Abstract
Long-acting injectable antiretroviral therapy (LAI-ART) is one of the latest advancements in HIV control with the potential to overcome oral ART barriers to adherence. The objective of this article is to anticipate and examine implementation considerations for LAI-ART using components of the PRISM model, a Practical, Robust Implementation and Sustainability Model for integrating research findings into practice. We conducted a scoping review from January to August 2020 of the growing literature on LAI-ART implementation and other fields using LAI therapies. Key considerations regarding LAI-ART were parsed from the searches and entered into the PRISM implementation science framework. The PRISM framework posed multiple questions for consideration in the development of an optimal implementation strategy for LAI-ART in the United States. These questions revealed the necessity for more data, including acceptability of LAI-ART among many different subgroups of people living with HIV (PLWH), cost effectiveness, patient satisfaction, and patient-reported outcomes, as well as more detailed information related to the external environment for optimal LAI-ART implementation. Ethical considerations of LAI-ART will also need to be considered. The anticipation of, and excitement for, LAI-ART represent the hope for a new direction for HIV treatment that reduces adherence barriers and improves prognoses for PLWH. We have a unique window of opportunity to anticipate implementation considerations for LAI-ART, so this new therapy can be used to its fullest potential. Outstanding questions remain, however, that need to be addressed to help achieve HIV suppression goals in diverse populations.
Keywords: HIV, antiretroviral therapy, long acting, injectables, implementation science, PRISM framework
Introduction
HIV is a manageable chronic disease because of the tremendous advancements in antiretroviral therapy (ART).1–3 People living with HIV (PLWH) now realize a life expectancy near that of the general population and do not have to fear sexual transmission of the disease if they achieve an undetectable viral load.2,4–8
Despite advances in ART, the Centers for Disease Control and Prevention estimates that 37% of PLWH in the United States remain virally unsuppressed and at-risk for transmitting HIV.9 Adherence to the lifelong, daily dosing of oral ART is required to maintain virologic suppression and often presents substantial and onerous barriers for some PLWH due to individual, social, and/or structural factors.10–19 Long-acting injectable antiretroviral therapy (LAI-ART) is the latest advancement in HIV control with the potential to obviate some barriers to oral ART adherence, but presents other barriers, such as the requirement to adhere to injection appointments.
Our objective with this scoping review is to anticipate and examine implementation considerations for LAI-ART using components of the PRISM framework, a Practical, Robust Implementation and Sustainability Model for integrating research findings into practice,20 based on the precedent set with HIV preexposure prophylaxis (PrEP) implementation.21 The PRISM model focuses on the context in which LAI-ART would be implemented as a framework for systematically anticipating potential issues that may arise during LAI-ART implementation into clinical care.20–24 While current HIV treatment is safe and effective, we know not all populations of PLWH are optimally benefitting from it.25,26 LAI-ART presents a unique opportunity to improve the HIV treatment landscape, but this will only be achieved through effective implementation.17,20,27,28
The implementation science literature reveals that less than half of effective interventions are used regularly by care providers and only one in five medical research dollars has the desired widespread public impact.22,25,29 This reality is the product of a dissonance between the effective implementation of interventions and the populations, contexts, and/or processes in which novel interventions are delivered.25,29
Clinical trials and studies traditionally examine the efficacy (performance in the research context) versus effectiveness (performance in the real-world) of interventions, while overlooking or only perfunctorily addressing downstream implementation aspects.20,30 Most empirical reports on LAI-ART have focused exclusively on pharmacology, as well as host and viral factors.12,31–33 To avoid the pitfalls in the roll out of new therapies, such as PrEP for HIV prevention,21 it is absolutely necessary to map the processes and potential bottlenecks that will affect the success of LAI-ART.34,35
Methods
We conducted a scoping review from January to August 2020 of the growing literature on LAI-ART and other fields using LAI therapies. Our scoping review uses the PRISMA Extension for Scoping Reviews (PRISMA-ScR) framework (distinct from the PRISM implementation model above).36 The purpose of a scoping of review is to summarize literature on a specific topic given that the nascent published literature does not allow for detailed qualitative or quantitative analyses of each study.37 A scoping review is ideal when the need is to organize information on an understudied topic by broadly mapping the literature to guide potential future research.37
As LAI-ART is a novel field, our review was exploratory with no clearly defined criteria for adjudicating the literature. We conducted our scoping review to identify gaps in the emerging LAI-ART literature and to focus future research on the barriers to be overcome when implementing this novel therapy.37,38
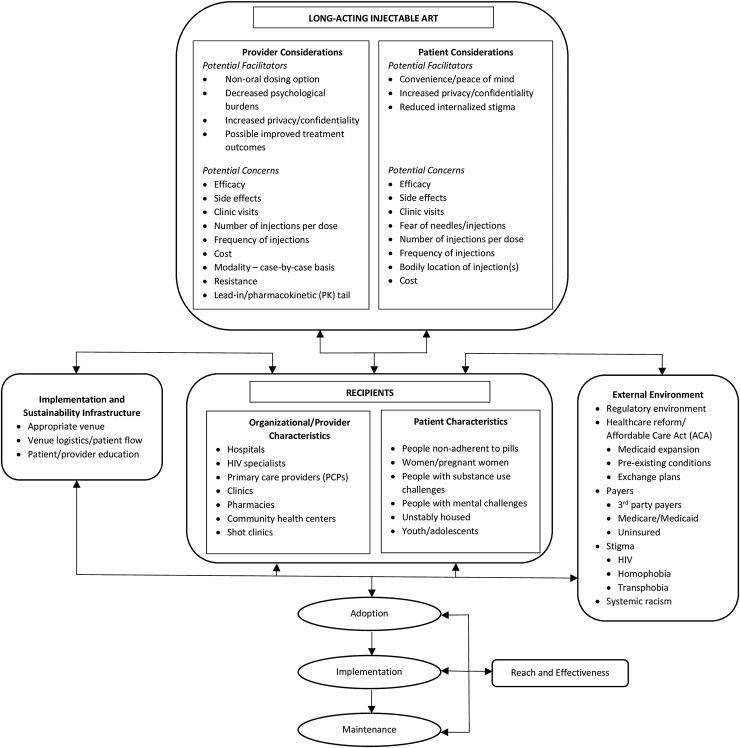
We focused our review on four domains of the PRISM implementation model: patient/provider considerations, recipient characteristics, external environments, and implementation and sustainability infrastructure (Fig. 1). The first domain, patient/provider considerations, concerns itself with the patient and provider perspectives or opinions of LAI-ART, such as its perceived benefits and drawbacks.20 The second domain, recipient characteristics, focuses on the age, sex and gender, socioeconomic status, and cultural characteristics of patient subgroups, as well as on organizational/provider characteristics of those who will administer LAI-ART.20 The third domain, external environments, recognizes that LAI-ART does not exist in a vacuum and must necessarily consider outside dynamics.20 The fourth domain, implementation and sustainability infrastructure, considers what logistical and educational structures need to be in place to assure sustainability of LAI-ART.20
FIG. 1.
The PRISM framework anticipating implementation considerations of LAI-ART in the United States. PRISM, Practical, Robust Implementation and Sustainability Model; LAI-ART, long-acting injectable antiretroviral therapy.
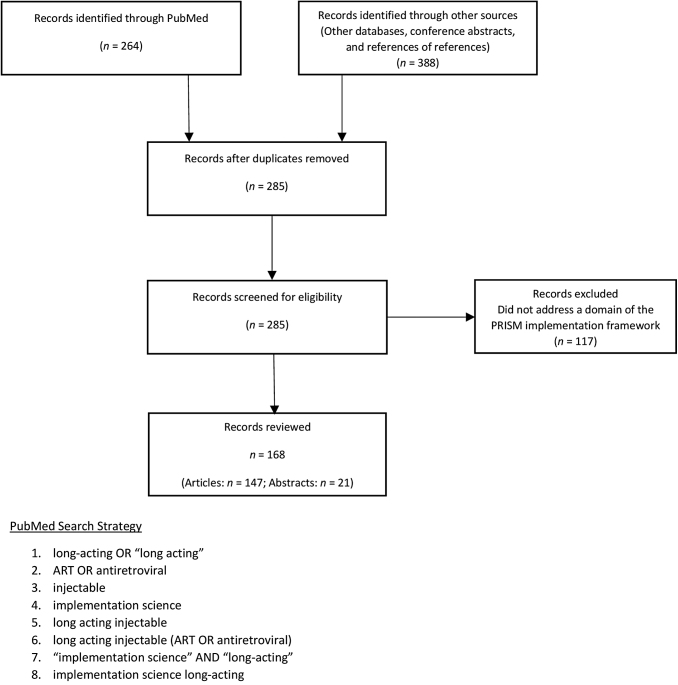
Using PubMed, the primary database where LAI-ART studies are indexed pursuant to the National Institutes of Health Public Access Policy, we searched terms in the English language such as long-acting, ART, and implementation science (n = 264) and pursued references from the articles we reviewed. In addition, we searched abstracts from major HIV/AIDS or infectious diseases conferences from the past 5 years (n = 21). We then ran our initial search through Scopus and ProQuest as a check on the thoroughness of our review (n = 388); these secondary searches produced duplicative results. We excluded those records that did not address at least one of the four domains of concern within the PRISM framework (n = 117).
