Abstract
Adeno-associated virus (AAV) vectors such as AAV6, which shows tropism for primary human CD4+ T cells in vitro, are being explored for delivery of anti-HIV therapeutic modalities in vivo. However, pre-existing immunity and sequestration in nontarget organs can significantly hinder their performance. To overcome these challenges, we investigated whether immunosuppression would allow gene delivery by AAV6 or targeted AAV6 derivatives in seropositive rhesus macaques. Animals were immune suppressed with rapamycin before intravenous (IV) or subcutaneous (SC) delivery of AAV, and we monitored vector biodistribution, gene transfer, and safety. Macaques received phosphate-buffered saline, AAV6 alone, or an equal dose of AAV6 and an AAV6-55.2 vector retargeted to CD4 through a direct ankyrin repeat protein (DARPin). AAV6 and AAV6-55.2 vector genomes were found in peripheral blood mononuclear cells and most organs up to 28 days postadministration, with the highest levels seen in liver, spleen, lymph nodes (LNs), and muscle, suggesting that retargeting did not prevent vector sequestration. Despite vector genome detection, gene expression from AAV6-55.2 was not detected in any tissue. SC injection of AAV6 facilitated efficient gene expression in muscle adjacent to the injection site, plus low-level gene expression in spleen, LNs, and liver, whereas gene expression following IV injection of AAV6 was predominantly seen in the spleen. AAV vectors were well tolerated, although elevated liver enzymes were detected in three of four AAV-treated animals 14 days after rapamycin withdrawal. One SC-injected animal had muscle inflammation proximal to the injection site, plus detectable T cell responses against transgene and AAV6 capsid at study finish. Overall, our data suggest that rapamycin treatment may offer a possible strategy to express anti-HIV therapeutics such as broadly neutralizing antibodies from muscle. This study provides important safety and efficacy data that will aid study design for future anti-HIV gene therapies.
Keywords: immunosupression, rapamycin, AAV, non-human primate, DARPin
Introduction
Recombinant adeno-associated virus (AAV) vectors have been widely used as in vivo gene transfer vectors, and extensively tested in many gene therapy applications. AAV has a favorable safety record, efficiently transduces various tissue types, can be produced at high titer, and has low immunogenicity, making it a vector of choice for many.1–3 AAV vectors are being explored as gene delivery platforms for anti-HIV therapeutics. For example, local administration of AAV to muscle has been used to systemically deliver broadly neutralizing antibodies (NAbs) or the soluble CD4-mimetic eCD4-Ig to block HIV infection.4–6 In addition, AAV6 has the potential to transduce CD4+ T cells based on its in vitro tropism,7–11 which gives AAV6 or variants engineered to bind CD4 the potential to deliver targeted anti-HIV therapeutics. These therapeutics could include HIV restriction factors or gene-editing enzymes that target the HIV provirus such as CRISPR/Cas9. Unfortunately, many of the natural evolved properties of AAV hinder its potential as an HIV gene therapy vehicle, with perhaps the two largest hurdles being pre-existing immunity and sequestration in nontarget tissues.
As natural hosts of AAV, humans and nonhuman primates (NHPs) have a high incidence of antibodies to many of the vectorized AAV serotypes,12,13 and studies have shown that up to 60% of humans possess anti-AAV antibodies.14–16 A subset of these are NAbs, which block viral infection by binding epitopes critical for cellular entry.17,18 Broadly cross-reactive antibody responses to multiple AAV serotypes can be induced after a single natural infection with AAV.19 Antibodies can have a profound effect on the efficiency of AAV tissue transduction, with even low antibody titers limiting gene transfer.20,21 Notably, intravenous (IV) delivery of vector is most susceptible to antibody inhibition.21,22 Anti-AAV cellular immune responses can also limit gene transfer, and while such responses are often weak, they are still capable of eliminating transduced cells.23 Immunosuppression has the potential to mitigate AAV- and transgene-specific humoral and cellular immune responses and improve vector transduction,24–29 although studies of immune responses in macaques or human liver and kidney transplant recipients have shown that pre-existing AAV-specific immune responses as well as the impact of immunosuppression on AAV-specific responses can be variable.30,31
Many natural AAV serotypes have been isolated from humans and NHPs,32,33 and although each has a comparatively unique tropism, in general, they all show tropism for multiple tissue types.34 A high AAV vector dose can be used to overcome vector sequestration in off-target tissue and cell types, but high-level exposure of off-target tissues to vector has the potential to induce vector- or transgene-specific immune responses that can limit efficacy.35–37 In addition, very high vector doses may lead to increased toxicity, and in some preclinical studies, this has proved lethal.38 Therefore, attempts to retarget AAV and concurrently reduce the effective dose have been ongoing. Modification and retargeting of AAV has enabled successful transduction of many different cell types,39 but in vivo retargeted vectors can still be taken up by irrelevant cell types and tissues. To minimize nonspecific tissue sequestration of AAV vectors, modification of natural receptor binding motifs within the capsid is required to substantially influence AAV vector biodistribution.40 Also, retargeting capsid modifications may also minimize immune responses to AAV.41–43
To realize the full potential of AAV vectors for HIV therapy, evasion of the host immune response and targeted cell transduction is key. In this study, we demonstrate that immunosuppressive treatment with rapamycin before and after AAV delivery can prevent onset of AAV capsid- and transgene-specific cellular immunity, and importantly allows for AAV6-mediated gene transfer following subcutaneous (SC) administration in AAV6 seropositive rhesus macaques. Immunosuppression enabled efficient transfer of vector genomes and subsequent transgene expression in muscle, liver, spleen, and lymph nodes (LNs). We also showed that despite being ablated for native heparin sulfate proteoglycan (HSPG) and sialic acid binding, a CD4-retargeted AAV6 vector (AAV6-55.2) had a similar biodistribution to unmodified AAV6 in vivo. Our results suggest that immunosuppressive rapamycin therapy was well tolerated and could be used to suppress anti-AAV immunity, and facilitate efficient localized gene delivery of AAV6-based anti-HIV therapeutics to muscle.
Materials and Methods
AAV Vectors
To enable production of AAV6 vectors that are detargeted and incorporate a modified VP2 with an N-terminal surface displayed direct ankyrin repeat protein (DARPin)-targeting ligand, mutations were introduced by Quikchange mutagenesis into the AAV6 cap gene of the plasmid and pRepCap6.44 A synonymous ACG to ACC mutation that deletes the T138 VP2 start codon was introduced to prevent expression of native VP2 protein. Previously identified V473D (GTT to GAT) and K531E (AAA to GAA) mutations that ablate sialic acid and heparin binding, respectively45,46 were introduced, creating the plasmid pAAV6-detarget. To generate the plasmid pDGM6-detarget, which contains the modified AAV6 cap gene plus the adenovirus genes needed for generating AAV vectors, the modified AAV6 cap gene was amplified by polymerase chain reaction (PCR) from pAAV6-detarget using primers AAV6cap-SwaI-F 5′CTTTGAACAATAAATGATTTAAATCAGGTATGGCTGCCGATGGTT3′ and AAV6cap-ClaI-R 5′CCGGACCCAAGGACATGCATCGATTGCTATGGTGACCAGATAAGATAAT3 ′, and then introduced into the plasmid pDGM647 by Gibson assembly following excisions of the wild-type AAV6 cap gene by SwaI/ClaI digestion.
To produce AAV6 vectors that display DARPins on the surface of their capsids, pcDNA3.1-derived plasmids were generated for expression in trans of VP2 with a CD4 targeted or control DARPin fused to its N-terminus. Plasmids incorporating DARPins specific for CD4 (55.2 and 57.2; Genbank accession Nos. JC982162 and JC982163), Her2/neu (G3; Genbank accession JC982169), or a Lactococcus lactis ABC transporter (LmrCD; Genbank accession JQ425607) were generated (Supplementary Fig. S1A).48–50 DARPins G3 and 55.2 contain two internal ankyrin repeat modules, and DARPins LmrCD and 57.2 contain three internal ankyrin repeat modules. VP2-DARPin constructs contained the following: an N-terminal 6-His tag, an IEGR factor Xa cleavage signal to remove the 6-His tag, each respective DARPin, an LYKYSDP linker, and the AAV6 VP2 sequence (starting at cap amino acid P140) from pAAV6-detarget (Supplementary Fig. S1B). To enable production of control detargeted sialic acid and HSPG binding ablated (AAV6) vectors that contain VP2 without an N-terminal DARPin, the V473D/K531E mutations were introduced into pRepCap6 by Quikchange mutagenesis to generate the plasmid pRepCap6-V473D-K531E.
AAV Production and Purification
Self-complementary AAV (scAAV) vectors expressing GFP or mCherry were generated using the AAV vector plasmids pscAAV-MND-GFP51 or pscAAV-MND-mCherry. pscAAV-MND-mCherry was generated by removing the GFP gene from pscAAV-MND-GFP by HindII/NotI digestion and inserting an mCherry PCR product through Gibson assembly. The MND promoter is a modified Moloney murine leukemia virus (MoMuLV) LTR-derived promoter with myeloproliferative sarcoma virus enhancer and deleted negative control region.52 AAV vectors were produced by transient transfection of HEK293 cells with plasmids containing vector, helper, and packaging sequences as previously described.53,54 The pDGM6 plasmid was used to package control AAV6 vectors with native tropism. The pRepCap6-V473D-K531E plasmid was used to generate control-detargeted AAV6 vectors.
