Abstract
Prominent clinical problems related to the skin-nerve interface include barrier dysfunction and erythema, but it is the symptoms of pain and itch that most often lead patients to seek medical treatment. Tissue-engineered innervated skin models provide an excellent solution for studying the mechanisms underlying neurocutaneous disorders for drug screening, and cutaneous device development. Innervated skin substitutes provide solutions beyond traditional monolayer cultures and have advantages that make them preferable to in vivo animal studies for certain applications, such as measuring somatosensory transduction. The tissue-engineered innervated skin models replicate the complex stratified epidermis that provides barrier function in native skin, a feature that is lacking in monolayer co-cultures, while allowing for a level of detail in measurement of nerve morphology and function that cannot be achieved in animal models. In this review, the advantages and disadvantages of different cell sources and scaffold materials will be discussed and a presentation of the current state of the field is reviewed.
Impact statement
A review of the current state of innervated skin substitutes and the considerations that need to be addressed when developing these models. Tissue-engineered skin substitutes are customizable and provide barrier function allowing for screening of topical drugs and for studying nerve function.
Keywords: skin, nerve, co-culture
Introduction
Prominent clinical problems related to the skin-nerve interface include barrier dysfunction and erythema, but it is the somatosensory symptoms of pain and itch that produce the impetus to seek medical treatment. Chronic itch is the most frequent symptom for which relief is sought in dermatology, with a lifetime prevalence of 22.6%.1 Clinically significant itch accompanies many common cutaneous diseases such as atopic dermatitis and psoriasis; it produces distress, inducing self-mutilation and relief-seeking behaviors leading to the consumption of conventional pharmaceutics as well as unproven alternative therapies.2,3 Chronic pain and chronic itch are common occurrences during wound healing, especially after thermal injury. Over 50% of patients with large burns suffer from chronic pain or itch.4,5
Pain from the skin, including from allodynia and hyperalgesia, is often related to neuropathies, including the small fiber neuropathies present in diabetes. Nociceptive unmyelinated C fibers have been linked to the pathology of skin inflammation and diseases.6 In addition to the transduction and transmission of somatosensory information, primary afferent fibers in the skin also act bidirectionally, releasing peptides and modulators into the skin, which mediate immune activation and interactions with vasculature and skin cells.7 These interactions are also involved in a host of skin disorders without somatosensory symptoms, such as vitiligo.8 Tissue engineering approaches are an excellent way to study these complex interactions and to screen drug treatments and develop cutaneous devices for neurocutaneous disorders. Below, the current state of the tissue-engineered models is reviewed along with a discussion of the advantages and disadvantages of the different cell sources and scaffold material choices.
Skin Anatomy
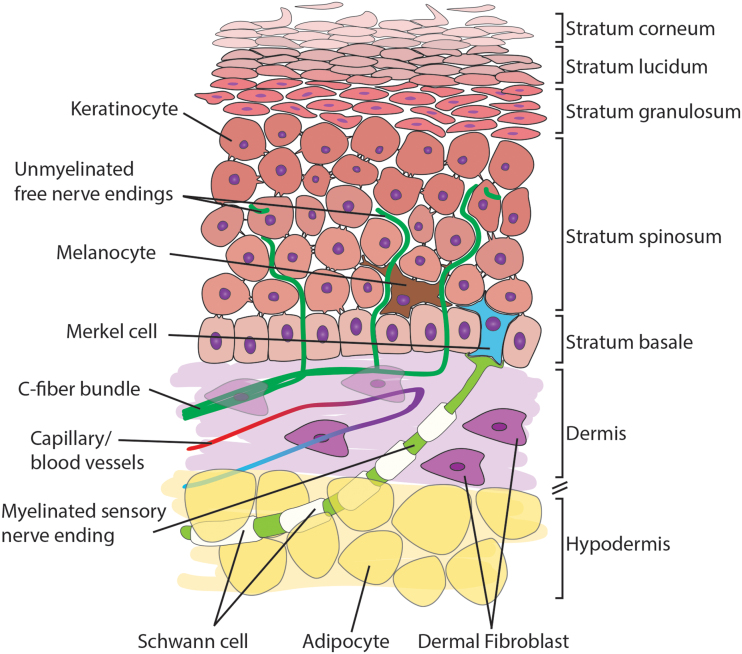
The skin is the largest organ, provides a protective barrier, and is critical for somatosensation. It comprises three layers: the epidermis, the dermis, and the hypodermis, also known as the subcutaneous layer (Fig. 1). Pseudo-unipolar axons originate from the somata of primary afferent neurons located in the dorsal root ganglia (DRG) and extend into the dermal and epidermal layers of the skin. There are three main types of afferent fibers: Aβ, Aδ, and C fibers. The C fibers are unmyelinated and terminate in the dermis and epidermis, while the larger, myelinated Aβ and Aδ tend to localize in the dermis.9,10 Nerve endings are responsible for bidirectional communication between the skin and nervous system, making possible somatosensation and neurogenic inflammation.7,11
FIG. 1.
Schematic illustration of skin anatomy. The skin comprises three major layers: the epidermis, the dermis, and the hypodermis. Select anatomical features and cell types are indicated. The layers of the stratified epidermis are labeled. Color images are available online.
The epidermis is the uppermost layer of the skin; it is highly stratified and composed primarily of keratinocytes in various stages of differentiation.12 The keratinocytes closest to the dermis are columnar and sit on top of a thin basement membrane. As they differentiate, the keratinocytes detach from the basement membrane and elongate, eventually terminally differentiating into corneocytes, which are anuclear keratinocytes. Keratinocytes express sensory receptors and secrete neuroactive substances,13 and re-epithelialization can be stimulated through neuronal signaling after an injury.14 Merkel cells are also found at the basement membrane among the cuboidal keratinocytes. The Merkel cells form complexes with neurites to produce touch receptors.15 Also, in the epidermis are the melanin-producing melanocytes that form the pigment in the skin. Melanocytes are neuromodulatory and secrete cytokines to regulate the immune response.
The dermis is the thickest layer of the skin imparting its mechanical properties such as strength and elasticity. The most abundant cell type in the dermis is the dermal fibroblast that produces the extracellular matrix, contains contractile apparatus to aid in wound closure, and secretes cytokines and growth factors.16 Larger numbers of fibroblasts are found closer to the epidermis than in the deep dermis, suggesting a role in signaling with keratinocytes or other cells of the epidermis. There are multiple other cell types found in the dermis due to the presence of capillaries, sweat glands, hair follicles, immune cells, and nerves.
The hypodermis is the deepest layer of the skin and is composed primarily of adipocytes. The hypodermis connects the skin to the surrounding facia and is the point for ingrowth of nerves and blood vessels. It acts as cushioning and insulation for the skin and secretes hormones, cytokines, and growth factors. Adipocytes communicate with neurons to modulate metabolism and neuropeptide production and are involved in regulating nociception.17
New understanding of communication between skin and sensory nerve terminals
Skin affects innocuous and noxious somatosensation by releasing neurotransmitters and neuromodulators that can signal directly to sensory neurons and influence neuronal excitability. Recently, keratinocytes were found to communicate directly with sensory nerve endings and contribute to mechanical (touch) responsiveness. Optogenetic silencing of keratinocytes in transgenic mice expressing archaerhodopsin in K14 expressing epidermal cells decreased behavioral responses to innocuous and noxious mechanical stimuli in the mouse hindpaw.18 These behavioral responses were mediated through the release of adenosine triphosphate (ATP), a purinergic signaling molecule, from keratinocytes.
