Abstract
Background:
Accurate survival estimates are important for cancer control planning. Although observed survival estimates are lacking for many countries, where available, wide variations are reported. Understanding the impact of specific treatment and imaging modalities can help decision-makers effectively allocate resources to improve survival in their own context.
Methods:
We developed a microsimulation model of stage-specific cancer survival in 200 countries/territories for 11 cancers comprising 60% of global diagnosed cancer cases. The model accounts for country-specific availability of treatment (chemotherapy, surgery, radiotherapy, targeted therapy) and imaging modalities (ultrasound, X-ray, computed tomography [CT], magnetic resonance imaging [MRI], positron-emission tomography [PET], single-photon emission computed tomography [SPECT]), as well as quality of care. We calibrated the model to reported survival estimates from CONCORD-3. We estimated 5-year net survival for diagnosed cancers in each country/territory and estimated potential survival gains from increasing the availability of individual treatment and imaging modalities, and more comprehensive packages of scale-up.
Findings:
Estimated global 5-year net survival for all 11 cancers combined is 42·6% (95% UI 40·3-44·3), with 10-times differences between low-income and high-income countries. Expanding availability of surgery, radiotherapy, and improving quality of care would yield the largest survival gains in low-income and lower-middle-income countries, while upper-middle and high-income countries are more likely to benefit from improved availability of targeted therapy. Investing in medical imaging will also be necessary to achieve substantial survival gains, with traditional modalities estimated to provide the largest gains in low-income settings, while MRI and PET would yield the largest gains in higher-income countries. Simultaneous expansion of treatment, imaging, and quality of care could improve 5-year net survival more than 10-times in low-income countries (3·8% to 45·2%), and more than double in lower-middle-income countries (20·1% to 47·1%).
Interpretation:
Scaling up both treatment and imaging availability could yield synergistic survival gains for cancer patients. Expanding traditional modalities in lower-income settings may be a feasible pathway to improve survival before scaling up more advanced technologies.
Funding:
Harvard T.H. Chan School of Public Health, National Cancer Institute P30 Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center
Introduction
Globally, it is estimated that there were over 18 million new cases of cancer and 9·6 million deaths from cancer in 2018.1 Although the burden of cancer is substantial for countries of all income levels, the rates of many cancers in high-income countries are declining due to decreasing risk factors (such as tobacco use), early detection, and improved treatment.2 Conversely, rates of cancer are rising in many low-income and middle-income countries due to increases in risk factors such as smoking and excess body weight, all while these countries continue to bear a disproportionate burden of infection-related cancers, such as stomach, liver, and uterine cervical cancers.2
The rising cancer burden in low-income and middle-income countries further stresses health systems that tend to be weak and poses unique challenges, especially because generalization of the experience of cancer control programs from high-income countries to other settings may not always be appropriate.3 Cancer control programs that increase the likelihood of timely cancer diagnosis, such as screening and surveillance, assume that identified cases will benefit from adequate treatment. However, while cancer survival among diagnosed cases has improved in recent years in high-income settings, wide disparities in survival persist globally, often due to constraints related to diagnosis and the availability and affordability of treatment.4
In addition to ensuring that cancer treatments are available, increasing the use of cancer diagnostics such as imaging can help improve the quality of treatment. For example, imaging may be used for cancer staging and treatment planning, to guide interventions, and to assess response to therapy.
We developed a simulation model to estimate the current impact of different treatment and imaging modalities on cancer survival in each country, and project the potential survival gains from scaling up the availability and quality of these modalities. These estimates will be used to inform the Lancet Oncology Commission on Imaging and Nuclear Medicine, and can help decision-makers prioritize policies with the greatest potential to improve cancer survival in their local context.
Methods
Overview
We developed a microsimulation (individual-level) model to simulate 5-year net survival for 11 cancers in 200 countries/territories. Specifically, we modelled the number of incident (diagnosed) cancer cases in 2018 in each country/territory and simulated individual-level survival for each cancer patient. Building on a conceptual framework developed for childhood cancer,5 the model simulates the clinical course of cancer for each modelled patient from diagnosis to five years after diagnosis, accounting for country-specific estimates of stage at diagnosis (TNM I-IV), availability of specific treatment and imaging modalities, and quality of care. Due to limited data, especially on total (i.e. diagnosed and undiagnosed) cancer incidence and stage distribution, we focus this analysis on the impact of treatment and imaging modalities on five-year net survival conditional on diagnosis and stage, and do not consider potential benefits of imaging on screening or early detection.
We modeled four treatment modalities (chemotherapy, radiotherapy, surgery, targeted therapy) and six imaging modalities (ultrasound [not including intracavitary techniques], X-ray [plain radiography only], computed tomography [CT], magnetic resonance imaging [MRI], positron-emission tomography [PET], and single-photon emission computed tomography [SPECT]).
We calibrated the model so that our predicted survival estimates were consistent with population-based survival estimates for each cancer and country from CONCORD.4 Using the calibrated model, we estimated current cancer survival for all countries and estimated the potential survival gains from improving the availability of each treatment and imaging modality, as well as more comprehensive packages of scale-up.
Data Sources
We modeled cancer survival in 200 countries/territories, derived from an exhaustive list of areas in the UN Population Projections (see appendix pg 6 for details). Countries/territories were classified into 22 UN geographical regions and four income groups based on the 2018 World Bank Income Groups [margin link URL: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups]. Country-specific model inputs were synthesized from multiple sources (see Table 1).
Table 1:
Model Data Sources Overview
| Model Parameter | Data Source | # of model countries reported |
Reference |
|---|---|---|---|
| Cancer Cases | |||
| Diagnosed cancer cases | Estimated annual diagnosed cases by cancer type and age group | Cancer-specific: 166-178 | GLOBOCAN 20186 (see Appendix pg 7-8) |
| Stage distribution | Literature review of stage distribution at diagnosis | Cancer-specific (mean: 32, range: 3-84) | Literature review (see Appendix pg 9-49) |
| Cancer Survival | |||
| Maximum achievable survival by cancer and stage | SEER 2010-2016 5-year relative survival used as initial proxy | 1 (USA) | SEER 2010-20167 (see Appendix pg 50) |
| Survival impact of treatment/imaging modalities | Prior probabilities based on survey of expert opinions; Prior probability of needing modern modalities based on SEER 1973-2014 | NA; 1 (USA) | Expert opinion; SEER 1973-20147 (see Appendix pg 51-81) |
| Cancer Treatment | |||
| Chemotherapy availability | Reported availability of chemotherapy agents | 94 | Cohen P et al. 20198 (see Appendix pg 82-85) |
| Radiotherapy availability | Radiotherapy coverage | 173 | Atun R et al, 20159 (see Appendix pg 86-89) |
| Surgery availability | Availability of general surgery | 184 | Alkire BC et al, 201510 (see Appendix pg 90-93) |
| Targeted therapy availability | Prior probabilities set by income group | NA | (see Appendix pg 94-95) |
| Availability of imaging modalities | Availability of: - Ultrasound - X-ray - CT - MRI - PET - SPECT |
− 29 − 65 − 174 − 172 − 186 − 185 |
IAEA IMAGINE database14 (see Appendix pg 96-103) |
| Quality of care | Prior probabilities set by income group | NA | (see Appendix pg 104) |
We modelled 11 cancer sites for which comparable ICD-O-3 topography codes were available in both GLOBOCAN and CONCORD-3: Oesophagus (C15), Stomach (C16), Colon (C18), Rectum (C19-20), Anus (C21), Liver (C22), Pancreas (C25), Lung (C33-34), Breast (C50), Cervix uteri (C53), Prostate (C61). Estimated cancer incidence was obtained from GLOBOCAN 20186 for each cancer site. These 11 diagnosis groups accounted for an estimated 10·8 million cases out of 18·1 million cases, comprising a majority (60%) of diagnosed global cancer cases. Estimated numbers of incident cancer cases were available from GLOBOCAN for 166-178 countries, depending on cancer site. Estimates were not available for countries with small populations, so we imputed incidence rates from similar countries (i.e. similar region and income group) – see appendix pg 7-8 for details.
Due to the paucity of data on stage distribution at diagnosis, we undertook a literature review. We screened 12,982 studies, of which 1,456 abstracts were obtained, yielding 485 final estimates of country- and cancer-specific stage distribution (see Appendix pg 9-10). We used a Bayesian hierarchical modelling approach to regularize the stage distribution estimates, and to make estimates for countries with no data (see Appendix pg 8, 11). We used raking to estimate the joint probabilities of age and stage at diagnosis (see Appendix pg 11).
