Abstract
The development of high-end targeted drugs and vaccines against modern pandemic infections, such as COVID-19, can take a too long time that lets the epidemic spin up and harms society. However, the countermeasures must be applied against the infection in this period until the targeted drugs became available. In this regard, the non-specific, broad-spectrum anti-viral means could be considered as a compromise allowing overcoming the period of trial. One way to enhance the ability to resist the infection is to activate the nonspecific immunity using a suitable driving-up agent, such as plant polysaccharides, particularly our drug Panavir isolated from the potato shoots. Earlier, we have shown the noticeable anti-viral and anti-bacterial activity of Panavir. Here we demonstrate the pro-inflammation activity of Panavir, which four-to-eight times intensified the ATP and MIF secretion by HL-60 cells. This effect was mediated by the active phagocytosis of the Panavir particles by the cells. We hypothesized the physiological basis of the Panavir proinflammatory activity is mediated by the indol-containing compounds (auxins) present in Panavir and acting as a plant analog of serotonin.
Keywords: Antiviral activity, Immune modulator, COVID-19
1. Introduction
The COVID-19 pandemic caused by the SARS-CoV-2 virus clearly showed that humanity does not yet have a reliable and effective means of rapidly responding to such global challenges. The standard protocol includes:
-
1)
enforcing quarantine and using personal protective equipment;
-
2)
development of the diagnostic tools and specific treatment;
-
3)
development of vaccines against a new pathogen.
The effectiveness and safety of the fastest measures (the first item in the list above) raise fierce debates, and the arguments of the opponents are rather valid. The rest of the items above take time, whereas a pandemic picks up the speed and can cause irreparable damage to society. The RNA viruses, including SARS-CoV-2, are highly variable, so the expenditures on developing novel means of specific prevention and treatment may become too significant [1]. Moreover, the development of an effective vaccine against many RNA viruses (including coronaviruses) could be additionally complicated by the effect of antigenic imprinting or the phenomenon of “original antigenic sin” (OAS, [[2], [3], [4]]) and the “antibody-dependent enhancement” (ADE, [[5], [6], [7], [8]]). The most striking example demonstrating the complexity of this problem is the time humanity has already spent on creating an effective vaccine against HIV infection and has not yet been able to create it, although 36 years have passed since discovering this virus. The Ebola vaccine was registered and recognized by WHO only in 2019, 43 years after discovering the virus. Also, specialists have made little progress in the development of a vaccine against the respiratory syncytial virus, despite the huge investment [1].
One of the approaches to solve the designated global problem is creating low-toxic agents for nonspecific activation of innate immunity. The latter would accelerate the primary response development and reduce the likelihood of the severe course of the disease. We assume the possibility of the targeted pharmacological impacting the innate immunity, which can transfer it to a certain hypothetical “state of increased responsibility” (SINRES). The innate immunity cells, in particular natural killer cells, can acquire the immune memory similarly to the adaptive immunity cells and enhance their activity in response to the antigenic stimulation [[9], [10], [11]]. Such trained or provoked immune cells could serve as a basis of the SINRES state [12,13], which is, however, was never introduced as a separate notion earlier.
We have previously shown that the particles of a high-molecular-weight polysaccharide extracted from potato shoots (Panavir) have a broad spectrum of antiviral activity [[14], [15], [16]]. The antiviral activity was demonstrated against the broad spectrum of germs, including both DNA and RNA-containing viruses, such as, for example, animal coronaviruses – the close relatives of SARS-CoV-2, and also against influenza virus, herpes, cytomegalovirus, tick-borne encephalitis virus, human papillomavirus, and the virus of hepatitis C. Such a broadness of the pharmacological activity of the drug forces to search its action mechanism among the non-specific immunological reactions.
Until now, we have considered the main mechanism of Panavir's antiviral activity to be caused by its ability to stimulate interferons' secretion, which are one of the coordinating factors of innate immunity [17]. Undoubtedly, this property is one of the key ones since the severity of pathologies caused by viral infections directly correlates with the defects in the interferon system, which has recently been shown in patients with COVID-19 [18,19]. Besides that, Panavir demonstrates neuroprotective and antibacterial activity and also suppresses autoimmune distresses [16], which is difficult to explain by the induction of interferons alone. At the same time, such a variety of pharmacological effects should be associated with some fundamental mechanism of the innate defense, where the infection, apparently, is one of, but not the only, potentially dangerous factor. Such a fundamental mechanism could be phagocytosis, as well as cascades of immunological reactions triggered by extracellular ATP and MIF exocytosis [20,21]. Moreover, these processes should occur primarily in the epithelial cells of the respiratory tract – the gateway to respiratory infections. A pool of mediators of the nonspecific immune response should form in these cells specifically to attract the nonspecific immunity cells, which would further participate in suppressing a viral infection [22].
2. Methods
2.1. Panavir
Panavir is a high-molecular-weight (~3 ⋅ 109 Da) fraction of the polysaccharides extracted from Solanum tuberosum (potato) shoots. The substance was isolated from the ground meristematic tissue by washing and subsequent filtration of the obtained liquid.