Cultured keratinocytes released ATP in a graded manner upon increasing mechanical stimulation, resulting in P2X2-mediated inward currents, and hydrolysis of local ATP in the hind paw decreased behavioral responses to both noxious and innocuous mechanical stimuli. These findings indicate that the release of ATP from epidermal keratinocytes contributes to skin-nerve signal processing and behavior and highlight the critical involvement of non-neuronal skin cells in somatosensation.18 Purinergic signaling has also been shown to be critical for thermal sensation.19
Merkel cells in the epidermis also communicate to sensory neurons through the release of neurotransmitters.20 Merkel cells express tyrosine hydrogenase, the rate-limiting enzyme for the production of catecholamines, and activate sensory neurons through adrenergic signaling, specifically the release of norepinephrine, in response to touch.21 Using ex vivo electrophysiology, Merkel cells and keratinocytes have been shown to excite sensory neurons and produce neuronal action potentials through optogenetic stimulation solely of the skin cells.22,23 Moreover, it has been seen that Merkel cells have the ability to transduce touch stimuli into excitatory responses without the presence of keratinocytes and sensory neurons through the mechanosensitive Piezo2 receptor.
In addition, Merkel cells exhibit an excitatory connection with sensory neurons to fine-tune mechanosensory responses.22 Merkel cells are one of the four types of mechanosensory end organs along with Meissner's corpuscles, Pacinian corpuscles, and Ruffini's endings that traditionally form units with Aβ Aδ, and C sensory neuron afferents to transduce touch stimuli.10,24–27 Keratinocytes are in close proximity with free nerve endings28 and are also capable of mediating sensory transduction.23,29,30 While the mechanisms by which keratinocytes communicate with sensory nerve endings in the epidermis still remain enigmatic, recently discovered functional “en passant” synapses between sensory nerve fibers and keratinocytes challenge the classical view of keratinocytes and shed light on their potential role as another touch transduction cell.31
Communication occurs bidirectionally between sensory neurons and immune cells in the skin and contributes to the inflammatory response and disease pathologies. Neurogenic inflammation occurs when activated sensory neurons release neuropeptides, such as substance P and calcitonin gene-related peptide (CGRP), into the periphery causing inflammation.32,33 Reciprocally, skin cells release neuropeptides that activate sensory neurons.32 In the epidermis, sensory nerve fibers activate keratinocytes that subsequently release pro-inflammatory cytokines such as IL-1a, IL-6, and IL-8.32 In the dermis, sensory neurons interact with blood vessels and mast cells, which play a role in chronic skin inflammation and disease.32 Therefore, the interactions between skin and nerve can produce feedback cycles that can promote the worsening or persistence of disease symptoms such as hypersensitivity, erythema, and itch.
Because of these intricate and important interactions between skin cells and neurons, investigating one or the other cell type in isolation is inadequate to gain a full understanding of the system.
Co-Culture Systems as a Tool to Investigate Skin-Nerve Interactions
Skin-nerve co-cultures have a high potential to be an effective way to recapitulate interactions in a setting in which variables can be controlled. The use of skin cells that are easily recovered from patients can allow precision in disease specificity. Recovered skin from various diseases that cause somatosensory complaints such as atopic dermatitis or psoriasis could be modeled in vitro with sensory neurons to determine the effects cells from diseased skin have on sensory neuron membrane excitability, transduction efficiency, nerve ending morphology, and changes to gene expression. Another benefit of skin-nerve co-cultures is they can be used in conjunction with cell-specific functional assays such as calcium imaging and patch-clamp electrophysiology. These techniques allow the measurement of cellular activity and physiology in vitro.
Barrier function and drug delivery
Due to the barrier function of the skin and the stratified epidermal layer, a tissue-engineered model is an important strategy for studying the treatment of neurocutaneous disorders, particularly for topically applied therapies. One of the main functions of the epidermis is to provide a barrier to the external environment. The stratum corneum layer of the epidermis provides the first physical barrier. The stratum corneum consists of corneocytes, a densely packed lipid layer, and other proteins such as filaggrin also contribute to barrier function. Tight junctions between cells in the stratum granulosum layer of the epidermis provide the second barrier, inhibiting molecules based on size. The final barrier layer of the epidermis is the basement membrane. The basement membrane is critical to epidermal development and may act as a physical barrier itself. Gorzelanny et al. provide an in-depth review of the skin barrier function.34
Benefits of tissue-engineered skin models over alternative culture techniques
Monolayer studies are commonly used to study cell biology; the study of interactions between keratinocytes and neurons is no exception. Monolayer co-cultures of skin and nerves have been created to study cutaneous neurogenic inflammation.35 Figure 2 shows monolayer co-cultures of human sensory neurons with epidermal keratinocytes. Monolayers have the benefit of being able to simplify the cultures; complexity can be added through micropatterning or use of chambered systems, which limit the interactions between different cell types.
FIG. 2.
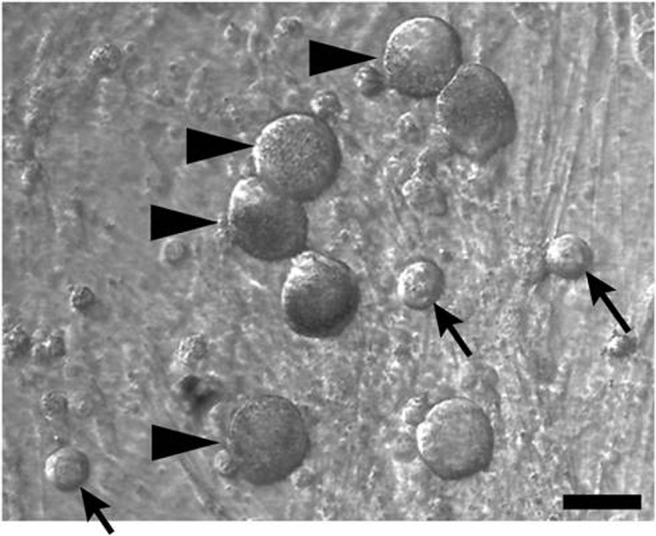
Human dorsal root ganglia neurons and human epidermal keratinocytes in co-culture. Arrowheads = neurons; arrows = keratinocytes. Scale bar = 50 μm.
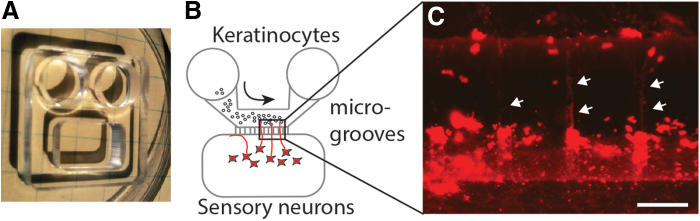
Microgrooved systems have been used for drug screening and to study nerve-keratinocyte communication. Kumamoto et al. cultured DRG and keratinocytes in separate chambers connected by a thin microgroove. This allowed nerve fibers to invade, but not the keratinocytes.36 Figure 3 shows an example of neurons cultured alone in a chamber with microgrooves for nerve fiber growth.
FIG. 3.
