative surface charge and thus help repel cationic AMPs such as neutrophil defensins (131). These processes are mediated by the S. aureus DltABCD system and multiple peptide resistance factor (129, 131).
O-acetylation of muramic acid by staphylococcal OatA confers resistance to lysozyme (10), and staphylokinase, aureolysin, and V8 protease inactivate AMPs by direct binding and/or proteolysis (74, 158). More recently, Stapels et al. discovered that S. aureus extracellular adherence protein (Eap) and homologs EapH1 and EapH2 inhibit the neutrophil serine proteases elastase, proteinase 3, and cathepsin G (167). These Eap proteins inhibit neutrophil serine protease activity in vitro and contribute to virulence in a mouse infection model (167). S. aureus has also evolved the means to escape NETs. For example, Berends et al. discovered that S. aureus nuclease (encoded by nuc) facilitates escape from NETs (11). Subsequent work by Thammavongsa et al. reported that nuclease coupled with S. aureus adenosine synthase A converts NETs into deoxyadenosine, which can initiate cell death in immune cells (177).
Although the majority of S. aureus ingested by neutrophils are killed, some of the ingested microbes can survive long enough to ultimately cause neutrophil lysis. This phenomenon varied depending on the S. aureus strain but is perhaps not unexpected considering the many mechanisms used by S. aureus to moderate the effects of neutrophil microbicides.
Neutrophil Lysis After Phagocytosis of S. aureus
As described above, many bacterial pathogens have the ability to alter the fate of neutrophils, and S. aureus is one such pathogen. In the early 1950s, Rogers and Tompsett reported that not all phagocytosed S. aureus are killed by neutrophils in vitro (148). Moreover, there was significant destruction/lysis of human neutrophils 3–4 h after phagocytosis of S. aureus (148). Subsequent work by Rogers and Melly showed that although 99% of S. aureus were ingested by human granulocytes in vitro, there was ∼50% bacterial survival (112, 147). Rogers also demonstrated that staphylococci contained within leukocytes cause persistent bacteremia in a rabbit model (146). The overarching idea was that any ingested staphylococci that survived after phagocytosis could serve as a source for persistent infection.
Almost 50 years later, Gresham et al. demonstrated that S. aureus-containing neutrophils can establish S. aureus infection in mice, suggesting release of the pathogen from neutrophils (60). Voyich et al. found that prominent CA-MRSA strains, especially the USA300 and USA400 strains, cause rapid lysis of human neutrophils following phagocytosis (185, 186). The enhanced ability of these CA-MRSA strains to evade killing by neutrophils suggests that they have enhanced virulence capacity, an attribute perhaps linked to their ability to cause infections in otherwise healthy individuals. Collectively, these previous observations have provided strong support to the idea that lysis of neutrophils after phagocytosis of S. aureus contributes to the pathogenesis of infection.
Given the potential importance of the S. aureus–neutrophil lysis phenomenon, there has been a recent effort to elucidate the mechanism for this process. The mechanism (or mechanisms) underlying neutrophil lysis after phagocytosis involves molecules produced by S. aureus within the phagosome, host cell processes triggered by S. aureus, or a combination of both. Kobayashi et al. showed that neutrophil lysis after phagocytosis of USA300 occurs independent of caspases and ROS produced by NADPH oxidase, thus eliminating the possibility of an apoptosis-like form of cell death (83). Furthermore, neutrophil lysis increased over time, was dependent largely on bacteria-to-neutrophil ratios, and required viable S. aureus (83). Interestingly, the authors found the phagosome membrane remained intact until the point of lysis (83). In subsequent work, Greenlee-Wacker et al. provided evidence that neutrophil lysis after S. aureus phagocytosis occurs by necroptosis or programmed necrosis (59). Further characterization of the neutrophil lysis phenomenon by the same authors indicated that lysis occurs independent of classical necroptosis molecules but requires RIPK-3 (58). Therefore, the role of specific neutrophil signal transduction pathways remains incompletely determined.
At least three S. aureus secreted toxins have been shown to contribute to lysis of neutrophils after phagocytosis. First, Ventura et al. found that an isogenic USA300 lukGH deletion strain caused significantly less neutrophil lysis after phagocytosis compared with that by the wild-type strain (182). The contribution of LukGH/LukAB to this process was verified and extended by DuMont et al. (42). Pang et al. compared USA300 wild-type and isogenic hla deletion strains to show that Hla contributes to PMN lysis after phagocytosis of S. aureus (125). It is noteworthy that Hla and LukGH/LukAB require interaction with specific surface receptors to form cytolytic membrane pores. Although these receptors would be present and in the correct orientation in the phagosome membrane, they are inaccessible from the cytoplasmic face of the plasma membrane and thus should be unable to cause cytolysis directly. However, these findings are compatible with the notion that interaction of Hla and/or LukGH/LukAB with host receptors lining the inside of the phagosome membrane triggers host cell death through an as yet undefined signaling pathway. Indeed, Hla has been shown recently to activate the inflammasome in macrophages, thus providing support to the idea that these cytolytic toxins can activate processes mediated by host signal transduction (29).
Finally, Surewaard et al. reported that PSMα peptides, which lack the requirement for membrane receptor interaction to effect cytolysis, contribute to neutrophil lysis after phagocytosis of S. aureus strain MW2, the prototype CA-MRSA strain (171). Whether the PSMα peptides target membranes directly or trigger signals that lead to neutrophil death remains incompletely determined. Clearly, more work is needed to fully understand the mechanism(s) that underlie rapid neutrophil lysis after phagocytosis.