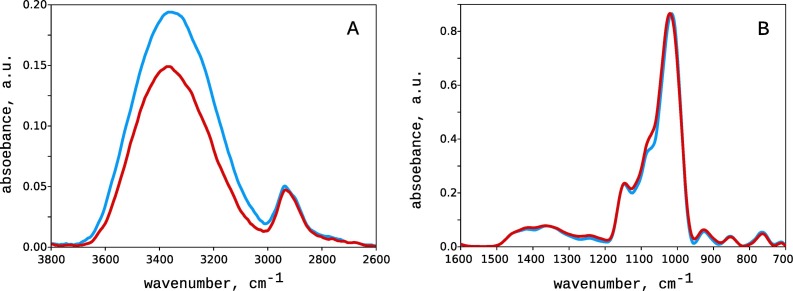
The Panavir particles have near a spherical shape with a diameter of about 300 nm. The electrokinetic (zeta) potential of the particles is negative and equals -25 mV. The chemical composition of Panavir is rather variable: glucose (10–67%), galactose (2–27%), arabinose (3–15%), rhamnose (2–10%), mannose (0.1–5%), and xylose (0.1–3%). It also contains some uronic acids (2–5%), traces of lipids, peptides, and proteins, first of all, RuBisCo (less than 1 % in total). The IR spectrum of Panavir is very close to the one of the starch as it is defined mainly by the hexoses (Fig. 1 ). The most intense spectral bands were 1150 cm-1 (C–O–C asymmetrical stretching), 1079 cm-1 (C–O bending and CH2 related modes), 1024 cm-1 (C–OH stretching vibration), and 928 and 854 cm-1 (C–O–C symmetrical stretching and C–H deformation). The spectrum also contained the broadened bands in the range of 1450–1200 cm-1, which can be attributed to deformation C–H bonds, asymmetric vibration in CH2 group, and C=O bond stretching. The absence of the iodine coloring indicates the cross-linking of the hexose polymers, which prevents the formation of the channel-type clathrates responsible for the characteristic blue coloring [15,23]. The X-Ray spectra of the dried Panavir demonstrated only amorphous halo without any signs of ordering (data not shown.
Fig. 1.
Infra-Red spectra of Panavir (red) and starch (blue). For clarity, the spectra were split into two regions of 3800–2600 cm-1 (A) and 1600–700 cm-1 (B). The arbitrary units in plots A and B are the same. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Cells cultivation
The HL-60 human myeloid leukemia cell line has been purchased from the Hungarian Cell Bank, Paseur Institute of Hungary, Szeged, NCBI Code C427. Cells were maintained in suspension culture at +37 °C under 5% CO2/air in RPMI 1640 (Gibco, UK) supplemented with 10% FCS and antibiotics: 100 U/ml of Penicillin and 100 μg/ml of Streptomycin. The cells were subcultured three times weekly, ATRA (Sigma, USA). This procedure has been originally adopted by Olins et al. [24] and then modified by Roy et al. [25].
The primary human nasal epithelial cells (NHEpC) isolated from healthy human nasal mucosa, male donors 18–25 years old (PromoCell, GmbH, Germany, Cat # C-14062) were also cultivated in standard conditions [26].
2.3. Phagocytic activity
Experiments were carried out using both original, immature HL-60 cells and mature neutrophils obtained by treating the HL-60 culture with 1.25% DMSO for 96 h [27,28]. Panavir solution in bidistilled water was added to the cell culture suspension (20–30 million cells per ml) and incubated for 2, 6, 12, and 24 h. The combined action of Parnavir and ATP was also investigated. The reagents were added into the culture medium simultaneously, and the ATP final concentration was 0.5 μg/ml. After the incubation, the cells were harvested by centrifugation (20 000 rpm, 20 min, 4 C) and rinsed with 50 mM PBS (pH 8.2). Then the cells were lysed with 2% Triton X-100 solution (10 min, 4 C) and subjected to ultracentrifugation (150 000 g, Beckman Coulter Optima L-90K, SW-27 rotor, 2 h, 4 °C).
The cytosol fraction (S150) was collected and treated with a mixture of nucleases: S1 nuclease (20 U/ml, Serva Heidelberg, Germany) and RNase A (5 μg/ml, Serva Heidelberg, Germany) for 40 min (37 C, pH 7.7). Then, 100 μl of a mixture of two proteases: proteinase K (10 μg/ml, Serva Heidelberg, Germany) and trypsin (10 μg/ml, Serva Heidelberg, Germany) was added to the obtained lysate and incubated for 60 min (37 °C, pH 8.0). The resulting lysate was ultrafiltered on a Diaflo Y5.0 filter at 800 psi (Amicon SQ600, Merck Millipore, USA). The obtained high molecular weight fraction resistant to nucleases and proteases was solved in 20 mM of Tris/EDTA buffer (20 mM/1.5 mM, pH 8.2) and analyzed calorimetrically. The volume of the obtained specimens was measured with an accuracy of ±1% by Microliter 700 syringes, Hamilton, USA. The protein concentration in the initial cytosol fraction was determined by the Bradford method [29].
2.4. Panavir concentration
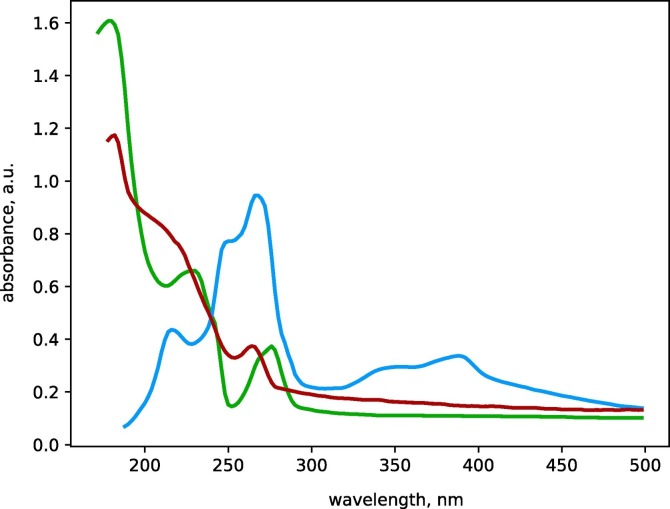
The absorption spectrum of Panavir has a characteristic peak in the region of 190 nm (Fig. 2 ), which allows calorimetric measuring of concentration. The Panavir extinction coefficient was determined for Panavir solutions in Tris/EDTA buffer (20 mM/1.5 mM, pH 8.2) using a Lambda 1050 spectrophotometer (Perkin Elmer, USA) in the concentration range of 100–1200 ng/ml. For the extinction coefficient we have obtained the value of (3.28 ± 0.09) ⋅ 103 ml/(ng⋅cm). To determine the Panavir concentration, we have compared the optical densities of the high molecular weight (HMW) and nuclease/protease-resistant fraction of the cells' cytoplasm obtained from cultures treated and not treated with Panavir. The difference in optical densities at 190 nm was fully attributed to Panavir particles consumed by the cells.