In total, we reviewed 147 articles (1994–2020) and 21 conference abstracts (2015–2020). Figure 2 is a flow diagram detailing our reference selection process, as well as our initial PubMed search strategy. We extracted and organized information relevant to the four domains via manual charting and then used narrative synthesis39 to integrate key findings into descriptive summaries and possible considerations for LAI-ART implementation. We then entered the parsed data into the PRISM framework (Fig. 1).
FIG. 2.
Flow diagram of reference selection and sample search strategy.
Results
The intervention: LAI-ART
LAI-ART is ART therapy that is administered by parenteral injection(s).40–44 A two-drug combination of cabotegravir (CAB), an integrase inhibitor, and rilpivirine (RPV), a non-nucleoside reverse transcriptase inhibitor, administered monthly in two separate injections, is awaiting United States Food and Drug Administration (FDA) approval and is currently the closest to market.14,40–43 Phase III clinical trials are underway to establish the efficacy of dosing bimonthly.14,45–47 Eliminating the daily pill burden of oral ART is the main benefit LAI-ART offers.1,12,14,46,48
The PRISM framework begins by focusing on patient and provider perspectives on LAI-ART. These two perspectives partially overlap and concerns surrounding LAI-ART are shaped by, and cannot be divorced from, occurrences in the external environment.
Patient perspectives
Although it is necessary to maximize the intervention's effectiveness and reach, the patient perspective is often the last to be considered, if not altogether excluded, when novel interventions are developed.20 Indeed, the need for patients' views to inform the development and implementation of new biomedical interventions has been repeatedly emphasized in the literature.18,49–51 To date, there has been meager research into patient acceptance of LAI-ART, even though this information is necessary for its successful implementation and uptake.12,18,28,49,52–57
Social science research shows that PLWH held a generally positive view of LAI-ART.1,12,16,18,28,34,49 Patients have identified several key facilitators regarding LAI-ART such as convenience and heightened peace of mind, increased privacy and confidentiality, and reduced internalized stigma.1,34,48,49,58 LAI-ART was seen as easy to integrate into one's daily life and as a stress reducer, particularly among women living with HIV.1 LAI-ART was also viewed as more discrete than oral medications with less potential for unintended disclosure of one's HIV status.1,12,34,49 Another widely agreed upon benefit of LAI-ART was its reduction of internalized stigma.59 Many PLWH expressed a desire for normality, and did not want to dwell on being HIV positive; taking a pill daily for HIV was seen as a constant reminder of one's status.1,16,34,58,59
Despite the potential benefits voiced by PLWH about LAI-ART, patients also expressed concerns that may affect implementation strategies for LAI-ART. Key patient concerns regarding LAI-ART included efficacy of LAI-ART versus standard oral ART, side effects, number of clinic visits, fear of needles/injections, number of injections per dose, frequency of injections, bodily location of injections(s), and cost.
First, for patients to consider LAI-ART, the LAI formulation must be at least as effective at suppressing the HIV viral load as oral ART.12,16,28,57 When making the critical decision to switch HIV control regimens, PLWH often make careful risk/benefit calculations based on their health status, available treatment options, their provider's recommendation, and current/past experiences with other medications.12,49,60–63 Their choice to try a new HIV therapy is heavily weighed because this may limit their future options and may cause uncertainty with regard to safety and effectiveness.61
In a cross-sectional survey conducted among 282 PLWH in the United States, participants who had an effective oral ART routine were reluctant to switching to a novel HIV therapy.49 In addition for some PLWH, switching to LAI-ART would not relieve their daily pill burden because they regularly took pills for other chronic morbidities.1,12,64 Further, some perceived that new modalities such as LAI-ART could lead to a loss of undetectable status and that this perceived “viral blip” could lead to forward transmission of HIV to sexual partners.49,60,63 Similarly, side effects, such as nausea, headache, or diarrhea, were often voiced concerns.12,58 Rash or bruising at or around injection sites was also considered unacceptable if easily visible.12
Fear of needles and aversion to injections were also prevalent concerns.1,12 Although many PLWH were not fond of needles, some viewed the temporary discomfort of injections as preferable over daily oral ART.1,12 Nevertheless, fear of needles remained a deterrent for many PLWH to take up LAI-ART.12 In a qualitative study of 36 PLWH, even mentioning that this new regimen involved needles elicited immediate visceral negative responses.12 Mitigating factors such as a lower dose volume or lower gauge needle often assuaged this concern.12
Other major deterrents included the number of injections per dose, frequency of injections, frequency of clinic visits, and associated costs.12,58 Currently, LAI-ART requires two injections in the gluteal muscles per dose; receiving more than one injection per visit was often considered a “deal breaker” for many, even among those who were generally comfortable with injections.12
The frequency of injections was also concerning for many PLWH.12,57 Anything more than once per week was considered unacceptable.12 In a recent United States-based study of 282 PLWH, 42% of respondents indicated a strong willingness to switching to LAI-ART if the dosing frequency were every 6 months,49 while currently available LAI-ART would require monthly or bimonthly dosing.40–42,45–47 For women, however, receiving an injection every 3 months to coincide with long-acting contraceptive shots may be convenient.13 Pharmaceutical developers, therefore, should consider multiple options for different populations of PLWH. Further, PLWH were concerned by the number of clinic visits involved due to the potential for unwanted HIV status disclosure and schedule disruption.12,64
The costs associated with LAI-ART was a significant barrier for many PLWH, particularly those receiving significant subsidies or obtaining oral ART for free through programs such as the AIDS Drug Assistance Program or the Ryan White Program.12 Transportation costs, parking fees, and loss of income caused by taking time off from work for increased clinic visits must also be considered when determining the LAI-ART cost-effectiveness to patients.
The patient perspective, although essential, is only one of the viewpoints that must be considered in designing an effective LAI-ART implementation plan. The frontline provider perspective must also be considered. These two perspectives work in tandem and any implementation strategy designed with only one view in mind will necessarily be lacking.
Provider perspectives
Provider buy-in is critical to the successful implementation of LAI-ART and to effecting sustainable change, according to the PRISM framework.20 The important role of provider attitudes has been demonstrated, and a precedent set, by the use of long-acting contraception and LAI antipsychotic agents to treat schizophrenia.46,65–67
In reviewing the LAI-ART literature, most providers recognized key facilitators and benefits of LAI-ART, such as providing a nonoral dosing option, decreasing the psychological burdens of HIV on their patients, increasing patient privacy and confidentiality, and the possibility of improving treatment outcomes.12,34 Providers, however, stressed that the use of LAI-ART must be decided on a case-by case basis because each PLWH presents a unique situation,34 and overwhelmingly insisted that they would not prescribe LAI-ART if it were not optimal for a given patient.12
Providers' perspectives were, in general, more cautious than patients' perspectives.34 Like their patients, providers were concerned with the number and frequency of injections, yet noted that patients generally grew accustomed to new treatments.12 Likewise, providers were trepidatious about the dosing frequency of LAI-ART, noting that odd numbered intervals or any interval that did not correspond to currently recommended health-related visits may lead to missed appointments and decreased engagement in HIV care.12
Additional provider concerns related to LAI-ART included length of the pharmacokinetic (PK) tail, drug–drug interactions (DDIs), oral ART lead-in, drug resistance, and clinical management.