Abbreviations Used
- AMPs
antimicrobial peptides
- BSI
bloodstream infection
- CA-MRSA
community-associated methicillin-resistant Staphylococcus aureus
- CC
clonal complex
- CGD
chronic granulomatous disease
- CHIPS
chemotaxis inhibitory protein of S. aureus
- ClfA
clumping factor A
- Eap
extracellular adherence protein
- fMLF
N-formyl-methionyl-leucyl-phenylalanine
- FPR
formyl peptide receptor
- FPR2
formyl peptide receptor 2
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- Hla
α-hemolysin or alpha-toxin
- IVIG
intravenous immune globulin
- LA-MRSA
livestock-associated MRSA
- Luk
leukocidin
- LukED
leukocidin ED
- mAb
monoclonal antibody
- MLST or ST
multilocus sequence typing
- MprF
multiple peptide resistance factor
- MRSA
methicillin-resistant S. aureus
- NADPH
nicotinamide adenine dinucleotide phosphate
- NETs
neutrophil extracellular traps
- PBP2a
penicillin-binding protein 2a
- PICD
phagocytosis-induced cell death
- PMN
polymorphonuclear leukocyte
- PRR
pattern recognition receptor
- PSGL-1
P-selectin glycoprotein ligand-1
- PSMα
α-type phenol soluble modulin
- PVL
Panton–Valentine leukocidin
- ROS
reactive oxygen species
- Sbi
second immunoglobulin-binding protein
- ScpA
Staphopain A
- SCV
small colony variant
- SEIX
staphylococcal enterotoxin-like toxin X
- SpA
S. aureus protein A
- SPIN
staphylococcal peroxidase inhibitor
- SSL5
superantigen-like 5
- SSTI
skin and soft tissue infections
- TLR
toll-like receptor
- VISA
vancomycin-intermediate S. aureus
- VRSA
vancomycin-resistant S. aureus
Funding Information
The authors are supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.***
References
- 1. Adlam C, Ward PD, McCartney AC, Arbuthnott JP, and Thorley CM. Effect immunization with highly purified alpha- and beta-toxins on staphylococcal mastitis in rabbits. Infect Immun 17: 250–256, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albrecht VS, Limbago BM, Moran GJ, Krishnadasan A, Gorwitz RJ, McDougal LK, Talan DA, and Group EMINS. Staphylococcus aureus colonization and strain type at various body sites among patients with a closed abscess and uninfected controls at U.S. emergency departments. J Clin Microbiol 53: 3478–3484, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alonzo F, 3rd, Kozhaya L, Rawlings SA, Reyes-Robles T, DuMont AL, Myszka DG, Landau NR, Unutmaz D, and Torres VJ. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493: 51–55, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson DJ, Moehring RW, Sloane R, Schmader KE, Weber DJ, Fowler VG Jr., Smathers E, and Sexton DJ.. Bloodstream infections in community hospitals in the 21st century: a multicenter cohort study. PLoS One 9: e91713, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arrecubieta C, Matsunaga I, Asai T, Naka Y, Deng MC, and Lowy FD. Vaccination with clumping factor A and fibronectin binding protein A to prevent Staphylococcus aureus infection of an aortic patch in mice. J Infect Dis 198: 571–575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Athens JW, Haab OP, Raab SO, Mauer AM, Ashenbrucker H, Cartwright GE, and Wintrobe MM. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest 40: 989–995, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bainton DF, Ullyot JL, and Farquhar MG. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med 134: 907–934, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barber M. Methicillin-resistant staphylococci. J Clin Pathol 14: 385–393, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Begier E, Seiden DJ, Patton M, Zito E, Severs J, Cooper D, Eiden J, Gruber WC, Jansen KU, Anderson AS, and Gurtman A. SA4Ag, a 4-antigen Staphylococcus aureus vaccine, rapidly induces high levels of bacteria-killing antibodies. Vaccine 35: 1132–1139, 2017 [DOI] [PubMed] [Google Scholar]
- 10. Bera A, Herbert S, Jakob A, Vollmer W, and Gotz F. Why are pathogenic staphylococci so lysozyme resistant? The peptidoglycan O-acetyltransferase OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Mol Microbiol 55: 778–787, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Berends ET, Horswill AR, Haste NM, Monestier M, Nizet V, and von Kockritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun 2: 576–586, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bestebroer J, Poppelier MJ, Ulfman LH, Lenting PJ, Denis CV, van Kessel KP, van Strijp JA, and de Haas CJ. Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood 109: 2936–2943, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Bhakdi S and Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev 55: 733–751, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boost M, Ho J, Guardabassi L, and O'Donoghue M. Colonization of butchers with livestock-associated methicillin-resistant Staphylococcus aureus. Zoonoses Public Health 60: 572–576, 2013 [DOI] [PubMed] [Google Scholar]
- 15. Bor DH, Woolhandler S, Nardin R, Brusch J, and Himmelstein DU. Infective endocarditis in the U.S., 1998–2009: a nationwide study. PLoS One 8: e60033, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, and Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48: 1–12, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, and Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Brouillette E, Lacasse P, Shkreta L, Belanger J, Grondin G, Diarra MS, Fournier S, and Talbot BG. DNA immunization against the clumping factor A (ClfA) of Staphylococcus aureus. Vaccine 20: 2348–2357, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, and Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13: 1405–1406, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Bubeck Wardenburg J and Schneewind O.. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 205: 287–294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cassini A, Hogberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C, Monnet DL, and Burden of AMRCG. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 19: 56–66, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease C and Prevention. Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb Mortal Wkly Rep 51: 565–567, 2002 [PubMed] [Google Scholar]
- 23. Chambers HF. Solving staphylococcal resistance to beta-lactams. Trends Microbiol 11: 145–148, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Chambers HF and Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7: 629–641, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen X, Sun Y, Missiakas D, and Schneewind O. Staphylococcus aureus decolonization of mice with monoclonal antibody neutralizing protein A. J Infect Dis 219: 884–888, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheung GY, Joo HS, Chatterjee SS, and Otto M. Phenol-soluble modulins—critical determinants of staphylococcal virulence. FEMS Microbiol Rev 38: 698–719, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chickering HT and Park JH. Staphylococcus aureus pneumonia. JAMA 72: 617–626, 1919 [Google Scholar]
