Graphical abstract
Keywords: Aerosol, Airborne particles, COVID-19, Environmental media, SARS-CoV-2
Abstract
Corona Virus Disease 2019 (COVID-19) caused by the novel coronavirus, results in an acute respiratory condition coronavirus 2 (SARS-CoV-2) and is highly infectious. The recent spread of this virus has caused a global pandemic. Currently, the transmission routes of SARS-CoV-2 are being established, especially the role of environmental transmission. Here we review the environmental transmission routes and persistence of SARS-CoV-2. Recent studies have established that the transmission of this virus may occur, amongst others, in the air, water, soil, cold-chain, biota, and surface contact. It has also been found that the survival potential of the SARS-CoV-2 virus is dependent on different environmental conditions and pollution. Potentially important pathways include aerosol and fecal matter. Particulate matter may also be a carrier for SARS-CoV-2. Since microscopic particles can be easily absorbed by humans, more attention must be focused on the dissemination of these particles. These considerations are required to evolve a theoretical platform for epidemic control and to minimize the global threat from future epidemics.
1. Introduction
In December 2019, an outbreak of pneumonia of unknown etiology emerged in the city of Wuhan China, from which the novel coronavirus strain was isolated (2019-nCoV/ SARS-CoV-2) (Li et al., 2020b). The virus is generally referred to as ‘novel Coronavirus’. On February 11th, 2020, the World Health Organization (WHO) announced that the disease was caused by the virus named Corona Virus Disease 2019 (COVID-19). It has infected more than 100 million individuals and resulted in more than 2 million deaths as of 14 March 2021 (WHO, 2021). Reddy et al. (2021) used previously validated phenomenological models and several non-linear growth curves to predict cases and deaths of COVID-19. Debate continues about how SARS-CoV-2 is spread in the air. However, growing evidence has highlighted that infective micro-droplets are small enough to remain suspended in the air (Anderson et al., 2020, Kohanski et al., 2020). The airborne transmission of aerosolized viruses will contribute towards the widespread distribution of COVID-19 in such a short time (Froum and Froum, 2020, Khan and Parab, 2020). Research has shown that the virus could be electrostatically attracted to charged nanofibers in filtration systems, with the potential of the virus attaching to free, potentially respirable, airborne fibers or particles in the atmosphere (Leung and Sun, 2020). This has led researchers to investigate the role of microscopic particles in the spread of SARS-CoV-2.
The British government announced the discovery of the new variant of the SARS-CoV-2 in mid-December 2020; evidence shows this new variant of SARS-CoV-2 transmits more easily than other strains (UK Government, 2020). In Brazil and South Africa, new mutations of viruses have also been reported (Koyama et al., 2020, Tegally et al., 2020, Sabino et al., 2021, Tang et al., 2021). The WHO stated that mutated SARS-CoV-2 will present new challenges. Because of the high infectivity and mutability of COVID-19, the environmental transmission routes of SARS-CoV-2 are a subject of urgent research. Some studies identify the main routes of transmission as respiratory droplets (Azuma et al., 2020, Marin-Garcia et al., 2021) and close contact (Martinez-Fierro et al., 2021). People may also become infected through physical contact of virus-contaminated objects such as solid waste (Al Huraimel et al., 2020, Ben-Shmuel et al., 2020, Suman et al., 2020). Aerosol transmission in a relatively closed environment (Azuma et al., 2020, Zhao et al., 2020, Tung et al., 2021), such as hospital locals have been confirmed (Shi et al., 2020). In addition, environmental pollution caused by feces and urine aerosols (Heller et al., 2020) or physical contact in toilets (Hoseinzadeh et al., 2020, Mehraeen et al., 2020, Pamplona et al., 2020) could pose a major transmission risk. This illustrates the importance of environmental factors in transmission routes. In previous studies, the exhaled droplet transmission and close contact (e.g., contaminated hands and surfaces) were considered the main mode of transmission (Li et al., 2020a, Wong et al., 2020b), and maintaining a social distance of two meters or more between people was stipulated to minimize the risk of transmission (Bian et al., 2020, Schroter, 2020). The available studies underpin the importance of environmental factors in transmission routes of COVID-19. In this review, we consider possible transmission routes of COVID-19 and different mutations of the virus via environmental media. The survival ability of SARS-CoV-2 in relation to the different environmental conditions is also discussed.
2. The biology of SARS-CoV-2
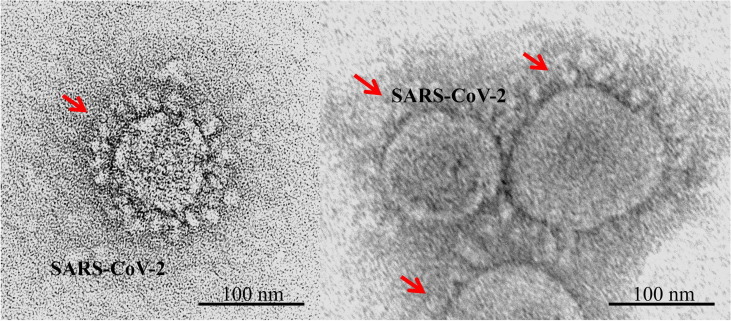
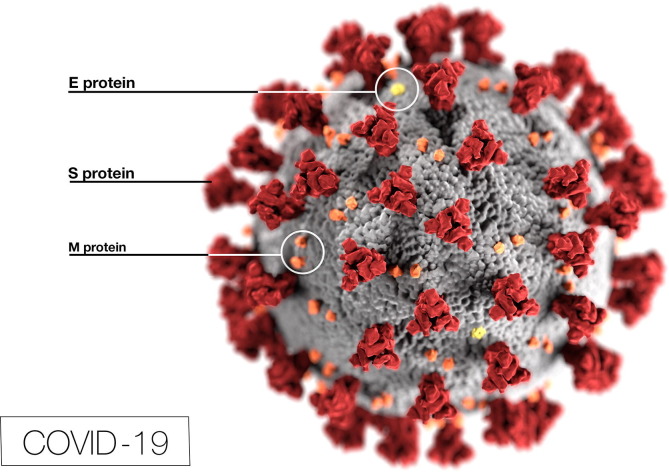
SARS-CoV-2 is a Betacoronavirus that is coated with a rigid envelope of protein to resist the antimicrobial enzymes in body fluids and lower inner shell disorder (Goh et al., 2020). Electron micrographs of SARS-CoV-2 (CDC, 2020) reveal a diverging spherical outline with some degree of pleomorphism, with virion diameters varying from 60 to 140 nm (Zhu et al., 2020b) (Fig. 1 ). CDC (2020) also reveals ultrastructural morphology exhibited by coronaviruses (Fig. 2 ). Specifically, the spikes that adorn the outer surface of the virus, which impart the look of a corona surrounding the virion, when viewed under electron microscopy (CDC, 2020). SARS-CoV-2 is constituted of structural proteins, including spike (S) glycoproteins, membrane (M) glycoproteins, envelope, and nucleocapsid (N) proteins. S glycoprotein binds to cell surface receptor angiotensin-converting enzyme II (ACE2) through receptor binding domain (RBD) (Yao et al., 2020). The initial symptoms of COVID-19 include respiratory symptoms, fever, cough, loss of taste and smell, and dyspnea. More severe infections can lead to pneumonia, severe acute respiratory syndrome, renal failure, and death (Huang et al., 2020a). A significant proportion of the patients also developed gastrointestinal symptoms such as abdominal pain, vomiting, nausea, anorexia, and diarrhea (Chen et al., 2020a).
Fig. 1.
Morphological characteristics of SARS-CoV-2 as viewed by transmission electron microscopy. The spherical viral particles may range from 60 to 140 nm in diameter (CDC, 2020).
Fig. 2.
Molecular architecture of SARS-CoV-2 which contains structural proteins (CDC, 2020).
Many studies have reported similarities between SARS-CoV-2 and other coronaviruses such as severe acute respiratory syndrome coronavirus-1, SARS-CoV-1; a bat coronavirus, and BatCoV RaTG13. In terms of nucleotide sequences, the homology of SARS-CoV-2 was 79% with SARS-CoV-1 and 96% with BatCoV RaTG13. This is markedly similar to BatCoV RaTG13. SARS-CoV-2 and SARS-CoV-1 are similar in that they both use angiotensin-converting enzyme II (ACE2) as the receptor of the virus (Zhou et al., 2020). The coronavirus binds to ACE2 receptors and enters the cells, causing infection and disease (Lu et al., 2020b, Tung et al., 2021). Studies on infectivity in cells show that SARS-CoV-2 may have a higher binding affinity to ACE2 than SARS-CoV-1 (Chen et al., 2020c), and this suggests that SARS-CoV-2 may infect human lung cells more effectively. SARS-CoV-2 and SARS-CoV-1 have similar receptor binding domains but SARS-CoV-2 and BatCoV RaTG13 receptor binding domains have low similarity, indicating that there is an intermediate host between bats and humans (Wong et al., 2020a). In terms of mortality, SARS-CoV-2 is less deadly but far more transmissible than SARS-CoV-1 (Koma et al., 2020, Petersen et al., 2020). The mortalities caused by COVID-19 vary between countries. Persons of color (POC, highest-risk phenotypes) developing COVID-19 infection are more likely to die disproportionately (Asare et al., 2020). The co-morbidities of diabetes, hypertension, cardiovascular disease, and the higher prevalence of obesity have provided hosts for viruses like COVID-19 to thrive and cause serious infections (Asare et al., 2020, Caci et al., 2020, Gasparotto et al., 2019, Giorgino et al., 2020). There is awareness of elderly people and those with immunocompromised conditions being more vulnerable to catching COVID-19 (Schmidt et al., 2020).
3. Overview of possible transmission pathways
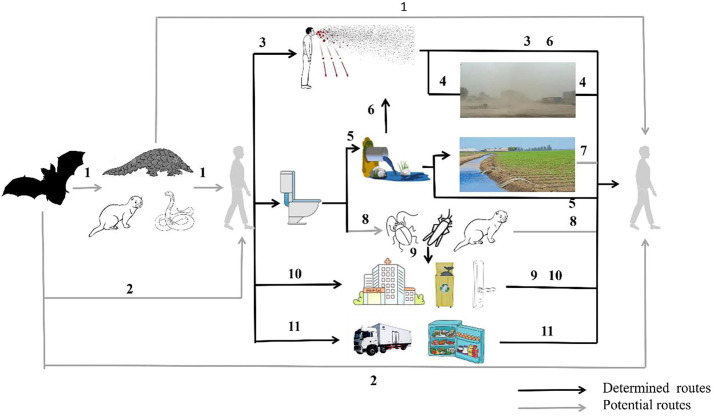
There is a plethora of studies that indicate the novel coronavirus transmission to be strongly influenced by environmental factors. The currently accepted pathways are respiratory droplets and close contact, touching virus-contaminated objects (Ben-Shmuel et al., 2020), aerosol transmission in closed environments (Shi et al., 2020), feces and urine aerosol, or contact in toilets (Pamplona et al., 2020), and cold-chain transmission (Han et al., 2020b, Liu et al., 2020b). In general, SARS-CoV-2 has been found in the environment, including air, water, soil, biota, surfaces, and cold-chain (Fig. 3 , Table 1 ). Some of these pathways overlap, resulting in potentially complex transmission pathways.
Fig. 3.
A schematic diagram to show the different environmental transmission routes of SARS-CoV-2 in animals and humans. Routes 1 and 2: Bats may either directly or indirectly act as a vector to pass on the SARS-CoV-2 viral infection to humans; Route 3: Infections are caused directly or indirectly by aerosols and droplets exhaled by the humans; Route 4: Falling aerosols or droplets may resuspend through the soil causing transmission; Route 5: The entry of excreta into sewage may cause water spread; Route 6: The excreta enters the sewage and the virus-containing air around the sewage may cause transmission; Route 7: Water containing the virus entering the soil can cause infection; Route 8: Animals (e.g., cockroaches) that can transmit pathogens to humans may cause the spread of SARS-CoV-2; Route 9: Animals can spread SARS-CoV-2 through contact with contaminated surfaces and secretions from sick people; Route 10: People can become infected by touching contaminated objects with their hands; Route 11: It may cause long-distance cold chain transmission through low temperature treatment of food, freezing storage and the cold-chain for transporting.
Table 1.