Fig. 2.
Area-normalized absorption spectra of an aqueous solution of Panavir (green), cytosolic fraction of HL-60 cells (blue) and HMW fractions of cytosol of HNEpC cells treated with Panavir (red). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.5. ATP release
After incubation of the cells with Panavir, ATP primer was added to the medium to a concentration of 2.5 mM, together with succinate (1.5 mM), and 32P-orthophosphate (1.0 mM) [30]. The samples were incubated for 60 min (37 °C), and immediately after the end of the incubation, they were mixed with ice-cold Triton X100 (2.0%, v/v)/10 mM Tris-HCl (pH 7.45) and incubated for 2 h. The pool of low-molecular-weight compounds was extracted with ice-cold acetone (10 v/v), while the debris was precipitated by centrifugation (20000 rpm, 20 min, QS70, Sorvall, USA). The extraction was repeated three times. The obtained extract was separated by HPLC: stationary phase ODS-S5CN, 10–60% linear gradient of pyridine based on 10% methanol, Altex-1800 column 15x280 mm, 22 °C, 2000 psi, 2.0 A254 in 50 μl of injected sample (Gilson W100 UV254 detecting HPLC System). ATP peaks were collected for further measurement of 32P concentration in a Koch Light Beta SL20 dioxane scintillator using a JR880 liquid scintillation counter (Wallac, Finland).
2.6. DNA polymerase activity
The catalytic activity was measured by the method of Sakaguchi and Boyd [31] adapted for 0.15 ml incubation volume: 50 mM Tris-HCl (pH 8.0)/8.0 mM dithiothreitol/15 mM MgCl2/15% glycerol (v/v)/27 μg act DNA, calf thymus/50 μg each of dATP, dCTP, dTTP, dGTP/0.25 μmole of [Methyl-1,2-3 H]dTTP (90–120 Ci/mmol, NET520A, NEN)/150 mM NaCl. The tritium-labeled nucleotide was purchased from New England Nuclear, USA. These compound concentration values were first optimized within both pH of 6.0–−9.0 and MgCl2 concentration of 5.0–50.0 mM. These mixture samples were pre-incubated at 37 °C for 60 min. Then 5.0–7.5 μg of the pure enzyme was added to each one of these running samples and they were incubated at 37 ° C for another 60 min. The ice-cold incubation samples as well as the trypsin treated samples (20 μg/mL trypsin, Merck GmbH, Germany, 37 °C) were taken as controls.
The post-incubation mixtures were subjected to the quantitative extraction of the DNA ultramicro–amounts using an AccuPrep Genomic DNA Extraction Kit (Bioneer Corp., Korea) as described by Mikami et al. [32] and modified by Haratian et al. [33]. The extracted DNA aliquots were used for electrophoretic determination of the DNApolβ-processed DNA chain sizes [34] and for [3H]-radioactivity measurements in Wallac 2200LX LS Counter (Wallac OY, Finland). The DNApolβ-specific catalytic activity values were expressed in [3H] cpmDNA/mg enzyme. The protein ultramicro amounts were estimated according to Fukami et al. [35]. The DNA ultramicro amounts measurements were performed in diluted water solutions as described by Müller et al. [36].
The kinetic constants, K M (mM) and Kcat ([μM dTTP/min]/mg enzyme), were estimated by the free dTTP pool depletion rates [37] measured using the HPLC analysis of acetone-soluble fractions of pre- and post-incubation mixtures: Altex 1800E (18 × 220 mm) column/ODS-S5CN stationary phase/mobile phase, 10–60% linear pyridine gradient based on 10% water-methanol/2 000 psi at 22 C/Waters DL600 HPLC Analytical System [37,38].
2.7. Indol-containing compounds
A total amount of the indol-containing compounds spread over the Panavir particles' surface was detected and quantitatively assessed using a multi-collector inductively coupled plasma mass spectrometer (Aurora M90 QS analytical system, Brucker, USA). The IUPAC MG410 software and database package were used to identify the amounts of the indol-derivatives.
3. Results and discussion
3.1. State of INcreased RESponsibility (SINRES)
It has been experimentally shown that the innate immunity cells, in particular natural killer cells, are able to acquire the immune memory similar to the adaptive immunity cells and subsequently enhance their activity [[9], [10], [11]]. de Laval et al. have shown that the “memory” of the nonspecific immunity can also be provided by myeloid cells (monocytes, macrophages, and neutrophils) demonstrating high phenotypic diversity and plasticity [39]. The latter is particularly characteristic for macrophages that acquire so-called “long-term antimicrobial skills” using epigenetic mechanisms that are fundamentally different from those triggering maturating of the adaptive immunity cells [12]. The cellular basis of such a “trained” immunity and heterologous protection against secondary infections consists in the functional reprogramming of the innate immune cells, which was first discovered in invertebrates [13]. Live attenuated vaccines (for example, BCG) or the pathogen cell's structures (such as beta-glucans or lipopolysaccharides) can provide for a long-term increase in the antimicrobial activity of the myeloid cells due to the conformational changes in chromatin packing altering the gene expression patterns [39]. Therefore, the stimulation of the innate immune cells can leave some kind of an “epigenetic scar”, a set of exposed enhancers and promoters of host defense genes. Each element of such a “scar”, due to its openness as a result of DNA methylation, can serve as the basis of the increased reactivity in the course of the realization of the acquired protection program [40]. Several epidemiological studies support these observations: for example, BCG has a pleiotropic effect reducing the incidence of viral infection [41].