Human sensory neurons and keratinocytes cultured in a microfluidic chamber with microgrooves. (A) Microfluidic chamber (approximate 2 cm square) made of PDMS. (B) Illustration of microfluidic chamber with keratinocytes in the flow-through compartment (arrow indicates flow direction) and sensory neurons in the apposed compartment separated by microgrooved channels. (C) When neurons are incubated with the lipophilic, crystalline red dye, DiI, axons (arrows) can be observed crossing the microgrooved channels to the keratinocyte side. Scale bar = 50 μm. PDMS, polydimethylsiloxane. Color images are available online.
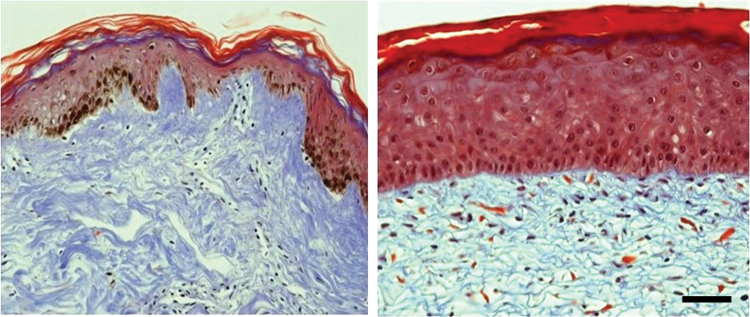
A chambered device has also been used to test the DRG neurons, keratinocytes, and dermal fibroblasts together.37 These models are valuable; however, they have a significant limitation in common with monolayers, at least in the present. Keratinocytes grown in monolayer culture are proliferative and do not differentiate as in vivo. Three-dimensional cultures proliferate more slowly, but can fully replicate the stratified epidermal layer. These multiple layers are critical to barrier function and are important when studying topical drug delivery. Figure 4 compares the epidermal layers from native skin and tissue-engineered skin.
FIG. 4.
Mason's trichrome staining of native skin (left) and tissue-engineered skin (right). Collagen is blue, cell nuclei are purple/black, and epithelial cell tissues stain red. The brown in the native skin is natural pigment due to melanin. The tissue-engineered skin substitute was fabricated with human primary cells seeded on a collagen-chondroitin 6 sulfate sponge and cultured for two and a half weeks. The epidermal layer of the engineered tissue is thicker, but contains the epidermal layers, including the stratum corneum. Scale bar = 50 μm. Color images are available online.
Organoid development from human pluripotent stem cells has recently been used to develop multilayered, multicellular skin organoids.38 Lee et al. started with a single cell from an embryonic stem cell line, forming aggregates and altering the culture medium to co-induce mesenchymal and ectodermal cells. Hair buds were found after 70 days in culture. After 100 days in culture, the organoids were multilayered with pigmentation due to evenly distributed melanocytes in the epidermal layer. The group suggests that this model can be used to study disease and drug delivery. While this is an exciting model for development, the length of culture, discrepancies between induced models and native tissues, and expense associated with this model make it less attractive for drug screening. Cell behavior and intracellular signaling can also change with age and responses to drugs may not be conserved between the cells in skin organoids and adult skin and nerve cells.
Engineered skin models can provide a balance between the more physiologic representation of the skin than monolayer cultures, while reducing the variation of ex vivo skin biopsy cultures or in vivo studies. Non-innervated skin or epidermal substitutes have been used clinically as graft material39–43 and for drug screening.44–46 Innervation of skin substitutes can be studied using animal models; the model of choice is the athymic mice so that innervation of skin substitutes composed of human skin cells can be evaluated.47 Using this system, Hahn et al. were able to identify Merkel cells in the substitutes that expressed the neuroendocrine marker synaptophysin and were spatially associated with the mouse nerves and the human Merkel cells.48
These models allow for control of the cell types incorporated; the addition of melanocytes from high or low Fitzpatrick scale skin, a measure of skin color, was used to show that melanin enhanced innervation.49 The immunocompromised mice are excellent models for studying wound healing and innervation and allow for use of human skin cells; however, they have limited utility to study pain and itch. Pain and itch can only be determined through observation of behavior, making it difficult to determine the severity of symptoms or degrees of relief. Scratching, a symptom of itch, can also damage engineered skin substitutes before significant healing occurs. While pain and itch cannot be measured directly in the innervated skin models, the activation of nociceptive and pruriceptive (pain and itch sensing) pathways can be studied using physiological means.
Current Methods in Tissue-Engineered Innervated Skin Models
There are many approaches to fabricating an innervated skin model, considering the choices of cell type and source and scaffold materials. There are additional considerations that must be taken into account when including the study of innervation or somatosensory function. A discussion of the choices in this context is included below and a list of the current models can be found in Table 1.
Table 1.
Summary of Tissue-Engineered Innervated Skin Models
| Skin cells | Neurons | Other cells | Scaffold | Objectives | Source |
|---|---|---|---|---|---|
| Human from breast reduction | Mouse DRG | HUVEC | Collagen I/III-chitosan sponge | Model for studying peripheral nerve growth | Gingras et al.57 |
| Murine | Murine motor neurons | Murine Schwann cells | Collagen I/III-chitosan sponge | Neurite growth and myelination | Gingras et al.50 |
| Human from breast reduction | Murine DRG | HUVEC & murine Schwann cells | Collagen I/II-chitosan sponge | Neurite growth improvement with Schwann cells | Blais et al.59 |
| Human: Normal and Atopic Dermatitis | Pig DRG | None | Collagen I hydrogel | Neurite outgrowth in diabetic skin | Roggenkamp et al.60 |
| Human neonatal | Mouse DRG | HUVEC and HDMEC | Collagen I/III + chitosan sponge | Sensory neurons effect on wound closure. | Blais et al.14 |
| Human | Mouse DRG | None | Collagen I/III-chitosan sponge | Study effects of AGEs on skin pathophysiology and to study effects of anti-AGE molecules. | Cadau et al.58 |
| Human upper arm: healthy and diabetic | Pig DRG | None | Collagen I hydrogel | Study diabetes-associated cutaneous denervation. | Reichert et al.61 |
| Human neonatal fibroblasts and HaCaT | Rat DRG | None | Cross-linked gelatin | Model to study neuronal signal transduction | Martorina et al.56 |
| Human | hiPSC-derived neurons or Mouse DRG (control) | HDMEC (neonatal)- and hiPSC-derived Schwann cells | Collagen I/III-chitosan sponge | Fully human innervated skin model | Muller et al.76 |
| Human neonatal | hiNSC | Some: cell mix isolated from adipose tissue in hypodermis | Dermis: collagen-silk Hypodermis: silk sponge with collagen coating |
Full-thickness innervated skin model | Vidal et al.78 |
| Human neonatal | hiNSC | Some: cell mix isolated from adipose tissue in hypodermis | Dermis: collagen-silk Hypodermis: silk; collagen coating |
Model to study neuro-immuno-cutaneous interactions | Vidal Yucha et al.79 |
AGE, advanced glycation end product; DRG, dorsal root ganglion; HDMEC, human dermal microvascular endothelial cell; hiNSC; human induced neuronal stem cell; hiPSC, human induced pluripotent stem cell; HUVEC, human umbilical vein endothelial cell.
