Abstract
Significance: Excessive and prolonged proinflammatory responses are associated with oxidative stress, which is commonly observed during chronic tuberculosis (TB). Such condition favors tissue destruction and consequently bacterial spread. A tissue remodeling program is also triggered in chronically inflamed sites, facilitating a wide spectrum of clinical manifestations.
Recent Advances: Since persistent and exacerbated oxidative stress responses have been associated with severe pathology, a number of studies have suggested that the inhibition of this augmented stress response by improving host antioxidant status may represent a reasonable strategy to ameliorate tissue damage in TB.
Critical Issues: This review summarizes the interplay between oxidative stress, systemic inflammation and tissue remodeling, and its consequences in promoting TB disease. We emphasize the most important mechanisms associated with stress responses that contribute to the progression of TB. We also point out important host immune components that may influence the exacerbation of cellular stress and the subsequent tissue injury.
Future Directions: Further research should reveal valuable targets for host-directed therapy of TB, preventing development of severe immunopathology and disease progression. Antioxid. Redox Signal. 34, 471–485.
Keywords: tuberculosis, reactive oxygen species, oxidative stress, heme oxygenase, matrix metalloproteinase, tissue remodeling
Introduction
Mycobacterium tuberculosis (Mtb), the etiological agent of tuberculosis (TB), infects approximately a quarter of the world's population and persists as a leading cause of death by a single pathogen (136). A prolonged battle between host and pathogen emerges once infection is established in the lung. From one side, alveolar macrophages (AMs) encounter Mtb, and trigger inflammatory host responses to restrict mycobacterial growth and spread to neighboring cells and/or tissue. On the other side, Mtb subverts the host immune cell activation by modifying its metabolic and transcriptional activities to thrive (71, 94). The balance between the host immune response activation against the pathogen and Mtb evasion mechanisms results in a wide spectrum of immunopathology, ranging from asymptomatic infection to disseminated disease and ultimately patient death (22, 106, 112).
Over time, Mtb infection triggers formation of immune cell structures, granulomas, in which different myeloid cells and lymphocytes are spatially organized to restrict further mycobacterial growth and dissemination to other tissues (106). A consequence of the interplay between the host and invading Mtb is a potent antimicrobial response that generates release of free radicals as well as production of cytokines. This host response helps the eradication of Mtb, although it may lead to bystander injury by promoting an unfettered necroimmunopathology (100). The latter scenario can be aggravated depending on pathogen virulence, which is most commonly associated with failure of the host immune response to prevent ongoing Mtb replication (Fig. 1).
FIG. 1.
TB-associated tissue necrosis. Upon infection with Mtb, host macrophages undergo potent activation, which results in excessive production of ROS and several proinflammatory mediators. This response is responsible for some mycobacterial killing but also induces cell death and collateral tissue damage (immunopathology). The dead cells and stressed environment result in formation of DAMPs that amplify immune activation signals leading to a chronic cycle of persistent inflammation and tissue damage, which we call herein a necroinflammatory response. Mtb is thought to take advantage of this scenario, and disseminate to the lungs and other tissues. DAMPs, damage-associated molecular patterns; Mtb, Mycobacterium tuberculosis; ROS, reactive oxygen species; TB, tuberculosis.
Furthermore, chronic inflammation leads to an imbalance between antioxidants as well as molecules with antioxidant properties such as glutathione (GSH), ferritin, coenzyme Q10, glutathione peroxidases (GPx) and free radical production, including superoxide (O2•−), hydroxyl radical (•OH), lipid hydroperoxide, and alkoxyl radicals, with subsequent tissue destruction and Mtb proliferation (118). This enables airway dissemination of Mtb through the lung and facilitates transmission to other susceptible hosts via cough. Persistent inflammation in response to different Mtb virulence factors occurs in parallel with resultant tissue remodeling, thereby leading to a wide spectrum of clinical manifestations.
Understanding how Mtb modulates the host immune response to evade host antimycobacterial effector functions is of critical importance to facilitate development of new therapies to treat TB. At present, antimycobacterial drugs are the mainstay of therapy focused on direct mycobactericidal activity or inhibition of bacterial replication. With the worldwide emergence of drug-resistant mycobacterial strains, alternative approaches are urgently needed. Innovative therapies modulating host immune responses may offer a promising approach to mitigate the necroinflammatory response, oxidative stress, and tissue remodeling. Novel therapies targeting unfettered immunopathology may avert the development of clinically severe TB and subsequent airborne transmission.
In this review, we discuss how the interplay between systemic inflammation, oxidative stress, and tissue remodeling influences Mtb infection and development of active TB. We also examine the current literature to highlight biochemical pathways and host immune mechanisms that may be useful as new targets for host-directed therapy of TB.
Mtb Infection Drives Systemic Inflammation
Perturbations in host tissue homeostasis triggered by infectious microorganisms result in activation of immune surveillance mechanisms that promote inflammation. In TB, the proinflammatory response is thought to play a crucial role in controlling Mtb infection. However, this antimicrobial host response may be detrimental when exacerbated, leading to unfettered inflammation and subsequent severe tissue injury (118). Systemic inflammation occurs in both pulmonary and extrapulmonary TB diseases, and is marked by increased concentrations of inflammatory markers in peripheral blood, such as acute phase proteins, lipid mediators (e.g., prostaglandin E2 [PGE2]), and a number of proinflammatory cytokines as well as chemokines (132).
This significant production of cytokines/chemokines induces migration of a large number of activated leukocytes, such as neutrophils, monocytes, dendritic cells (DCs), and effector lymphocytes into the affected tissue, thereby favoring establishment of immunopathology (106). Most of these cells can also be detected in the blood vasculature, as evidenced by a strong inflammatory signature in the case of extrapulmonary TB.
When Mtb is inhaled, it lodges in the lower respiratory tract where it is phagocytosed by AMs and DCs. To evade the antimicrobial response from those mononuclear cells, Mtb blocks phagosome fusion with lysosomes by inducing host expression of coronin-1/TACO, and thus favoring proliferation inside phagosome and continuous secretion of mycobacterial products, such as 6 kDa early secretory antigenic target (ESAT-6) (26, 62, 122). Cytosolic ESAT-6 triggers host sensors recognition of an infection eliciting proinflammatory response marked by the inflammasome activation and mature interleukin (IL)-1β generation (6). Recently, Mtb-infected AMs were shown to migrate into the lung interstitium and facilitate Mtb dissemination to other immune cells in the tissue (33).
AM translocation from alveoli into lung parenchyma depends on IL-1 signaling, since IL1R−/− and MyD88−/− mice display substantial accumulation of AMs in the alveolar space. Furthermore, Mtb ESX-1 secretion system, which is required for ESAT-6 release, has been shown to play a role in AM migration into lung interstitium (33).
Interestingly, other studies have demonstrated that Mtb escapes from the phagosome into the cytosol through a mechanism dependent on ESX-1/ESAT-6, resulting in the subsequent induction of macrophage necrosis (59, 122). The exact mechanism by which Mtb escapes from the phagosome into the cytosol is not yet well understood. One possible mechanism for Mtb escape is through a pore formation on the phagosomal membrane mediated by ESAT-6, since this Mtb protein has been described to exhibit pore-forming capability (74).
The presence of Mtb as well as its ESAT-6 antigen triggers activation of several molecular pathways, including the NLRP3-inflammasome assembly required for cleavage of pro-IL-1β (6), an essential cytokine for host defense against Mtb (85, 86). These findings suggest a possible role of mycobacterial escape into cytosol and IL-1β production by the NLRP3-inflammasome, promoting AM translocation into lung parenchyma and further mycobacterial dissemination.
A wide spectrum of individual lesions is formed in the lungs that vary from a solid structure to necrotic, caseous tubercles, and ultimately cavitary granulomas. Different stages of granuloma progression are concurrently apparent during active TB. Granuloma formation begins with an aggregate and ill-defined mass of immune cells resulting from cell recruitment to the site of infection, becoming more organized over time. The granuloma is composed of a macrophage-enriched center, which later differentiates into specialized cell types including multinucleated giant cells and epithelioid macrophages, surrounded by B and T cells, creating an efficient bacterial containment barrier to prevent dissemination of infection (22, 100, 106).
Tumor necrosis factor alpha (TNF-α) and IL-12, produced early in Mtb infection by antigen-presenting cells, play a critical role in the establishment of a Mtb-specific adaptative immune response and granuloma formation. Interferon (IFN)-γ, which is initially produced by natural killer cells during the early stage of infection, is subsequently enhanced secondary to expansion and activation of a Mtb-specific host adaptative immune response. IFN-γ plays an essential role in the host defense against Mtb infection by potentiating macrophage ability to phagocytose and kill the pathogen (94).
Mice deficient in IFN-γ production are extremely susceptible to mycobacterial infection (103). Recently, the production of this protective cytokine has shown to be regulated by adenosine receptors (A1, A2a, A2b, and A3), as a result of extracellular ATP degradation by ectonucleotidases that are enhanced during massive lung destruction caused by hypervirulent mycobacterial strains (5). The optimal generation of IFN-γ will determine the course of TB pathogenesis, in which either low or inappropriately high production of this cytokine is detrimental for the host (13, 14).
Interestingly, a study combining human disease and experimental models with nonhuman primates reported that the heterogeneity of granulomas may impact the extent of immune response and efficiency in controlling Mtb infection (130). A recent study has demonstrated that the immune response against Mtb varies in anatomically distinct compartments within granulomas through sophisticated high-resolution imaging and mass spectrometry imaging.
Proinflammatory enzymes responsible for the generation of lipid-derived inflammatory factors, such as eicosanoids, are highly expressed both in necrotic centers and in cells bordering the caseum. Moreover, high concentrations of eicosanoid precursors were found within the granuloma when compared with normal lung tissue, with marked accumulation of these mediators at the border of the caseum. Enhanced expression of leukotriene A4 hydrolase and lipoxygenases has also been observed at the caseous granuloma when compared with solid granulomas, suggesting increased generation of inflammatory eicosanoids, such as leukotriene B4 and lipoxins (81). In the following section, we discuss the implications of inflammatory eicosanoids and their generation/modulation on TB pathogenesis.