To generate detargeted and DARPin retargeted AAV6 vectors, cells were co-transfected with AAV vector plasmid, pDGM6-detarget, and a pcDNA-DARPin-AAV6-VP2 plasmid. For in vitro studies, AAV vectors were purified by iodixanol gradient separation (Supplementary Fig. S2), and then concentrated into phosphate-buffered saline (PBS) using Amicon Ultra-15 100K MW columns as previously described.53 For in vivo studies, AAV6 vectors were purified by HPLC affinity chromatography (Supplementary Fig. S2) using a HiTrap heparin column (GE Healthcare), followed by dialysis against HBSS as described.54 The detargeted AAV6-55.2 vector used for in vivo studies that displays a CD4-specific DARPin on the capsid was concentrated by precipitating combined clarified cell lysate and culture supernatant from virus cultures by adding 40% PEG 8000 to a final concentration of 8% and then incubating on ice for 2 h. Virus was then pelleted by centrifugation and resuspended, digested with Factor Xa for 16 h at 37°C to remove the N-terminal His tag, and purified using first a step cesium chloride gradient and then an isopycnic cesium chloride gradient (Supplementary Fig. S2), before dialysis against HBSS as described previously.54 All AAV vectors were quantified by quantitative PCR (qPCR) according to the method of Aurnhammer et al.55 or Southern blot as described previously.47
Cell Lines
HEK293 and HEK293T cells have been described previously.56,57 Stable cell lines 293T-hCD4 and 293T-RhCD4 that express human CCR5 plus human or rhesus CD4 were generously provided by Dr. Julie Overbaugh.58 All cells were grown in Dulbecco's modified Eagle's medium +10% fetal bovine serum (FBS).
Primary CD4+ T Cells and In Vitro AAV Transduction
Primary CD4+ T cells were extracted from human peripheral blood mononuclear cells (PBMCs) using the EasySep human CD4 T cell isolation kit (Stemcell) and from rhesus macaque peripheral blood using a custom rhesus CD4 T cell isolation kit (Stemcell). Cells were cultured in Iscove's modified Dulbecco medium plus 10% FBS containing 30 U/mL of rhIL-2 (human and rhesus cells). Before AAV transductions, cells were plated at 2.5 × 106 cells/well in 24-well plates and activated for 72 h with CD3/CD28 Dynabeads for human CD4+ T cells (Thermo Fisher) and according to the protocol of Munoz et al. for rhesus CD4+ T cells,59 using paramagnetic beads (Dynabeads M-450 Tosylactivated; Thermo Fisher) coated with mouse monoclonal antibodies anti-human CD3 (clone SP34-2; BD Biosciences, San Jose, CA) and anti-human CD28 (CD28.2 obtained from Dr. Daniel Olive, INSERM, France, through the NIH Nonhuman Primate Reagent Resource). Activated primary CD4+ T cells were incubated with iodixanol-purified AAV vectors at the indicated multiplicity of infection (MOI) for 2.5 h before media were changed, and levels of gene transfer were measured at 48 h post-transduction. IRB approval by University of Washington, Study 000004312, “Protocol for the collection of laboratory research specimens.”
Anti-AAV Enzyme-Linked Immunosorbent Assay
MaxiSorp Immunoplates (Nunc) were coated with AAV6 particles overnight at 4°C in a volume of 50 μL using 1 × 109 vector genomes/well diluted in carbonate-bicarbonate coating buffer (Cat. No. c3041-50CAP; Sigma-Aldrich). Wells were then washed three times with PBS-T (0.05% Tween 20 in PBS), incubated with blocking/binding buffer (PBS-T + 6% bovine serum albumin [BSA]) for 2 h at room temperature, and then washed three more times with PBS-T. Macaque serum was heat inactivated at 56°C for 30 min, serially diluted in blocking/binding buffer, and added to wells for 1 h at 37°C in a volume of 50 μL. IV immunoglobulin (Gammagard; Cat. No. 1500190; Baxter) was used as a positive control, and guinea pig serum (Cat. No. GTX27482; Gene Tex) was used as a negative control. Wells were then washed three times with PBS-T before incubation with 50 μL of a 1:500 dilution in blocking/binding buffer of mouse anti-human IgG Fc HRP-conjugated antibody (clone JDC-10,Cat. No. 555788; SouthernBiotech) for 1 h at 37°C. Wells were washed three more times with PBS-T, and then 100 μL of TMB substrate solution (Cat. No. N301; Thermo Fisher) was added for 15–30 min at room temperature before stopping the reaction by adding 100 μL of TMB stop solution (Cat. No. N600; Thermo Fisher) and reading the plate absorbance at 450 nm.
Animal Welfare Statement
The protocol was approved by the Institutional Animal Care and Use Committee of the University of Washington (Protocol No. 2370-30), and was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH (“The Guide”). All animals were housed at the Washington National Primate Research Center (WaNPRC) and were housed using standard WaNPRC recommendations and monitoring procedures.
Study Details
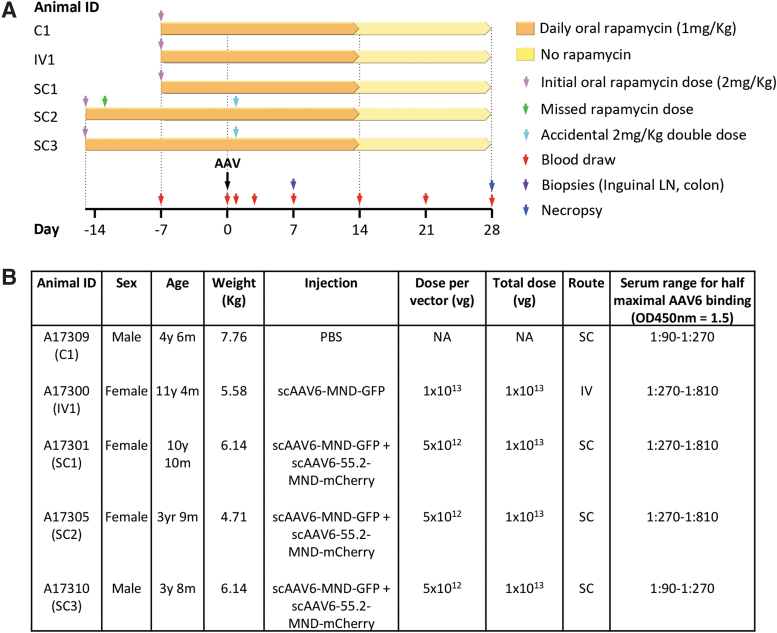
Studies were performed in male and female rhesus macaques 3–11 years of age, and 4–8 kg in size. Rapamycin (Selleck Chemicals) was administered orally from 7 or 15 days pre-AAV administration with an initial single dose of 2 mg/kg, followed by daily doses of 1 mg/kg up to 14 days post-AAV administration. Drug was administered daily as powder in an applesauce treat, or through oral gavage under sedation. Animals were administered AAV through IV injection in the saphenous vein or through interscapular SC injection in the back in a volume of 10 mL diluted in 1 × UPS grade PBS (Quality Biological, Inc.). Animals were administered a total dose of 1 × 1013 vector genomes per animal of either scAAV6-MND-GFP or a 50:50 mixed-dose preparation of scAAV6-MND-GFP and scAAV6-55.2-MND-mCherry vectors. Blood draws were performed on days 0, 1, 3, 7, 14, 21, and 28 post-AAV administration. On day 7 post-AAV administration, a single inguinal LN was removed and punch biopsies were taken from the colon. Animals were humanely euthanized on day 28 post-AAV administration.
Blood, LN, Colon, and Spleen Processing
Complete blood count (CBC) and T cell subset analysis was performed by the WaNPRC virology core. Blood chemistry analysis was performed at the University of Washington Medical Center clinical laboratory. PBMCs were isolated from whole blood by Ficoll density gradient separation and cryopreserved in freezing medium (90% FBS and 10% dimethyl sulfoxide [DMSO]). All tissues for lymphocyte extraction were collected into R10 media (RPMI 1640 and 10% FBS) and stored on ice until processing. For lymphoid cell extraction, colonic tissue was digested in R10 media containing 0.1 U/mL liberase (Roche) and 20 μg/mL Dnase I (Sigma-Aldrich) at 37°C for 1 h with agitation. Digested tissue was then filtered through a 70 μM cell strainer, pelleted, and then washed with fresh R10 media before cell counting and cryopreservation in freezing medium. For LNs and spleen, fat was trimmed before tissue was cut into small pieces and ground through a 70 μM cell strainer. Cells were pelleted and then washed with fresh R10 media before cell counting and cryopreservation in freezing medium. For spleen, a 5-min incubation in ACK lysing solution (Thermo Fisher) was performed immediately after the wash step to remove contaminating erythrocytes.
Flow Cytometry and Cell Sorting
Flow cytometry was performed using cryopreserved PBMCs or tissue-derived mononuclear cells. Cells were stained with the following antibodies: BV570 mouse anti-human CD20 (No. 302332, 1:20 dilution; Biolegend), BV605 mouse anti-human CD4 (No. 317438, 1:20 dilution; Biolegend), BV785 mouse anti-human CD14 (No. 301840, 1:20 dilution; Biolegend), BV650 mouse anti-human CD3 (No. 563916, 1:20 dilution; BD Biosciences), ApcCy7 mouse anti-human CD8 (No. 557760, 1:20 dilution; BD Biosciences), BV421 mouse anti-human CD95 (No. 562616, 1:10 dilution; BD Biosciences), PE mouse anti-human CD28 (No. 556622, 1:10 dilution; BD Biosciences), PE-Cy7 mouse anti-rhesus macaque CD45 (No. 561294, 1:20 dilution; BD Biosciences), and PerCP-eFluor710 rat anti-human CCR7 (No. 46-1979-42, 1:10 dilution; ThermoFisher). Stained cells were acquired on an FACSARIA III (BD Biosciences) and analyzed using FlowJo software v10.5.3 (FlowJo, LLC).
Quantitative PCR
Before DNA extraction, tissue was snap frozen, PBMCs were cryopreserved in freezing media, and tissue-derived mononuclear cells were snap frozen. Before RNA extraction, tissue samples or lymphocyte populations were placed in RNA Later and PBMCs were cryopreserved in freezing media. DNA was extracted using the Roche MagNA Pure 96 system. AAV vector genomes were detected using primer probe sets specific for GFP and mCherry, and copies per cell were calculated using a primer/probe set designed for the rhesus RPP30 (Supplementary Table S1). Levels of GFP or mCherry messenger RNA (mRNA) were also detected by quantitative reverse transcription (qRT)-PCR using the same primer/probe sets.