To account for the curability of different cancers, we estimated maximum achievable survival probabilities using 2010-16 data from the Surveillance, Epidemiology, and End Results (SEER) Program by cancer type and stage.7 We inflated the SEER estimates to account for the possibility of non-optimal service delivery in the USA (see Appendix pg 50). This model parameter is used to estimate relative differences in survival by cancer site/stage, and represents the highest possible survival given current knowledge and medical technology. This approach also helps to guard against overestimating the survival benefit of expanding the availability of treatment and imaging modalities by setting a ceiling on achievable survival based on recent empirical data.
To set prior probability distributions for the impact of treatment and imaging modalities on stage-specific cancer survival we used a two-stage survey to elicit expert opinion (see Appendix pg 51-52). A sample of actively practicing physicians was selected from collaborating institutions based on demonstrable expertise in their field (imaging or treatment of cancer patients), both in high-income and low-resource settings. Respondents were asked to indicate the impact of each treatment/imaging modality on five-year net survival for each cancer and stage using a four-point scale. We received between 17-35 responses for each modality (see Appendix pg 53-62). Based on the responses we estimated priors for the probability that the modality was necessary to achieve 5-year survival, given diagnosis and stage (see Appendix pg 63-73). To provide consensus results, responses with at least 75% agreement were accepted as final responses, while responses with lower levels of agreement were discussed by a panel of experts to forge final consensus (see Appendix pg 74-78 for results). However, when calibrating the model we sampled from the estimated priors accounting for the uncertainty from all responses, even for modalities with over 75% agreement. The survival impact (i.e. probability that the modality is needed for the patient to survive) was modelled independently for each modality, with the interaction of multiple modalities (i.e. joint probability) modelled as a relative effect modifier on individual-level survival.
We also estimated the proportion of cancer cases expected to benefit from modern modalities (targeted therapy, CT, MRI, PET, and SPECT). Because these modalities were generally not available until the late 1970s-early 1980s, we analyzed trends in stage-specific survival using SEER data7 between 1973-2014 to estimate the level of survival achievable before the introduction of modern modalities (see Appendix pg 79-81).
To estimate the availability of traditional treatment modalities (chemotherapy, radiotherapy, surgery) we relied on previously published estimates. We estimated priors of the availability of chemotherapy based on data from a global survey of oncologists (see Appendix pg 82-85).8 While the survey was of pediatric oncologists, we assumed that the relative availability of adult chemotherapy agents would be similar and used these estimates as a proxy to inform the prior distributions. Estimates of radiotherapy coverage were based on the Lancet Oncology Commission on Expanding Global Access to Radiotherapy (see Appendix pg 86-89).9 For surgery, we used estimates from a modelling study of the Lancet Commission on Global Surgery (see Appendix pg 90-93).10
Data on the global availability of targeted therapy are scarce, but estimates that are available suggest that patients in many countries have poor access to targeted therapy, usually because of the high cost.11 For example, patients in only 6 countries had access to at least half of 49 new oncology medicines launched between 2010-2014.12 Furthermore, such drugs are often only accessible for a privileged minority of the population with private health insurance.13 We therefore set priors by income group for targeted therapy availability, and ensured that the calibrated probabilities of targeted therapy availability were lower than for chemotherapy in each country (see Appendix pg 94-95).
For each imaging modality we obtained coverage estimates (equipment per million population) from the International Atomic Energy Agency (IAEA) IMAGINE database.14 Ultrasound and x-ray were classified as traditional imaging modalities in the model, with CT, MRI, PET, and SPECT considered modern modalities. To estimate probabilities of availability we set thresholds of minimum coverage density needed to ensure availability. Because there are no general guidelines regarding the ideal number of imaging units per population, we set thresholds based on observed data in high-income countries with relatively low coverage so as not to overestimate the thresholds needed to ensure availability (see Appendix pg 96-103).
Lastly, we included a parameter for quality of care, defined as the “degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge”.15 This parameter captures health-system and facility-level factors that account for residual differences in survival not explained by cancer stage or treatment and imaging availability, and has been found to be an important consideration in global cancer survival (see Appendix pg 104).5
For all treatment-related parameters we used Bayesian hierarchical models with 4 levels (income group, geographic area [i.e., continent], geographic region, country) to synthesize all available estimates and generate prior probability distributions (see Appendix pg 8, 82-104). This approach allowed us to regularize the reported estimates and estimate priors for countries for which no data were available. We used these priors as initial sampling distributions for model calibration and enforced non-decreasing income group intercepts when sampling from the hierarchical models.
Model Calibration
Calibration involves comparing the model predictions with empirical data in order to identify parameter sets that achieve a good fit (see Appendix pg 105 for more details).16 As calibration targets, we obtained country-specific 5-year net survival estimates for the 11 cancers included in the model from CONCORD-3.4 Rectum and anus cancers were combined into one group in CONCORD-3, so estimates were available for 10 cancer groups. Survival estimates were available for between 55-61 countries, yielding 583 estimates in total (see Appendix pg 105-106). Most of the CONCORD-3 survival estimates obtained (n=576) were for the period 2010-14, with a few estimates (n=7) for 2005-09 used for countries/cancers lacking more recent estimates. To evaluate the predictive accuracy of our model, we removed 50 estimates at random and reserved them as a validation test set. These estimates were not used to calibrate the model, so we scored our parameter sets against 533 total targets during calibration.
We used the most recent data on global cancer incidence and survival to calibrate the model. Although our estimates of cancer incidence from GLOBOCAN 2018 are more recent than the CONCORD-3 estimates (mostly for 2010-2014), because cancer survival tends to change slowly over time (see Appendix pg 79-81 for SEER estimates, for example), we assume that the CONCORD-3 survival estimates used to calibrate the model are close to the survival probabilities for patients diagnosed in 2018.
We calibrated the model using a Bayesian approach in which the observed data (i.e. CONCORD survival estimates) are considered fixed, and the model parameters are random variables. To fit the parameters, we used a simulated annealing search algorithm (a stochastic optimization approach) to identify good-fitting parameter sets. A goodness-of-fit score for each proposed parameter set was calculated as the sum of the squared distance between the predicted and reported 5-year net survival estimates. We weighted each survival target inversely proportional to the width of its confidence interval to allow more precise estimates to have larger influence in the calibration. We ran 2,000 independent search chains of 1,000 iterations each, selecting the final 100 best-fitting parameter sets to account for uncertainty around the model parameters (see Appendix pg 106-107). Using the top 100 parameter sets is less computationally intensive than running the model with all of the parameter sets, and is consistent with the directed search algorithm which aims to return samples from the mode of the posterior (i.e. maximum a posteriori, or MAP estimates).
As a posterior predictive check of the calibrated model, we compared our predicted 5-year net survival to the CONCORD estimates used in our calibration training set. To evaluate the predictive accuracy of our model we also compared our predictions to the test set of CONCORD estimates not used in model calibration. We calculated how often our prediction intervals (95% uncertainty intervals) overlapped the CONCORD 95% CIs, how often they contained the reported point estimate (i.e. coverage probability), the correlation coefficient of the predicted means vs reported estimates, the general R2 (i.e. one minus the fraction of unexplained variance), and the mean absolute error.
Policy Scenarios
With our calibrated model, we then ran 1,000 simulations to estimate current levels of survival, in each iteration sampling a parameter set from the best-fitting 100 calibrated sets and sampling the number of cancer cases from the GLOBOCAN estimates. We simulated 5-year net survival for each individual cancer patient in the model, allowing us to account for both stochastic (first-order) and parameter (second-order) uncertainty to estimate the posterior predictive distributions of our model outcomes. We estimated survival for all 11 cancers combined by pooling all simulated cancer cases in the model within each country, thus accounting for the relative incidence and survival of each cancer site.
To estimate the impact of each treatment and imaging modality on survival, we simulated counterfactual policy scenarios (1,000 simulations each) in which we increased the relevant parameter for each country to the mean estimated parameter among high-income countries (if higher than the baseline parameter value). We also simulated packages of policy interventions in which we scaled up combinations of parameters to estimate the impact of expanding treatment vs imaging, and traditional modalities vs all modalities. Policy scenarios are described in the appendix (pg 139). For all model outcomes we report the mean and 95% uncertainty intervals, calculated as the 2·5 and 97·5 percentiles of the simulation results. The simulation model was developed in Java (version 1.8.0).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
We found that our simulated estimates have a high degree of accuracy. Comparing our model results with the CONCORD-3 estimates (most of which are for 2010-2014), our posterior predictive checks of our training set (used for calibration) found that our prediction intervals (95% UIs) overlapped with the CONCORD 95% CIs 94·2% of the time, and contained the reported point estimate 80·9% of the time. Summary indicators by continent are available in the Appendix pg 108. Our mean survival estimates were highly correlated (r=0·971) with the calibration targets, with an R2 of 0·942 and mean absolute error of 4·49 percentage points (see Appendix pg 109-131).
