Abstract
Background
The topic of prognosis in COVID-19 research may be important in adopting appropriate clinical decisions. Multidimensional prognostic index (MPI) is a frailty assessment tool widely used for stratifying prognosis in older people, but data regarding inpatients, affected by COVID-19, are not available.
Objectives
To evaluate whether MPI can predict in-hospital mortality and the admission to intensive care unit (ICU) in older inpatients hospitalized for COVID-19 infection.
Methods
In this longitudinal, Italian, multi-center study, older patients with COVID-19 were included. MPI was calculated using eight different domains typical of comprehensive geriatric assessment and categorized in three groups (MPI 1 ≤ 0.33, MPI 2 0.34–0.66, MPI 3 > 0.66). A multivariable Cox's regression analysis was used reporting the results as hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
227 older patients hospitalized for SARS-CoV-2 infection were enrolled (mean age: 80.5 years, 59% females). Inpatients in the MPI 3 were subjected less frequently than those in the MPI 1 to non-invasive ventilation (NIV). In the multivariable analysis, people in MPI 3 experienced a higher risk of in hospital mortality (HR = 6.30, 95%CI: 1.44–27.61), compared to MPI 1. The accuracy of MPI in predicting in hospital mortality was good (Area Under the Curve (AUC) = 0.76, 95%CI: 0.68–0.83). People in MPI 3 experienced a significant longer length of stay (LOS) in hospital compared to other participants. No association between MPI and ICU admission was found.
Conclusions
Frailty- as assessed by high MPI score - was associated with a significant higher risk of in-hospital mortality, longer LOS, and lower use NIV, whilst the association with ICU admission was not significant. These findings suggest that prognostic stratification by using the MPI could be useful in clinical decision making in older inpatients affected by COVID-19.
Keywords: COVID-19, Multidimensional prognostic index, Mortality, Intensive care unit, Prognosis, Frailty, Comprehensive geriatric assessment
1. Introduction
During 2020, more than 83 million people were affected by coronavirus-19 disease (COVID-19), with about 2 million of deaths (Organization, 2019). The epidemiological data available indicated that COVID-19 could be considered as an emerging geriatric condition (Lloyd-Sherlock et al., 2020), as the prevalence and mortality are higher in old compared to young adults (Onder, Rezza, & Brusaferro, 2020). Among all the National Health Systems, a source of great concern is the number of hospital and intensive care unit (ICU) beds available during the different pandemic phases to face with the great number of older patients needing hospitalization for COVID-19 (Haas, de Lange, van Dijk, & van Delden, 2020). This indicates the need of a precise stratification of older people by prognostic factors other than age and disease-specific determinants, including also functional, physical and psycho-social factors that can be best addressed by a geriatric multidimensional assessment (Polidori, Maggi, Mattace-Raso, & Pilotto, 2020).
The Multidimensional Prognostic Index (MPI) is a prognostic tool based on a standard Comprehensive Geriatric Assessment (CGA) useful to address short and long-term mortality risk validated in hospitalised older patients (Pilotto et al., 2008). Multicentre studies have extensively demonstrated that MPI has an excellent accuracy and calibration in predicting negative clinical outcomes (e.g., mortality) during hospitalization (Pilotto et al., 2019, Volpato, Bazzano, Fontana, & Ferrucci, 2015). The MPI has been validated in over 54,000 older adults suffering from the most common chronic and acute age-related diseases in over 50 international studies (Pilotto et al., 2020) and is thus considered one of the most commonly tool used to assess frailty in older people (Dent et al., 2019).
As frailty is a main factor related to short and long-term prognosis in older people, evaluating its role in COVID-19 disease is pivotal for a proper patients’ stratification. In this regard, a large study on this topic reported an higher risk of mortality in frailer patients (evaluated with clinical frailty scale, CFS) among 1564 hospitalized patients (Hewitt et al., 2020). Other studies, including more limited sample sizes, substantially confirmed these findings (Bellelli, Rebora, Valsecchi, Bonfanti, & Citerio, 2020; De Smet et al., 2020) Two recent meta-analyses have further confirmed that CFS is related to a significantly higher risk of mortality in older people affected by COVID-19 (Pranata et al., 2020; Zhang et al., 2021). Even if these studies advanced our knowledge regarding the importance of frailty as prognostic factor in older people affected by COVID-19, a main limitation of previous studies is the use of CFS that it is not a product of the CGA (Hubbard et al., 2020). Moreover, no one of these studies assessed if frailty could be associated with a different allocation of ICU beds, taking only in hospital mortality as outcome (Bellelli, Rebora, Valsecchi, Bonfanti, & Citerio, 2020; De Smet et al., 2020).
Given this background, the aim of this study (MPI COVID-19) is to evaluate if the MPI can predict in older inpatients affected by COVID-19 in hospital mortality and the admission to ICU in different Italian centres.