Transmission codes of SARS-CoV-2 in different environments.
| Types of virus transmission | Virus transmission mode |
|---|---|
| Airborne transmission | The potential transmission of SARS-CoV-2 in the air is mainly from respiratory droplets and aerosols by coughing, sneezing, talking, and singing |
| Water transmission | Water transmission refers to bacteria and viruses discharged from the body through excreta to pollute the environment, followed by entry into the human respiratory and/or GIT to infect patients |
| Soil transmission | For one thing, contaminated soils are resuspended and inhaled by humans and animals. For another, water containing virus is discharged into soil or sludge may cause indirect diffusion of the soil near the water flow |
| Surface transmission | Infection may occur when humans make contact with the surface of contaminated objects and then the mucosal parts. Cold-chain transmission is a special surface propagation mode by frozen food’s packaging materials |
| Biota transmission | On the one hand, some insects transmit the virus by contacting contaminated surfaces and secretions of patients. On the other hand, it is necessary to study whether animals (e.g., minks) infected with virus can be transmitted to humans |
3.1. Airborne transmission of SARS-CoV-2
3.1.1. Modes of airborne transmission
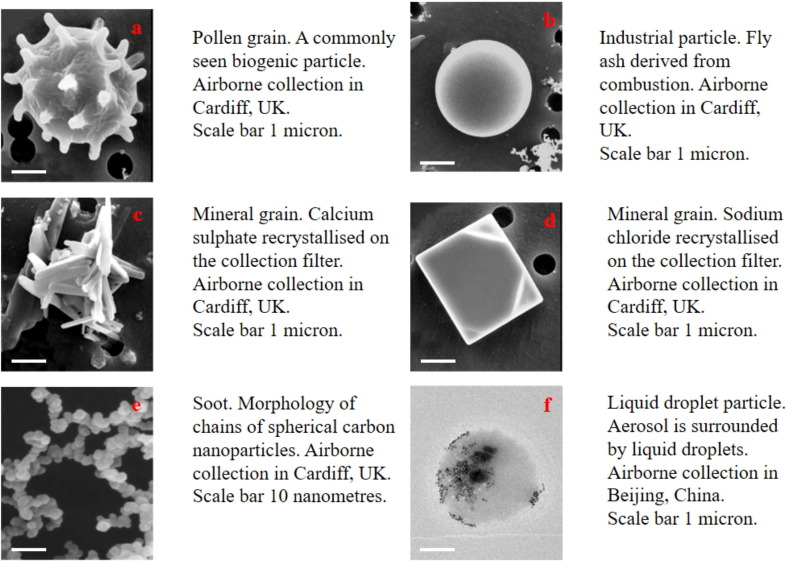
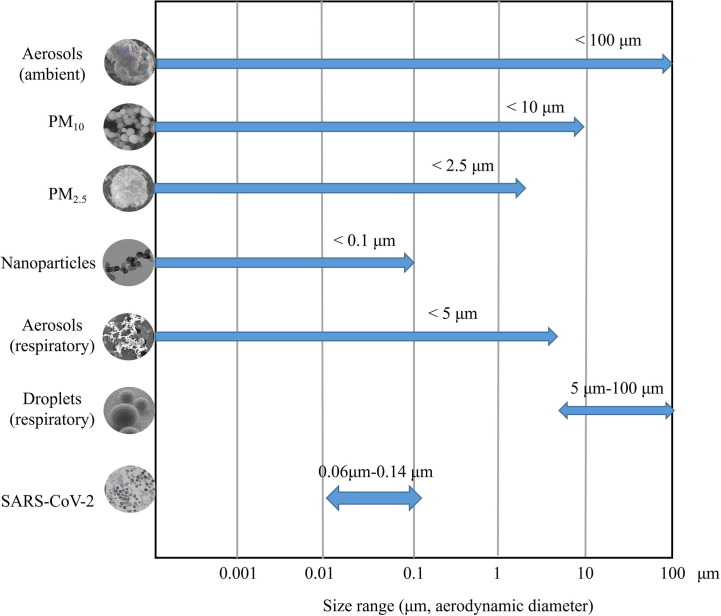
The virus may be enveloped in the interior or adhere to the surface of respiratory droplets and aerosols. In the ambient air, the liquid droplets can encrust and absorb finer aerosol particles (Freney et al., 2010, Yuan et al., 2019). Fig. 4 showed some typical images of ambient aerosol particles including biogenic particle, fly ash, mineral, soot, and liquid droplets. According to their aerodynamic diameter, the ambient aerosol particles are divided into PM10 (particulate matter less than 10 μm), PM2.5 (particulate matter less than 2.5 μm), and nanoparticles (Shao et al., 2018, Boongla et al., 2020, Chen et al., 2020b). Nano-aerosol particles are airborne aerosols up to 100 nm (Leung et al., 2018) (Fig. 4). The respiratory aerosols are classified into droplets and aerosol, with the main differences between respiratory aerosols and droplets and various ambient aerosol particles being the particle sizes (Fig. 5 ). The WHO defined the respiratory particles larger than 5 μm as droplets and those smaller than 5 μm as respiratory aerosols (WHO, 2014). However, the respiratory aerosol is different from an ambient aerosol defined in atmospheric science where it generally refers to the suspension system of solid particles, liquid particles, or both of them in a gaseous medium (Tang et al., 2006, Freney et al., 2010). Ambient aerosol is considered potentially harmful to human health as it can contain not only hazardous elements and chemicals (Shao et al., 2017, Feng et al., 2020, Rao et al., 2020), but also pathogens such as bacteria, fungi, and viruses (Han et al., 2021). Airborne fine particles (PM2.5) are considered of greater health significance with their large surface area and strong adsorption capability (Ding and Zhu, 2003, Shao et al., 2018). Respiratory droplets are produced by any action resulting in the energetic release of air from the mouth or nose, such as coughing, sneezing, talking, and singing (emission jets) (Xie et al., 2009). The potential transmission of SARS-CoV-2 in the air is mainly from respiratory droplets and aerosols (Azuma et al., 2020, Jayaweera et al., 2020). Studies have indicated that aerosols containing SARS-CoV-2 are mainly distributed in two size ranges that include 0.25–1.0 μm and > 2.5 μm, respectively (Liu et al., 2020a).
Fig. 4.
Morphology and composition of different types of particles. (a–e) Scanning electron microscope [SEM] image. (f) Transmission electron microscope [TEM] image.
Fig. 5.
The size range of different types of particles. The graph denotes the size ranges between ambient aerosol (e.g., Ca aluminium silicate Grossular) with irregular morphology; scanning electron microscope [SEM] image), PM10 (e.g., coal fly ash particles; SEM), PM2.5 (e.g., an aggregate of carbon black particles, SEM image), nanoparticles (ultrafine particles) (e.g., aggregate, transmission electron microscope [TEM] image), respiratory aerosol (e.g. soot particles, SEM), respiratory droplets (SEM), virus SARS-CoV-2 (TEM). The SARS-CoV-2 viral particles with aerodynamic diameters of 0.06–0.14 μm fall within the “highly respirable” size range.
It is generally accepted that the larger respiratory particles travel shorter distances, dropping out of the air sooner, and potentially increasing the risk of contamination on proximal surfaces. Epidemiological and pathogen infection experiments conclude that the range of respiratory droplet infection is typically within 1 m away from the source of infection (WHO, 2014). In contrast, respiratory aerosols may be suspended in the air for long periods of time due to Brownian motion, and thus, travel over greater distances; therefore, aerosol propagation is possible (Jayaweera et al., 2020). However, this capacity of respiratory droplet and aerosol transmission can be influenced by environmental parameters (e.g., evaporation, humidity, gravity, wind speed, and wind direction). Respiratory droplet transmission induces mucocutaneous and respiratory tract infections, which has been of great concern to medical practitioners, whereas aerosol can transmit deeply into the respiratory tract including the alveolar region (Drossinos and Stilianakis, 2020). Microscopic particles can contain virus that can be absorbed to cause infection by (1) oral ingestion of contaminated food and water; (2) inhalation through the airway; and (3) dermal absorption (Bakshi, 2020, Leung and Sun, 2020, Silva et al., 2021, Romer et al., 2011). Virus-containing aerosol is most likely to penetrate deep into the distal respiratory airways of the lung. From there, they can penetrate the alveolar membrane, enter the bloodstream directly and translocate to vital organs (Drossinos and Stilianakis, 2020, Silva et al., 2020, Silva et al., 2021). Substances attached to smaller particles may have more harmful health effects than those attached to larger particles (Leung et al., 2018). Therefore, droplet and aerosol, especially finer aerosol, transmission cannot be ignored.
3.1.2. Examples of aerosols containing SARS-CoV-2
Evidence for the quantification and assessment of SARS-CoV-2 around the disease concentration areas is lacking, although there is evidence for the detection of virus-containing aerosols (Cao et al., 2021, Liu et al., 2020a). At present, there is no direct evidence that SARS-CoV-2 can be transmitted through the air. There are indications of viral-containing aerosols in many places. This may confirm that such a transmission pathway may be possible.
In hospital environments, the supportive therapy may produce aerosols containing viruses. The associated medical operations in relation to the virus transmission include bronchoscopy, cardiopulmonary resuscitation (CPR), non-invasive positive pressure ventilation (NPPV), tracheal intubation and extubation, tracheostomy, surgery, the suction of body fluids, nebulizer treatment, otolaryngology medical, and dental treatment (Table 2 ). The SARS-CoV-2 in dentistry and otolaryngology is found to be associated with the aerosol produced by mechanical oral transmission or coughing (Becker et al., 2020, Ralli et al., 2020). The airway operation (non-invasive positive, tracheal intubation and extubation, tracheostomy) during surgery will also produce the aerosols containing SARS-CoV-2 (Loth et al., 2020, Dhillon et al., 2021). The aerosols may also be produced when the anesthesiologist assists with airway management (e.g., mask ventilation, chest cavity) and CPR (Wong et al., 2020c). Health care workers performing bronchoscopy in emergency situations are at risk of aerosol transmission (Yamamoto et al., 2021). The non-invasive positive pressure ventilation (NPPV) patients infected with SARS-CoV-2 are at a high risk of transmitting the virus (Fujita and Suzuki, 2020). Aerosols containing SARS-CoV-2 RNA were detected in a hospital in Wuhan, China, and the viral load in ambient aerosols in the patient toilets was found to be highest, followed by the waiting-room for patient visitors (Liu et al., 2020a). Aerosol in these environments is usually produced by cough, surgery, mechanical transmission, and medical interventions that may cause infection of relevant supporting medical staff.
Table 2.
Medical operation and induction mechanism of aerosol generation in hospital.
| Medical operation | Mechanism | References |
|---|---|---|
| Bronchoscopy | Cough | Yamamoto et al., 2021 |
| Cardiopulmonary resuscitation (CPR) | Cough | Wong et al., 2020c |
| Non-invasive positive pressure ventilation (NPPV) | Mechanical transmission | Fujita and Suzuki, 2020 |
| Tracheal intubation and extubation | Mechanical transmission | Dhillon et al., 2021 |
| Tracheostomy | Mechanical transmission | Loth et al., 2020 |
| Surgery | Surgery operation and mechanical transmission | Ralli et al., 2020 |
| The suction of body fluids | Cough | Tran et al., 2012 |
| Nebulizer treatment | Mechanical transmission | Tran et al., 2012 |
| Otolaryngology medical | Mechanical transmission | Ralli et al., 2020 |
| Dental treatment | Cough | Becker et al., 2020 |
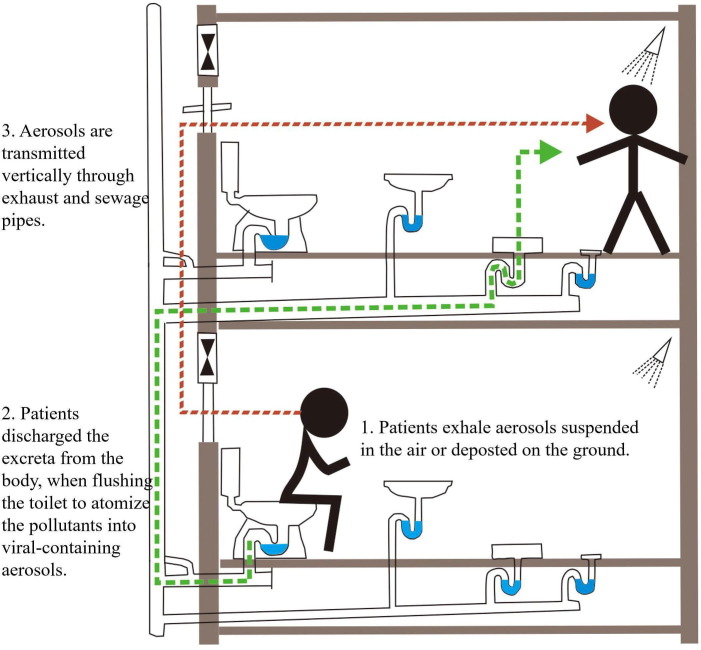
Indoor transmission of aerosols containing coronaviruses has been reported in a variety of cases. The case of the SARS-CoV-1 infection in Amoy Gardens, Hong Kong, in 2003, where a total of 321 people were infected, serves as a possible example of aerosol transmission of virus (Peiris et al., 2003, Lau et al., 2004). The aerosols exhaled by the residents were deposited on the indoor surface or in the suspended air. Firstly, virus-containing aerosols in the air were transmitted vertically through exhaust pipes (e.g., toilet and shower; Fig. 6 ). Secondly, when the residents discharged their waste (feces and urine) after using toilets, airflow was generated upon flushing the toilet and produced viral-containing aerosol transmission through the sewage pipe (Fig. 6) (Gormley et al., 2017). This in turn, increased the risk of infection, as novel coronavirus has been isolated from the feces of COVID-19 patients (Jones et al., 2020). Therefore, it is important to implement effective measures to prevent aerosol transmission caused by using “sitting” style toilets, such as increasing the toilet room ventilation and disinfection routines. Toilet and bathroom ventilation systems are also a potential route for airborne transmission, which draw air into the apartment and expedites long-distance transmission. Although there is no evidence that the virus can spread through air conditioning systems, it has been observed in some cases of “cluster outbreaks” that the airflow of air conditioning in a closed environment will affect the transmission of novel coronavirus (Lacatusu et al., 2020). Three families in a restaurant in Guangzhou who were seated according to the Worldwide social distance rule of “2 m separation between persons” developed COVID-19 symptoms (Lu et al., 2020a). The strong airflow atomization of air conditioning may have carried virus particles and caused transmission (Chirico et al., 2020). These case studies indicate that human generated aerosols containing novel coronavirus may exist in a relatively closed interior space for extended periods of time. Therefore, it is speculated that the aerosols and droplets produced by human respiratory performance may lead to the rapid spread of disease. Thus, further studies on the indoor transmission characteristics of aerosols containing the SARS-CoV-2 are required.