Skin cells: dermal fibroblast and keratinocytes
Due to the availability of discard skin, most tissue-engineered models utilize primary human skin cells. The only exception to date for the innervated skin models is Gingras et al.,50 who developed an innervated model with all primary murine cells. This reduces any species difference that occurs with the use of murine DRG and allows the results to be compared easily to animal studies. Human skin biopsies are most frequently obtained from skin that is discarded after an elective procedure such as a breast reduction, panniculectomy, or circumcision.
Neonatal foreskin is a common source of primary skin cells, so much so that several tissue-engineered skin or dermal substitutes containing neonatal cells have been approved as temporary wound dressings.39–41 While this is a highly available source for skin cells, several additional factors need to be taken into account when using the neonatal tissues. Neonatal fibroblasts, from the foreskin or face, are more proliferative and less susceptible to apoptosis.51–53 They express greater levels of smooth muscle α-actin, nestin, and basal antioxidant levels, and have a higher migratory potential than adult fibroblasts.54,55 Similar patterns in cell proliferation and viability are seen in neonatal keratinocytes.53 In addition, neonatal keratinocytes are poorly differentiated, expressing keratins 8, 14, and 19.54 This may not be of concern when studying inherited diseases, but is a likely factor when screening drugs for adult patients or when studying adult-onset disorders such as diabetes-associated neuropathy.
To reduce patient- and age-related variation, some researchers choose to use immortalized cell lines. The HacaT cell line is an immortalized keratinocyte line that has been heavily utilized in skin research. There has been significant validation showing similar functions to normal adult keratinocytes; Martorina et al. utilized these cells in an innervated skin model along with neonatal dermal fibroblasts.56 As with any immortalized cell line, additional validation is needed when investigating a different cellular function.
Neuron sources
Due to the challenges in obtaining and maintaining primary human nerve cells in culture, which are postmitotic and must be acquired rapidly from organ donors, animal sources are often the choice for innervated skin models. Mouse,14,50,57–59 rat,56 and pig60,61 DRG neurons have all been used. Rodent DRGs are relatively accessible and well-established protocols exist for culture11,62; however, the neurons from rodent DRGs can differ in their expression levels or types of receptors, genes, and signaling pathways compared to human DRG neurons, for which much is still unknown.63 For example, human DRG neurons express lower levels of Cav2.3, the excitability and neurotransmitter release-regulating voltage-gated calcium channel, compared to mouse DRG neurons.64
Pig DRG are more expensive and not as accessible, but may share more properties with human DRGs, specifically in cutaneous nociception and pain signaling.63 For instance, “silent,” mechano-insensitive C fiber nociceptors, which become sensitive to mechanical stimuli under inflammatory pain conditions, are present in human and pig sensory neurons, but are rare in rodents.65 Moreover, these silent nociceptors are the key mediators of the axon-reflex vasodilation that occurs in the skin of both humans and pigs,66 but this effect is mediated in rodents by a different type of polymodal nociceptors instead.65 Likewise, pigs and humans share similar vascular flares from skin injury, while rodents exhibit significantly smaller cutaneous vascular responses.67 Together, the use of pig skin and sensory neurons may be a more translational model for somatosensation, neurogenic inflammation, and other skin diseases seen in humans.
Due to the difficulties in obtaining primary adult neurons, human sources of neurons for these innervated models currently have used different methods, including the use of human stem cells (hSCs) reprogrammed from adult cells or human induced pluripotent stem cells (hiPSCs), which can be derived from fibroblasts, and increase the feasibility of utilizing human neuron-like cells.68 By the expression of specific transcription factors, hiPSCs can be differentiated into different classes of cells that share characteristics with sensory neurons such as nociceptors, mechanoreceptors, and proprioceptors.69,70 hIPSCs can be made to express nociceptive markers such as TAC1 (propeptide to Substance P), SCN9A (Nav1.7), and SCN10A (Nav1.8), and can be an effective tool to study sensory neuron function and receptor physiology and pharmacology.71
While hSCs and hiPSCs are useful tools to study human disease and drug efficacy, there can be inconsistencies in cellular function compared to native human or rodent cells. Despite expressing the canonical markers of sensory neurons, some hiPSC-derived neurons do not respond to capsaicin,72 an agonist of the TRPV1 receptor that is one of the gold standard markers of nociceptors. Inconsistencies in regard to function can also occur in disease models, for example, patients with inherited erythromelalgia (IEM) have a mutation in the Nav1.7 receptor, which results in decreased action potential threshold and increased firing in rat DRG neurons.73,74 However, this altered physiology was not consistent in hiPSCs from a cohort of patients with IEM.74,75 This discrepancy points to potential limitations for the use of hiPSCs; however, it also emphasizes the need to better understand individual differences from mutations and gene expression.
Investigating differential gene expression can help validate the transcriptional profile of sensory neuron-derived hSCs or hiPSCs.72 Adding Schwann cells increases axon outgrowth of hiPSC DRGs76 and preserves neuron-glia interactions in vitro.77 hiPSC-derived sensory neuron and human induced neural stem cell (hiNSC) models have the potential to effectively recapitulate disease phenotypes. To date, hiPSC-derived neurons76 or hiNSCs, directly reprogrammed from neonatal fibroblasts or adult adipose-derived stem cells,78,79 have been used in the innervated engineered skin models.
DRG neurons from rodents, pigs, and humans have all been used to investigate sensory neuron function in conjunction with the periphery or in the context of disease. Utilizing sensory neurons in culture allows for a more targeted and efficient way to study disease modeling and drug screening. This approach will allow researchers to expand to other areas such as investigating the role of sensory neurons in wound healing and burn recovery and building new systems of end-organ-nerve interaction such as colon and bladder, other common sources of pain, and inflammatory disease.
Incorporation of other cell types
The complexity of innervated skin substitutes has been enhanced by the incorporation of additional cell types that are thought to be involved in neurocutaneous signaling. Vascular endothelial cells have been added to the engineered dermis in several models14,57,76; these cells form capillary-like structures in the engineered skin that is often associated with the neurites. Schwann cells, either isolated from murine nerves50,59 or derived from hiPSCs,76 have also been added to the engineered dermal layers. Schwann cells produce laminin to ensheath the neurons once they come in contact with the axons80; Schwann cells were found to be necessary for neuron myelination50 and for establishing neuronal networks that spanned the entire engineered skin up to the epithelial layer.76
The addition of a hypodermis layer complete with lipid aspirate has been used.78,79 The lipid aspirate results in a mixture of cells that are found in the hypodermis, including adipocytes, endothelial cells, pericytes, and immune cells.81 The incorporation of the hypodermis containing lipoaspirate resulted in an increase in cytokine expression compared to skin substitutes containing only a dermis and epidermis.78
Scaffold choices
The first “artificial skin” described by Yannas and Burke, and used on patients, was an engineered dermal substitute82 that would later become Integra™ Dermal Regeneration Template. The scaffold for the engineered dermis consisted of bovine dermal collagen with chondroitin 6-sulfate. Collagen I is abundant in skin, biocompatible, and bioresorbable making it an excellent choice in scaffold material. It is also easily modified for drug delivery,83 something for future consideration. Collagen III is added at ratios similar to native skin. Mutations in collagen III are often found in patients with type IV Ehlers-Danlos syndrome, a disorder that results in fragile skin.84
Chondroitin 6-sulfate is added to increase the mechanical properties of the collagen scaffolds. While other natural and synthetic materials have been used for skin substitutes, this combination of collagen I and collagen III is a very popular scaffold choice due the abundance of these proteins in the body.85 This is especially true for those developing an innervated model. Regardless of the fabrication choice, freeze drying, electrospinning, or collagen self-assembly, the result is a relatively soft hydrogel with physical properties similar to that of native nerve tissue that can promote nerve ingrowth.86 While the term hydrogel is often used to describe the collagen self-assembly method, in this study, we are talking about any polymer network that is significantly hydrophilic and may increase in volume when hydrated, but does not lose its structure.