2. Materials and methods
2.1. Study population
This was a prospective, observational study conducted according to the World Medical Association's 2008 Declaration of Helsinki, the guidelines for Good Clinical Practice, and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (von Elm, Altman, Egger, & Pocock, 2008).
Inclusion criteria were: a. Patients ≥ 65 years of age; b. Consecutively admitted to the hospital with ascertained diagnosis of COVID-19 through nasopharyngeal swab with RT-PCR (real time-polymerase chain reaction); c. Willing to participate to the study. Exclusion criteria were age <65 years, unwillingness to participate to the study and inability to give the informed consent. The period of enrollment and follow-up was between 31 January and 31 December 2020. Patients were hospitalized in units for SARS-CoV-2 infection in five Italian Hospitals. At baseline, all patients underwent a CGA to collect information on functional, biological and psychosocial domains to calculate MPI; moreover clinical, radiological, treatments and intervention information during hospitalization were also recorded (see above).
The Ethical Committees of each of the five centres approved the study. Informed consent was given by participants who underwent initial evaluation and/or their proxies for their clinical records to be used in clinical studies. All patient records and information were anonymized and de-identified prior to the analysis.
2.2. Multidimensional prognostic index (MPI)
MPI, administered during the first 24–48 h from the admission in the correspondent ward, was developed including information from eight different domains of the CGA (Pilotto et al., 2008):
-
1.
Functional status was evaluated by Activities of Daily Living (ADL) index (Katz, Downs, Cash, & Grotz, 1970);
-
2.
Independence in the Instrumental Activities of Daily Living (IADL) (Lawton & Brody, 1969);
-
3.
Cognitive status through the Short Portable Mental Status Questionnaire (SPMSQ) (Pfeiffer, 1975);
-
4.
Co-morbidity was examined using the Cumulative Illness Rating Scale (CIRS) (Linn, Linn, & Gurel, 1968) The CIRS uses a 5-point ordinal scale (score 1–5) to estimate the severity of pathology in each of 13 systems. Based on the ratings, the Comorbidity Index (CIRS-CI) score, reflecting the number of concomitant diseases, were derived from the total number of categories in which moderate or severe levels (grade from 3 to 5) of disease were identified (range from 0 to 13).
-
5.
Nutritional status was investigated with the Mini Nutritional Assessment (MNA) short form (SF) (Guigoz & Vellas, 1999), which includes information on several nutritional aspects;
-
6.
Risk of developing pressure sores was evaluated through the Exton Smith Scale (ESS) (Bliss, McLaren, & Exton-Smith, 1966);
-
7.
Medication use was defined according to the Anatomical Therapeutics Chemical Classification code system (ATC classification) and the number of drugs used by patients at admission was also recorded;
-
8.
Social domain was categorized in living alone, in family (or with other support) and in institution.
For each domain, a tripartite hierarchy was used, i.e. 0 = no problems, 0.5 = minor problems, and 1 = major problems, based on conventional cut-off points derived from the literature for the singular items. (Pilotto et al., 2008) The sum of the calculated scores from the eight domains was divided by 8 to obtain a final MPI risk score ranging from 0 = no risk to 1 = higher risk of mortality. The MPI was expressed as three categories of risk: MPI-1 low risk (MPI value ≤ 0.33), MPI-2 moderate risk (MPI value between 0.34 and 0.66) and MPI-3 severe risk (MPI value > 0.66). (Pilotto et al., 2008) MPI requires between 15 and 25 min for its complete execution. (Pilotto et al., 2019) In case of impossibility of doing the CGA (e.g., hyperactive delirium), the evaluation was postponed to the following day, however within the 48 h from the admission.
2.3. Outcomes
The primary outcome of our research was in hospital mortality, whilst intensive care unit (ICU) admission was treated as secondary outcome. Dates of death were identified from death certificates, whilst dates of admission in the ICU were identified from clinical records.
2.4. Clinical and radiological parameters
In the MPI COVID-19 study, we recorded several clinical and radiological information, typical of COVID-19. X-ray findings were categorized as bilateral ground-glass opacities vs. other findings, whilst CT findings in pneumonia suggestive of COVID-19 vs. others, according to a standardized classification (Wong, Wong, Tang, Au, & Wai, 2020).
Among clinical signs and symptoms, we recorded information regarding fever (body temperature ≥ 37.5 °C), cough, diarrhea and dyspnea. Moreover, we investigated the presence of (prevalent) delirium, also using the 4AT score, a short tool for delirium assessment designed to be easy to use in clinical care (Bellelli et al., 2014).
2.5. Therapy and interventions during the recovery
In the MPI COVID-19 study, we recorded the data regarding therapeutical interventions for COVID-19 during the hospitalization, such as the use of antibiotics, corticosteroids, anti IL-1 and IL-6, chloroquine/hydroxychloroquine, and anti-retroviral medications. Finally, data regarding non-invasive (NIV) and invasive (oro-tracheal intubation) ventilation were recorded.
