Abstract
Background
The prevalence of fibromyalgia (FM) in pharmacy students and professionals is unknown. This study identifies the prevalence of FM in pharmacy students and professionals using three screening tools and factors associated with its development. Furthermore, this study assesses the level of agreement between the tools and the magnitude of the participants’ responses to each item in the screening tools.
Methods
This was a cross-sectional survey conducted on members of the Saudi Pharmaceutical Society using an online questionnaire. The participants were asked to fill three questionnaires: the London Fibromyalgia Epidemiology Study Screening Questionnaire (LFESSQ), Fibromyalgia Rapid Screening Tool (FiRST) and Fibromyalgia Survey Questionnaire (FSQ). Demographic data and factors affecting FM in pharmacy students and professionals were collected and analysed.
Results
Two hundred ninety-three participants accessed the survey: most of them were Saudi (93.5%) and females (78.8%) with a mean (standard deviation) age of 29 (8) years. Furthermore, 52% of the participants had generalised body pain. The prevalence of FM using FiRST, LFESSQ Pain, LFESSQ with fatigue criteria and FSQ was 27.1%, 34.9%, 50.9% and 68.4%, respectively. Fleiss’ kappa coefficient revealed fair agreement among all three screening tools (kappa = 0.350; p < 0.001). After adjusting for significant variables, the resulting adjusted odds ratio of developing FM was 4.86 in people working for 41–45 h weekly (95% confidence interval [CI], 1.32–17.84; p = 0.017), 5.16 in people who frequently wake up during sleep (95% CI, 1.85–14.40; p = 0.002) and 12.99 in people with sleep apnea or other sleeping disorders (95% CI, 2.07–81.68; p = 0.006).
Conclusion
FM was prevalent among pharmacy students and professionals and was much more than data reported on the general population or other healthcare workers. Traditional factors along with higher working hours were identified as significant variables.
Keywords: fibromyalgia, epidemiology prevalence, pharmacy.
Introduction
Fibromyalgia (FM) is defined according to the American College of Rheumatology (ACR) as chronic widespread pain and tenderness in at least 11 of 18 defined tender points for at least three months.1 FM is considered the second most common rheumatic disorder after osteoarthritis, affecting 5% of all women in the USA and 4.7% in European countries.2,3 The specific aetiology of FM is not clearly understood.4 People with FM report a wide array of somatic and psychological symptoms, including pain, fatigue, headache, unrefreshing sleep, depression and cognitive dysfunction.4 These symptoms have a substantial effect on physical functioning and may result in disabilities, resulting in limited social participation.5,6 Moreover, a massive impact on the quality of life,7–9 productivity and absenteeism10–12 has been found with a substantial economic burden on healthcare.13–15 Other conditions can coexist with FM, such as osteoarthritis, inflammatory arthritis, systemic lupus erythematosus, irritable bowel syndrome, anxiety, depression and headache.16,17
Patients with FM often lack a diagnosis for years due to the discrepancies in recognising symptoms and in the validity of FM as a diagnosis.18 The wide array of FM symptoms that may exist with a diversity of rheumatologic, medical or psychological comorbid conditions presents a diagnostic challenge for healthcare practitioners (HCP). The diagnosis is primarily clinically performed by HCPs using the 1990 ACR criteria considered the gold standard.19 The tender point examination in the 1990 ACR is difficult, rarely performed and mostly conducted incorrectly.20 The diagnosis of FM has evolved with the development of more practical diagnostic tools. The new tools overcoming the challenge of tender point examination made diagnosing FM possible using simple self-reported questionnaires, such as the preliminary and modified 2010 ACR,21,22 the 2016 ACR,23 the London Fibromyalgia Epidemiology Study Screening Questionnaire (LFESSQ),24 the Fibromyalgia Rapid Screening Tool (FiRST),25 and the Fibromyalgia Survey Questionnaire (FSQ).26
The increased awareness of FM with its related socioeconomic burden has led to a rise in epidemiological studies in the general or specific population. Pharmacy professionals and students are at increased risk of depression,27,28 anxiety,29 burnout,30,31 and stress.32–34 All these factors are considered as psychological determinants of FM;35 thus, investigation on the prevalence of FM in this population is highly warranted. To our knowledge, no studies have assessed the prevalence of FM in pharmacy students and professionals. Therefore, this study 1) estimates the prevalence of FM and its associated risk factors among pharmacists and pharmacy students registered in the Saudi Pharmaceutical Society (SPS) using three validated screening tools, 2) assesses the level of agreement in the three tools among identified people with FM and 3) examines the magnitude of the participants’ responses to each item in the three screening tools.
Materials and Methods
This was a cross-sectional observational study conducted using online questionnaires in the English language. The survey was sent to participants using the SPS mailing list. The SPS is a non-profit professional organisation with the aim of contributing to developing the pharmacy profession.36 The society offers membership to pharmacy professionals and students around the Kingdom of Saudi Arabia.
Participants were eligible if they were workers or students (academic year 2 to 6) for at least three months in the pharmacy field. The exclusion criteria included pharmacists or students working or studying for less than three months in the field and practitioners of other healthcare professions. Two reminders were sent to the participants after 2–4 weeks. The collection period was approximately two months. The average time to complete the questionnaire was 6 min based on a pilot study conducted on 10 participants. The study was conducted between May 2019 and June 2019.