All of the innervated models to date have used collagen I or gelatin, which is partially hydrolyzed collagen, in the scaffolds, often with collagen III. The compositions are listed in Table 1. Unlike Burke et al.,82 the addition of chondroitin sulfate proteoglycans is omitted as Zuo et al., showed that it inhibits the growth-promoting action of laminin.87 Instead, the polysaccharide chitosan is often used in its place; this was first used in the fabrication of skin substitutes by Berthod et al.88 Chitosan, a linear polysaccharide, has been shown to enhance tissue remodeling and may have antibacterial and antiviral properties.89,90 The addition of chitosan also improves Schwann cell attachment and proliferation,91 which would enhance nerve ingrowth and axon stability.
Silk has also been used to enhance the mechanical properties of the collagen scaffolds; it also reduces fibroblast-mediated scaffold contraction.81 The hypodermis has been fabricated separately, but uses the same scaffold materials as the dermis when included. The scaffold for the epidermis is cell secreted. Keratinocytes are seeded onto the top of the dermis and lifted to the air-liquid interface where the keratinocytes will begin to proliferate and differentiate forming a stratified epidermis similar to that of native skin. Adding laminin to a tissue-engineered skin scaffold was shown to improve neuronal ingrowth.92
Current research on neurons and peripheral nerve conduits can also provide some insight into scaffold modifications that may be beneficial for an innervated skin model. Polydopamine-modified scaffolds are currently gaining popularity. Polydopamine, formed by oxidative self-polymerization of dopamine, promotes cell adhesion and improves the proliferation and differentiation of neural stem cells.93 Neurotrophic growth factors have been studied and the most promising currently is glial cell-derived neurotrophic factor (GDNF). GDNF was found to improve nerve sprouting when conjugated to the scaffold.94
Current Work, Drug Delivery, and Disease Modeling
Many of the current tissue-engineered models have focused on the study of the innervation of the skin; however, several have begun to use the models to study disease and drug delivery. Proper neural networks and the ability of the neurons to function after receiving sensory stimuli are important for both disease modeling and drug screening for neurocutaneous disorders. Roggenkamp et al. used an innervated skin model to study atopic dermatitis.60 Skin cells were isolated from sites of eczema in patients who were diagnosed with atopic dermatitis; a volunteer control group of similar ages was assembled. Skin substitutes comprising cells from patients with atopic dermatitis had a thicker epidermal layer, were more innervated, and end released more CGRP than those comprising cells from healthy controls. This model was later used to study the effects of menthoxypropanediol on nerve sprouting, a phenomenon linked to several pruritic diseases.95
Reichert et al. compared neurite outgrowth in an innervated skin model fabricated with cells either from diabetic patients or from healthy controls.61 Neurite outgrowth was impaired in the diabetic skin models; keratinocytes in these models were less sensitive to insulin and had reduced glyoxalase 1 activity. Cadau et al. promoted glycation to study the effects of advanced glycation end products (AGEs) on skin pathophysiology.58 Glyoxal treatment, to induce the formation of AGEs, led to impaired nerve fiber growth into the collagen sponge. Treatment with aminoguanidine, an anti-AGE molecule, led to reduced AGE expression and improved capillary-like structure formation.
To study nociception and pruriception, it is necessary to have functional sensory networks. Martorina et al. demonstrated sensory function of their model 8 days after the addition of DRG neurons.56 Capsaicin was added to the epidermal layer and nerve function was assessed by measuring Ca++ waves. While the response was not as robust as what is typically seen in monolayer studies, this is the first model to demonstrate nerve function within the model and to look at a topically applied stimulus. Models such as this have significant application for studying chronic pain and chronic itch, and for screening potential therapeutic drugs.
Future Directions for Drug Screening and Disease Modeling
The field of innervated skin models is still quite young; however, there have been significant advances. Further development of disease-based models will allow for the identification of mechanisms behind the diseases and testing of possible molecular interventions. Drug screening was a long-term goal for many of the models that have been developed to date and significant work is expected in this area. In addition, innervated skin models can be used to develop small cutaneous or transcutaneous devices with sensors to detect axon reflex or inflammatory flare-ups with biofeedback controlled electrical or chemical release capabilities to treat local symptoms of skin disease. One of the benefits of the tissue-engineered innervated skin models is the ability to customize the cultures by selecting the cell types to be included.
Several studies have already used this approach; further studies can be used to study the role of other cell types, such as melanocytes, or other structures such as hair follicles, which have been implicated in itch sensation. Mehnert et al. cultured mouse and rat DRG with human sweat gland-derived stem cells (hSGSCs).96 The paracrine effect of the hSGSCs significantly improved neurite outgrowth; co-culture in a 3D environment or along with other skin cells may lead to further improvements and a more physiologic model.
One advancement that would be useful will be the development of an innervated skin model composed entirely from primary human cells. The major limitation that has impeded the use of human DRGs of this is their availability. While research teams have been able to obtain human sensory neurons,68,97,98 this is not a common practice and depends on the availability of organ donors. For functional studies, DRG must be recovered shortly after cardiac death and cultured shortly thereafter. As neural tissues do not undergo mitosis, the primary tissue cultures are only useful for the lifetime of the original cells in culture, a matter of days or only a couple of weeks.
Cultured human DRGs have allowed researchers to understand the physiological properties of receptors on functional sensory neurons in the context of pain and tissue injury and to appreciate the differences between rodent and human DRGs. Research teams have studied voltage-gated sodium channels,99,100 nicotinic acetylcholine receptors,101 and opioid receptors102 in cultured human sensory neurons. Although induced sensory-like neurons from human precursor cells are an intriguing alternative, it is still unknown just how similar the genetic and proteomic expression is between these cells and true primary neurons. Skin-human DRG co-cultures provide a new translationally powerful avenue to test therapeutics and advanced models and culture conditions may increase the longevity of the human DRGs.
Conclusion
Tissue-engineered innervated skin substitutes are excellent models for studying neurocutaneous diseases and cutaneous device development, and for drug screening, especially of topical applications. These co-cultures include a level of complexity that cannot be found in monolayer cultures and 3D models have been found to more closely predict the in vivo results. In the case of skin, the tissue-engineered model also recapitulates the complexity of the epidermal layer, which is key to barrier function and transduction of sensory stimuli. The functionality along with the ability to customize the cell types and sources makes this model indispensable for the study of neurocutaneous disorders.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Institutes of Health award number R01NS107356 (to S.D.)