First, a major concern for providers was LAI-ART's prolonged PK tail, or the concentrations of the long-acting drug(s) after a single injection that remain in the body for extended periods of time.15,34,68,69 Because current LAI-ART formulations cannot be removed from the body once injected, even if adverse reactions occur, providers were loath to switch their patients from oral ART to LAI-ART without additional data.69–72 Further, the extensive PK tail requires more complex clinical management and consistent follow-up, and the fact that PK tails were found to vary among patients exacerbated provider queries.1,68,73 For instance, body mass index and gender seemed to play a role in determining the length of PK tail, as well as local injection site fat content and distribution.72,74
Likewise, DDIs were another prevalent concern expressed by providers.34,75 Although newer ART are less likely to cause adverse reactions when combined with other medications, questions still remained around LAI-ART's potential for DDIs.34,75 The two drugs currently in Phase III studies, CAB and RPV, have limited effect as perpetrators of DDIs.34 Parenteral administration, because it bypasses the gastrointestinal tract, further reduces the likelihood of DDIs.34
The requirement of an oral ART lead-in before the administration of LAI-ART is another prominent topic in the literature.41,69,73 LAI-ART formulations are best used for maintenance than for initial viral suppression.41,42 Therefore, newly diagnosed patients would need to be virally suppressed with oral ART before starting LAI-ART. The intent of an oral lead-in is to prevent prolonged exposure in PLWH who have severe reactions or intolerability to the administered drugs.73 Although somewhat antithetical to the purpose of developing LAI-ART, an oral ART lead-in will likely be required until the safety of the long-acting formulation(s) can be established.73 A recent study of 566 PLWH on long-acting CAB and RPV indicates an oral ART lead-in, currently one pill daily for 4 weeks, will be required for virally suppressed patients,41 although this requirement is likely to be rescinded once more data become available.
Additional factors that may lead to provider hesitancy in prescribing LAI-ART include drug intolerability, long-term toxicity, and drug resistance, as well as clinical maintenance and lack of data on LAI-ART in specific populations of interest.15,34,69,76,77 It has been noted that, paradoxically, populations most at need for this intervention are underrepresented in clinical trials and, thus, the safety of LAI-ART in these populations remains largely unknown.69,71
Moving from patient and provider perspectives, the characteristics of the recipients of LAI-ART must also be considered. In the PRISM framework, the patient and provider perspectives inform the recipient characteristics,20 which will be explored next.
The recipients
The PRISM framework also concerns itself with the characteristics of the patients at which the intervention is best directed, as well as the characteristics of organizations/providers at which and by whom the intervention is to be administered. Each will be addressed in turn, the former by describing the patient characteristics and the latter by describing the organizational/provider characteristics.
Patient characteristics
Patients optimally adherent to oral ART may benefit from switching to LAI-ART because of increased convenience.2,78 However, LAI-ART for many other populations could be transformative, potentially leading to greater viral suppression rates and increased retention in HIV care. Among them are persons who face adherence challenges, such as pregnant women, youth, and adolescents,13 and populations such as PLWH experiencing homelessness,79,80 PLWH engaged in substance abuse,13,81,82 PLWH with mental health challenges,13,82–84 or PLWH with disabilities.1,12
Adherence to medication is essential to the treatment of HIV, and LAI-ART is potentially a viable alternative to oral ART for PLWH who miss clinic visits and/or have difficulty achieving viral suppression.16,69,70,85 In fact, difficulty adhering to daily oral ART spurred the development of LAI-ART based on successful similar implementation of long-acting formulations in patients with schizophrenia and as contraceptives.70,86,87 Thus, LAI-ART is likely to increase adherence and effectiveness by reducing or eliminating the daily pill burden and increasing the overall quality of life of PLWH who have difficulty adhering to daily oral ART.13,15,16 Data, however, are lacking on how LAI-ART could most optimally increase adherence or viral suppression in these groups.
Mental disorders and substance abuse are significantly higher among PLWH compared to the general population, and are correlated with HIV-related stigma.2,82,84,88 Mental disorders, including depression, are one of the strongest predictors of ART nonadherence, and this finding is consistent among all socioeconomic statuses and countries.83,88–91 LAI-ART may serve to quell some of the internal and external stigma related to HIV by eliminating the daily pill burden of oral ART.1,34,49
Adolescent and young PLWH may also benefit greatly from LAI-ART. In a recent study of 303 young PLWH, 88.1% of youth expressed a great interest in LAI-ART.68 This number illustrates that interest in LAI-ART among youth/adolescent PLWH parallels or surpasses that of adult PLWH.56 Youth and adolescents comprise more than 20% of all incident HIV cases in the United States, have poor suppression rates, and are particularly susceptible to suboptimal adherence.68,92–96 Many young PLWH lack social support and experience high rates of mental health disorders, particularly as they transition into adult care.2,97–99 Data remain largely lacking on diverse youth/adolescent populations' acceptability of LAI-ART.71
Patient characteristics are only one half of a dyad. The PRISM framework also requires analysis of the organizational/provider characteristics to ensure optimal implementation of LAI-ART.
Organizational/provider characteristics
According to the PRISM model, initiating novel interventions in the context of key organizational (e.g., HIV clinics) values is critical to achieving implementation success.20 Therefore, where and by whom LAI-ART is administered must be driven by patient and provider perspectives.
These considerations will vary greatly by setting, country and resource levels. Globally, health systems continue to evolve away from traditional care models for HIV to models that vary the location of care, reduce the frequency, and expand the roles of nurses, lay health care workers, and pharmacists.100 Acceptability research within the United States revealed that patients generally agreed that skilled or trained professionals should administer the injections and, recently, primary care providers (PCPs) have assumed a greater role in the long-term care of PLWH.1,76,101 Within the United States, it remains unclear if LAI-ART should be administered through a patient's PCP, through an HIV-specific care provider, or in alternative settings.
Besides PCP and specialist offices, academic hospitals, pharmacies, community health centers, and other nontraditional venues such as stand-alone or mobile shot clinics should be considered.21,77,102,103 Many pharmacies already provide preventive services such as vaccines.21 Offering LAI-ART through pharmacies would increase the reach and convenience of LAI-ART administration as well as the populations served. Pharmacies would, however, require a separate physical space, in which to administer the gluteal injections, and the privacy and confidentiality of patients would need to be safeguarded.21,71 Some PLWH, particularly those living in small communities, were averse to receiving LAI-ART injections at a pharmacy because of the increased potential for unintended status disclosure.12
Community health centers could be viable venues, particularly in resource-limited communities and communities of color.21,102 Finally, mobile or free-standing shot clinics offer other venues to consider, especially for PLWH who are unstably housed or resource-limited.77,103
Considerations of patient/provider perspectives and recipient characteristics are alone not enough to optimally implement a novel intervention such as LAI-ART. The PRISM framework further illustrates that the external environment and infrastructure are integral components to implementation design.
The external environment
The PRISM framework interrogates the external factors that must be considered when designing an optimal implementation strategy for LAI-ART. Market forces, such as variable drug costs, distribution concerns, and insurance coverage, as well as elements relevant to the external environment, such as health care reform and stigma, may be among the most powerful predictors of an intervention's success.20
As of September 2020, LAI-ART still awaits regulatory approval in the United States from the FDA.43 LAI-ART has, however, already been approved for use in Canada and important lessons may be learned from the early implementation of LAI-ART in this country.104 The current external climate is rife with other dynamic situations that may facilitate or hinder LAI-ART implementation, such as health care reform, cost and payment for LAI-ART, stigma, and systemic racism that will act as a barrier to uptake in communities of color.
The constitutionality of the Patient Protection and Affordable Care Act (ACA) of 2010105 is being challenged in court.106,107 Should the Supreme Court rule to repeal the ACA, as did a lower federal appeals court, more than 20 million Americans are likely to lose their insurance coverage over the next decade, according to the Congressional Budget Office.21,108,109 Millions of low income adults would become uninsured because states cannot continue Medicaid expansion without the federal funds that the ACA provides.109,110 In addition, the number of uninsured Americans would spike because, without the ACA in place, insurance companies would be free to deny coverage or charge drastically higher premiums to people with preexisting conditions such as HIV.109,110 Finally, people who purchased insurance through the federal marketplace would almost certainly lose their coverage.109,110
To contextualize these figures, 34% of PLWH have private insurance coverage, either through their employer or bought on the federal exchange, and only 5% of PLWH were uninsured in Medicaid expansion states compared to 19% in states that did not expand Medicaid under the ACA.111 Combined with the fact that the lesbian, gay, bisexual, and transgender population and populations of color are far more likely to be uninsured than the general population,112,113 the cost and payment of LAI-ART must be given the utmost consideration.