Eicosanoids are lipid mediators derived from the enzymatic or nonenzymatic oxidation of arachidonic acid, including prostaglandins, resolvins, lipoxins, and leukotrienes, which play an important role in regulating the immune response (116).
Two groups of enzymes, cyclooxygenases (COXs) and lipoxygenases, compete with each other for arachidonic acid generating prostaglandins, lipoxins, and leukotrienes. The latter two are bioproducts exclusively resulting from lipoxygenase activity. Mice deficient in 5-lipoxygenase are more resistant to Mtb infection, whereas prostaglandin E synthase-deficient mice display high susceptibility to infection (12, 29, 40). It is possible that lipid mediators may differentially regulate host protection against Mtb by interfering directly or indirectly with the regulation of programmed cell death in infected macrophages, but this issue is still not fully understood (15, 85, 128).
In infected macrophages, lipoxin A4 (LXA4) has a deleterious effect on host Mtb containment, promoting macrophage necrosis and Mtb dissemination into adjacent cells. Conversely, PGE2 appears to prevent cell necrosis and simultaneously stimulate apoptosis in Mtb-infected macrophages, thereby containing mycobacteria and reducing bacillary burden through a process called efferocytosis, in which apoptotic bodies are engulfed and removed from the milieu by neighboring tissue cells. Efferocytosis of Mtb trapped within an apoptotic body delivers it to the lysosomal compartment, where the pathogen will be killed (12, 29, 64, 83, 87, 97, 132, 138).
Mycobacterial virulence factors were shown to significantly influence the generation of different lipid mediators, in which LXA4 is provoked by virulent Mtb strains, whereas PGE2 was revealed to be triggered by avirulent strains (40). Interestingly, Mtb-infected mice genetically lacking IL-1 receptor signaling have been shown to display a profound reduction of PGE2 generation along with increased levels of LXA4 and LTB4 (both products of 5-LO) in the bronchoalveolar lavage fluid (BALF) (85).
In humans, plasma levels of prostaglandins and lipoxins are both higher in patients with active TB than in uninfected individuals (97, 132). Besides the role of PGE2 in inducing apoptosis of Mtb-infected macrophages, this lipid mediator has been reported to trigger several tissue remodeling enzymes such as the extracellular matrix (ECM) metalloproteinase-1 (MMP-1), highlighting the role of PGE2 in tissue repair during TB (49).
Leukocytes generate reactive oxygen species (ROS) such as hydrogen peroxides and nitric oxide to control Mtb growth in the hyperinflammatory environment of the granuloma (81, 118). To prevent collateral cellular damage from excessive ROS, infected and activated cells concurrently induce production of antioxidants and transcription of antioxidant enzymes.
With persistence of infection, when excessive or sustained ROS production overwhelms the available antioxidant defense systems, key molecules are denatured, with subsequent dampening of immune cellular functions, leading to cell damage and death (Fig. 2) (11, 118, 133). This inefficient control of excessive ROS-mediated toxicity occurs due to reduced activity of endogenous antioxidative enzymes and reduced intake or absorption of antioxidants usually obtained from the diet (104). Mtb thrives in this proinflammatory environment and increases its metabolic activity leading to unrestrained proliferation and augmentation of mycobacterial burden.
FIG. 2.
Excessive cellular stress in Mtb-infected macrophages. In conditions of low mycobacterial loads, infected macrophages fully preserve its effector functions, such as production of ROS and inflammatory mediators, and the capacity to present antigen to T cells via MHC class II. This scenario is associated with restrain of mycobacterial growth and induction of the Mtb metabolic dormancy. With increasing mycobacterial replication, excessive activation leads to oxidative stress of infected cells, affecting key molecules that account for cellular integrity. With defective effector functions, the infected macrophage now becomes unable to restrain Mtb growth, leading to cell death and pathogen replication and dissemination.
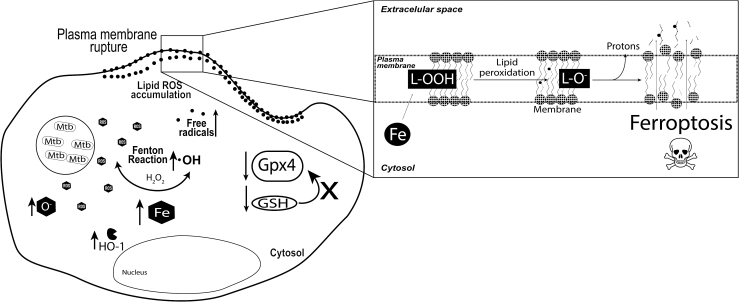
We have recently shown that increased Mtb replication in macrophages is associated with intracellular iron overload and cell death that facilitates Mtb dissemination (Fig. 3) (4). Moreover, when ferrostatin-1, a drug known to prevent lipid peroxidation (41), is used to treat both macrophages in vitro and Mtb-infected mice in vivo it is possible to detect a drastic suppression in macrophage death, tissue necrosis, and reduced Mtb loads in lungs of infected mice (4). Interestingly, several reports have associated vitamin E deficiency, an antioxidant that dampens lipid ROS (41), with increased host susceptibility and disease severity, supporting the idea that the loss of antioxidant response and excessive ROS generation are both detrimental to the host (2, 38, 70, 75, 96, 131).
FIG. 3.
Mtb infection leads to death of infected macrophages via ferroptosis. With progression of infection inside macrophages, Mtb leads to significant ROS production. The consequences of such ROS accumulation are several: (i) excessive ROS lead to induction of HO-1, an enzyme that catabolizes heme and releases free iron inside the cell; (ii) there is a substantial accumulation of intracellular free iron, which further increases the oxidative stress; and (iii) a decrease in GSH and GPX4 is observed, which possibly dampens the capacity of the infected cell to fight against ROS accumulation. Iron is highly reactive and amplifies the oxidative stress via Fenton's reaction. Excessive ROS cause rupture of the plasma membrane through lipid peroxidation, leading cells to die through and iron-dependent cell death named ferroptosis. GPX4, glutathione peroxidase 4; GSH, glutathione; HO-1, heme oxygenase-1.
Thus, manipulation of oxidative stress may potentially serve as an adjunct therapy for TB, when added to conventional long-term treatment. In support of this approach, a body of clinical studies has shown that patients given vitamin E (a major fat-soluble antioxidant that scavenges peroxyl radicals and dismisses the oxidation of polyunsaturated fatty acids [PUFAs]), selenium [a micronutrient important for the function of some antioxidant enzymes, such as Gpx4 (61)], and/or N-acetylcysteine [a precursor of GSH, an important antioxidant (3)] as adjunct to Mtb antibiotics demonstrated improved treatment outcomes when compared with those receiving placebo, which was coincident with an improved immune response against Mtb (23, 25, 55, 60, 65, 76, 117).
Along with the detrimental effect of excessive ROS production, type I IFNs (IFN-α and IFN-β) have been shown to promote Mtb infection. In addition to well-characterized antiviral effects, type I IFN response generates proinflammatory immune signatures in the context of active TB infection (17). Type I IFN response promotes unrestrained inflammation-driven tissue damage in mice and patients with more severe forms of TB (97, 115, 132). Absence of type I IFN signaling in ifnar-deficient mice results in increased PGE2 and IL-1β production in BALF, suggesting that type I IFNs antagonize the protective effect of the IL-1 pathway during Mtb infection (84).
We have recently demonstrated that the overall disturbances in cytokine plasma levels are heavily influenced by IFN-α, the signal of which has been found elevated in blood from TB patients (97). These findings argue that type I IFNs act to downregulate protective host immune functions against Mtb.
IL-1 has an important role in host resistance against Mtb through an inflammatory cascade that stimulates PGE2 production via a pathway requiring COX-2, as described previously (85). Aberrant levels of this cytokine, however, may be detrimental to host defense control of Mtb infection by increasing tissue damage (90). Specifically, elevated production of IL-1β is associated with more extensive radiographic disease (27, 134) as well as with larger cavitary lesions (7, 32, 120, 129). Furthermore, IL-1 is involved in fibroblast activation (19) and recruitment of neutrophils, which has been in turn shown to augment tissue damage, promoting loss of pulmonary function and host death (16, 17, 51, 72, 89, 123).
As a major modulator of inflammation, IL-10 is a potent cytokine in modulating exaggerated antimycobacterial immune responses. The induction of IL-10 production during infection inhibits macrophage functions and suppresses proinflammatory cytokine production including TNF-α, another cytokine required for optimal granuloma formation (1, 94). Interestingly, TNF-α blockade has been shown to facilitate granuloma disintegration and thus promote Mtb dissemination (28, 44, 56, 121). IL-10 production occurs within the granuloma and may also facilitate mycobacterial persistence by preventing Mtb-phagosome maturation in macrophages (35). In contrast, production of IL-10 by B cells may aid counterbalancing of chronic inflammation in the lungs of those with more advanced stages of TB disease characterized by intense production of ROS (108).
The complex interplay between cytokines, chemokines, and lipid mediators promotes loss of optimal regulation of the inflammatory cascade. This chronic process leads to cell stress, inducing deregulation in the redox reactions (imbalance of oxidant and antioxidant products) affecting tissue remodeling response, thereby culminating in the destruction of pulmonary parenchyma.
Tissue Remodeling in Pulmonary TB
TB initially triggers an intense inflammatory response that in most cases is followed by a chronic process of inflammation mediated by numerous cell types, proinflammatory cytokines, and chemokines. This inflammatory response results in significant tissue remodeling of the ECM with destruction of pulmonary parenchyma leading to bronchiectasis, restrictive and obstructive lung disease (109). How Mtb promotes development of cavitary lesions is not completely understood. Mycobacterial virulence factors are directly responsible for significant tissue remodeling, reinforcing the perception that Mtb needs to promote ECM disruption to disseminate.