Histology and Immunohistochemistry
Tissues were formalin fixed and then paraffin embedded after necropsy before sectioning at 5 μm. For basic histology, sections were stained with hematoxylin and eosin. To detect expression of GFP or mCherry in different tissues, immunohistochemistry was performed on the Leica Bond RX automated stainer (Leica Biosystems). GFP was detected using rabbit polyclonal anti-GFP (No. A11122; 1:1,000 dilution; ThermoFisher) primary antibody, TCT blocking buffer (Leica Biosystems), H2 antigen retrieval buffer (Leica Biosystems), and H2O2 peroxidase block (Leica Biosystems). mCherry was detected using mouse monoclonal anti-mCherry (Clone G6; 1:2,000 dilution; Fred Hutch Experimental Histopathology) primary antibody, TCT blocking buffer (Leica Biosystems), H1 antigen retrieval buffer (Leica Biosystems), and H2O2 peroxidase block (Leica Biosystems). Primary antibodies were detected with PowerVision Poly-HRP anti-rabbit (Leica Biosystems) or PowerVision Poly-HRP anti-mouse (Leica Biosystems), respectively, and DAB chromagen substrate (Leica Biosystems). Sections were counterstained with hematoxylin.
For fluorescent labeling of GFP-positive cells in combination with CD8 or CD20 cells in trapezius muscle, antigen retrieval was performed on deparaffinized sections using sodium citrate buffer (10 mM sodium citrate and 0.05% Tween 20, pH 6.0). Sections were then blocked with 10% donkey serum +1% BSA in TBS before incubation with goat anti-GFP (No. NB100-1770; 1:200; Novus) in combination with rabbit anti-CD8 (No. ab4055; 1:200; Abcam) or rabbit polyclonal anti-CD20 (No. ab9475; 1:1,000; Abcam) antibody in TBS +1% BSA. Donkey anti-goat Alexa Fluor 488 (No. ab150129; 1:200; Abcam) and donkey anti-rabbit Alexa Fluor 594 (No. A-21207; 1:200; ThermoFisher) secondary antibodies were diluted in TBS +1% BSA.
Enzyme-Linked Immunospot Assay
IFNγ-specific enzyme-linked immunospot (ELISpot) assays were performed in triplicate using the human IFNγ single-color 384-well enzymatic ELISPot assay (ImmunoSpot) according to the manufacturer's recommendations. Briefly, 105 PBMCs were stimulated with the indicated mitogens/antigens for 24 h before incubation with IFNγ-capture antibody and subsequent detection of IFNγ-specific responses. PBMCs were stimulated with DMSO, 5 μg/mL Concanavalin A resuspended in sterile water, recombinant mCherry (No. TP790040; Origene), and recombinant eGFP (No. TP790050; Origene) readjusted to 1 μg/mL in DMSO, or 1 μg/mL of pooled 15mer peptides with 11 amino acid overlap spanning the entire length of eGFP (No. PM-EGFP; JPT Peptide Technologies GmbH) or AAV6 VP1 (No. PM-AAV6-VP1; JPT Peptide Technologies GmbH) resuspended in DMSO.
Results
Detection of Anti-AAV6 Antibodies in Rhesus Macaques
Pre-existing capsid-specific antibodies pose a hurdle to AAV-mediated gene delivery as they can prevent transduction through direct neutralization and may play a role in other cell-mediated AAV vector clearance mechanisms.60,61 In the healthy human population, pre-existing anti-AAV6 antibodies have been reported at levels of up to 46%,62 and in a study of 50 rhesus macaques, the prevalence of anti-AAV6 NAbs was also high (68%).13 We therefore developed an enzyme-linked immunosorbent assay (ELISA) to quantify capsid-specific antibodies against AAV6 and used this ELISA to screen a panel of 11 rhesus macaques that were available for our study for pre-existing antibodies that could potentially impede gene transfer by AAV6-derived vectors. We screened sera from several different species, including sheep, mouse, and guinea pig, to identify one that did not bind to AAV6 virions and found that guinea pig sera was the best internal negative control serum (data not shown). Unexpectedly, moderate to high levels of anti-AAV6 capsid-specific binding antibodies were detected in all animals, with half maximal binding (OD 450 nm = 1.5) seen between a 1:90 and 1:2,430 dilution of serum (Supplementary Fig. S3). The five animals with the lowest levels of AAV6-specific antibodies (half maximal binding over an approximately threefold dilution range, 1:90–1:810) were selected for use in our study (A17309 < A17310 < A17305 < A17300 < A17301).
Generation of CD4 Retargeted AAV Vectors
Recent studies have shown that AAV6 vectors transduce primary human T cells in vitro at higher rates than other AAV serotypes.7–11 It is not clear what properties of AAV6 facilitate this transduction, but it could be due to AAV6 receptor usage in T cells or faster uncoating of AAV6 capsids than other serotypes in some cell types.63,64 When administered systemically in vivo, however, AAV6 displays broad tropism for multiple tissues, including liver, heart and skeletal muscle, suggesting that vector sequestration in nontarget organs and cell types47,65–70 would pose a hurdle to using AAV6 to target CD4+ T cells when delivered systemically. To harness the T cell transduction properties of the AAV6 virion, while also reducing off-target transduction, we used a previously described approach for redirecting AAV tropism to CD4-expressing cells through capsid surface display of one of two different CD4 binding DARPins71–73 (Supplementary Fig. S1A–C). We generated both AAV2 and AAV6 vectors that display each CD4-binding DARPin (55.2 or 57.2) on the capsid surface and determined their ability to transduce cells through human and rhesus CD4 in HEK293T cells (Supplementary Data and Supplementary Figs. S4 and S5).
Based on our observations in HEK293T cells expressing CD4, we next incubated CD3-/CD28-activated primary human or rhesus macaque CD4+ T cells with control or CD4-targeted AAV6 vectors at an MOI of 500,000 or 750,000 genomes/cell. In primary human CD4+ T cells, only AAV6, AAV6-55.2, and AAV6-57.2 vectors transduced human CD4+ T cells, with up to 39%, 39%, and 18% of cells GFP positive, respectively, at 48 h postinfection (Supplementary Fig. S6A). Surprisingly, in primary rhesus macaque CD4+ T cells from two independent control donors, gene delivery was low and was only seen with the AAV6-55.2 and AAV6-57.2 vectors, with GFP expression detected in up to 5.6% of cells incubated with both vectors at 48 h postinfection (Supplementary Fig. S6A, B). Based on its overall superior performance, the AAV6-55.2 vector was chosen for subsequent studies.
In Vivo Evaluation of AAV6- and CD4-Retargeted AAV6 Vectors
The primary aim of our study was to compare the biodistribution and gene transfer of AAV6- and CD4-retargeted AAV6 vectors, leading to a study design of 4 weeks of observation followed by analysis at necropsy. Since all the macaques tested were AAV6 seropositive, we elected to study the five animals with the lowest levels of anti-AAV6 binding antibodies, which were then immunosuppressed with rapamycin. This would be expected to limit clearance of AAV vector by pre-existing NAbs, and to minimize the effect of pre-existing transgene or AAV-specific cytotoxic T cells (CTLs) on tissue biodistribution and gene transfer. Rapamycin has been shown to reduce AAV capsid- or transgene-specific humoral and cellular immune responses,24,27–29,74 and we used a previously described rapamycin dosing regimen shown to reduce humoral and cellular responses against AAV capsids and transgenes.27
Animals received a daily oral rapamycin dose for 7 or 15 days pre-AAV administration, to determine whether longer pretreatment is beneficial and received rapamycin daily until 14 days post-AAV delivery. Due to the limited number of animals, a single animal was chosen to be a no-AAV control and was administered rapamycin to control for the effects of immune suppression on various study parameters. Another animal was administered AAV6 intravenously, to control for how SC delivery affects biodistribution and gene transfer of an unmodified AAV vector when compared to this more commonly used route of AAV administration. The remaining animals were administered both AAV vectors through SC injection to determine whether transduction of LNs would be increased through this route of administration, to compare vector performance head-to-head, and to determine how animal to animal variability can affect biodistribution and gene transfer. Since all animals were seropositive for anti-AAV6 antibodies, a no rapamycin+AAV control animal was not included, as immune clearance of AAV in this animal would be highly likely, and inclusion would have limited our understanding of AAV vector biodistribution and gene transfer.
Animals given 7 days of rapamycin pretreatment were administered PBS subcutaneously (C1), AAV6 (scAAV-MND-GFP) vector intravenously (IV1), or a 50:50 mixture of AAV6 (scAAV-MND-GFP) and AAV6-55.2 (scAAV-MND-mCherry) vectors subcutaneously (SC1) (Fig. 1). Animals given 15 days of rapamycin pretreatment received the same mixed AAV vector treatment as animal SC1 subcutaneously (SC2–3). AAV6-55.2 vectors were administered subcutaneously to determine whether drainage of vector from the SC space at the site of injection into lymphatic vessels delivery would enable systemic targeting of CD4+ T lymphocytes in both blood and lymphoid tissues. Blood samples were drawn on days −15, −7, 0, 1, 3, 7, 14, 21, and 28 for CBC, blood chemistry analysis, and PBMC isolation (Fig. 1). Colonic punch biopsies and inguinal LN biopsies were collected on day 7. On day 28, animals were humanely euthanized and multiple tissues taken for analysis.
Figure 1.
Experimental details. (A) Timeline for individual animal treatments. Rapamycin was given daily by oral administration to all animals, and blood and tissue sampling were performed as indicated. (B) Animal sex, age, weight, injection details, and levels of pre-existing anti-AAV6 antibodies at study initiation. Animals IDs from Supplementary Fig. S6 were changed for clarity to reflect the control PBS-injected (C1), intravenously injected (IV1), and subcutaneously injected (SC1-SC3) animals. IV, intravenous; NA, not applicable; PBS, phosphate-buffered saline; SC, subcutaneous; vg, vector genomes.