Although the exact cost of LAI-ART has yet to be determined, it will most likely cost more than oral ART because it is a novel market modality.69 Because LAI-ART will likely be considered clinically equivalent to oral ART, payers will decide whether and for whom LAI-ART will be supplied.69 Thus, switching to LAI-ART will not be up to the provider or the patient, but rather the policy maker(s).69 Insurance companies and Medicare/Medicaid officials will decide whether to add LAI-ART to their formularies and how to classify it.
Structural stigma is another issue that cannot be disregarded when designing an implementation strategy for any HIV treatment.114 HIV is different from other chronic conditions in that it invokes vast stigma that results in rampant discrimination.34,115 In PLWH, fears of structural discrimination are still a major barrier to HIV treatment.1,16,34,83,115 HIV-related stigma is far greater than the stigma associated with sexual orientation; homophobia, and transphobia, however, exponentially compound stigma and discrimination.1,116–120 Likewise, systemic racism intersects with HIV-related stigma, homophobia, and/or transphobia to create additional barriers to HIV treatment and prevention.116,121,122 Further, there is no evidence to suggest that LAI-ART will not exhibit racial or other disparities in uptake as we have seen with other novel biomedical interventions for HIV prevention and treatment.
The external environment will always be dynamic. Thus, a carefully crafted infrastructure that can be modified when external forces shift is a necessary consideration to a sustainable and effective LAI-ART implementation strategy.
Implementation and sustainability infrastructure
The infrastructural design for LAI-ART poses unique challenges.123 New modalities must make ART viable, feasible, acceptable, and sustainable if they are to be worth their investment.123 The PRISM framework describes infrastructure as a necessary component to the implementation of any intervention, and planning for sustainability of the intervention is key.20 Because LAI-ART shifts the paradigm from one of adherence to pills to one of adherence to injection or clinic visits,69 determinations of where the treatment is administered, how to arrange venue logistics and patient flow through them, and the types of patient/provider educational materials to be developed and the methods of deploying them are but a few fundamental considerations.54
The administration of LAI-ART would almost certainly pose a challenging conundrum because of the dramatic increase in patient visits at already busy infectious disease clinics due to monthly or bimonthly dosing required by current LAI-ART.46 Providers have expressed concern over the increase in numbers of patients in their clinics, as well as where in the clinic to administer the injections and who within the clinic would be best suited to administer said injections.1 This could potentially involve training/retraining of, or task shifting among, health care workers.
Materials to support patient–provider communication around risks and benefits of LAI-ART, guidelines and assessment tools to determine the best candidates for LAI-ART, and when/how to transition from oral ART to LAI-ART also need to be developed.1,16,64 Shared decision-making between the patient and their provider can build trust and strengthen partnerships, an integral part of treating patients with chronic conditions such as HIV.124,125 As such, materials should be developed with not only the clinician's view of what is best for the patient, but also the patient's view.
Discussion
This scoping review resulted in many questions that need answers for designing an optimal implementation strategy for LAI-ART in the United States via the PRISM model. Table 1 lists key questions and their relevant considerations by PRISM domain (intervention, recipients, external environment, and infrastructure). Particularly, there are questions around the acceptability of LAI-ART among many different subgroups of PLWH, especially those who would benefit the most from it but who are often overlooked, which include subgroups of age, sex and gender, socioeconomic status, and race and ethnicity.1,17,57 The field of LAI-ART implementation will also require data on cost, patient satisfaction, and patient-reported outcomes.18,57
Table 1.
Long-Acting Injectable Antiretroviral Therapy Key Questions and Relevant Considerations by Practical, Robust Implementation and Sustainability Model Domain
| PRISM domain | Key questions | Considerations |
|---|---|---|
| Intervention | What concerns do patients have about LAI-ART? | • Efficacy |
| • Side effects | ||
| • Clinic visits | ||
| • Fear of needles/injections | ||
| • Number of injections per dose | ||
| • Frequency of injections | ||
| • Bodily location of injections | ||
| • Cost | ||
| What concerns do providers have about LAI-ART? | • Efficacy | |
| • Side effects | ||
| • Clinic visits | ||
| • Number of injections per dose | ||
| • Frequency of injections | ||
| • Cost | ||
| • Modality | ||
| • Resistance | ||
| • Oral lead-in/PK tail | ||
| • DDIs | ||
| • Clinical management/resources | ||
| Recipients | At which patient populations is LAI-ART best directed? | • People non-adherent to pills |
| • Women/pregnant women | ||
| • People with substance abuse challenges | ||
| • People with mental challenges | ||
| • Unstably housed | ||
| • Youth/adolescents | ||
| Which organizations/providers are best suited to administer LAI-ART? | • Hospitals | |
| • HIV specialists | ||
| • PCPs | ||
| • Clinics | ||
| • Pharmacies | ||
| • Community health centers | ||
| • Shot clinics | ||
| External environment | What external factors bear on the implementation of LAI-ART? | • Regulatory environment |
| • Health care reform/ACA | ||
| • Medicaid expansion | ||
| • Preexisting conditions | ||
| • Exchange plans | ||
| • Payers | ||
| • Third party payers | ||
| • Medicare/Medicaid | ||
| • Uninsured | ||
| • Stigma | ||
| • HIV | ||
| • Homophobia | ||
| • Transphobia | ||
| • Systemic racism | ||
| Infrastructure | What is needed to craft a sustainable infrastructure for LAI-ART? | • Appropriate venue |
| • Venue logistics/patient flow | ||
| • Patient/provider education |
PRISM, Practical, Robust Implementation and Sustainability Model; LAI-ART, long-acting injectable antiretroviral therapy; PK, pharmacokinetic; DDI, drug–drug interaction; PCP, primary care provider; ACA, Affordable Care Act.
As LAI-ART is implemented in the real-world, it will be important to study how the external environment and organizational characteristics influence implementation, as well as how potential facilitators may be used to maximize its acceptability and uptake. Not discussed in this article, but still important to consider, are the ethical considerations of LAI-ART implementation, such as its potential for coercive use among susceptible populations and what services, other than the injection(s), providers should be obligated to provide to their patients at the point of care.
The preceding paragraph is a description of future research possibilities, although it is by no means exhaustive. Table 2 summarizes possible future research directions predicated on our findings for the field of LAI-ART implementation, such as acceptability among diverse populations of PLWH and providers, external and organizational factors, prospective consideration of real-world implementation, and ethical considerations related to implementation. Mapping our key findings onto the PRISM framework necessitated the answers to these future research questions before designing an implementation protocol for LAI-ART.
Table 2.
Suggested Future Research Questions for the Field of Long-Acting Injectable Antiretroviral Therapy Implementation
| Patient perspectives: LAI-ART acceptability among diverse populations of PLWH |
|---|
| • What factors affect acceptability of LAI-ART among diverse populations of PLWH? |
| • How do demographic characteristics (age, sex/gender, race/ethnicity, socio-economic status) affect LAI-ART acceptability, perceptions, and understanding? |
| • What is the acceptability of LAI-ART among susceptible populations, such as the homeless/unstably housed, substance abusers/IV drug users, the mentally ill, the disabled, youth/adolescents, pregnant women, prisoners, etc.? What effect does LAI-ART have on the uptake and adherence among these populations? |
| • How do PROs on experiences and satisfaction with LAI-ART affect the implementation strategy? |
| Provider perspectives: LAI-ART acceptability among care providers |
| • What factors affect acceptability of LAI-ART among HIV care providers? |
| • What clinical data are needed to adequately inform HIV care providers on LAI-ART, specifically on susceptible populations and populations currently underrepresented in clinical trials? |
| • How do patient demographics vary drug intolerability, long-term toxicity, drug resistance, etc. in LAI-ART vs. oral ART? |
| External and organizational factors |
| • What is the actual cost of LAI-ART? How does the actual cost relate to third-party payer cost (Medicare, Medicaid, private insurers, etc.)? |
| • How does HIV-related stigma (both internal and external), homophobia, transphobia, systemic racism, and intersectionality affect LAI-ART uptake and retention? |
| • What organizational characteristics affect patient willingness to switch to, and continue with, LAI-ART? |
| • Are there distinctions between urban and rural organizational characteristics that affect uptake and adherence of LAI-ART? Is so, what are these distinctions? |
| • What variations in organizational contexts by geographic region hinder or facilitate LAI-ART uptake and retention? |
| Prospective real-world implementation |
| • What are the actual realized costs of LAI-ART to patients, including travel and parking costs, loss of work, etc.? |
| • How do patient preferences change over time? What cause these longitudinal shifts in views? |
| Ethical Considerations of LAI-ART Implementation |
| • What are the ethical considerations that must be anticipated with LAI-ART implementation? |
| • What is LAI-ART's potential for coercive use among susceptible populations? How can it be eliminated or minimized? |
| • What are the provider obligations of LAI-ART to their patients at the point of care, besides the injection (e.g., mental health assessments)? |
PRO, patient-reported outcome; PLWH, people living with HIV.