- 28. Clauditz A, Resch A, Wieland KP, Peschel A, and Gotz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun 74: 4950–4953, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen TS, Boland ML, Boland BB, Takahashi V, Tovchigrechko A, Lee Y, Wilde AD, Mazaitis MJ, Jones-Nelson O, Tkaczyk C, Raja R, Stover CK, and Sellman BR. S. aureus evades macrophage killing through NLRP3-dependent effects on mitochondrial trafficking. Cell Rep 22: 2431–2441, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cosgrove K, Coutts G, Jonsson IM, Tarkowski A, Kokai-Kun JF, Mond JJ, and Foster SJ. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J Bacteriol 189: 1025–1035, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Creech CB, Frenck RW, Fiquet A, Feldman R, Kankam MK, Pathirana S, Baber J, Radley D, Cooper D, Eiden J, Gruber WC, Jansen KU, Anderson AS, and Gurtman A. Persistence of immune responses through 36 months in healthy adults after vaccination with a novel Staphylococcus aureus 4-antigen vaccine (SA4Ag). Open Forum Infect Dis 7: ofz532, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cui L, Ma X, Sato K, Okuma K, Tenover FC, Mamizuka EM, Gemmell CG, Kim MN, Ploy MC, El-Solh N, Ferraz V, and Hiramatsu K. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J Clin Microbiol 41: 5–14, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. David MZ and Daum RS. Treatment of Staphylococcus aureus infections. Curr Top Microbiol Immunol 409: 325–383, 2017 [DOI] [PubMed] [Google Scholar]
- 34. de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJ, Heezius EC, Poppelier MJ, Van Kessel KP, and van Strijp JA. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 199: 687–695, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Jong NWM, Ramyar KX, Guerra FE, Nijland R, Fevre C, Voyich JM, McCarthy AJ, Garcia BL, van Kessel KPM, van Strijp JAG, Geisbrecht BV, and Haas PA. Immune evasion by a staphylococcal inhibitor of myeloperoxidase. Proc Natl Acad Sci U S A 114: 9439–9444, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Oliveira S, Rosowski EE, and Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 16: 378–391, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DeJonge M, Burchfield D, Bloom B, Duenas M, Walker W, Polak M, Jung E, Millard D, Schelonka R, Eyal F, Morris A, Kapik B, Roberson D, Kesler K, Patti J, and Hetherington S. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr 151: 260–265, 265 e261, 2007 [DOI] [PubMed] [Google Scholar]
- 38. DeLeo FR and Nauseef WM. Granulocytic phagocytes. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, edited by Bennett JE, Dolin R, and Blaser MJ. Ninth ed, Vol. 1, Philedelphia, PA: Elsevier, Inc., 2020, pp. 83–98 [Google Scholar]
- 39. Diep BA, Hilliard JJ, Le VT, Tkaczyk C, Le HN, Tran VG, Rao RL, Dip EC, Pereira-Franchi EP, Cha P, Jacobson S, Broome R, Cheng LI, Weiss W, Prokai L, Nguyen V, Stover CK, and Sellman BR. Targeting alpha toxin to mitigate its lethal toxicity in ferret and rabbit models of Staphylococcus aureus necrotizing pneumonia. Antimicrob Agents Chemother 61, e02456–16, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dossett JH, Kronvall G, Williams RC Jr., and Quie PG. Antiphagocytic effects of staphylococcal protein A. J Immunol 103: 1405–1410, 1969 [PubMed] [Google Scholar]
- 41. DuMont AL, Yoong P, Day CJ, Alonzo F, 3rd, McDonald WH, Jennings MP, and Torres VJ. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A 110: 10794–10799, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DuMont AL, Yoong P, Surewaard BG, Benson MA, Nijland R, van Strijp JA, and Torres VJ. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect Immun 81: 1830–1841, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. El-Ahdab F, Benjamin DK Jr., Wang A, Cabell CH, Chu VH, Stryjewski ME, Corey GR, Sexton DJ, Reller LB, and Fowler VG Jr. Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med 118: 225–229, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Falugi F, Kim HK, Missiakas DM, and Schneewind O. Role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. MBio 4: e00575-00513, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fevre C, Bestebroer J, Mebius MM, de Haas CJ, van Strijp JA, Fitzgerald JR, and Haas PJ. Staphylococcus aureus proteins SSL6 and SElX interact with neutrophil receptors as identified using secretome phage display. Cell Microbiol 16: 1646–1665, 2014 [DOI] [PubMed] [Google Scholar]
- 46. Fishovitz J, Hermoso JA, Chang M, and Mobashery S. Penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. IUBMB Life 66: 572–577, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Forsgren A and Nordstrom K. Protein A from Staphylococcus aureus: the biological significance of its reaction with IgG. Ann N Y Acad Sci 236: 252–266, 1974 [DOI] [PubMed] [Google Scholar]
- 48. Foucault ML, Courvalin P, and Grillot-Courvalin C. Fitness cost of VanA-type vancomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 53: 2354–2359, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fowler VG Jr., Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS, and Investigators ICE.. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293: 3012–3021, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hansel NN, Perl T, Ticehurst JR, Carroll K, Thomas DL, Nuermberger E, and Bartlett JG. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis 40: 100–107, 2005 [DOI] [PubMed] [Google Scholar]
- 51. François B, Garcia Sanchez M, Eggimann P, Dequin P, Laterre P, Huberlant V, Escudero D, Boulain T, Bretonniere C, Pugin J, Trenado Alvarez J, Ali O, Shoemaker K, Ruzin A, Vandamme D, Colbert S, Bellamy T, Dubiovsky F, Jafri H, and Group SS. Suvratoxumab reduces Staphylococcus aureus pneumonia in highrisk ICU patients: results of the SAATELLITE study. In: American Thoracic Society 2019 International Conference. Dallas, TX: ATS Journals: A7358 [Google Scholar]
- 52. Friaes A, Resina C, Manuel V, Lito L, Ramirez M, and Melo-Cristino J. Epidemiological survey of the first case of vancomycin-resistant Staphylococcus aureus infection in Europe. Epidemiol Infect 143: 745–748, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, Vandenesch F, Piemont Y, Brousse N, Floret D, and Etienne J. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359: 753–759, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Goerge T, Lorenz MB, van Alen S, Hubner NO, Becker K, and Kock R. MRSA colonization and infection among persons with occupational livestock exposure in Europe: prevalence, preventive options and evidence. Vet Microbiol 200: 6–12, 2017 [DOI] [PubMed] [Google Scholar]