The mechanisms described above can serve as the substrate for the development of SINRES. The SINRES state should provide for adequate immunological reactivity in response to any kind of infection, but at the same time not harm the organism, limiting its activity on the feedback principle base. Indeed, in order to maintain homeostasis, the on-time termination of the immune response is as important as its onset, which was clearly demonstrated by the COVID-19 pandemic. Long-term inflammatory processes going far beyond the adequate response have significant “intrinsic toxicity” and can cause tissue damage [42]. Certain elements of innate immunity, particularly macrophages, play a key role in restoring homeostasis in various pathological processes, both infectious and non-infectious diseases, such as oncological, autoimmune, neurodegenerative, chronic inflammatory bowel and lung diseases [[43], [44], [45]]. Therefore, it became reasonable to propose that, in the SINRES state, the body should be potentially able to resist any damaging factors, including viruses that cause viral respiratory infections, and should also be able to control chronic viral diseases.
3.2. ATP and MIF secretion
Cellular stress (caused, for example, by infection) leads to the release of ATP, ADP, and other nucleotides into the extracellular space [46,47]. Extracellular nucleotides activate the immune cells through the system of purinergic receptors [[48], [49], [50], [51], [52], [53]]. Purinergic receptors are present in the membranes of many cell types, particularly in the membranes of all immune, epithelial and endothelial cells. Thus, extracellular ATP can activate all the elements of the nonspecific (innate) immunity, including the antiviral immunity [54,55].
Extracellular nucleotides function as autocrine and paracrine signal activating P2 purinergic receptors that elicit pro-inflammatory immune responses, which is an acute inflammation phase lasting minutes to hours. Over time, extracellular nucleotides are metabolized to adenosine, which leads to a decrease in the signal from P2 and an increase in the signal through the anti-inflammatory adenosine P1 purinergic receptors, which is a subacute phase lasting from hours to several days [20]. Relatively high concentrations of extracellular ATP (in the millimolar range) cause predominantly pro-inflammatory effects due to the activation of the low-affinity P2X7 receptor, while the low (the micromolar) ATP concentrations have a predominantly tolerogenic/immunosuppressive effect provided by the activation of the high-affinity P2Y11 receptor [56,57]. Therefore, the extracellular ATP can serve both as an initiator and as a terminator of immune responses.
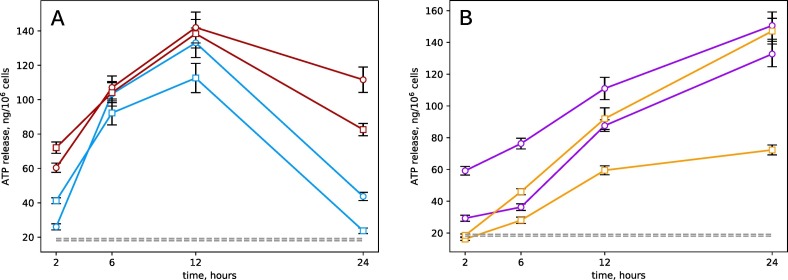
Following our expectations, treatment of the HL-60 cell culture with Panavir resulted in the secretion of ATP into the extracellular environment (Fig. 3 ). Note that this effect was observed both for mature neutrophils and for the initial HL-60 cells. The effect was observed both in the physiological range of Panavir concentrations (2.5–10 ng/ml, Fig. 3A) corresponding to the pharmacological dose of 200 μg for an adult [16] and at the elevated concentrations (Fig. 3B). In all cases, in the presence of Panavir, the ATP concentration in the extracellular medium was an order of magnitude higher than the background value of 18.5 ± 0.6 ng per million cells.
Fig. 3.
Release of ATP by immature (circles) and mature (squares) HL-60 cells under the influence of physiological (A) and elevated (B) Panavir concentrations of: 2.5 (blue), 10 (red), 100 (orange), and 1000 ng/ml (purple). The gray dotted lines show the background concentration of ATP in the culture medium. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
At the therapeutic concentration of Panavir, the extracellular ATP concentration reached the maximum at 12 h after treatment. Therefore, the characteristic time of the ATP-mediated effect of Panavir on the immune system cells can be assumed to be of at least one day. Note that the effect was 12 ± 0.6% more prominent for mature neutrophils than the immature HL-60 cells.
The extracellular ATP concentration in the epithelial cells of the nasal cavity (HNEpC) cell culture was 680 ± 50 ng per million cells, which is more than 4 times higher than the ATP concentration in the HL-60 cell culture. The HNEpC cells form the gateway for SARS-CoV-2 and other airborne infections, so their susceptibility to Panavir is noteworthy. On the other hand, the phagocytosis of foreign particles, for example, Panavir particles, can stimulate nonspecific immunity, which was previously described for many elements of the nonspecific immunity system [58]. The latter creates an opportunity for the SINRES state development and increasing the resistivity of the organism.
The average ATP content in HL-60 cells was 4.4 ± 0.2 fmol per cell [59]. Therefore, one million HL-60 cells contain about 4.4 nmol or 2.2 μg ATP. Thus, in our experiments, cells secreted into the environment something about 5–6% of the ATP contained in them, when treated with physiological concentrations of Panavir. This is a very significant amount, and such a loss of energy equivalents should affect the intensity of synthetic processes in the cell, which was discovered experimentally (Table 1 ). Treatment of the isolated nuclei of HL-60 cells with Panavir at high concentrations led to more than a two-fold decrease in nucleic acid synthesis rate.
Table 1.
The rate of DNA and RNA synthesis (3H cpm/mg DNA) by isolated nuclei of HL-60 cells.
| Sample | Synthesis rate |
|
|---|---|---|
| DNA | RNA | |
| Control | 70 021 ± 836 | 38 834 ± 630 |
| PNR, 0.2 μg/ml | 42 037 ± 588 | 16 321 ± 584 |
| PNR, 1.0 μg/ml | 31 221 ± 489 | 28 011 ± 489 |
A significant difference between HNEpC cells and immature HL-60 cells is the different activity of the gene encoding the macrophage migration inhibition factor (MIF), which is an evolutionarily ancient protein whose homologs were found in a wide range of organisms from vertebrates to cyanobacteria [21]. MIF is a multipotent protein implicated in the pathogenesis of many infectious and autoimmune diseases. Unlike many other cytokines that are secreted during antigenic stimulation, MIF is constantly expressed and stored in intercellular pools [21]. In general, MIF seems to be the most important regulator of many cellular processes, and its violation can lead to pathological states. For this reason, MIF is an obvious target for the study of potential therapeutic agents against infectious, inflammatory, and proliferative disorders [60,61]. Note, that MIF affects, particularly the response of immune-competent cells to viral and bacterial infection [62].