Why is it imperative that we address these issues?
The public health implications of LAI-ART are immense. The discrete use of LAI-ART eliminates daily oral ART adherence, which has the potential to greatly increase the proportion of PLWH who present to care with suboptimal adherence.14,48,74 This dovetails with the United Nations goal of 90% of PLWH initiating ART achieving and sustaining viral suppression via adherence to ART.126,127 Likewise, the United States' Ending the Epidemic goals of 75% reduction in new HIV infections by 2025 and at least 90% reduction in new HIV cases by 2030128 may only be achieved by increasing the percentage of PLWH who achieve and sustain viral suppression, thus, preventing forward transmission. The goals have been set both nationally and internationally; only through sound leadership in anticipating implementation challenges and ensuring successful and sustainable LAI-ART implementation will these goals ever come to fruition.
To fulfill the promise of LAI-ART in increasing viral suppression, it is imperative that we proactively anticipate and address potential implementation issues related to patients, providers, and the external environment as detailed in this article. For example, while daily oral ART adherence may be obviated, it is imperative to develop systems to track adherence to injection appointments, which are currently expected to occur every 1–2 months. Given that when novel biomedical technologies are introduced there is also a potential to exacerbate entrenched inequities, it is also important to consider the circumstances and interventions for adherence to LAI-ART among the most vulnerable groups.
With LAI-ART, as with other HIV prevention and treatment modalities, populations who may benefit the most are also those least likely to participate in HIV clinical trials and only take up novel interventions when they become widely available. Redressing these critical implementation bottlenecks is imperative to bridging the gap between research and practice, and to ensuring implementation success of novel interventions with the potential for high public health impact.
New modalities for administering long-acting ART, such as patches and implants, are already underway.2,14,15,17 LAI-ART is merely the first to reach Phase III clinical trials.2,129,130 Each novel HIV control strategy will require the same detailed analysis and foresight as that posed for LAI-ART. Moving forward, long-acting ART should be effective, easy to administer, and affordable so that it can be optimally implemented in the United States, as well as resource-limited settings that may eventually benefit the most from the advent of long-acting formulations to achieve higher viral suppression rates.14,70
What have we learned from prior research on other clinical products and how do these findings intersect?
PrEP research has shown the importance of well-reasoned and patient-centered implementation strategies. Even though PrEP has the potential to be a “prevention as cure” strategy, it has been met with implementation barriers such as cost, provider hesitance, and suboptimal provision to marginalized communities who could benefit the most from PrEP.21 These barriers remain today. In 2018, uptake and adherence to PrEP was estimated to be only 10% of those who might be expected to benefit from it.21 LAI-ART will face the same implementation barriers as PrEP—including long-acting PrEP;131 thus, only by learning from prior interventions like PrEP can LAI-ART be used to its maximum potential.
Implementation of long-acting agents, such as those used in the fields of schizophrenia and contraception,52,65–67,86,87 reveals that while long-acting formulations can reduce adherence barriers, attention must be paid to lead-in periods, PK tails, DDIs, drug resistance, and clinical management. The adherence conundrum shifts from one of adherence to tablets/pills to one of adherence to clinic visits to receive injections. As outlined in this article, factors such as efficacy, acceptability, side effects, psychological impacts, and stigma must be considered. When novel biomedical technologies are introduced, as recently shown with novel Hepatitis C cure regimens,132,133 predictors of intervention success also lie in the external environment and include considerations for cost, distribution, medical insurance, and health systems preparedness.
Limitations
We must acknowledge important limitations to this review. There is the potential that our review was subject to selection bias due to the scoping methods used. In addition, the quality of references/studies and the strength of the evidence within them were not assessed.
These limitations notwithstanding, we believe that we have catalogued some of the most critical considerations for LAI-ART implementation success following the PRISM framework.
Conclusion
LAI-ART represents the genesis of a new direction for HIV treatment that offers the possibility of being patient-centered and focused on bettering the quality of life of PLWH by reducing barriers and improving prognoses.14,101,134–138 Emphasis should be on the long-term health of PLWH and all that encompasses, not merely on the dispensing of medicines.139,140 We have a unique window of opportunity to anticipate implementation considerations for LAI-ART. Of greatest importance will be to determine how LAI-ART can be used to its fullest potential to maintain and advance durable HIV suppression rather than simply becoming an optional modality for those already adherent to ART.
Authors' Contributions
J.T.K. drafted the article. K.D., P.S., and J.A.S. reviewed the article for intellectual contents. All authors approved the final version of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
K.D. received support from the National Institute of Mental Health, R21MH118120.
References
- 1. Kerrigan D, Mantsios A, Gorgolas M, et al. : Experiences with long acting injectable ART: A qualitative study among PLHIV participating in a phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One 2018;13:e0190487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Masters MC, Krueger KM, Williams JL, et al. : Beyond one pill, once daily: Current challenges of antiretroviral therapy management in the United States. Expert Rev Clin Pharmacol 2019;12:1129–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palella FJ, Delaney KM, Moorman AC, et al. : Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338:853–860 [DOI] [PubMed] [Google Scholar]
- 4. Cohen MS, Chen YQ, McCauley M, et al. : Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCray E: CDC Letter Approving Usage of U = U Communications (2019). Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 6. Eisinger RW, Fauci AS: Ending the HIV/AIDS pandemic. Emerg Infect Dis 2018;24:413–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cook R, Davidson P, Martin R: Antiretroviral treatment can reduce the risk of HIV transmission between male partners to “zero.” BMJ 2019;366:l4915. [DOI] [PubMed] [Google Scholar]
- 8. Rodger AJ, Cambiano V, Bruun T, et al. : Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): Final results of a multicentre, prospective, observational study. Lancet 2019;393:2428–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. CDC: Ending HIV transmission-test, treat, and prevent. Available at https://cdc.gov/nchhstp/newsroom/2019/ending-HIV-transmission-test-treat-and-prevent.html, accessed March3, 2020
- 10. Iacob SA, Iacob DG, Jugulete G: Improving the adherence to antiretroviral therapy, a difficult but essential task for a successful HIV treatment-clinical points of view and practical considerations. Front Pharmacol 2017;8:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giordano TP, Suarez-Almazor ME, Grimes RM: The population effectiveness of highly active antiretroviral therapy: Are good drugs good enough? Curr HIV/AIDS Rep 2005;2:177–183 [DOI] [PubMed] [Google Scholar]
- 12. Simoni JM, Beima-Sofie K, Mohamed ZH, et al. : Long-acting injectable antiretroviral treatment acceptability and preferences: A qualitative study among US providers, adults living with HIV, and parents of youth living with HIV. AIDS Patient Care STDS 2019;33:104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benning L, Mantsios A, Kerrigan D, et al. : Examining adherence barriers among women with HIV to tailor outreach for long-acting injectable antiretroviral therapy. BMC Womens Health 2020;20:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cobb DA, Smith NA, Edagwa BJ, et al. : Long-acting approaches for delivery of antiretroviral drugs for prevention and treatment of HIV: A review of recent research. Expert Opin Drug Deliv 2020;17:1227–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Labh R, Gupta R: Emerging trends in the long-acting antiretroviral therapy: current status and therapeutic challenges. Curr HIV Res 2020. [Epub ahead of print]; DOI: 10.2174/1570162X18666200824104140 [DOI] [PubMed] [Google Scholar]