References
- 1. Weisshaar, E., and Dalgard, F.. Epidemiology of itch: adding to the burden of skin morbidity. Acta Derm Venereol 89, 339, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Weisshaar, E. Examination of Patients. In: Misery L., Stander S., eds. Pruritis. London: Springer-Verlag London, 2016, p. 79 [Google Scholar]
- 3. Oaklander, A.L., Cohen, S.P., and Raju, S.V.. Intractable postherpetic itch and cutaneous deafferentation after facial shingles. Pain 96, 9, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Dauber, A., Osgood, P.F., Breslau, A.J., Vernon, H.L., and Carr, D.B.. Chronic persistent pain after severe burns: a survey of 358 burn survivors. Pain Med 3, 6, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Mauck, M.C., Smith, J., Liu, A.Y., et al. Chronic pain and itch are common, morbid sequelae among individuals who receive tissue autograft after major thermal burn injury. Clin J Pain 33, 627, 2017 [DOI] [PubMed] [Google Scholar]
- 6. Pinho-Ribeiro, F.A., Verri, W.A.Jr., and Chiu, I.M.. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol 38, 5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roosterman, D., Goerge, T., Schneider, S.W., Bunnett, N.W., and Steinhoff, M.. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 86, 1309, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Lotti, T., Zanardelli, M., and D'Erme, A.M.. Vitiligo: what's new in the psycho-neuro-endocrine-immune connection and related treatments. Wien Med Wochenschr 164, 278, 2014 [DOI] [PubMed] [Google Scholar]
- 9. Provitera, V., Nolano, M., Pagano, A., Caporaso, G., Stancanelli, A., and Santoro, L.. Myelinated nerve endings in human skin. Muscle Nerve 35, 767, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Abraira, V.E., and Ginty, D.D.. The sensory neurons of touch. Neuron 79, 618, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le Gall-Ianotto, C., Andres, E., Hurtado, S.P., Pereira, U., and Misery, L.. Characterization of the first coculture between human primary keratinocytes and the dorsal root ganglion-derived neuronal cell line F-11. Neuroscience 210, 47, 2012 [DOI] [PubMed] [Google Scholar]
- 12. Moreci, R.S., and Lechler, T.. Epidermal structure and differentiation. Curr Biol 30, R144, 2020 [DOI] [PubMed] [Google Scholar]
- 13. Talagas, M., Lebonvallet, N., Berthod, F., and Misery, L.. Cutaneous nociception: role of keratinocytes. Exp Dermatol 28, 1466, 2019 [DOI] [PubMed] [Google Scholar]
- 14. Blais, M., Mottier, L., Germain, M.A., Bellenfant, S., Cadau, S., and Berthod, F.. Sensory neurons accelerate skin reepithelialization via substance P in an innervated tissue-engineered wound healing model. Tissue Eng Part A 20, 2180, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yao, M., and Wang, R.. Neurodynamic analysis of Merkel cell-neurite complex transduction mechanism during tactile sensing. Cogn Neurodyn 13, 293, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Driskell, R.R., and Watt, F.M.. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol 25, 92, 2015 [DOI] [PubMed] [Google Scholar]
- 17. Sun, L., Li, H., Tai, L.W., Gu, P., and Cheung, C.W.. Adiponectin regulates thermal nociception in a mouse model of neuropathic pain. Br J Anaesth 120, 1356, 2018 [DOI] [PubMed] [Google Scholar]
- 18. Moehring, F., Cowie, A.M., Menzel, A.D., et al. Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. Elife 7, e31684, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sadler, K.E., Moehring, F., and Stucky, C.L.. Keratinocytes contribute to normal cold and heat sensation. Elife 9, e58625, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maksimovic, S., Baba, Y., and Lumpkin, E.A.. Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor. Ann N Y Acad Sci 1279, 13, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoffman, B.U., Baba, Y., Griffith, T.N., et al. Merkel cells activate sensory neural pathways through adrenergic synapses. Neuron 100, 1401, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maksimovic, S., Nakatani, M., Baba, Y., et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baumbauer, K.M., DeBerry, J.J., Adelman, P.C., et al. Keratinocytes can modulate and directly initiate nociceptive responses. Elife 4, e09674, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janig, W. The afferent innervation of the central pad of the cat's hind foot. Brain Res 28, 203, 1971 [DOI] [PubMed] [Google Scholar]
- 25. Zimmerman, A., Bai, L., and Ginty, D.D.. The gentle touch receptors of mammalian skin. Science 346, 950, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chambers, M.R., Andres, K.H., von Duering, M., and Iggo, A.. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci 57, 417, 1972 [DOI] [PubMed] [Google Scholar]
- 27. Johnson, K.O. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 11, 455, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Hilliges, M., Wang, L., and Johansson, O.. Ultrastructural evidence for nerve fibers within all vital layers of the human epidermis. J Invest Dermatol 104, 134, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Lumpkin, E.A., and Caterina, M.J.. Mechanisms of sensory transduction in the skin. Nature 445, 858, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Talagas, M., Lebonvallet, N., Berthod, F., and Misery, L.. Lifting the veil on the keratinocyte contribution to cutaneous nociception. Protein Cell 11, 239, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Talagas, M., Lebonvallet, N., Leschiera, R., et al. Keratinocytes communicate with sensory neurons via synaptic-like contacts. Ann Neurol 88, 1205, 2020 [DOI] [PubMed] [Google Scholar]
- 32. Choi, J.E., and Di Nardo, A.. Skin neurogenic inflammation. Semin Immunopathol 40, 249, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ansel, J.C., Armstrong, C.A., Song, I., et al. Interactions of the skin and nervous system. J Invest Dermatol Symp Proc 2, 23, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Gorzelanny, C., Mess, C., Schneider, S.W., Huck, V., and Brandner, J.M.. Skin barriers in dermal drug delivery: which barriers have to be overcome and how can we measure them? Pharmaceutics 12, 684, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pereira, U., Boulais, N., Lebonvallet, N., Lefeuvre, L., Gougerot, A., and Misery, L.. Development of an in vitro coculture of primary sensitive pig neurons and keratinocytes for the study of cutaneous neurogenic inflammation. Exp Dermatol 19, 931, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Kumamoto, J., Nakatani, M., Tsutsumi, M., et al. Coculture system of keratinocytes and dorsal-root-ganglion-derived cells for screening neurotrophic factors involved in guidance of neuronal axon growth in the skin. Exp Dermatol 23, 58, 2014 [DOI] [PubMed] [Google Scholar]
- 37. Roggenkamp, D., Falkner, S., Stab, F., Petersen, M., Schmelz, M., and Neufang, G.. Atopic keratinocytes induce increased neurite outgrowth in a coculture model of porcine dorsal root ganglia neurons and human skin cells. J Invest Dermatol 132, 1892, 2012 [DOI] [PubMed] [Google Scholar]
- 38. Lee, J., Rabbani, C.C., Gao, H., et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 582, 399, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Towler, M.A., Rush, E.W., Richardson, M.K., and Williams, C.L.. Randomized, Prospective, Blinded-Enrollment, Head-To-Head Venous Leg Ulcer Healing Trial Comparing Living, Bioengineered Skin Graft Substitute (Apligraf) with Living, Cryopreserved, Human Skin Allograft (TheraSkin). Clin Podiatr Med Surg 35, 357, 2018 [DOI] [PubMed] [Google Scholar]