Fig. 6.
Aerosol propagation in sewage pipes (modified after Gormley et al., 2017).
The outdoor transmission of SARS-CoV-2 is another possible route of the airborne transmission of virus. The highest concentration of a positive sample of novel coronavirus was detected in ventilation grills at a hospital in the United States treating patients with SARS-CoV-2 (Santarpia et al., 2020). This increases the likelihood that aerosols can travel outdoors. A study also suggests that conditions of greater atmospheric stability and dryness are conducive to the spread of SARS-CoV-2 (Sanchez-Lorenzo et al., 2020). A retrospective cohort study in the United States showed that incidence of COVID-19 cases was significantly higher in 14 out of 20 counties that had a large outdoor gathering 15 days prior (Miron et al., 2020). Therefore, it is important to note that infections are possible outdoors, and protective measures should be strengthened in outdoors.
In offices and outdoor environments, the number of nanoparticles is as high as 60–300 million count/m3 (Leung et al., 2018). However, due to the difficulty of observation and sampling, there is still no evidence of virus-containing nanoparticles or nanoparticle transmission.
Therefore, available epidemiological implicates airborne transmission of SARS-CoV-2 as a potential route for the spreading of the disease. The possibility of long-distance aerosol transmission needs further and urgent epidemiological and experimental studies.
3.1.3. The relationship between air quality and SARS-CoV-2
Studies have shown that the high infection rate of COVID-19 is related to poor air quality (Cazzolla Gatti et al., 2020, Coccia, 2020, Conticini et al., 2020). Air pollution can cause inflammations, cell damage, and respiratory diseases (Shao et al., 2017, Feng et al., 2020, Rao et al., 2020, Wang et al., 2021). High concentrations of volatile organic compounds (VOCs) can cause health risks (Yan et al., 2015). Inflammation is the hall mark of human diseases, such as the co-morbidities (e.g., obesity) in persons that become infected with COVID-19 (Caci et al., 2020). The rise of inflammation may increase the mortality rate and the severity of expression of the disease in the most polluted areas (Comunian et al., 2020).
The positive relationship between air pollution and COVID-19 infection rate has been confirmed in many countries. For example, a study conducted in Italy showed that the infection rate of COVID-19 was associated with PM10 exceeding the limits in urban air (Coccia, 2020). Another study observed two regions in Europe, Lombardy, and Emilia-Romagna, which have the highest mortality rate of the virus in the World and are typified by the most polluted area in Europe (Conticini et al., 2020). Cazzolla Gatti et al. (2020) also believed that PM2.5 was related to transmission dynamics and the infectivity of SARS-CoV-2. They projected that, with an increase of 5%–10% in air pollution, similar future pathogens may inflate the epidemic toll of Italy by 21%–32% (Cazzolla Gatti et al., 2020). In 235 Chinese cities, the relationship between short-term exposure to air pollution and daily confirmed cases were assessed, revealing a positive relationship (Zhang et al., 2021).
Other studies have shown that the concentration of air pollutants was affected by the COVID-19 lockdown. Reduction in aerosol loading over a majority of the aerosol hotspots were observed during the COVID-19 lockdown (Sanap, 2021). A study found the air pollutants PM10, PM2.5, NOx, CO, and SO2 decreased whereas O3 increased in Hangzhou, China (Liu et al., 2020c). Shi et al. (2021) also observed NO2 and PM2.5 concentration decreased and O3 increased in 11 cities globally with the exception of London and Paris. The lockdown not only decreased the air pollution significantly but also affected through social distancing and isolation of the COVID-19 cases from the unaffected population. A study informed that the lowest rate of COVID-19 confirmed cases were found in full lockdown (Rahman et al., 2021). The COVID-19 lockdown certainly is expected to have a synergistic effect in bringing down transmission of virus. However, more systematic epidemiological studies are needed.
Ambient PM concentrations were significantly associated with cases of human influenza and other respiratory diseases (Liang et al., 2014, Zhou et al., 2021a). Studies on COVID-19 have also shown that PM can help transmission of novel coronavirus (Barakat et al., 2020, Tung et al., 2021). PM can be exacerbated by the inflammation induced by COVID-19. Therefore, PM may play a role as a booster of COVID-19 (Mescoli et al., 2020). It is assumed that PM could be the transmission model of SARS-CoV-2 infection and could be the “carrier” of SARS-CoV-2, which enters the human body directly (Tung et al., 2021). Exposure to PM increases ACE2 expression which indirectly promotes the entry of SARS-CoV-2 into the human body (Tung et al., 2021). For SARS-CoV-2, the minimum size is approximately 60 nm (Zhu et al., 2020b). The above research stressed that the presence of ambient PM is involved in the spread of SARS-CoV-2 is crucial.
3.1.4. The survival potential of SARS-CoV-2 in the air
The survival potential and transmission routes of viruses in airborne transmission are affected by environmental factors. Studies have shown that temperature and humidity in the air, microbial resistance to external physical and biological stresses, and ultraviolet intensity all affect the viability and distribution of microorganisms in the air (Pyankov et al., 2012, Trancossi et al., 2021). An experimental study suggested that the greater the humidity, the larger the airborne particle size, and the less easy the particle diffusion (Pawar et al., 2009). Atmospheric humidity effects on viral spread have been identified by many studies, but the conclusions are still controversial. High air humidity seems to be the most important climatic factor in viral spreading in the rainforest region (Crema, 2021). In two humid regions of Iran, the rate of SARS-CoV-2 spreading is high (Ahmadi et al., 2020). However, in arid areas, humidity has a reverse relationship with the disease infection rate (Ahmadi et al., 2020). Ma et al. (2020) showed the risk of SARS-CoV-2 was inversely proportional to the relative humidity and directly proportional to the diurnal temperature range. The novel coronavirus can maintain its activity in aerosol for three hours (van Doremalen et al., 2020). The WHO pointed out that high temperature, high or low pH value and sunlight may inactivate the coronavirus (WHO, 2020b). However, in a bathroom, it was found that novel coronavirus was infective despite being in an environment with both high humidity and temperature (Luo et al., 2020). A study on the infection of COVID-19 in 122 cities in China from January to February 2020 has revealed that the infection rate did not decrease when the weather became warm (Xie and Zhu, 2020). The relationship between COVID-19 transmission parameters and environmental factors is used to elucidate whether they are related to viral mutation rate. The transmission speed of the first wave of the virus decreased with temperature, and high ultraviolet light would reduce the transmission speed, but the second wave of mutant COVID-19 adapted to a higher temperature (Seligmann et al., 2020). Therefore, the ability of the virus to survive in the air has not yet been agreed upon, but it has been shown that the mutant virus has a higher survivability.
At present, there are still doubts about the mode of airborne transmission of novel coronavirus. Although there is growing evidence to prove the transmission route of SARS-CoV-2 in the air, further proofs are needed. Aerosol transmission could occur in some cluster cases when the environmental factors are suitable. However, understanding the outdoor transmission of aerosol is an urgent priority. In addition, to explain the relationship between air pollution and infection rate of COVID-19, the studies reviewed here demonstrate that exposure to air pollution especially PM2.5 and PM10 may contribute significantly to higher rates of COVID-19. However, there are several viewpoints without reliable supporting evidence. The results of these investigations (e.g., Zhu et al., 2020a) may be affected by the issue of spurious correlation due to the omission of a common factor, namely, population density (Copiello and Grillenzoni, 2020). Therefore, human factors should be considered when evaluating the relationship between infection rate and air pollution. There have been conflicting reports on the role of temperature and humidity in the viability of novel coronavirus. Maybe global scale research will be able to allow more accurate data of larger ranges of temperature and humidity. Although there are still controversies about the airborne transmission mode and survival potential of novel coronavirus, the activity of SARS-CoV-2 in the air has been substantiated, and this mode of transmission should not be ignored.
3.2. Water transmission of SARS-CoV-2
The rapid spread of coronaviruses during outbreaks suggests the primary mode of transmission of human coronaviruses is respiratory droplets and close contact. However, their fecal elimination also suggests the possible spread via water. SARS-CoV-2 has been found in the fecal samples and anal swabs of some patients, and thus, demonstrating the possibility of fecal-oral (including waterborne, e.g., sewage pipes, wastewater, and agricultural run-off) transmission (La Rosa et al., 2020). For example, novel coronavirus RNA has been detected in the feces of symptomatic and asymptomatic COVID-19 patients (Jones et al., 2020). The scarcity of information on coronaviruses in the environment suggests that further studies are needed to understand the fate of these viruses in the water cycle.
3.2.1. Potential fecal-oral transmission
Digestive tract (Gastrointestinal tract; GIT) transmission is also known as fecal-oral transmission. Fecal-oral transmission refers to bacteria and viruses discharged from the body through excreta to pollute the environment, followed by entry into the human respiratory and/or GIT to infect patients. Numerous studies have established that the GIT is a potential transmission route of SARS-CoV-2 (Arslan et al., 2020, Chen et al., 2020c, Wu et al., 2020). It is reported that the receptor ACE2 was positively stained for gastrointestinal epithelial cells (Xiao et al., 2020). An analysis of data from COVID-19 patients in Hong Kong demonstrated that viral RNA was detected in 48.1% of the patients' fecal samples, and the fecal samples collected even after respiratory samples tested negative were still positive (Cheung et al., 2020). A similar study comparing respiratory and fecal samples from patients with SARS-CoV-2 indicated that fecal samples remained positive for an average of 27 ± 9 days after first symptom onset (i.e., an average of 11 ± 2 days longer than respiratory samples) (Wu et al., 2020). Therefore, strict precautionary measures are essential to prevent fecal transmission.
Novel coronavirus has been detected in sewage from wastewater treatment plants (e.g., Wang et al., 2020). These studies demonstrated that the novel coronavirus was able to invade the human digestive tract and utilize the fecal-oral transmission route to enter the water cycle (e.g., Arslan et al., 2020). At present, there is no direct evidence that the aerosolized SARS-CoV-2 viral particles may be contracted from domestic wastewater. However, untreated sewage water containing the virus may be a dissemination medium. Contaminated fecal particles may enter natural water bodies through leaky sewers or due to contaminated wastewater treatment plants, especially those in developing countries (Majumdar et al., 2019). Novel coronavirus in water transmission may pose several risks, including the airborne spread of virus-containing aerosols from toilet flushing by household members (Fig. 6), the contact transmission of contaminated hands and inanimate surfaces due to poor personal hygiene behaviors, and the surface contact transmission caused by arthropods (e.g., flies, cockroaches) (Arslan et al., 2020, Heller et al., 2020).
3.2.2. Wastewater monitoring as an early warning system
Novel coronavirus has been found in water sources, but there is no direct evidence to confirm whether the waterborne virus has the capacity to cause infection. However, regular wastewater monitoring can be used as a means of early warning (Foladori et al., 2020). Firstly, utilizing the concentration of the virus in a body of water, the infection level of a local population may be estimated. Secondly, because patients may defecate before symptoms appear, the virus can be detected before spreading. The study also found novel coronavirus in feces 3 days post-infection (Han et al., 2020a), but the diagnosis time of COVID-19 has been two weeks. In addition, some patients excreted SARS-CoV-2 RNA via feces post-recovery. For example, a patient's feces sample was still positive 33 days after the respiratory tract sample was negative (Wu et al., 2020). Therefore, a rigorous monitoring program for the detection of COVID-19 in wastewater could provide a way forward in terms of an advanced warning system.