Demographic Data and Screening Tools
The questionnaire asked about the participants’ demographic characteristics including age, sex, marriage, nationality and body mass index (BMI). Additionally, it enquired about average hours of work per week. Other secondary measures included smoking status, family history of FM, presence of chronic comorbidities, sleeping pattern (time of sleep, frequent wakeups during sleep, any naps, snoring, sleeping pill intake, and changes in sleeping pattern during weekends or vacations) and having sleep apnea or other sleep disorders. The participants who answered “yes” to the screening question “Do you have body pain” were invited to complete three validated diagnostic tools for FM, which was designed to be self-administered. The first tool was LFESSQ,24 which was designed to assess pain (4 items) and fatigue (2 items). A positive response defined when all pain items were met alone or met with the fatigue criteria. The second screening tool is the FiRST consisting of six questions related to different dimensions of FM (widespread pain, fatigue, pain characteristics, non-painful abnormal sensation, functional somatic symptoms and sleep and cognitive problems).25 Each item corresponds to one point and a cut-off score of 5 of 6 is considered positive of FM.25 The third tool was the FSQ, which consists of three parts: the Widespread Pain Index (WPI) in 19 painful areas, Symptom Severity Score (SSS) and additional criteria asking whether SSS exists for at least three months.26 Participants who satisfy the FSQ should meet the following conditions: 1) WPI in ≥7/19 pain sites and SSS of ≥5/12 or WPI between 3/19 and 6/19 and SSS of ≥9/12 and 2) symptoms have been present for at least three months.26
Statistical Analysis
Demographic characteristics and the prevalence of FM based on the three screening tools were stratified by gender and analysed using descriptive analysis. The descriptive analysis includes chi-square for categorical variable such as gender and educational status, and independent-sample t-test for continuous and categorical variables such as age and presence of FM, if the continuous data were normally distributed. The prevalence of FM was reported in percentages using the three screening tools. The concordance correlation coefficient (Cohen’s kappa) was used to assess inter-rater reliability between the tools. As proposed by Landis and Koch,37 the standards for the strength of agreement for the kappa coefficient are as follows: ≤0 = poor, 0.01–0.20 = slight, 0.21–0.40 = fair, 0.41–0.60 = moderate, 0.61–0.80 = substantial and 0.81–1 = almost perfect.
The LFESSQ was used as a binary independent variable in the logistic regression analysis to investigate the association between different demographic variables and FM. Unadjusted and adjusted odds ratio (OR) values were presented along with 95% CI, and p-values of <0.05 were used to denote statistical significance. Finally, radar chart analysis was performed to visualise the magnitude of the participants’ responses to each item of the three screening tools. Microsoft Excel 2010 was used to draw radar plots and Statistical Package for the Social Sciences (version 26; IBM Corp., Armonk, N.Y., USA) was used for all statistical analyses.
Results
Characteristics of the Participants
Two hundred and ninety-three participants accessed the survey, with 292 participants started to provide responses to the questions. Table 1 represents the demographic data of the respondents, stratified by gender. Most respondents were Saudi (93.5%) and females (78.8%) with a mean (standard deviation) age of 29 (8) years, and 45.3% of the respondents had the ideal BMI. Most responders were unmarried (61%). Among them, 32.5% had children and 10.3% admitted smoking. Almost half of the respondents (48%) were Bachelor’s degree holders, and a few (23%) were students, not yet graduated. The largest field of work was hospital (35.3%), followed by academia (18.6%). In addition, 61.1% of the respondents were satisfied with their work/study, and 36.1% work/study for 41–45 h per week. Six percent reported positive family history to FM and 17% had chronic comorbidities. Reported comorbidities included cardiovascular (n=11), psychological/neurological (n=7), thyroid disorders (n=6), asthma/allergy-related disorders (n=5), chronic diseases (n=4), and inflammatory disorders (n=2). Seven participants reported they had comorbidities without specifying the type of the disease.
Table 1.
Baseline Demographic Characteristics of the Participants, Stratified by Gender
| Female n = 228 | Male n = 64 | Total | p- value | |||
|---|---|---|---|---|---|---|
| Nationality, n (%) | n = 292 | Saudi | 215 (78.8) | 58 (21.2) | 273 (93.5) | 0.216 |
| Age, years mean (standard deviation) | n = 292 | 27 (7) | 43 (9) | 29 (8) | <0.001* | |
| Body mass index kg/m2, n (%) | n = 292 | <18.5 | 24 (82.8) | 5 (17.2) | 29 (10.0) | 0.002* |
| 18.5–24.9 | 113 (86.3) | 18 (13.7) | 131 (45.3) | |||
| 25.0–29.9 | 61 (71.8) | 24 (28.2) | 85 (29.4) | |||
| ≥30 | 27 (61.4) | 17 (38.6) | 44 (15.2) | |||
| Marital status, n (%) | n = 292 | Unmarried | 155 (87.1) | 23 (12.9) | 178 (61.0) | <0.001* |
| Married | 73 (64.0) | 41 (36.0) | 114 (39.0) | |||
| Children, n (%) | n = 292 | Yes | 62 (65.3) | 33 (34.7) | 95 (32.5) | <0.001* |
| Smoking, n (%) | n = 292 | Yes | 7 (23.3) | 23 (76.7) | 30 (10.3) | <0.001* |