- 40. Centanni, J.M., Straseski, J.A., Wicks, A., et al. StrataGraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumatic wounds: results from a prospective, randomized, controlled dose escalation trial. Ann Surg 253, 672, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marston, W.A., Hanft, J., Norwood, P., Pollak, R., and Dermagraft Diabetic Foot Ulcer Study, G.. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 26, 1701, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Sood, R., Roggy, D., Zieger, M., et al. Cultured epithelial autografts for coverage of large burn wounds in eighty-eight patients: the Indiana University experience. J Burn Care Res 31, 559, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Moris, V., Cristofari, S., Stivala, A., et al. Recell in post-traumatic cases: preliminary results. J Plast Reconstr Aesthet Surg 73, 1897, 2020 [DOI] [PubMed] [Google Scholar]
- 44. Osborne, R., and Perkins, M.A.. An approach for development of alternative test methods based on mechanisms of skin irritation. Food Chem Toxicol 32, 133, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Gabbanini, S., Lucchi, E., Carli, M., Berlini, E., Minghetti, A., and Valgimigli, L.. In vitro evaluation of the permeation through reconstructed human epidermis of essentials oils from cosmetic formulations. J Pharm Biomed Anal 50, 370, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Ackermann, K., Borgia, S.L., Korting, H.C., Mewes, K.R., and Schafer-Korting, M.. The Phenion full-thickness skin model for percutaneous absorption testing. Skin Pharmacol Physiol 23, 105, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Gingras, M., Paradis, I., and Berthod, F.. Nerve regeneration in a collagen-chitosan tissue-engineered skin transplanted on nude mice. Biomaterials 24, 1653, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Hahn, J.M., Combs, K.A., Lloyd, C.M., McFarland, K.L., Boyce, S.T., and Supp, D.M.. Identification of Merkel cells associated with neurons in engineered skin substitutes after grafting to full thickness wounds. PLoS One 14, e0213325, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Biedermann, T., Klar, A.S., Bottcher-Haberzeth, S., Reichmann, E., and Meuli, M.. Myelinated and unmyelinated nerve fibers reinnervate tissue-engineered dermo-epidermal human skin analogs in an in vivo model. Pediatr Surg Int 32, 1183, 2016 [DOI] [PubMed] [Google Scholar]
- 50. Gingras, M., Beaulieu, M.M., Gagnon, V., Durham, H.D., and Berthod, F.. In vitro study of axonal migration and myelination of motor neurons in a three-dimensional tissue-engineered model. Glia 56, 354, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Jelaska, A., and Korn, J.H.. Anti-Fas induces apoptosis and proliferation in human dermal fibroblasts: differences between foreskin and adult fibroblasts. J Cell Physiol 175, 19, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Varani, J., Perone, P., Griffiths, C.E., Inman, D.R., Fligiel, S.E., and Voorhees, J.J.. All-trans retinoic acid (RA) stimulates events in organ-cultured human skin that underlie repair. Adult skin from sun-protected and sun-exposed sites responds in an identical manner to RA while neonatal foreskin responds differently. J Clin Invest 94, 1747, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stanulis-Praeger, B.M., and Gilchrest, B.A.. Growth factor responsiveness declines during adulthood for human skin-derived cells. Mech Ageing Dev 35, 185, 1986 [DOI] [PubMed] [Google Scholar]
- 54. Mateu, R., Zivicova, V., Krejci, E.D., et al. Functional differences between neonatal and adult fibroblasts and keratinocytes: donor age affects epithelial-mesenchymal crosstalk in vitro. Int J Mol Med 38, 1063, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saguet, T., Robin, S., Nicod, L., et al. Comparison of ultraviolet B-induced imbalance of antioxidant status in foreskin- and abdominal skin-derived human fibroblasts. Eur J Dermatol 16, 368, 2006 [PubMed] [Google Scholar]
- 56. Martorina, F., Casale, C., Urciuolo, F., Netti, P.A., and Imparato, G.. In vitro activation of the neuro-transduction mechanism in sensitive organotypic human skin model. Biomaterials 113, 217, 2017 [DOI] [PubMed] [Google Scholar]
- 57. Gingras, M., Bergeron, J., Dery, J., Durham, H.D., and Berthod, F.. In vitro development of a tissue-engineered model of peripheral nerve regeneration to study neurite growth. FASEB J 17, 2124, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Cadau, S., Leoty-Okombi, S., Pain, S., Bechetoille, N., Andre-Frei, V., and Berthod, F.. In vitro glycation of an endothelialized and innervated tissue-engineered skin to screen anti-AGE molecules. Biomaterials 51, 216, 2015 [DOI] [PubMed] [Google Scholar]
- 59. Blais, M., Grenier, M., and Berthod, F.. Improvement of nerve regeneration in tissue-engineered skin enriched with schwann cells. J Invest Dermatol 129, 2895, 2009 [DOI] [PubMed] [Google Scholar]
- 60. Roggenkamp, D., Kopnick, S., Stab, F., Wenck, H., Schmelz, M., and Neufang, G.. Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J Invest Dermatol 133, 1620, 2013 [DOI] [PubMed] [Google Scholar]
- 61. Reichert, O., Fleming, T., Neufang, G., et al. Impaired glyoxalase activity is associated with reduced expression of neurotrophic factors and pro-inflammatory processes in diabetic skin cells. Exp Dermatol 26, 44, 2017 [DOI] [PubMed] [Google Scholar]
- 62. Ulmann, L., Rodeau, J.L., Danoux, L., Contet-Audonneau, J.L., Pauly, G., and Schlichter, R.. Trophic effects of keratinocytes on the axonal development of sensory neurons in a coculture model. Eur J Neurosci 26, 113, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Haberberger, R.V., Barry, C., Dominguez, N., and Matusica, D.. Human dorsal root ganglia. Front Cell Neurosci 13, 271, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Castro, J., Harrington, A.M., Garcia-Caraballo, S., et al. alpha-Conotoxin Vc1.1 inhibits human dorsal root ganglion neuroexcitability and mouse colonic nociception via GABAB receptors. Gut 66, 1083, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Obreja, O., Klusch, A., Ponelies, N., Schmelz, M., and Petersen, M.. A subpopulation of capsaicin-sensitive porcine dorsal root ganglion neurons is lacking hyperpolarization-activated cyclic nucleotide-gated channels. Eur J Pain 12, 775, 2008 [DOI] [PubMed] [Google Scholar]
- 66. Schmelz, M., Schmid, R., Handwerker, H.O., and Torebjork, H.E.. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 123 (Pt 3), 560, 2000 [DOI] [PubMed] [Google Scholar]
- 67. Lynn, B., Schutterle, S., and Pierau, F.K.. The vasodilator component of neurogenic inflammation is caused by a special subclass of heat-sensitive nociceptors in the skin of the pig. J Physiol 494 (Pt 2), 587, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Viventi, S., and Dottori, M.. Modelling the dorsal root ganglia using human pluripotent stem cells: a platform to study peripheral neuropathies. Int J Biochem Cell Biol 100, 61, 2018 [DOI] [PubMed] [Google Scholar]