Our validation checks of our test set (not used for calibration) found that 96·0% of our prediction intervals overlapped the CONCORD 95% CIs, with a coverage probability of 82·0%. Our predictions were highly correlated (r=0·962) with the mean estimates in the test set, with an R2 of 0·922 and mean absolute error of 5·36 percentage points (see Appendix pg 132-134). In general, we find that the model performs better in areas where more data are available, as we would expect. While the coverage probabilities are generally lower in lower-income settings, the correlation values suggest that the model does capture relative variation in survival.
Posterior estimates of the model parameters are plotted in the appendix (pg 135-138), and country-specific parameters and survival estimates by cancer site and stage are also available in the appendix (pg 142-342), as well as a public data repository [NOTE: Please include a margin link to the data repository: https://doi.org/10.7910/DVN/C7WE5L which is hosted at https://dataverse.harvard.edu/dataverse/zward. This DOI is currently unpublished but we will activate it if the manuscript is accepted].
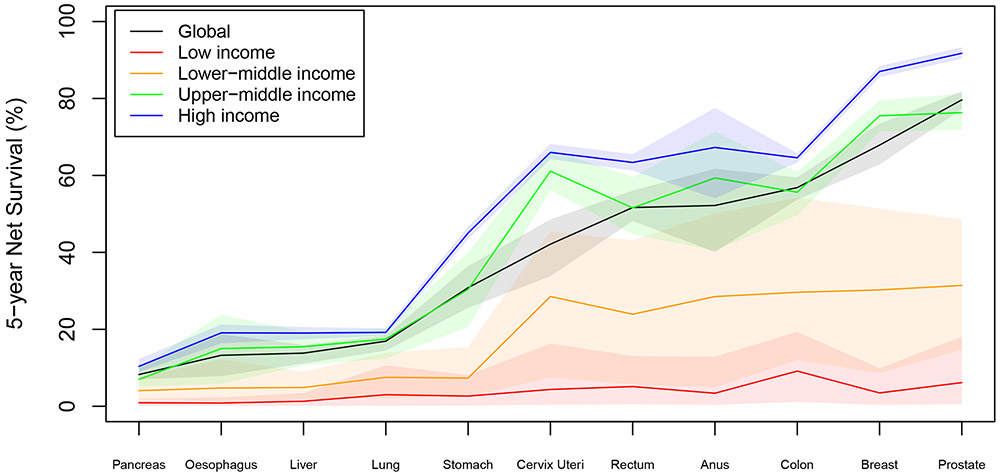
We estimate that, for all 11 cancers combined, global 5-year net survival for cases diagnosed in 2018 was 42·6% (95% UI 40·3-44·3), with large variation by country (see appendix pg 139) and diagnosis (see Figure 1).
Figure 1: 5-Year Net Survival by Cancer and Country Income Group.
Shaded regions indicate 95% uncertainty intervals
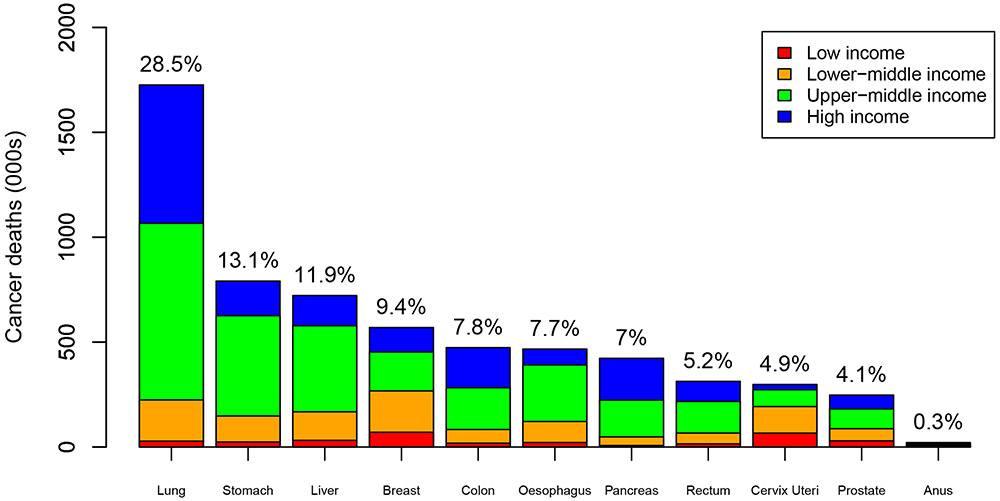
We find that among the 11 modeled cancers diagnosed in 2018, there will be 6·2 (95% UI 6·0-6·5) million deaths within 5 years, with lung cancer comprising nearly a third of these deaths globally (see Figure 2). These estimates are similar to numbers from GLOBOCAN, which estimates 6·4 million deaths from these cancers in 2018.6 We estimate that 5·2% [322,000/6,210,000] (95% UI 4·8%-5·5%) of these global cancer deaths occur in low-income countries, with 20·9% [1,299,000/6,210,000] (95% UI 18·4%-23·6%) in lower-middle-income countries, 46·1% [2,864,000/6,210,000] (95% UI 43·8%-47·8%) in upper-middle-income countries, and 27·8% [1,725,000/6,210,000] (95% UI 26·6%-28·8%) in high-income countries. Estimated cancer deaths per 100,000 population by income group are presented in the Appendix, pg 140.
Figure 2: Cancer deaths (among 11 modeled cancers) within 5 years of diagnosis (2018), by cancer and country income group, and as a percentage of total.
Note: % indicate percentage of total cancer deaths for the 11 cancers
We estimate that the majority (65·7% [95% UI 55·6-76·4]) of cancer cases are diagnosed at late stage (III-IV) in low-income countries, compared to less than half (44·3% [95% UI 40·5-48·7]) in high-income countries. Even after accounting for stage at diagnosis, we find large differences in survival by income group, with 10-times differences in 5-year net survival between low-income and high-income countries (see Table 2).
Table 2:
Overall stage distribution and 5-year net survival for all 11 cancers combined (global and by country income group), Mean (95% UI)
| Stage I | Stage II | Stage III | Stage IV | |||||
|---|---|---|---|---|---|---|---|---|
| % of cases | 5-year net survival |
% of cases | 5-year net survival |
% of cases | 5-year net survival |
% of cases | 5-year net survival |
|
| GLOBAL | 19·6 (15·4-24·1) | 76·9 (70·2-82·7) | 26·6 (21·5-32·5) | 59·4 (53·1-65·4) | 28·5 (24·5-33·1) | 34·8 (30·2-40·6) | 25·4 (21·4-29·4) | 7·1 (5·9-8·5) |
| Low Income | 11·8 (4·6-23·5) | 8·4 (0·6-23·5) | 22·6 (13·5-33·3) | 4·6 (0·6-13·3) | 37·4 (27·9-47·6) | 2·8 (0·4-7·6) | 28·3 (20·4-36·4) | 2·5 (0·3-6·0) |
| Lower-Middle Income | 10·5 (5·0-17·7) | 36·8 (11·9-66·7) | 28·0 (21·1-37·4) | 29·0 (7·6-54·0) | 37·5 (29·8-46·8) | 17·8 (6·1-29·6) | 24·4 (17·2-30·8) | 6·4 (3·7-9·3) |
| Upper-Middle Income | 15·1 (9·5-21·1) | 72·6 (62·3-80·0) | 26·4 (18·3-35·2) | 59·1 (50·4-67·4) | 31·8 (25·2-41·2) | 37·0 (31·2-44·1) | 26·7 (19·8-33·8) | 6·4 (4·9-8·0) |
| High Income | 29·3 (22·8-35·4) | 87·2 (85·0-89·9) | 26·4 (18·6-31·9) | 76·6 (70·1-81·9) | 20·3 (16·9-24·5) | 48·1 (39·8-57·3) | 24·0 (20·7-26·6) | 8·9 (7·2-11·4) |
Among single policy interventions to scale up treatment, we find that improving the quality of care would yield the largest survival gains in low-income countries, while increasing the availability of surgery would yield the largest gains in lower-middle-income countries, with increasing targeted therapy yielding the largest gains for upper-middle-income and high-income countries (see Table 3). In general, we find that expanding availability of surgery, radiotherapy, and improving quality of care are the most important priorities for low-income and lower-middle-income countries, while upper-middle-income and high-income countries are more likely to benefit from improving availability of targeted therapy, as availability of traditional treatment modalities is already relatively high.