| Scientific degree, n (%) | n = 269 | Not yet graduated | 58 (93.5) | 4 (6.5) | 62 (23.0) | 0.001* |
| Diploma | 0 (0.0) | 2 (100.0) | 2 (0.7) | |||
| Bachelor/Pharm D | 96 (74.4) | 33 (25.6) | 129 (48.0) | |||
| Master | 35 (81.4) | 8 (18.6) | 43 (16.0) | |||
| Doctor of philosophy | 23 (69.7) | 10 (30.3) | 33 (12.3) | |||
| Field of work, n (%) | n = 269 | Hospital | 70 (73.7) | 25 (26.3) | 95 (35.3) | <0.001* |
| Industry | 15 (50.0) | 15 (50.0) | 30 (11.2) | |||
| Academia | 41 (82.0) | 9 (18.0) | 50 (18.6) | |||
| Student | 86 (91.5) | 8 (8.5) | 94 (34.9) | |||
| Satisfaction with your work/training/study, n (%) | n = 269 | Highly satisfied | 34 (72.3) | 13 (27.7) | 47 (17.4) | 0.137 |
| Satisfied | 89 (75.4) | 29 (24.6) | 118 (43.7) | |||
| Partially satisfied | 75 (87.2) | 11 (12.8) | 86 (31.9) | |||
| Not satisfied | 11 (73.3) | 4 (26.7) | 15 (5.6) | |||
| Highly unsatisfied | 4 (100.0) | 0 (0.0) | 4 (1.5) | |||
| Number of hours in work/study per week, n (%) | n = 269 | 35–39 h | 66 (85.7) | 11 (14.3) | 77 (28.6) | 0.086 |
| 40 h | 47 (83.9) | 9 (16.1) | 56 (20.8) | |||
| 41–45 h | 69 (71.1) | 28 (28.9) | 97 (36.1) | |||
| Other | 30 (76.9) | 9 (23.1) | 39 (14.5) | |||
| Family history for FM, n (%) | n= 234 | Yes | 14 (7.2) | 1 (2.5) | 15 (6.4%) | 0.099 |
| Chronic comorbidities, n (%) | n= 243 | Yes | 33 (16.4) | 9 (21.4) | 42 (17.3%) | 0.282 |
| Sleep time, n (%) | n = 223 | Before 12 AM | 114 (84.4) | 21 (15.6) | 135 (60.5) | 0.166 |
| After 12 AM | 69 (78.4) | 19 (21.6) | 88 (39.5) | |||
| Frequently wake up during sleep, n (%) | n = 223 | Yes | 74 (81.3) | 17 (18.7) | 91 (40.8) | 0.472 |
| Take naps during the day, n (%) | n = 223 | Yes | 54 (80.6) | 13 (19.4) | 67 (30.0) | 0.421 |
| Take sleeping pills, n (%) | n = 223 | Yes | 14 (87.5) | 2 (12.5) | 16 (7.2) | 0.426 |
| Snore during sleep, n (%) | n = 223 | Yes | 25 (58.1) | 18 (41.9) | 43 (19.3) | <0.001* |
| Sleep apnea or other sleeping disorders, n (%) | n = 223 | Yes | 9 (69.2) | 4 (30.8) | 13 (5.8) | 0.187 |
| Sleeping pattern changes during vacation or weekends, n (%) | n = 223 | Sleep earlier | 11 (84.6) | 2 (15.4) | 13 (5.8) | 0.700 |
| Sleep later | 141 (82.9) | 29 (17.1) | 170 (76.2) | |||
| No change | 31 (77.5) | 9 (22.5) | 40 (17.9) | |||
Note: *Significance level of p value <0.050.
With regard to sleeping patterns, 60.5% sleep before midnight and 76.2% sleep later during the weekends. Less than half (40.1%) of the respondents have reported that they frequently wake up during sleep, almost one-third take naps during the day (30.0%) and less than a quarter take sleeping pills, snore and have a sleeping disorder (7.2%, 19.3 and 5.8%, respectively).
Univariate analysis showed that a significant difference in age, BMI, marital status, having children, smoking, scientific degree and field of work between males and females where females were younger, had a lower BMI, were less likely to be married or have children, were more likely to smoke, had less scientific degree and were more likely to work in the industry. The resulting p-value of univariate analysis is shown in Table 1.
Prevalence of Fibromyalgia
With regard to FM prevalence, only 220 participants completed the survey among whom 52.3% had generalised body pain and possible FM (Table 2). The prevalence of FM (number of participants who filled the tool) using the FiRST, LFESSQ Pain, LFESSQ Fatigue and FSQ was 27.1% (n = 118), 34.9% (n = 106), 50.9% (n = 108) and 68.4% (n = 98), respectively. When the prevalence of FM was stratified by gender, female respondents had a statistically significant risk to have generalised body pain with 101 (87.7%) of “yes” responses with a p-value of 0.012. The data of the surveys stratified by gender with univariate analysis and the resulting p-value are shown in Table 2.
Table 2.
Fibromyalgia Surveys Stratified by Gender and Resulting p-value of Univariate Analysis and Kappa Correlation Coefficient (n = 220)
| Female n = 180 | Male n = 40 | Total n = 220 | % of Total | p-value | ||
|---|---|---|---|---|---|---|
| Do you have any body pain? n (%) | 101 (87.8) | 14 (12.2) | 115 | 52.3 | 0.012* | |
| FiRST, n (%) | 30 (93.8) | 2 (6.3) | 32 | 14.5 | 0.257 | |
| LFESSQ pain, n (%) | 34 (91.9) | 3 (8.1) | 37 | 16.8 | 0.256 | |
| LFESSQ fatigue, n (%) | 51 (92.7) | 4 (7.3) | 55 | 25.0 | 0.104 | |
| FSQ, n (%) | 60 (89.6) | 7 (10.4) | 67 | 30.5 | 0.481 | |
| Cohen’s Kappa Coefficient | Female, n (%) n = 180 | Male, n (%) n = 40 | Total, n (%) n = 220 | % of Total | p-value | |
| FiRST*FSQ | 0.262 | 26 (92.9) | 2 (7.1) | 28 | 12.7 | 0.001* |
| FiRST*LFESSQ pain | 0.350 | 18 (94.7) | 1 (5.3) | 19 | 8.6 | <0.001* |
| FSQ*LFESSQ pain | 0.249 | 28 (93.3) | 2 (6.7) | 30 | 13.6 | 0.002* |
| FiRST*FSQ*LFESSQ pain | 0.350 | 16 (94.1) | 1 (5.9) | 17 | 7.7 | <0.001* |
Note: *Significance level <0.050.
Abbreviations: FiRST, Fibromyalgia Rapid Screening Tool; LFESSQ, London Fibromyalgia Epidemiology Study Screening Questionnaire; FSQ, Fibromyalgia Survey Questionnaire; CI, confidence interval.
Agreement and Correlation Between the Screening Tools
Cohen’s kappa was run to determine the agreement between the three screening tools on diagnosing FM. The FiRST versus the FSQ, the FiRST versus LFESSQ pain and the FSQ versus the LFESSQ pain revealed fair agreement (k = 0.249–0.350; p < 0.001–0.002) (Table 2). Fleiss’ kappa coefficient revealed fair agreement among all three screening tools (kappa = 0.350; p < 0.001).