Tissue remodeling is a physiological process initiated after cellular damage that aims to restore tissue function. Intracellular components released into the extracellular milieu trigger immune responses promoting the recruitment of cell types such as neutrophils and macrophages into the tissue parenchyma. After the initial stage, a nonspecific type of collagen is deposited in the tissue being repaired, which is replaced later by tissue-specific collagen in a slow and gradual process, involving the organization of collagen fibers. This process is necessary for repairing damaged ECM in response to innumerable infectious and noninfectious pulmonary diseases, including pneumonia, chronic obstructive pulmonary disease, sarcoidosis, and acute respiratory distress syndrome (49).
Through immune evasion mechanisms, Mtb begins its struggle to survive in the midst of the host immune system resulting in dysregulated production of cytokines, chemokines, and lipid mediators. Chronic persistence of Mtb leads to a host response that increases bystander tissue destruction involving MMPs, cathepsins, and kallikreins, which outpaces collagen deposition, TIMPs (metalloproteinase inhibitors), and other protease inhibitors, resulting in tissue remodeling. Elucidation of this process is crucial to understanding TB immunopathology and may highlight novel adjunctive treatment of pulmonary TB (46).
In particular, cathepsins play an important role in bone, cartilage, and collagen processing, with cathepsin K being the most studied. Cathepsin K has the capacity to cleave collagen triple helix, and its activity differs from MMPs. Generally, pathological collagen degradation is associated with high levels of cathepsin K (66, 139). Other cathepsins such as cathepsins B, L, H, and S have been identified as potential targets for tissue remodeling based on in vitro studies (135), although there is no evidence of involvement of these cathepsins in tissue remodeling in animal models of TB.
In the lungs, cathepsin K is highly expressed by pulmonary epithelial cells as well as AMs, playing an important role in remodeling the ECM (20, 21). This finding is supported by studies that found cathepsin B and K upregulation in serum/plasma of pulmonary TB patients (6, 66). Employing an animal model of lung cavitation, Kubler et al. infected rabbits with a virulent Mtb strain and evaluated levels of cathepsin K as well as MMPs. Upregulation of several MMPs including MMP-1, MMP-13, MMP-14 as well as cathepsin K was found in the lungs of Mtb-infected animals (66). These findings suggest that inhibition of MMPs and cathepsin K may serve as a potential target for adjuvant therapies in TB as blockade of these enzymes may reduce pulmonary pathology.
MMPs are a family of proteases (which can be associated with either zinc or calcium) that are able to degrade all components of the ECM. Moreover, only certain MMPs are capable of cleaving lung fibrillar collagens at neutral pH, which facilitates tissue remodeling (45, 47). MMPs can be classified on the basis of their substrate specificity into different subfamilies, namely collagenases (MMP-1, MMP-8, and MMP-13), gelatinases (MMP-2 and MMP-9), stromelysins (MMP-3, MMP-10, and MMP-11), elastases (MMP-7 and MMP-12), and membrane-type MMP (MMP-14, MMP-15, MMP-16, and MMP-17) (111).
In the lungs, MMP-1 (known as interstitial collagenase), MMP-8 (referred to as neutrophil collagenase), MMP-13 (collagenase 3), and MMP-14 play important roles in cleaving the primary architectural collagen in the tissue parenchyma (43). Most MMPs are tightly regulated requiring gene transcription induction before their secretion, except MMP-8 and MMP-9 in neutrophils. The majority of MMPs are not expressed in healthy tissues, with their expression being observed only in inflamed tissues, or in those undergoing repair or remodeling (102). These enzymes are usually secreted by monocytes-derived cells, neutrophils, and stromal cells (98).
Epithelial cells in the lungs are a significant source of MMPs since they express MMP-1, MMP-2, MMP-7, and MMP-9. In addition, highly differentiated macrophages express a broader profile of MMPs than monocytes. Under all circumstances, cytokines, exogenous stimuli, and cell–cell contact are required for MMP expression (24). MMPs have several physiological functions in development, reproduction, maintenance of homeostasis as well as facilitation of cell migration, cleavage of cytokines and activation of defensins.
In TB, exaggerated generation of mainly MMP1 is associated with progression of consolidated regions to cavities with high bacterial loads as demonstrated in a rabbit model of pulmonary TB cavitation (67). In addition, reduced level of a metallopeptidase inhibitor TIMP-1 is found in consolidated areas, leading to a potent activity of metalloproteinases and promoting tissue destruction (48, 67). Several studies have reported significant increase in MMP-1 levels in blood of TB patients (8, 67), although the exact mechanism by which this happens is poorly understood.
Interestingly, MMP-1 has been shown to be regulated by mitogen-activated protein kinase (MAPK) signaling in human macrophages after Mtb infection (8, 49, 95, 107). In addition, a number of studies have shown that increased MMP-1 levels are critical for cavitation in TB pathogenesis (45). It has been shown that mice do not express MMP-1, impeding in vivo study on how this enzyme contributes to TB pathogenesis. Of note, murine granulomas rarely progress to necrosis as observed in humans, suggesting that expression of MMPs, such as MMP-1, might be important for tissue damage induction. This issue was addressed by infecting transgenic mice overexpressing human MMP-1 with virulent Mtb. The upregulation of this enzyme has been shown to be detrimental via significant destruction of alveolar walls and lung parenchyma, through enzymatic collagen degradation (45).
Furthermore, Mtb-infected mice deficient in MMP-9 display reduced macrophage migration into the lung and defective granuloma formation, while being counterintuitively more resistant to infection (126). Elevated migration of neutrophils is also observed during early stages of Mtb infection in mice and humans, with the corresponding high MMP-9 levels after infection in both host species (57). Mtb promotes MMP activity and collagen degradation by eliciting cytokines such as TNF-α, which was shown previously to augment levels of MMP-1 as well as MMP-10 and MMP-3, thus propagating collagenase activity and causing tissue damage. Interestingly, active TB patients display higher levels of both heme oxygenase-1 (HO-1) and MMP-1 in plasma compared with those with latent disease (8).
HO-1 cleaves heme, which is generally obtained from heme-containing proteins, and generates three by-products such as biliverdin, carbon monoxide (CO), and free iron. HO-1 is induced in Mtb-infected macrophages by a mechanism associated with ROS production and increased activity of NRF-2 (Fig. 4) (110). Human monocyte-derived macrophages, when exposed to Mtb or intracellular ESAT-6, were shown to upregulate HO-1 and simultaneously downregulate MMP-1. Inhibition of MMP-1 is associated with CO production as a result of HO-1 activity (8). However, free iron, another by-product of HO-1 activity, was shown to trigger high levels of MMP-9 through activation of AP-1/ERK/AKT pathway (63), but did not impact induction of MMP-1 (8, 63). Notably, increased cellular iron was associated with enhanced secretion of both MMP-9 and MMP-1 (79).
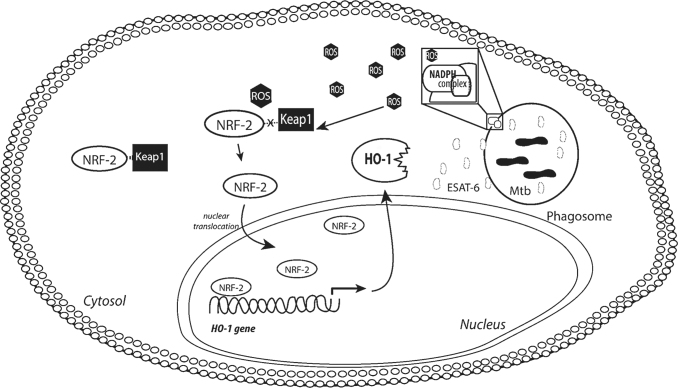
FIG. 4.
Induction of HO-1 in Mtb-infected macrophages. HO-1 is a key antioxidant enzyme, which is produced in response to cellular stress. In steady state conditions, the HO-1 transcription factor NRF-2 is not found at higher concentrations in the nucleus, and is rather in the cytosol, where it interacts with the molecule Keap-1, which leads to proteasome-mediated degradation. Inside macrophages, Mtb induces HO-1 through a mechanism dependent on the release of the virulence factor ESAT-6. ESAT-6 is secreted by Mtb inside the phagolysosome and creates pores in the membrane, leaking to the cytosol. This process leads to potent activation of the NADPH oxidase complex (subunits p22phox and gp91phox) and results in significant ROS production, including superoxide and hydrogen peroxides. This accumulated ROS interferes the interaction between NRF-2 and Keap-1, releasing NRF-2 to be translocated to the nucleus and to induce HO-1 transcription. ESAT-6, 6 kDa early secretory antigenic target; NADPH, nicotinamide adenine dinucleotide phosphate; NRF-2, nuclear factor erythroid 2-related factor 2.
With recruitment of inflammatory cells and their subsequent death as a result of cell stress, caseous necrosis appears within days after Mtb infection, creating an optimal environment for bacterial proliferation. Granuloma formation is an effective way to contain Mtb proliferation and with an appropriate host immune response maintain an intact ECM, thereby preventing TB transmission by infected individuals. However, when an imbalance between MMP-1 and TIMPs occurs, the granuloma progresses to instability, facilitating bacterial dissemination through tissue as well as between individuals (98). MMP inhibitors have been shown to enhance both delivery and/or retention of TB drugs in the lungs resulting in improved drug efficacy (137). In contrast, the inhibition of MMP-7 in mice has been associated with more severe pathology (99), suggesting a different role for MMPs in regulating host resistance against Mtb.