Table 3:
Estimated 5-year net survival, 11 cancers combined: Single policy interventions – Mean (95% UI)
| Treatment | Imaging | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | Baseline | Chemotherapy | Radiotherapy | Surgery | Targeted therapy | Quality | Ultrasound | X-ray | CT | MRI | PET | SPECT |
| Global | 42·6 (40·3-44·3) | 43·1 (40·6-45·0) | 43·3 (41·1-45·2) | 43·8 (41·5-45·8) | 43·1 (40·6-44·9) | 43·1 (40·8-45·1) | 42·6 (40·4-44·3) | 42·6 (40·3-44·5) | 43·0 (40·7-44·9) | 43·1 (40·7-45·4) | 43·1 (40·7-45·2) | 42·8 (40·4-44·3) |
| Low income | 3·8 (0·5-9·2) | 4·8 (0·6-11·3) | 6·3 (0·8-14·4) | 6·4 (0·8-18·6) | 3·8 (0·5-9·3) | 7·2 (2·9-14·7) | 4·4 (0·5-10·9) | 4·1 (0·5-10·1) | 3·9 (0·5-9·6) | 3·9 (0·5-9·7) | 3·9 (0·5-9·3) | 3·8 (0·5-9·2) |
| Lower middle income | 20·1 (7·2-31·7) | 21·8 (7·4-35·0) | 23·6 (10·5-36·4) | 26·2 (12·9-35·8) | 20·4 (7·3-31·9) | 22·5 (7·8-33·8) | 20·5 (7·3-31·9) | 20·4 (7·2-32·0) | 20·8 (7·4-32·5) | 20·9 (7·6-32·9) | 20·5 (7·3-32·1) | 20·2 (7·2-31·8) |
| Upper middle income | 39·9 (37·7-42·2) | 40·3 (38·0-43·0) | 40·1 (37·9-42·3) | 40·2 (38·0-42·7) | 40·6 (38·1-43·4) | 40·0 (37·8-42·3) | 39·9 (37·7-42·2) | 39·9 (37·7-42·2) | 40·4 (38·1-42·9) | 40·7 (38·3-43·1) | 40·7 (38·3-43·7) | 40·2 (38·0-42·7) |
| High income | 57·8 (57·3-58·3) | 57·9 (57·3-58·3) | 57·9 (57·3-58·4) | 57·9 (57·3-58·5) | 58·2 (57·5-58·8) | 57·9 (57·4-58·4) | 57·8 (57·3-58·3) | 57·8 (57·3-58·3) | 58·0 (57·4-58·7) | 57·9 (57·3-58·4) | 58·1 (57·4-58·9) | 58·0 (57·4-58·5) |
| Africa | 15·4 (9·2-21·6) | 17·3 (10·9-24·1) | 19·1 (11·4-27·0) | 19·1 (11·5-28·9) | 15·7 (9·3-22·0) | 18·1 (12·1-26·5) | 15·9 (9·4-22·7) | 15·7 (9·2-21·7) | 15·8 (9·3-22·0) | 16·1 (9·3-22·5) | 15·7 (9·3-22·1) | 15·5 (9·2-21·6) |
| Eastern Africa | 5·9 (0·9-14·9) | 6·8 (0·9-16·7) | 9·5 (1·6-22·1) | 8·8 (0·9-24·5) | 5·9 (0·9-14·9) | 9·7 (4·1-24·1) | 6·5 (1·0-17·8) | 6·1 (0·9-15·8) | 6·0 (0·9-15·3) | 6·1 (0·9-15·3) | 6·0 (1·0-15·0) | 5·9 (0·9-14·9) |
| Middle Africa | 6·1 (0·3-18·4) | 8·0 (0·4-21·6) | 9·0 (0·6-24·5) | 9·1 (0·5-23·8) | 6·1 (0·3-18·5) | 8·5 (1·7-24·6) | 6·6 (0·3-22·2) | 6·3 (0·3-21·1) | 6·2 (0·3-18·7) | 6·4 (0·3-19·1) | 6·2 (0·3-18·6) | 6·1 (0·3-18·5) |
| Southern Africa | 26·5 (12·0-47·5) | 29·2 (12·1-50·3) | 32·9 (12·2-51·4) | 34·8 (15·2-50·8) | 26·9 (12·3-48·8) | 27·8 (12·0-48·7) | 26·8 (12·0-47·5) | 26·5 (12·0-47·5) | 27·2 (12·2-47·5) | 27·9 (12·2-50·7) | 27·0 (12·8-50·1) | 26·7 (12·0-47·5) |
| Western Africa | 10·7 (0·7-22·5) | 13·3 (0·9-26·7) | 15·5 (1·3-29·1) | 14·6 (1·4-27·6) | 10·8 (0·7-22·6) | 12·7 (3·0-25·1) | 11·4 (0·7-23·2) | 11·2 (0·8-22·5) | 10·9 (0·7-22·8) | 11·2 (0·7-23·7) | 10·9 (0·7-22·9) | 10·8 (0·7-22·5) |
| Northern Africa | 30·0 (14·0-42·6) | 31·9 (14·0-44·6) | 31·6 (16·0-42·8) | 32·5 (15·6-46·3) | 30·8 (14·3-44·7) | 32·9 (17·9-44·1) | 30·3 (14·0-42·6) | 30·3 (14·0-43·0) | 30·6 (14·0-43·4) | 31·1 (14·5-44·4) | 30·5 (14·2-43·9) | 30·2 (14·2-42·8) |
| Asia | 36·5 (32·5-39·5) | 37·0 (32·8-40·2) | 37·3 (33·7-40·1) | 38·1 (34·1-41·9) | 37·0 (32·6-40·1) | 37·0 (33·1-40·1) | 36·6 (32·5-39·5) | 36·6 (32·5-39·8) | 36·8 (33·3-39·9) | 37·2 (32·8-40·7) | 37·0 (32·6-40·5) | 36·7 (33·0-39·6) |
| Central Asia | 19·1 (5·1-37·1) | 20·8 (5·9-38·2) | 24·7 (5·7-38·2) | 23·0 (10·6-39·5) | 19·4 (5·1-37·4) | 20·2 (5·3-37·8) | 19·3 (5·1-37·1) | 19·2 (5·1-37·4) | 19·8 (5·3-38·5) | 19·5 (5·3-37·6) | 19·6 (5·1-38·7) | 19·3 (5·1-37·7) |
| Eastern Asia | 41·0 (38·6-43·7) | 41·3 (38·6-44·3) | 41·0 (38·6-43·7) | 41·0 (38·6-44·2) | 41·5 (38·8-44·6) | 41·1 (38·6-43·8) | 41·0 (38·6-43·8) | 41·0 (38·6-43·7) | 41·2 (38·9-44·2) | 41·7 (38·6-44·4) | 41·6 (39·1-44·6) | 41·3 (38·8-44·2) |
| South-Eastern Asia | 23·1 (11·2-35·5) | 24·9 (11·2-36·5) | 26·2 (12·1-37·8) | 25·9 (15·4-36·3) | 23·4 (11·5-35·7) | 24·2 (12·8-35·8) | 23·7 (11·3-35·6) | 23·6 (11·2-35·9) | 23·9 (11·3-36·2) | 24·1 (11·5-37·2) | 23·5 (11·9-36·5) | 23·2 (11·2-35·7) |
| Southern Asia | 21·9 (5·0-36·6) | 23·1 (5·1-37·8) | 24·5 (6·1-40·7) | 31·0 (11·2-43·0) | 22·3 (5·1-37·1) | 24·2 (5·2-40·8) | 22·0 (5·1-36·6) | 22·1 (5·2-36·9) | 22·7 (5·2-38·2) | 22·8 (5·2-38·5) | 22·4 (5·1-37·5) | 22·1 (5·1-36·8) |
| Western Asia | 45·9 (42·9-49·2) | 46·4 (43·6-49·5) | 46·5 (43·6-49·4) | 47·1 (43·2-51·7) | 46·6 (43·3-50·3) | 46·4 (43·4-50·3) | 46·0 (43·0-49·4) | 46·0 (42·9-49·2) | 46·1 (43·1-49·5) | 46·1 (43·0-49·5) | 46·2 (43·4-49·7) | 46·2 (43·1-49·4) |
| Europe | 52·1 (50·6-53·2) | 52·3 (51·0-53·6) | 52·5 (51·0-53·5) | 52·4 (51·0-53·6) | 52·8 (51·1-54·0) | 52·6 (51·4-53·7) | 52·2 (50·6-53·2) | 52·1 (50·6-53·2) | 52·5 (51·1-54·2) | 52·6 (50·9-54·1) | 52·8 (51·3-54·4) | 52·2 (51·0-53·4) |