Association Between Fibromyalgia and the Participants’ Characteristics
Since the surveys were fairly co-related, the most completed survey FiRST was used as a binary variable (FM yes or no) in the unadjusted and adjusted binary logistic regression. The unadjusted ORs of having FM according to the FiRST with regard to gender, nationality, marital status, having children, smoking, scientific degree, field of work, satisfaction, sleep time, taking naps, snore during sleep and having changed sleeping patterns during the weekends were all insignificant (Table 3). The unadjusted OR of having FM in participants working for 41–45 h/week was significant (OR, 4.83; 95% CI, 1.45–16.10), and the resulting p-value was 0.010. In addition, participants who frequently wake up during night time had an unadjusted OR of 3.72 (95% CI, 1.54–9.00), with a p-value of 0.004, which was significant. Furthermore, participants who take sleeping pills and have sleep apnea or other sleeping disorders were more likely to have FM with reported unadjusted ORs of 3.74 (95% CI, 1.05–13.26; p = 0.041) and 11.76 (95% CI, 2.30–60.24; p = 0.003), respectively. The significant OR was then adjusted to the most common factors that might affect FM development such as age, gender, BMI and smoking resulting in a significant adjusted OR of having FM in people working for 41–45 h weekly (4.86; 95% CI, 1.32–17.84; p = 0.017) and in people who frequently wake up during sleep (5.16; 95% CI, 1.85–14.40; p = 0.002) or have sleep apnoea or sleeping disorders (12.99; 95% CI, 2.07–81.68; p = 0.006). The adjusted and unadjusted ORs with resulting 95% CI and p-values are shown in Table 3.
Table 3.
Binary Logistic Regression and Resulting Unadjusted and Adjusted Odds Ratio of Fibromyalgia Indicators (FiRST n = 85) with Different Baseline Factors
| Unadjusted | ||||
|---|---|---|---|---|
| Factors | Group | Odds Ratio | 95% CI | p- value |
| Gender | Female | 2.20 | 0.46–10.52 | 0.323 |
| Nationality | Saudi | 2.21 | 0.45–10.05 | 0.344 |
| Body mass index, kg/m2 | 18.5–24.9 | 2.42 | 0.28–21.17 | 0.425 |
| 25.0–29.9 | 3.48 | 0.38–31.63 | 0.268 | |
| ≥30 | 5.10 | 0.52–50.00 | 0.163 | |
| Marital status | Married | 1.02 | 0.45–2.29 | 0.972 |
| Have children | Yes | 1.69 | 0.74–3.83 | 0.211 |
| Smoker | Yes | 2.80 | 0.38–20.77 | 0.314 |
| Degree | Bachelor or pharm D | 1.24 | 0.40–3.79 | 0.712 |
| Masters | 1.66 | 0.50–5.53 | 0.411 | |
| PhD | 1.59 | 0.40–6.41 | 0.514 | |
| Work | Academia | 2.22 | 0.78–6.32 | 0.134 |
| Industry | 0.86 | 0.15–4.82 | 0.861 | |
| Hospital | 2.14 | 0.68–6.75 | 0.193 | |
| Work satisfaction | Satisfied | 1.29 | 0.36–4.64 | 0.701 |
| Partially satisfied | 1.55 | 0.43–5.65 | 0.505 | |
| Unsatisfied | 1.25 | 0.18–8.73 | 0.822 | |
| Highly unsatisfied | 7.50 | 0.53–105.28 | 0.135 | |
| Working hours per week | 40 | 2.54 | 0.66–9.83 | 0.178 |
| 41–45 | 4.83 | 1.45–16.10 | 0.010* | |
| Other | 1.45 | 0.23–9.16 | 0.693 | |
| Family history for FM | Yes | 1.39 | 0.39–4.99 | 0.611 |
| Chronic comorbidities | Yes | 3.70 | 1.45–9.48 | 0.006* |
| Sleep time | After 12 AM | 1.31 | 0.57–2.99 | 0.518 |
| Frequently wake up during sleep | Yes | 3.72 | 1.54–9.00 | 0.004* |
| Take naps during the day | Yes | 0.37 | 0.12–1.16 | 0.089 |
| Take sleeping pills | Yes | 3.74 | 1.05–13.26 | 0.041* |
| Snore during sleep | Yes | 1.35 | 0.52–3.53 | 0.537 |
| Have sleep apnoea or other sleeping disorders | Yes | 11.76 | 2.30–60.24 | 0.003* |
| Does your sleeping pattern change during vacation/weekends? | Sleep later | 0.48 | 0.12–1.89 | 0.296 |
| No change | 0.67 | 0.15–3.03 | 0.600 | |
| Adjusteda | ||||
| Working hours per week | 40 | 2.59 | 0.62–10.84 | 0.192 |
| 41–45 | 4.86 | 1.32–17.84 | 0.017* | |
| Other | 1.45 | 0.22–9.69 | 0.701 | |
| Frequently wake up during sleep | Yes | 5.16 | 1.85–14.40 | 0.002* |
| Take sleeping pills | Yes | 2.49 | 0.61–10.06 | 0.202 |
| Have sleep apnoea or other sleeping disorders | Yes | 12.99 | 2.07–81.68 | 0.006* |
Notes: aAdjusted to age, gender, body mass index, and smoking; chronic comorbidities; *Significance level <0.050.
Abbreviation: CI, confidence intervals.