- 55. Golding GR, Bryden L, Levett PN, McDonald RR, Wong A, Wylie J, Graham MR, Tyler S, Van Domselaar G, Simor AE, Gravel D, and Mulvey MR. Livestock-associated methicillin-resistant Staphylococcus aureus sequence type 398 in humans, Canada. Emerg Infect Dis 16: 587–594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goncalves VL, Nobre LS, Vicente JB, Teixeira M, and Saraiva LM. Flavohemoglobin requires microaerophilic conditions for nitrosative protection of Staphylococcus aureus. FEBS Lett 580: 1817–1821, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, Jensen BJ, Killgore G, Tenover FC, and Kuehnert MJ. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis 197: 1226–1234, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Greenlee-Wacker MC, Kremserova S, and Nauseef WM. Lysis of human neutrophils by community-associated methicillin-resistant Staphylococcus aureus. Blood 129: 3237–3244, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Greenlee-Wacker MC, Rigby KM, Kobayashi SD, Porter AR, DeLeo FR, and Nauseef WM. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J Immunol 192: 4709–4717, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, and Lindberg FP. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol 164: 3713–3722, 2000 [DOI] [PubMed] [Google Scholar]
- 61. Haaber J, Friberg C, McCreary M, Lin R, Cohen SN, and Ingmer H. Reversible antibiotic tolerance induced in Staphylococcus aureus by concurrent drug exposure. MBio 6: e02268-14, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hageman JC, Uyeki TM, Francis JS, Jernigan DB, Wheeler JG, Bridges CB, Barenkamp SJ, Sievert DM, Srinivasan A, Doherty MC, McDougal LK, Killgore GE, Lopatin UA, Coffman R, MacDonald JK, McAllister SK, Fosheim GE, Patel JB, and McDonald LC. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–2004 influenza season. Emerg Infect Dis 12: 894–899, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hampton MB, Kettle AJ, and Winterbourn CC. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect Immun 64: 3512–3517, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Harkins CP, Pichon B, Doumith M, Parkhill J, Westh H, Tomasz A, de Lencastre H, Bentley SD, Kearns AM, and Holden MTG. Methicillin-resistant Staphylococcus aureus emerged long before the introduction of methicillin into clinical practice. Genome Biol 18: 130, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Higgins J, Loughman A, van Kessel KP, van Strijp JA, and Foster TJ. Clumping factor A of Staphylococcus aureus inhibits phagocytosis by human polymorphonuclear leucocytes. FEMS Microbiol Lett 258: 290–296, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Hilliard JJ, Datta V, Tkaczyk C, Hamilton M, Sadowska A, Jones-Nelson O, O'Day T, Weiss WJ, Szarka S, Nguyen V, Prokai L, Suzich J, Stover CK, and Sellman BR. Anti-alpha-toxin monoclonal antibody and antibiotic combination therapy improves disease outcome and accelerates healing in a Staphylococcus aureus dermonecrosis model. Antimicrob Agents Chemother 59: 299–309, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, and Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother 40: 135–136, 1997 [DOI] [PubMed] [Google Scholar]
- 68. Holland SM and Gallin JI. Evaluation of the patient with suspected immunodeficiency. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, edited by Bennett JE, Dolin R, and Blaser MJ. Ninth ed, Vol. 1, Philede: Elsevier, Inc., 2020, pp. 142–153 [Google Scholar]
- 69. Howden BP, Smith DJ, Mansell A, Johnson PD, Ward PB, Stinear TP, and Davies JK. Different bacterial gene expression patterns and attenuated host immune responses are associated with the evolution of low-level vancomycin resistance during persistent methicillin-resistant Staphylococcus aureus bacteraemia. BMC Microbiol 8: 39, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, Keller A, O'Day T, Du Q, Cheng L, Damschroder M, Robbie G, Suzich J, Stover CK, and Sellman BR. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 58: 1108–1117, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huang SS, Diekema DJ, Warren DK, Zuccotti G, Winokur PL, Tendolkar S, Boyken L, Datta R, Jones RM, Ward MA, Aubrey T, Onderdonk AB, Garcia C, and Platt R. Strain-relatedness of methicillin-resistant Staphylococcus aureus isolates recovered from patients with repeated infection. Clin Infect Dis 46: 1241–1247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huijsdens XW, van Dijke BJ, Spalburg E, van Santen-Verheuvel MG, Heck ME, Pluister GN, Voss A, Wannet WJ, and de Neeling AJ. Community-acquired MRSA and pig-farming. Ann Clin Microbiol Antimicrob 5: 26, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jevons MP, Rolinson GN, and Knox R. “Celbenin”-resistant staphylococci. BMJ 1: 124–126, 1961 [Google Scholar]
- 74. Jin T, Bokarewa M, Foster T, Mitchell J, Higgins J, and Tarkowski A. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J Immunol 172: 1169–1176, 2004 [DOI] [PubMed] [Google Scholar]
- 75. Josefsson E, Hartford O, O'Brien L, Patti JM, and Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis 184: 1572–1580, 2001 [DOI] [PubMed] [Google Scholar]
- 76. Juhasz-Kaszanyitzky E, Janosi S, Somogyi P, Dan A, van der Graaf-van Bloois L, van Duijkeren E, and Wagenaar JA. MRSA transmission between cows and humans. Emerg Infect Dis 13: 630–632, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kalinka J, Hachmeister M, Geraci J, Sordelli D, Hansen U, Niemann S, Oetermann S, Peters G, Loffler B, and Tuchscherr L. Staphylococcus aureus isolates from chronic osteomyelitis are characterized by high host cell invasion and intracellular adaptation, but still induce inflammation. Int J Med Microbiol 304: 1038–1049, 2014 [DOI] [PubMed] [Google Scholar]
- 78. Katayama Y, Sekine M, Hishinuma T, Aiba Y, and Hiramatsu K. Complete reconstitution of the vancomycin-intermediate Staphylococcus aureus phenotype of strain Mu50 in vancomycin-susceptible S. aureus. Antimicrob Agents Chemother 60: 3730–3742, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, and DeLeo FR. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 202: 1050–1058, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Khanna T, Friendship R, Dewey C, and Weese JS. Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Vet Microbiol 128: 298–303, 2008 [DOI] [PubMed] [Google Scholar]
- 81. Kirby WM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science 99: 452–453, 1944 [DOI] [PubMed] [Google Scholar]