| Eastern Europe | 40·7 (37·0-43·4) | 41·2 (37·4-44·5) | 41·5 (37·2-44·7) | 41·2 (37·2-44·3) | 41·5 (37·7-44·5) | 41·7 (38·9-45·5) | 40·8 (37·0-43·4) | 40·7 (37·0-43·4) | 41·8 (37·0-46·3) | 42·0 (37·9-46·1) | 41·7 (37·8-45·3) | 40·9 (37·2-43·6) |
| Northern Europe | 57·9 (56·9-59·3) | 58·0 (56·9-59·3) | 58·2 (57·0-60·1) | 58·0 (57·0-59·9) | 58·4 (57·0-60·2) | 58·1 (56·9-60·1) | 57·9 (56·9-59·3) | 57·9 (56·9-59·3) | 58·1 (57·0-60·1) | 58·0 (56·9-59·3) | 58·6 (57·2-60·9) | 58·1 (56·9-59·3) |
| Southern Europe | 54·4 (51·5-56·2) | 54·6 (52·1-56·2) | 54·6 (51·5-56·4) | 54·6 (51·9-56·4) | 55·2 (52·2-57·6) | 54·6 (52·1-56·3) | 54·4 (51·7-56·2) | 54·4 (51·5-56·2) | 54·5 (51·5-56·3) | 54·4 (51·7-56·4) | 55·1 (52·9-57·6) | 54·4 (51·5-56·2) |
| Western Europe | 59·0 (57·5-60·4) | 59·0 (57·6-60·4) | 59·0 (57·5-60·5) | 59·1 (57·5-60·5) | 59·5 (57·7-61·6) | 59·1 (57·5-61·1) | 59·0 (57·5-60·4) | 59·0 (57·5-60·4) | 59·1 (57·5-61·2) | 59·0 (57·5-60·4) | 59·2 (57·8-61·2) | 59·1 (57·6-60·4) |
| Latin America and the Caribbean | 51·3 (47·9-54·3) | 51·9 (49·1-55·2) | 51·5 (48·4-54·6) | 51·7 (49·1-54·4) | 52·2 (49·2-56·1) | 51·5 (48·2-54·7) | 51·3 (47·9-54·3) | 51·3 (47·9-54·3) | 52·9 (49·1-58·0) | 51·8 (48·2-57·0) | 52·4 (48·2-56·7) | 51·6 (48·1-54·7) |
| Caribbean | 48·3 (44·1-52·3) | 48·4 (44·1-52·5) | 49·0 (44·1-53·6) | 49·1 (44·8-53·8) | 49·1 (44·6-53·5) | 48·8 (44·8-53·3) | 48·3 (44·1-52·3) | 48·3 (44·1-52·4) | 48·8 (44·4-53·3) | 48·7 (44·3-53·1) | 49·3 (44·8-53·9) | 48·9 (44·5-53·5) |
| Central America | 52·3 (37·9-59·4) | 53·1 (38·0-60·1) | 52·8 (37·9-60·1) | 54·3 (47·8-60·3) | 53·4 (37·9-60·3) | 52·7 (38·2-59·7) | 52·3 (37·9-59·4) | 52·3 (37·9-59·4) | 52·7 (37·9-59·9) | 52·5 (38·4-59·5) | 52·7 (38·2-59·5) | 52·6 (37·9-60·0) |
| South America | 51·4 (48·8-54·4) | 52·0 (48·8-55·9) | 51·4 (48·8-54·4) | 51·4 (48·8-54·4) | 52·2 (48·8-56·2) | 51·6 (48·8-54·5) | 51·4 (48·8-54·4) | 51·4 (48·8-54·4) | 53·4 (49·4-58·7) | 51·9 (48·9-57·7) | 52·7 (48·8-57·0) | 51·7 (49·0-54·6) |
| Northern America | 61·0 (59·8-61·9) | 61·0 (59·8-61·9) | 61·0 (59·8-61·9) | 61·0 (59·8-62·3) | 61·0 (59·8-62·1) | 61·0 (59·8-62·3) | 61·0 (59·8-61·9) | 61·0 (59·8-61·9) | 61·0 (59·8-62·0) | 61·0 (59·8-61·9) | 61·0 (59·9-62·2) | 61·2 (60·2-62·6) |
| Oceania | 59·2 (56·0-62·6) | 59·3 (56·1-62·6) | 59·7 (56·5-63·2) | 59·9 (57·1-63·9) | 59·8 (56·6-62·9) | 59·8 (56·2-62·8) | 59·2 (56·0-62·6) | 59·3 56·0-62·7) | 59·4 56·0-63·4) | 59·3 56·0-62·7) | 59·5 56·0-62·8) | 59·7 (56·9-63·3) |
| Australia/New Zealand | 63·1 (60·3-66·2) | 63·2 (60·3-66·5) | 63·1 (60·3-66·3) | 63·4 (60·6-66·8) | 63·7 (60·6-66·8) | 63·4 (60·3-66·5) | 63·1 (60·3-66·2) | 63·1 (60·3-66·2) | 63·3 (60·5-66·6) | 63·1 (60·3-66·3) | 63·4 (60·4-66·4) | 63·6 (60·4-66·6) |
| Melanesia | 13·7 (1·9-39·0) | 14·5 (2·2-39·0) | 19·4 (2·9-45·9) | 18·9 (2·8-46·2) | 13·8 (2·0-39·2) | 18·7 (3·9-46·9) | 14·3 (2·0-39·0) | 14·6 (2·0-42·7) | 14·2 (1·9-41·3) | 14·2 (2·1-41·8) | 14·1 (2·0-39·4) | 13·9 (1·9-39·4) |
| Micronesia | 25·5 (4·9-50·6) | 26·6 (4·9-51·6) | 34·3 (9·7-54·9) | 28·1 (5·3-53·4) | 25·7 (4·9-51·1) | 30·4 (9·6-54·4) | 26·2 (4·9-50·9) | 26·1 (6·0-51·7) | 26·0 (5·0-50·6) | 26·6 (5·3-53·4) | 26·0 (5·0-52·2) | 25·7 (4·9-51·4) |
| Polynesia | 25·1 (3·8-47·3) | 26·0 (3·8-47·4) | 36·4 (6·8-50·1) | 25·4 (3·8-47·3) | 25·3 (3·8-47·3) | 28·2 (9·7-48·1) | 26·1 (3·8-47·3) | 25·8 (3·8-47·4) | 25·6 (3·8-48·4) | 26·7 (4·1-50·3) | 25·7 (3·9-48·3) | 25·4 (3·8-47·5) |
Among imaging modalities, we find that expanding the availability of ultrasound would yield the largest survival gains in low-income countries, especially in Africa. In contrast, we find that expanding availability of MRI and PET would yield the largest survival gains in middle-income and high-income countries, with expanded availability of CT estimated to yield the largest benefits in Latin America. Overall, however, we find that small survival gains are expected from increasing the availability of any single treatment or imaging modality alone.
Among policy packages we find that expanding treatment availability yields greater survival benefits for low-income and lower-middle-income countries, while expanding availability of imaging yields higher benefits for upper-middle-income and high-income countries (see Table 4). However, we find that expanding both treatment and imaging yields synergistic gains in survival. For example, in low-income countries, expanding treatment availability alone, while important, would yield roughly half the gains of expanding both treatment and imaging. In addition, increasing the availability of treatment and imaging modalities but neglecting to improve the quality of care will yield small gains, especially in low-income countries.