3.2.3. The survival potential of SARS-CoV-2 in water
The transmission of viruses through water is not only affected by the properties of virus itself (e.g., enveloped, and lower shell disorder, Gwenzi, 2021), but also by the environmental conditions (e.g., water matrix and temperature) (Ahmed et al., 2020). The lower shell disorder of SARS-CoV-2 may lead to the possibility of fecal-oral transmission (Gwenzi, 2021). Water matrix and temperature, as the environmental conditions, can affect the viability of the virus (Table 3 ). A study has confirmed that at 4 °C, SARS-CoV-2 can survive for 27.8 ± 4.45 days in untreated wastewater and 43.2 ± 5.95 days in autoclaved wastewater. At 15 °C, SARS-CoV-2 can survive for 20.4 ± 2.13 days in untreated wastewater and 29.9 ± 2.39 days in autoclaved wastewater. At 25 °C, SARS-CoV-2 can survive for 12.6 ± 0.59 days in untreated wastewater and 13.5 ± 0.85 days in autoclaved wastewater. At 37 °C, SARS-CoV-2 can survive for 8.04 ± 0.23 days in untreated wastewater and 5.71 ± 0.50 days in autoclaved wastewater (Ahmed et al., 2020). For safety reasons, researchers tend to use the substitute virus including human coronaviruses (i.e., SARS-CoV-1) and other influenza viruses (i.e., Murine hepatitis virus, MHV) to conduct the experiments (Wang et al., 2005, Ahmed et al., 2020). SARS-CoV-1, which is very similar to SARS-CoV-2, is used in the experiments, and it is found that, at 4 °C, SARS-CoV-1 can survive in all types of water (domestic sewage and dechlorinated tap water) for more than 14 days. Compared to other types of water, in feces and urine, this virus can survive for more than 17 days at 4 °C, but at 20 °C, SARS-CoV-1 only can survive 3 days (Wang et al., 2005). MHV, as another similar virus, has also been used in the experiments, and it is found that, in untreated wastewater, the survival days of the viruses are different at different temperatures (Ahmed et al., 2020). Survival days are 56.6 ± 14.2 days at 4 °C, 28.5 ± 4.43 days at 15 °C, 17.3 ± 2.46 days at 25 °C, 7.44 ± 0.61 days at 37 °C (Ahmed et al., 2020). In autoclaved wastewater, the virus has a shorter survival period. At 4 °C, MHV can survive 43.1 ± 4.02 days. At 15 °C, it can survive 33.9 ± 1.97 days. At 25 °C, MHV can survive 17.6 ± 2.46 days. And at 37 °C, MHV can survive 5.58 ± 0.25 days (Table 3) (Ahmed et al., 2020). From these experiments, it can be inferred that SARS-CoV-2 has a strong vitality in the water environment of low temperature, the isotonic human body, and/or wastewater.
Table 3.
Environmental viability of SARS-CoV-2 and other similar viruses in different water environments.
| Species of virus | Temperature (°C) | Water matrix | Survival potential | References |
|---|---|---|---|---|
| SARS-CoV-1 | 4 | Feces and urine | More than 17 days | Wang et al., 2005 |
| All types of water | More than 14 days | |||
| 20 | Feces and urine | 3 days | ||
| SARS-CoV-2 | 4 | Untreated wastewater | 27.8 ± 4.45 days | |
| Autoclaved wastewater | 43.2 ± 5.95 days | |||
| 15 | Untreated wastewater | 20.4 ± 2.13 days | ||
| Autoclaved wastewater | 29.9 ± 2.39 days | |||
| 25 | Untreated wastewater | 12.6 ± 0.59 days | ||
| Autoclaved wastewater | 13.5 ± 0.85 days | |||
| 37 | Untreated wastewater | 8.04 ± 0.23 days | ||
| Autoclaved wastewater | 5.71 ± 0.50 days | |||
| Murine hepatitis virus(MHV) | 4 | Untreated wastewater | 56.6 ± 14.2 days | Ahmed et al., 2020 |
| Autoclaved wastewater | 43.1 ± 4.02 days | |||
| 15 | Untreated wastewater | 28.5 ± 4.43 days | ||
| Autoclaved wastewater | 33.9 ± 1.97 days | |||
| 25 | Untreated wastewater | 17.3 ± 2.46 days | ||
| Autoclaved wastewater | 17.6 ± 2.46 days | |||
| 37 | Untreated wastewater | 7.44 ± 0.61 days | ||
| Autoclaved wastewater | 5.58 ± 0.25 days |
SARS-CoV-2 in the water environment may come from untreated sewage, poorly performing sewage treatment plants, and waterworks that lack sanitation facilities. SARS-CoV-2 is an enveloped virus which can survive for several days in wastewater and surface water (Han et al., 2020a). Compared with the non-enveloped viruses (e.g., coxsackieviruses, rotavirus, or poliovirus), which can survive for extended periods on surfaces, the enveloped viruses, including H1N1 and human coronaviruses, remain infectious on surfaces after several days (Firquet et al., 2015). In contrast, the enveloped viruses have a faster inactivation rate, poorer environmental stability, and higher sensitivity to oxidants, such as chlorine (Gundy et al., 2009, Ye et al., 2016, Pinon and Vialette, 2018). Therefore, oxidant containing chlorine can be used for virus inactivation in sewage treatment plants. The method recommended by WHO is that disinfectants with chlorinated free chlorine concentration greater than 0.5 mg/l should be exposed for at least 30 min at pH 8.0 (WHO, 2020b). However, organic matters and suspended solids in the wastewater can be adhered to and protected from the virus (Pinon and Vialette, 2018). Thus, the inactivation efficiency of disinfectants can be reduced. When the viral load was high, the disinfectant might not be enough to inactivate SARS-CoV-2 (Zhang et al., 2020a). Therefore, the effective purification measures of viruses require further studies.
The novel coronavirus transmission in water takes place after the virus enters the GIT and is discharged from the body into the water source, causing the putative transmission route. At present, it is still unclear what the concentration limit of the virus is in water. At the same time, there are a limited number of studies on the survival potential of novel coronavirus in water. The risk of novel coronavirus in sewage cannot be ignored, especially in developing countries with poor sewage treatment facilities.
3.3. Soil transmission of SARS-CoV-2
Soil transmission is partly similar to the airborne transmission route, whereby contaminated soils have the potential to be resuspended and inhaled by humans and animals (Donado et al., 2021, Trejos et al., 2021). Another component of transmission is water runoff to the soil, where wastewater treatment is lacking. Untreated wastewater is used directly to irrigate the soil, or it may cause indirect diffusion of the soil near the water flow (Conde-Cid et al., 2020). Some studies are investigating the detection and quantification of SARS-CoV-2 in sewage sludge, which provides an approach to estimate changes in COVID-19 prevalence on a population level (Peccia et al., 2020). However, the infection of novel coronavirus in soil has not been proved, and the virulence of SARS-CoV-2 in sewage sludge and soil has not been determined (Collivignarelli et al., 2020). Despite this, the expression of coronavirus proteins with hydrophobic structure can bind to soil components (Zhang et al., 2020b) which may allow the coronavirus to enter the soil. After passing through 70 cm of soil in the column test and field test, fecal bacteria can still survive in leachate (Periago et al., 2002), implying that the infection of novel coronavirus in soil is possible.
The factors affecting the transmission of virus in soil mainly include soil types, temperature, and pH values (Collivignarelli et al., 2020, Nunez-Delgado, 2020, Steffan et al., 2020). However, there is a lack of data on the infectivity of SARS-CoV-2 in soil. On one hand, vitro experiments show that the resistance of SARS-CoV-2 to high temperature is decreased (Collivignarelli et al., 2020). On the other hand, it has been shown that even significant changes in pH (from 3 to 10) do not seem to confirm the disappearance of SARS-CoV-2 (Chin et al., 2020). A study examining the survival of enveloped virus (e.g., H5N1) in soil demonstrated that the virus did not survive in sandy topsoil but did survive in purchased construction sand and compost suggesting that different soil characteristics greatly impact virus survival (Steffan et al., 2020).
It is difficult to find SARS-CoV-2 RNA in different types of soil, resulting in the lack of data on the survival of SARS-CoV-2. The effective transmission ability of pathogens in soil has not been confirmed. Therefore, there is a risk of virus transmission in soil, so further research should be carried out on different types of soil receiving sewage and the different plants growing within the soil.
3.4. Biota transmission of SARS-CoV-2
Recent studies have recognized that SARS-CoV-2 can originate from biota (Rodriguez-Morales et al., 2020, Zhou and Shi, 2021). Due to the high homology between SARS-CoV-2 and bat-CoV, bats are considered as the original hosts of SARS-CoV-2, and minks, or pangolins may be intermediate hosts, albeit further investigation is needed (Luan et al., 2020, Zhang et al., 2020c). Much remains to be elucidated regarding which species may act as natural reservoirs or transmission vectors for the virus. Our current knowledge indicates that biota may play a major role in the evolution of novel coronavirus.
Biota also plays a role in the spread of novel coronavirus. Some studies have found that beetles and domestic insects are carriers of pathogens, and these insects transmit the virus by contacting contaminated surfaces and secretions of patients (Vazirianzadeh et al., 2014). At present, it has been proved that the fecal particles of patients contain SARS-CoV-2 (Wu et al., 2020). The municipal government of Guanzhou has also ordered the mass extermination of rats, cockroaches, flies, and mosquitoes, although there has been no conclusive evidence that insects are involved in the spread of SARS-CoV-1 (Parry, 2004). They can transmit more than 100 pathogens through legs, hair, oral, feces, and vomit (Hazeleger et al., 2008). Therefore, feeding domestic insects and beetles with feces may play an important role in disease spread by mechanical transmission of virus. Insects lack a receptor that can bind SARS-CoV-2, thus preventing the virus from replicating in insects (Dicke et al., 2020). However, if the domestic waste and sewage are not treated properly, the risk of mechanical transmission of SARS-CoV-2 by insects may be increased (Dehghani and Kassiri, 2020). Recently, the rapid spread of SARS-CoV-2 infection was reported in mink farms and the associated mutations resulted in a new mink-associated variant that was identified in both minks and humans, thereby providing evidence of mink-to-human transmission (Sharun et al., 2020). Measures should be taken to prevent the spread of novel coronavirus and mutant viruses. WHO has declared that mosquitoes cannot transmit SARS-CoV-2 (WHO, 2020a). Mosquitoes are not carriers of novel coronavirus, even if a small amount of virus is inhaled into mosquitoes with blood, it cannot survive and not enter saliva through the digestive system. In an experimental study of the possibility of SARS-CoV-2 infection and its ability to transmit through mosquitoes, it was revealed that SARS-CoV-2 could not replicate in these mosquitoes under extreme conditions (Huang et al., 2020b). Therefore, it is considered impossible to transmit SARS-CoV-2 to humans even when mosquitoes feed on the virus-host.
It can be summarized that biota has played a role in the evolution and transmission of novel coronavirus, and there is a close relationship between humans, animals, and the environment. Therefore, policymakers should pay attention to the cross-species transmission of SARS-CoV-2 and other potential transmission problems caused by biota.
3.5. Surface transmission of SARS-CoV-2
Coronavirus can survive on the surfaces of objects. Infection may occur when humans make contact with the surface of contaminated objects and then the mucosal parts (e.g., the oral cavity or nasal cavity) when the virus reaches a certain load. Surfaces contact usually occurs in environments involving medical treatments, visiting friends and family, and food transportation.
Frequent surface contact contamination in the medical environment is a potential source of the virus (Tran et al., 2012, Zhou et al., 2021b). A study detected SARS-CoV-2 in surface swabs collected from hospitals of Wuhan using both RT-PCR and digital PCR, and the positive detection rate was 3.1% (Zhou et al., 2021b). The viruses have also been detected in the samples collected on the surface of an ICU ventilator (Ong et al., 2020). Although the body fluids and blood of patients are contained in medical wastes, they are usually classified and incinerated by medical centers and hospitals to prevent the transmission of the virus.
With the spread of the epidemic situation and the implementation of self-segregation measures, solid waste containing surviving SARS-CoV-2 may be generated at the household level, which brings infection risk to front-line garbage disposal workers. For example, in a case study by Dhakal and Karki (2020), active surveillance and contact were traced in patients being asymptomatic in Nepal. Epidemiological studies show that the vast majority of the cases in Nepal were young males (Dhakal and Karki, 2020). The contaminated solid waste may spread to the scavengers and garbage disposal workers, resulting in the second spread of COVID-19. Additionally, some of the waste materials are sold in informal markets to poor people, posing additional risk of infection (Kharel, 2020). Therefore, it is suggested that different stages of waste management should be adopted to prevent the surface transmission of the virus (CDC, 2021). However, there is no evidence that SARS-CoV-2 is transmitted through domestic solid waste and has not attracted the attention of the scientific community.
Transmission of the virus through materials such as doorknobs, doorbells, or inhalers is crucial. Household gloves and medical mask waste also pose a risk of infection and environmental contamination (Kraay et al., 2018). Under laboratory-controlled conditions, SARS-CoV-2 on non-porous surfaces gradually lost its infectivity completely at ambient temperatures on day 4 (Ben-Shmuel et al., 2020). A similar study was carried out in another experiment in which the viability of viruses on different surfaces was assessed (Chin et al., 2020). SARS-CoV-2 was more stable on glass and paper money (4 days) and stainless steel and plastic (7 days). After 7 days of culture, detectable infectious virus levels were found on the outer layer of surgical masks (Chin et al., 2020). These studies indicated that different surfaces also affected the survival potential of the virus.
Environmental conditions may facilitate the surface transmission of the virus. Some studies have shown that relative humidity and temperature will have influences on the survival of coronavirus on inanimate surfaces (Aboubakr et al., 2021, Kratzel et al., 2020). The data showed that the survival time of some human coronaviruses was longer under low temperature and low humidity (Aboubakr et al., 2021). An experiment showed that the increase of temperature and relative humidity accelerated the inactivation of SARS-CoV-2 on the surface (Biryukov et al., 2020). Yet, a study showed that the infectivity of SARS-CoV-2 was low in the initial drying process, and the survival potential was not related to the ambient temperature (Kratzel et al., 2020). Therefore, it is still controversial whether the surface viability of novel coronavirus is related to temperature, warranting further investigations.