- 82. Knox R. A new penicillin (BRL 1241) active against penicillin-resistant staphylococci. Br Med J 2: 690–693, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kobayashi SD, Braughton KR, Palazzolo-Ballance AM, Kennedy AD, Sampaio E, Kristosturyan E, Whitney AR, Sturdevant DE, Dorward DW, Holland SM, Kreiswirth BN, Musser JM, and DeLeo FR. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J Innate Immun 2: 560–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kobayashi SD and DeLeo FR. Staphylococcus aureus protein A promotes immune suppression. MBio 4: e00764-00713, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kobayashi SD, Voyich JM, Braughton KR, Whitney AR, Nauseef WM, Malech HL, and DeLeo FR. Gene expression profiling provides insight into the pathophysiology of chronic granulomatous disease. J Immunol 172: 636–643, 2004 [DOI] [PubMed] [Google Scholar]
- 86. Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, and Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 128: 3854–3862, 2005 [DOI] [PubMed] [Google Scholar]
- 87. Kollef MH, Zilberberg MD, Shorr AF, Vo L, Schein J, Micek ST, and Kim M. Epidemiology, microbiology and outcomes of healthcare-associated and community-acquired bacteremia: a multicenter cohort study. J Infect 62: 130–135, 2011 [DOI] [PubMed] [Google Scholar]
- 88. Kourtis AP, Hatfield K, Baggs J, Mu Y, See I, Epson E, Nadle J, Kainer MA, Dumyati G, Petit S, Ray SM, Emerging Infections Program Mag, Ham D, Capers C, Ewing H, Coffin N, McDonald LC, Jernigan J, and Cardo D. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections - United States. MMWR Morb Mortal Wkly Rep 68: 214–219, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kremers HM, Nwojo ME, Ransom JE, Wood-Wentz CM, Melton LJ, 3rd, and Huddleston PM, 3rd. Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Joint Surg Am 97: 837–845, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, Schoneberg T, Rabiet MJ, Boulay F, Klebanoff SJ, van Kessel KA, van Strijp JA, Otto M, and Peschel A. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe 7: 463–473, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Krismer B, Weidenmaier C, Zipperer A, and Peschel A. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat Rev Microbiol 15: 675–687, 2017 [DOI] [PubMed] [Google Scholar]
- 92. Kristian SA, Durr M, Van Strijp JA, Neumeister B, and Peschel A. MprF-mediated lysinylation of phospholipids in Staphylococcus aureus leads to protection against oxygen-independent neutrophil killing. Infect Immun 71: 546–549, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Laarman AJ, Mijnheer G, Mootz JM, van Rooijen WJ, Ruyken M, Malone CL, Heezius EC, Ward R, Milligan G, van Strijp JA, de Haas CJ, Horswill AR, van Kessel KP, and Rooijakkers SH. Staphylococcus aureus staphopain A inhibits CXCR2-dependent neutrophil activation and chemotaxis. EMBO J 31: 3607–3619, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Landrum ML, Neumann C, Cook C, Chukwuma U, Ellis MW, Hospenthal DR, and Murray CK. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA 308: 50–59, 2012 [DOI] [PubMed] [Google Scholar]
- 95. Laupland KB, Ross T, and Gregson DB. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis 198: 336–343, 2008 [DOI] [PubMed] [Google Scholar]
- 96. Lee AS, de Lencastre H, Garau J, Kluytmans J, Malhotra-Kumar S, Peschel A, and Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers 4: 18033, 2018 [DOI] [PubMed] [Google Scholar]
- 97. Lee BY, Singh A, David MZ, Bartsch SM, Slayton RB, Huang SS, Zimmer SM, Potter MA, Macal CM, Lauderdale DS, Miller LG, and Daum RS. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Clin Microbiol Infect 19: 528–536, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lee KH, Kronbichler A, Park DD, Park Y, Moon H, Kim H, Choi JH, Choi Y, Shim S, Lyu IS, Yun BH, Han Y, Lee D, Lee SY, Yoo BH, Lee KH, Kim TL, Kim H, Shim JS, Nam W, So H, Choi S, Lee S, and Shin JI. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun Rev 16: 1160–1173, 2017 [DOI] [PubMed] [Google Scholar]
- 99. Li M, Cha DJ, Lai Y, Villaruz AE, Sturdevant DE, and Otto M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol Microbiol 66: 1136–1147, 2007 [DOI] [PubMed] [Google Scholar]
- 100. Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, and Otto M. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 106: 5883–5888, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li M, Lai Y, Villaruz AE, Cha DJ, Sturdevant DE, and Otto M. Gram-positive three-component antimicrobial peptide-sensing system. Proc Natl Acad Sci U S A 104: 9469–9474, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li X, Wang X, Thompson CD, Park S, Park WB, and Lee JC. Preclinical efficacy of clumping factor A in prevention of Staphylococcus aureus infection. mBio 7: e02232-02215, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, M JR, Talan DA, Chambers HF, and Infectious Diseases Society of A. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52: e18–e55, 2011 [DOI] [PubMed] [Google Scholar]
- 104. Liu CI, Liu GY, Song Y, Yin F, Hensler ME, Jeng WY, Nizet V, Wang AH, and Oldfield E. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science 319: 1391–1394, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, and Nizet V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med 202: 209–215, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lowy FD. Staphylococcus aureus infections. N Engl J Med 339: 520–532, 1998 [DOI] [PubMed] [Google Scholar]
- 107. Lu T, Porter AR, Kennedy AD, Kobayashi SD, and DeLeo FR. Phagocytosis and killing of Staphylococcus aureus by human neutrophils. J Innate Immun 6: 639–649, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Magill SS, O'Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE, Kainer MA, Lynfield R, Greissman S, Ray SM, Beldavs Z, Gross C, Bamberg W, Sievers M, Concannon C, Buhr N, Warnke L, Maloney M, Ocampo V, Brooks J, Oyewumi T, Sharmin S, Richards K, Rainbow J, Samper M, Hancock EB, Leaptrot D, Scalise E, Badrun F, Phelps R, Edwards JR, and Emerging Infections Program Hospital Prevalence Survey T. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 379: 1732–1744, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Magyarics Z, Leslie F, Bartko J, Rouha H, Luperchio S, Schorgenhofer C, Schwameis M, Derhaschnig U, Lagler H, Stiebellehner L, Firbas C, Weber S, Campanaro E, Jilma B, Nagy E, and Stevens C. Randomized, double-blind, placebo-controlled, single-ascending-dose study of the penetration of a monoclonal antibody combination (ASN100) targeting Staphylococcus aureus cytotoxins in the lung epithelial lining fluid of healthy volunteers. Antimicrob Agents Chemother 63, e00350–19, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mandell GL. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal—leukocyte interaction. J Clin Invest 55: 561–566, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. McGuinness WA, Malachowa N, and DeLeo FR. Vancomycin resistance in Staphylococcus aureus. Yale J Biol Med 90: 269–281, 2017 [PMC free article] [PubMed] [Google Scholar]