- 69. Marmigere, F., and Ernfors, P.. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci 8, 114, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Chambers, S.M., Qi, Y., Mica, Y., et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol 30, 715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Eberhardt, E., Havlicek, S., Schmidt, D., et al. Pattern of Functional TTX-resistant sodium channels reveals a developmental stage of human iPSC- and ESC-derived nociceptors. Stem Cell Rep 5, 305, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guimaraes, M.Z.P., De Vecchi, R., Vitoria, G., et al. Generation of iPSC-derived human peripheral sensory neurons releasing substance P elicited by TRPV1 agonists. Front Mol Neurosci 11, 277, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Han, C., Dib-Hajj, S.D., Lin, Z., et al. Early- and late-onset inherited erythromelalgia: genotype-phenotype correlation. Brain 132, 1711, 2009 [DOI] [PubMed] [Google Scholar]
- 74. Meents, J.E., Bressan, E., Sontag, S., et al. The role of Nav1.7 in human nociceptors: insights from human induced pluripotent stem cell-derived sensory neurons of erythromelalgia patients. Pain 160, 1327, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cao, L., McDonnell, A., Nitzsche, A., et al. Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia. Sci Transl Med 8, 335ra56, 2016 [DOI] [PubMed] [Google Scholar]
- 76. Muller, Q., Beaudet, M.J., De Serres-Berard, T., Bellenfant, S., Flacher, V., and Berthod, F.. Development of an innervated tissue-engineered skin with human sensory neurons and Schwann cells differentiated from iPS cells. Acta Biomater 82, 93, 2018 [DOI] [PubMed] [Google Scholar]
- 77. Melli, G., and Hoke, A.. Dorsal Root Ganglia Sensory Neuronal Cultures: a tool for drug discovery for peripheral neuropathies. Expert Opin Drug Discov 4, 1035, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vidal, S.E.L., Tamamoto, K.A., Nguyen, H., Abbott, R.D., Cairns, D.M., and Kaplan, D.L.. 3D biomaterial matrix to support long term, full thickness, immuno-competent human skin equivalents with nervous system components. Biomaterials 198, 194, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vidal Yucha, S.E., Tamamoto, K.A., Nguyen, H., Cairns, D.M., and Kaplan, D.L.. Human skin equivalents demonstrate need for neuro-immuno-cutaneous system. Adv Biosyst 3, e1800283, 2019 [DOI] [PubMed] [Google Scholar]
- 80. Clark, M.B., and Bunge, M.B.. Cultured Schwann cells assemble normal-appearing basal lamina only when they ensheathe axons. Dev Biol 133, 393, 1989 [DOI] [PubMed] [Google Scholar]
- 81. Abbott, R.D., Wang, R.Y., Reagan, M.R., et al. The use of silk as a scaffold for mature, sustainable unilocular adipose 3D tissue engineered systems. Adv Healthc Mater 5, 1667, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yannas, I.V., and Burke, J.F.. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res 14, 65, 1980 [DOI] [PubMed] [Google Scholar]
- 83. Qiu, H., Guo, H., Li, D., Hou, Y., Kuang, T., and Ding, J.. Intravesical hydrogels as drug reservoirs. Trends Biotechnol 38, 579, 2020 [DOI] [PubMed] [Google Scholar]
- 84. Prockop, D.J., and Kivirikko, K.I.. Heritable diseases of collagen. N Engl J Med 311, 376, 1984 [DOI] [PubMed] [Google Scholar]
- 85. Stadelmann, W.K., Digenis, A.G., and Tobin, G.R.. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 176, 26S, 1998 [DOI] [PubMed] [Google Scholar]
- 86. Samadian, H., Maleki, H., Fathollahi, A., et al. Naturally occurring biological macromolecules-based hydrogels: potential biomaterials for peripheral nerve regeneration. Int J Biol Macromol 154, 795, 2020 [DOI] [PubMed] [Google Scholar]
- 87. Zuo, J., Ferguson, T.A., Hernandez, Y.J., Stetler-Stevenson, W.G., and Muir, D.. Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite-inhibiting chondroitin sulfate proteoglycan. J Neurosci 18, 5203, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Berthod, F., Saintigny, G., Chretien, F., Hayek, D., Collombel, C., and Damour, O.. Optimization of thickness, pore size and mechanical properties of a biomaterial designed for deep burn coverage. Clin Mater 15, 259, 1994 [DOI] [PubMed] [Google Scholar]
- 89. Muzzarelli, R., Baldassarre, V., Conti, F., et al. Biological activity of chitosan: ultrastructural study. Biomaterials 9, 247, 1988 [DOI] [PubMed] [Google Scholar]
- 90. Nishimura, K., Nishimura, S., Nishi, N., Saiki, I., Tokura, S., and Azuma, I.. Immunological activity of chitin and its derivatives. Vaccine 2, 93, 1984 [DOI] [PubMed] [Google Scholar]
- 91. Wach, R.A., Adamus-Wlodarczyk, A., Olejnik, A.K., Matusiak, M., Tranquilan-Aranilla, C., and Ulanski, P.. Carboxymethylchitosan hydrogel manufactured by radiation-induced crosslinking as potential nerve regeneration guide scaffold. React Funct Polym 152, 104588, 2020 [Google Scholar]
- 92. Caissie, R., Gingras, M., Champigny, M.F., and Berthod, F.. In vivo enhancement of sensory perception recovery in a tissue-engineered skin enriched with laminin. Biomaterials 27, 2988, 2006 [DOI] [PubMed] [Google Scholar]
- 93. Yan, J., Wu, R., Liao, S., Jiang, M., and Qian, Y.. Applications of polydopamine-modified scaffolds in the peripheral nerve tissue engineering. Front Bioeng Biotechnol 8, 590998, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ziv-Polat, O., Shahar, A., Levy, I., et al. The role of neurotrophic factors conjugated to iron oxide nanoparticles in peripheral nerve regeneration: in vitro studies. Biomed Res Int 2014, 267808, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Roggenkamp, D., Worthmann, A.C., Sulzberger, M., Wenck, H., Stab, F., and Neufang, G.. Menthoxypropanediol inhibits nerve growth factor-induced nerve fibre sprouting in coculture models of sensory neurons and skin cells. Exp Dermatol 25, 824, 2016 [DOI] [PubMed] [Google Scholar]
- 96. Mehnert, J.M., Kisch, T., and Brandenburger, M.. Co-culture systems of human sweat gland derived stem cells and peripheral nerve cells: an in vitro approach for peripheral nerve regeneration. Cell Physiol Biochem 34, 1027, 2014 [DOI] [PubMed] [Google Scholar]
- 97. Valtcheva, M.V., Copits, B.A., Davidson, S., et al. Surgical extraction of human dorsal root ganglia from organ donors and preparation of primary sensory neuron cultures. Nat Protoc 11, 1877, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Davidson, S., Copits, B.A., Zhang, J., Page, G., Ghetti, A., and Gereau, R.W.. Human sensory neurons: membrane properties and sensitization by inflammatory mediators. Pain 155, 1861, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang, X., Priest, B.T., Belfer, I., and Gold, M.S.. Voltage-gated Na(+) currents in human dorsal root ganglion neurons. Elife 6, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Han, C., Estacion, M., Huang, J., et al. Human Na(v)1.8: enhanced persistent and ramp currents contribute to distinct firing properties of human DRG neurons. J Neurophysiol 113, 3172, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang, X., Hartung, J.E., Friedman, R.L., Koerber, H.R., Belfer, I., and Gold, M.S.. Nicotine evoked currents in human primary sensory neurons. J Pain 20, 810, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Moy, J.K., Hartung, J.E., Duque, M.G., et al. Distribution of functional opioid receptors in human dorsal root ganglion neurons. Pain 161, 1636, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]