Oxidative Stress Mechanisms in Pulmonary TB
Oxidative stress results from the imbalance of total oxidant over total antioxidant status. After Mtb infection, the first immune response is characterized by significant production of oxidants by AMs (18), a critical process for the initial destruction of Mtb. During the initial stages of infection, immune cells, primarily macrophages, produce ROS to promote the death of Mtb. Under physiological conditions, high levels of ROS occur concurrent with increased production of antioxidants to neutralize potentially harmful effects of ROS in tissues (Fig. 5A). The sensitive balance between antioxidant and oxidants is the key of tissue homeostasis and, when disturbed, results in irreversible cell damage with pathological consequences.
FIG. 5.
Oxidative stress during infection. In a balanced response found when a pathogen is eliminated by infected cells (A), initially there is a substantial increase in ROS generation, followed by a counter-response characterized by increased production of antioxidant molecules, which prevents excessive stress and damage. When a pathogen persists, the ROS production may increase incessantly over time. In this setting, the antioxidant responses could either fail to increase proportionally (B) or be insufficient to dampen the pathological oxidation regardless of its induction capacity (C). The persistent and unbalanced accumulation of ROS is called oxidative stress, and has important deleterious consequences in cell metabolism and viability.
While initial infection with Mtb triggers intense production of ROS, in certain situations there is no commensurate and proportional increase in antioxidants, thereby leading to an imbalance between ROS and antioxidants in the tissue (Fig. 5B). Through its continuous interaction with the host immune system, Mtb triggers progressive increases in ROS production by macrophages that culminate in excessive oxidation and lipid peroxidation (Fig. 5C) (3, 4). Human monocyte-derived macrophages infected with Mtb in vitro exhibit increased lipid peroxidation, DNA oxidation, and more frequent cell death resulting from dramatic accumulation of intracellular ROS (3, 4, 110).
Via an unknown mechanism, macrophages lose their primary antioxidant enzymatic functions involved in the regulation of lipid peroxide accumulation, resulting in increased pathogen burden and proliferation. Excessive macrophage stress leads to DNA peroxidation, which, consequently, downregulates cellular metabolism resulting in a cascade of alterations that increases ROS generation along with reduction of antioxidants, culminating in significant tissue damage and pathogen dissemination.
Peroxidation of lipids is a natural process that occurs in steady state in small proportion in the body mainly by the effect of several ROS, including hydroxyl radical, hydrogen peroxide, and others. These ROS attack most often the PUFAs initiating a self-propagating chain reaction. In brief, lipid peroxidation is initiated by the hydrogen abstraction or addition of an oxygen radical causing a rearrangement of the double bonds in polyunsaturated lipids, which eventually culminates in destruction of membrane lipids. Due to the presence of methylene CH2-groups, which contain hydrogen that is particularly reactive to ROS, PUFAs are considered to be more sensitive than saturated lipids to this peroxidation process (34).
Several by-products such as malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE) are generated during homolytic decomposition of lipid hydroperoxides in this complex process. The accumulation of these by-products has severe consequences for cell function. Both MDA and 4-HNE have mutagenicity properties due to their high ability to form DNA-adducts (82). As mentioned above, failure of the natural biological membrane repair leads to destruction of these cellular membranes, favoring the accumulation of lipid peroxides (lipid ROS), which are dangerous for cellular and tissue integrity. Interestingly, high levels of lipid peroxidation by-products have been reported in several noninfectious maladies, including Alzheimer's disease, renal failure, hepatotoxicity, fibrosis as well as infectious diseases such as HIV/AIDS, malaria, and TB (3, 39, 91, 114).
HO-1 is an antioxidant enzyme highly expressed in lung tissue that functions as a key stress-response component involved in degradation of heme molecules resulting in generation of three by-products free iron, CO, and biliverdin, as mentioned previously (30, 36, 119, 127). HO-1 displays a dual role in the host stress response as it may work as an anti-inflammatory component that improves cell viability through generation of two important antioxidant molecules, CO and biliverdin, or may conversely augment oxidative stress, induce cell death, and stimulate pathogen growth by releasing free iron from heme (4, 53). HO-1 has emerged as an important mediator in several diseases, such as malaria (88), leishmaniasis (73), TB (8, 30, 36, 110, 119), and sepsis (113), being frequently associated with severity of disease (9).
As mentioned above, Mtb drives production and secretion of HO-1 and oxidant factors, such as MMPs (8). HO-1 and MMP-1 are differentially regulated in infected macrophages as a result of different inflammatory responses leading to distinct clinical presentations, and the balance between these molecules influences the extent of lung lesions and bacterial loads. Mtb infection is associated with substantial increase in total oxidation status in pulmonary TB patients when compared with those with latent infection and uninfected controls (3, 8). HO-1 has also been implicated in the promotion of lipid peroxidation by liberating free iron and thus regulating ferroptotic cell death (68, 80). Cells overexpressing HO-1 undergo ferroptosis, whereas cells deficient in HO-1 display increased resistance to this form of necrotic cell death in several in vitro models (68). How HO-1 is involved in the induction of lipid ROS as well as cell death triggered by Mtb remains unclear.
Lungs are continually exposed to high levels of free radicals under stress conditions. Excessive production of ROS has been highly associated with the oxidation of proteins, DNA, and lipids, causing direct lung injury. Biological membrane lipids are highly susceptible to free radical damage, which is detrimental to cell functions and viability. Lung functions are significantly impaired by accumulation of free radicals. Initial oxidation of few lipid molecules is sufficient to result in significant tissue damage, as lipid peroxidation is a self-propagating chain reaction. Interestingly, lipid ROS are also involved in the induction of MMP-1 expression (105).
The depletion of GSH, an important antioxidant, triggers the accumulation of ROS along with increased levels of MMP-1. Inhibition of lipid peroxidation through treatment of cells with Trolox (a potent antioxidant) and by free iron chelation reduced the expression of MMP-1 in vitro (105). Despite extensive efforts in the field of lipid peroxidation, it is not clear whether lipid peroxide accumulation is the cause or consequence of pathological conditions. It seems that tissue damage is initiated by excessive ROS production, and thus contributes to the generation of several MMPs culminating in ECM degradation, which in turn amplifies lipid ROS accumulation as well as widespread necrosis. Since MMPs, HO-1, and lipid peroxidation are all associated with severe TB disease (10, 30, 36), it is reasonable to consider the prevention of excessive ROS production through augmentation of host antioxidant response as a target for host-directed therapy in TB.
Interplay Between Oxidative Stress and Tissue Remodeling: Therapeutic Perspectives
Despite the fact that Mtb infects humans over millennia, and that intense research toward identification of new therapies has been done, TB treatment remains ineffective, with multidrug therapy required for long periods focused on halting replication and driving Mtb death. This treatment strategy has limitations with ongoing significant mortality resulting from TB disease and emergence of resistant strains. Alternative and adjunct therapies are thus needed against Mtb infection. A thorough understanding of the three main drivers of pathology in TB, as shown in this review, highlights areas for new therapeutic strategies that target aberrant host responses to Mtb infection, an approach that will complement pathogen death by reducing pulmonary injury.
Several studies have associated oxidative stress with various lung diseases, such as chronic obstructive pulmonary diseases, asthma, acute pulmonary distress syndrome, and TB (93). Prior clinical trials have evaluated the efficacy of therapies aiming to dampen inflammation in TB through decreased ROS secretion and tissue remodeling in pulmonary TB, focused primarily on steroids that have been used as adjunctive treatment for several decades (37, 42, 123) to reduce IFN-γ, TNF-α, and IL-1β (77). Dampening inflammation through reduction of proinflammatory cytokines may impact the ability of immune cells to control Mtb infection.
Although ROS production is important for pathogen death, it can also trigger a series of oxidative reactions of PUFAs on host cells inducing cell death by accumulating lipid peroxides on biological membranes (4, 50). High levels of lipid peroxidation have been reported in patients with pulmonary TB compared with healthy individuals (3, 52). As mentioned previously, lipid peroxides can also induce MMPs, which in turn promote ECM degradation. Thus, it seems that targeting excess generation of ROS is a promising therapeutic strategy for TB, since overproduction of these molecules initiates a series of chemical reactions and causes damage to cellular components and excessive immune response activation by interfering transcription factors, kinases activation, and expression of proinflammatory mediators (31, 58, 92).
Convergence of these pathological pathways during TB disease promotes oxidative stress and tissue remodeling through mediation of the immune response. MMP activity is balanced by TIMP function, while other mechanisms including the prostaglandin signaling cascade and extracellular signal-regulated kinase (ERK) MAPKs also regulate MMP-1. In this context, p-aminosalicylic acid (PAS) has been shown to be effective as one of the first treatments for TB. Despite the absence of a well-understood mechanism of action, PAS is now known to immunomodulate PGE2 production, suppressing the release of MMP1 (107). These findings point PAS as a promising novel approach to adjunctly treat TB.
Similarly, doxycycline has been shown to modulate MMP secretion, primarily MMP-1 and MMP-3, in Mtb-infected primary human macrophages at 72 h in a dose-dependent manner as well as in bronchial epithelial cells (134). Moreover, doxycycline suppresses TNF-α secretion by macrophages, downregulating host inflammation in response to Mtb infection (134). Concurrent with this anti-inflammatory function, doxycycline has a direct bacteriostatic action against Mtb. By acting on multiple pathways involved in TB pathogenesis, doxycycline may serve as a potential adjunctive drug to combat TB and reduce tissue complications related to excessive inflammation. Interestingly, several studies have shown that broad spectrum inhibition of MMPs improves TB drug treatment and also reduces tissue damage in different animal models (78, 125). To date, there is one clinical trial investigating the effect of doxycycline in the modulation of tissue destruction in pulmonary TB patients (retrieved from clinicaltrials.gov ID: NCT02774993).
Statins are widely used as inhibitors of cholesterol biosynthesis. It is known that these drugs have broad anti-inflammatory effects, such as preventing excessive ROS generation and also inducing autophagy in vitro, a host strategy for eliminating the pathogen without triggering a necroinflammatory response (54, 101). Of note, there is evidence of an association between statin use and reduced risk of developing active pulmonary TB (69, 124). In murine models of pulmonary disease, statin treatment reduces lung pathology. Nevertheless, there is still no direct evidence that this drug prevents tissue damage in TB patients. Thus far, there are two clinical trials of statin use as adjunctive therapy for TB and TB-IRIS (retrieved from clinicaltrials.gov ID: NCT03882177 and ID: NCT03456102).