Table 4:
Estimated 5-year net survival, 11 cancers combined: Policy package interventions – Mean (95% UI)
| Estimated 5-year Net Survival, 11 Cancers Combined | |||||||
|---|---|---|---|---|---|---|---|
| No Quality Improvements | Plus Quality Improvements | ||||||
| Baseline | Treatment Only |
Imaging Only |
Comprehensive | Treatment Only |
Imaging Only |
Comprehensive | |
| Global | 42·6 (40·3-44·3) | 46·0 (43·7-48·1) | 44·8 (42·2-47·1) | 49·1 (46·2-51·1) | 47·1 (45·4-48·7) | 45·5 (42·7-48·0) | 50·7 (49·2-52·0) |
| Low income | 3·8 (0·5-9·2) | 14·3 (2·7-32·6) | 5·7 (0·7-14·6) | 23·1 (3·5-45·5) | 27·4 (19·9-37·0) | 11·2 (3·9-22·3) | 45·2 (40·2-52·1) |
| Lower middle income | 20·1 (7·2-31·7) | 34·0 (23·9-40·9) | 24·2 (8·7-37·2) | 42·2 (27·2-48·3) | 38·0 (30·7-43·4) | 27·0 (9·6-40·4) | 47·1 (42·8-50·8) |
| Upper middle income | 39·9 (37·7-42·2) | 41·6 (39·0-44·6) | 42·7 (40·4-45·4) | 44·8 (42·3-47·4) | 41·7 (39·1-45·2) | 42·8 (40·5-45·6) | 44·9 (42·4-47·6) |
| High income | 57·8 (57·3-58·3) | 58·4 (57·7-59·0) | 58·5 (57·7-59·3) | 59·1 (58·4-59·9) | 58·5 (57·8-59·1) | 58·6 (57·9-59·4) | 59·2 (58·5-59·9) |
| Africa | 15·4 (9·2-21·6) | 28·4 (17·3-41·4) | 18·4 (10·5-26·4) | 36·8 (23·1-51·3) | 36·4 (29·3-43·4) | 22·2 (13·8-32·0) | 49·5 (45·2-53·6) |
| Eastern Africa | 5·9 (0·9-14·9) | 17·6 (1·6-43·8) | 8·0 (1·3-23·6) | 26·6 (2·7-53·9) | 30·9 (21·3-45·7) | 13·9 (5·4-31·4) | 48·9 (42·3-55·9) |
| Middle Africa | 6·1 (0·3-18·4) | 19·6 (1·4-46·2) | 8·3 (0·3-27·5) | 29·3 (2·3-53·8) | 31·1 (17·5-47·9) | 12·1 (3·0-37·2) | 48·3 (39·6-57·5) |
| Southern Africa | 26·5 (12·0-47·5) | 45·6 (30·5-52·9) | 30·6 (13·2-53·8) | 53·3 (35·8-60·2) | 47·5 (40·4-53·3) | 32·0 (13·2-55·4) | 55·4 (50·3-60·6) |
| Western Africa | 10·7 (0·7-22·5) | 27·4 (2·5-42·0) | 13·7 (1·0-27·6) | 37·6 (3·7-53·8) | 34·9 (19·7-44·6) | 16·5 (5·4-30·8) | 49·5 (44·0-54·8) |
| Northern Africa | 30·0 (14·0-42·6) | 37·6 (18·2-47·9) | 33·8 (15·4-46·7) | 43·4 (19·5-52·4) | 41·6 (31·8-50·3) | 37·1 (19·7-48·5) | 48·0 (41·5-53·9) |
| Asia | 36·5 (32·5-39·5) | 40·4 (37·0-44·1) | 38·9 (34·7-42·8) | 43·9 (39·6-46·6) | 41·4 (38·2-44·5) | 39·5 (35·2-44·1) | 45·1 (42·7-47·2) |
| Central Asia | 19·1 (5·1-37·1) | 33·8 (19·3-42·6) | 21·8 (6·1-40·8) | 40·4 (21·1-48·3) | 36·5 (28·0-44·0) | 23·1 (6·5-42·4) | 44·0 (40·7-48·7) |
| Eastern Asia | 41·0 (38·6-43·7) | 41·8 (38·8-45·1) | 42·9 (40·6-45·4) | 43·9 (41·3-46·6) | 41·9 (38·9-45·4) | 43·0 (40·6-45·5) | 44·0 (41·5-46·7) |
| South-Eastern Asia | 23·1 (11·2-35·5) | 32·8 (19·6-40·8) | 28·1 (12·6-42·5) | 41·1 (21·9-47·7) | 35·0 (24·2-41·4) | 29·5 (14·1-43·0) | 44·1 (40·2-48·4) |
| Southern Asia | 21·9 (5·0-36·6) | 37·1 (14·2-45·2) | 25·5 (5·8-43·2) | 44·4 (15·2-51·7) | 40·9 (30·5-46·7) | 28·2 (5·9-47·1) | 49·1 (42·7-53·1) |
| Western Asia | 45·9 (42·9-49·2) | 49·2 (45·2-53·0) | 47·0 (43·8-50·7) | 51·0 (46·8-55·1) | 50·3 (46·7-54·6) | 47·7 (44·5-53·1) | 52·8 (49·9-55·9) |
| Europe | 52·1 (50·6-53·2) | 53·6 (52·1-55·3) | 53·9 (52·0-55·8) | 55·6 (53·7-57·1) | 54·1 (52·7-55·9) | 54·3 (52·8-55·9) | 56·2 (54·9-57·6) |
| Eastern Europe | 40·7 (37·0-43·4) | 43·2 (38·5-47·5) | 44·7 (40·0-48·9) | 48·1 (41·2-51·2) | 44·4 (42·0-49·3) | 45·8 (42·3-49·7) | 49·5 (47·1-51·7) |
| Northern Europe | 57·9 (56·9-59·3) | 58·7 (57·2-60·5) | 59·0 (57·4-61·1) | 59·8 (58·2-61·2) | 58·9 (57·2-60·6) | 59·1 (57·4-61·1) | 59·9 (58·5-61·3) |
| Southern Europe | 54·4 (51·5-56·2) | 55·9 (53·2-58·6) | 55·3 (53·0-58·1) | 57·0 (55·3-59·0) | 56·1 (53·9-58·6) | 55·6 (53·4-58·1) | 57·2 (55·6-59·1) |
| Western Europe | 59·0 (57·5-60·4) | 59·6 (57·7-61·6) | 59·5 (57·9-61·6) | 60·1 (58·6-61·8) | 59·8 (57·7-61·7) | 59·6 (58·0-61·8) | 60·2 (58·6-62·0) |
| Latin America and the Caribbean | 51·3 (47·9-54·3) | 53·6 (50·6-56·8) | 55·3 (51·0-59·7) | 58·2 (55·5-60·9) | 54·0 (51·0-57·5) | 55·6 (51·4-60·1) | 58·7 (56·1-61·2) |
| Caribbean | 48·3 (44·1-52·3) | 51·3 (45·9-57·5) | 51·5 (47·0-56·1) | 55·6 (49·9-60·0) | 53·0 (48·6-58·1) | 52·2 (47·2-56·8) | 58·0 (54·2-61·1) |
| Central America | 52·3 (37·9-59·4) | 57·0 (51·8-61·6) | 54·0 (38·7-60·7) | 59·1 (55·3-62·5) | 57·6 (51·9-61·9) | 54·5 (38·7-61·0) | 59·8 (55·6-62·8) |
| South America | 51·4 (48·8-54·4) | 53·0 (48·9-57·7) | 56·1 (51·4-60·5) | 58·3 (54·8-61·3) | 53·2 (48·9-58·1) | 56·3 (52·4-60·7) | 58·5 (55·3-61·4) |
| Northern America | 61·0 (59·8-61·9) | 61·1 (59·8-62·3) | 61·2 (60·3-62·6) | 61·4 (60·4-62·6) | 61·1 (59·8-62·4) | 61·3 (60·3-62·7) | 61·4 (60·4-62·7) |
| Oceania | 59·2 (56·0-62·6) | 61·4 (58·0-64·6) | 60·5 (57·1-64·8) | 63·2 (59·5-66·1) | 62·4 (58·9-65·5) | 61·3 (57·1-65·0) | 64·5 (62·5-66·8) |
| Australia/New Zealand | 63·1 (60·3-66·2) | 64·2 (61·0-67·3) | 64·1 (61·4-67·2) | 65·3 (63·0-67·6) | 64·5 (61·0-67·5) | 64·4 (61·4-67·3) | 65·6 (63·5-68·0) |
| Melanesia | 13·7 (1·9-39·0) | 28·8 (3·3-48·1) | 18·2 (2·3-50·0) | 38·5 (4·3-56·0) | 38·2 (20·3-49·3) | 25·0 (5·2-51·5) | 51·5 (45·9-57·3) |
| Micronesia | 25·5 (4·9-50·6) | 40·2 (10·0-59·0) | 30·9 (6·9-59·4) | 50·1 (12·5-64·7) | 48·0 (26·7-60·4) | 36·9 (13·2-61·1) | 59·9 (53·7-67·2) |
| Polynesia | 25·1 (3·8-47·3) | 38·2 (6·9-51·2) | 31·7 (4·4-56·4) | 48·7 (8·1-59·9) | 42·4 (21·2-52·5) | 35·3 (16·1-57·0) | 53·7 (47·3-60·3) |
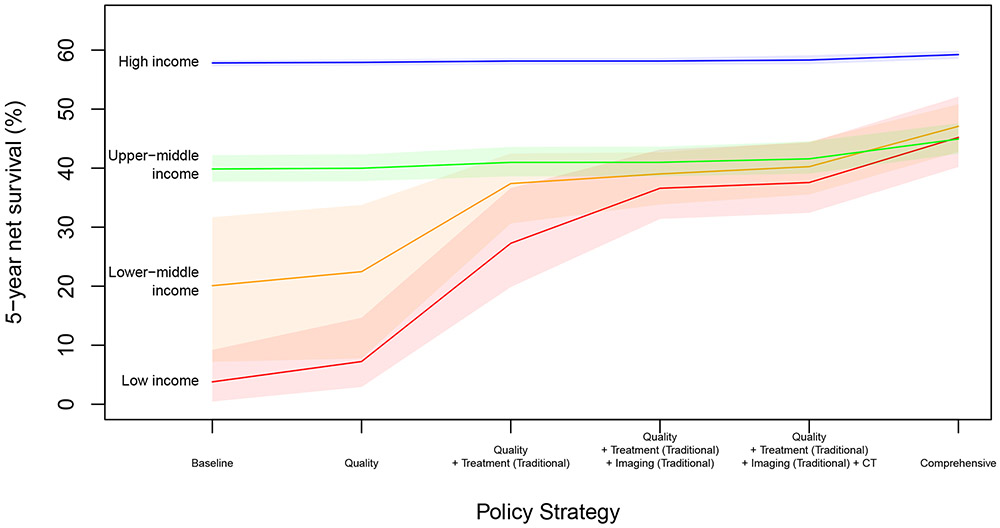
For low-income and lower-middle-income countries, scaling up traditional treatment (surgery, radiotherapy, chemotherapy) and imaging modalities (in addition to improving quality of care) yields a large proportion of the survival gains that could be achieved by comprehensive scale-up of all (traditional and more advanced) treatment and imaging modalities (see Figure 3). However, even at comprehensive scale-up there still exists a gap in survival compared to high-income countries due to worse cancer stage at diagnosis, and poorer prognosis for types of cancer more common in lower-income countries (e.g. stomach, liver).