- 112. Melly MA, Thomison JB, and Rogers DE. Fate of staphylococci within human leukocytes. J Exp Med 112: 1121–1130, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Menegazzi R, Decleva E, and Dri P. Killing by neutrophil extracellular traps: fact or folklore? Blood 119: 1214–1216, 2012 [DOI] [PubMed] [Google Scholar]
- 114. Menzies BE and Kernodle DS. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect Immun 64: 1839–1841, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mermel LA, Cartony JM, Covington P, Maxey G, and Morse D. Methicillin-resistant Staphylococcus aureus colonization at different body sites: a prospective, quantitative analysis. J Clin Microbiol 49: 1119–1121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA, and Group EMINS. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 355: 666–674, 2006 [DOI] [PubMed] [Google Scholar]
- 117. Morgan E, Hohmann S, Ridgway JP, Daum RS, and David MZ. Decreasing incidence of skin and soft-tissue infections in 86 US emergency departments, 2009–2014. Clin Infect Dis 68: 453–459, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Murphy E and Novick RP. Physical mapping of Staphylococcus aureus penicillinase plasmid pI524: characterization of an invertible region. Mol Gen Genet 175: 19–30, 1979 [DOI] [PubMed] [Google Scholar]
- 119. Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, Richardson P, Bruce D, Rubin E, Myers E, Siggia ED, and Tomasz A. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U S A 104: 9451–9456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV, Latif H, Kim JN, Lockhart A, Artis K, David G, Taylor P, Streib J, Dorrestein PC, Grier A, Gill SR, Zengler K, Hata TR, Leung DY, and Gallo RL.. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 9, eaah4680, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Nanra JS, Buitrago SM, Crawford S, Ng J, Fink PS, Hawkins J, Scully IL, McNeil LK, Aste-Amezaga JM, Cooper D, Jansen KU, and Anderson AS. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum Vaccin Immunother 9: 480–487, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Newsom SW. Ogston's coccus. J Hosp Infect 70: 369–372, 2008 [DOI] [PubMed] [Google Scholar]
- 123. Otto M. Staphylococcal biofilms. Microbiol Spectr 6: GPP-0023-2018, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Palmqvist N, Foster T, Tarkowski A, and Josefsson E. Protein A is a virulence factor in Staphylococcus aureus arthritis and septic death. Microb Pathog 33: 239–249, 2002 [DOI] [PubMed] [Google Scholar]
- 125. Pang YY, Schwartz J, Thoendel M, Ackermann LW, Horswill AR, and Nauseef WM. agr-Dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J Innate Immun 2: 546–559, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pantosti A, Sanchini A, and Monaco M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol 2: 323–334, 2007 [DOI] [PubMed] [Google Scholar]
- 127. Patel AH, Nowlan P, Weavers ED, and Foster T. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun 55: 3103–3110, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pauli NT, Kim HK, Falugi F, Huang M, Dulac J, Henry Dunand C, Zheng NY, Kaur K, Andrews SF, Huang Y, DeDent A, Frank KM, Charnot-Katsikas A, Schneewind O, and Wilson PC. Staphylococcus aureus infection induces protein A-mediated immune evasion in humans. J Exp Med 211: 2331–2339, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, Kalbacher H, Nieuwenhuizen WF, Jung G, Tarkowski A, van Kessel KP, and van Strijp JA. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med 193: 1067–1076, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Peschel A and Otto M. Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol 11: 667–673, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, and Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 274: 8405–8410, 1999 [DOI] [PubMed] [Google Scholar]
- 132. Peterson PK, Verhoef J, Sabath LD, and Quie PG. Extracellular and bacterial factors influencing staphylococcal phagocytosis and killing by human polymorphonuclear leukocytes. Infect Immun 14: 496–501, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Petri B and Sanz MJ. Neutrophil chemotaxis. Cell Tissue Res 371: 425–436, 2018 [DOI] [PubMed] [Google Scholar]
- 134. Postma B, Poppelier MJ, van Galen JC, Prossnitz ER, van Strijp JA, de Haas CJ, and van Kessel KP. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J Immunol 172: 6994–7001, 2004 [DOI] [PubMed] [Google Scholar]
- 135. Prat C, Bestebroer J, de Haas CJ, van Strijp JA, and van Kessel KP. A new staphylococcal anti-inflammatory protein that antagonizes the formyl peptide receptor-like 1. J Immunol 177: 8017–8026, 2006 [DOI] [PubMed] [Google Scholar]
- 136. Prat C, Haas PJ, Bestebroer J, de Haas CJ, van Strijp JA, and van Kessel KP. A homolog of formyl peptide receptor-like 1 (FPRL1) inhibitor from Staphylococcus aureus (FPRL1 inhibitory protein) that inhibits FPRL1 and FPR. J Immunol 183: 6569–6578, 2009 [DOI] [PubMed] [Google Scholar]
- 137. Price LB, Stegger M, Hasman H, Aziz M, Larsen J, Andersen PS, Pearson T, Waters AE, Foster JT, Schupp J, Gillece J, Driebe E, Liu CM, Springer B, Zdovc I, Battisti A, Franco A, Zmudzki J, Schwarz S, Butaye P, Jouy E, Pomba C, Porrero MC, Ruimy R, Smith TC, Robinson DA, Weese JS, Arriola CS, Yu F, Laurent F, Keim P, Skov R, and Aarestrup FM. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. MBio 3, e00305–11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rao N, Ziran BH, and Lipsky BA. Treating osteomyelitis: antibiotics and surgery. Plast Reconstr Surg 127 Suppl 1: 177S–187S, 2011 [DOI] [PubMed] [Google Scholar]
- 139. Reyes J, Rincon S, Diaz L, Panesso D, Contreras GA, Zurita J, Carrillo C, Rizzi A, Guzman M, Adachi J, Chowdhury S, Murray BE, and Arias CA. Dissemination of methicillin-resistant Staphylococcus aureus USA300 sequence type 8 lineage in Latin America. Clin Infect Dis 49: 1861–1867, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Reyes-Robles T, Alonzo F, 3rd, Kozhaya L, Lacy DB, Unutmaz D, and Torres VJ. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe 14: 453–459, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Richardson AR, Dunman PM, and Fang FC. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol Microbiol 61: 927–939, 2006 [DOI] [PubMed] [Google Scholar]
- 142. Richmond MH, Parker MT, Jevons MP, and John M. High penicillinase production correlated with multiple antibiotic resistance in Staphylococcus aureus. Lancet 1: 293–296, 1964 [DOI] [PubMed] [Google Scholar]