Conclusions
TB infection has persisted as a significant cause of morbidity and mortality despite the availability of antimicrobials that aim to inhibit replication or cause mycobacterial death. While much research to date has focused on novel antimycobacterial therapies to address emerging drug resistance, it is now clear that a shift in focus is needed to modulate aberrant host responses. Emerging pathways involved in tissue remodeling, excess lipid ROS generation, and systemic inflammation offer novel targets for adjunctive therapies to temper host immune responses. Methods to dampen systemic inflammation must be balanced against the need for a robust initial immune response driven by infected macrophages to promote granuloma formation and Mtb containment (Fig. 6).
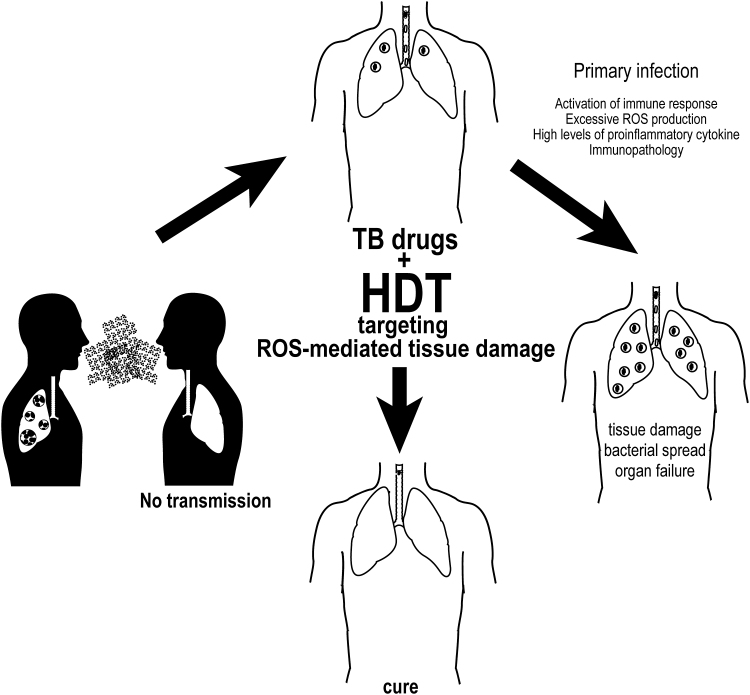
FIG. 6.
HDTs focused on control of oxidative stress may control TB. To be transmitted, Mtb requires lung tissue damage and cough. Targeting key processes, such as unbalanced oxidative responses, may result in decreased loss of cellular metabolism and better preservation of effector functions as well as in diminished cell death and dampened tissue damage. Together with antimicrobial drugs, such therapy may optimize treatment by reducing its length, by promoting better pathogen clearance, and by minimizing chances of residual tissue damage after treatment. Novel clinical trials testing HDTs are warranted to test this hypothesis. HDTs, host-directed therapies.
Prolonged macrophage activation in the course of chronic infection leads to imbalance of oxidants and antioxidants. Existing therapies including PAS and doxycycline, in addition to systemic steroids, are currently available options that may modulate the host immune response via inhibition of MMP-1 activity in chronic Mtb infection. Moreover, novel therapies that target inflammatory pathways early in infection may represent an effective strategy to avoid disease progression and tissue damage.
Abbreviations Used
- •OH
hydroxyl radical
- 4-HNE
4-hydroxy-2-nonenal
- 5-LO
5-lipoxygenase
- AKT
serine/threonine kinase 1
- AM
alveolar macrophage
- AP-1
activator protein 1
- ATP
adenosine triphosphate
- BALF
bronchoalveolar lavage fluid
- CO
carbon monoxide
- COX
cyclooxygenase
- DAMPs
damage-associated molecular patterns
- DCs
dendritic cells
- ECM
extracellular matrix
- ERK
extracellular signal-regulated kinase
- ESAT-6
6 kDa early secretory antigenic target
- ESX-1
Mtb secretion system
- GPx
glutathione peroxidases
- GPX4
glutathione peroxidase 4
- GSH
glutathione
- HO-1
heme oxygenase-1
- IFN
interferon
- IL
interleukin
- IRIS
immune reconstitution inflammatory syndrome
- LTB4
leukotriene B4
- LXA4
lipoxin A4
- MAPK
mitogen-activated protein kinase
- MDA
malondialdehyde
- MMP
matrix metalloproteinase
- Mtb
Mycobacterium tuberculosis
- MyD88
myeloid differentiation primary response 88
- NADPH
nicotinamide adenine dinucleotide phosphate
- NLRP3
NACHT, LRR and PYD domains-containing protein 3
- NRF-2
nuclear factor erythroid 2-related factor-2
- O2•−
superoxide
- PAS
p-aminosalicylic acid
- PGE2
prostaglandin E2
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- TB
tuberculosis
- TIMP
tissue inhibitor of metalloproteinase
- TNF-α
tumor necrosis factor alpha
Funding Information
No funding was received for this article.
References
- 1. Abdalla AE, Lambert N, Duan X, and Xie J. Interleukin-10 family and tuberculosis: an old story renewed. Int J Biol Sci 12: 710–717, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aibana O, Franke MF, Huang CC, Galea JT, Calderon R, Zhang Z, Becerra MC, Smith ER, Contreras C, Yataco R, Lecca L, Murray MB. and Vitamin E status is inversely associated with risk of incident tuberculosis disease among household contacts. J Nutr 148: 56–62, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amaral EP, Conceicao EL, Costa DL, Rocha MS, Marinho JM, Cordeiro-Santos M, D'Imperio-Lima MR, Barbosa T, Sher A, and Andrade BB. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol 16: 251, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amaral EP, Costa DL, Namasivayam S, Riteau N, Kamenyeva O, Mittereder L, Mayer-Barber KD, Andrade BB, and Sher A. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J Exp Med 216: 556–570, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amaral EP, Machado de Salles E, Barbosa Bomfim CC, Salgado RM, Almeida FM, de Souza PC, Alvarez JM, Hirata MH, Lasunskaia EB, and D'Imperio-Lima MR. Inhibiting adenosine receptor signaling promotes accumulation of effector CD4+ T cells in the lung parenchyma during severe tuberculosis. J Infect Dis 219: 964–974, 2019 [DOI] [PubMed] [Google Scholar]
- 6. Amaral EP, Riteau N, Moayeri M, Maier N, Mayer-Barber KD, Pereira RM, Lage SL, Kubler A, Bishai WR, D'Imperio-Lima MR, Sher A, and Andrade BB. Lysosomal cathepsin release is required for NLRP3-inflammasome activation by Mycobacterium tuberculosis in infected macrophages. Front Immunol 9: 1427, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ameglio F, Casarini M, Capoluongo E, Mattia P, Puglisi G, and Giosue S. Post-treatment changes of six cytokines in active pulmonary tuberculosis: differences between patients with stable or increased fibrosis. Int J Tuberc Lung Dis 9: 98–104, 2005 [PubMed] [Google Scholar]
- 8. Andrade BB, Pavan Kumar N, Amaral EP, Riteau N, Mayer-Barber KD, Tosh KW, Maier N, Conceicao EL, Kubler A, Sridhar R, Banurekha VV, Jawahar MS, Barbosa T, Manganiello VC, Moss J, Fontana JR, Marciano BE, Sampaio EP, Olivier KN, Holland SM, Jackson SH, Moayeri M, Leppla S, Sereti I, Barber DL, Nutman TB, Babu S, and Sher A. Heme oxygenase-1 regulation of matrix metalloproteinase-1 expression underlies distinct disease profiles in tuberculosis. J Immunol 195: 2763–2773, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrade BB, Pavan Kumar N, Mayer-Barber KD, Barber DL, Sridhar R, Rekha VV, Jawahar MS, Nutman TB, Sher A, and Babu S. Plasma heme oxygenase-1 levels distinguish latent or successfully treated human tuberculosis from active disease. PLoS One 8: e62618, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andrade BB, Pavan Kumar N, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Sher A, and Babu S. Heightened plasma levels of heme oxygenase-1 and tissue inhibitor of metalloproteinase-4 as well as elevated peripheral neutrophil counts are associated with TB-diabetes comorbidity. Chest 145: 1244–1254, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anthony D, Papanicolaou A, Wang H, Seow HJ, To EE, Yatmaz S, Anderson GP, Wijburg O, Selemidis S, Vlahos R, and Bozinovski S. Excessive reactive oxygen species inhibit IL-17A(+) gammadelta T cells and innate cellular responses to bacterial lung infection. Antioxid Redox Signal 32: 943–956, 2020 [DOI] [PubMed] [Google Scholar]
- 12. Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, and Aliberti J. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest 115: 1601–1606, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, and Sher A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol 186: 1598–1607, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barber DL, Sakai S, Kudchadkar RR, Fling SP, Day TA, Vergara JA, Ashkin D, Cheng JH, Lundgren LM, Raabe VN, Kraft CS, Nieva JJ, Cheever MA, Nghiem PT, and Sharon E. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci Transl Med 11: eaat2702, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Behar SM, Divangahi M, and Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol 8: 668–674, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berrocal-Almanza LC, Goyal S, Hussain A, Klassert TE, Driesch D, Grozdanovic Z, Sumanlatha G, Ahmed N, Valluri V, Conrad ML, Dittrich N, Schumann RR, Lala B, and Slevogt H. S100A12 is up-regulated in pulmonary tuberculosis and predicts the extent of alveolar infiltration on chest radiography: an observational study. Sci Rep 6: 31798, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, and O'Garra A. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466: 973–977, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhat SA, Singh N, Trivedi A, Kansal P, Gupta P, and Kumar A. The mechanism of redox sensing in Mycobacterium tuberculosis. Free Radic Biol Med 53: 1625–1641, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Borthwick LA. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin Immunopathol 38: 517–534, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buhling F, Gerber A, Hackel C, Kruger S, Kohnlein T, Bromme D, Reinhold D, Ansorge S, and Welte T. Expression of cathepsin K in lung epithelial cells. Am J Respir Cell Mol Biol 20: 612–619, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Buhling F, Waldburg N, Gerber A, Hackel C, Kruger S, Reinhold D, Bromme D, Weber E, Ansorge S, Welte T. and Cathepsin K expression in human lung. Adv Exp Med Biol 477: 281–286, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Cadena AM, Fortune SM, and Flynn JL. Heterogeneity in tuberculosis. Nat Rev Immunol 17: 691–702, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campa A, Baum MK, Bussmann H, Martinez SS, Farahani M, van Widenfelt E, Moyo S, Makhema J, Essex M, and Marlink R. The effect of micronutrient supplementation on active TB incidence early in HIV infection in Botswana. Nutr Diet Suppl 2017: 37–45, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Campbell EJ, Cury JD, Shapiro SD, Goldberg GI, and Welgus HG. Neutral proteinases of human mononuclear phagocytes. Cellular differentiation markedly alters cell phenotype for serine proteinases, metalloproteinases, and tissue inhibitor of metalloproteinases. J Immunol 146: 1286–1293, 1991 [PubMed] [Google Scholar]