2.6. Statistical analysis
Data on continuous variables were normally distributed according to the Kolmogorov-Smirnov test. Data were presented as means and standard deviation values (SD) for quantitative measures and absolute numbers (and percentages) for the discrete variables, by MPI categories (≤0.33; 0.34–0.66; >0.66). Levene's test was used to test the homoscedasticity of variances and, if its assumption was violated, Welch's ANOVA was used. P values for trends were calculated using the Jonckheere-Terpstra test for continuous variables and the Mantel-Haenszel Chi-square test for categorical ones.
The association between MPI and the primary outcome of our investigation (i.e., in hospital mortality) was made using different approaches. First, we reported the incidence of the outcome of interest, per 1000 persons-days, by MPI categories. Moreover, we assessed the effect of MPI (in categories and as increase in 0.10 points) with the primary outcome using a Cox's regression analysis, adjusted for age (categorized in 65–75 [reference], 75–85, ≥ 85 years), gender, center. Multivariable Cox regression analysis was then conducted to assess the association between MPI and mortality. The results were consequently reported as Hazard Ratios (HRs) with their 95% confidence intervals (95%CI). Similar analyses were run, taking ICU admission as outcome and the single domains of the MPI as exposure. The accuracy of MPI was evaluated with the 5-fold Cross-validated Area Under the Curve (AUC), with the correspondent 95% CIs.
All analyses were performed using the SPSS 21.0 for Windows (SPSS Inc., Chicago, Illinois). All statistical tests were two-tailed and statistical significance was assumed for a p-value < 0.05.
3. Results
3.1. Sample selection
Overall, 250 subjects were recruited. We have then excluded 15 participants for which MPI was not calculable and 8 without data regarding hospitalization and ICU admission, leaving 227 participants eligible for this study.
3.2. Baseline characteristics
The 227 participants aged a mean of 80.5 years (range: 65–99), mainly females (59.0%). After dividing the participants by their MPI values at the admission, 100 (=44%) were classified frail, since they belonged to the MPI 3 group. Participants in the MPI 3 group were significantly older, more frequently females than their counterparts (Table 1 ). Participants in the MPI 3 group scored significantly worse than the other participants in all MPI domains, being less frequently alone (Table 1).
Table 1.
Baseline clinical characteristics by multidimensional prognostic index (MPI) values.
| Parameter | MPI 1 (≤ 0.33) (n = 60) | MPI 2 (0.33–0.66) (n = 67) | MPI 3 (>0.66) (n = 100) | p-value |
|---|---|---|---|---|
| Mean age | 75 (8) | 80 (7) | 84 (8) | <0.0001 |
| Female gender | 26 (43.3) | 40 (59.7) | 68 (68.0) | 0.009 |
| MPI domains | ||||
| ADL score | 5.9 (0.4) | 3.3 (2.0) | 0.6 (1.0) | <0.0001 |
| IADL score | 6.9 (1.5) | 3.2 (2.1) | 0.4 (0.7) | <0.0001 |
| SPMSQ score | 1.3 (2.1) | 2.5 (2.4) | 7.1 (3.0) | <0.0001 |
| ESS score | 18 (2) | 15 (2) | 10 (3) | <0.0001 |
| MNA-SF score | 12 (3) | 8 (3) | 5 (3) | <0.0001 |
| CIRS-CI score | 2.2 (1.6) | 4.0 (2.2) | 6.0 (2.2) | <0.0001 |
| Number of medications | 4 (3) | 5 (3) | 7 (3) | <0.0001 |
| Living alone | 52 (86.7) | 42 (62.7) | 50 (50.0) | 0.01 |
| MPI score | 0.20 (0.08) | 0.51 (0.10) | 0.77 (0.07) | <0.0001 |
| Clinical and radiological presentation | ||||
| Bilateral ground-glass opacities (X-ray) | 18 (30.0) | 11 (16.4) | 19 (19.0) | 0.06 |
| Pneumonia suggestive of COVID-19 (CT) | 43 (71.7) | 46 (68.79 | 74 (74.0) | 0.38 |
| Fever | 35 (59.3) | 40 (60.6) | 50 (50.0) | 0.07 |
| cough | 31 (53.4) | 23 (34.3) | 30 (30.0) | 0.005 |
| diarrhoea | 6 (10.5) | 4 (6.1) | 10 (10.0) | 0.97 |
| dyspnoea | 42 (71.2) | 44 (65.7) | 71 (71.0) | 0.93 |
| Delirium | 2 (3.3) | 5 (7.5) | 16 (16.0) | 0.02 |
| 4AT score | 0.6 (1.5) | 2.5 (3.0) | 6.5 (4.2) | <0.0001 |
| Therapy and interventions during the recovery | ||||
| Antibiotics | 56 (93.3) | 61 (91.0) | 97 (97.0) | 0.24 |
| Corticosteroids | 50 (83.3) | 58 (88.6) | 88 (88.0) | 0.62 |
| Anti IL-1 | 5 (8.3) | 6 (9.0) | 4 (4.0) | 0.35 |
| Anti IL-6 | 3 (5.0) | 3 (4.5) | 2 (2.0) | 0.57 |
| Chloroquine/hydroxychloroquine | 23 (38.3) | 19 (28.4) | 40 (40.0) | 0.28 |
| Anti-retroviral medications | 1 (1.7) | 1 (1.5) | 5 (5.0) | 0.27 |
| Non-invasive ventilation | 21 (35.0) | 19 (28.4) | 11 (11.0) | 0.001 |
| Invasive ventilation | 1 (1.7) | 1 (1.5) | 0 (0) | 0.19 |
Abbreviations: MPI: multidimensional prognostic index; ADL: activities of daily living; IADL: instrumental activities of daily living; SPMSQ: short portable mental state questionnaire; ESS: Exton-Smith Scale; MNA-SF: Mini Nutritional Assessment-Short Form; CIRS-CI: Cumulative Illness Rating Scale-Comorbidity Index; IL: interleukin.