- 16. Mantsios A, Murray M, Karver TS, et al.: Efficacy and freedom: Patient experiences with the transition from daily oral to long-acting injectable antiretroviral therapy to treat HIV in the context of phase 3 trials. AIDS Behav 2020. [Epub ahead of print]; DOI: 10.1007/s10461-020-02918-x [DOI] [PubMed] [Google Scholar]
- 17. Rana AI, Castillo-Mancilla JR, Tashima KT, et al. : Advances in long-acting agents for the treatment of HIV infection. Drugs 2020;80:535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murray M, Antela A, Mills A, et al.: Patient-reported outcomes in ATLAS and FLAIR participants on long-acting regimens of cabotegravir and rilpivirine over 48 weeks. AIDS Behav 2020. [Epub ahead of print]; DOI: 10.1007/s10461-020-02929-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kerrigan D, Sanchez Karver T, Muraleetharan O, et al. : A dream come true: Perspectives on long-acting injectable antiretroviral therapy among female sex workers living with HIV from the Dominican Republic and Tanzania. PLoS One 2020;15:e0234666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feldstein AC, Glasgow RE: A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt Comm J Qual Patient Saf 2008;34:228–243 [DOI] [PubMed] [Google Scholar]
- 21. Mayer KH, Chan PA, Patel R, et al. : Evolving models and ongoing challenges for HIV preexposure prophylaxis implementation in the United States. J Acquir Immune Defic Syndr 2018;77:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bauer MS, Kirchner J: Implementation science: What is it and why should I care? Psychiatry Res 2020;283:112376. [DOI] [PubMed] [Google Scholar]
- 23. Eccles MP, Mittman BS: Welcome to implementation science. Implement Sci 2006;1:1 [Google Scholar]
- 24. Glasgow RE, Vinson C, Chambers D, et al. : National Institutes of Health approaches to dissemination and implementation science: Current and future directions. Am J Public Health 2012;102:1274–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Czarnogorski M: Using implementation science to better integrate novel long-acting injectable therapy into routine HIV care. J Acquir Immune Defic Syndr 2019;82 Suppl 3:S286–S288 [DOI] [PubMed] [Google Scholar]
- 26. Eron J: Long-acting ART and other treatment products. Presented at the: Looking to the Future: Behavioral Aspects of Long-Acting and Extended Delivery HIV Prevention and Treatment Regimens. Rockville, MD, May 13, 2019 [Google Scholar]
- 27. Geng E: Learning from HIV care retention: Rates, patterns, and interventions. Presented at the: Looking to the Future: Behavioral aspects of long-acting and extended delivery HIV prevention and treatment regimens. Rockville, MD, May 13, 2019 [Google Scholar]
- 28. Dandachi D, Dang BN, Lucari B, et al.: Acceptability and preferences for long-acting antiretroviral formulations among people with HIV infection. AIDS Care 2020:1–9 [DOI] [PubMed] [Google Scholar]
- 29. Beres LK, Simbeza S, Holmes CB, et al. : Human-centered design lessons for implementation science: Improving the implementation of a patient-centered care intervention. J Acquir Immune Defic Syndr 2019;82(Suppl 3):S230–S243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lenfant C: Shattuck lecture—clinical research to clinical practice—lost in translation? N Engl J Med 2003;349:868–874 [DOI] [PubMed] [Google Scholar]
- 31. Benítez-Gutiérrez L, Soriano V, Requena S, et al. : Treatment and prevention of HIV infection with long-acting antiretrovirals. Expert Rev Clin Pharmacol 2018;11:507–517 [DOI] [PubMed] [Google Scholar]
- 32. Matt Barnhart LM: Long-acting, injectable antiretroviral therapy for the management of HIV infection: An update on a potential game-changer. J AIDS Clin Res 2015;6 [Epub ahead of print]; DOI: 10.4172/2155-6113.1000466. [DOI] [Google Scholar]
- 33. Spreen W, Williams P, Margolis D, et al. : Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr 2014;67:487–492 [DOI] [PubMed] [Google Scholar]
- 34. D'Amico R, Margolis DA: Long-acting injectable therapy: An emerging paradigm for the treatment of HIV infection. Curr Opin HIV AIDS 2020;15:13–18 [DOI] [PubMed] [Google Scholar]
- 35. Purcell D: CDC perspective. Presented at the: Looking to the Future: Behavioral Aspects of Long-Acting and Extended Delivery HIV Prevention and Treatment Regimens. Rockville, MD, May 14, 2019 [Google Scholar]
- 36. Tricco AC, Lillie E, Zarin W, et al. : PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med 2018;169:467–473 [DOI] [PubMed] [Google Scholar]
- 37. Armstrong R, Hall BJ, Doyle J, et al. : Cochrane update. “Scoping the scope” of a cochrane review. J Public Health 2011;33:147–150 [DOI] [PubMed] [Google Scholar]
- 38. Peters MDJ, Godfrey CM, Khalil H, et al. : Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015;13:141–146 [DOI] [PubMed] [Google Scholar]
- 39. Popay J, Roberts H, Sowden A, et al.: Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. ESRC Methods Programme. 2001 [Google Scholar]
- 40. Margolis DA, Gonzalez-Garcia J, Stellbrink H-J, et al. : Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017;390:1499–1510 [DOI] [PubMed] [Google Scholar]
- 41. Orkin C, Arasteh K, Hernández-Mora MG, et al.: Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020. [Epub ahead of print]; DOI: 10.1056/NEJMoa1909512 [DOI] [PubMed] [Google Scholar]
- 42. Swindells S, Andrade-Villanueva J-F, Richmond GJ, et al.: Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020. [Epub ahead of print]; DOI: 10.1056/NEJMoa1904398 [DOI] [PubMed] [Google Scholar]
- 43. FDA defers approval of first long-acting HIV therapy, surprising everyone (2019). NEJM Journal Watch (WWW Document). Available at https://blogs.jwatch.org/hiv-id-observations/index.php/fda-defers-approval-of-first-long-acting-hiv-therapy-surprising-everyone/2019/12/23/, accessed February26, 2020
- 44. Abbasi J: Promising early results for potent, long-acting HIV injection. JAMA 2020;324:539. [DOI] [PubMed] [Google Scholar]
- 45. ViiV HealthCare presents positive, 48-week from phase III study showing every-two-month regimen of investigational long-acting, injectable cabotegravir and rilpivirine has similar efficacy to once-monthly dosing (2020). ViiV Healthcare (WWW Document). Available at https://viivhealthcare.com/en-us/us-news/us-articles/2020/viiv-healthcare-presents-positive--48-week-data-from-phase-iii-s/, accessed March22, 2020
- 46. Currier JS: Monthly injectable antiretroviral therapy-version 1.0 of a new treatment approach. N Engl J Med 2020;382:1164–1165 [DOI] [PubMed] [Google Scholar]
- 47. ViiV Healthcare reports positive phase III study results of investigational, long-acting, injectable HIV-treatment regimen administered every two months (2019). ViiV Healthcare (WWW Document). Available at https://viivhealthcare.com/en-gb/media/press-releases/2019/august/viiv-healthcare-reports-positive-phase-iii-study-results-of-inve/, accessed March6, 2020
- 48. Cohen J: Long-acting drug acts like a short-term AIDS vaccine. Science 2020;368:807. [DOI] [PubMed] [Google Scholar]
- 49. Dubé K, Eskaf S, Evans D, et al. : The dose response: Perceptions of people living with HIV in the United States on alternatives to oral daily antiretroviral therapy. AIDS Res Hum Retroviruses 2020;36:324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dubé K, Sylla L, Dee L, et al. : Research on HIV cure: Mapping the ethics landscape. PLoS Med 2017;14:e1002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grossman CI, Ross AL, Auerbach JD, et al. : Towards multidisciplinary HIV-cure research: Integrating social science with biomedical research. Trends Microbiol 2016;24:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Owen A, Rannard S: Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: Insights for applications in HIV therapy. Adv Drug Deliv Rev 2016;103:144–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rusconi S, Marcotullio S, Cingolani A: Long-acting agents for HIV infection: Biological aspects, role in treatment and prevention, and patient's perspective. New Microbiol 2017;40:75–79 [PubMed] [Google Scholar]