- 25. Cao R, Teskey G, Islamoglu H, Abrahem R, Munjal S, Gyurjian K, Zhong L, and Venketaraman V. Characterizing the effects of glutathione as an immunoadjuvant in the treatment of tuberculosis. Antimicrob Agents Chemother 62: e01132-18, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carranza C and Chavez-Galan L. Several routes to the same destination: inhibition of phagosome-lysosome fusion by Mycobacterium tuberculosis. Am J Med Sci 357: 184–194, 2019 [DOI] [PubMed] [Google Scholar]
- 27. Casarini M, Ameglio F, Alemanno L, Zangrilli P, Mattia P, Paone G, Bisetti A, and Giosue S. Cytokine levels correlate with a radiologic score in active pulmonary tuberculosis. Am J Respir Crit Care Med 159: 143–148, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Chakravarty SD, Zhu G, Tsai MC, Mohan VP, Marino S, Kirschner DE, Huang L, Flynn J, and Chan J. Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs. Infect Immun 76: 916–926, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, Serhan CN, Behar SM, and Remold HG. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med 205: 2791–2801, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chinta KC, Rahman MA, Saini V, Glasgow JN, Reddy VP, Lever JM, Nhamoyebonde S, Leslie A, Wells RM, Traylor A, Madansein R, Siegal GP, Antony VB, Deshane J, Wells G, Nargan K, George JF, Ramdial PK, Agarwal A, and Steyn AJC. Microanatomic distribution of myeloid heme oxygenase-1 protects against free radical-mediated immunopathology in human tuberculosis. Cell Rep 25: 1938–1952.e5, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chong WC, Shastri MD, and Eri R. Endoplasmic reticulum stress and oxidative stress: a vicious nexus implicated in bowel disease pathophysiology. Int J Mol Sci 18: 771, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chowdhury IH, Ahmed AM, Choudhuri S, Sen A, Hazra A, Pal NK, Bhattacharya B, and Bahar B. Alteration of serum inflammatory cytokines in active pulmonary tuberculosis following anti-tuberculosis drug therapy. Mol Immunol 62: 159–168, 2014 [DOI] [PubMed] [Google Scholar]
- 33. Cohen SB, Gern BH, Delahaye JL, Adams KN, Plumlee CR, Winkler JK, Sherman DR, Gerner MY, and Urdahl KB. Alveolar macrophages provide an early Mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe 24: 439–446.e4, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Conrad M and Pratt DA. The chemical basis of ferroptosis. Nat Chem Biol 15: 1137–1147, 2019 [DOI] [PubMed] [Google Scholar]
- 35. Cooper AM, Mayer-Barber KD, and Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol 4: 252–260, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Costa DL, Namasivayam S, Amaral EP, Arora K, Chao A, Mittereder LR, Maiga M, Boshoff HI, Barry CE 3rd, Goulding CW, Andrade BB, and Sher A. Pharmacological inhibition of host heme oxygenase-1 suppresses Mycobacterium tuberculosis infection in vivo by a mechanism dependent on T lymphocytes. mBio 7: e01675-16, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Critchley JA, Young F, Orton L, and Garner P. Corticosteroids for prevention of mortality in people with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 13: 223–237, 2013 [DOI] [PubMed] [Google Scholar]
- 38. Dalvi SM, Patil VW, Ramraje NN, Phadtare JM, and Gujarathi SU. Nitric oxide, carbonyl protein, lipid peroxidation and correlation between antioxidant vitamins in different categories of pulmonary and extra pulmonary tuberculosis. Malays J Med Sci 20: 21–30, 2013 [PMC free article] [PubMed] [Google Scholar]
- 39. Das BS and Nanda NK. Evidence for erythrocyte lipid peroxidation in acute falciparum malaria. Trans R Soc Trop Med Hyg 93: 58–62, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Divangahi M, Chen M, Gan H, Desjardins D, Hickman TT, Lee DM, Fortune S, Behar SM, and Remold HG. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol 10: 899–906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, and Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dooley DP, Carpenter JL, and Rademacher S. Adjunctive corticosteroid therapy for tuberculosis: a critical reappraisal of the literature. Clin Infect Dis 25: 872–887, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Dunsmore SE and Rannels DE. Extracellular matrix biology in the lung. Am J Physiol 270: L3–L27, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Ehlers S. Tumor necrosis factor and its blockade in granulomatous infections: differential modes of action of infliximab and etanercept? Clin Infect Dis 41 Suppl 3: S199–S203, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, Saraiva L, Pedersen B, Mauri F, Lipman M, Edwards DR, Robertson BD, D'Armiento J, and Friedland JS. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest 121: 1827–1833, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Elkington PT. Tuberculosis: time for a new perspective? J Infect 66: 299–302, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Elkington PT, D'Armiento JM, and Friedland JS. Tuberculosis immunopathology: the neglected role of extracellular matrix destruction. Sci Transl Med 3: 71ps6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Elkington PT and Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax 61: 259–266, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Elkington PT, Nuttall RK, Boyle JJ, O'Kane CM, Horncastle DE, Edwards DR, and Friedland JS. Mycobacterium tuberculosis, but not vaccine BCG, specifically upregulates matrix metalloproteinase-1. Am J Respir Crit Care Med 172: 1596–1604, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Ezraty B, Gennaris A, Barras F, and Collet JF. Oxidative stress, protein damage and repair in bacteria. Nat Rev Microbiol 15: 385–396, 2017 [DOI] [PubMed] [Google Scholar]
- 51. Gopal R, Monin L, Torres D, Slight S, Mehra S, McKenna KC, Fallert Junecko BA, Reinhart TA, Kolls J, Baez-Saldana R, Cruz-Lagunas A, Rodriguez-Reyna TS, Kumar NP, Tessier P, Roth J, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Cumming B, Kasprowicz VO, Steyn AJ, Babu S, Kaushal D, Zuniga J, Vogl T, Rangel-Moreno J, and Khader SA. S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am J Respir Crit Care Med 188: 1137–1146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goyal N, Kashyap B, Singh NP, and Kaur IR. Neopterin and oxidative stress markers in the diagnosis of extrapulmonary tuberculosis. Biomarkers 22: 648–653, 2017 [DOI] [PubMed] [Google Scholar]
- 53. Gozzelino R, Jeney V, and Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50: 323–354, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Hennessy E, Adams C, Reen FJ, and O'Gara F. Is there potential for repurposing statins as novel antimicrobials? Antimicrob Agents Chemother 60: 5111–5121, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hernandez J, Garibay-Escobar A, Mendoza-Mendoza A, Pinelli-Saavedra A, and Valenzuela O. Effect of exogenous vitamin E on proliferation and cytokine production in peripheral blood mononuclear cells from patients with tuberculosis. Br J Nutr 99: 224–229, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Hess S, Hospach T, Nossal R, Dannecker G, Magdorf K, and Uhlemann F. Life-threatening disseminated tuberculosis as a complication of TNF-alpha blockade in an adolescent. Eur J Pediatr 170: 1337–1342, 2011 [DOI] [PubMed] [Google Scholar]
- 57. Hoheisel G, Sack U, Hui DS, Huse K, Chan KS, Chan KK, Hartwig K, Schuster E, Scholz GH, and Schauer J. Occurrence of matrix metalloproteinases and tissue inhibitors of metalloproteinases in tuberculous pleuritis. Tuberculosis (Edinb) 81: 203–209, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Holguin F. Oxidative stress in airway diseases. Ann Am Thorac Soc 10 Suppl: S150–S157, 2013 [DOI] [PubMed] [Google Scholar]
- 59. Houben D, Demangel C, van Ingen J, Perez J, Baldeon L, Abdallah AM, Caleechurn L, Bottai D, van Zon M, de Punder K, van der Laan T, Kant A, Bossers-de Vries R, Willemsen P, Bitter W, van Soolingen D, Brosch R, van der Wel N, and Peters PJ. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol 14: 1287–1298, 2012 [DOI] [PubMed] [Google Scholar]
- 60. Hussain MI, Ahmed W, Nasir M, Mushtaq MH, Sheikh AA, Shaheen AY, and Mahmood A. Immune boosting role of vitamin E against pulmonary tuberculosis. Pak J Pharm Sci 32: 269–276, 2019 [PubMed] [Google Scholar]
- 61. Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T, Mehr L, Aichler M, Walch A, Lamp D, Jastroch M, Miyamoto S, Wurst W, Ursini F, Arner ESJ, Fradejas-Villar N, Schweizer U, Zischka H, Friedmann Angeli JP, and Conrad M. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172: 409–422.e21, 2018 [DOI] [PubMed] [Google Scholar]
- 62. Janssen S, Jayachandran R, Khathi L, Zinsstag J, Grobusch MP, and Pieters J. Exploring prospects of novel drugs for tuberculosis. Drug Des Devel Ther 6: 217–224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kaomongkolgit R, Cheepsunthorn P, Pavasant P, and Sanchavanakit N. Iron increases MMP-9 expression through activation of AP-1 via ERK/Akt pathway in human head and neck squamous carcinoma cells. Oral Oncol 44: 587–594, 2008 [DOI] [PubMed] [Google Scholar]