AAV Vector Biodistribution
The biodistribution of AAV6 (scAAV6-MND-GFP) and AAV6-55.2 (scAAV6-55.2-MND-mCherry) vectors delivered intravenously or subcutaneously was measured using GFP- and mCherry-specific qPCR. Longitudinal blood samples, tissue biopsies from day 7, and necropsy tissue samples from day 28 were sampled as indicated (Fig. 1) so that vector genome levels could be quantified in each location. Vector genome levels were first analyzed in PBMCs isolated on days 0, 1, 3, 7, 14, 21, and 28 post-AAV delivery (Fig. 2A). In all three animals receiving both vectors subcutaneously (SC1-SC3), AAV6 vector DNA was detected at higher levels than AAV6-55.2 vector DNA at all time points (range 1.4–18.7-fold higher, days 1–7), and AAV6 was persistently detected in PBMCs up to day 28 post-AAV administration. In contrast, AAV6-55.2 was not detectable in PBMCs beyond day 7 post-AAV administration. AAV6 levels in PBMCs from animal IV1 that only received AAV6 were comparable at all time points with those in the three animals receiving both vectors subcutaneously.
Figure 2.
AAV vector biodistribution. Quantification of AAV6-GFP and AAV6-55.2-mCherry vector genomes in PBMCs on days 0–28 (A), mononuclear cells from day 7 colon and inguinal LN biopsies (B), day 28 lymphoid necropsy tissues (C), and day 28 non-lymphoid necropsy tissues (D, E). All tissue DNA samples were treated with Chelex to remove PCR inhibitors. eGFP and mCherry copies are shown per two copies of the rhesus RPP30 housekeeping gene. Asterisk, PCR reaction inhibited despite Chelex treatment. AAV, adeno-associated virus; LN, lymph node; PBMCs, peripheral blood mononuclear cells; PCR, polymerase chain reaction.
We next quantified AAV vector DNA levels in colonic punch biopsies and inguinal LNs biopsied on day 7 post-AAV administration (Fig. 2B). Again, AAV6 was detected at higher levels than AAV6-55.2 (2.06–95.8-fold higher—colon and 1.57–8.45-fold higher—inguinal LN) in animals receiving both vectors. In animal IV1, AAV6 levels were at least 2.75-fold (colon) and 1.56-fold (inguinal LN) higher than those seen in any of the subcutaneously AAV-injected animals. Interestingly, day 7 levels of AAV6 in the colon of animals SC2 and SC3 were ≥15.58-fold higher than those seen in animal SC1, despite receiving the same combination and dose of AAV vectors, suggesting shorter rapamycin pretreatment (7 vs. 15 days) may influence vector sequestration in some organs.
AAV vector was also quantified in DNA extracted from lymphoid (Fig. 2C) and non-lymphoid (Fig. 2D, E) necropsy tissues isolated on day 28 post-AAV administration. In animals SC1, SC2, and SC3, AAV6 and AAV6-55.2 were detected in all lymphoid tissues, and AAV6 was detected at higher levels than AAV6-55.2 (range 1.56–255-fold higher). AAV6 levels in animal IV1 were comparable to those seen in SC1, SC2, and SC3, except in the axillary LN, where levels were significantly higher in animals SC1-SC3 (≥18-fold). The higher levels of AAV vector seen in axillary LNs following interscapular SC AAV delivery are likely due to uptake of vector by lymphatic vessels close to the injection site followed by drainage into the proximal axillary LNs. As in the colon on day 7, day 28 levels of AAV6 in the colon and spleen of animals SC2 and SC3 were ≥15-fold and ≥3-fold higher, respectively, than those seen in animal SC1, again suggesting that length of rapamycin pretreatment may play a role in AAV sequestration within some organs.
AAV transgenes were detected in all non-lymphoid tissues, except for the hippocampus, basal ganglia, and thalamus, which did not contain AAV6-55.2, and AAV6 levels were higher than AAV6-55.2 in all tissues for animals SC1-SC3 at all time points. The highest levels of both AAV vectors were detected in the trapezius muscle of animals injected subcutaneously, followed by the liver, and interscapular skin of animals injected subcutaneously. Notably, animal IV1 had >3 logs less AAV6 and AAV6-55.2 in the trapezius muscle than animals SC1-SC3, indicating that injection site proximity was likely the cause of trapezius muscle AAV sequestration. Overall, levels of AAV were comparable in most organs from animals that received AAV6 intravenously compared with those receiving AAV6 subcutaneously, except in tissue adjacent to the SC injection site (interscapular skin, axillary LN, and trapezius muscle), where levels were higher in animals SC1-SC3, or in the brain, where IV delivery resulted in 1–2 logs more AAV6 in the cerebrum (hippocampus, thalamus, basal ganglia, and parietal cortex), but not the cerebellum. The duration of rapamycin treatment also appeared to influence sequestration levels for both AAV6 and AAV6-55.2 vectors in some tissues, as animal SC1, which received 1 week less rapamycin pretreatment than SC2 and SC3, had significantly lower genome levels for both AAV6 and AAV6-55.2 (3–160-fold less AAV6 and 16–163-fold less AAV6-55.2) in liver (quadrate lobe, caudate lobe, left lateral lobe, and right lateral lobe), colon, spleen, lung, heart, kidney, and cerebellum.
Tissue Gene Transfer
Gene expression by AAV6 and AAV6-55.2 vectors was analyzed by immunohistochemical staining for GFP and mCherry, respectively, in paraffin sections from liver (left and right lateral lobes), thalamus, trapezius muscle, interscapular skin, abdominal skin, spleen, and axillary, retropharyngeal, and submandibular LNs. For mCherry, mRNA expression was also analyzed in numerous tissues by qRT-PCR using RNA extracted from flash-frozen tissue. AAV6-55.2 mCherry expression was not detected in any of the analyzed organs at necropsy by either immunohistochemistry or qRT-PCR (data not shown), whereas AAV6-GFP expression was detected by immunohistochemistry in several organs.
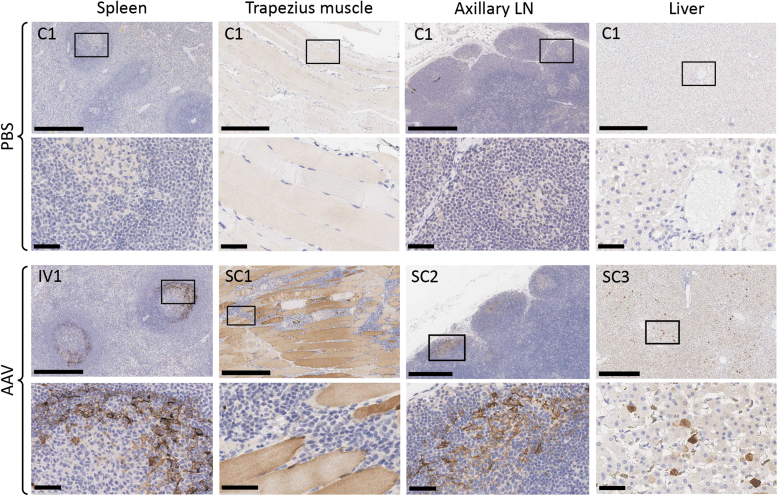
The highest levels of AAV6-GFP expression were detected in the trapezius muscle of animals SC1-SC3, but none was seen in animals C1 and IV1 (Fig. 3 and Supplementary Fig. S7). AAV6-GFP expression was detected in cells within the mantle zone surrounding splenic germinal centers for all animals receiving AAV6 vector, with significantly higher levels seen in animal IV1 (Fig. 3 and Supplementary Fig. S7). In the axillary, submandibular, and retropharyngeal LNs, low levels of AAV6-GFP expression were seen in the mantle zone and within some lymphoid follicles of all animals receiving AAV. Sporadic AAV6-GFP-positive cells were also seen in the paracortex of axillary, submandibular, and retropharyngeal LNs (Supplementary Fig. S8). Surprisingly, given the high levels of AAV DNA found 28 days after administration in animals IV1, SC2, and SC3, AAV6-GFP expression was only detected in the liver of animal SC3, which had low levels of GFP-positive hepatocytes visible throughout both the left and right lateral lobes, and at very low levels in animal SC2 (Fig. 3 and Supplementary Figs. S7 and S9).
Figure 3.
AAV6 gene transfer. Spleen, trapezius muscle, axillary LN, and liver were analyzed for GFP expression on day 28 post-AAV administration. Paraffin sections were stained by immunohistochemistry with anti-GFP antibody. Scale bar = 500 μm (upper panels); 50 μm (lower inset panels). Individual animal IDs are indicated in the upper left corner.
Lymphoid Gene Transfer
To determine which hematopoietic cell types could be transduced by AAV6 and AAV6-55.2 vectors in vivo, an 11-color flow cytometry panel consisting of antibodies for GFP, mCherry, CD3, CD4, CD8, CD14, CD20, CD28, CD45, CD95, and CCR7 was used for identification of monocytes, B lymphocytes, CD4+ helper T lymphocytes, naive CD4+ T lymphocytes, central memory CD4+ T lymphocytes, effector memory CD4+ T lymphocytes, and CD8+ cytotoxic T lymphocytes. Flow cytometry was performed on PBMCs isolated on days 0, 1, 3, 7, 14, 21, and 28, and on cells isolated from inguinal LNs or colonic biopsies on day 7. At all time points post-AAV delivery and in all cell types tested, AAV-transduced cells (GFP+ or mCherry+) were not detected above the background frequency seen in either PBS-injected animal C1 or day 0 PBMC samples (data not shown). A representative t-distributed stochastic neighbor embedding plot of this multicolor flow analysis is shown for pooled PBMC samples from all animals collected on days 0 and 3 post-AAV (Supplementary Fig. S10).