Magnitude of Participants’ Responses to Items of Fibromyalgia Screening Tools
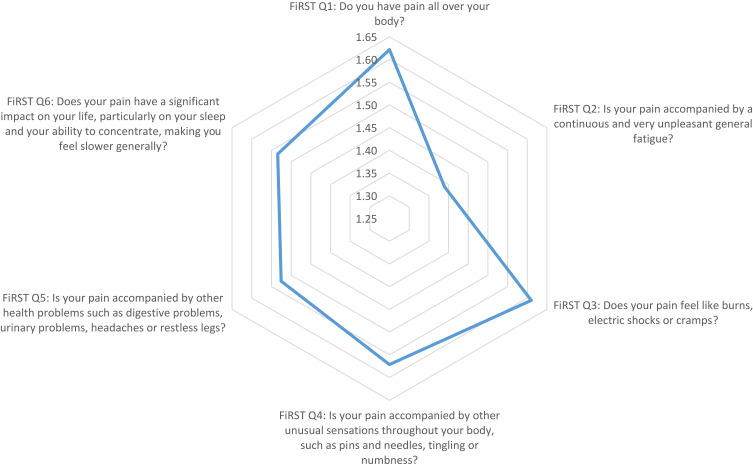
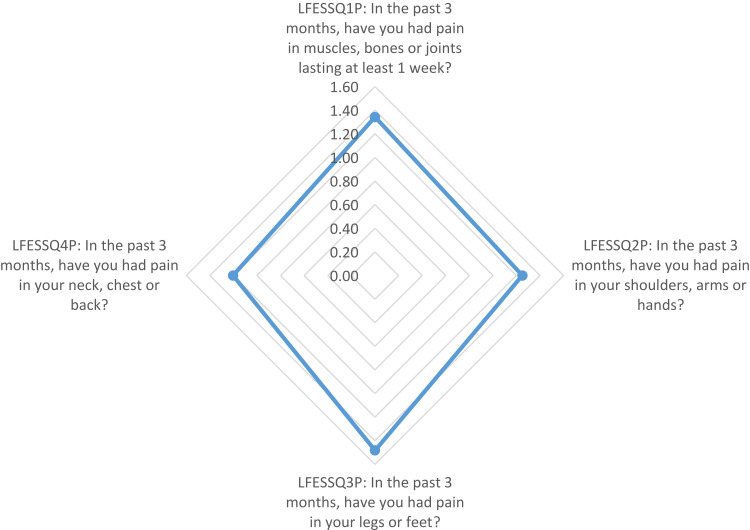
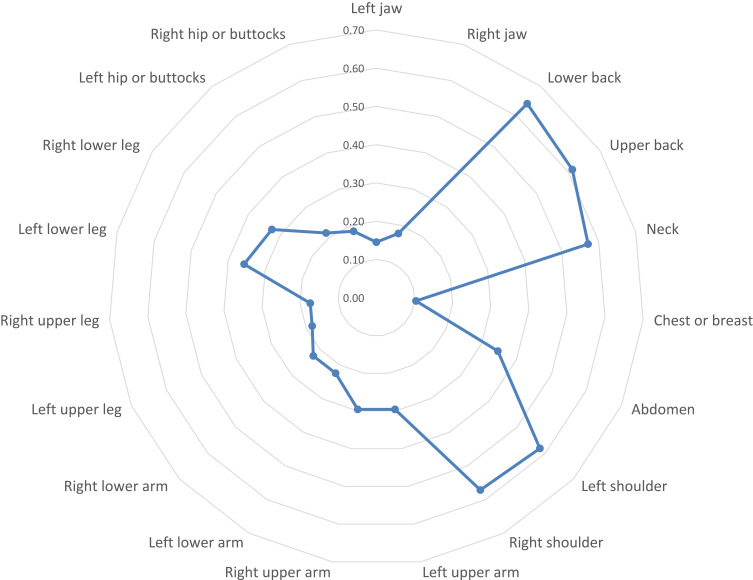
Using radar chart graphing, the resulting plot of the FiRST (Figure 1) showed that the most influential question of the survey was the first question (Do you have pain all over your body?). The LFESSQ Pain was evenly influenced by all four items (Figure 2). Finally, the FSQ WPI was influenced by back pain, neck pain and shoulder pain (Figure 3).
Figure 1.
Graphical representation using radar chart of the mean values of the FiRST items. Note: Items for the data in the graph were based on FiRST questionnaire; item data from Perrot et al.25
Figure 2.
Graphical representation using radar chart of the mean values of LFESSQ Pain items. Note: Items for the data in the graph were based on LFESSQ questionnaire; item data from White et al.24
Figure 3.
Graphical representation using radar chart of mean values of FSQ-WPI items. Note: Items for the data in the graph were based on FSQ questionnaire; item data from Häuser et al.26
Discussion
This study investigated the prevalence of FM in pharmacy professionals and students and found that FM is highly prevalent in this population. Various studies have estimated the prevalence of FM in general populations to be between 2% and 9%,38,39 with prevalence data reaching 4.43% in the Eastern Mediterranean region.40 However, limited research has been done among healthcare providers. In Saudi Arabia, only one study has focused on FM prevalence among the working population and reported a prevalence of 6%, 11.6% and 8.2% using the FiRST, LFESSQ and FSQ, respectively, in physicians in training.41 Scientific evidence revealed that female gender has three to six fold rate of developing FM.42 This might be the main influence of the high FM prevalence in the current study in which 78.8% of participants were females when compared to 42.9% in Omair and colleagues.41 Our findings revealed that the female gender was significantly associated with body pain with a prevalence of 87.8% compared with 12.2% in males. This association conforms to Omair et al’s study in which physicians in training in Saudi Arabia were assessed for FM.41
When the level of agreement between the FM screening tools was evaluated, a fair but significant degree of agreement between these tools in diagnosing FM was found. Furthermore, a fair to moderate agreement was found previously.41 The small sample size, small percentage of positive responses, components of each tool and weight of each component are the main possible factors for the fair association.41
With respect to factors contributing to FM, the number of working hours per week was associated with an increase in odds for developing FM in both unadjusted and adjusted regression. Studies have reported that workload is associated with FM and its major symptoms, such as widespread body pain.43,44 Moreover, frequent waking up during sleep and having sleep apnoea or other sleeping disorders were significantly associated with increasing odds of developing FM. This finding conforms to the findings of numerous studies in which disturbed sleep is a major symptom of patients with FM,45,46 which has been related to both depression and pain in FM.47 In addition, unrefreshing sleep is linked to the increase in inflammatory markers, thus, it contributes to inflammatory-related disorders such as FM.48,49
Radar charts permit the examination of the most influential factors in the three questionnaires. The results showed that the most influential question in the FiRST was “do you have pain all over your body”, which is corresponding to the most frequent complaint in FM. FM occurs in response to reduced pain inhibition and even pain amplification.50 The radar chart on the LFESSQ Pain illustrated that the four items were evenly distributed, which is in parallel to the FiRST’s radar chart, since all items were intended to measure pain in different parts of the body. The radar chart for the FSQ showed that the pain items were for the neck, shoulders and back sites. This finding can be explained by the sedentary work or study behaviour by setting and using computers to perform daily working and studying duties for pharmacists and pharmacy students. This finding was supported by Aminan et al,51 who reported that 50% of male pharmacists and 76% of male dentists experienced at least one episode of neck and upper extremity symptoms in the last 12 months (adjusted OR = 3.2; p < 0.001).