- 54. Havlir D, Gandhi M: Implementation challenges for long-acting antivirals as treatment. Curr Opin HIV AIDS 2015;10:282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Derrick CB, Ostermann J, Weissman SB, et al. : Who wants to switch? gauging patient interest in novel antiretroviral therapies. Open Forum Infect Dis 2018;5:ofy247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saberi P, Eskaf S, Sauceda J, et al. : Perceptions of HIV virologic control strategies among younger and older age groups of people living with HIV in the United States: A cross-sectional survey. AIDS Res Hum Retroviruses 2020;36:606–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Simoni JM, Tapia K, Lee S-J, et al.: A conjoint analysis of the acceptability of targeted long-acting injectable antiretroviral therapy among persons living with HIV in the U.S. AIDS Behav 2020;24:1226–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dube K, Campbell DM, Perry KE, et al.: Reasons people living with HIV might prefer oral daily antiretroviral therapy, long-acting formulations, or future HIV remission options. AIDS Res Hum Retroviruses 2020 [Epub ahead of print]; DOI: 10.1089/AID.2020.0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Philbin MM, Parish C, Kinnard EN, et al.: A multi-site study of women living with HIV's perceived barriers to, and interest in, long-acting injectable anti-retroviral therapy. J Acquir Immune Defic Syndr 2020. [Epub ahead of print]; DOI: 10.1097/QAI.0000000000002337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dubé K, Taylor J, Sylla L, et al. : “Well, it's the risk of the unknown… right?” A qualitative study of perceived risks and benefits of HIV cure research in the united states. PLoS One 2017;12:e0170112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. U.S. Food and Drug Administration. The voice of the patient (2014). U.S. Food and Drug Administration, Washington, DC [Google Scholar]
- 62. Dubé K, Simoni J, Louella M, et al. : Acceptability of cell and gene therapy for curing HIV infection among people living with HIV in the Northwestern United States: A qualitative study. AIDS Res Hum Retroviruses 2019;35:649–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dubé K, Evans D, Dee L, et al. : We need to deploy them very thoughtfully and carefully”: Perceptions of analytical treatment interruptions in HIV cure research in the United States-a qualitative inquiry. AIDS Res Hum Retroviruses 2018;34:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Campbell DM: Keeping HIV under control: A cross-sectional survey on preferences for virologic control strategies among people living with HIV (PLWHIV) in the United States. Presented at the: American Public Health Association Annual Meeting. Philadelphia, PA, 2019 [Google Scholar]
- 65. Potkin S, Bera R, Zubek D, et al. : Patient and prescriber perspectives on long-acting injectable (LAI) antipsychotics and analysis of in-office discussion regarding LAI treatment for schizophrenia. BMC Psychiatry 2013;13:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kavanaugh ML, Frohwirth L, Jerman J, et al. : Long-acting reversible contraception for adolescents and young adults: Patient and provider perspectives. J Pediatr Adolesc Gynecol 2013;26:86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tolley EE, McKenna K, Mackenzie C, et al. : Preferences for a potential longer-acting injectable contraceptive: Perspectives from women, providers, and policy makers in Kenya and Rwanda. Glob Health Sci Pract 2014;2:182–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weld ED, Rana MS, Dallas RH, et al. : Interest of youth living with HIV in long-acting antiretrovirals. J Acquir Immune Defic Syndr 2019;80:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Giordano T: Treatment provider perspective. Presented at the: Looking to the Future: Behavioral Aspects of Long-Acting and Extended Delivery HIV Prevention and Treatment Regimens. Rockville, MD, May 14, 2019 [Google Scholar]
- 70. Kovarova M, Benhabbour SR, Massud I, et al. : Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat Commun 2018;9:4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Struble K: FDA perspective. Presented at the: Looking to the Future: Behavioral Aspects of Long-Acting and Extended Delivery HIV Prevention and Treatment Regimens. Rockville, MD, May 14, 2019 [Google Scholar]
- 72. Grinsztejn B: Addressing the long-tail of injectable PrEP. Presented at the: Looking to the Future: Behavioral Aspects of Long-Acting and Extended Delivery HIV Prevention and Treatment Regimens. Rockville, MD, May 13, 2019 [Google Scholar]
- 73. Landovitz RJ, Kofron R, McCauley M: The promise and pitfalls of long-acting injectable agents for HIV prevention. Curr Opin HIV AIDS 2016;11:122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Landovitz RJ, Li S, Grinsztejn B, et al. : Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med 2018;15:e1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf, accessed March3, 2020
- 76. Kerrigan D: User and provider perspectives on long-acting PrEP and ART. Presented at the: Looking to the Future: Behavioral Aspects of Long-Acting and Extended Delivery HIV Prevention and Treatment Regimens. Rockville, MD, May 13, 2019 [Google Scholar]
- 77. Healey L: HRSA perspective. Presented at the: Looking to the Future: Behavioral Aspects of Long-Acting and Extended Delivery HIV Prevention and Treatment Regimens. Rockville, MD, May 14, 2019 [Google Scholar]
- 78. Clay PG, Yuet WC, Moecklinghoff CH, et al. : A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Res Ther 2018;15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Davila JA, Cabral HJ, Maskay MH, et al. : Risk factors associated with multi-dimensional stigma among people living with HIV/AIDS who are homeless/unstably housed. AIDS Care 2018;30:1335–1340 [DOI] [PubMed] [Google Scholar]
- 80. Aidala AA, Wilson MG, Shubert V, et al. : Housing status, medical care, and health outcomes among people living with HIV/AIDS: A systematic review. Am J Public Health 2016;106:e1–e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Altice FL, Bruce RD, Lucas GM, et al. : HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: Results from a multisite study. J Acquir Immune Defic Syndr 2011;56 Suppl 1:S22–S32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chander G, Himelhoch S, Moore RD: Substance abuse and psychiatric disorders in HIV-positive patients: Epidemiology and impact on antiretroviral therapy. Drugs 2006;66:769–789 [DOI] [PubMed] [Google Scholar]
- 83. Remien RH, Stirratt MJ, Nguyen N, et al. : Mental health and HIV/AIDS: The need for an integrated response. AIDS 2019;33:1411–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Whetten K, Reif S, Whetten R, et al. : Trauma, mental health, distrust, and stigma among HIV-positive persons: Implications for effective care. Psychosom Med 2008;70:531–538 [DOI] [PubMed] [Google Scholar]
- 85. Osterberg L, Blaschke T: Adherence to medication. N Engl J Med 2005;353:487–497 [DOI] [PubMed] [Google Scholar]
- 86. Bahamondes L, Fernandes A, Monteiro I, et al.: Long-acting reversible contraceptive (LARCs) methods. Best Pract Res Clin Obstet Gynaecol 2019. [Epub ahead of print]; DOI: 10.1016/j.bpobgyn.2019.12.002 [DOI] [PubMed] [Google Scholar]
- 87. McEvoy JP: Risks versus benefits of different types of long-acting injectable antipsychotics. J Clin Psychiatry 2006;67 Suppl 5:15–18 [PubMed] [Google Scholar]
- 88. Robertson KR, Smurzynski M, Parsons TD, et al. : The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007;21:1915–1921 [DOI] [PubMed] [Google Scholar]
- 89. Gonzalez JS, Batchelder AW, Psaros C, et al. : Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. J Acquir Immune Defic Syndr 2011;58:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pence BW, O'Donnell JK, Gaynes BN: Falling through the cracks: The gaps between depression prevalence, diagnosis, treatment, and response in HIV care. AIDS 2012;26:656–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Uthman OA, Magidson JF, Safren SA, et al. : Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: A systematic review and meta-analysis. Curr HIV/AIDS Rep 2014;11:291–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rudy BJ, Lindsey JC, Flynn PM, et al. : Immune reconstitution and predictors of virologic failure in adolescents infected through risk behaviors and initiating HAART: Week 60 results from the PACTG 381 cohort. AIDS Res Hum Retroviruses 2006;22:213–221 [DOI] [PubMed] [Google Scholar]
- 93. Centers for Disease Control and Prevention. HIV Surveillance Report, 2018 (Preliminary); vol. 30 (2019). Centers for Disease Control and Prevention, Atlanta, GA. Available at http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
- 94. Ryscavage P, Anderson EJ, Sutton SH, et al. : Clinical outcomes of adolescents and young adults in adult HIV care. J Acquir Immune Defic Syndr 2011;58:193–197 [DOI] [PubMed] [Google Scholar]
- 95. MacDonell K, Naar-King S, Huszti H, et al. : Barriers to medication adherence in behaviorally and perinatally infected youth living with HIV. AIDS Behav 2013;17:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rudy BJ, Murphy DA, Harris DR, et al. : Prevalence and interactions of patient-related risks for nonadherence to antiretroviral therapy among perinatally infected youth in the United States. AIDS Patient Care STDS 2010;24:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Straub DM, Tanner AE: Health-care transition from adolescent to adult services for young people with HIV. Lancet Child Adolesc Health 2018;2:214–222 [DOI] [PubMed] [Google Scholar]
- 98. Naar-King S, Templin T, Wright K, et al. : Psychosocial factors and medication adherence in HIV-positive youth. AIDS Patient Care STDS 2006;20:44–47 [DOI] [PubMed] [Google Scholar]