Blood Cell Counts and Blood Chemistry
No major changes in blood cell counts were observed, although white blood cell (WBC) and lymphocyte (T and B lymphocytes) counts in four of five animals were below reported normal ranges for rhesus macaques75,76 for the duration of the experiment (Supplementary Figs. S11 and S12). Notably, WBC and lymphocyte counts for all animals were low before rapamycin treatment or AAV administration. Slight decreases in hemoglobin, hematocrit, and red blood cell (RBC) counts were seen for the duration of rapamycin treatment.
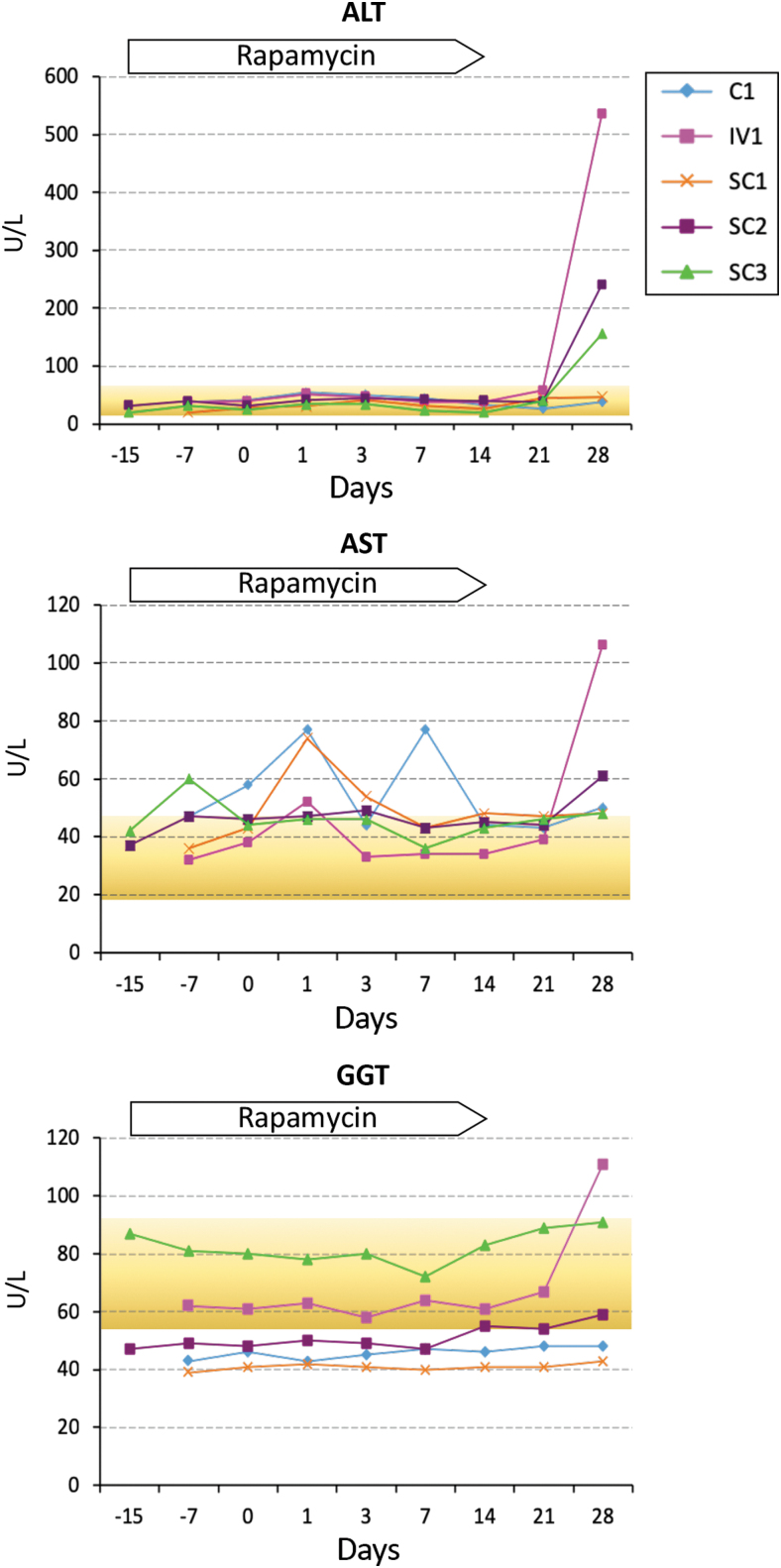
Longitudinal blood chemistry values in all five animals were predominantly within normal ranges, with the exception of ALT and AST elevations observed in multiple animals (ALT/AST >60 U/L) (Fig. 4). ALT levels remained within normal ranges in all animals for the duration of the experiment until day 28 when levels rapidly spiked in three of the four AAV-treated animals (IV1, SC2, and SC3). On day 28, the livers of these three animals contained 1–2 logs more AAV than animal SC1 (Fig. 2E), suggesting that AAV sequestration in the liver could be responsible for the observed peaks in ALT. The highest spike in ALT (535 U/L) was seen in animal IV1, which also had a shorter duration of rapamycin pretreatment than SC2 and SC3 (241 and 156 U/L, respectively). Notably, the two animals with the highest levels of ALT (IV1 and SC2) also had the highest levels of circulating T lymphocytes throughout the course of the experiment, and on the day 28 time point, these animals also had >35% increase in the number of CTL numbers when compared to day 21 (Supplementary Fig. S12). For AST, minor fluctuations above the normal range were seen in all animals (Fig. 4), and coincided with WBC, monocyte, and/or neutrophil count elevations (Supplementary Figs. S11 and S12). The peak AST value (106 U/L) was seen on day 28 in animal IV1, which also had the highest spike in ALT levels observed. Serum GGT levels in animal IV1 also spiked above normal on day 28 post-AAV administration to 111 U/L (Fig. 4, normal range 72.8 ± 19.577), further suggesting that liver damage was ongoing in this animal at the time of necropsy.
Figure 4.
Longitudinal liver enzyme levels in blood. Time points are relative to administration of PBS control or AAV vectors. Reference ranges for Macaca mulatta (mean ± SD) are indicated using data from Lee et al.77 (yellow). SD, standard deviation.
Histopathology
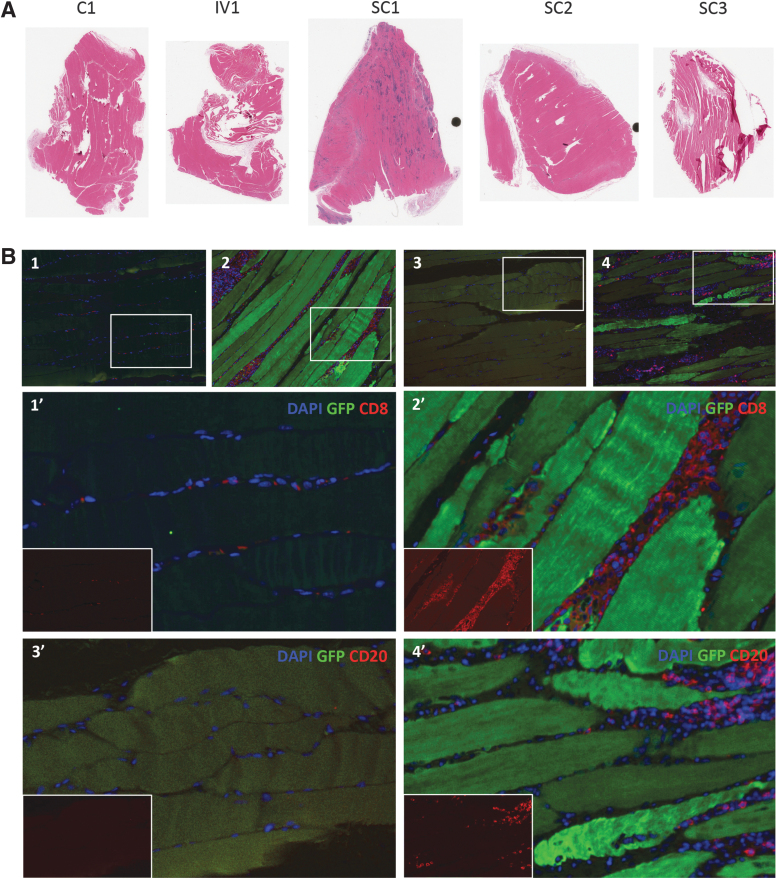
No significant changes in gross pathology were seen for all animals (Supplementary Fig. S13). Animal SC1 had megabacteria (Macrorhabdus ornithogaster) overgrowth in the cardiac stomach, which is rarely found in immunocompetent animals and likely was a consequence of rapamycin treatment. Animals IV1 and SC1 had mild to moderately extensive, multifocal, granulomatous, and fibrosing myocarditis. While a cause for the myocarditis was not identified, these were the two animals that received AAV in combination with a shorter immunosuppressive pretreatment with rapamycin. Diffuse mild-to-moderate hepatic Kupffer cell pigment deposition was seen in all animals receiving AAV (most notably in animal SC3), and was sometimes seen in canaliculi as well. This change is interpreted as representing biliary stasis, and likely was secondary to the AAV vectors, particularly as rapamycin is not known to be hepatotoxic. Notably, no influx of CD8+ or CD20+ cells above background levels in the PBS-injected control animal was seen in the livers of any animal receiving AAV (Supplementary Fig. S9). All animals that received AAV through interscapular SC injection (SC1, SC2, and SC3) had mild to multifocal, to extensive, multifocal, and coalescing granulomatous myositis of the trapezius muscle. In contrast, the gastrocnemius and bicep muscles were normal (data not shown). The trapezius muscle of animal SC1 had extensive immune cell infiltration when compared with the other animals (Figs. 3 and 5A and Supplementary Figs. S7 and S13), with CD8+ and CD20+ cells detected throughout the infiltrate by immunohistochemistry (Fig. 5B). Of note, animal SC1 received both the shortest length of rapamycin pretreatment before AAV administration and had the highest levels of anti-AAV6 antibodies in its serum at study onset, both likely contributing to the robust inflammation.
Figure 5.