FM has been associated with various psychological factors, including stress and hassles of daily life and workplace,43 in which alterations in the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system were found in patients with FM and related conditions.52 Stress in pharmacy professionals and students was extensively reported previously.32,53–56 Stressors include increased coursework, lack of sleep, financial burden, grades, family or social issues, daily hassles, increased independence and financial burdens57,58 in students. Increased workload, staffing shortage, inadequate breaks, inadequate pay, few opportunities for job advancement, interruptions by phone calls or other work while performing normal duties55,59,60 were key stressors among pharmacists and pharmacy technicians.
This is the first study to estimate the prevalence of FM in pharmacy professionals and students. The design of the methodology was similar to daily clinical practice in which only patients experiencing body pain seek medical assistance for diagnosis. Our study reflects the daily clinical practice in which any participant not complaining of general body pain was ruled out when answering the screening questions. Radar charts to examine the impact of individual questions on total survey in the FM tools were not used previously in FM literature. The importance of this is highlighted by providing the baseline for qualitative analysis to explore pharmacy students’ and professionals’ views related to FM.
This study has some limitations. First, the number of participants who filled the questionnaire completely was low. However, the method for administering the survey using the SPS mailing list was the most suitable method to invite the target sample. Reasons such as engagement with work or absence of interest in the research topic might be the reasons behind the incompleteness of surveys. Second, selection bias in the questionnaire responders in whom they felt that they are prone or interested in FM is possible. Third, the diagnosis of FM was not confirmed using the gold standard clinical assessments of the sample. Thus, measurement error might be introduced to the study.
Since FM results in a greater clinical burden leading to a significant economic impact on workplace by affecting employees and their employers.61 This problem is magnified considering the current COVID-19 pandemic as it might affect the role of pharmacists as frontline workers.62 Using this work to encourage stakeholders to implement stress control strategies for pharmacists and pharmacy students might positively impact the work provided by pharmacists as an integral part of the healthcare system. Further studies with larger sample sizes are needed to confirm the findings of this study in addition to performing qualitative assessments to explore other reasons behind the presence of FM in pharmacy professionals and students.
Conclusions
In conclusion, FM is highly prevalent among pharmacists and pharmacy students and is related to working hours and sleep patterns.
Data Sharing Statement
The datasets related to the current study are available upon request from the corresponding author.
Acknowledgments
The authors thank the Deanship of Scientific Research and the Research Support and Services Unit at King Saud University for their technical support. Also, we acknowledge Hannan Abouzaid from King Saud University for her assistance in preparing the manuscript for submission.
Funding Statement
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Ethics Approval and Informed Consent
The study protocol approval was obtained from the Institutional Review Board of King Khalid Medical City (E-19-4111), and all participants provided informed consent to answer the survey. The research was carried out in compliance with the Declaration of Helsinki and respecting the confidentiality and anonymization of patient data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203 [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branco JC, Bannwarth B, Failde I, et al. Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum. 2010;39(6):448–453. doi: 10.1016/j.semarthrit.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 4.Rahman A, Underwood M, Carnes D. Fibromyalgia. BMJ. 2014;348:g1224. doi: 10.1136/bmj.g1224 [DOI] [PubMed] [Google Scholar]
- 5.Choy E, Perrot S, Leon T, et al. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv Res. 2010;10:102. doi: 10.1186/1472-6963-10-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ullrich A, Farin E, Jackel WH. [Restrictions in participation in women with fibromyalgia syndrome. An explorative pilot study]. Schmerz. 2012;26(1):54–60. German. doi: 10.1007/s00482-011-1123-3 [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz F, Sahin F, Ergoz E, et al. Quality of life assessments with SF 36 in different musculoskeletal diseases. Clin Rheumatol. 2008;27(3):327–332. doi: 10.1007/s10067-007-0717-8 [DOI] [PubMed] [Google Scholar]
- 8.Verbunt JA, Pernot DH, Smeets RJ. Disability and quality of life in patients with fibromyalgia. Health Qual Life Outcomes. 2008;6(1):1–8. doi: 10.1186/1477-7525-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birtane M, Uzunca K, Taştekin N, Tuna H. The evaluation of quality of life in fibromyalgia syndrome: a comparison with rheumatoid arthritis by using SF-36 health survey. Clin Rheumatol. 2007;26(5):679–684. doi: 10.1007/s10067-006-0359-2 [DOI] [PubMed] [Google Scholar]
- 10.Grodman I, Buskila D, Arnson Y, Altaman A, Amital D, Amital H. Understanding fibromyalgia and its resultant disability. Isr Med Assoc J. 2011;13(12):769–772. [PubMed] [Google Scholar]