The intracellular synthesis of MIF in HNEpC cell culture treated with Panavir solution (10 ng/ml) was 1850 ± 60 versus 450 ± 50 pg per million cells in control. Thus, stimulation of epithelial cells of the nasal epithelium with Panavir can stimulate the activity of the immune cells, which in turn should reduce the time required for the development of the response.
3.3. Phagocytic activity
One of the crucial issues regarding the Panavir physiological action is the ability of rather large Panavir particles to penetrate the cells' cytoplasm. Considering the size of these particles' condensed nucleus equaling ∼150 nm [15], the most probable way of penetration should be phagocytosis.
In the immature HL-60 cell culture, 89% of the cells are myeloblasts and promyelocytes, and only 1% are premature banded neutrophils [27]. Treatment of the culture with DMSO and some other polar agents for 3–6 days leads to their maturation [27,28]. In the process of maturation, The intensity of the specific immune response, in particular the ability to phagocytosis, increases manyfold [27].
In our experiments, even immature HL-60 cells exhibited significant phagocytic activity towards Panavir particles at a high concentration (20 μg/ml), which in mature cells increased by only 40% (Table 2 ). Note that the treatment of both mature and immature cells with Panavir in the presence of ATP leads to an additional increase in phagocytic activity by another 40–50%, which is no longer associated with cell maturation, but is a physiological response to the presence of purine trinucleotides in the medium [[48], [49], [50], [51], [52], [53]]. Since the treatment of HL-60 cells with Panavir induces ATP secretion, and ATP promotes phagocytosis, there is a positive feedback loop that enhances the immunostimulatory effect of Panavir.
Table 2.
Panavir and Ge nanoparticles uptake (particles number per cell) by immature and DMSO maturated HL-60 cells.
| Sample | Panavir |
Ge |
|
|---|---|---|---|
| Mature | Immature | ||
| Control | Not detected | Not detected | Not detected |
| Panavir (2.5 ng/ml) | No data | 1.2 ± 0.1 | Not detected |
| Panavir (20 μg/ml) | 9.6 ± 1.0 | 6.8 ± 0.8 | No data |
| Panavir (20 μg/ml) +ATP (0.5 μg/ml) | 11.5 ± 1.0 | 10.7 ± 1.0 | No data |
| Ge (0.1 μg/ml) | No data | Not detected | 0.20 ± 0.01 |
| Ge (0.1 μg/ml) +Panavir (2.5 ng/ml) | No data | 4.8 ± 0.5 | 0.22±0.02 |
Similar results were obtained when the culture of mature HL-60 cells was treated with neutral germanium particles (diameter of about 400 nm), which, like the Panavir particles, were consumed by the cells. Moreover, the presence of large germanium particles in the medium leads to a two-to-five fold increase in the phagocytosis of Panavir particles, depending on the concentration (Table 2). The opposite effect was also observed, but it was much less explicit: comparatively smaller Panavir particles enhanced the phagocytosis of germanium particles by only 14%. It is remarkable that when treated with Panavir at a concentration of 2.5 ng/ml, HNEpC cells consumed the drug particles more actively than HL-60 cells, and on average, absorbed 3.8 ± 0.2 particles per cell.
3.4. Indole containing Panavir components
The chemical composition of the Panavir particles inherits some features of the plant tissue they were isolated from (potato shoots' meristem, [15]). Actively dividing meristematic tissues act as a source of auxins, plant hormones regulating almost all aspects of plant growth and development, including cell growth and differentiation [63,64]. Auxins are indole-containing compounds, which allows them to be considered as chemical analogs of serotonin [65], which was also found in plant tissues. The functions of plant serotonin have not been precisely established, but it was reported that serotonin is a functional inhibitor of auxin and involved in roots differentiation [65].
In mammals and humans, serotonin is involved in the processes of allergy and inflammation, increases vascular permeability, enhances chemotaxis, increases the migration of leukocytes to the inflammation areas, increases the content of eosinophils in the blood, enhances the degranulation of mast cells, and the release of other mediators of allergy and inflammation [66]. Thus, the immunostimulating properties of Panavir could appear due to the presence of plant analogs of serotonin (auxins and other indole-containing compounds).
According to the results of plasma mass spectrometry, the amount of indole-containing groups on the surface of Panavir particles was 38.2 ± 2.6 μg per gram of Panavir. Taking into account the molecular weight of Panavir particles (~3 GDa [15]), the number of indole groups on the surface of a single particle would be about 300 units. Therefore, in one therapeutic dose of Panavir (∼200 μg), the number of such groups would be ∼10−11 mol, and their concentration in human blood after Paravir application would be about 2 ⋅ 10−9 g/l or about 10−11 M. This value is five orders of magnitude lower than the native physiological concentration of serotonin, which is about 150–200 ng/ml or (1.3–1.8)⋅10−6 M [9]. However, if at least one Panavir particle enters the cell, the concentration of indole groups inside the cell appears to be a thousand times greater (∼10−8 M), which is already comparable to the physiological concentrations of serotonin. The assessment made implies that the proposed biological activity of indole radicals, which can be released during intracellular degradation of the particles, cannot be excluded when analyzing the Panavir physiological effects. Therefore, here we observe the interaction between the purinergic and serotonergic systems explicitly, enhancing the positive feedback effect of inflammation and the suppression of infection.