- 64. Kelly DM, ten Bokum AM, O'Leary SM, O'Sullivan MP, and Keane J. Bystander macrophage apoptosis after Mycobacterium tuberculosis H37Ra infection. Infect Immun 76: 351–360, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kranzer K, Elamin WF, Cox H, Seddon JA, Ford N, and Drobniewski F. A systematic review and meta-analysis of the efficacy and safety of N-acetylcysteine in preventing aminoglycoside-induced ototoxicity: implications for the treatment of multidrug-resistant TB. Thorax 70: 1070–1077, 2015 [DOI] [PubMed] [Google Scholar]
- 66. Kubler A, Larsson C, Luna B, Andrade BB, Amaral EP, Urbanowski M, Orandle M, Bock K, Ammerman NC, Cheung LS, Winglee K, Halushka M, Park JK, Sher A, Friedland JS, Elkington PT, Bishai WR. and Cathepsin K Contributes to cavitation and collagen turnover in pulmonary tuberculosis. J Infect Dis 213: 618–627, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kubler A, Luna B, Larsson C, Ammerman NC, Andrade BB, Orandle M, Bock KW, Xu Z, Bagci U, Molura DJ, Marshall J, Burns J, Winglee K, Ahidjo BA, Cheung LS, Klunk M, Jain SK, Kumar NP, Babu S, Sher A, Friedland JS, Elkington PT, and Bishai WR. Mycobacterium tuberculosis dysregulates MMP/TIMP balance to drive rapid cavitation and unrestrained bacterial proliferation. J Pathol 235: 431–444, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kwon MY, Park E, Lee SJ, and Chung SW. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget 6: 24393–24403, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lai CC, Lee MT, Lee SH, Hsu WT, Chang SS, Chen SC, and Lee CC. Statin treatment is associated with a decreased risk of active tuberculosis: an analysis of a nationally representative cohort. Thorax 71: 646–651, 2016 [DOI] [PubMed] [Google Scholar]
- 70. Lamsal M, Gautam N, Bhatta N, Toora BD, Bhattacharya SK, and Baral N. Evaluation of lipid peroxidation product, nitrite and antioxidant levels in newly diagnosed and two months follow-up patients with pulmonary tuberculosis. Southeast Asian J Trop Med Public Health 38: 695–703, 2007 [PubMed] [Google Scholar]
- 71. Liu CH, Liu H, and Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol 14: 963–975, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lowe DM, Redford PS, Wilkinson RJ, O'Garra A, and Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends Immunol 33: 14–25, 2012 [DOI] [PubMed] [Google Scholar]
- 73. Luz NF, Andrade BB, Feijo DF, Araujo-Santos T, Carvalho GQ, Andrade D, Abanades DR, Melo EV, Silva AM, Brodskyn CI, Barral-Netto M, Barral A, Soares RP, Almeida RP, Bozza MT, and Borges VM. Heme oxygenase-1 promotes the persistence of Leishmania chagasi infection. J Immunol 188: 4460–4467, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ma Y, Keil V, and Sun J. Characterization of Mycobacterium tuberculosis EsxA membrane insertion: roles of N- and C-terminal flexible arms and central helix-turn-helix motif. J Biol Chem 290: 7314–7322, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Madebo T, Lindtjorn B, Aukrust P, and Berge RK. Circulating antioxidants and lipid peroxidation products in untreated tuberculosis patients in Ethiopia. Am J Clin Nutr 78: 117–122, 2003 [DOI] [PubMed] [Google Scholar]
- 76. Mahakalkar SM, Nagrale D, Gaur S, Urade C, Murhar B, and Turankar A. N-acetylcysteine as an add-on to Directly Observed Therapy Short-I therapy in fresh pulmonary tuberculosis patients: a randomized, placebo-controlled, double-blinded study. Perspect Clin Res 8: 132–136, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mahuad C, Bay ML, Farroni MA, Bozza V, Del Rey A, Besedovsky H, and Bottasso OA. Cortisol and dehydroepiandrosterone affect the response of peripheral blood mononuclear cells to mycobacterial antigens during tuberculosis. Scand J Immunol 60: 639–646, 2004 [DOI] [PubMed] [Google Scholar]
- 78. Maiga MC, Ahidjo BA, Maiga M, and Bishai WR. Roflumilast, a type 4 phosphodiesterase inhibitor, shows promising adjunctive, host-directed therapeutic activity in a mouse model of tuberculosis. Antimicrob Agents Chemother 59: 7888–7890, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mairuae N, Connor JR, and Cheepsunthorn P. Increased cellular iron levels affect matrix metalloproteinase expression and phagocytosis in activated microglia. Neurosci Lett 500: 36–40, 2011 [DOI] [PubMed] [Google Scholar]
- 80. Malfa GA, Tomasello B, Acquaviva R, Genovese C, La Mantia A, Cammarata FP, Ragusa M, Renis M, and Di Giacomo C. Betula etnensis Raf. (Betulaceae) extract induced HO-1 expression and ferroptosis cell death in human colon cancer cells. Int J Mol Sci 20: 2723, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Marakalala MJ, Raju RM, Sharma K, Zhang YJ, Eugenin EA, Prideaux B, Daudelin IB, Chen PY, Booty MG, Kim JH, Eum SY, Via LE, Behar SM, Barry CE 3rd, Mann M, Dartois V, and Rubin EJ. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat Med 22: 531–538, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res 424: 83–95, 1999 [DOI] [PubMed] [Google Scholar]
- 83. Martin CJ, Booty MG, Rosebrock TR, Nunes-Alves C, Desjardins DM, Keren I, Fortune SM, Remold HG, and Behar SM. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe 12: 289–300, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, and Sher A. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 35: 1023–1034, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, Derrick SC, Shi R, Kumar NP, Wei W, Yuan X, Zhang G, Cai Y, Babu S, Catalfamo M, Salazar AM, Via LE, Barry CE 3rd, and Sher A. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511: 99–103, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, Schlueter D, Flavell RA, Sutterwala FS, and Sher A. Caspase-1 independent IL-1beta production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol 184: 3326–3330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mayer-Barber KD, and Sher A. Cytokine and lipid mediator networks in tuberculosis. Immunol Rev 264: 264–275, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mendonca VR, Luz NF, Santos NJ, Borges VM, Goncalves MS, Andrade BB, and Barral-Netto M. Association between the haptoglobin and heme oxygenase 1 genetic profiles and soluble CD163 in susceptibility to and severity of human malaria. Infect Immun 80: 1445–1454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mishra BB, Lovewell RR, Olive AJ, Zhang G, Wang W, Eugenin E, Smith CM, Phuah JY, Long JE, Dubuke ML, Palace SG, Goguen JD, Baker RE, Nambi S, Mishra R, Booty MG, Baer CE, Shaffer SA, Dartois V, McCormick BA, Chen X, and Sassetti CM. Nitric oxide prevents a pathogen-permissive granulocytic inflammation during tuberculosis. Nat Microbiol 2: 17072, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mishra BB, Rathinam VA, Martens GW, Martinot AJ, Kornfeld H, Fitzgerald KA, and Sassetti CM. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat Immunol 14: 52–60, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Morris D, Guerra C, Donohue C, Oh H, Khurasany M, and Venketaraman V. Unveiling the mechanisms for decreased glutathione in individuals with HIV infection. Clin Dev Immunol 2012: 734125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mosca A, Onelli E, Rosti E, Paleari R, Luzzana M, and Imbimbo BP. A patient-side technique for real-time measurement of acetylcholinesterase activity during monitoring of eptastigmine treatment. Ther Drug Monit 17: 230–238, 1995 [DOI] [PubMed] [Google Scholar]
- 93. Nambi S, Long JE, Mishra BB, Baker R, Murphy KC, Olive AJ, Nguyen HP, Shaffer SA, and Sassetti CM. The oxidative stress network of Mycobacterium tuberculosis reveals coordination between radical detoxification systems. Cell Host Microbe 17: 829–837, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, and Berry MP. The immune response in tuberculosis. Annu Rev Immunol 31: 475–527, 2013 [DOI] [PubMed] [Google Scholar]
- 95. O'Kane CM, Elkington PT, Jones MD, Caviedes L, Tovar M, Gilman RH, Stamp G, and Friedland JS. STAT3, p38 MAPK, and NF-kappaB drive unopposed monocyte-dependent fibroblast MMP-1 secretion in tuberculosis. Am J Respir Cell Mol Biol 43: 465–474, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Oh J, Choi R, Park HD, Lee H, Jeong BH, Park HY, Jeon K, Kwon OJ, Koh WJ, and Lee SY. Evaluation of vitamin status in patients with pulmonary tuberculosis. J Infect 74: 272–280, 2017 [DOI] [PubMed] [Google Scholar]
- 97. Oliveira-de-Souza D, Vinhaes CL, Arriaga MB, Kumar NP, Cubillos-Angulo JM, Shi R, Wei W, Yuan X, Zhang G, Cai Y, Barry CE 3rd, Via LE, Sher A, Babu S, Mayer-Barber KD, Nakaya HI, Fukutani KF, and Andrade BB. Molecular degree of perturbation of plasma inflammatory markers associated with tuberculosis reveals distinct disease profiles between Indian and Chinese populations. Sci Rep 9: 8002, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ong CW, Elkington PT, and Friedland JS. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am J Respir Crit Care Med 190: 9–18, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ordonez AA, Pokkali S, Sanchez-Bautista J, Klunk MH, Urbanowski ME, Kubler A, Bishai WR, Elkington PT, and Jain SK. Matrix metalloproteinase inhibition in a murine model of cavitary tuberculosis paradoxically worsens pathology. J Infect Dis 219: 633–636, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pagan AJ and Ramakrishnan L. Immunity and immunopathology in the tuberculous granuloma. Cold Spring Harb Perspect Med 5: a018499, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Parihar SP, Guler R, Khutlang R, Lang DM, Hurdayal R, Mhlanga MM, Suzuki H, Marais AD, and Brombacher F. Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis 209: 754–763, 2014 [DOI] [PubMed] [Google Scholar]