- 11.Mannerkorpi K, Gard G. Hinders for continued work among persons with fibromyalgia. BMC Musculoskelet Disord. 2012;13:96. doi: 10.1186/1471-2474-13-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White LA, Birnbaum HG, Kaltenboeck A, Tang J, Mallett D, Robinson RL. Employees with fibromyalgia: medical comorbidity, healthcare costs, and work loss. J Occup Environ Med. 2008;50(1):13–24. doi: 10.1097/JOM.0b013e31815cff4b [DOI] [PubMed] [Google Scholar]
- 13.Wolfe F, Anderson J, Harkness D, et al. A prospective, longitudinal, multicenter study of service utilization and costs in fibromyalgia. Arthritis Rheum. 1997;40(9):1560–1570. doi: 10.1002/art.1780400904 [DOI] [PubMed] [Google Scholar]
- 14.Robinson RL, Birnbaum HG, Morley MA, Sisitsky T, Greenberg PE, Claxton AJ. Economic cost and epidemiological characteristics of patients with fibromyalgia claims. J Rheumatol. 2003;30(6):1318–1325. [PubMed] [Google Scholar]
- 15.Hughes G, Martinez C, Myon E, Taïeb C, Wessely S. The impact of a diagnosis of fibromyalgia on health care resource use by primary care patients in the UK: an observational study based on clinical practice. Arthritis Rheum. 2006;54(1):177–183. doi: 10.1002/art.21545 [DOI] [PubMed] [Google Scholar]
- 16.Weir PT, Harlan GA, Nkoy FL, et al. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th revision codes. JCR J Clin Rheumatol. 2006;12(3):124–128. doi: 10.1097/01.rhu.0000221817.46231.18 [DOI] [PubMed] [Google Scholar]
- 17.Cohen H. Controversies and challenges in fibromyalgia: a review and a proposal. Ther Adv Musculoskelet Dis. 2017;9(5):115–127. doi: 10.1177/1759720X17699199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes SM, Myhal GC, Thornton JF, et al. Fibromyalgia and the therapeutic relationship: where uncertainty meets attitude. Pain Res Manag. 2010;15:15. doi: 10.1155/2010/354868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glennon P. Fibromyalgia syndrome: management in primary care. Rep Rheum Dis. 2010;6(7):1–6. [Google Scholar]
- 20.Fitzcharles M, Boulos P. Inaccuracy in the diagnosis of fibromyalgia syndrome: analysis of referrals. Rheumatology. 2003;42(2):263–267. doi: 10.1093/rheumatology/keg075 [DOI] [PubMed] [Google Scholar]
- 21.Wolfe F, Clauw DJ, Fitzcharles M, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62(5):600–610. doi: 10.1002/acr.20140 [DOI] [PubMed] [Google Scholar]
- 22.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol. 2011;38(6):1113–1122. doi: 10.3899/jrheum.100594 [DOI] [PubMed] [Google Scholar]
- 23.Wolfe F, Clauw DJ, Fitzcharles M-A, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–329. doi: 10.1016/j.semarthrit.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 24.White KP, Harth M, Speechley M, Ostbye T. Testing an instrument to screen for fibromyalgia syndrome in general population studies: the London Fibromyalgia Epidemiology Study Screening Questionnaire. J Rheumatol. 1999;26(4):880–884. [PubMed] [Google Scholar]
- 25.Perrot S, Bouhassira D, Fermanian J. Development and validation of the fibromyalgia rapid screening tool (FiRST). Pain. 2010;150(2):250–256. doi: 10.1016/j.pain.2010.03.034 [DOI] [PubMed] [Google Scholar]
- 26.Häuser W, Jung E, Erbslöh-Möller B, et al. Validation of the Fibromyalgia Survey Questionnaire within a cross-sectional survey. PLoS One. 2012;7(5):e37504. doi: 10.1371/journal.pone.0037504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamasha AA-H, Kareem YM, Alghamdi MS, Algarni MS, Alahedib KS, Alharbi FA. Risk indicators of depression among medical, dental, nursing, pharmacology, and other medical science students in Saudi Arabia. Int Rev Psychiatry. 2019;31(7–8):646–652. doi: 10.1080/09540261.2019.1584095 [DOI] [PubMed] [Google Scholar]
- 28.Abbas A, Rizvi SA, Hasan R, et al. The prevalence of depression and its perceptions among undergraduate pharmacy students. Pharm Educ. 2015;15. [Google Scholar]
- 29.Silva RG, Figueiredo-Braga M. Evaluation of the relationships among happiness, stress, anxiety, and depression in pharmacy students. Curr Pharm Teach Learn. 2018;10(7):903–910. doi: 10.1016/j.cptl.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 30.Durham ME, Bush PW, Ball AM. Evidence of burnout in health-system pharmacists. Am J Heal Pharm. 2018;75(23_Supplement_4):S93–S100. doi: 10.2146/ajhp170818 [DOI] [PubMed] [Google Scholar]
- 31.Jones GM, Roe NA, Louden L, Tubbs CR. Factors associated with burnout among US hospital clinical pharmacy practitioners: results of a nationwide pilot survey. Hosp Pharm. 2017;52(11):742–751. doi: 10.1177/0018578717732339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dutta AP, Pyles MA, Miederhoff P. Measuring and understanding stress in pharmacy students. Stress Ment Heal Coll Students. 2006;1:28. [Google Scholar]
- 33.Marshall LL, Allison A, Nykamp D, Lanke S. Perceived stress and quality of life among doctor of pharmacy students. Am J Pharm Educ. 2008;72:6. doi: 10.5688/aj7206137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCann L, Hughes CM, Adair CG, Cardwell C. Assessing job satisfaction and stress among pharmacists in Northern Ireland. Pharm World Sci. 2009;31(2):188. doi: 10.1007/s11096-008-9277-5 [DOI] [PubMed] [Google Scholar]
- 35.Winfield JB. Psychological determinants of fibromyalgia and related syndromes. Curr Rev Pain. 2000;4(4):276–286. doi: 10.1007/s11916-000-0104-5 [DOI] [PubMed] [Google Scholar]
- 36.Saudi Pharmaceutical Society. No Title; 2018. Available from: https://www.saudipharmsociety.com/. Accessed November2, 2020.