Figure 3: Pathways to Scale-Up by Income Group.
Shaded areas indicate 95% uncertainty intervals
Discussion
We developed a simulation model of cancer survival for 11 cancers in 200 countries/territories, synthesizing data from multiple sources, which finds large variation in 5-year net survival by country and cancer due to differences in the availability of treatment and imaging modalities and quality of care. Even after considering stage at diagnosis, we find that 5-year net survival varies 10-times between low-income and high-income countries. We find that our model has a high degree of accuracy, and our validation checks of our test set of CONCORD survival estimates help build confidence in our modeled estimates. We incorporated the uncertainty around model parameters when calibrating the model, so our estimated 95% uncertainty intervals (reported for all model outcomes) indicate the sensitivity of our results to different parameter values, and account for their joint distribution.
While cancer survival is much lower in low-income countries, we find that reported cancer deaths in low-income countries are a relatively small proportion of global cancer deaths. In addition to having a smaller population (low-income countries comprise only about 10% of the global population), higher competing mortality risks in these countries mean that less adults survive to older ages where cancer incidence is highest, thus lowering the overall incidence of cancer in these populations. Also, it is important to note that this analysis only considers reported (diagnosed) cases of cancer, and thus likely underestimates total cancer cases and deaths in the population that are not diagnosed due to health system weaknesses.
In addition to ensuring availability of treatment and imaging modalities, we find that improving the quality of care will also be needed to substantially improve cancer survival. Indeed, we find that in low-income countries, improving quality of care may be the single most effective policy to improve survival, and may need to be established first before implementing treatment protocols from high-income settings to reduce the potential for harm from toxic deaths.
However, and importantly, although improving quality and ensuring availability of treatment or imaging modalities separately is projected to yield improvements in survival, we find that simultaneous scale-up of both treatment and imaging modalities has a synergistic effect, leading to larger gains. In general, we find that ensuring availability of traditional treatment and imaging modalities would yield the largest survival gains in low-income countries, with priorities in higher-income countries shifting to expanding availability of more advanced technologies such as targeted therapy, MRI, and PET. These findings have good face validity for these different contexts and help to provide new evidence and quantitative justifications for priority-setting for investments in resource-constrained settings. In all settings, investments in workforce training (eg, radiologists, technicians, oncologists, etc) will also be needed to ensure that appropriate protocols are followed, and imaging equipment is well-maintained. In addition to this analysis of comparative effectiveness, we plan to perform more comprehensive economic analyses in follow-up work to estimate the required investments and resulting benefits of different scale-up strategies.
We find that for low-income countries, scaling up traditional treatment (chemotherapy, radiation, surgery) and imaging modalities (ultrasound, X-ray), while improving quality of care yields substantial survival gains and is likely more achievable in the short-term. Scaling up CT also yields gains, and given its importance for radiotherapy planning, CT is considered essential for any dedicated cancer center.17 However, countries without adequate financial and human resources may have to use alternative modalities until CT capabilities become available, as was done in high-income countries before the development of CT (see Appendix pg 79). Resource-stratified clinical practice guidelines, such as those developed by the American Society of Clinical Oncology18 and the National Comprehensive Cancer Network19 can help to provide specific guidance for cancer care in resource-poor settings.
While substantial survival gains in low-income and lower-middle-income countries can be achieved by a comprehensive approach that achieves parity with high-income countries in treatment, imaging, and quality of care, we still find a gap in survival due to worse stage at diagnosis and types of incident cancers. We find that two-thirds of diagnosed cancers in low-income countries are detected at an advanced stage, compared to less than half in high-income countries. In addition to improving treatment and accurate diagnosis, upstream health system, behavioral, and environmental factors that result in worse stage at diagnosis must also be addressed. Cancer prevention and early detection efforts will therefore also be needed for global cancer survival to achieve parity with high-income countries.
In order to substantially improve cancer survival, we find that investments in medical imaging will be necessary in addition to expanding treatment availability. Indeed, our estimates of the impact of imaging modalities are likely conservative, since we focus only on the impact of imaging on 5-year net survival, conditional on diagnosis and stage. This does not take into account other potential benefits of imaging, such as improved cancer screening and staging, as well as identifying complications of cancer treatment, all of which may impact costs and quality of life in addition to survival. As more data become available in the future our model can be extended to include these other factors.
We are not aware of other modelling studies that have estimated the impact of imaging modalities on global cancer survival, but our results regarding treatment availability are broadly similar to other modelled estimates. For example, we find that expanding treatment availability in Eastern Africa would increase breast cancer survival by about 13 percentage points (data not shown), which is similar to the 10 percentage point survival increase estimated by Birnbaum et al.20 from expanding the availability of chemotherapy and endocrine therapy for currently detected ER+ cases in the region. Although these estimates are not directly comparable due to differences in modelled outcomes, this type of broad model benchmarking can help to build confidence in the general results.
While we find that our model has a high degree of accuracy, we had limited data with which to fit the model, especially for low-income countries. Similarly, due to limited comparability between GLOBOCAN and CONCORD cancer groupings, we could only model 11 cancers, which do not include brain tumours or haematological malignancies. Nevertheless, these cancers represent a majority (60%) of total cancers worldwide, and may therefore provide insight into overall cancer survival and priority-setting.
Although we synthesized data from multiple sources, data limitations required us to make assumptions when developing the model. For example, because estimates of cancer incidence were missing for some countries we used matching to impute incidence rates from similar countries. However, as the countries with missing incidence estimates all have small populations we would not expect the matching to affect our results. To maximize the amount of survival data available for model calibration, we used all estimates available from CONCORD-3, including estimates that were flagged due to concerns around data quality. Survival estimates were flagged due to insufficient sample size to allow for age-standardisation, or because the estimates were derived from data with other quality issues.4 We present these estimates in the appendix (pgs 109-134) as posterior predictive checks to evaluate the fit of our calibrated model, but care should be taken when interpreting these plots.
Due to lack of data on adult chemotherapy availability we used data on paediatric chemotherapy agents to inform the prior probability distributions for model calibration, assuming that the factors which impact chemotherapy availability (e.g. cost, clinical expertise, etc) would be similar for adult and paediatric treatments. Also, we estimated minimum coverage thresholds for each imaging modality based on country-level data, which does not take into account how imaging equipment and human resources may be distributed within countries, or potential differences in the availability of imaging for diagnostics vs treatment planning. Estimates of ultrasound coverage were also only available for a small number of countries, and were very general given the low-cost and decentralized use of ultrasound. Empirical estimates of targeted therapy availability and quality of care indicators would also be useful for informing our model estimates, since these parameters are currently fit solely via model calibration. Data on the distribution of cancer biological subtypes – currently modelled implicitly via the treatment impact parameters of targeted therapy – would also help to refine our estimates of targeted therapy survival impact. Lastly, in this analysis we estimate survival for diagnosed cases only, which likely underestimates the actual cancer burden in lower-income countries due to underdiagnosis.21
Further research to provide country-level and sub-national estimates of cancer incidence, survival, stage at diagnosis, treatment and imaging availability, and quality of care indicators would improve the data used for our modelling, and provide important information for global oncology in general. The collection and reporting of stage data in population-based cancer registries (across and within countries) would be especially useful, as some of our estimates are based on hospital data, which may be less representative. While simulation models can help to synthesize data from disparate sources and provide useful information for policy-makers (especially regarding counterfactual scenarios), modelling ultimately relies on – and cannot replace – real world cancer data.