3.5. Toxicity and effectiveness of Panavir
In mice, LD50 of Panavir for intraperitoneal injection is 1240 mg/kg, which makes it possible to classify Panavir as a low-toxic drug [67]. In the studied therapeutic doses, Panavir does not exhibit allergic, local irritating, teratogenic, embryo-toxic, and mutagenic effects. The therapeutic index (the ratio of LD50 to ED50) of Panavir is as much as 413 000 [67]. Consequently, Panavir has one of the broadest “therapeutic windows” among registered drugs. Such a large therapeutic index is not common in classical pharmacology since low-toxic drugs usually have low therapeutic activity. However, in the case of Panavir, the drug is represented by rather large nanoparticles, and the therapeutic dose (200 μg) of such particles contains only about 1010 of them, which is a million times less than the values typical for conventional, low-molecular-weight drugs. This value corresponds to a concentration of 10−13 M and refers to the range of ultra-low doses of biologically active substances, usually demonstrating extremely low toxic effects [68]. However, many drugs in ultra-low doses still demonstrate significant therapeutic and/or physiological effects [68].
4. Conclusion
Summarizing the obtained results, we can specify several major points. The physiological activity of Panavir on the cellular level is mediated by the phagocytosis of its nanoparticles (by HL-60 cells in our experiments). We tend to consider the act of phagocytosis as a primary event providing for all of the consequent effects. The phagocytosis of the Panavir particles is followed by the active ATP and MIF exocytosis that in its turn activates all kinds of immune cells and mature neutrophils, in particular, and intensifies the phagocytosis. This interrelation makes for the positive feed-back loop necessary for the development of any significant physiological effect.
At the intracellular level, the physiological activity of Panavir can be associated with the metabolism of the indol-containing auxins discovered in the Panavir particles and usually abundant in actively dividing meristematic plant cells. Auxins and their metabolites can possess a serotonin-like activity, which explains the observed pro-inflammatory activity of Panavir. Therefore, the supramolecular nature of the Panavir represented by rather large (several MDa) particles becomes essential as smaller particles would not be effectively phagocyted, while the tissue of Panavir origin becomes crucial due to the high auxins level usual for meristems.
The two factors described above fortunately combined in Panavir and made it a good driving-up agent for the immune system. The non-specific stimulation of the immune system could induce a so-called SINRES state (State of INcreased RESponsibility), forcing the immune system to form sharper responses to any kind of antigenic stimulation compared to the “normal” state [[9], [10], [11], [12]]. We propose the transfer of the immune system to the SINRES state could be an effective way of resisting the infections allowing decreasing the incidence and gaining some time on elaboration and production of the targeted drugs. Such kind of immuno-modulating drugs seems to be of particular importance in the cases of pandemics such as modern COVID-19.
CRediT authorship contribution statement
Sergey V. Stovbun: Conceptualization, Reviewing and Editing;
Tatiana S. Kalinina: Writing, Indol-containing compounds investigation;
Dmitry V. Zlenko: Writing, visualization;
Aleksei V. Kiselev: Panavir isolation and purification;
Alexander A. Litvin: Conceptualization;
Alexander A. Bukhvostov: Cell culture experiments;
Sergey V. Usachev: FT IR experiments;
Dmitry A. Kuznetsov: Conceptualization;
Footnotes
The work was completed as a part of the state assignment (theme number AAAA-A20-120013190076-0).
References
- 1.Mullard A. COVID-19 vaccine development pipeline gears up. Lancet. 2020;395:1751–1752. doi: 10.1016/S0140-6736(20)31252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma J., Dushoff J., Earn D.J.D. Age-specific mortality risk from pandemic influenza. J. Theor. Biol. 2013;288:29–34. doi: 10.1016/j.jtbi.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Kim J.H., Skountzou I., Compans R., Jacob J. Original antigenic sin responses to influenza viruses. J. Immunol. 2009;183:3294–3301. doi: 10.4049/jimmunol.0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsons M.S., Muller S., Kohler H., Grant M.D., Bernard N.F. On the benefits of sin. Can greater understanding of the 1F7-idiotypic repertoire freeze enhance hiv vaccine development? Human Vaccines & Immunotherapeutics. 2013;9:1532–1538. doi: 10.4161/hv.24460. [DOI] [PubMed] [Google Scholar]
- 5.Tirado S.M.C., Yoon K.-J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16:69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- 6.Halstead S.B., Mahalingam S., Marovich M.A., Ubol S., Mosser D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. Lancet Infect. Dis. 2010;10:712–722. doi: 10.1016/S1473-3099(10)70166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takano T., Kawakami C., Yamadaa S., Satoh R., Hohdatsu T. Antibody-dependent enhancement occurs upon re-infection with the identical serotype virus in feline infectious peritonitis virus infection. J. Vet. Med. Sci. 2008;70:1315–1321. doi: 10.1292/jvms.70.1315. [DOI] [PubMed] [Google Scholar]