In general, SARS-CoV-2 RNA may be detected on many surfaces in the community and hospital where the outbreak occurred. It has been shown that viruses can exist on different surfaces, but the environmental survival conditions still need to be examined. According to this, the routes of transmission through the surface of articles are complex.
3.6. Cold-chain transmission of SARS-CoV-2
There is no evidence that the novel coronavirus can be transmitted through food, but during the current pandemic, frozen foods served as a vehicle for remote transmission (Han et al., 2020b). Since July 12, 2020, SARS-CoV-2 RNA contaminations in frozen food mostly on their packaging materials imported from the countries with ongoing epidemics have been reported in several provinces in China (Han et al., 2020b). Therefore, cold-chain transmission is a special surface propagation mode.
Novel coronavirus may cause long-distance cold-chain transmission through low temperature treatment of food, freezing storage, and the cold-chain for transporting, which seriously threatens food safety. In the Xinfadi Market of Beijing, novel coronavirus was detected on the cutting board of imported salmon, and subsequently, 40 environmental positive samples were found (Pang et al., 2020). The virus genome sequence analysis showed that the virus sequence had obvious mutation characteristics (Pang et al., 2020). The results of the epidemiological investigation showed that a single outbreak source took place in Beijing. An epidemiological investigation was conducted on the stevedores with no contact history of COVID-19 cases at Qingdao Port, and the SARS-CoV-2 was first isolated from the surface of the outer packaging of imported cod (Liu et al., 2020b). Combined with the above cases, it is inferred that the cause of the outbreak of COVID-19 may be the contamination of the external packaging of imported food during cold-chain transportation.
A recent study found that the infectivity of SARS-CoV-2 on the surface of frozen food (e.g., chicken, salmon, and pork) did not decrease after 21 days at 4 °C and –20 °C (Fisher et al., 2020). This suggested that the cold chain environment may prolong the life span of the novel coronavirus and cause secondary infection. However, since cold-chain food usually contains water, it is necessary to study the influence of the permeability of virus on the surface of substrate material on the survival time of virus.
Although it is not clear whether novel coronavirus concentrations in frozen food packaging can cause infection, there is an urgent need to curb community communication in the early days. There is a high possibility that cold-chain transmission may become a pathway of novel coronavirus transmission. Policymakers should regularly supervise and inspect the implementation of the normalization, prevention, and control measures for the novel coronavirus epidemic in the cold-chain industry, elucidate the weak links and expedite the blocking of risk points.
4. Insufficient control measures
As the survival potential of viruses in different environmental media are diverse (Table 4 ), our understanding of the environmental transmission of novel coronavirus is still limited, which makes the disease difficult to control. In particular, the following measures must be implemented:
-
(1)
The survival potential of the virus in different environments requires more systematic investigations in order to facilitate our understanding of environmental transmission routes.
-
(2)
At present, there are no proper methods to prove the transmission pathways of the viral aerosol in outdoor and fecal-oral transmission.
-
(3)
Solid waste, biota, soil, and other environmental research fields have not received sufficient attention. Although there are a few relevant studies, additional investigations should be undertaken for these different environments.
-
(4)
There is a lack of mathematical models to verify the relationship between novel coronavirus and environmental factors. Collaboration of scientists from different areas is necessary for this urgent task.
-
(5)
Some research results are only a snapshot of the current situation, while the environment has a cumulative effect over a long period of time. The cumulative effects must be considered when carrying on the identification of the pathways of the SARS-CoV-2.
-
(6)
Most of the simulation experiments used the extrapolation method, in which the viruses with similar characteristics to SARS-CoV-2 (e.g., SARS-CoV-1, murine hepatitis virus [MHV]) were used. The application of these methods is often limited by different properties and different gene sequencing of viruses.
Table 4.
SARS-CoV-2 survival potential in different environments.
| Environmental media | Survival potential of SARS-CoV-2 in environment |
|---|---|
| Air | SARS-CoV-2 can maintain active in the air, but its viability remains controversial. The variant of virus has higher survival ability |
| Water | At the low temperature, the virus can survive in human excreta, and an isotonic environment, and wastewater |
| Soil | There is a lack of experiments on virus survival ability in soil. Vitro experiments showed that the resistance of the virus was low under high temperature and was not affected by pH |
| Surface contact | Different surfaces also affected the survival potential of the virus. It is controversial whether the surface viability of virus is related to temperature |
| Cold-chain | The virus can survive for a long time at a low temperature |
5. Conclusion and prospects
In this overview, we have summarized the potential environmental transmission routes and environmental persistence of the SARS-CoV-2 and propose some suggestions for further prevention and in-depth study. The main conclusions and prospects are as follows.
-
(1)
The environmental transmission pathways of SARS-CoV-2 are mainly through air, water, cold-chain, and surface, with potential transmission in soil and by biota. SARS-CoV-2 RNA was detected in the air, water, sludge, and surface of the epidemic areas. Droplet transmission in the air has been identified, and aerosol and fecal-oral transmission could occur in some cluster cases.
-
(2)
The survival potential of SARS-CoV-2 varies in the different environmental media. There is still a contradiction between the survival potential of the virus in air and/or on inanimate surfaces. Low temperature and isotonic environment are more suitable for virus to survive in water. In the cold-chain environment, SARS-CoV-2 can survive for several days at low temperatures.
-
(3)
SARS-CoV-2 can be a biological nanoparticle on its own, which can directly enter the human body. It is possible nanoparticles at the top end of the size range could act as SARS-CoV-2 carriers to influence the spread of virus. However, a composite carrier nanoparticle + virus will be too large to be classified as a single nanoparticle. It is plausible that these composite particles could be inhaled into human lungs and then to the circulatory system causing infection.
-
(4)
Regular environmental monitoring (e.g., air, sewage, soil) can be used to assess the future trend of COVID-19 infection, to understand the relationship between the virus and the environment, and to provide a means of early warning for potential virus outbreaks.
-
(5)
In general, detailed research on solid waste, biota, soil, and other environments has not received sufficient attention in terms of virus transmission. More investigations should be undertaken focusing on the environmental transmission pathways of virus, which could contribute to the prevention and control of the COVID-19 epidemic.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study is supported by the National Natural Science Foundation of China (NSFC) (Grant No. 42075107), the Projects of International Cooperation and Exchanges NSFC (Grant No. 41571130031) and the Yueqi Scholar fund of China University of Mining and Technology (Beijing).
Biographies

Longyi Shao is Professor in College of Geoscience and Surveying Engineering at China University of Mining and Technology (Beijing). He is currently working on sedimentology, environmental geology, and atmospheric environment linked to health effects. He has been the board member of several important academic societies including the Committee of Sedimentology of the Chinese Society for Mineralogy, Petrology and Geochemistry (CSMPG), Committee of Environmental Mineralogy of CSMPG, Committee of Aerosol Science of the Chinese Meteorological Society, Indoor Environment and Health Branch of the Chinese Society of Environmental Sciences. He has been named on the editorial board of some important Chinese journals including Acta Sedimentologica Sinica, Journal of Palaeogeography, Acta Geologica Sinica, and Earth Science Frontiers. Published more than 400 papers in Chinese and more than 130 papers in English. The H-index is 34 by Scopus.

Shuoyi Ge is a MSc student majoring in Geoscience at College of Geoscience and Surveying Engineering of China University of Mining and Technology (Beijing). She received her BSc from North China University of Science and Technology in 2019. She is currently working on physicochemical characterization of airborne particles linked to health effects.

Tim Jones is a Reader in Environmental Geology in the School of Earth and Environmental Sciences at Cardiff University. He has research interests in human health and geological materials, concentrating on respiratory exposures to airborne particulate matter. His specific expertise lies in pollution collection and chemical characterization. Relevant funded research projects have included: airborne volcanic ash from the on-going Montserrat eruption; particulate emissions from opencast coal mining in south Wales; urban PM source apportionment and toxicity; indoor air quality and bioreactive emissions from UK and Hong Kong landfills. He has an H-index of 31 and has 186 outputs listed in the Cardiff University ORCA research archive.

M. Santosh is Professor at the China University of Geosciences Beijing (China), Specially Appointed Foreign Expert of China, Professor at the University of Adelaide, Australia and Emeritus Professor at the Faculty of Science, Kochi University, Japan. PhD (Cochin University of Science and Technology, India), D.Sc. (Osaka City University, Japan) and D.Sc. (University of Pretoria, South Africa). He is the Founding Editor of Gondwana Research as well as the Editorial Advisor of Geoscience Frontiers. Research fields include petrology, fluid inclusions, geochemistry, geochronology, metallogeny and supercontinent tectonics. Published over 1,500 research papers, edited several memoir volumes and journal special issues, and co-author of the book ‘Continents and Supercontinents’ (Oxford University Press, 2004). Recipient of National Mineral Award, Outstanding Geologist Award, Thomson Reuters 2012 Research Front Award, and Thomson Reuters/Clarivate High Cited Researcher recognition during the last six years.

Luis F.O. Silva is Professor in Department of Civil and Environmental, Universidad de la Costa, Colombia. B.Sc. in Chemistry and Ph.D. in Environmental Science. Scientific Specialization: Water, soil and air quality assessment and management including nanoparticles investigations. Fate and behavior of emerging contaminants in air pollution reductions by green systems; surface waters and soil; wastewaters and groundwater. Application, analysis, fate and risk of nanomaterials in the environment and new construction budding materials. The H-index is 48 by Scopus. He is Associate Editor for Energy Geoscience and Editorial Board Member of Environment International.

Yaxin Cao is a MSc student majoring in Geological Resources and Geological Engineering at College of Geoscience and Surveying Engineering of China University of Mining and Technology (Beijing). She received her BSc from Henan Polytechnic University in 2019. She is currently working on physicochemical characterization of airborne particles linked to health effects.

Marcos L.S. Oliveira is Professor in Department of Civil and Architecture, University of Lima, Peru. B.Sc. in History and Ph.D. in Environmental Science. The research interests are focused on the following areas: natural and anthropogenic nanoparticles; edifications; water waste depuration. In specific, prof. Oliveira has a specialty in nanoparticles. Actually, have more than 90 published articles. The H-index is 30 by Scopus.

Mengyuan Zhang is a PhD student majoring in Environmental Geology at College of Geoscience and Surveying Engineering of China University of Mining and Technology (Beijing). She received her BSc from Capital Normal University in 2017. She is currently working on physicochemical characterization of airborne particles linked to health effects in a lung cancer village.

Kelly BéruBé is a Reader in Cell Biology and the Director of the Lung & Particle Research Group at the School of Biosciences, Cardiff University, UK. With a background in electron microscopy and lung toxicology, she has built an international reputation in the field air pollution and human health. Her research focuses on the determination of intelligent biomarkers of exposure and harm in the respiratory system, with a particular interest in understanding how air pollutants compromise lung health. She has 165 published items in the Cardiff University online research repository and has a H-index of 38. Her key work on developing ‘human tissue equivalents of respiratory epithelia’, as in vitro alternatives to animals for in vivo inhalation toxicology, was awarded the UK NC3Rs/MRC ‘Replacement Prize’ and the ‘Science & Technology Innovation Prizes’ in 2007 and 2010 and 2012. http://orca.cf.ac.uk/view/allcardiffauthors/A040411Y.html.