Regarding clinical and radiological presentation, we observed no significant difference in presence of bilateral ground-glass opacities at the x-ray examination and pneumonia suggestive of COVID-19 at the CT scan. People in the MPI, from a clinical point of view, reported less frequently cough (p = 0.005), but more frequently delirium (p = 0.02) and higher 4AT score (p < 0.0001) than their counterparts with lower MPI values.
Finally, we failed to observe any significant difference in the medical therapies proposed, as shown in Table 1, whilst people in the MPI 3 were subjected less frequently than those in the MPI 1 to NIV (p = 0.001), without any difference in invasive ventilation (p = 0.19).
3.3. Follow-up data
Over a median of 21 days (range: 1–208), we observed 43 deaths (=18.9%) and 11 (=4.8%) admissions to the ICU. Of these 11 people admitted in the ICU, 10 died during the recovery: the only one survivor had an MPI score of 0.31 (MPI 1 group), whilst the people died in the ICU belonged to the MPI 2 (=5 participants) or MPI 3 (=5) groups.
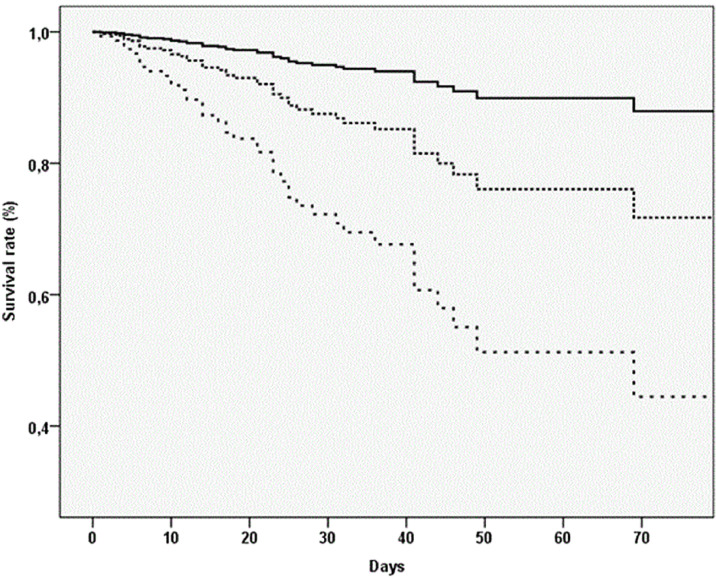
Table 2 shows the analyses regarding follow-up outcomes, using MPI as exposure. People in MPI 3 had a significant higher in hospital mortality rate (10.78 vs. 1.60 per 1000 persons-days) compared to MPI 1 (Fig. 1 ). In the multivariable analysis, adjusting for age, gender, center, people in MPI 3 experience a significant higher risk of in hospital mortality (HR=6.30, 95%CI: 1.44–27.61; p = 0.02). Of importance, age (categorized as 65–75, 75–85, ≥ 85) was not associated with any risk of in hospital mortality. Each increase in 0.10 points of MPI was associated with a significant increase in-hospital death of 41% (HR=1.41; 95%CI: 1.17–1.70). Supplementary Table 1 reports the data according to single domains of the MPI. Taking people with no problems severity as reference and after adjusting for age, gender, center, all participants in the severe problems severity group reported a significant higher risk of mortality, independently from the domain considered.
Table 2.