- 143. Robertson L, Caley JP, and Moore J. Importance of Staphylococcus aureus in pneumonia in the 1957 epidemic of influenza A. Lancet 2: 233–236, 1958 [DOI] [PubMed] [Google Scholar]
- 144. Robinson DA, Kearns AM, Holmes A, Morrison D, Grundmann H, Edwards G, O'Brien FG, Tenover FC, McDougal LK, Monk AB, and Enright MC. Re-emergence of early pandemic Staphylococcus aureus as a community-acquired meticillin-resistant clone. Lancet 365: 1256–1258, 2005 [DOI] [PubMed] [Google Scholar]
- 145. Rodriguez D, Pigrau C, Euba G, Cobo J, Garcia-Lechuz J, Palomino J, Riera M, Del Toro MD, Granados A, Ariza X, and Group R. Acute haematogenous prosthetic joint infection: prospective evaluation of medical and surgical management. Clin Microbiol Infect 16: 1789–1795, 2010 [DOI] [PubMed] [Google Scholar]
- 146. Rogers DE. Studies on bacteriemia. I. Mechanisms relating to the persistence of bacteriemia in rabbits following the intravenous injection of staphylococci. J Exp Med 103: 713–742, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rogers DE and Melly MA. Further observations on the behavior of staphylococci within human leukocytes. J Exp Med 111: 533–558, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rogers DE and Tompsett R. The survival of staphylococci within human leukocytes. J Exp Med 95: 209–230, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Rossi F, Garcia P, Ronzon B, Curcio D, and Dowzicky MJ. Rates of antimicrobial resistance in Latin America (2004–2007) and in vitro activity of the glycylcycline tigecycline and of other antibiotics. Braz J Infect Dis 12: 405–415, 2008 [DOI] [PubMed] [Google Scholar]
- 150. Rouha H, Weber S, Janesch P, Maierhofer B, Gross K, Dolezilkova I, Mirkina I, Visram ZC, Malafa S, Stulik L, Badarau A, and Nagy E. Disarming Staphylococcus aureus from destroying human cells by simultaneously neutralizing six cytotoxins with two human monoclonal antibodies. Virulence 9: 231–247, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rountree PM and Freeman BM. Infections caused by a particular phage type of Staphylococcus aureus. Med J Aust 42: 157–161, 1955 [PubMed] [Google Scholar]
- 152. Ruzin A, Wu Y, Yu L, Yu XQ, Tabor DE, Mok H, Tkaczyk C, Jensen K, Bellamy T, Roskos L, Esser MT, and Jafri HS. Characterisation of anti-alpha toxin antibody levels and colonisation status after administration of an investigational human monoclonal antibody, MEDI4893, against Staphylococcus aureus alpha toxin. Clin Transl Immunol 7: e1009, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Savill JS, Henson PM, and Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel “charge-sensitive” recognition mechanism. J Clin Invest 84: 1518–1527, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, and Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest 83: 865–875, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Schwartz JT, Barker JH, Kaufman J, Fayram DC, McCracken JM, and Allen LA. Francisella tularensis inhibits the intrinsic and extrinsic pathways to delay constitutive apoptosis and prolong human neutrophil lifespan. J Immunol 188: 3351–3363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Schwarzmann SW, Adler JL, Sullivan RJ Jr., and Marine WM. Bacterial pneumonia during the Hong Kong influenza epidemic of 1968–1969. Arch Intern Med 127: 1037–1041, 1971 [PubMed] [Google Scholar]
- 157. Seilie ES and Bubeck Wardenburg J. Staphylococcus aureus pore-forming toxins: The interface of pathogen and host complexity. Semin Cell Dev Biol 72: 101–116, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sieprawska-Lupa M, Mydel P, Krawczyk K, Wojcik K, Puklo M, Lupa B, Suder P, Silberring J, Reed M, Pohl J, Shafer W, McAleese F, Foster T, Travis J, and Potempa J. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother 48: 4673–4679, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Simons MP, Nauseef WM, Griffith TS, and Apicella MA. Neisseria gonorrhoeae delays the onset of apoptosis in polymorphonuclear leukocytes. Cell Microbiol 8: 1780–1790, 2006 [DOI] [PubMed] [Google Scholar]
- 160. Smith EJ, Visai L, Kerrigan SW, Speziale P, and Foster TJ. The Sbi protein is a multifunctional immune evasion factor of Staphylococcus aureus. Infect Immun 79: 3801–3809, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Smith TC, Male MJ, Harper AL, Kroeger JS, Tinkler GP, Moritz ED, Capuano AW, Herwaldt LA, and Diekema DJ. Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern U.S. swine and swine workers. PLoS One 4: e4258, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Spaan AN, Henry T, van Rooijen WJM, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJC, van Kessel KPM, Vandenesch F, Lina G, and van Strijp JAG. The staphylococcal toxin Panton-Valentine leukocidin targets human C5a receptors. Cell Host Microbe 13: 584–594, 2013 [DOI] [PubMed] [Google Scholar]
- 163. Spaan AN, van Strijp JAG, and Torres VJ. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol 15: 435–447, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Spaan AN, Vrieling M, Wallet P, Badiou C, Reyes-Robles T, Ohneck EA, Benito Y, de Haas CJ, Day CJ, Jennings MP, Lina G, Vandenesch F, van Kessel KP, Torres VJ, van Strijp JA, and Henry T. The staphylococcal toxins gamma-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat Commun 5: 5438, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Spink WW and Ferris V. Penicillin-resistant staphylococci: mechanisms involved in the development of resistance. J Clin Invest 26: 379–393, 1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Spink WW, Hall WH, and Ferris V. Clinical significance of staphylococci with natrual or acquired resistance to the sulfonamides or to penicillin. JAMA 128: 555–559, 1945 [Google Scholar]
- 167. Stapels DA, Ramyar KX, Bischoff M, von Kockritz-Blickwede M, Milder FJ, Ruyken M, Eisenbeis J, McWhorter WJ, Herrmann M, van Kessel KP, Geisbrecht BV, and Rooijakkers SH. Staphylococcus aureus secretes a unique class of neutrophil serine protease inhibitors. Proc Natl Acad Sci U S A 111: 13187–13192, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Stapleton PD and Taylor PW. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci Prog 85: 57–72, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Stulik L, Rouha H, Labrousse D, Visram ZC, Badarau A, Maierhofer B, Gross K, Weber S, Kramaric MD, Glojnaric I, Nagy G, Croisier D, and Nagy E. Preventing lung pathology and mortality in rabbit Staphylococcus aureus pneumonia models with cytotoxin-neutralizing monoclonal IgGs penetrating the epithelial lining fluid. Sci Rep 9: 5339, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Sully EK, Malachowa N, Elmore BO, Alexander SM, Femling JK, Gray BM, DeLeo FR, Otto M, Cheung AL, Edwards BS, Sklar LA, Horswill AR, Hall PR, and Gresham HD. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog 10: e1004174, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Surewaard BG, de Haas CJ, Vervoort F, Rigby KM, DeLeo FR, Otto M, van Strijp JA, and Nijland R. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol 15: 1427–1437, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Surewaard BGJ, Thanabalasuriar A, Zeng Z, Tkaczyk C, Cohen TS, Bardoel BW, Jorch SK, Deppermann C, Bubeck Wardenburg J, Davis RP, Jenne CN, Stover KC, Sellman BR, and Kubes P. Alpha-toxin induces platelet aggregation and liver injury during Staphylococcus aureus sepsis. Cell Host Microbe 24: 271–284 e273, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Takeuchi O, Hoshino K, and Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 165: 5392–5396, 2000 [DOI] [PubMed] [Google Scholar]