Handling Editor: V.O. Samuel
References
- Aboubakr, H.A., Sharafeldin, T.A., Goyal, S.M., 2021. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transbound. Emerg. Dis. 68, 296–312. https://doi.org/10.1111/tbed.13707. [DOI] [PMC free article] [PubMed]
- Ahmadi M., Sharifi A., Dorosti S., Ghoushchi S.J., Ghanbari N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddell S., Sherchan S.P., Simpson S.L., Sirikanchana K., Symonds E.M., Verhagen R., Vasan S.S., Kitajima M., Bivins A. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Huraimel K., Alhosani M., Kunhabdulla S., Stietiya M.H. SARS-CoV-2 in the environment: modes of transmission, early detection and potential role of pollutions. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.L., Turnham P., Griffin J.R., Clarke C.C. Consideration of the aerosol transmission for COVID-19 and public health. Risk Anal. 2020;40:902–907. doi: 10.1111/risa.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan M., Xu B., El-Din M.G. Transmission of SARS-CoV-2 via fecal-oral and aerosols-borne routes: environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asare S., Sandio A., Opara I.N., Riddle-Jones L., Palla M., Renny N., Ayers E. Higher obesity trends among African Americans are associated with increased mortality in infected COVID-19 patients within the city of Detroit. SN Compr. Clin. Med. 2020;2:1045–1047. doi: 10.1007/s42399-020-00385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma K., Yanagi U., Kagi N., Kim H., Ogata M., Hayashi M. Environmental factors involved in SARS-CoV-2 transmission: effect and role of indoor environmental quality in the strategy for COVID-19 infection control. Environ. Health Prev. 2020;25:66. doi: 10.1186/s12199-020-00904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi M.S. Impact of nanomaterials on ecosystems: mechanistic aspects in vivo. Environ. Res. 2020;182 doi: 10.1016/j.envres.2019.109099. [DOI] [PubMed] [Google Scholar]
- Barakat T., Muylkens B., Su B.L. Is particulate matter of air pollution a vector of Covid-19 pandemic? Matter. 2020;3:977–980. doi: 10.1016/j.matt.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K., Brunello G., Gurzawska-Comis K., Becker J., Sivolella S., Schwarz F., Klinge B. Dental care during COVID-19 pandemic: survey of experts' opinion. Clin. Oral Implant. Res. 2020;31:1253–1260. doi: 10.1111/clr.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shmuel A., Brosh-Nissimov T., Glinert I., Bar-David E., Sittner A., Poni R., Cohen R., Achdout H., Tamir H., Yahalom-Ronen Y., Politi B., Melamed S., Vitner E., Cherry L., Israeli O., Beth-Din A., Paran N., Israely T., Yitzhaki S., Levy H., Weiss S. Detection and infectivity potential of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) environmental contamination in isolation units and quarantine facilities. Clin. Microbiol. Infect. 2020;26:1658–1662. doi: 10.1016/j.cmi.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S., Zhou B., Lukowicz P. Social distance monitor with a wearable magnetic field proximity sensor. Sensors. 2020;20:5101. doi: 10.3390/s20185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biryukov J., Boydston J.A., Dunning R.A., Yeager J.J., Wood S., Reese A.L., Ferris A., Miller D., Weaver W., Zeitouni N.E., Phillips A., Freeburger D., Hooper I., Ratnesar-Shumate S., Yolitz J., Krause M., Williams G., Dawson D.G., Herzog A., Dabisch P., Wahl V., Hevey M.C., Altamura L.A. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. Msphere. 2020;5:e00441–e520. doi: 10.1128/mSphere.00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boongla, Y., Chanonmuang, P., Hata, M., Furuuchi, M., Phairuang, W., 2020. The characteristics of carbonaceous particles down to the nanoparticle range in Rangsit city in the Bangkok Metropolitan Region, Thailand. Environ. Pollut. (Barking, Essex: 1987) 272, 115940. [DOI] [PubMed]
- Caci G., Albini A., Malerba M., Noonan D.M., Pochetti P., Polosa R. COVID-19 and obesity: dangerous liaisons. J. Clin. Med. 2020;9:2511. doi: 10.3390/jcm9082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.X., Shao L.Y., Jones T., Oliveira M.L.S., Ge S.Y., Feng X.L., Silva L.F.O., BeruBe K. Multiple relationships between aerosol and COVID-19: A framework for global studies. Gondwana Res. 2021;93:243–251. doi: 10.1016/j.gr.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzolla Gatti R., Velichevskaya A., Tateo A., Amoroso N., Monaco A. Machine learning reveals that prolonged exposure to air pollution is associated with SARS-CoV-2 mortality and infectivity in Italy. Environ. Pollut. 2020;267 doi: 10.1016/j.envpol.2020.115471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention), Public Health Image Library. ID: 23640, 23641,23313. https://phil.cdc.gov/Details.aspx?pid/, 2020.
- CDC (Centers for Disease Control and Prevention), Interim laboratory biosafety guidelines for handling and processing specimens associated with coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html/, 2021 (accessed 6 January 2021).
- Chen A., Agarwal A., Ravindran N., To C., Zhang T., Thuluvath P.J. Are gastrointestinal symptoms specific for coronavirus 2019 infection? A prospective case-control study from the United States. Gastroenterology. 2020;159:1161. doi: 10.1053/j.gastro.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Guo Y., Pan Y., Zhao Z. Structure analysis of the receptor binding of 2019-nCoV. Biochem. Biophys. Res. Commun. 2020;525:135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.L., Li C.P., Tang C.S., Lung S.C.C., Chuang H.C., Chou D.W., Chang L.T. Risk assessment for people exposed to PM2.5 and constituents at different vertical heights in an urban area of Taiwan. Atmosphere. 2020;11(11):1145. [Google Scholar]
- Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T.W.H., Tam A.R., Yip C.C.Y., Leung K.-H., Fung A.Y.F., Zhang R.R., Lin Y., Cheng H.M., Zhang A.J.X., To K.K.W., Chan K.H., Yuen K.-Y., Leung W.K. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1 doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico F., Sacco A., Bragazzi N.L., Magnavita N. Can air-conditioning systems contribute to the spread of SARS/MERS/COVID-19 infection? Insights from a rapid review of the literature. Int. J. Environ. Res. Public Health. 2020;17:6052. doi: 10.3390/ijerph17176052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collivignarelli M.C., Collivignarelli C., Miino M.C., Abba A., Pedrazzani R., Bertanza G. SARS-CoV-2 in sewer systems and connected facilities. Process Saf. Environ. 2020;143:196–203. doi: 10.1016/j.psep.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comunian S., Dongo D., Milani C., Palestini P. Air pollution and COVID-19: the role of particulate matter in the spread and increase of COVID-19's morbidity and mortality. Int. J. Env. Res. Pub. He. 2020;17:4487. doi: 10.3390/ijerph17124487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Cid M., Arias-Estevez M., Nunez-Delgado A. How to study SARS-CoV-2 in soils? Environ. Res. 2020;110464–110464 doi: 10.1016/j.envres.2020.110464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020;261 doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copiello S., Grillenzoni C. The spread of 2019-nCoV in China was primarily driven by population density. Comment on “Association between short-term exposure to air pollution and COVID-19 infection: evidence from China” by Zhu et al. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.141028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crema E. The SARS-COV-2 outbreak around the Amazon rainforest: The relevance of the airborne transmission. Sci. Total Environ. 2021;759 doi: 10.1016/j.scitotenv.2020.144312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani R., Kassiri H. A brief review on the possible role of houseflies and cockroaches in the mechanical transmission of coronavirus disease (COVID-19) Arch. Clin. Infect. Dis. 2020;15 [Google Scholar]
- Dhakal S., Karki S. Early epidemiological features of COVID-19 in Nepal and public health response. Front. Med. 2020;7:524. doi: 10.3389/fmed.2020.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon, R.S., Rowin, W.A., Humphries, R.S., Kevin, K., Ward, J.D., Phan, T.D., Nguyen, L.V., Wynne, D.D., Scott, D.A., Clinical Aerosolisation Study Group, 2021. Aerosolisation during tracheal intubation and extubation in an operating theatre setting. Anaesthesia 76, 182–188. 10.1111/anae.15301. [DOI] [PMC free article] [PubMed]
- Dicke M., Eilenberg J., Salles J.F., Jensen A.B., Lecocq A., Pijlman G.P., van Loon J.J.A., van Oers M.M. Edible insects unlikely to contribute to transmission of coronavirus SARS-CoV-2. J. Insects Food Feed. 2020;6:333–339. [Google Scholar]
- Ding J., Zhu T. Heterogeneous reactions on the surface of fine particles in the atmosphere. Chin. Sci. Bull. 2003;48:2267–2276. [Google Scholar]
- Donado E.P., Oliveira M.L.S., Goncalves J.O., Dotto G.L., Silva L.F.O. Soil contamination in Colombian playgrounds: effects of vehicles, construction, and traffic. Environ. Sci. Pollut. Res. 2021;28:166–176. doi: 10.1007/s11356-020-09965-w. [DOI] [PubMed] [Google Scholar]
- Drossinos Y., Stilianakis N.I. What aerosol physics tells us about airborne pathogen transmission. Aerosol Sci. Tech. 2020;54:639–643. [Google Scholar]
- Feng X.L., Shao L.Y., Xi C.X., Jones T., Zhang D.Z., BeruBe K. Particle-induced oxidative damage by indoor size-segregated particulate matter from coal-burning homes in the Xuanwei lung cancer epidemic area, Yunnan Province, China. Chemosphere. 2020;256 doi: 10.1016/j.chemosphere.2020.127058. [DOI] [PubMed] [Google Scholar]
- Firquet S., Beaujard S., Lobert P.E., Sane F., Caloone D., Izard D., Hober D. Survival of enveloped and non-enveloped viruses on inanimate surfaces. Microbes Environ. 2015;30:140–144. doi: 10.1264/jsme2.ME14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, D., Reilly, A., Zheng, A., Cook, A., Anderson, D., 2020. Seeding of outbreaks of COVID-19 by contaminated fresh and frozen food. bioRxiv. https://doi.org/10.1101/2020.08.17.255166.
- Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know. A review. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freney E.J., Adachi K., Buseck P.R. Internally mixed atmospheric aerosol particles: hygroscopic growth and light scattering. J. Geophys. Res.-Atmos. 2010;115:D19210. [Google Scholar]
- Froum S.H., Froum S.J. Incidence of COVID-19 virus transmission in three dental offices: A 6-month retrospective study. Int. J. Periodont. 2020;40:853–860. doi: 10.11607/prd.5455. [DOI] [PubMed] [Google Scholar]
- Fujita H., Suzuki K. NPPV treatment for neuromuscular diseases during the COVID-19 pandemic. Brain Nerve. 2020;72:1085–1089. doi: 10.11477/mf.1416201653. [DOI] [PubMed] [Google Scholar]
- Gasparotto J., Chaves P.R., da Boit Martinello K., Silva L.F.O., Gelain D.P., Fonseca Moreira J.C. Obesity associated with coal ash inhalation triggers systemic inflammation and oxidative damage in the hippocampus of rats. Food Chem. Toxicol. 2019;133 doi: 10.1016/j.fct.2019.110766. [DOI] [PubMed] [Google Scholar]
- Giorgino F., Bhana S., Czupryniak L., Dagdelen S., Galstyan G.R., Janez A., Lalic N., Nouri N., Rahelic D., Stoian A.P., Raz I. Management of patients with diabetes and obesity in the COVID-19 era: experiences and learnings from south and east Europe, the Middle East, and Africa. Diabetes Res. Clin. Pr. 2020;172:108617. doi: 10.1016/j.diabres.2020.108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G.-K.-M., Dunker A.K., Foster J.A., Uversky V.N. Shell disorder analysis suggests that pangolins offered a window for a silent spread of an attenuated SARS-CoV-2 precursor among humans. J. Proteome Res. 2020;19:4543–4552. doi: 10.1021/acs.jproteome.0c00460. [DOI] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A., Rodriguez-Gil C. Pathogen cross-transmission via building sanitary plumbing systems in a full scale pilot test-rig. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1:10–14. [Google Scholar]
- Gwenzi W. Leaving no stone unturned in light of the COVID-19 faecal-oral hypothesis? A water, sanitation and hygiene (WASH) perspective targeting low-income countries. Sci. Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.141751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C.Q., Duan C.H., Zhang S.Y., Spiegel B., Shi H.Y., Wang W.J., Zhang L., Lin R., Liu J., Ding Z., Hou X.H. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am. J. Gastroenterol. 2020;115:916–923. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.P., Li L., Wang Y., Ma J.W., Li P.Y., Han C., Liu J.X. Composition, dispersion, and health risks of bioaerosols in wastewater treatment plants: a review. Front. Environ. Sci. Eng. 2021;15:38. [Google Scholar]
- Han J., Zhang X., He S., Jia P.Q. Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environ. Chem. Lett. 2020;19:5–16. doi: 10.1007/s10311-020-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeleger W.C., Bolder N.M., Beumer R.R., Jacobs-Reitsma W.F. Darkling beetles (alphitobius diaperinus) and their larvae as potential vectors for the transfer of Campylobacter jejuni and Salmonella enterica serovar paratyphi B variant Java between successive broiler flocks. Appl. Environ. Microb. 2008;74:6887–6891. doi: 10.1128/AEM.00451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller L., Mota C.R., Greco D.B. COVID-19 faecal-oral transmission: are we asking the right questions? Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoseinzadeh E., Javan S., Farzadkia M., Mohammadi F., Hossini H., Taghavi M. An updated min-review on environmental route of the SARS-CoV-2 transmission. Ecotox. Environ. Safe. 2020;202 doi: 10.1016/j.ecoenv.2020.111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.J.S., Vanlandingham D.L., Bilyeu A.N., Sharp H.M., Hettenbach S.M., Higgs S. SARS-CoV-2 failure to infect or replicate in mosquitoes: an extreme challenge. Sci. Rep. 2020;10:4. doi: 10.1038/s41598-020-68882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., other 29 authors Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.L., Baluja M.Q., Graham D.W., Corbishley A., McDonald J.E., Malham S.K., Hillary L.S., Connor T.R., Gaze W.H., Moura I.B., Wilcox M.H., Farkas K. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.M., Parab S.R. Simple face shield for public as a crucial factor to slow aerosol transmission during unlock phase of COVID pandemic. Indian J. Otolaryngol. 2020 doi: 10.1007/s12070-020-02078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharel T.P. Risk of COVID-19 for household waste workers in Nepal. Int. J. Multidisciplinary Sci. Adv. Technol. 2020;1:116–123. [Google Scholar]