Outcomes of interest by multidimensional prognostic index (MPI) values at baseline.
| In hospital mortality | Intensive care unit admission | |||||||
|---|---|---|---|---|---|---|---|---|
| MPI values | Number of participants | Number of events | Incidence rate (per 1000) (95% CI) | Fully-adjusted modela,b (HR, 95%CI) | Number of events | Incidence rate (per 1000) (95% CI) | Fully-adjusted modela,b (HR, 95%CI) | Days of hospitalizationc (median, IQR) |
| MPI 1 | 60 | 2 | 1.60 (0.40–6.42) | 1 [reference] | 1 | 0.80 (0.11–5.70) | 1 [reference] | 16 (11–30) |
| MPI 2 | 67 | 8 | 3.99 (1.99–7.98) | 2.58 (0.53–12.48) P = 0.24 | 5 | 2.50 (1.04–5.99) | 2.46 (0.26–23.26) P = 0.43 | 23 (12–43) |
| MPI 3 | 100 | 33 | 10.78 (7.67–15.16) | 6.30 (1.44–27.61) P = 0.02 | 5 | 1.63 (0.68–3.93) | 1.03 (0.09–11.26) P = 0.98 | 27 (16–41) |
| Increase in 0.10 points | – | – | – | 1.41 (1.17–1.70) P<0.0001 | – | – | 0.96 (0.70–1.32) P = 0.82 | – |
| AUC (95%CI) | 0.76 (0.68–0.83) | 0.55 (0.43–0.68) | – | |||||
Data are reported as hazard ratios (HRs) and their 95% confidence intervals (CIs).
Fully adjusted model included age (in categories, 65–75, 75–85, ≥85), gender, center.
After excluding people died during hospitalization.
Abbreviations: MPI: multidimensional prognostic index; AUC: area under the curve, IQR: interquartile range.
Fig. 1.
Association between multidimensional prognostic index (MPI) and in-hospital mortality.
Notes: the highest curve represents participants in MPI-1, in the middle MPI-2, the lowest MPI-3.
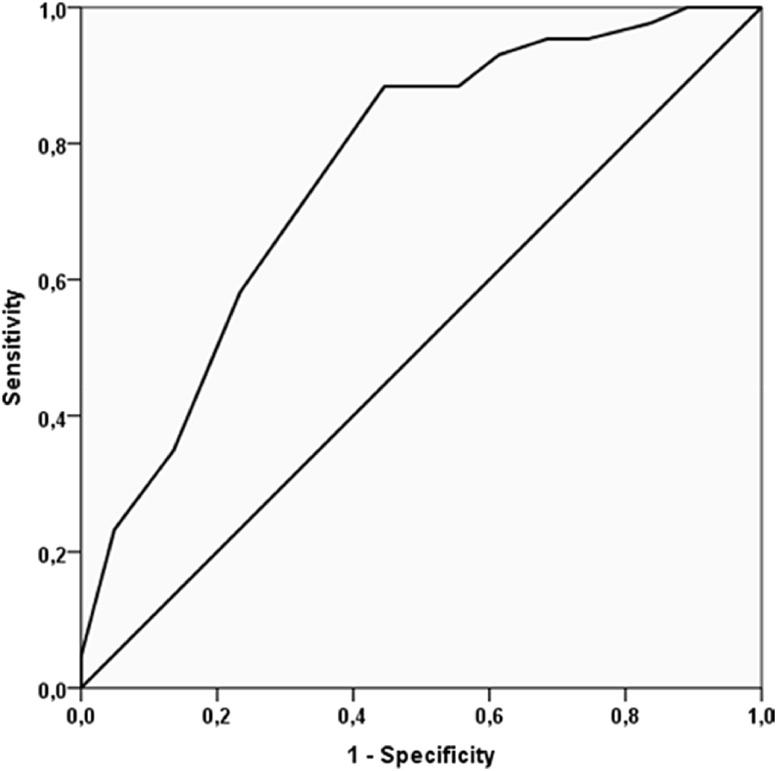
The accuracy of MPI in predicting in hospital mortality was good (AUC = 0.76, 95%CI: 0.68–0.83; p < 0.0001) (Fig. 2 ). No significant association was, on the contrary, found between MPI and ICU admission, as shown in Table 2.
Fig. 2.
Accuracy in predicting in-hospital mortality of the multidimensional prognostic index (MPI).
Notes: the continuous line represents the receiving operator curve (ROC) taking MPI as continuous variable as exposure and in hospital mortality as outcome.
Finally, after excluding people deceased, people in MPI 3 experienced a significant longer length of stay in hospital (MPI 3, median, 27 days vs. 16 days in MPI 1; p < 0.0001) (Table 2).
4. Discussion
In this multicentre research, involving 227 older inpatients affected by COVID-19, we found that higher MPI values at the admission were associated with a higher in hospital mortality, whilst no association was found with ICU admission. Single domains of the MPI have similar power in predicting mortality. The accuracy of MPI in predicting in hospital mortality was good, as showed by the AUC of 0.76, very similar to previous studies carried-out in hospitalized older patients for acute diseases (Pilotto et al., 2008, Pilotto et al., 2019). Finally, in agreement with previous data (Volpato, Bazzano, Fontana, & Ferrucci, 2015), the median length of stay in hospital was significantly higher in patients with higher MPI score compared to inpatients in lower MPI class.