Using a model-based approach, we provide estimates of cancer survival for 11 cancers in 200 countries/territories, and estimate the impact of specific treatment and imaging modalities on 5-year net survival, as well as quality of care. We find that cancer survival varies widely by country, largely due to differences in the availability and quality of treatment and imaging modalities, but also due to worse stage of cancer at diagnosis in lower-income countries. We find that scaling up both treatment and imaging availability could yield synergistic survival gains, and that focusing on traditional modalities in lower-income settings may be a viable pathway to feasibly achieving substantial survival gains before scaling up more advanced imaging technologies and targeted therapy.
Supplementary Material
Research in context.
Evidence before this study
Recent data on 5-year net cancer survival for 18 cancers from 322 population-based cancer registries in 71 countries are provided by the CONCORD-3 study. In addition, GLOBOCAN 2018, produced by the International Agency for Research on Cancer, provides modeled mortality estimates for 36 cancers in 185 countries. We searched PubMed for studies on the impact of imaging on global cancer survival using the search terms “cancer”, “survival”, “global”, and “imaging” on Apr 28, 2020, without language or publication date restrictions. Although resource-stratified guidelines provide guidance for cancer care and imaging in different settings, we found no estimates of how imaging impacts global cancer survival. While few observed data are available from low-income and lower-middle-income countries, reported survival varies substantially by region.
Added value of this study
This study provides estimates of stage distribution at diagnosis based on a global literature review, as well as survival estimates for 11 cancers in 200 countries and territories. We provide global, regional, and country-level estimates of 5-year net survival and estimate the potential impact of various policy scenarios that scale up specific treatment and imaging modalities. These results can help guide priority-setting efforts in different contexts aimed at improving cancer survival.
Implications of all the available evidence
Stage distribution at diagnosis varies substantially by income group, with two-thirds of cancer cases diagnosed at advanced stages in low-income countries, compared to less than half in high-income countries. Even after controlling for stage, we find that cancer survival varies 10-times between low-income and high-income countries. We find that in addition to expanding the availability of treatment (chemotherapy, surgery, radiotherapy and targeted therapy), investing in imaging (ultrasound, X-ray, computed tomography [CT], magnetic resonance imaging [MRI], positron-emission tomography [PET], and single-photon emission computed tomography [SPECT]) and improving quality of care will be necessary to achieve considerable survival gains in lower-income countries. While higher-income countries may benefit most from investments in advanced imaging modalities such as MRI and PET, and advanced treatments such as targeted therapy, investing in traditional imaging modalities along with chemotherapy, radiotherapy, and surgery can yield substantial survival gains in lower-income settings and may be a feasible pathway to improving cancer outcomes before scaling up more advanced imaging technologies and targeted therapy.
Acknowledgements
This study was funded by the Harvard TH Chan School of Public Health and the National Cancer Institute P30 Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center.
Footnotes
Declaration of interests
We declare no competing interests. HH receives annual compensation for serving on the Board of Directors of Ion Beam Applications (IBA).
Contributor Information
Zachary J. Ward, Center for Health Decision Science, Harvard TH Chan School of Public Health, Harvard University, Boston, MA, USA.
Andrew M. Scott, Olivia Newton-John Cancer Research Institute, Melbourne, Australia; Department of Molecular Imaging and Therapy, Austin Health, Melbourne, Australia; School of Cancer Medicine, La Trobe University, Melbourne, Australia; Department of Medicine, University of Melbourne, Melbourne, Australia
Hedvig Hricak, Department of Radiology, Memorial Sloan Kettering Cancer Center, NY, USA.
May Abdel-Wahab, International Atomic Energy Agency (IAEA) Department of Nuclear Sciences and Applications, Division of Human Health, Nuclear Medicine and Diagnostic Imaging Section, Vienna, Austria.
Diana Paez, International Atomic Energy Agency (IAEA) Department of Nuclear Sciences and Applications, Division of Human Health, Nuclear Medicine and Diagnostic Imaging Section, Vienna, Austria.
Miriam Mikhail Lette, International Atomic Energy Agency (IAEA) Department of Nuclear Sciences and Applications, Division of Human Health, Nuclear Medicine and Diagnostic Imaging Section, Vienna, Austria.
H. Alberto Vargas, Department of Radiology, Memorial Sloan Kettering Cancer Center, NY, USA.
T. Peter Kingham, Department of Surgery, Memorial Sloan Kettering Cancer Center, NY, USA.
Rifat Atun, Department of Global Health and Population, Harvard TH Chan School of Public Health, Harvard University, Boston, MA, USA; Department of Global Health and Social Medicine, Harvard Medical School, Harvard University, Boston, MA, USA.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends – an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1): 16–27. [DOI] [PubMed] [Google Scholar]
- 3.Shah SC, Kayamba V, Peek RM Jr, Heimburger D. Cancer control in low- and middle-income countries: is it time to consider screening? J Glob Oncol 2019; 5: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Girardi F, Atun R. Global childhood cancer survival estimates and priority-setting: a simulation-based analysis. Lancet Oncol 2019; 20(7):972–983. [DOI] [PubMed] [Google Scholar]
- 6.Global Cancer Observatory. International Agency for Research on Cancer. http://gco.iarc.fr/today/online-analysis-table [Accessed Feb 13, 2020]. [Google Scholar]
- 7.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (2000-2016) - Linked To County Attributes - Total U.S., 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission.
- 8.Cohen P, Friedrich P, Lam C, et al. Global access to essential medicines for childhood cancer: a cross-sectional survey. J Glob Oncol 2018; 4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol 2015; 16: 1153–86. [DOI] [PubMed] [Google Scholar]
- 10.Alkire BC, Raykar NP, Shrime MG, et al. Global access to surgical care: a modelling study. Lancet Glob Health 2015; 3: e316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yip CH, Buccimazza I, Hartman M, Deo SV, Cheung PS. Improving outcomes in breast cancer for low and middle income countries. World J Surg 2015; 39(3): 686–92. [DOI] [PubMed] [Google Scholar]
- 12.IMS Institute for Healthcare Informatics. Global Oncology Trend Report: A Review of 2015 and Outlook to 2020. 2016. Available at: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-oncology-trend-report-2016.pdf [Accessed Feb 13, 2020].
- 13.Ruiz R, Strasser-Weippl K, Touya D, et al. Improving access to high-cost cancer drugs in Latin America: Much to be done. Cancer 2017; 123(8): 1313–1323. [DOI] [PubMed] [Google Scholar]
- 14.International Atomic Energy Agency. IMAGINE – IAEA Medical imAGIng and Nuclear mEdicine global resources database. https://humanhealth.iaea.org/HHW/DBStatistics/IMAGINE.html [Accessed Feb 13, 2020].
- 15.Institute of Medicine. Medicare: a strategy for quality assurance. Washington, DC: National Academy Press, 1990. [Google Scholar]
- 16.Vanni T, Karnon J, Madan J, et al. Calibrating models in economic evaluation: a seven step approach. Pharmacoeconomics 2011; 29: 35–49. [DOI] [PubMed] [Google Scholar]
- 17.International Atomic Energy Agency. Planning National Radiotherapy Services: A Practical Tool. IAEA Human Health Series, No. 14. Vienna, 2010. https://www-pub.iaea.org/MTCD/Publications/PDF/Pub1462_web.pdf [Accessed Mar 12, 2020]. [Google Scholar]
- 18.Al-Sukhun S, Temin S, Chavez-MacGregor M, et al. ASCO Resource-Stratified Guidelines: Methods and Opportunities. J Glob Oncol 2018; 4: 1–8.Carlson RW, Scavone JL, Koh WJ, et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NCCN Framework for Resource Stratification: A Framework for Providing and Improving Global Quality Oncology Care. J Natl Compr Canc Netw 2016; 14(8): 961–9. [DOI] [PubMed] [Google Scholar]
- 20.Birnbaum JK, Duggan C, Anderson BO, Etzioni R. Early detection and treatment strategies for breast cancer in low-income and upper middle-income countries: a modelling study. Lancet Glob Health 2018; 6(8): e885–e893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Atun R. Estimating the total incidence of global childhood cancer – a simulation-based analysis. Lancet Oncol 2019; 20(4):483–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.