Inflammation proximal to the injection site. (A) Trapezius muscle from each animal was stained with hematoxylin and eosin to visualize inflammation proximal to the SC injection site. (B) Immunohistochemistry of trapezius muscle sections from animals C1 (control, B1, B3) or SC1 (AAV+short-dose rapamycin, B2, B4) were performed to detect GFP transgene expression in combination with infiltrating CD8+ T cells (B1, B2) or CD20+ B cells (B3, B4). Inset images (B1′, B2′, B3′, B4′) show infiltrating CD8+ or CD20+ immune cells.
AAV Capsid- and Transgene-Specific T Cell Responses
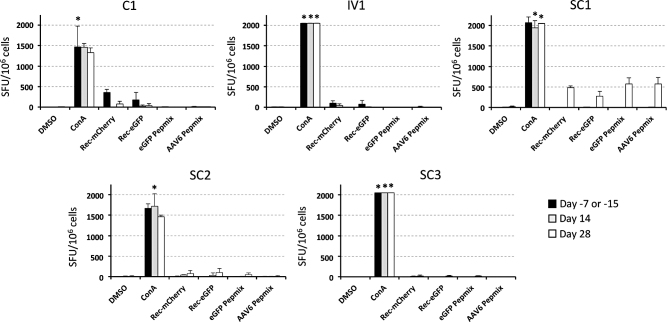
Previous studies have shown that rapamycin treatment can influence the magnitude of capsid or transgene-specific cellular and humoral immune responses. We therefore used a rhesus macaque cross-reactive IFNγ ELISpot assay to determine the levels of capsid- and transgene-specific CTLs in PBMCs isolated from each animal, before rapamycin treatment (days −7 or −15), on the last day of rapamycin treatment (day 14), and 2 weeks after the cessation of rapamycin treatment (day 28). PBMCs were incubated with concanavalin A, recombinant eGFP protein, recombinant mCherry protein, pooled eGFP peptides, or pooled AAV6 VP1 peptides for 24 h, and the number of spot-forming units (SFU) per well were determined the following day (Fig. 6 and Supplementary Fig. S14). Transgene and AAV6-specific T cell responses were only detected above the threshold for positivity (>50 SFU/106 cells) after AAV administration in a single animal (SC1), and only on day 28 post-AAV administration once rapamycin had been withdrawn for 14 days. In day 28 PBMCs from animal SC1, moderate IFNγ T cell responses of similar magnitudes (270–570 SFU/106 cells) were detected following stimulation with recombinant mCherry and eGFP protein, and with eGFP or AAV6 peptide pools. Notably, animal SC1 received 1 week less rapamycin pretreatment before AAV vector delivery, and also had the highest levels of pre-existing AAV6-specific humoral immunity in serum collected before study initiation (Supplementary Fig. S3).
Figure 6.
Capsid- and transgene-specific cytotoxic T cell responses. AAV6-, eGFP-, and mCherry-specific T cell responses were measured for each animal by IFNγ ELISpot using PBMCs isolated before rapamycin treatment, at the termination of rapamycin treatment, and 14 days post-cessation of rapamycin treatment. Rhesus macaque PBMCs from day −7 (animals C1, IV1, and SC1), day −15 (animals SC2 and SC3), day 14 (all animals), and day 28 (all animals) were incubated with the indicated mitogen or antigen for 24 h at 37°C. In each well, 100,000 PBMCs were then added to the IFNγ capture antibody-coated plate in triplicate to detect antigen-specific effector cells. Asterisks represent samples where at least one replicate had too many spots to count, and these samples were set at the highest detected spot count within the experiment (205 counts per well). All values were converted to SFU per million cells. ConA, concanavalin A; DMSO, dimethyl sulfoxide; ELISpot, enzyme-linked immunospot; Pepmix, tiled 15mer peptides with 11 amino acid overlap; Rec, recombinant; SFU, spot-forming units.
Discussion
Two of the main barriers to AAV use for system wide in vivo gene delivery are pre-existing humoral and cellular immunity due to previous exposures to naturally occurring AAV serotypes and the sequestration of AAV vector in nontarget tissues and cell types following systemic or localized delivery. In this study, we tested whether approaches to avoid these hurdles would enable either widespread or target cell-specific gene transfer in rhesus macaques. We also performed one of the first studies to assess the gene transfer efficiency of an engineered AAV vector in a non-human primate.
Pre-existing AAV capsid-specific NAbs and CTLs pose a significant barrier to AAV vector-mediated gene transfer. Pre-existing AAV6-specific antibodies were detected in serum from all 11 macaques screened for this study. We selected the five animals with the lowest levels of AAV6-specific antibodies for our study, and none of these animals had AAV6-specific T cells at the onset of the study. We administered the immunosuppressive drug rapamycin before and after AAV administration, as several studies have shown that rapamycin or other immunosuppressive therapies can increase the efficiency of AAV gene transfer through modulation of humoral and cellular immune responses, which may lead to tolerance to AAV capsid and transgene products.24,25,27–29 Our study used a rapamycin dosing regimen that was previously able to suppress levels of anti-AAV antibodies efficiently,27 but longitudinal serum samples were not collected, precluding analysis of the degree to which rapamycin treatment affected the levels of anti-AAV6 antibodies over time. On the other hand, we measured transgene- and capsid-specific T cell responses in PBMCs for all animals before, during, and after rapamycin treatment. In three of the four animals that received AAV, no AAV6- or transgene-specific T cell responses were detected at either 14 or 28 days post-AAV administration, despite delivery of a moderately high dose of 1013 AAV vector genomes per animal. This suggests that rapamycin may have suppressed cellular immune responses against the capsid and transgenes in these three animals during therapy. In animal SC1, robust T cell responses against both capsid and transgene were detected in macaque PBMCs (270–570 SFU/million cells), but only on day 28 post-AAV administration, when high levels of CD8- and CD20-positive cells were also seen in trapezius muscle proximal to the injection site. Notably, animal SC1 received the shortest rapamycin pretreatment period, had the highest levels of AAV6-specific antibodies before study onset, and only developed CTLs 14 days after the cessation of rapamycin treatment. Despite these encouraging effects of rapamycin on cellular immunity, the lack of available serum samples prevents comparison of the relative effects on cellular versus humoral immunity. Efficient suppression of anti-AAV antibody levels by rapamycin would be expected to improve initial AAV biodistribution and gene transfer, and rapamycin could also blunt the boosting of circulating anti-AAV6 antibodies after AAV6 or AAV6-55.2 administration. Future studies should address the degree to which both cellular and humoral responses are impacted by similar immune suppressive regimens, particularly in a setting where subjects are effectively being “readministered” an AAV vector to which they already show seropositivity.
In an attempt to prevent nonspecific AAV sequestration and facilitate gene transfer into CD4+ cells throughout the body, we generated detargeted and CD4-retargeted vectors based on the AAV6 serotype, which is able to efficiently transduce primary human CD4+ T cells in vitro.7–11 Like AAV6, CD4-retargeted AAV6 vectors were able to transduce human CD4+ T cells, but unexpectedly neither vector performed as well in primary rhesus macaque CD4+ T cells isolated from two independent donors. This species difference could be due to lower activity from the MND promoter in rhesus CD4+ T cells than in human CD4+ T cells, although the MND promoter has shown activity in other rhesus hematopoietic cell populations previously.78 CD4-retargeted AAV6-55.2 and AAV6-57.2 vectors were able to transduce HEK293 cells through human and rhesus CD4 in vitro (Supplementary Fig. S5), so it is unlikely that receptor binding is the reason for this observation. It is possible that species-specific differences in vector uptake, trafficking, and uncoating occur between rhesus and human CD4+ T cells, and might account for the observed differences. Alternatively, the species disparity may be due to differences in primary CD4+ T cell activation status following stimulation by human versus rhesus CD3/CD28 beads in culture. Further studies will be required to determine which factors influence these differences.
Despite our attempt to prevent nonspecific sequestration, our AAV6-55.2 vector was found in almost all organs after SC delivery, a delivery route that was chosen to facilitate more rapid drainage of vector into LNs that are rich in tissue-resident CD4+ T cells based on previous studies using nanoparticles of a similar size to AAV.79,80 While SC delivery of AAV between the shoulder blades did lead to efficient local delivery of both AAV6 and AAV-55.2 to skin and trapezius muscle, overall, the biodistribution of AAV6-55.2 following SC delivery was similar to that seen for unmodified AAV6, although at lower levels. This implies that much of the in vivo biodistribution of AAV6 could be independent of its ability to utilize either HSPGs or sialic acid for cell entry, as known receptor usage for AAV6 and AAV6-55.2 does not overlap (HSPG/sialic acid vs. CD4). Since our study was small, all animals were sacrificed on day 28 post-AAV administration, and no tissue biodistribution data are available from earlier time points, it is not possible to determine whether the higher levels of AAV6 observed were a result of AAV6-55.2 detargeting, or whether cross competition between AAV6 and AAV6-55.2 vectors through an unknown mechanism may have contributed to the observed differences. The biodistribution of AAV6 and AAV6-55.2 may be less distinct early after AAV administration, and it is possible that AAV6-55.2 was cleared from organs more rapidly than AAV6 by day 28 post-AAV delivery. Furthermore, even though AAV6-55.2 was able to transduce activated human and rhesus macaque CD4+ T cells in vitro, and vector genomes were detected in macaque PBMCs and lymphoid organs, no gene transfer was detected from this vector in lymphoid cells from any animal. Additional studies are needed to determine which mechanisms influence the observed differences in biodistribution in vivo.