A first important finding of our research is that frail patients, identified in MPI 3 group, did not differ in terms of radiological or clinical presentation compared to the other inpatients in MPI 1 and 2 groups. However, it should be acknowledged that frail patients had a greater prevalence of delirium than their counterparts. This result is confirmed in other setting, such as in emergency department, in which frailty was significantly associated with the signs and symptoms of delirium (Choutko-Joaquim, Tacchini-Jacquier, D'Alessio, & Verloo, 2019; Verloo, Goulet, Morin, & von Gunten, 2016). In our opinion, another relevant clinical finding to remark is that, even if the severity of COVID-19 is similar across MPI groups as shown by x-ray and CT findings, we found that cough (and even fever) are less frequently present in frail people. In this sense, we suggest that older frail people might have an atypical clinical presentation of COVID-19 represented by delirium, and lower prevalence of cough and fever that future studies should confirm.
Another point regards the medical therapies proposed during recovery that did not differ across MPI groups, suggesting that the attitude of physicians in using medications for COVID-19, including corticosteroids, antibiotics, anti-inflammatory drugs and anti-retrovirals is independent from the degree of frailty. On the contrary, non-invasive ventilation was more prescribed in people more robust indicating that the choice to ventilate or not also depends on frailty status, as confirmed in other studies. A retrospective observational study of 231 patients older than 70 years admitted to an acute geriatric unit for acute respiratory failure (ARF) reported that higher MPI scores at admission predicted overall mortality and NIV failure among NIV users (Custodero et al., 2021). One interesting study in ICU older patients showed that frailty was associated with higher NIV problems, failure and mortality risk. All these findings suggests that multidimensional determinants of prognosis should be assessed in older patients (Kara et al., 2018; Pilotto et al., 2019, Pilotto et al., 2020; Pilotto, Daragjati, & Veronese, 2021; Volpato, Bazzano, Fontana, & Ferrucci, 2015). Unfortunately no other specific studies were made in the context of COVID-19 indicating the scarcity of data in this area.
Moreover, our study has confirmed other research made in COVID-19 topic regarding the importance of prognostic factors vs. age in determining mortality and ICU beds utilization. As several authors have proposed, “age is just a number”, adding little in the prognostic evaluation of older patients affected by COVID-19 (Fisman, Greer, & Tuite, 2020; Panagiotou et al., 2021). Our study further confirms this impression since, in the multivariable analyses, the presence of frailty quantified with the MPI is significantly associated with in hospital mortality, whilst age was not. In this sense, we believe that our findings could be of importance to facilitate clinical decisions on better using the hospital and ICU resources confirming the necessity of assessing frailty, more than age, for correctly stratifying prognosis in older patients (National Institute for Health and Care, 2020; Hubbard et al., 2020).
Finally, our study has reported some potentially novel results. The first one is that the other studies made so far did not report the accuracy of the tools assessing frailty in their works. Moreover, many of them have investigated frailty using CFS without mentioning accuracy that is an important characteristic for a prognostic tool (Yourman, Lee, Schonberg, Widera, & Smith, 2012). CFS should be used as a screening tool since it has not the characteristics for competing with the tools that have become available in geriatric medicine for stratifying prognosis (Chong, Chan, Tan, & Lim, 2020; Rockwood & Theou, 2020). In our opinion, especially in the setting of the COVID-19 pandemic, a multidimensional approach to frailty is necessary to better define prognosis. Among the pros of MPI we can cite the fact that this tool is a product of the CGA and, consequently, can give a multidimensional picture of an older patient hospitalized for COVID-19 also indicating the areas in which we can do tailored interventions. On the contrary, among the cons, we can indicate that it needs the appropriate time for doing a CGA. Another important finding is that higher MPI values are associated with a longer length of stay in hospital, even if still alive. Our finding is in agreement with other studies in this regard (McAdams-DeMarco et al., 2017; Volpato, Bazzano, Fontana, & Ferrucci, 2015), even when made in COVID-19 setting (Kundi, EHÖ, Canpolat, & Aras, 2020), but the research in this sense is still limited. Finally, our study has also reported that the attitude of physicians in transferring and accepting older patients in the ICU setting or undergoing invasive ventilation does not depend on frailty severity, but probably by other factors. One possible explanation is that very few older people were intubated (only 2 over 227 patients) or transferred to the ICU (only 11), limiting the power of analyses. However, of importance, we observed that 10/11 older people admitted to ICU died during the recovery in this ward indicating, again, the importance of assessing prognosis for better using ICU beds. Future studies regarding this important point should be made specifically for COVID-19.
The findings of our study must be interpreted within its shortcomings. First, the observational nature of this research. Second, we did not explore the results after hospitalization, but they could be interesting for better understanding the long-term consequences of frailty in COVID-19 patients. Finally, frailty was evaluated in the studies so far with the CFS that we did not assess in our study. Therefore, we were not able to compare the accuracy of the MPI vs. CFS that future studies should assess.