- Kohanski M.A., Lo L.J., Waring M.S. Review of indoor aerosol generation, transport, and control in the context of COVID-19. Int. Forum. Allergy Rh. 2020;10:1173–1179. doi: 10.1002/alr.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koma T., Adachi S., Doi N., Adachi A., Nomaguchi M. Toward understanding molecular bases for biological diversification of human Coronaviruses: present status and future perspectives. Front. Microbiol. 2020;11:2016. doi: 10.3389/fmicb.2020.02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T., Platt D., Parida L. Variant analysis of SARS-CoV-2 genomes. Bull. WHO. 2020;98:495–504. doi: 10.2471/BLT.20.253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraay A.N.M., Hayashi M.A.L., Hernandez-Ceron N., Spicknall I.H., Eisenberg M.C., Meza R., Eisenberg J.N.S. Fomite-mediated transmission as a sufficient pathway: a comparative analysis across three viral pathogens. BMC Infect Dis. 2018;18:540. doi: 10.1186/s12879-018-3425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzel A., Steiner S., Todt D., V'Kovski P., Brueggemann Y., Steinmann J., Steinmann E., Thiel V., Pfaender S. Temperature-dependent surface stability of SARS-CoV-2. J. Infection. 2020;81:474–476. doi: 10.1016/j.jinf.2020.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179:11. doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacatusu G.A., Vasilescu C., Mihai I.F., Filip-Ciubotaru F., Vata A., Manciuc C. COVID-19 and air conditioning - is there an environmental link? Environ. Eng. Manag. J. 2020;19:1255–1260. [Google Scholar]
- Lau J.T.F., Tsui H., Lau M., Yang X.L. SARS transmission, risk factors, and prevention in Hong Kong. Emerg. Infect. Dis. 2004;10:587–592. doi: 10.3201/eid1004.030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.W.F., Sun Q.Q. Electrostatic charged nanofiber filter for filtering airborne novel coronavirus (COVID-19) and nano-aerosols. Sep. Purif. Technol. 2020;250:17. doi: 10.1016/j.seppur.2020.116886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.W.F., Hau C.W.Y., Choy H.F. Microfiber-nanofiber composite filter for high-efficiency and low pressure drop under nano-aerosol loading. Sep. Purif. Technol. 2018;206:26–38. [Google Scholar]
- Li Y.Q., Ren B., Peng X., Hu T., Li J.Y., Gong T., Tang B.Y., Xu X., Zhou X.D. Saliva is a non-negligible factor in the spread of COVID-19. Mol. Oral Microbiol. 2020;35:141–145. doi: 10.1111/omi.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.W., Wu C.B., Zhang X., Chen L., Wang X.Y., Guan X.L., Li J.H., Lin Z.C., Xiong N. Post-pandemic testing of SARS-CoV-2 in Huanan Seafood Market area in Wuhan, China. Clin. Infect Dis. 2020;ciaa1043 doi: 10.1093/cid/ciaa1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.J., Fang L.Q., Pan H., Zhang K.Z., Kan H.D., Brook J.R., Sun Q.H. PM2.5 in Beijing – temporal pattern and its association with influenza. Environ. Health. 2014;13:102. doi: 10.1186/1476-069X-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K.-F., Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Liu P.P., Yang M.J., Zhao X., Guo Y.Y., Wang L., Zhang J., Lei W.W., Han W.F., Jiang F.C., Liu W.J., Gao G.F., Wu G.Z. Cold-chain transportation in the frozen food industry may have caused a recurrence of COVID-19 cases in destination: successful isolation of SARS-CoV-2 virus from the imported frozen cod package surface. Biosaf. Health. 2020;2:199–201. doi: 10.1016/j.bsheal.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Zhang J., Du R.G., Teng X.M., Li W.J. Chemistry of atmospheric fine particles during the COVID pandemic in a megacity of eastern China. Geophys. Res. Lett. 2020;48:e2020. doi: 10.1029/2020GL091611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loth A.G., Guderian D.B., Haake B., Zacharowski K., Stover T., Leinung M. Aerosol exposure during surgical tracheotomy in SARS-CoV-2 positive patients. Shock. 2020;55(4):472–478. doi: 10.1097/SHK.0000000000001655. [DOI] [PubMed] [Google Scholar]
- Lu J.Y., Gu J.N., Li K.B., Xu C.H., Su W.Z., Lai Z.S., Zhou D.Q., Yu C., Xu B., Yang Z.C. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg. Infect Dis. 2020;26:1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R.J., Zhao X., Li J., Niu P.H., Yang B., Wu H.L., Wang W.L., Song H., Huang B.Y., Zhu N., Bi Y.H., Ma X.J., Zhan F.X., Wang L., Hu T., Zhou H., Hu Z.H., Zhou W.M., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J.Y., Xie Z.H., Ma J.M., Liu W.J., Wang D.Y., Xu W.B., Holmes E.C., Gao G.F., Wu G., Chen W.J., Shi W.F., Tan W.J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J.W., Jin X.L., Lu Y., Zhang L.L. SARS-CoV-2 spike protein favors ACE2 from Bovidae and Cricetidae. J. Med. Virol. 2020;92:1649–1656. doi: 10.1002/jmv.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Yao L., Zhang L. Possible transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a public bath center in Huai'an, Jiangsu Province, China. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. L., Zhao, Y. D., Liu, J. T., He, X. T., Wang, B., Fu, S. H., Yan, J., Niu, J. P., Luo, B., 2020. Effects of temperature variation and humidity on the mortality of COVID-19 in Wuhan. medRxiv. https://doi.org/10.1101/2020.03.15.20036426.
- Majumdar S.A., Nayak A.K., Misra S.N. The perception study of water supply and drainage in the city of Bhubaneswar. Int. J. Eng. Res. Appl. 2019;10:664–670. [Google Scholar]
- Marin-Garcia, D., Moyano-Campos, J.J., Bienvenido-Huertas, J.D., 2021. Distances of transmission risk of COVID-19 inside dwellings and evaluation of the effectiveness of reciprocal proximity warning sounds. Indoor Air 31, 335–347. https://doi.org/10.1111/ina.12738. [DOI] [PubMed]
- Martinez-Fierro M.L., Rios-Jasso J., Garza-Veloz I., Reyes-Veyna L., Cerda-Luna R.M., Duque-Jara I., Galvan-Jimenez M., Ramirez-Hernandez L.A., Morales-Esquivel A., Ortiz-Castro Y., Gutierrez-Camacho J.R., Valdes-Aguayo J.J., Vargas-Rodriguez J.R. The role of close contacts of COVID-19 patients in the SARS-CoV-2 transmission: an emphasis on the percentage of nonevaluated positivity in Mexico. Am. J. Infect Control. 2021;49:15–20. doi: 10.1016/j.ajic.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehraeen E., Salehi M.A., Behnezhad F., Moghaddam H.R., SeyedAlinaghi S. Transmission modes of COVID-19: a systematic review. Infect. Disord. Drug Targets. 2020;20:1. doi: 10.2174/1871526520666201116095934. [DOI] [PubMed] [Google Scholar]
- Mescoli A., Maffei G., Pillo G., Bortone G., Marchesi S., Morandi E., Ranzi A., Rotondo F., Serra S., Vaccari M., Zauli Sajani S., Mascolo M.G., Jacobs M.N., Colacci A. The Secretive liaison of particulate matter and SARS-CoV-2. A hypothesis and theory investigation. Front. Genet. 2020;11 doi: 10.3389/fgene.2020.579964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron, O., Yu, K.-H., Wilf-Miron, R., Davidovitch, N., 2020. COVID-19 infections following outdoor mass gatherings in low incidence areas: retrospective cohort study. medRxiv. https://doi.org/10.1101/2020.10.22.20184630.
- Nunez-Delgado A. SARS-CoV-2 in soils. Environ. Res. 2020;190 doi: 10.1016/j.envres.2020.110045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Sutjipto S., Chia P.Y., Young B.E., Gum M., Lau S.K., Chan M., Vasoo S., Mendis S., Toh B.K., Leong J., Barkham T., Ang B.S.P., Tan B.H., Leo Y.-S., Marimuthu K., Wong M.S.Y., Ng O.T. Absence of contamination of personal protective equipment (PPE) by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Infect. Control Hosp. Epidemiol. 2020;41:614–616. doi: 10.1017/ice.2020.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona, J., Solano, R., Soler, C., Sabat, M., 2020. Epidemiological approximation of the enteric manifestation and possible fecal-oral transmission in COVID-19: a preliminary systematic review. Eur. J. Gastroenterology Hepat. https://doi.org/10.1097/MEG.0000000000001934. [DOI] [PubMed]
- Pang X.H., Ren L.L., Wu S.S., Ma W.T., Yang J., Di L., Li J., Xiao Y., Kang L., Du S., Du J., Wang J., Li G., Zhai S.G., Chen L.J., Zhou W.X., Lai S.J., Gao L., Pan Y., Wang Q.Y., Li M.K., Wang J.B., Huang Y.Y., Wang J.W. Cold-chain food contamination as the possible origin of COVID-19 resurgence in Beijing. Nat. Sci. Rev. 2020;7:1861–1864. doi: 10.1093/nsr/nwaa264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry J. WHO queries culling of civet cats. BMJ (Clinical research ed.) 2004;328:128. doi: 10.1136/bmj.328.7432.128-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar S.D., Murugavel P., Lal D.M. Effect of relative humidity and sea level pressure on electrical conductivity of air over Indian Ocean. J. Geophys. Res.-Atmos. 2009;114:D02205. [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Arnold W., Omer S.B. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38:1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L.M., Law K.I., Tang B.S.F., Hon T.Y.W., Chan C.S., Chan K.H., Ng J.S.C., Zheng B.J., Ng W.L., Lai R.W.M., Guan Y., Yuen K.Y., Grp H.U.S.S. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periago E.L., Delgado A.N., Diaz-Fierros F. Attenuation of groundwater contamination caused by cattle slurry: a plot-scale experimental study. Bioresour. Technol. 2002;84:105–111. doi: 10.1016/s0960-8524(02)00041-x. [DOI] [PubMed] [Google Scholar]
- Petersen E., Koopmans M., Go U., Hamer D.H., Petrosillo N., Castelli F., Storgaard M., Al Khalili S., Simonsen L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020;20:E238–E244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon A., Vialette M. Survival of viruses in water. Intervirology. 2018;61:214–222. doi: 10.1159/000484899. [DOI] [PubMed] [Google Scholar]
- Pyankov O.V., Pyankova O.G., Agranovski I.E. Inactivation of airborne influenza virus in the ambient air. J. Aerosol Sci. 2012;53:21–28. [Google Scholar]
- Rahman M.S., Azad M.A.K., Hasanuzzaman M., Salam R., Islam A.R.M.T., Rahman M.M., Hoque M.M.M. How air quality and COVID-19 transmission change under different lockdown scenarios? A case from Dhaka city, Bangladesh. Sci. Total Environ. 2021;762 doi: 10.1016/j.scitotenv.2020.143161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralli M., Greco A., de Vincentiis M. The effects of the COVID-19/SARS-CoV-2 pandemic outbreak on otolaryngology activity in Italy. Ent-Ear Nose Throat J. 2020;99:565–566. doi: 10.1177/0145561320923893. [DOI] [PubMed] [Google Scholar]
- Rao L.F., Zhang L.Y., Wang X.Z., Xie T.T., Zhou S.M., Lu S.L., Liu X.C., Lu H., Xiao K., Wang W.Q., Wang Q.Y. Oxidative potential induced by ambient particulate matters with acellular assays: a review. Various Technol. Environ. Pollut. Control. 2020;8:1410. [Google Scholar]
- Reddy T., Shkedy Z., Janse van Rensburg C., Mwambi H., Debba P., Zuma K., Manda S. Short-term real-time prediction of total number of reported COVID-19 cases and deaths in South Africa: a data driven approach. BMC Med. Res. Methodol. 2021;21:15. doi: 10.1186/s12874-020-01165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Morales A.J., Bonilla-Aldana D.K., Balbin-Ramon G.J., Rabaan A.A., Sah R., Paniz-Mondolfi A., Pagliano P., Esposito S. History is repeating itself: probable zoonotic spillover as the cause of the 2019 novel Coronavirus Epidemic. Infez. Med. 2020;28:3–5. [PubMed] [Google Scholar]
- Sanap S.D. Global and regional variations in aerosol loading during COVID-19 imposed lockdown. Atmos. Environ. 2021;246 doi: 10.1016/j.atmosenv.2020.118132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lorenzo A., Vaquero-Martinez J., Calbo J., Wild M., Santurtun A., Lopez-Bustins J.A., Vaquero J.M., Folini D., Anton M. Did anomalous atmospheric circulation favor the spread of COVID-19 in Europe? Environ. Res. 2020;194:110626. doi: 10.1016/j.envres.2020.110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer, I., White, T.A., Baalousha, M., Chipman, K., Viant, M.R., Lead, J.R., 2011. Aggregation and dispersion of silver nanoparticles in exposure media for aquatic toxicity tests. J. Chromatogr. A 1218, 4226–4233. [DOI] [PubMed]
- Sabino, E.C., Buss, L.F., Carvalho, M.P.S.,Prete, Jr.C.A., Crispim, M.A.E., Fraiji, N.A., Pereira, R.H.M., Parag, K.V., Peixoto, P.S., Kraemer, M.U.G., Oikawa, M.K., Salomon, T., Cucunuba, Z.M., Castro, M.C., Santos, A.A.S., Nascimento, V.H., Pereira, H.S., Ferguson, N.M., Pybus, O.G., Kucharski, A., Busch, M.P., Dye, C., Faria, N.R., 2021. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. The Lancet 397(10273), 452–455. https://doi.org/10.1016/S0140-6736(21)00183-5 [DOI] [PMC free article] [PubMed]
- Santarpia, J.L., Rivera, D.N., Herrera, V., Morwitzer, M.J., Creager, H., Santarpia, G.W.., Crown, K.K., Brett-Major, D.M., Schnaubelt, E., Broadhurst, M.J., Lawler, J.V., 2020. Transmission potential of SARS-CoV-2 in viral shedding observed at the university of Nebraska Medical Center. medRxiv. https://doi.org/10.1101/2020.03.23.20039446.