Previously, Munch et al. showed that enrichment for DARPin containing virions through iMac His purification increases the specificity of AAV2-55.2 vectors for human CD4+ T cells both in vitro and in vivo.73 Our AAV6-55.2 vector was not iMac purified so a fraction of the vector likely did not display DARPin 55.2 on the capsid surface. As much as 68% of virions within an AAV-DARPin vector prep are thought to display DARPins on their surface,73 but for our AAV6-55.2 vector preparation, it is unknown what percentage of virions displayed DARPin 55.2. This may explain why the biodistribution of AAV6-55.2 was not more restricted to tissues containing CD4+ lymphocytes, and was similar to AAV6 vector biodistribution. Previous targeting studies showing that iMac-purified AAV2-55.2 vectors can selectively transduce CD4+ T cells in vivo were performed in humanized NSG mice that contain CD4+ T cells. Unlike humanized NSG mice, macaques contain a full complement of immune cells and other cell types that express CD4, such as monocytes, macrophages, and microglia, which may have acted as a sink for AAV6-55.2.
A recent study in rhesus macaques and pigs found that a very high AAV dose of 2 × 1014 genomes/kg caused severe toxicity, transaminitis, and in extreme cases, liver failure, or proprioceptive deficits and ataxia that impaired movement, all of which necessitated euthanasia.38 Our animals received a significantly lower, but still moderately high AAV dose, but were nevertheless monitored for hallmarks of AAV-mediated toxicity such as transaminitis, or fluctuations in blood cell counts. Previous studies in humans and macaques have seen transaminitis following delivery of AAV2, AAV8, or variant AAV9 vectors at doses ranging from 2 × 1012 to 2 × 1014 vector genomes (vg)/kg,20,21,26,38,81 which is thought to be caused by CTL-mediated lysis of AAV-transduced hepatocytes after systemic AAV administration.23 The commonly accepted explanation is that CTLs involved in this process are predominantly directed against vector transgenes and not the AAV capsid, although the main target for CTL responses following AAV gene therapy can vary between transgene and capsid across different species, including macaques.37,82–85 In macaques, transgene-specific T cells have been detected at high frequency in PBMCs and livers of some animals, and at higher levels than capsid-specific T cells.85,86 On the other hand, capsid-specific CD4+ and CD8+ T cells are detected at high frequency in both rhesus macaques and humans exposed to natural AAV infections, although their ability to differentiate and function differs from those found in humans.30 In our study, no clinically significant toxicity was observed, although ALT and AST levels above normal were seen in multiple animals, but only after rapamycin had been withdrawn for 14 days, and histologic evidence of biliary stasis was seen in all animals that received AAV. The highest elevations of hepatic enzymes were seen in the animal that received AAV intravenously, received a shorter duration of rapamycin pretreatment, had the highest baseline T lymphocyte levels, and had elevated CD8+CD4− T cell counts, concurrently with transaminitis. Interestingly, no correlation was seen between liver toxicity and treatment-related T cell responses in any animal. Moreover, transaminitis was not observed in the only animal (SC1) with detectable capsid- or transgene-specific T cells on day 28 post-AAV administration. Beyond the transaminitis observed in three of four animals receiving AAV, no other major hallmark of toxicity was detected aside from inflammation adjacent to the delivery site within the trapezius muscle of all animals receiving SC AAV, and the aforementioned biliary stasis in all animals receiving AAV.
A number of in vivo studies suggest that AAV6 has a broad tissue tropism in vivo, but the widespread transduction of muscle and other organs previously seen in mice47,65,67,69 was not observed in NHPs beyond the trapezius muscle proximal to the AAV injection site. AAV6 can transduce hepatocytes in mice,67 sheep,87 and dogs,66 but in our study, hepatocyte transduction was only detected at low levels in the two animals receiving AAV subcutaneously after the longest rapamycin pretreatment (SC2 and SC3). These animals had no AAV- or transgene-specific CTLs on day 28 post-AAV administration. Animal SC1, which also received AAV subcutaneously, but had shorter rapamycin pretreatment, had the lowest vector genome levels in liver, the highest anti-transgene and anti-AAV T cell responses on day 28 post-AAV administration, and no detectable gene transfer in liver.
In this study, we present a preclinical evaluation of an AAV6 vector and an engineered CD4-retargeted AAV6 vector in immune-suppressed rhesus macaques that had pre-existing antibodies against AAV6 at the onset of the study. This setting is conceivable in humans, where anti-AAV antibodies are prevalent. Despite the evidence of pre-existing anti-AAV6 immunity, both vectors were well tolerated, with only minor evidence of clinically insignificant toxicity. Immunosuppressive rapamycin treatment enabled high-level localized gene expression from AAV6 in trapezius muscle, and other organs following SC delivery, whereas no gene transfer was detected from the CD4-retargeted AAV6 vector in any organ. Overall, our study demonstrates that immunosuppressive therapy can enable efficient gene transfer in rhesus macaques despite the presence of pre-existing immunity to AAV, and offers hope for future AAV-mediated gene therapies that target diseases such as HIV in patients that previously may not have been considered for treatment.
Supplementary Material
Acknowledgments
We thank Jeff Chamberlain, James Allen, and Christine Halbert of the Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center vector core at the University of Washington for production of in vivo grade AAV vectors. We thank Sandra Dross and Deb Fuller for advice developing multicolor rhesus macaque flow cytometry panels. We thank David Russell for generously providing the plasmids pRepCap6 and pDGM6. We thank Julie Overbaugh for generously providing cell lines 293T-hCD4 and 293T-RhCD4.
Author Disclosure
The authors declare no competing financial interests.
Funding Information
This work was funded by grants from amfAR (ARCHE-GT grant No. 109575-62-RGRL), the National Institute of Allergy and Infectious Diseases (UM1 AI126623), and, in part, by a developmental grant from the University of Washington Center for AIDS Research (CFAR), an NIH-funded program under award number P30 AI 027757, which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK), and, in part, by NIH/NCI Cancer Center Support Grant P30 CA015704.
Supplementary Material
REFERENCES
- 1. Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet 2011;12:341–355 [DOI] [PubMed] [Google Scholar]
- 2. Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet 2014;15:445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buning H, Perabo L, Coutelle O, et al. . Recent developments in adeno-associated virus vector technology. J Gene Med 2008;10:717–733 [DOI] [PubMed] [Google Scholar]
- 4. Gardner MR, Fetzer I, Kattenhorn LM, et al. . Anti-drug antibody responses impair prophylaxis mediated by AAV-delivered HIV-1 broadly neutralizing antibodies. Mol Ther 2019;27:650–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson PR, Schnepp BC, Zhang J, et al. . Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med 2009;15:901–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez-Navio JM, Fuchs SP, Pantry SN, et al. . Adeno-associated virus delivery of anti-HIV monoclonal antibodies can drive long-term virologic suppression. Immunity 2019;50:567.e5–575.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sather BD, Romano Ibarra GS, Sommer K, et al. . Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template. Sci Transl Med 2015;7:307ra156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang J, DeClercq JJ, Hayward SB, et al. . Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery. Nucleic Acids Res 2016;44:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bak RO, Porteus MH. CRISPR-mediated integration of large gene cassettes using AAV donor vectors. Cell Rep 2017;20:750–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hale M, Lee B, Honaker Y, et al. . Homology-directed recombination for enhanced engineering of chimeric antigen receptor T cells. Mol Ther Methods Clin Dev 2017;4:192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacLeod DT, Antony J, Martin AJ, et al. . Integration of a CD19 CAR into the TCR alpha chain locus streamlines production of allogeneic gene-edited CAR T cells. Mol Ther 2017;25:949–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calcedo R, Morizono H, Wang L, et al. . Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol 2011;18:1586–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang D, Zhong L, Li M, et al. . Adeno-associated virus neutralizing antibodies in large animals and their impact on brain intraparenchymal gene transfer. Mol Ther Methods Clin Dev 2018;11:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li C, Narkbunnam N, Samulski RJ, et al. . Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther 2012;19:288–294 [DOI] [PubMed] [Google Scholar]
- 15. Erles K, Sebokova P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol 1999;59:406–411 [DOI] [PubMed] [Google Scholar]
- 16. Fitzpatrick Z, Leborgne C, Barbon E, et al. . Influence of pre-existing anti-capsid neutralizing and binding antibodies on AAV vector transduction. Mol Ther Methods Clin Dev 2018;9:119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gurda BL, DiMattia MA, Miller EB, et al. . Capsid antibodies to different adeno-associated virus serotypes bind common regions. J Virol 2013;87:9111–9124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gurda BL, Raupp C, Popa-Wagner R, et al. . Mapping a neutralizing epitope onto the capsid of adeno-associated virus serotype 8. J Virol 2012;86:7739–7751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calcedo R, Wilson JM. AAV Natural infection induces broad cross-neutralizing antibody responses to multiple AAV serotypes in chimpanzees. Hum Gene Ther Clin Dev 2016;27:79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manno CS, Pierce GF, Arruda VR, et al. . Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 [DOI] [PubMed] [Google Scholar]
- 21. Jiang H, Couto LB, Patarroyo-White S, et al. . Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 2006;108:3321–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scallan CD, Jiang H, Liu T, et al. . Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 2006;107:1810–1817 [DOI] [PubMed] [Google Scholar]