- 102. Parks WC and Shapiro SD. Matrix metalloproteinases in lung biology. Respir Res 2: 10–19, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pearl JE, Saunders B, Ehlers S, Orme IM, and Cooper AM. Inflammation and lymphocyte activation during mycobacterial infection in the interferon-gamma-deficient mouse. Cell Immunol 211: 43–50, 2001 [DOI] [PubMed] [Google Scholar]
- 104. Poljsak B, Suput D, and Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013: 956792, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Polte T and Tyrrell RM. Involvement of lipid peroxidation and organic peroxides in UVA-induced matrix metalloproteinase-1 expression. Free Radic Biol Med 36: 1566–1574, 2004 [DOI] [PubMed] [Google Scholar]
- 106. Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol 12: 352–366, 2012 [DOI] [PubMed] [Google Scholar]
- 107. Rand L, Green JA, Saraiva L, Friedland JS, and Elkington PT. Matrix metalloproteinase-1 is regulated in tuberculosis by a p38 MAPK-dependent, p-aminosalicylic acid-sensitive signaling cascade. J Immunol 182: 5865–5872, 2009 [DOI] [PubMed] [Google Scholar]
- 108. Rao M, Valentini D, Poiret T, Dodoo E, Parida S, Zumla A, Brighenti S, and Maeurer M. B in TB: B cells as mediators of clinically relevant immune responses in tuberculosis. Clin Infect Dis 61(Suppl 3): S225–S234, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ravimohan S, Kornfeld H, Weissman D, and Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 27: 170077, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rockwood N, Costa DL, Amaral EP, Du Bruyn E, Kubler A, Gil-Santana L, Fukutani KF, Scanga CA, Flynn JL, Jackson SH, Wilkinson KA, Bishai WR, Sher A, Wilkinson RJ, and Andrade BB. Mycobacterium tuberculosis induction of heme oxygenase-1 expression is dependent on oxidative stress and reflects treatment outcomes. Front Immunol 8: 542, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rohlwink UK, Walker NF, Ordonez AA, Li YJ, Tucker EW, Elkington PT, Wilkinson RJ, and Wilkinson KA. Matrix metalloproteinases in pulmonary and central nervous system tuberculosis—a review. Int J Mol Sci 20: 1350, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sabir N, Hussain T, Mangi MH, Zhao D, and Zhou X. Matrix metalloproteinases: expression, regulation and role in the immunopathology of tuberculosis. Cell Prolif 52: e12649, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Saukkonen K, Lakkisto P, Kaunisto MA, Varpula M, Voipio-Pulkki LM, Varpula T, Pettila V, and Pulkki K. Heme oxygenase 1 polymorphisms and plasma concentrations in critically ill patients. Shock 34: 558–564, 2010 [DOI] [PubMed] [Google Scholar]
- 114. Schwarzer E, Arese P, and Skorokhod OA. Role of the lipoperoxidation product 4-hydroxynonenal in the pathogenesis of severe malaria anemia and malaria immunodepression. Oxid Med Cell Longev 2015: 638416, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Scriba TJ, Coussens AK, and Fletcher HA.. Human immunology of tuberculosis. Microbiol Spectr 4, 2016. DOI: 10.1128/microbiolspec.TBTB2-0016-2016 [DOI] [PubMed] [Google Scholar]
- 116. Serhan CN, Chiang N, and Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8: 349–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Seyedrezazadeh E, Ostadrahimi A, Mahboob S, Assadi Y, Ghaemmagami J, and Pourmogaddam M. Effect of vitamin E and selenium supplementation on oxidative stress status in pulmonary tuberculosis patients. Respirology 13: 294–298, 2008 [DOI] [PubMed] [Google Scholar]
- 118. Shastri MD, Shukla SD, Chong WC, Dua K, Peterson GM, Patel RP, Hansbro PM, Eri R, and O'Toole RF. Role of oxidative stress in the pathology and management of human tuberculosis. Oxid Med Cell Longev 2018: 7695364, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Shiloh MU, Manzanillo P, and Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe 3: 323–330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sigal GB, Segal MR, Mathew A, Jarlsberg L, Wang M, Barbero S, Small N, Haynesworth K, Davis JL, Weiner M, Whitworth WC, Jacobs J, Schorey J, Lewinsohn DM, and Nahid P. Biomarkers of tuberculosis severity and treatment effect: a directed screen of 70 host markers in a randomized clinical trial. EBioMedicine 25: 112–121, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Silva D, Silva MVD, Barros CCO, Alexandre PBD, Timoteo RP, Catarino JS, Sales-Campos H, Machado JR, Rodrigues DBR, Oliveira CJ, and Rodrigues V. TNF-alpha blockade impairs in vitro tuberculous granuloma formation and down modulate Th1, Th17 and Treg cytokines. PLoS One 13: e0194430, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Simeone R, Bobard A, Lippmann J, Bitter W, Majlessi L, Brosch R, and Enninga J. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog 8: e1002507, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Stek C, Allwood B, Walker NF, Wilkinson RJ, Lynen L, and Meintjes G. The immune mechanisms of lung parenchymal damage in tuberculosis and the role of host-directed therapy. Front Microbiol 9: 2603, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Su VY, Su WJ, Yen YF, Pan SW, Chuang PH, Feng JY, Chou KT, Yang KY, Lee YC, and Chen TJ. Statin use is associated with a lower risk of TB. Chest 152: 598–606, 2017 [DOI] [PubMed] [Google Scholar]
- 125. Subbian S, Tsenova L, O'Brien P, Yang G, Koo MS, Peixoto B, Fallows D, Zeldis JB, Muller G, and Kaplan G. Phosphodiesterase-4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am J Pathol 179: 289–301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Taylor JL, Hattle JM, Dreitz SA, Troudt JM, Izzo LS, Basaraba RJ, Orme IM, Matrisian LM, and Izzo AA. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect Immun 74: 6135–6144, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tenhunen R, Marver HS, and Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A 61: 748–755, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tobin DM and Ramakrishnan L. TB: the Yin and Yang of lipid mediators. Curr Opin Pharmacol 13: 641–645, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tsao TC, Hong J, Li LF, Hsieh MJ, Liao SK, and Chang KS. Imbalances between tumor necrosis factor-alpha and its soluble receptor forms, and interleukin-1beta and interleukin-1 receptor antagonist in BAL fluid of cavitary pulmonary tuberculosis. Chest 117: 103–109, 2000 [DOI] [PubMed] [Google Scholar]
- 130. Via LE, Schimel D, Weiner DM, Dartois V, Dayao E, Cai Y, Yoon YS, Dreher MR, Kastenmayer RJ, Laymon CM, Carny JE, Flynn JL, Herscovitch P, and Barry CE 3rd. Infection dynamics and response to chemotherapy in a rabbit model of tuberculosis using [(1)(8)F]2-fluoro-deoxy-D-glucose positron emission tomography and computed tomography. Antimicrob Agents Chemother 56: 4391–4402, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Vijayamalini M and Manoharan S. Lipid peroxidation, vitamins C, E and reduced glutathione levels in patients with pulmonary tuberculosis. Cell Biochem Funct 22: 19–22, 2004 [DOI] [PubMed] [Google Scholar]
- 132. Vinhaes CL, Oliveira-de-Souza D, Silveira-Mattos PS, Nogueira B, Shi R, Wei W, Yuan X, Zhang G, Cai Y, Barry CE 3rd, Via LE, Fukutani KF, Andrade BB, and Mayer-Barber KD. Changes in inflammatory protein and lipid mediator profiles persist after antitubercular treatment of pulmonary and extrapulmonary tuberculosis: a prospective cohort study. Cytokine 123: 154759, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Virag L, Jaen RI, Regdon Z, Bosca L, and Prieto P. Self-defense of macrophages against oxidative injury: fighting for their own survival. Redox Biol 26: 101261, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Walker NF, Clark SO, Oni T, Andreu N, Tezera L, Singh S, Saraiva L, Pedersen B, Kelly DL, Tree JA, D'Armiento JM, Meintjes G, Mauri FA, Williams A, Wilkinson RJ, Friedland JS, and Elkington PT. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am J Respir Crit Care Med 185: 989–997, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wheater G, Elshahaly M, Tuck SP, Datta HK, and van Laar JM. The clinical utility of bone marker measurements in osteoporosis. J Transl Med 11: 201, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. WHO. World Health Organization. Global Tuberculosis Report, 2019. Geneva: World Health Organization, 2019 [Google Scholar]
- 137. Xu Y, Wang L, Zimmerman MD, Chen KY, Huang L, Fu DJ, Kaya F, Rakhilin N, Nazarova EV, Bu P, Dartois V, Russell DG, and Shen X. Matrix metalloproteinase inhibitors enhance the efficacy of frontline drugs against Mycobacterium tuberculosis. PLoS Pathog 14: e1006974, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, and Ramakrishnan L. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe 12: 301–312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yasuda Y, Kaleta J, and Bromme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev 57: 973–993, 2005 [DOI] [PubMed] [Google Scholar]