- Schmidt T., Cloete A., Davids A., Makola L., Zondi N., Jantjies M. Myths, misconceptions, othering and stigmatizing responses to Covid-19 in South Africa: a rapid qualitative assessment. PLoS One. 2020;15 doi: 10.1371/journal.pone.0244420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroter R.C. Social distancing for covid-19: is 2 metres far enough? Br. Med. J. 2020;369 doi: 10.1136/bmj.m2010. [DOI] [PubMed] [Google Scholar]
- Seligmann H., Iggui S., Rachdi M., Vuillerme N., Demongeot J. Inverted covariate effects for first versus mutated second wave Covid-19: high temperature spread biased for young. Biology-Basel. 2020;9:226. doi: 10.3390/biology9080226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L.Y., Hu Y., Shen R., Schäfer K., Wang J., Wang J., Schnelle-Kreis J., Zimmermann R., Bérubé K., Suppan P. Seasonal variation of particle-induced oxidative potential of airborne particulate matter in Beijing. Sci. Total Environ. 2017;579:1152–1160. doi: 10.1016/j.scitotenv.2016.11.094. [DOI] [PubMed] [Google Scholar]
- Shao L.Y., Wang W.H., Xing J.P., Li W.J., Niu H.Y., Hou C., Yang S.S. Physicochemical characteristics and effects of airborne particles: Research progress and prospects. Earth Sci. 2018;43(5):1691–1708. (in Chinese with English abstract) [Google Scholar]
- Sharun K., Tiwari R., Natesan S., Dhama K. SARS-CoV-2 infection in farmed minks, associated zoonotic concerns, and importance of the one health approach during the ongoing COVID-19 pandemic. Vet. Q. 2020;41:50–60. doi: 10.1080/01652176.2020.1867776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q.F., Chen X., Lin J.B., Hu B.J., Gao X.D. Aerosol transmission of coronavirus in hospital. Shanghai J. Prevent. Med. 2020;32:851–856. (in Chinese with English abstract) [Google Scholar]
- Shi Z.B., Song C.B., Liu B.W., Lu G.D., Xu J.S., Van Vu T., Elliott R.J.R., Li W.J., Bloss W.J., Harrison R.M. Abrupt but smaller than expected changes in surface air quality attributable to COVID-19 lockdowns. Sci. Adv. 2021;7:eabd6696. doi: 10.1126/sciadv.abd6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva L.F.O., Crissien T.J., Schneider I.L., Blanco E.P., Sampaio C.H. Nanometric particles of high economic value in coal fire region: opportunities for social improvement. J. Clean Prod. 2020;256 [Google Scholar]
- Silva L.F.O., Santosh M., Schindler M., Gasparotto J., Dotto G., Oliveira M.L.S., Hochella M. Nanoparticles in fossil and mineral fuel sectors and their impact on environment and human health: a review and perspective. Gondwana Res. 2021;92:184–201. [Google Scholar]
- Steffan J.J., Derby J.A., Brevik E.C. Soil pathogens that may potentially cause pandemics, including severe acute respiratory syndrome (SARS) coronaviruses. Curr. Opin. Environ. Sci. Health. 2020;17:35–40. doi: 10.1016/j.coesh.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suman R., Javaid M., Haleem A., Vaishya R., Bahl S., Nandan D. Sustainability of coronavirus on different surfaces. J. Clin. Exp. Hepatol. 2020;10:386–390. doi: 10.1016/j.jceh.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W., Toovey O.T.R., Harvey K.N., Hui D.D.S. Introduction of the South African SARS-CoV-2 variant 501Y.V2 into the UK. J. Infect. 2021 doi: 10.1016/j.jinf.2021.1001.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.Y., Zhang Y.H., Shao M. Higher Education Press; Beijing: 2006. Atmospheric Environmental Chemistry; p. 789. [Google Scholar]
- Tegally, H., Wilkinson, E., Giovanetti, M., and another 44 authors, 2020. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv. 10.1101/2020.1112.1121.2024864
- Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trancossi M., Carli C., Cannistraro G., Pascoa J., Sharma S. Could thermodynamics and heat and mass transfer research produce a fundamental step advance toward and significant reduction of SARS-COV-2 spread? Int. J. Heat Mass Transf. 2021;170 doi: 10.1016/j.ijheatmasstransfer.2021.120983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejos E.M., Silva L.F.O., Hower J.C., Flores E.M.M., Gonzalez C.M., Pachon J.E., Aristizabal B.H. Volcanic emissions and atmospheric pollution: a study of nanoparticles. Geosci. Front. 2021;12:746–755. [Google Scholar]
- Tung N.T., Cheng P.C., Chi K.H., Hsiao T.C., Jones T., BeruBe K., Ho K.F., Chuang H.C. Particulate matter and SARS-CoV-2: a possible model of COVID-19 transmission. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.141532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Government, 2020. New COVID-19 (SARS-CoV-2) variants. https://www.gov.uk/government/collections/new-sars-cov-2-variant/, 2020 (accessed 21 December 2020).
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazirianzadeh B., Dehghani R., Mehdinejad M., Sharififard M., Nasirabadi N. The first report of drug resistant bacteria isolated from the brown-banded cockroach, supella longipalpa, in Ahvaz, South-western Iran. J. Arthropod-Borne. Dis. 2014;8:53–59. [PMC free article] [PubMed] [Google Scholar]
- Wang J., Feng H.T., Zhang S., Ni Z.W., Ni L.M., Chen Y., Zhuo L.X., Zhong Z.F., Qu T.T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Jin M., Zhen B., Kong Q.X., Song N., Xiao W.J., Yin J., Wei W., Wang G.J., Si B.Y., Guob B.Z., Liu C., Ou G.R., Wang M.N., Fang T.Y., Chao F.H., Li J.W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.Y., Qiu X.H., Cao J.Y., Peng L., Zhang N.N., Yan Y.L., Li R.M. Policy-driven changes in the health risk of PM2.5 and O-3 exposure in China during 2013–2018. Sci. Total Environ. 2021;757 doi: 10.1016/j.scitotenv.2020.143775. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. WHO Guidelines. 2014:156. [PubMed] [Google Scholar]
- WHO (World Health Organization). The COVID-19 virus cannot be spread through mosquito bites. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters#mosquito-bites/, 2020a (accessed 19 February 2020).
- WHO (World Health Organization). Water, sanitation, hygiene, and waste management for SARS-CoV-2, the virus that causes COVID-19. https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-WASH-2020.4/, 2020b (accessed 29 July 2020).
- WHO (World Health Organization). WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/, 2021 (accessed 14 March 2021).
- Wong, M.C., Javornik Cregeen, S.J., Ajami, N.J., Petrosino, J.F., 2020a. Evidence of recombination in coronaviruses implicating pangolin origins of nCoV-2019. bioRxiv. 10.1101/2020.02.07.939207. [DOI]
- Wong S.C.Y., Kwong R.T.S., Wu T.C., Chan J.W.M., Chu M.Y., Lee S.Y., Wong H.Y., Lung D.C. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J. Hosp. Infect. 2020;105:119–127. doi: 10.1016/j.jhin.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P., Ong S.G., Lim W.Y. COVID-19 and cardiopulmonary resuscitation: an N95 respirator mask may not be adequate. Brit. J. Anaesth. 2020;125:E319–E322. doi: 10.1016/j.bja.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.J., Guo C., Tang L.T., Hong Z.S., Zhou J.H., Dong X., Yin H., Xiao Q., Tang Y.P., Qu X.J., Kuang L.J., Fang X.M., Mishra N., Lu J.H., Shan H., Jiang G.M., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M.W., Zheng X.B., Liu Y., Li X.F., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X.J., Li Y.G., Sun H.Q., Liu L. Exhaled droplets due to talking and coughing. J. R. Soc. Interface. 2009;6:S703–S714. doi: 10.1098/rsif.2009.0388.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.G., Zhu Y.J. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Nakayama M., Tsubochi H., Endo S. A novel protective barrier enclosure for performing bronchoscopy. Respir. Investig. 2021;59(2):260–262. doi: 10.1016/j.resinv.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.L., Peng L., Cheng N., Bai H., Mu L. Health risk assessment of toxic VOCs species for the coal fire well drillers. Environ. Sci. Pollut. R. 2015;22:15132–15144. doi: 10.1007/s11356-015-4729-7. [DOI] [PubMed] [Google Scholar]
- Yao H.P., Song Y.T., Chen Y., Wu N.P., Xu J.L., Sun C.J., Zhang J.X., Weng T.H., Zhang Z.Y., Wu Z.G., Cheng L.F., Shi D.R., Lu X.Y., Lei J.L., Crispin M., Shi Y.G., Li L.J., Li S. Molecular architecture of the SARS-CoV-2 virus. Cell. 2020;183(3):730–738. doi: 10.1016/j.cell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y.Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Yuan Q., Xu J.Z., Wang Y.Y., Zhang X.H., Pang Y.E., Liu L., Bi L., Kang S.C., Li W.J. Mixing state and fractal dimension of soot particles at a remote site in the southeastern Tibetan Plateau. Environ. Sci. Technol. 2019;53:8227–8234. doi: 10.1021/acs.est.9b01917. [DOI] [PubMed] [Google Scholar]
- Zhang D.Y., Ling H.B., Huang X., Li J., Li W.W., Yi C., Zhang T., Jiang Y.Z., He Y.N., Deng S.Q., Zhang X., Wang X.Z., Liu Y., Li G.H., Qu J.H. Potential spreading risks and disinfection challenges of medical wastewater by the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang hospital. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Tang M., Guo F., Wei F., Yu Z., Gao K., Jin M., Wang J., Chen K. Associations between air pollution and COVID-19 epidemic during quarantine period in China. Environ. Pollut. 2021;268:115897. doi: 10.1016/j.envpol.2020.115897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.Z., Yao L.L., Liu Q., Qu G.B., Hu L.G., Zhou Q.F., Shi J.B., Jiang G.B. Environmental behavior and transmission of representative human coronaviruses. Environ. Chem. 2020;39:1464–1472. (in Chinese with English abstract) [Google Scholar]
- Zhang C.X., Zheng W., Huang X.Q., Bell E.W., Zhou X.G., Zhang Y. Protein structure and sequence reanalysis of 2019-nCoV genome refutes snakes as its intermediate host and the unique similarity between its spike protein insertions and HIV-1. J. Proteome Res. 2020;19:1351–1360. doi: 10.1021/acs.jproteome.0c00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Qi Y.H., Luzzatto-Fegiz P., Cui Y., Zhu Y.Y. COVID-19: Effects of environmental conditions on the propagation of respiratory droplets. Nano Lett. 2020;20:7744–7750. doi: 10.1021/acs.nanolett.0c03331. [DOI] [PubMed] [Google Scholar]
- Zhou H., Geng H., Dong C., Bai T. The short-term harvesting effects of ambient particulate matter on mortality in Taiyuan elderly residents: A time-series analysis with a generalized additive distributed lag model. Ecotox. Environ. Safe. 2021;207 doi: 10.1016/j.ecoenv.2020.111235. [DOI] [PubMed] [Google Scholar]
- Zhou P., Shi Z.L. SARS-CoV-2 spillover events. Science. 2021;371:120–122. doi: 10.1126/science.abf6097. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Yao M.S., Zhang X., Hu B.C., Li X.Y., Chen H.X., Zhang L., Liu Y., Du M., Sun B.C., Jiang Y.Y., Zhou K., Hong J., Yu N., Ding Z., Xu Y., Hu M., Morawska L., Grinshpun S.A., Biswas P., Flagan R.C., Zhu B.L., Liu W.Q., Zhang Y.H. Breath-, air- and surface-borne SARS-CoV-2 in hospitals. J. Aerosol. Sci. 2021;152 doi: 10.1016/j.jaerosci.2020.105693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.J., Xie J.G., Huang F.M., Cao L.Q. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D.Y., Wang W.L., Li X.W., Yang B., Song J.D., Zhao X., Huang B.Y., Shi W.F., Lu R.J., Niu P.H., Zhan F.X., Ma X.J., Wang D.Y., Xu W.B., Wu G.Z., Gao G.G.F., Tan W.J., China Novel C. A novel coronavirus from patients with pneumonia in China, 2019. New Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]