- 174. Talan DA, Krishnadasan A, Gorwitz RJ, Fosheim GE, Limbago B, Albrecht V, Moran GJ, and Group EMINS. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis 53: 144–149, 2011 [DOI] [PubMed] [Google Scholar]
- 175. Tattevin P, Schwartz BS, Graber CJ, Volinski J, Bhukhen A, Bhukhen A, Mai TT, Vo NH, Dang DN, Phan TH, Basuino L, Perdreau-Remington F, Chambers HF, and Diep BA. Concurrent epidemics of skin and soft tissue infection and bloodstream infection due to community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis 55: 781–788, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Thakker M, Park JS, Carey V, and Lee JC. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun 66: 5183–5189, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Thammavongsa V, Missiakas DM, and Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 342: 863–866, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Tong SY, Davis JS, Eichenberger E, Holland TL, and Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28: 603–661, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Tran VG, Venkatasubramaniam A, Adhikari RP, Krishnan S, Wang X, Le VTM, Le HN, Vu TTT, Schneider-Smith E, Aman MJ, and Diep BA. Efficacy of active immunization with attenuated alpha-hemolysin and Panton-Valentine leukocidin in a rabbit model of Staphylococcus aureus necrotizing pneumonia. J Infect Dis 221: 267–275, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Tuchscherr L, Medina E, Hussain M, Volker W, Heitmann V, Niemann S, Holzinger D, Roth J, Proctor RA, Becker K, Peters G, and Loffler B. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med 3: 129–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Uhlemann AC, Porcella SF, Trivedi S, Sullivan SB, Hafer C, Kennedy AD, Barbian KD, McCarthy AJ, Street C, Hirschberg DL, Lipkin WI, Lindsay JA, DeLeo FR, and Lowy FD. Identification of a highly transmissible animal-independent Staphylococcus aureus ST398 clone with distinct genomic and cell adhesion properties. MBio 3: e00027-12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ventura CL, Malachowa N, Hammer CH, Nardone GA, Robinson MA, Kobayashi SD, and DeLeo FR. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One 5: e11634, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. von Eiff C, Becker K, Machka K, Stammer H, and Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 344: 11–16, 2001 [DOI] [PubMed] [Google Scholar]
- 184. Voss A, Loeffen F, Bakker J, Klaassen C, and Wulf M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg Infect Dis 11: 1965–1966, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, and DeLeo FR. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol 175: 3907–3919, 2005 [DOI] [PubMed] [Google Scholar]
- 186. Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, Long RD, Dorward DW, Gardner DJ, Lina G, Kreiswirth BN, and DeLeo FR. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis 194: 1761–1770, 2006 [DOI] [PubMed] [Google Scholar]
- 187. Vu TTT, Nguyen NTQ, Tran VG, Gras E, Mao Y, Jung DH, Tkaczyk C, Sellman BR, and Diep BA.. Protective efficacy of monoclonal antibodies neutralizing alpha-hemolysin and bicomponent leukocidins in a rabbit model of Staphylococcus aureus necrotizing pneumonia. Antimicrob Agents Chemother 64, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Walters MS, Eggers P, Albrecht V, Travis T, Lonsway D, Hovan G, Taylor D, Rasheed K, Limbago B, and Kallen A. Vancomycin-resistant Staphylococcus aureus - Delaware, 2015. MMWR Morb Mortal Wkly Rep 64: 1056, 2015 [DOI] [PubMed] [Google Scholar]
- 189. Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, and Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13: 1510–1514, 2007 [DOI] [PubMed] [Google Scholar]
- 190. Watkins RR, Holubar M, and David MZ. Antimicrobial resistance in methicillin-resistant Staphylococcus aureus to newer antimicrobial agents. Antimicrob Agents Chemother 63: e01216-19, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Watson RW, Redmond HP, Wang JH, Condron C, and Bouchier-Hayes D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol 156: 3986–3992, 1996 [PubMed] [Google Scholar]
- 192. Weems JJ Jr., Steinberg JP, Filler S, Baddley JW, Corey GR, Sampathkumar P, Winston L, John JF, Kubin CJ, Talwani R, Moore T, Patti JM, Hetherington S, Texter M, Wenzel E, Kelley VA, and Fowler VG Jr. Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 50: 2751–2755, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Whyte MK, Meagher LC, MacDermot J, and Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. J Immunol 150: 5124–5134, 1993 [PubMed] [Google Scholar]
- 194. Wright JS, 3rd, Jin R, and Novick RP. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc Natl Acad Sci U S A 102: 1691–1696, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Wulf MW, Sorum M, van Nes A, Skov R, Melchers WJ, Klaassen CH, and Voss A. Prevalence of methicillin-resistant Staphylococcus aureus among veterinarians: an international study. Clin Microbiol Infect 14: 29–34, 2008 [DOI] [PubMed] [Google Scholar]
- 196. Wyllie DH, Walker AS, Miller R, Moore C, Williamson SR, Schlackow I, Finney JM, O'Connor L, Peto TE, and Crook DW. Decline of meticillin-resistant Staphylococcus aureus in Oxfordshire hospitals is strain-specific and preceded infection-control intensification. BMJ Open 1: e000160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Yoshiie K, Kim HY, Mott J, and Rikihisa Y. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect Immun 68: 1125–1133, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zhang B, Hirahashi J, Cullere X, and Mayadas TN. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J Biol Chem 278: 28443–28454, 2003 [DOI] [PubMed] [Google Scholar]
- 199. Zhang S, Sun X, Chang W, Dai Y, and Ma X. Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. PLoS One 10: e0136082, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]