- 99. Mellins CA, Brackis-Cott E, Leu C-S, et al. : Rates and types of psychiatric disorders in perinatally human immunodeficiency virus-infected youth and seroreverters. J Child Psychol Psychiatry 2009;50:1131–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Grimsrud A, Bygrave H, Doherty M, et al. : Reimagining HIV service delivery: The role of differentiated care from prevention to suppression. J Int AIDS Soc 2016;19:21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Safreed-Harmon K, Anderson J, Azzopardi-Muscat N, et al. : Reorienting health systems to care for people with HIV beyond viral suppression. Lancet HIV 2019;6:e869–e877 [DOI] [PubMed] [Google Scholar]
- 102. Research fact sheets and infographics. National Association of Community Health Centers (WWW Document). Available at http://nachc.org/research-and-data/research-fact-sheets-and-infographics/, (accessed March5, 2020)
- 103. Yu SWY, Hill C, Ricks ML, et al. : The scope and impact of mobile health clinics in the United States: A literature review. Int J Equity Health 2017;16:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Stulpin C. Health Canada approves long-acting HIV treatment cabenuva (2020). Healio (WWW Document). Available at https://www.healio.com/infectious-disease/hiv-aids/news/online/%7Bd7210f4f-c7b1-478f-addb-6079f49ccfab%7D/health-canada-approves-long-acting-hiv-treatment-cabenuva, accessed May14, 2020
- 105. Read the affordable care act, health care law HealthCare.gov (WWW Document). Available at https://www.healthcare.gov/where-can-i-read-the-affordable-care-act/, accessed March11, 2020
- 106. Liptak A, Goodnough A. Supreme court to hear obamacare appeal (2020). NYTimes.com (WWW Document). Available at https://www.nytimes.com/2020/02/02/us/supreme-court-obamacare-appeal.html, accessed March4, 2020
- 107. Texas v. United States. (5th Cir. 2020). Available at https://www.ca5.uscourts.gov/opinions/pub/19/19-10011-CV0.pdf
- 108. Congressional Budget Office. Congressional Budget Office Cost Estimate: Congressional Budget Office. Available at https://cbo.gov/system/files/115th-congress-2017-2018/costestimate/americanhealthcareact.pdf (2017), accessed March5, 2020
- 109. Turner A. Here's who will lose their insurance if Obamacare is overturned (2019). CNBC (WWW Document). Available at https://cnbc.com/2019/05/03/heres-who-will-lose-their-insurance-if-obamacare-is-overturned.html, accessed March5, 2020
- 110. HEALTH INSURANCE EXCHANGES 2019 OPEN ENROLLMENT REPORT (2019). Centers for Medicare & Medicaid Services (WWW Document). Available at https://cms.gov/newsroom/fact-sheets/health-insurance-exchanges-2019-open-enrollment-report, accessed March5, 2020
- 111. Dawson L, Kates J. An Update on Insurance Coverage Among People wih HIV in the United States (2020). The Henry J. Kaiser Family Foundation (WWW Document). Available at https://kff.org/hivaids/issue-brief/an-update-on-insurance-coverage-among-people-with-hiv-in-the-united-states/, accessed March23, 2020
- 112. Gates GJ. In U.S., LGBT More Likely Than Non-LGBT to be Uninsured (2014). GALLUP (WWW Document). Available at https://news.gallup.com/poll/175445/lgbt-likely-non-lgbt-uninsured.aspx, accessed February26, 2020
- 113. Census - Table Results. United States Census Bureau (WWW Document). Available at https://data.census.gov/cedsci/table?q=United%20States&tid=PEPPOP2019.PEPANNRES&hidePreview=false, accessed February26, 2020
- 114. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. : State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS 2015;29:837–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Katz IT, Ryu AE, Onuegbu AG, et al. : Impact of HIV-related stigma on treatment adherence: Systematic review and meta-synthesis. J Int AIDS Soc 2013;16(3 Suppl 2):18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Arnold EA, Rebchook GM, Kegeles SM: “Triply cursed”: Racism, homophobia and HIV-related stigma are barriers to regular HIV testing, treatment adherence and disclosure among young Black gay men. Cult Health Sex 2014;16:710–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Frye V, Paige MQ, Gordon S, et al. : Impact of a community-level intervention on HIV stigma, homophobia and HIV testing in New York City: Results from project CHHANGE. Stigma Health 2019;4:72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Safer JD, Coleman E, Feldman J, et al. : Barriers to healthcare for transgender individuals. Curr Opin Endocrinol Diabetes Obes 2016;23:168–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. White Hughto JM, Reisner SL, Pachankis JE: Transgender stigma and health: A critical review of stigma determinants, mechanisms, and interventions. Soc Sci Med 2015;147:222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Health & HIV | National Center for Transgender Equality. National Center for Transgender Equality (WWW Document). Available at https://transequality.org/issues/health-hiv, accessed January14, 2019
- 121. Arscott J, Humphreys J, Merwin E, et al. : “That guy is gay and black. That's a red flag.” How HIV stigma and racism affect perception of risk among young black men who have sex with men. AIDS Behav 2020;24:173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Watkins-Hayes C: Intersectionality and the sociology of HIV/AIDS: Past, present, and future research directions. Annu Rev Sociol 2014;40:431–457 [Google Scholar]
- 123. Tolley E: Informing future product design. Presented at the: Looking to the Future: Behavioral Aspects of Long-Acting and Extended Delivery HIV Prevention and Treatment Regimens. Rockville, MD, May 13, 2019 [Google Scholar]
- 124. Breslin M, Mullan RJ, Montori VM: The design of a decision aid about diabetes medications for use during the consultation with patients with type 2 diabetes. Patient Educ Couns 2008;73:465–472 [DOI] [PubMed] [Google Scholar]
- 125. International Patient Decision Aids Standards (IPDAS) collaboration (2019). (WWW Document). Available at http://ipdas.ohri.ca/, accessed March23, 2020
- 126. UNAIDS: 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Presented at the: Joint United Nations Programme on HIV/AIDS; Geneva, Switzerland, 2014
- 127. Granich R, Gupta S, Hall I, et al. : Status and methodology of publicly available national HIV care continua and 90-90-90 targets: A systematic review. PLoS Med 2017;14:e1002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. What is ‘Ending the HIV epidemic: A plan for America?’. HIV.gov. (WWW Document). Available at https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview, accessed March21, 2020
- 129. Moffatt K, Wang Y, Raj Singh TR, et al. : Microneedles for enhanced transdermal and intraocular drug delivery. Curr Opin Pharmacol 2017;36:14–21 [DOI] [PubMed] [Google Scholar]
- 130. Stewart SA, Domínguez-Robles J, Donnelly RF, et al. : Implantable polymeric drug delivery devices: Classification, manufacture, materials, and clinical applications. Polymers (Basel) 2018;10:1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Grinsztejn B: HPTN 083 interim results: Efficacy of pre-exposure prophylaxis (PrEP) containing long-acting injectable cabotegravir (CAB-LA) is maintained across regions and key populations. Presented at the: AIDS 2020 Virtual. July 2020
- 132. Millman AJ, Ntiri-Reid B, Irvin R, et al. : Barriers to treatment access for chronic hepatitis C virus infection: A case series. Top Antivir Med 2017;25:110–113 [PMC free article] [PubMed] [Google Scholar]
- 133. Zuckerman A, Douglas A, Nwosu S, et al. : Increasing success and evolving barriers in the hepatitis C cascade of care during the direct acting antiviral era. PLoS One 2018;13:e0199174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Harris TG, Rabkin M, El-Sadr WM: Achieving the fourth 90: Healthy aging for people living with HIV. AIDS 2018;32:1563–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lazarus JV, Safreed-Harmon K, Barton SE, et al. : Beyond viral suppression of HIV-the new quality of life frontier. BMC Med 2016;14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Miners A, Phillips A, Kreif N, et al. : Health-related quality-of-life of people with HIV in the era of combination antiretroviral treatment: A cross-sectional comparison with the general population. Lancet HIV 2014;1:e32–e40 [DOI] [PubMed] [Google Scholar]
- 137. Sheikh K, Ranson MK, Gilson L: Explorations on people centredness in health systems. Health Policy Plan 2014;29 Suppl 2:ii1–ii5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Richards T, Coulter A, Wicks P: Time to deliver patient centred care. BMJ 2015;350:h530. [DOI] [PubMed] [Google Scholar]
- 139. Deeks SG, Lewin SR, Havlir DV: The end of AIDS: HIV infection as a chronic disease. Lancet 2013;382:1525–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kall M, Marcellin F, Harding R, et al. : Patient-reported outcomes to enhance person-centred HIV care. Lancet HIV 2020;7:e59–e68 [DOI] [PubMed] [Google Scholar]