- 37.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 38.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38(1):19–28. doi: 10.1002/art.1780380104 [DOI] [PubMed] [Google Scholar]
- 39.Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17(8):356. doi: 10.1007/s11916-013-0356-5 [DOI] [PubMed] [Google Scholar]
- 40.Heidari F, Afshari M, Moosazadeh M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int. 2017;37(9):1527–1539. [DOI] [PubMed] [Google Scholar]
- 41.Omair MA, Alobud S, Al-Bogami MH, et al. Prevalence of fibromyalgia in physicians in training: a cross-sectional study. Clin Rheumatol. 2019;38(1):165–172. doi: 10.1007/s10067-018-4313-x [DOI] [PubMed] [Google Scholar]
- 42.Marcus DA. Fibromyalgia: diagnosis and treatment options. Gend Med. 2009;6:139–151. doi: 10.1016/j.genm.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 43.Kivimaki M, Leino-Arjas P, Virtanen M, et al. Work stress and incidence of newly diagnosed fibromyalgia: prospective cohort study. J Psychosom Res. 2004;57(5):417–422. doi: 10.1016/j.jpsychores.2003.10.013 [DOI] [PubMed] [Google Scholar]
- 44.McBeth J, Harkness EF, Silman AJ, MacFarlane GJ. The role of workplace low-level mechanical trauma, posture and environment in the onset of chronic widespread pain. Rheumatology. 2003;42(12):1486–1494. doi: 10.1093/rheumatology/keg399 [DOI] [PubMed] [Google Scholar]
- 45.Harding SM. Sleep in fibromyalgia patients: subjective and objective findings. Am J Med Sci. 1998;315(6):367–376. doi: 10.1097/00000441-199806000-00005 [DOI] [PubMed] [Google Scholar]
- 46.Shaver JLF, Lentz M, Landis CA, Heitkemper MM, Buchwald DS, Woods NF. Sleep, psychological distress, and stress arousal in women with fibromyalgia. Res Nurs Health. 1997;20(3):247–257. doi: [DOI] [PubMed] [Google Scholar]
- 47.Anch AM, Lue FA, MacLean AW, Moldofsky H. Sleep physiology and psychological aspects of the fibrositis (fibromyalgia) syndrome. Can J Psychol Can Psychol. 1991;45(2):179. doi: 10.1037/h0084280 [DOI] [PubMed] [Google Scholar]
- 48.Metyas S, Rezk T, Arkfeld D, Leptich T. Autoinflammation and immunomodulation in inflammatory fibromyalgia syndrome – a review. Curr Rheumatol Rev. 2017;13(2):98–102. doi: 10.2174/1573397112666160919120530 [DOI] [PubMed] [Google Scholar]
- 49.Benlidayi IC. Role of inflammation in the pathogenesis and treatment of fibromyalgia. Rheumatol Int. 2019;39(5):781–791. doi: 10.1007/s00296-019-04251-6 [DOI] [PubMed] [Google Scholar]
- 50.Staud R. Biology and therapy of fibromyalgia: pain in fibromyalgia syndrome. Arthritis Res Ther. 2006;8(3):208. doi: 10.1186/ar1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aminian O, Alemohammad ZB, Hosseini MH. Neck and upper extremity symptoms among male dentists and pharmacists. Work. 2015;51:863–868. doi: 10.3233/WOR-141969 [DOI] [PubMed] [Google Scholar]
- 52.Cohen H, Neumann L, Shore M, Amir M, Cassuto Y, Buskila D. Autonomic dysfunction in patients with fibromyalgia: application of power spectral analysis of heart rate variability. In: Seminars in Arthritis and Rheumatism. Vol. 29. Elsevier; 2000:217–227. [DOI] [PubMed] [Google Scholar]
- 53.Henning K, Ey S, Shaw D. Perfectionism, the impostor phenomenon and psychological adjustment in medical, dental, nursing and pharmacy students. Med Educ. 1998;32(5):456–464. doi: 10.1046/j.1365-2923.1998.00234.x [DOI] [PubMed] [Google Scholar]
- 54.Dutta AP, Pyles MA, Miederhoff PA. Stress in health professions students: myth or reality? A review of the existing literature. J Natl Black Nurses’ Assoc JNBNA. 2005;16(1):63–68. [PubMed] [Google Scholar]
- 55.Lapane K, Hughes C. Baseline job satisfaction and stress among pharmacists and pharmacy technicians participating in the Fleetwood Phase III Study. Consult Pharm. 2004;19(11):1029–1037. doi: 10.4140/TCP.n.2004.1029 [DOI] [PubMed] [Google Scholar]
- 56.Wolfgang AP, Perri III M, Wolfgang CF. Job-related stress experienced by hospital pharmacists and nurses. Am J Hosp Pharm. 1988;45(6):1342–1345. [PubMed] [Google Scholar]
- 57.Votta RJ, Benau EM. Sources of stress for pharmacy students in a nationwide sample. Curr Pharm Teach Learn. 2014;6(5):675–681. doi: 10.1016/j.cptl.2014.05.002 [DOI] [Google Scholar]
- 58.Garber MC, Huston SA, Breese CR. Sources of stress in a pharmacy student population. Curr Pharm Teach Learn. 2019;11(4):329–337. doi: 10.1016/j.cptl.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 59.Lea VM, Corlett SA, Rodgers RM. Workload and its impact on community pharmacists’ job satisfaction and stress: a review of the literature. Int J Pharm Pract. 2012;20(4):259–271. doi: 10.1111/j.2042-7174.2012.00192.x [DOI] [PubMed] [Google Scholar]
- 60.McCann L, Adair CG, Hughes CM. An exploration of work‐related stress in Northern Ireland community pharmacy: a qualitative study. Int J Pharm Pract. 2009;17(5):261–267. doi: 10.1211/ijpp.17.05.0002 [DOI] [PubMed] [Google Scholar]
- 61.Laroche F, Azoulay D, Trouvin AP, Coste J, Perrot S. Fibromyalgia in the workplace: risk factors for sick leave are related to professional context rather than fibromyalgia characteristics – a French national survey of 955 patients. BMC Rheumatol. 2019;3(1):44. doi: 10.1186/s41927-019-0089-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Visacri MB, Figueiredo IV, de Lima TM. Role of pharmacist during the COVID-19 pandemic: a scoping review. Res Soc Adm Pharm. 2020. doi: 10.1016/j.sapharm.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]