- 8.Yip M.S., Leung N.H.L., Cheung C.Y., Li P.H., Lee H.H.Y., Daëron M., Peiris J.S.M., Bruzzone R., Jaume M. Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol. J. 2014;11:82. doi: 10.1186/1743-422X-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Gonzalez I., Mathä L., Steer C.A., Ghaedi M., Poon G.F.T., Takei F. Allergen-experienced group 2 innate lymphoid cells acquire memory-like properties and enhance allergic lung inflammation. Immunity. 2016;45:198–208. doi: 10.1016/j.immuni.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 10.O'Leary J.G., Goodarzi M., Drayton D.L., von Andrian U.H. T cell and B cell-independent adaptive immunity mediated by natural killer cells. Nature Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 11.Sun J.C., Beilke J.N., Lanier L.L. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netea M.G., Domínguez-Andres J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L.A.B., van der Meer J.W.M., Mhlanga M.M., Mulder W.J.M., Riksen N.P., Schlitzer A., Schultze J.L., Benn C.S., Sun J.C., Xavier R.J., Latz E. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz J., Franz K. Innate defence: evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. doi: 10.1038/425037a. [DOI] [PubMed] [Google Scholar]
- 14.Stovbun S., Berlin A., Mikhailov A., Sergienko V., Govorun V., Demina I., Kalinina T. Physicochemical properties of high-molecular-weight plant polysaccharide of hexose glycoside class (Panavir) with antiviral activity. Nanotechnologies in Russia. 2012;7:539–543. [Google Scholar]
- 15.Stovbun S.V., Yakovenko L.V. The physicochemical basis of the biological activity and pharmacological properties of the antiviral agent Panavir. Mosc. Univ. Phys. Bull. 2014;69:542–547. [Google Scholar]
- 16.Kalinina T.S., Zlenko D.V., Kiselev A.V., Litvin A.A., Stovbun S.V. Antiviral activity of the high-molecular-weight plant polysaccharides. Panavir®Int. J. Biol. Macromol. 2020;161:936–938. doi: 10.1016/j.ijbiomac.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolbukhina L., Nosik N., Merkulova L., Braginskii D., Lavrukhina L., Kalinina T., Stovbun S., Litvin A., Sergienko V. Time course of leukocyte interferon induction after single and repeated application of Panavir. Cytokines and Inflammation. 2009;8:49–52. [Google Scholar]
- 18.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., et al. The genetics underlying severe COVID-19. Science. 2020;370:eabd4585. [Google Scholar]
- 19.Zhang Q., Bastard P., Liu Z., Pen J.L., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cekic C., Linden J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 21.Calandra T., Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picher M. Springer; Dordrecht, Germany: 2011. Purinergic Regulation of Respiratory Diseases. [Google Scholar]
- 23.Stovbun S., Safronov D., Kucherov V., Farzaliev T., Chekameyeva V. Anti-inflamatory Panavir effect in simulation experiments and clinical practice. Bulletin MSRU. 2011;3:82–85. [Google Scholar]
- 24.Olins A.L., Herrmann H., Lichter P., Olins D.E. Retinoic acid differentiation of HL-60 cells promotes cytoskeletal polarization. Exp. Cell Res. 2000;254:130–142. doi: 10.1006/excr.1999.4727. [DOI] [PubMed] [Google Scholar]
- 25.Roy M.K., Thalang V.N., Trakoontivakorn G., Nakahara K. Mechanism of mahanine-induced apoptosis in human leukemia cells. HL-60Biochem. Pharmacol. 2004;67:41–51. doi: 10.1016/j.bcp.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Schagen J., Sly P.D., Fantino E. Characterizing well-differentiated culture of primary human nasal epithelial cells for use in wound healing assays. Lab. Investig. 2018;98:1478–1486. doi: 10.1038/s41374-018-0100-1. [DOI] [PubMed] [Google Scholar]
- 27.Collins S., Ruscetti F., Gallagher R., Gallo R. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc. Natl. Acad. Sci. U. S. A. 1978;75:2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F., Gallo R. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–733. [PubMed] [Google Scholar]
- 29.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Buchachenko A.L., Kouznetsov D.A., Arkhangelsky S.E., Orlova M.A., Markarian A.A. Spin biochemistry. Magnetic 24mg – 25mg – 26mg isotope effect in mitochondrial ADP phosphorylation. Cell Biochem. Biophys. 2005;43:243–251. doi: 10.1385/CBB:43:2:243. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi K., Boyd J.B. Purification and characterization of a DNA polymerase beta from Drosophila. J. Biol. Chem. 1985;260:10406–10411. [PubMed] [Google Scholar]
- 32.Mikami T., Satoh N., Hatayama I., Nakane A. Buthionine sulfoximine inhibits cytopathic effect and apoptosis induced by infection with human echovirus 9. Arch. Virol. 2004;149:1117–1128. doi: 10.1007/s00705-003-0283-6. [DOI] [PubMed] [Google Scholar]
- 33.Haratian K., Shahrabadi M.S., Sardari S. Buthionine sulfoximine inhibits cytopathic effects and apoptosis induced by infection with AIK-HDC strain of measles virus. Iran Biomedical Journal. 2007;11:229–235. [PubMed] [Google Scholar]
- 34.Reichmann M.E., Rice S.A., Thomas C.A., Doty P. A further examination of the molecular weight and size of desoxypentose nucleic acid. Proc. Natl. Acad. Sci. U. S. A. 1954;76:3047–3053. [Google Scholar]
- 35.Fukami T., Uchiyama K., Yoshimura Y., Watanabe T., Nakazawa H. Ultramicro-analysis by use of light-scanning photoacoustic densitometry for electrophoresed protein in human hair. Anal. Biochem. 1996;238:60–64. doi: 10.1006/abio.1996.0251. [DOI] [PubMed] [Google Scholar]
- 36.Müller W.E., Obermeiera J., Totsuka A., Zahn R.K. Influence of template inactivators on the binding of DNA polymerase to DNA. Nucleic Acids Res. 1974;1:63–74. doi: 10.1093/nar/1.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varfolomeyev S.D. Chemical and Biological Kinetics. Part 2: Biological Kinetics Chemistry Publ. Moscow. 2005. Enzymatic catalysis: kinetics, catalytic site structures, bioinformatics. [Google Scholar]
- 38.Kuznetsov D.A., Govorkov A.V., Zavijalov N.V., Sibileva T.M., Richter V., Drawczek J.A. Fast estimation of ATP/ADP ratio as a special step in pharmacological and toxicological studies using the cell-free translation systems. J. Biochem. Biophys. Methods. 1986;13:53–56. doi: 10.1016/0165-022x(86)90008-4. [DOI] [PubMed] [Google Scholar]
- 39.de Laval B., Maurizio J., Kandalla P.K., Brisou G., Simonnet L., Huber C., Gimenez G., Matcovitch-Natan O., Reinhardt S., David E., Mildner A., Leutz A., Nadel B., Bordi C., Amit I., Sarrazin S., Sieweke M.H. C/EBPβ-dependent epigenetic memory induces trained immunity in hematopoietic stem cells. Cell Stem Cell. 2020;26:657–674. doi: 10.1016/j.stem.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani A., Netea M.G. Trained innate immunity, epigenetics, and COVID-19. N. Engl. J. Med. 2020;383:1078–1080. doi: 10.1056/NEJMcibr2011679. [DOI] [PubMed] [Google Scholar]