5. Conclusions
Our study showed that prognostic stratification, as assessed by the MPI, was associated with a significant different risk of in hospital mortality, LOS and NIV among older inpatients affected by COVID-19, whilst the association with ICU admission was not significant. We believe that our findings are of importance for finally introducing prognostic factors derived from comprehensive geriatric assessment in daily clinical practice in hospital, and not only age that our study, again, has indicated as poor prognostic factor.
Author contributions
Conceptualized and designed the study: Pilotto Alberto, Cella. Performed data acquisition: all authors. Performed analysis and interpretation: Veronese, Pilotto Alberto. Prepared the manuscript: Veronese, Pilotto Alberto, Custodero. Critically revised the paper: Azzini, Cenderello, Castagna, Pilotto Andrea. All authors approved the final version for submission.
Sources of funding
None.
Declaration of Competing Interest
The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.archger.2021.104415.
Appendix. Supplementary materials
References
- Bellelli G., Morandi A., Davis D.H., Mazzola P., Turco R., Gentile S., et al. Validation of the 4AT, a new instrument for rapid delirium screening: A study in 234 hospitalised older people. Age and Ageing. 2014;43(4):496–502. doi: 10.1093/ageing/afu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellelli G., Rebora P., Valsecchi M.G., Bonfanti P., Citerio G., et al. COVID-19 Monza Team members Frailty index predicts poor outcome in COVID-19 patients. Intensive Care Medicin. 2020;1:1634–1636. doi: 10.1007/s00134-020-06087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss M.R., McLaren R., Exton-Smith A.N. Mattresses for preventing pressure sores in geriatric patients. Monthly bulletin of the Ministry of Health and the Public Health Laboratory Servic. 1966;25:238–268. [PubMed] [Google Scholar]
- Chong E., Chan M., Tan H.N., Lim W.S. COVID-19: Use of the clinical frailty scale for critical care decisions. Journal of the American Geriatrics Society. 2020;68(6):E30–E32. doi: 10.1111/jgs.16528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choutko-Joaquim S., Tacchini-Jacquier N., D'Alessio G.P., Verloo H. Associations between frailty and delirium among older patients admitted to an emergency department. Dementia and Geriatric Cognitive Disorders Extra. 2019;9(2):236–249. doi: 10.1159/000499707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custodero C., Gandolfo F., Cella A., Cammalleri L.A., Custoreri R., Dini S., et al. Multidimensional prognostic index (MPI) predicts non-invasive ventilation failure in older adults with acute respiratory failure. Archives of Gerontology and Geriatric. 2021;94 doi: 10.1016/j.archger.2020.104327. [DOI] [PubMed] [Google Scholar]
- De Smet R., Mellaerts B., Vandewinckele H., Lybeert P., Frans E., Ombelet S., et al. Frailty and mortality in hospitalized older adults with COVID-19: Retrospective observational study. Journal of the American Medical Directors Association. 2020;21(7):928–932. doi: 10.1016/j.jamda.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E., Martin F.C., Bergman H., Woo J., Romero-Ortuno R., Walston J. Management of frailty: Opportunities, challenges, and future directions. The Lancet. 2019;394(10206):1376–1386. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- Fisman D.N., Greer A.L., Tuite A.R. Age is just a number: a critically important number for COVID-19 case fatality. Annals of Internal Medicin. 2020;173(9):762–763. doi: 10.7326/M20-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigoz Y., Vellas B. The mini nutritional assessment (MNA) for grading the nutritional state of elderly patients: Presentation of the MNA, history and validation. Nestle Nutrition Workshop Series Clinical & Performance Programm. 1999;1:3–11. doi: 10.1159/000062967. discussion 11-12. [DOI] [PubMed] [Google Scholar]
- Haas L.E., de Lange D.W., van Dijk D., van Delden J.J. Should we deny ICU admission to the elderly? Ethical considerations in times of COVID-19. Critical Care. 2020;24(1):1–3. doi: 10.1186/s13054-020-03050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (2020). NICE updates rapid COVID-19 guideline on critical care. Available at: https://www.nice.org.uk/guidance/ng191. [PubMed]
- Hewitt J., Carter B., Vilches-Moraga A., Quinn T.J., Braude P., Verduri A., et al. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. The Lancet Public Health. 2020;5(8):e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R.E., Maier A.B., Hilmer S.N., Naganathan V., Etherton-Beer C., Rockwood K. Frailty in the face of COVID-19. Age and Ageing. 2020;49(4):499–500. doi: 10.1093/ageing/afaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara I., Yildirim F., Zerman A., Gullu Z., Boyaci N., Aydogan B., B., et al. The impact of frailty on noninvasive mechanical ventilation in elderly medical intensive care unit patients. Aging clinical and experimental research. 2018;30(4):359–366. doi: 10.1007/s40520-017-0774-z. [DOI] [PubMed] [Google Scholar]