- 23. Pien GC, Basner-Tschakarjan E, Hui DJ, et al. . Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest 2009;119:1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meliani A, Boisgerault F, Hardet R, et al. . Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat Commun 2018;9:4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corti M, Elder M, Falk D, et al. . B-cell depletion is protective against anti-AAV capsid immune response: a human subject case study. Mol Ther Methods Clin Dev 2014;1:14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nathwani AC, Tuddenham EG, Rangarajan S, et al. . Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramsingh AI, Gray SJ, Reilly A, et al. . Sustained AAV9-mediated expression of a non-self protein in the CNS of non-human primates after immunomodulation. PLoS One 2018;13:e0198154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Velazquez VM, Meadows AS, Pineda RJ, et al. . Effective depletion of pre-existing anti-AAV antibodies requires broad immune targeting. Mol Ther Methods Clin Dev 2017;4:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, et al. . Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood 2007;110:2334–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li H, Lasaro MO, Jia B, et al. . Capsid-specific T-cell responses to natural infections with adeno-associated viruses in humans differ from those of nonhuman primates. Mol Ther 2011;19:2021–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parzych EM, Li H, Yin X, et al. . Effects of immunosuppression on circulating adeno-associated virus capsid-specific T cells in humans. Hum Gene Ther 2013;24:431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao G, Alvira MR, Somanathan S, et al. . Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci U S A 2003;100:6081–6086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao G, Vandenberghe LH, Alvira MR, et al. . Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol 2004;78:6381–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaufmann KB, Buning H, Galy A, et al. . Gene therapy on the move. EMBO Mol Med 2013;5:1642–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buning H, Srivastava A. Capsid modifications for targeting and improving the efficacy of AAV vectors. Mol Ther Methods Clin Dev 2019;12:248–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vandamme C, Adjali O, Mingozzi F. Unraveling the complex story of immune responses to AAV vectors trial after trial. Hum Gene Ther 2017;28:1061–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herzog RW. Complexity of immune responses to AAV transgene products—example of factor IX. Cell Immunol 2019;342:103658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hinderer C, Katz N, Buza EL, et al. . Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther 2018;29:285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hacker U, Buning H. Optimizing the adeno-associated viral vector system: a brief summary. Ther Deliv 2011;2:967–970 [DOI] [PubMed] [Google Scholar]
- 40. Reul J, Muik A, Buchholz CJ. Ligand coupling to the AAV capsid for cell-specific gene transfer. Methods Mol Biol 2019;1950:35–50 [DOI] [PubMed] [Google Scholar]
- 41. van Lieshout LP, Domm JM, Rindler TN, et al. . A novel triple-mutant AAV6 capsid induces rapid and potent transgene expression in the muscle and respiratory tract of mice. Mol Ther Methods Clin Dev 2018;9:323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martino AT, Basner-Tschakarjan E, Markusic DM, et al. . Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood 2013;121:2224–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huttner NA, Girod A, Perabo L, et al. . Genetic modifications of the adeno-associated virus type 2 capsid reduce the affinity and the neutralizing effects of human serum antibodies. Gene Ther 2003;10:2139–2147 [DOI] [PubMed] [Google Scholar]
- 44. Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol 1998;72:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang LY, Patel A, Ng R, et al. . Characterization of the adeno-associated virus 1 and 6 sialic acid binding site. J Virol 2016;90:5219–5230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu Z, Asokan A, Grieger JC, et al. . Single amino acid changes can influence titer, heparin binding, and tissue tropism in different adeno-associated virus serotypes. J Virol 2006;80:11393–11397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gregorevic P, Blankinship MJ, Allen JM, et al. . Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 2004;10:828–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schweizer A, Rusert P, Berlinger L, et al. . CD4-specific designed ankyrin repeat proteins are novel potent HIV entry inhibitors with unique characteristics. PLoS Pathog 2008;4:e1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seeger MA, Mittal A, Velamakanni S, et al. . Tuning the drug efflux activity of an ABC transporter in vivo by in vitro selected DARPin binders. PLoS One 2012;7:e37845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zahnd C, Wyler E, Schwenk JM, et al. . A designed ankyrin repeat protein evolved to picomolar affinity to Her2. J Mol Biol 2007;369:1015–1028 [DOI] [PubMed] [Google Scholar]
- 51. De Silva Feelixge HS, Stone D, Pietz HL, et al. . Detection of treatment-resistant infectious HIV after genome-directed antiviral endonuclease therapy. Antiviral Res 2016;126:90–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Challita PM, Skelton D, el-Khoueiry A, et al. . Multiple modifications in cis elements of the long terminal repeat of retroviral vectors lead to increased expression and decreased DNA methylation in embryonic carcinoma cells. J Virol 1995;69:748–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Choi VW, Asokan A, Haberman RA, et al. . Production of recombinant adeno-associated viral vectors for in vitro and in vivo use. Curr Protoc Mol Biol 2007;Chapter 16:Unit 16.25 [DOI] [PubMed] [Google Scholar]
- 54. Halbert CL, Allen JM, Chamberlain JS. AAV6 vector production and purification for muscle gene therapy. Methods Mol Biol 2018;1687:257–266 [DOI] [PubMed] [Google Scholar]
- 55. Aurnhammer C, Haase M, Muether N, et al. . Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther Methods 2012;23:18–28 [DOI] [PubMed] [Google Scholar]
- 56. DuBridge RB, Tang P, Hsia HC, et al. . Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol 1987;7:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Graham FL, Smiley J, Russell WC, et al. . Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 1977;36:59–74 [DOI] [PubMed] [Google Scholar]
- 58. Nahabedian J, Sharma A, Kaczmarek ME, et al. . Owl monkey CCR5 reveals synergism between CD4 and CCR5 in HIV-1 entry. Virology 2017;512:180–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Munoz NM, Trobridge GD, Kiem HP. Ex vivo expansion and lentiviral transduction of Macaca nemestrina CD4+ T cells. J Med Primatol 2009;38:438–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parren PW, Burton DR. The antiviral activity of antibodies in vitro and in vivo. Adv Immunol 2001;77:195–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mingozzi F, High KA. Overcoming the host immune response to adeno-associated virus gene delivery vectors: the race between clearance, tolerance, neutralization, and escape. Annu Rev Virol 2017;4:511–534 [DOI] [PubMed] [Google Scholar]
- 62. Boutin S, Monteilhet V, Veron P, et al. . Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010;21:704–712 [DOI] [PubMed] [Google Scholar]
- 63. Sipo I, Fechner H, Pinkert S, et al. . Differential internalization and nuclear uncoating of self-complementary adeno-associated virus pseudotype vectors as determinants of cardiac cell transduction. Gene Ther 2007;14:1319–1329 [DOI] [PubMed] [Google Scholar]
- 64. Thomas CE, Storm TA, Huang Z, et al. . Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol 2004;78:3110–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blankinship MJ, Gregorevic P, Allen JM, et al. . Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther 2004;10:671–678 [DOI] [PubMed] [Google Scholar]
- 66. Jiang H, Lillicrap D, Patarroyo-White S, et al. . Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood 2006;108:107–115 [DOI] [PubMed] [Google Scholar]
- 67. Zincarelli C, Soltys S, Rengo G, et al. . Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 2008;16:1073–1080 [DOI] [PubMed] [Google Scholar]
- 68. Towne C, Raoul C, Schneider BL, et al. . Systemic AAV6 delivery mediating RNA interference against SOD1: neuromuscular transduction does not alter disease progression in fALS mice. Mol Ther 2008;16:1018–1025 [DOI] [PubMed] [Google Scholar]
- 69. Zincarelli C, Soltys S, Rengo G, et al. . Comparative cardiac gene delivery of adeno-associated virus serotypes 1–9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci 2010;3:81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gao G, Bish LT, Sleeper MM, et al. . Transendocardial delivery of AAV6 results in highly efficient and global cardiac gene transfer in rhesus macaques. Hum Gene Ther 2011;22:979–984 [DOI] [PubMed] [Google Scholar]
- 71. Munch RC, Janicki H, Volker I, et al. . Displaying high-affinity ligands on adeno-associated viral vectors enables tumor cell-specific and safe gene transfer. Mol Ther 2013;21:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hartmann J, Munch RC, Freiling RT, et al. . A library-based screening strategy for the identification of DARPins as ligands for receptor-targeted AAV and lentiviral vectors. Mol Ther Methods Clin Dev 2018;10:128–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Munch RC, Muth A, Muik A, et al. . Off-target-free gene delivery by affinity-purified receptor-targeted viral vectors. Nat Commun 2015;6:6246. [DOI] [PubMed] [Google Scholar]
- 74. Nayak S, Sarkar D, Perrin GQ, et al. . Prevention and reversal of antibody responses against factor IX in gene therapy for hemophilia B. Front Microbiol 2011;2:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen Y, Qin S, Ding Y, et al. . Reference values of clinical chemistry and hematology parameters in rhesus monkeys (Macaca mulatta). Xenotransplantation 2009;16:496–501 [DOI] [PubMed] [Google Scholar]
- 76. Caldwell RG, Marshall P, Fishel J. Method validation and reference range values for a peripheral blood immunophenotyping assay in non-human primates. J Immunotoxicol 2016;13:64–76 [DOI] [PubMed] [Google Scholar]
- 77. Lee JI, Shin JS, Lee JE, et al. . Reference values of hematology, chemistry, electrolytes, blood gas, coagulation time, and urinalysis in the Chinese rhesus macaques (Macaca mulatta). Xenotransplantation 2012;19:244–248 [DOI] [PubMed] [Google Scholar]
- 78. Singh S, Khan I, Khim S, et al. . Safe and effective gene therapy for murine Wiskott-Aldrich syndrome using an insulated lentiviral vector. Mol Ther Methods Clin Dev 2017;4:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Freeling JP, Koehn J, Shu C, et al. . Long-acting three-drug combination anti-HIV nanoparticles enhance drug exposure in primate plasma and cells within lymph nodes and blood. AIDS 2014;28:2625–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Freeling JP, Koehn J, Shu C, et al. . Anti-HIV drug-combination nanoparticles enhance plasma drug exposure duration as well as triple-drug combination levels in cells within lymph nodes and blood in primates. AIDS Res Hum Retroviruses 2015;31:107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Greig JA, Limberis MP, Bell P, et al. . Non-clinical study examining AAV8.TBG.hLDLR vector-associated toxicity in chow-fed wild-type and LDLR(+/−) rhesus macaques. Hum Gene Ther Clin Dev 2017;28:39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mays LE, Wilson JM. The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol Ther 2011;19:16–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Basner-Tschakarjan E, Mingozzi F. Cell-mediated immunity to AAV vectors, evolving concepts and potential solutions. Front Immunol 2014;5:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Verdera HC, Kuranda K, Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol Ther 2020;28:723–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang L, Calcedo R, Wang H, et al. . The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther 2010;18:126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gao G, Wang Q, Calcedo R, et al. . Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses. Hum Gene Ther 2009;20:930–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Davey MG, Riley JS, Andrews A, et al. . Induction of immune tolerance to foreign protein via adeno-associated viral vector gene transfer in mid-gestation fetal sheep. PLoS One 2017;12:e0171132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.