- 41.Escobar L.E., Molina-Cruz A., Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019. COVID-19Proc. Natl. Acad. Sci. U. S. A. 2020;117:17720–17726. doi: 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorini S., Gatta L., Pontecorvo L., Vitiello L., la Sala A. Regulation of innate immunity by extracellular nucleotides. American Journal of Blood Research. 2013;3:14–28. [PMC free article] [PubMed] [Google Scholar]
- 43.Byrne A.J., Mathie S.A., Gregory L.G., Lloyd C.M. Pulmonary macrophages: key players in the innate defence of the airways. Thorax. 2015;70:1189–1196. doi: 10.1136/thoraxjnl-2015-207020. [DOI] [PubMed] [Google Scholar]
- 44.Murray P.J., Wynn T.A. Obstacles and opportunities for understanding macrophage polarization. J. Leukoc. Biol. 2011;89:557–563. doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbracchio M.P., Burnstock G., Verkhratsky A., Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends in Neurosciences. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Corriden R., Insel P.A. Basal release of atp: an autocrine-paracrine mechanism for cell regulation. Science Signaling. 2010;3:re1. doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bours M., Swennen E., Virgilio F., Cronstein B., Dagnelie P. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci. Signal. 2009;2 doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 50.Junger W.G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barletta K.E., Ley K., Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler. Thromb. Vasc. Biol. 2012;32:856–864. doi: 10.1161/ATVBAHA.111.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virgilio F.D., Vuerich M. Purinergic signaling in the immune system. Auton. Neurosci. 2015;191:117–123. doi: 10.1016/j.autneu.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Dosch M., Gerber J., Jebbawi F., Beldi G. Mechanisms of ATP release by inflammatory cells. Int. J. Mol. Sci. 2018;19:1222. doi: 10.3390/ijms19041222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Passos D.F., Schetinger M.R.C., Leal D.B. Purinergic signaling and human immunodeficiency virus/acquired immune deficiency syndrome: from viral entry to therapy. World Journal of Virology. 2015;4:285–294. doi: 10.5501/wjv.v4.i3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrari D., Idzko M., Müller T., Manservigi R., Marconi P. Purinergic signaling: a new pharmacological target against viruses? Trends Pharmacol. Sci. 2018;39:926–936. doi: 10.1016/j.tips.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Boeynaems J.-M., Communi D. Modulation of inflammation by extracellular nucleotides. J. Investig. Dermatol. 2006;126:943–944. doi: 10.1038/sj.jid.5700233. [DOI] [PubMed] [Google Scholar]
- 57.Virgilio F.D., Boeynaems J.-M., Robson S.C. Extracellular nucleotides as negative modulators of immunity. Curr. Opin. Pharmacol. 2009;9:507–513. doi: 10.1016/j.coph.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gordon S. Phagocytosis: an immunobiologic process. Immunity. 2016;44:463–475. doi: 10.1016/j.immuni.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 59.Kuzmits R., Rumpold H., Müller M.M., Schopf G. The use of bioluminescence to evaluate the influence of chemotherapeutic drugs on ATP-levels of malignant cell lines. J. Clin. Chem. Clin. Biochem. 1986;24:293–298. doi: 10.1515/cclm.1986.24.5.293. [DOI] [PubMed] [Google Scholar]
- 60.Stosic-Grujicic S., Stojanovic I., Nicoletti F. MIF in autoimmunity and novel therapeutic approaches. Autoimmunity Review. 2009;8:244–249. doi: 10.1016/j.autrev.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 61.Trivedi-Parmar V., Jorgensen W.L. Advances and insights for small molecule inhibition of macrophage migration inhibitory factor. Journal of Medical Chemistry. 2018;61:8104–8119. doi: 10.1021/acs.jmedchem.8b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bloom J., Sun S., Al-Abed Y. MIF, a controversial cytokine: a review of structural features, challenges, and opportunities for drug development. Expert Opin. Ther. Targets. 2016;20:1463–1475. doi: 10.1080/14728222.2016.1251582. [DOI] [PubMed] [Google Scholar]
- 63.Vanneste S., Friml J. Auxin: a trigger for change in plant development. Cell. 2009;136:1005–1016. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Brumos J., Robles L.M., Yun J., Vu T.C., Jackson S., Alonso J.M., Stepanova A.N. Local auxin biosynthesis is a key regulator of plant development. Dev. Cell. 2018;47:306–318. doi: 10.1016/j.devcel.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 65.Pelagio-Flores R., Ortiz-Castro R., Mendez-Bravo A., Macias-Rodriguez L., Lopez-Bucio J. Serotonin, a tryptophan-derived signal conserved in plants and animals, regulates root system architecture probably acting as a natural auxin inhibitor in Arabidopsis thaliana. Plant Cell Physiol. 2011;52:490–508. doi: 10.1093/pcp/pcr006. [DOI] [PubMed] [Google Scholar]
- 66.Wu H., Denna T.H., Storkersen J.N., Gerriets V.A. Beyond a neurotransmitter: the role of serotonin in inflammation and immunity. Pharmacology Research. 2019;140:100–114. doi: 10.1016/j.phrs.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 67.Stovbun S.V., Kiselev A.V., Zanin A.M., Kalinina T.S., Voronina T.A., Mikhailov A.I., Berlin A.A. Effects of physicochemical forms of phenazepam and panavir on their action at ultra-low doses. Bull. Exp. Biol. Med. 2012;153:455–458. doi: 10.1007/s10517-012-1739-z. [DOI] [PubMed] [Google Scholar]
- 68.Molodavkin G.M., Voronina T.A., Chernyavskaya L.I., Burlakova E.B., Khorseva N.I., Seredenin S.B. Pharmacological activity of phenazepam and flunitrazepam in ultralow doses. Bull. Exp. Biol. Med. 2003;135:39–41. doi: 10.1023/a:1024710225445. [DOI] [PubMed] [Google Scholar]