- Katz S., Downs T.D., Cash H.R., Grotz R.C. Progress in development of the index of ADL. The Gerontologis. 1970;10(1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- Kundi H., EHÖ Çetin, Canpolat U., Aras S., et al. The role of frailty on adverse outcomes among older patients with COVID-19. Journal of Infection. 2020;81(6):944–951. doi: 10.1016/j.jinf.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M.P., Brody E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologis. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Linn B.S., Linn M.W., Gurel L. Cumulative illness rating scale. Journal of the American Geriatrics Society. 1968;16(5):622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Lloyd-Sherlock P.G., Kalache A., McKee M., Derbyshire J., Geffen L., Gomez-Olive Casas F. WHO must prioritise the needs of older people in its response to the covid-19 pandemic. BMJ. 2020;368:m1164. doi: 10.1136/bmj.m1164. [DOI] [PubMed] [Google Scholar]
- McAdams-DeMarco M.A., King E.A., Luo X., Haugen C., DiBrito S., Shaffer A., et al. Frailty, length of stay, and mortality in kidney transplant recipients: A national registry and prospective cohort study. Annals of Surgery. 2017;266(6):1084–1090. doi: 10.1097/SLA.0000000000002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Panagiotou O.A., Kosar C.M., White E.M., Bantis L.E., Yang X., Santostefano C., M., et al. Risk Factors Associated With All-Cause 30-Day Mortality in Nursing Home Residents With COVID-19. JAMA Internal Medicine. 2021;181(4):439–448. doi: 10.1001/jamainternmed.2020.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire (SPMSQ) Journal of the American Geriatrics Society. 1975;23(10):1975. doi: 10.1111/j.1532-5415.1975.tb00927.x. -1975. [DOI] [PubMed] [Google Scholar]
- Pilotto A., Custodero C., Maggi S., Polidori M.C., Veronese N., Ferrucci L. A multidimensional approach to frailty in older people. Ageing Research Review. 2020;60:101047. doi: 10.1016/j.arr.2020.101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Daragjati J., Veronese N. Comprehensive geriatric assessment. 2021. CGA and clinical decision-making: the multidimensional prognostic index. [Google Scholar]
- Pilotto A., Ferrucci L., Franceschi M., D’Ambrosio L.P., Scarcelli C., Cascavilla L., et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Research. 2008;11(1):151–161. doi: 10.1089/rej.2007.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Veronese N., Daragjati J., Cruz-Jentoft A.J., Polidori M.C., Mattace-Raso F., et al. Using the multidimensional prognostic index to predict clinical outcomes of hospitalized older persons: A prospective, multicenter, international study. The Journals of Gerontology: Series. 2019;74(10):1643–1649. doi: 10.1093/gerona/gly239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polidori M.C., Maggi S., Mattace-Raso F., Pilotto A. The unavoidable costs of frailty: A geriatric perspective in the time of COVID-19. Geriatric Care. 2020;6(1) doi: 10.4081/gc.2020.8989. [DOI] [Google Scholar]
- Pranata R., Henrina J., Lim M.A., Lawrensia S., Yonas E., Vania R., et al. Clinical frailty scale and mortality in COVID-19: A systematic review and dose-response meta-analysis. Archives of Gerontology And Geriatric. 2020;93 doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K., Theou O. Using the clinical frailty scale in allocating scarce health care resources. Canadian Geriatrics Journa. 2020;23(3):210. doi: 10.5770/cgj.23.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verloo H., Goulet C., Morin D., von Gunten A. Association between frailty and delirium in older adult patients discharged from hospital. Clinical interventions in agin. 2016;11:55. doi: 10.2147/CIA.S100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato S., Bazzano S., Fontana A., Ferrucci L., MPI-TriVeneto Study Group Multidimensional prognostic index predicts mortality and length of stay during hospitalization in the older patients: A multicenter prospective study. Journals of Gerontology Series A: Biomedical Sciences and Medical Science. 2015;70(3):325–331. doi: 10.1093/gerona/glu167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E., Altman D.G., Egger M., Pocock S.J., STROBE Initiative The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guildelines for reporting observational studies. Journal of Clinical Epidemiology. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Wong C.K., Wong J.Y., Tang E.H., Au C.H., Wai A.K.C. Clinical presentations, laboratory and radiological findings, and treatments for 11, 028 COVID-19 patients: A systematic review and meta-analysis. Scientific Report. 2020;10(1):1–16. doi: 10.1038/s41598-020-74988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Coronavirus disease 2019 (COVID-19): Situation report. Coronavirus disease 2019 (COVID-19): Situation report Situation report. 45 2019 2020.
- Yourman L.C., Lee S.J., Schonberg M.A., Widera E.W., Smith A.K. Prognostic indices for older adults: A systematic review. Jam. 2012;307(2):182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-M., Jiao J., Cao J., Huo X.-P., Zhu C., Xin-Juan W., et al. Frailty as a predictor of mortality among patients with COVID-19: A systematic review and meta-analysis. BMC Geriatric. 2021;21(1):1–11. doi: 10.1186/s12877-021-02138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.